Graphical abstract
Keywords: Pandemic, SARS-CoV-2, Medicaments, Emerging micropollutants, Environmental pollution
Abstract
On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a pandemic. The outbreak caused a worldwide impact, becoming a health threat to the general population and its professionals. To date, there are no specific antiviral treatments or vaccines for the COVID-19 infection, however, some drugs are being clinically tested. The use of these drugs on large scale raises great concern about their imminent environmental risk, since the elimination of these compounds by feces and urine associated with the inefficiency of sewage treatment plants in their removal can result in their persistence in the environment, putting in risk the health of humans and of other species. Thus, the goal of this work was to conduct a review of other studies that evaluated the presence of the drugs chloroquine, hydroxychloroquine, azithromycin, ivermectin, dexamethasone, remdesivir, favipiravir and some HIV antivirals in the environment. The research indicated the presence of these drugs in the environment in different regions, with concentration data that could serve as a basis for further comparative studies following the pandemic.
1. Introduction
In December 2019, a new coronavirus (COVID-19), correlated to respiratory illnesses in humans, was detected as the cause of pneumonia and death in the city of Wuhan, China (V. Kumar et al., 2020a, 2020b). On March 7, 2020, the World Health Organization (WHO) published a consolidated package of existing guidelines covering preparedness, readiness and response actions for different transmission scenarios. On March 11, 2020, WHO declared COVID-19 a pandemic, fact followed by an increase of more than tenfold in cases in less than a month, and according to a situation report (W.H.O., 2020) in early April of the same year more than 1 million cases of COVID-19 had already been confirmed worldwide. The outbreak has had a major environmental and socioeconomic impact and has become a health threat to the population and health workers (V. Kumar et al., 2020a, 2020b). Rotating data as of November 13th, 2020 confirmed 49,106,931 cases of COVID-19, out of which 1,239,157 had not survived the infection (Paital et al., 2020).
This infectious disease is caused by a new strain of CoV, a mutation (ID-19) of its two previous forms and is called SARS-CoV-2 or CoV-19 and the disease it causes is called COVID-19 (Jin et al., 2020). To date, there are no exact specific antiviral treatments or vaccines for the COVID-19 disease (M. Kumar et al., 2020a, 2020b). Therefore, to treat or alleviate its symptoms, some drugs are being clinically evaluated, including chloroquine (CQ) and hydroxychloroquine (HCQ) (Borba et al., 2020; Gao et al., 2020; Magagnoli et al., 2020; Tang et al., 2020), azithromycin (AZT) (Arshad et al., 2020; Dubernet et al., 2020), ivermectin (IVM) (Caly et al., 2020; Yang et al., 2020), dexamethasone (DEX) (Hassan et al., 2020; Johnson and Vinetz, 2020; Lester et al., 2020; Selvaraj, 2020) and some HIV antivirals (Agrawal et al., 2020; Alvi et al., 2020).
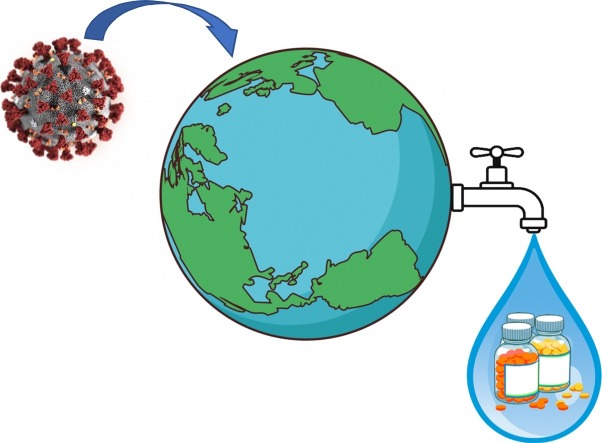
The increase in use of some of the clinically tested drugs in the treatment of COVID-19 during the pandemic has already been reported in some countries, such as Italy (Sartor et al., 2020) and Brazil (Martins et al., 2020) which raises concern of their usage on such a large scale, potentializing an important threat to the quality of water supply for human consumption (Kolpin et al., 2002; Kümmerer, 2009). Since most of these drugs are not fully metabolized by the body, they end up being eliminated through feces and urine in their active form or as metabolites (Heberer, 2002). Consequently, these compounds reach the sewage system and are sent to sewage treatment plants which mostly rely on conventional treatment systems, which have limitations in their removal and therefore cause them to end up in the water supply that, in many cases, are points of water capture from water treatment plants (WTP) for public consumption, which also have limitations in the removal of the compounds, leading them to consequently end up in tap water (Eggen et al., 2014; Gruchlik et al., 2018; Nippes et al., 2021; Rivera-Utrilla et al., 2013) (Fig. 1 ). Some of the compounds, which are currently being clinically tested in the treatment of COVID-19, have already been detected in the environment (Chen et al., 2013; Herrero et al., 2012; M. Kumar et al., 2020a, 2020b; Miège et al., 2009; Olatunde et al., 2014; Syslová et al., 2019).
Fig. 1.
Environmental risk of large-scale use of pharmaceutical drugs.
From the same environmental perspective, different considerations must be made for the different drugs used in the therapies of COVID-19, since the future impacts associated with the disease are issues that need to be well understood scientifically. The real concern related to the use of these drugs and their environmental persistence, in analogy to the development of increasingly potent antibiotics, is the potential formation of resistant strains by chronic exposure (Loureiro et al., 2016). Consequently, this can have more adverse effects on human health than other classes of drugs (Jain et al., 2013). In addition, other disadvantages are represented by the low biodegradability of some compounds and their increased use during outbreaks of other infectious diseases (Russo et al., 2017; Funke et al., 2016; Hill et al., 2014).
As with the drug Tamiflu during the H1N1 outbreak, when there was a large increase in its consumption and, subsequently, an increase in the environmental concentrations of the drug and its metabolites (Chen et al., 2020, 2014), the same environmental risk can occur with the drugs now in question. According to M. Kumar et al. (2020a, 2020b), the intense search for effective drugs against the new coronavirus (SARS-CoV-2) has progressed worldwide, and several antivirals and antiparasitic drugs have been clinically tested in patients with COVID-19, which can lead to the disposal of these drugs into the environment, since these drugs and their metabolites are eliminated mainly by urine.
Thus, in order to raise awareness about this imminent problem, the goal of this work was to carry out a systematic review of the literature of the existing studies from 2006 to the present year, using the PRISMA methodology, for the evaluation of the main drugs being tested against COVID-19.
2. Methodology
The PRISMA methodology was used to carry out a systematic literature research (Moher et al., 2009), which included eligibility and exclusion criteria, only for studies on drug detection in the environment. Clinical and ecotoxicological studies were not included in the PRISMA methodology, as the first goals were to demonstrate the use of these drugs in the treatment of COVID-19, while the second ones were to emphasize problems that involve the environmental risks of their presence.
2.1. Sources of information and search
The systematic review process was carried out directly on the publications databases such as the Science Direct, Pubmed, Springer, ACS and Taylor & Francis, through scientific articles that evaluated the detection of drugs used in the treatment of COVID-19. In the systematic review, articles published from 2006 to the present year were included. The search included the keywords: azithromycin, chloroquine, hydroxychloroquine, ivermectin, dexamethasone, remdesivir, favipiravir, antivirals for HIV, water, sewage, sewage, effluents, river, soils, detection and environmental matrices. The searches were performed using the “AND” connector. After this stage, a set of inclusion and exclusion criteria was employed.
2.2. Eligibility and exclusion criteria
Eligibility criteria were applied to each publication, which consisted of the scope and availability of the data. Initially, the titles and abstracts of the articles were evaluated to see if the article included the detection of drugs in the environment. In this stage, review articles, letters to the editor, books, book chapters, book series, presentations, conference proceedings and articles in languages other than English were excluded. Research articles and papers on the development of drug detection methods have been included. After the first stage, information was extracted from each article, such as: first author, year of publication, study location, analyzed environment and detected available concentration values.
3. Results
3.1. Selection of studies
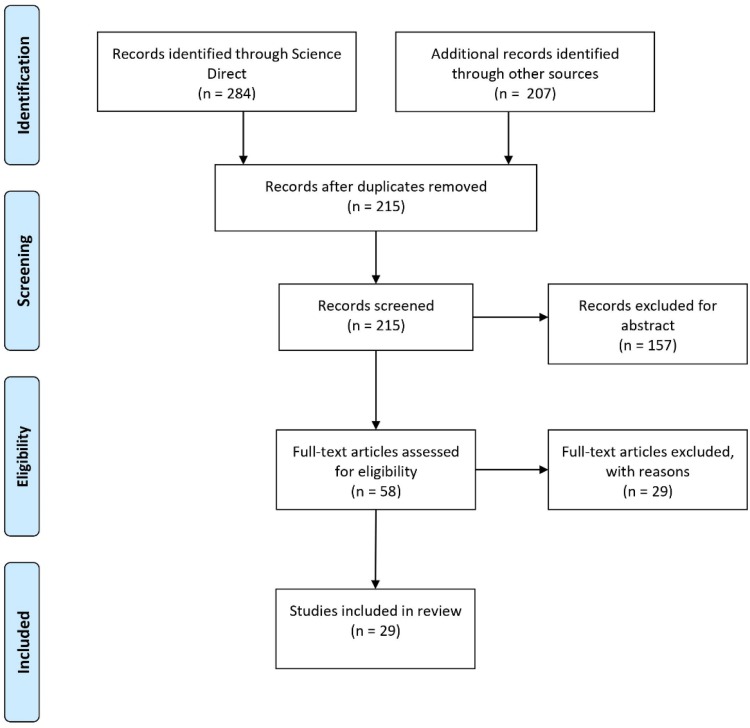
The initial research on the databases revealed a total of 491 articles. All of these studies were selected by their titles or abstracts. From this selection, 462 studies were excluded according to the eligibility criteria mentioned above in item 2.2. and 29 original studies remained for systematic review. These studies were included to answer the main question of the review. The systematic review process, based on the PRISMA methodology, is available in Fig. 2 .
Fig. 2.
The PRISMA flowchart of the search and selection of papers.
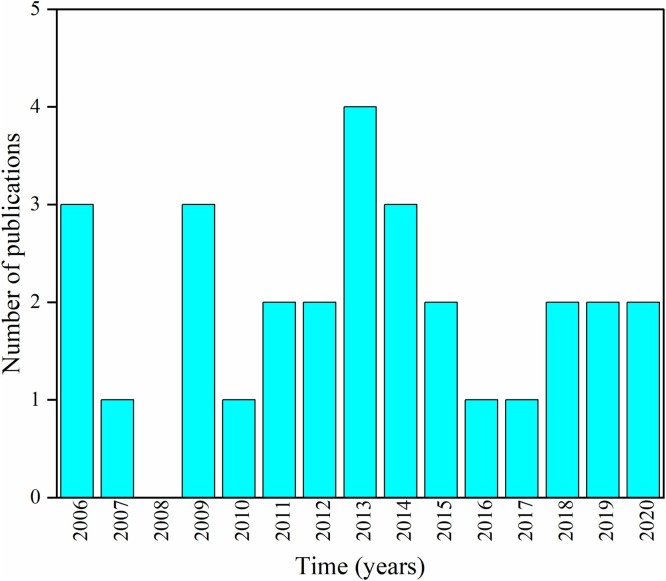
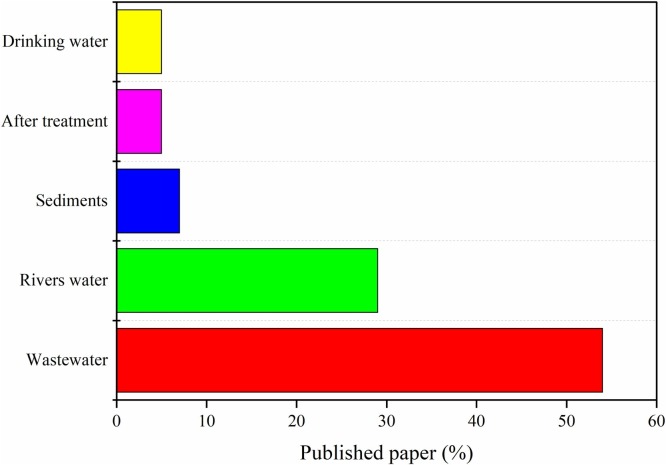
Fig. 3 shows the number of publications chosen for this present work from the last 15 years. The classification of published articles according to the presence of drugs in certain aquatic matrices is shown in Fig. 4 . The selected studies and their respective information were compiled in Table 1 .
Fig. 3.
Number of publications from the last fifteen years by year.
Fig. 4.
Classification of published papers (percentual) based on water matrices.
Table 1.
Detection studies of drugs clinically tested in the treatment of COVID-19 in environmental matrices.
| Drugs | Country | Results |
Reference | |
|---|---|---|---|---|
| Samples | Concentration | |||
| Azithromycin (AZT) | Brazil | Effluent | 10–107 ng L–1 | Jank et al. (2014) |
| USA | Final effluent | 15.9 ng L−1 | Blair et al. (2013) | |
| Sediment from Lake Michigan | 3.7 ng L−1 | |||
| Spain | Influential | 129 ng L−1 | Collado et al. (2014) | |
| After undergoing primary and secondary treatment of the WWTP | 134 ng L−1 | |||
| After tertiary treatment with UV | 115 ng L−1 | |||
| Italy | Effluent | 17 ng L−1 | Al Aukidy et al. (2012) | |
| Japan | Influent | 160−1,347 ng L−1 | Ghosh et al. (2009) | |
| Affluent | 260 ng L−1 | Yasojima et al. (2006) | ||
| Portugal | University hospital | 3748 ng L−1 | Santos et al. (2013) | |
| General hospital | 1889 ng L− 1 | |||
| – | below 1 μg L−1 | Sousa et al. (2011) | ||
| WWE | 200 ng L−1 | Pereira et al. (2015) | ||
| – | Influent | 0.260 μg L−1 | Miège et al. (2009) | |
| – | Wastewater matrix | 70 ng L−1 | Petrovic et al. (2006) | |
| Chloroquine (CQ) and Hydroxychloroquine (HCQ) | China | Traces in the surface sediments of tidal sections of rivers | – | Chen et al. (2013) |
| Nigeria | CQ in groundwater | 5.014 μg L−1 | Olatunde et al. (2014) | |
| CQ in surface water | 0.11 μg L−1 | |||
| Ivermectin (IVM) | Argentina | Feces of cattle | – | Iglesias et al. (2006) |
| France | Water resources and tap water | 5−20 ng L−1 | Charuaud et al. (2019) | |
| Spain | Irrigation waters | 0.093 μg L−1 | Rodriguez-Gil et al. (2013) | |
| Dexamethasone | Spain | Affluent sewage | – | Herrero et al. (2012) |
| China | Sewage samples | – | Fan et al. (2011) | |
| Urban river | 52 ng L−1 | Chang et al. (2009) | ||
| Discharge point | 390 ng L−1 | |||
| Surface waters | 0.33 ng L−1 | Gong et al. (2019) | ||
| Hungry | River and drinking water | – | Tölgyesi et al. (2010) | |
| Japan | Sewers, treatment plants and river. | – | Chang et al. (2007) | |
| Malaysia | Lui river | 0.02 ng L−1 | Praveena et al. (2018) | |
| Gombak river | 6.32 ng L−1 | |||
| Selangor river | 0.73 ng L−1 | |||
| Antivirals | South Africa | Lopinavir - surface waters | 305 ng L−1 | Wood et al. (2015) |
| WWTPs and a wastewater treatment plant | 69−43.000 ng L−1 | Abafe et al. (2018) | ||
| Atazanavir - wastewater | 75.12 ng L−1 | Mhuka et al. (2020) | ||
| Norway | Atazanavir - wastewater | 510.1 ng L−1 | Ferrando-Climent et al. (2016) | |
| Greece | Atazanavir - WWTP | – | Ibáñez et al. (2017) | |
| Atazanavir - influential | 0.02 μg L−1 | Gago-Ferrero et al. (2020) | ||
| Darunavir - influential | 0.15 μg L−1 | |||
| Emtricitabane - influential | 0.33 μg L−1 | |||
3.2. Pharmaceutical drugs
The main drugs clinically tested to combat COVID-19 and their occurrence in environmental matrices, which are analyzed in this paper, are described below.
3.2.1. Azithromycin
Azithromycin (AZT, C38H72N2O12) is a broad-spectrum antibiotic of the macrolides group that acts against several Gram-positive and Gram-negative bacteria such as Propionibacterium acnes and Neisseria gonorrhoeae, a fact which has promoted its use in the treatment of various respiratory infections and sexually transmitted infections (Kanoh and Rubin, 2010; Amsden, 2005). Its large application potential has made AZT one of the most consumed drugs in the world. To gather a perspective on AZT consumption Wirtz et al. (2010) carried out a survey on the consumption of antibiotics in eight countries in Latin America (Argentina, Brazil, Chile, Colombia, Mexico, Peru, Uruguay and Venezuela) from 1997 to 2007. According to the authors, among the group of macrolide antibiotics, AZT was the second most consumed one in all eight countries.
AZT is being clinically tested to treat the coronavirus disease, alone or in combination with other drugs, such as HCQ (Arshad et al., 2020; Dubernet et al., 2020; Fiolet et al., 2019; Gautret et al., 2020a; Mori et al., 2020). However, its effectiveness in the treatment of COVID-19 is still uncertain and its use is viewed with caution by the scientific community, since the drug has potential side effects and the evidence on its safe use is still controversial (Furtado et al., 2020; Molina et al., 2020; Pani et al., 2020). Despite the uncertainties about the effectiveness of AZT for the treatment of COVID-19, alone or even combined with other drugs, consumption has increased given the global levels of the pandemic, including according to (Usman et al., 2020) by a large number of people who self-medicate with antibiotics in the wrong attempt to protect themselves from the virus, a fact which raises concern.
The presence of AZT in environmental matrices has been evidenced in recent years, because, as with other compounds, some AZT leaves the body in its unchanged form through feces and urine and consequently reaches the waste treatment plants. In addition, AZT's slow metabolism indicates possible poor degradation in treatment plants (Koch et al., 2005). According to Brazil’s National Sanitary Regulatory Agency (ANVISA) 75 % of AZT leaves the body through urine in its original unmetabolized form (Cano et al., 2020; Christian et al., 2003). To contextualize the problem involving the presence of AZT, most recent studies which evaluated its presence (isolated or with other compounds) in different environmental matrices in various countries were reviewed.
Jank et al. (2014) carried out a study that evaluated the presence of eight antibiotics of different classes, including AZT, in samples of wastewater, tributaries and effluents from a treatment plant that uses conventional biological treatment in the city of Porto Alegre, Brazil. According to the authors, macrolide antibiotics, AZT and erythromycin, are largely excreted into the sewage in their unchanged forms at excretion rates greater than 60 % and found at high values, such as 1.5 μg L−1 in WWTP (Wastewater Treatment Plant) samples (Karthikeyan and Meyer, 2006). Based on the results, the authors concluded that AZT was detected in surface water at two points of a water stream named Dilúvio (39.7 and 23.7 ng L−1). AZT was detected in the WWTP samples in all months in which the study was carried out (except July) with very similar concentrations in the affluent and effluent samples of the WWTPs that use activated sludge as treatment. The level of AZT concentration in effluent samples (10−107 ng L−1) is in agreement with other studies available in literature. The authors pointed out that few reports dealing with the removal of AZT at WWTPs have been published.
Blair et al. (2013) carried out a study in which they evaluated the concentration and the corresponding risk of medications and personal hygiene products from a source of wastewater effluent at varying distances on Lake Michigan (USA). Fifty-four products and hormones were evaluated on six different dates over a two-year period in surface water and sediment samples. The authors concluded that 38 of the 54 compounds were detected in surface water samples from Lake Michigan or its effluents, including AZT. In the sediment samples, 30 compounds were detected above the minimum detection levels, including AZT, which was one of the most detected compounds during the study with the sediment samples. In their conclusions, the authors state that the detection of such a large number of high and medium risk medications and personal care products in the Great Lakes region is new and worrying.
Collado et al. (2014) evaluated the presence and removal of 81 Active Pharmaceutical Compounds from a municipal WWTP located in a highly industrialized area, with partial water reuse after tertiary UV treatment and discharge into a Mediterranean river. Water monitoring was carried out in an integrated manner at different points of the WWTP and the river. According to the authors, AZT was detected in concentrations of 129 ng L−1 in the uptake samples, 134 ng L−1 after the primary and secondary treatment of the WWTP and 115 ng L−1 after the tertiary treatment with UV, demonstrating the difficulty of the processes applied in removing this compound.
Al Aukidy et al. (2012) evaluated the monitoring of 27 pharmaceutical drugs, belonging to different classes, in the effluent of two WWTPs and their respective receptive water bodies in the sensitive area of Vale do Pó (Northern Italy). These channels were monitored upstream and downstream of the effluent discharge points, in order to assess the impact of the effluent on the quality of surface water, which is commonly used for irrigation. From the results obtained, the authors concluded that of the 27 compounds analyzed, 19 were detected in the effluent of the WWTP-A and, of these, 12 drugs were always detected, including AZT. In WWTP-B, 21 of the 27 compounds were detected, 17 with a frequency of 100 %, including AZT. Regarding the occurrence of the chemicals in the water environments in general, 22 of the 27 selected substances were detected. Among the most detected compounds, AZT was detected 7 times. In addition, according to the authors, AZT together with sulfamethoxazole and clarithromycin were the most critical pharmaceutical compounds due to their high RQ (risk quotient) values, based on the guidelines of the European Agency for the Evaluation of Medicinal Products (EMEA).
Pereira et al. (2015) evaluated the occurrence, destination, geographical and seasonal influence and environmental risk assessment of eleven of the most consumed medicines in Portugal from samples of different WWTPs throughout the country during the summer and spring of 2013. The most commonly found medicines were lipids (bezafibrate, genfibrozil and simvastatin), anti-inflammatory drugs (diclofenac and ibuprofen) and antibiotics (AZT and ciprofloxacin).
Chen et al. (2021) investigated the occurrence, spatial and seasonal distribution of pharmaceutical and personal care products (PPCPs), including azithromycin, in surface water and lake sediments and the WWTP-river-estuary system around hospitals in Wuhan, China, and evaluated the pandemic ecological risk of these compounds. According to the authors, sulfamethoxazole and azithromycin were considered potential risks to aquatic organisms according to a semi-probabilistic approach and were classified as priority pollutants based on an optimized risk assessment and highlighted to the fact that the increased occurrence of certain drugs and their potential risks to ecological systems need more attention. A strict source control policy, advanced risk monitoring and early warning system for emergency response and long-term risk control of PPCPs in waste is urgently needed. In addition to the reported works, other studies available in literature report the presence of ATZ in the environment from different locations on the planet: Ghosh et al., 2009; Miège et al., 2009; Petrovic et al., 2006; Santos et al., 2013; Sousa et al., 2011; Verlicchi et al., 2012; Yasojima et al., 2006.
3.2.2. Chloroquine and hydroxychloroquine
Chloroquine (CQ) and hydroxychloroquine (HCQ) are drugs belonging to the 4-aminoquinoline group and are widely used in the prophylaxis and treatment of malaria (Price et al., 2014; Tanenbaum and Tuffanelli, 1980), lupus (Lee et al., 2011), amoebiasis (Singh et al., 2011) and rheumatoid arthritis (Chung et al., 2007; Haque et al., 2008), being considered a potential broad-spectrum antiviral agent (Savarino et al., 2006). Its mechanism of action consists of inhibiting the fusion of the virus to the cell membrane by modulating the endosomal pH (Goodarzi et al., 2020). Both drugs have between 70%–80% bioavailability after oral administration and their half-life is 40–50 days. Because of this, 96 % steady therapeutic levels are not reach until approximately 6 months of continuous use (Tett et al., 1989).
Recently, some international health organizations have allowed patients affected by Coronavirus (COVID-19) to be treated with CQ or HCQ, in an isolated manner (Borba et al., 2020; Gao et al., 2020; Magagnoli et al., 2020; Tang et al., 2020) or combined with AZT (Arshad et al., 2020; Gautret et al., 2020b). The emergency authorization of antimalarial drugs ended up requiring large-scale manufacturing to fight the virus that ended up infecting millions of people worldwide in a few months (Midassi et al., 2020) and data on the behavior and monitoring of these drugs in the environment are still very scarce.
Several authors claim these drugs are persistent and bioaccumulative, considering them as emerging pharmaceutical contaminants (Daughton, 2014; Howard and Muir, 2011; Zurita et al., 2005). Ramesh et al. (2018) reported in his study many histopathological changes in vital organs, such as gills, liver and kidneys in groups of Cyprinus carpio organisms treated with CQ, indicating that the drug has toxic effects on non-target organisms. Rendal et al. (2011) studied the toxicity of CQ in the species Salix viminalis and Daphnia magna reporting a higher toxic potential when the environment had an elevated pH level.
A study by Chen et al. (2013) in the top layer sediments of tidal sections of rivers located in southeastern China detected a total of 330 pharmaceutical compounds and among them, traces of CQ and HCQ. Olatunde et al. (2014) detected the presence of CQ in samples of surface and groundwater in an industrial pharmaceutical area of Sango Ota, in the state of Ogun, Nigeria. The average concentration of CQ detected by the authors was 5.014 μg L−1 in groundwater and 0.11 μg L−1 in surface water. One of the major concerns expressed by the authors is that the presence of this drug in drinking water causes the drug to lose its therapeutic efficiency and leads bacteria to develop natural resistance to it.
Jjemba (2002) reports a study on the effects of three antimicrobial agents on the soil, where soybean planting was later carried out. Their results show that a low amount of CQ (2−4 mg g−1 of soil) caused most of the seeds to germinate normally, however, when the dosage was increased (8−16 mg g−1 of soil), the dosage had become lethal, causing some plants to have stunted development, or to not develop after 13 days of planting.
For Midassi et al. (2020) the high persistence potential of these drugs in the environment can lead to bioaccumulation and its transfer to living beings in more intensified and toxic forms, due to their antiviral and antibacterial characteristics. Coelho et al. (2017) studied the stability of CQ, in which they submitted samples of the drug to degradation by acid, alkaline and neutral hydrolysis, oxidation, metal ions, heat and light and observed that CQ is susceptible to degradation only in alkaline environments and oxidation, resulting in unknown end products.
Studies like these should serve as a warning to regulatory authorities, because, in a short time, the consequences of high consumption of medicines around the world can cause immeasurable losses. It is noteworthy that the monitoring of these drugs should become highly relevant, since the effluent and water treatment systems do not have adequate processes for the elimination of emerging contaminants. CQ and HCQ are derived from quinolones, which due to their good solubility and low biodegradation, have become common contaminants in groundwater and end up attacking non-target organisms (Gosu et al., 2016).
3.2.3. Ivermectin
Ivermectin (IVM) is a macrocyclic lactone, belonging to the avermectin family, officially isolated in 1975 as a fermentation product of the bacterium Streptomyces avermitilis, belonging to the phylum Actinomycete (Atakisi et al., 2009). This compound is used in veterinary and human medicine as an antiparasitic drug (Campbell, 1985). Currently, more than one hundred IVM-based formulations are registered, in injectable, cutaneous and oral forms in different concentrations. Up to 90 % of the drug is excreted by the body through feces, milk and urine, causing changes in the invertebrate organisms which participate of the fecal degradation process (Tišler and Kožuh Eržen, 2006).
This drug has been approved by the FDA as an effective and quick remedy for COVID-19 (Caly et al., 2020; Yang et al., 2020), however, its use is still controversial. Some authors have reported a low degradation of IVM in the environment, being considered a persistent drug that can cause negative impacts on the environment (Löffler et al., 2005; Oppel et al., 2004; Prasse et al., 2009; Sanderson et al., 2007).
For Edwards et al. (2001) IVM is a compound that presents a broad spectrum of activity, is hydrophobic, has low volatility and solubility and has a strong affinity for lipids, soil and organic matter, and can persist for several months or even years in the soil. The authors also mention that significant amounts of IVM are excreted in the animals' feces, which end up retaining its biological activity for more than a year and can be considered a toxic compound for a variety of insects that participate in the degradation of manure. When it reaches aquatic systems, it can affect fish and invertebrates that live there, such as Daphnia. Iglesias et al. (2006) found that IVM remains at high levels in cattle feces even when exposed to the environment for 60 days, which affects the degradation of the organic matter and the subsequent recycling of soil nutrients.
Halley et al. (1989) evaluated the toxicity of IVM in aquatic organisms and found that Daphnia magna was the most sensitive species for the 48 h exposure period, however, due to the high sorption of sediments and rapid dissipation of the drug in water, this evaluation concluded that only a very small amount of IVM would reach surface water and, consequently, the drug would not pose a significant ecological risk.
In a more recent bench-scale survey, Schweitzer et al. (2010) concluded that the direct deposition of feces containing small amounts of IVM in water bodies was considered very toxic for the species Daphnia magna and Chironomus riparius, with negative impacts on the survival, growth and abundance of the species. For the authors, IVM concentrations persisted for a long period, which indicates a possible risk for aquatic systems. Lopes et al. (2009) also reported toxicity to organisms of the species Daphnia and Ceriodaphnia, in which they confirm that IVM concentrations smaller than 1 ng L−1 had drastic effects on the development of these freshwater invertebrates and reinforced that efforts must be made to limit contamination of aquatic ecosystems. Syslová et al. (2019) detected 6 metabolites of IVM in the roots of Arabidopsis thaliana and concluded that this could be considered as a possible negative impact of the plant's physiology.
In aquatic matrices, Charuaud et al. (2019) conducted a study on water resources and tap water in Britain, where they found the drug in a range of 5–20 ng L−1. This study demonstrates the importance of monitoring and quantifying drugs for veterinary and human use due to the population's exposure to contaminants through tap water and presents a contrast with other studies that state that the compound is not very persistent (Löffler et al., 2005; Oppel et al., 2004; Prasse et al., 2009; Sanderson et al., 2007), alerting for the need of a better assessment regarding the presence of IVM in treated water. Similarly, Rodriguez-Gil et al. (2013) evaluated the quality of irrigation water in western Spain and quantified 0.093 μg L−1 of IVM. The value found by these authors is above the value of the risk quotient, which implies a moderate potential risk of toxicity in the lower waters of the studied rivers.
Recent studies by Mesa et al. (2020) and Essid et al. (2020) highlight the importance of quantifying IVM in aqueous systems. Mesa et al. (2020) conducted a field study in the wetlands of the Paraná River, in Argentina, with the goal of determining the presence, accumulation and persistence of the drug in the sediments and aquatic fauna of the wet areas exposed to cattle previously treated with IVM. They concluded that the accumulation of IVM in aquatic communities is alarming because they play a fundamental role in the food chains of the aquatic ecosystem and that there is a difference in the growth of macrophytes that grow in places with higher concentrations of drugs in the sediment and water, therefore a greater control over the use of the medication and better management strategies for animals must be studied in order to reduce the introduction and accumulation of IVM to aquatic systems. Essid et al. (2020) reported a growing concern about the intense release of IVM in the marine areas of the Mediterranean Sea, where three European epicenters of COVID-19 are located (Italy, Spain and France). The authors' experimental study reported that IVM causes a great reduction in the abundance of half-benthic nematodes and diversity indexes decreases when environments are exposed to high doses of it. Due to this bioaccumulative power, it is necessary to carefully monitor seafood, including those intended for human consumption, especially if they are caught near the coast of countries seriously affected by COVID-19.
3.2.4. Dexamethasone
Dexamethasone (DEX) is a synthetic glucocorticoid of the corticosteroids class (Hassan et al., 2020) also known as corticosteroid or steroid. This type of medication has a high anti-inflammatory and immunosuppressive power, being essentially a synthetic version of the hormones produced by the suprarenal (adrenal) glands, located in the upper region of the kidneys (Cain and Cidlowski, 2017) used for intensive and short-term treatments (Rhen and Cidlowski, 2005), in situations such as rheumatological diseases, skin illnesses, ocular, glandular, pulmonary, gastrointestinal, neurological and blood problems, allergies, organ transplants and tumors, and in addition to these applications, DEX has been evaluated for the treatment of patients infected with COVID-19 (Hassan et al., 2020; Johnson and Vinetz, 2020; Lester et al., 2020; Selvaraj, 2020). According to Sharun et al. (2020), DEX may be beneficial in patients with COVID-19 due to its ability to inhibit excessive cytokine production, consequence of the body’s immune response, and reduce its destructive effects.
The preliminary results of an important randomized clinical trial carried out by the RECOVERY group (Randomized Evaluation of COVID-19 Therapy) compared the effects of using low doses of DEX (6 mg orally or parenterally) for 10 days in 2104 patients with COVID-19 in multiple hospital facilities in the United Kingdom, with 4321 patients undergoing usual treatment then adopted in the country. The numbers showed a reduction in mortality of 35 % for patients who needed treatment with ventilators and 20 % for those who needed oxygen therapy. It has been reported that the drug is effective in less severe cases (RECOVERY (Randomised Evaluation of COVid-19 thERapY), 2020).
The use of DEX in the treatment of patients infected with the new corona virus puts it as part of the same problem reported in item 1 of this present study and is reinforced by the fact that DEX is the most potent cortisone derivative used in hospitals and clinics, and relatively high levels DEX have already been detected in sewage systems (Arsand et al., 2013; Herrero et al., 2012). Glucocorticoids such as DEX are poorly absorbed by the body, which means that about 50–90% of these drugs are rapidly eliminated through urine and feces and end up directly in municipal sewage systems which are then sent to sewage treatment plants. In addition to DEX persistence in surface waters, it has great polluting potential as a xenobiotic and the potential for bioaccumulation in the environment (Herrero et al., 2012).
The effects of the presence of DEX in the environment are already reported in literature (DellaGreca et al., 2004; Lalone et al., 2012; Lorenz et al., 2009; Margiotta-Casaluci et al., 2016; Shen et al., 2020). Due to its potential of affecting the development, reproduction, growth and expression of mRNA of amphibians and fish, the presence of this chemical in water can also cause toxicity to aquatic organisms. In their study Xu et al. (2011) found that prenatal exposure to DEX resulted in toxicity during fetal growth.
Like Herrero et al. (2012), who carried out a study on the presence of glucocorticoids in the wastewater and in Catalan rivers by ultra-high performance liquid chromatography, many other studies have been carried out recently trying to determine the presence and quantity of glucocorticoids in biological means, given their wide use in veterinary treatments. The authors themselves confirmed the presence of DEX in sewage samples and point out that there are few studies performed in environmental matrices, but those that were carried out confirmed the presence of these drugs.
Fan et al. (2011) investigated the behavior of seven glucocorticoids, eight androgens and nine progestogens compared to six estrogens at a municipal sewage treatment plant in Beijing, China, and found DEX in sewage samples. Chang et al. (2007) evaluated the occurrence of natural and synthetic glucocorticoids in sewers, treatment plants and rivers in Japan and detected the presence of DEX in the evaluated environmental matrices. The authors point out that DEX was detected only occasionally in effluents from sewage treatment plants and very often in samples from the two rivers studied, although at relatively low levels. Chang et al. (2009) found that the concentrations of the glucocorticoid hormone in the urban part of the river and at the discharge point were 52 and 390 ng L−1, respectively. Tölgyesi et al. (2010) detected DEX in samples from the Danube River and in drinking water sources from different regions of Hungary in two sampling periods from 2008 and 2009. Gong et al. (2019) evaluated the occurrence, time and space distribution and potential risks of 21 glucocorticoids (GCs) and 3 mineralocorticoids (MCs) in four rivers investigating the surface waters of the Pearl River Delta (PRD) in southern China, identifying the presence of DEX in the concentrations of 0.33 ng L−1. According to the authors, the concentrations of the most common corticosteroids in the Pearl River system, including DEX, were comparable to those of the Beijing rivers at similar levels ranging from <1 ng L−1 to tens of ng L−1 and higher than in German rivers (Chang et al., 2009, 2007; Weizel et al., 2018).
Praveena et al. (2018) investigated the occurrence of nine drugs, including DEX, to measure potential risks (human health and ecotoxicological) in Lui, Gombak and Selangor (Malaysia). DEX was detected in average concentrations of 0.02 ng L−1, 6.32 ng L−1 and 0.73 ng L−1, respectively in the Lui, Gombak and Selangor rivers. The Ministry of Health Malaysia (2014) reported that DEX is the second most common administered anti-inflammatory drug for the immune system in Malaysia. DEX concentrations in tropical surface water, on this study, were considered to be higher when compared to the concentrations in surface water at the temperatures typically found in Spain and Hungary. The authors argue that it is crucial to continuously monitor surface water bodies for pharmaceutical products using an economic prioritization approach to assess the risk for sensitive populations.
3.2.5. Antivirals (Remdesivir, Favipiravir, HIV Antivirals)
Antiviral drugs are a class of drugs used exclusively to treat viral infections, inhibiting the development of the virus (De Clercq, 2004). Most of them are designed to deal with the herpes virus, hepatitis B and C virus, influenza virus and the human immunodeficiency virus (HIV) (He, 2013). For Sanderson et al. (2004) according to modeling and toxicity data, antivirals occupy the eighth place among the drugs with the greatest dangerous potential for aquatic organisms, such as algae, Daphnias and fish. This fact further reinforces the need for studies on the toxic effects of these substances.
Remdesivir (RMD) known commercially as Veklury, GS-5734, is a prodrug with a wide spectrum of antiviral properties and potent in vitro activity against a diverse panel of RNA viruses, such as the Ebola virus, MERS-CoV and SARS-CoV (Sheahan et al., 2017; Wang et al., 2020). Favipiravir (FAV), T-705, commercially known as Avigan, is also classified as a broad-spectrum antiviral drug against RNA viruses (Hossen et al., 2020), and also has an effective in vitro activity against a wide range of virus, including Influenza H1N1/A, H1N1/pdm09, H5N1 and Avian Virus A (H7N9) (Furuta et al., 2013).
Both FAV and RMD are members of one of the oldest and most important classes of antiviral drugs, known as nucleoside analogs, which are incorporated into nascent viral RNA chains and inhibit the viral RNA polymerase (Eastman et al., 2020; Eyer et al., 2018). In the absence of any other effective treatment for SARS-CoV-2 infection (COVID-19), the drugs favipiravir and remdesivir have been tested and approved for use in severe cases of COVID-19 (Agrawal et al., 2020; Alvi et al., 2020; Grein et al., 2020; Singh et al., 2020).
Although there is still little published on the environmental risk related to both drugs, regarding ecotoxicity and degradability, some antivirals are highly bioactive and can negatively affect non-target organisms persisting in aquatic environments (Azuma et al., 2017). In addition, they can react with organic and inorganic matter during wastewater treatment and can be transformed into different molecules with greater environmental persistence (Funke et al., 2016; Jain et al., 2013).
The possible increase of human waste containing RMD and FAV and consequently their subsequent presence in surface waters of effluents from sewage treatment plants represents a worrying environmental issue, as both are poorly soluble in water and because there’s little data on the octanol/water partition coefficient (log Kow) (Sheahan et al., 2017). According to the European Medicines Agency (EMEA) guideline, this coefficient is a relevant factor for the assessment of the environmental risk of the medicine for human use, which makes experimental studies that aim at obtaining the log Kow really important (EMEA, 2020).
Studies available in literature indicate that these drugs are only partially transformed in the human body and can be eliminated through urine and feces mostly unchanged. RMD has been reported to remain unchanged at 74 % in the urine and 18 % in the feces, with 49 % of this dose recovered in the form of its active metabolite, nucleoside triphosphate, GS-441,524 (Sheahan et al., 2017). Meanwhile AVF is eliminated predominantly through urine in the form of metabolites (Madelain et al., 2016).
Thus, the antivirals remdesivir and faviparivir can be considered emerging contaminant candidates, considering a large part of their active form still remains unchanged when disposed in aquatic environments. Although information about their related environmental risks is scarce, they continue to be widely released on water environments due to their use in the pandemic (Jain et al., 2013).
Despite their alarming consumption rates, several antiviral drugs have been detected, but not systematically monitored in the aquatic environment (Aminot et al., 2017; Azuma et al., 2019, 2017; Funke et al., 2016; Mosekiemang et al., 2019; Prasse et al., 2010). However, data on the concentrations of RMD and FAV in the environment are still very scarce. M. Kumar et al. (2020a, 2020b) made a preliminary estimate of how much rivers and lakes receive from the metabolite favipiravir hydroxide, GS-441,524, the active form of RMD, which resulted in 430−2120 ng L−1 and 54–270 ng L−1, respectively.
In Europe, combination therapy for HIV is responsible for 54 % of all antivirals, and in South Africa, three million people use these drugs, which would be approximately 12.7 % of the population (Abafe et al., 2018; Nannou et al., 2020). The daily rate is estimated at 991 mg/day, out of which 30 % is eliminated through urine and it is estimated that 326 tons per year of HIV treatment drugs reach treatment plants in South Africa (Abafe et al., 2018; Nannou et al., 2020). Some of the antivirals used to treat HIV are being tested to treat or reduce the symptoms of COVID-19, including Lopinavir, Darunavir, Atazanavir, Saquinavir, Emtricitabine, Azvudina (Frediansyah et al., 2020; Liu et al., 2020; Liu and Wang, 2020). Due to the widespread use of these antivirals in the treatment of HIV, these chemicals have already been detected in environmental matrices, fact which can be enhanced due to their current use for the treatment of COVID-19.
Wood et al. (2015) reported on the presence of the drug Lopinavir in surface water near a treatment plant in South Africa, in 23 points. This was one of the most commonly found drugs, with the highest value found being 305 ng L−1. Abafe et al. (2018) documented the presence of antiretroviral drugs (ARVDs) in water samples from two main WWTPs in Thekwini municipality, KwaZulu-Natal, South Africa, between 15th and 19th August 2016 using the LC–MS/MS method. Thirteen ARVDs used to treat HIV were detected, from which Darunavir and Lopinavir had the highest concentrations (69−43,000 ng L−1). The authors also determined the amount of ARVDs introduced into the WWTP and its removal capacity before final discharge. The treatment techniques applied in the three WWTPs proved to be effective for the removal of Darunavir and Saquinavir, however Atazanavir and Lopinavir were persistent in the effluents of all WWTPs.
Mhuka et al. (2020) collected water samples on different days from December 2016 to March 2018 at the Daspoort Wastewater Treatment Plant in Pretoria, South Africa. Affluent and effluent samples were collected, as well as at sampling points located along of the Apies River, both upstream and downstream of the Treatment Station. The evaluation was done by liquid chromatography coupled to high resolution mass spectrometry. Atazanavir was detected in some effluent samples in the order of 75.12 ng L−1, however it was not detected in the affluents. This suggests a possible accumulation of this chemical in some compartments during the effluent treatment process.
Ferrando-Climent et al. (2016) conducted a study at the WWTPs in Oslo, one named VEAS and another in Bekkelaget, located in Oslofjord, which receive city wastewater from the main city and several neighboring ones. VEAS receives sewage from several sources, including the largest hospital in the region, where most chemotherapy treatments take place. Factory and domestic sewage are also processed at VEAS in the western part of the city. 24 samples of the affluent effluent from both WWTPs were collected and by means of chromatography, Atazanavir was detected at the amount of 510.1 ng L−1.
Ibáñez et al. (2017) performed analyzes of wastewater samples treated at the WWTP in Athens, Greece in October 2014 for seven consecutive days and detected the chemical Atazanavir in six of the samples analyzed. The authors Gago-Ferrero et al. (2020) conducted their studies at the same WWTP in Athens, were able to quantify Atazanavir (0.02 μg L−1), Darunavir (0.15 μg L−1) and Emtricitabine (0.33 μg L−1) at intake water.
It is of great importance to continue the monitoring of rivers and seas around the world, as some antivirals are highly bioactive and in recent years their continued release and persistence in aquatic systems have been reported by several authors (Jain et al., 2013).
3.3. Effects on environmental matrices and treatment technologies
To better understand the problem in question, a table was elaborated containing the effects of the drugs studied, in the environmental matrices, based on information reported in literature. Table 2 shows the resulting effects from the presence of these drugs in environmental matrices, according to their pharmacological group. The studies in question evaluated aspects such as the persistence of drugs in the environment and/or toxic effects on life. According to the studies, the drugs in question showed persistence in the environment and, as an aggravating factor, the drugs azithromycin, chloroquine and hydroxychloroquine also showed potential for bioaccumulation, which raises greater concern. Regarding possible effects on human beings, the literature is still scarce and more studies need to be carried out to assess this aspect, as the greatest concern is the long term exposure to these drugs from two main sources: drinking water and food made of organisms which have accumulated such drug residues (Wang et al., 2021).
Table 2.
Effects on environmental matrices for each drug.
| Drugs | Therapeutic class | Effect on environmental matrices | Reference |
|---|---|---|---|
| Azithromycin | Macrolide antibiotic | Potential bioaccumulation | Grabicova et al. (2015) |
| May compromise the growth, development and health of animals | da Luz et al. (2021) | ||
| Accumulation in non-target species (caddisfly larvae) | Vermillion Maier and Tjeerdema (2018) | ||
| Inhibition of p-glycoprotein | Asakura et al. (2004) | ||
| Contribution to the growing worldwide epidemic of antibiotic resistance | Verlicchi et al. (2012) | ||
| Significant inhibition of bacterial growth and chlorophyll content | González-Pleiter et al. (2021) | ||
| Chloroquine | Antimicrobial | Potentially persistent and bioaccumulative properties | Howard and Muir (2011) |
| Good solubility and low biodegradation | Gosu et al. (2016) | ||
| Hydroxychloroquine | |||
| Toxic effects on non-target organisms | Ramesh et al. (2018) | ||
| Ivermectin | Antiparasitic | The use of ivermectin might pose a risk to local aquatic ecosystems | Garric et al. (2007) |
| It was pointed out to be locally hazardous for soil and water organisms | Van Wezel and Jager (2002) | ||
| Toxic ivermectin concentrations persisted for an extended period | Schweitzer et al. (2010) | ||
| It caused a great reduction in abundance of nematodes of Mediterranean Sea | Essid et al. (2020) | ||
| Dexamethasone | Glucocorticoid | Inhibition of population growth in organisms in the freshwater chain | DellaGreca et al. (2004) |
| Osteoporosis in vertebrates | De Vrieze et al. (2014) | ||
| Reduced fertility, spawning frequency and morphological abnormalities in fish | Lalone et al. (2012) | ||
| Developmental deficiencies in molluscs and reduced fertility and growth in cladocerans | Sitre et al. (2009) | ||
| Remdesivir | Antivirals | Persistence in the environment due to stability to photodegradation | Dunge et al. (2004); Russo et al. (2018) |
| Favipiravir | |||
| HIV Antivirals | Show low sorption trend | Azuma et al. (2017) | |
| Toxic effects on bacteria, algae, water fleas, fish, planktonic crustaceans | M. Kumar et al. (2021a, 2021b) | ||
| Evidence of absorption in plants, which may induce hormonal and toxic effects | Akenga et al. (2021) |
Facing the risk of permanence of the drugs in question in aquatic matrices and the difficulty of conventional treatments in removing these compounds, studies have been carried out in order to propose processes that can effectively remove these pollutants. For this, it is ideal that the process should be efficient, with relatively low cost and environmentally friendly. Table 3 shows the studies available in literature, which evaluated the application of some processes to remove the drugs tested in the treatment of COVID-19, together with the operational parameters that were used and the resulting achieved efficiency of each. However, most of the reported studies were carried out in a laboratory setting and the incorporation of these processes in effluent treatment plants would require first an evaluation on a pilot scale, as well as an economic feasibility study.
Table 3.
Treatment technologies for each drug.
| Drugs | Treatment technologies | Operational conditions | Efficiency of removal* | Reference |
|---|---|---|---|---|
| Azithromycin | Photocatalytic degradation | 30 mg of Ag@Bi4O5I2/SPION/Calg | 98.4% | A. Kumar et al. (2021a, 2021b) |
| Xe lamp 300W | ||||
| 90 min of reaction | ||||
| 5 mg og ZrO2/Ag@TiO2 | 90% | Naraginti et al. (2019) | ||
| Xe lamp 250W | ||||
| 8 hours of reaction | ||||
| 1000 mg of GO@Fe3O4/ZnO/SnO2 | 90.06% | Sayadi et al. (2019) | ||
| UV-C lamp 6W | ||||
| 120 min | ||||
| Membrane bioreactor | Pilot plant (anaerobic MBR, 100 PE) | 25% | Göbel et al. (2007) | |
| hydraulic retention time 13 hours | ||||
| solid retention time 16 ± 2 d, 33 ± 3 d or 60 – 80 d | ||||
| Adsorption | Saponin-modified nano diatomite | 99.8% | Davoodi et al. (2019) | |
| 1 g L-1; pH 9; 25 °C; agitation 450 rpm | ||||
| 60 min | ||||
| FAU-type zeolites | 79% | de Sousa et al. (2018) | ||
| 10 mg L-1 of adsorbent; pH 6,5 | ||||
| 30 min | ||||
| Nanofiltration | Composite polyamide membrane | 99% | Li et al. (2020) | |
| pH 5; 25 °C; 8 bar | ||||
| 120 min | ||||
| Ozonation | Municipal sewage treatment plant | 92.6% | Nakada et al. (2007) | |
| 1.7 × 105 m3 of sewage per day | ||||
| Concentration of ozone 3 mg L-1 | ||||
| Retention time 27 min | ||||
| Chloroquine Hydroxychloroquine | Photocatalysis-activated degradation | 400 mg of PDINH/MIL-88A(Fe) composite | 95.7% | Yi et al. (2021) |
| irradiation of 300 ± 50 mW LED visible light | ||||
| 30 min | ||||
| Electrochemical oxidation | Boron doped diamond (BDD) anodes | 100% | Bensalah et al. (2020) | |
| UV lamp mercury 15 W | ||||
| Sonication (sono-assisted electrochemical) | ||||
| 300 min | ||||
| Photodegradation | Simulated solar radiation (Xe lamp) | - | Dabić et al. (2019) | |
| Solutions of HCQ in spring, river and sea water | ||||
| 50 hours | ||||
| Membrane bioreactor | Membrane with melanized E. coli | 98.2% | Lindroos et al. (2019) | |
| Permeate flow 0.02 L min-1 | ||||
| 20 hours | ||||
| Electron-Fenton oxidation | Boron-doped diamond (BDD) anode | 100% | Midassi et al. (2020) | |
| H2O2 = 60 mA cm-2; O2 = 80 mL min-1; pH = 3 | ||||
| 300 min | ||||
| Ivermectin | Adsorption | Kaolinite biochar composite | 83.5% | Olu-Owolabi et al. (2021) |
| 100 mg of adsorbent; 30 °C | ||||
| 180 min | ||||
| Graphene oxide-polyaniline (GO/PANI) | - | Rezazadeh et al. (2018) | ||
| pH = 7; 700 rpm; salt addition of 2.0 M | ||||
| 45 min | ||||
| Photocatalytic degradation | 2 g L-1 TiO2 | 92.1% | Havlíková et al. (2016) | |
| UV Camag lamp; pH = 5 | ||||
| 5 hours | ||||
| Ferrate (VI) treatment | 3 mg L-1 of Fe in Jar test | 25% | Patibandla et al. (2018) | |
| sample pH at 6 | ||||
| fast mixing 2 min + slow mixing 20 min | ||||
| Dexamethasone | Electrocoagulation | Aluminum electrodes; NaCl as electrolyte | 38% | Arsand et al. (2013) |
| Sampling of hospital wastewater | ||||
| 45 min | ||||
| Photocatalysis | 0.75–2.5 g L−1 Ag/TiO2 and 10–20 mg L−1 H2O2 | 82.3% | Pazoki et al. (2016) | |
| UV and visible-light irradiation | ||||
| DXM (5–30 mg L−1); pH (3-11); 30–80 °C | ||||
| 240 min | ||||
| Adsorption | 0.1 – 0.5 g/50 ml Clinoptilolite (CP) modified zeolite | 78% | Mohseni et al. (2016) | |
| pH 4-7-9; 25 °C | ||||
| 120 min | ||||
| Multi-wall carbon nanotube and activated carbon | - | Vadi et al. (2013) | ||
| 0.005 g of adsorbent; 25 ± 2 °C | ||||
| 10 min | ||||
| Remdesivir | Photocatalytic degradation | 1 – 10 mg of TiO2 | - | Woche et al. (2016) |
| Mercury vapor lamp (Hg-UV) | ||||
| 140 min | ||||
| Catalytic ozonation | 1.5 g L-1 of Titanium-doped mesoporous γ-Al2O3 (γ-Ti-Al2O3) | - | Bing et al. (2017) | |
| 30 mg L-1 of gaseous O3 (ozone); 20 °C | ||||
| 60 min | ||||
| Favipiravir | Ozonation followed by activated carbon and biological filters | Pilot scale ozonation | - | Knopp et al. (2016) |
| 0.87 ± 0.29 g O3 | ||||
| Hydraulic retention time 17 ± 3 min | ||||
| HIV Antivirals | Ozonation | Analyte-ozone-rations (1:0, 10:1, 5:1, 2:1, 1:1, 1:2, 1:5, 1:10) | - | Funke et al. (2021) |
| Effluent from conventional WWTP was used with the addition of 5 mg L-1 of antiviral | ||||
| Electrochemical degradation | Ti/SnO2-Sb anode | 97% | Zhou et al. (2019) | |
| 10 min | ||||
| Adsorption | 10-30 g L-1 non-modified expanded perlite (E-perlite) | 58.5% | Babas et al. (2021) | |
| pH 3 – 11; 25 °C | ||||
| 250 min | ||||
| 5 mg L-1 Carbon nanotubes (CNTs) | 90% | Wang et al. (2015) | ||
| pH 2 – 12; 25 °C | ||||
| 48 hours |
Some data of efficiency removal was not provided specifically by the authors.
4. Conclusions
From the review of articles on the detection of the main drugs tested in the treatment of COVID-19, it was possible to collect data that demonstrate that these drugs are already present in environmental matrices, especially aquatic ones, and a potential increase in their environmental concentrations may occur, given the example of a similar problem related to the large-scale use of Tamiflu during the H1N1 outbreak along with the inefficiency of current wastewater treatments in eliminating these compounds. In perspective, it is necessary and extremely urgent to carry out studies to monitor the presence of the drugs mentioned in the environmental matrices, as well as to carry out studies that evaluate the use of efficient and economically viable processes for the removal of these compounds with the potential to be incorporated in already existing wastewater treatment plants. It is also necessary to evaluate the short- and long-term toxic effects of these drugs’ exposure in humans. Starting with the performance of new detection studies of these drugs in environmental matrices after the pandemic, it will be very useful to carry out the compilation of new data and thus determine a quantitative and qualitative comparison. These compounds must be included in water quality priority lists around the world to prepare for future challenges.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) (Process Number 88887.185200/2018-00).
References
- Abafe O.A., Späth J., Fick J., Jansson S., Buckley C., Stark A., Pietruschka B., Martincigh B.S. LC-MS/MS determination of antiretroviral drugs in influents and effluents from wastewater treatment plants in KwaZulu-Natal, South Africa. Chemosphere. 2018;200:660–670. doi: 10.1016/j.chemosphere.2018.02.105. [DOI] [PubMed] [Google Scholar]
- Agrawal U., Raju R., Udwadia Z.F. Favipiravir: a new and emerging antiviral option in COVID-19. Med. J. Armed Forces India. 2020 doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akenga P., Gachanja A., Fitzsimons M.F., Tappin A., Comber S. Uptake, accumulation and impact of antiretroviral and antiviral pharmaceutical compounds in lettuce. Sci. Total Environ. 2021;766 doi: 10.1016/j.scitotenv.2020.144499. [DOI] [PubMed] [Google Scholar]
- Al Aukidy M., Verlicchi P., Jelic A., Petrovic M., Barcelò D. Monitoring release of pharmaceutical compounds: occurrence and environmental risk assessment of two WWTP effluents and their receiving bodies in the Po Valley. Italy. Sci. Total Environ. 2012;438:15–25. doi: 10.1016/j.scitotenv.2012.08.061. [DOI] [PubMed] [Google Scholar]
- Alvi M.M., Sivasankaran S., Singh M. Pharmacological and non-pharmacological efforts at prevention, mitigation, and treatment for COVID-19. J. Drug Target. 2020;28:1–13. doi: 10.1080/1061186X.2020.1793990. [DOI] [PubMed] [Google Scholar]
- Aminot Y., Litrico X., Chambolle M., Arnaud C., Pardon P., Budzinski H. Erratum to: development and application of a multi-residue method for the determination of 53 pharmaceuticals in water, sediment, and suspended solids using liquid chromatography-tandem mass spectrometry (Analytical and Bioanalytical Chemistry 10.1007/s00. Anal. Bioanal. Chem. 2017;407:8623. doi: 10.1007/s00216-015-9017-3. [DOI] [PubMed] [Google Scholar]
- Amsden G.W. Anti-inflammatory effects of macrolides - an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J. Antimicrob. Chemother. 2005;55:10–21. doi: 10.1093/jac/dkh519. [DOI] [PubMed] [Google Scholar]
- Arsand D.R., Kümmerer K., Martins A.F. Removal of dexamethasone from aqueous solution and hospital wastewater by electrocoagulation. Sci. Total Environ. 2013;443:351–357. doi: 10.1016/j.scitotenv.2012.10.100. [DOI] [PubMed] [Google Scholar]
- Arshad S., Kilgore P., Chaudhry Z.S., Jacobsen G., Wang D.D., Huitsing K., Brar I., Alangaden G.J., Ramesh M.S., McKinnon J.E., O’Neill W., Zervos M., Nauriyal V., Hamed A.A., Nadeem O., Swiderek J., Godfrey A., Jennings J., Gardner-Gray J., Ackerman A.M., Lezotte J., Ruhala J., Fadel R., Vahia A., Gudipati S., Parraga T., Shallal A., Maki G., Tariq Z., Suleyman G., Yared N., Herc E., Williams J., Lanfranco O.A., Bhargava P., Reyes K. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura E., Nakayama H., Sugie M., Lan Zhao Y., Nadai M., Kitaichi K., Shimizu A., Miyoshi M., Takagi Kenji, Takagi Kenzo, Hasegawa T. Azithromycin reverses anticancer drug resistance and modifies hepatobiliary excretion of doxorubicin in rats. Eur. J. Pharmacol. 2004;484:333–339. doi: 10.1016/j.ejphar.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Atakisi E., Atakisi O., Topcu B., Uzun M. Effects of therapeutic dose of ivermectin on plasma nitric oxide and total antioxidant capacity in rabbits. Eur. Rev. Med. Pharmacol. Sci. 2009;13:425–429. [PubMed] [Google Scholar]
- Azuma T., Ishida M., Hisamatsu K., Yunoki A., Otomo K., Kunitou M., Shimizu M., Hosomaru K., Mikata S., Mino Y. Fate of new three anti-influenza drugs and one prodrug in the water environment. Chemosphere. 2017;169:550–557. doi: 10.1016/j.chemosphere.2016.11.102. [DOI] [PubMed] [Google Scholar]
- Azuma T., Otomo K., Kunitou M., Shimizu M., Hosomaru K., Mikata S., Ishida M., Hisamatsu K., Yunoki A., Mino Y., Hayashi T. Environmental fate of pharmaceutical compounds and antimicrobial-resistant bacteria in hospital effluents, and contributions to pollutant loads in the surface waters in Japan. Sci. Total Environ. 2019;657:476–484. doi: 10.1016/j.scitotenv.2018.11.433. [DOI] [PubMed] [Google Scholar]
- Babas H., Kaichouh G., Khachani M., Karbane M.E., Chakir A., Guenbour A., Bellaouchou A., Warad I., Zarrouk A. Equilibrium and kinetic studies for removal of antiviral sofosbuvir from aqueous solution by adsorption on expanded perlite: experimental, modelling and optimization. Surf. Interfaces. 2021;23 doi: 10.1016/j.surfin.2021.100962. [DOI] [Google Scholar]
- Bensalah N., Midassi S., Ahmad M.I., Bedoui A. Degradation of hydroxychloroquine by electrochemical advanced oxidation processes. Chem. Eng. J. 2020;402 doi: 10.1016/j.cej.2020.126279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing J., Hu C., Zhang L. Enhanced mineralization of pharmaceuticals by surface oxidation over mesoporous γ-Ti-Al2O3 suspension with ozone. Appl. Catal. B Environ. 2017;202:118–126. doi: 10.1016/j.apcatb.2016.09.019. [DOI] [Google Scholar]
- Blair B.D., Crago J.P., Hedman C.J., Klaper R.D. Pharmaceuticals and personal care products found in the Great Lakes above concentrations of environmental concern. Chemosphere. 2013;93:2116–2123. doi: 10.1016/j.chemosphere.2013.07.057. [DOI] [PubMed] [Google Scholar]
- Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., Mourão M.P.G., Brito-Sousa J.D., Baía-da-Silva D., Guerra M.V.F., Hajjar L.A., Pinto R.C., Balieiro A.A.S., Pacheco A.G.F., Santos J.D.O., Naveca F.G., Xavier M.S., Siqueira A.M., Schwarzbold A., Croda J., Nogueira M.L., Romero G.A.S., Bassat Q., Fontes C.J., Albuquerque B.C., Daniel-Ribeiro C.T., Monteiro W.M., Lacerda M.V.G. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw. open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- Cain D.W., Cidlowski J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017;17:233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:3–6. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W.C. Ivermectin: an update. Parasitol. Today. 1985;1:10–16. doi: 10.1016/0169-4758(85)90100-0. [DOI] [PubMed] [Google Scholar]
- Cano P.A., Jaramillo-Baquero M., Zúñiga-Benítez H., Londoño Y.A., Peñuela G.A. Use of simulated sunlight radiation and hydrogen peroxide in azithromycin removal from aqueous solutions: optimization & mineralization analysis. Emerg. Contam. 2020;6:53–61. doi: 10.1016/j.emcon.2019.12.004. [DOI] [Google Scholar]
- Chang H., Hu J., Shao B. Occurrence of natural and synthetic glucocorticoids in sewage treatment plants and receiving river waters. Environ. Sci. Technol. 2007;41:3462–3468. doi: 10.1021/es062746o. [DOI] [PubMed] [Google Scholar]
- Chang H., Wan Y., Hu J. Determination and source apportionment of five classes of steroid hormones in urban rivers. Environ. Sci. Technol. 2009;43:7691–7698. doi: 10.1021/es803653j. [DOI] [PubMed] [Google Scholar]
- Charuaud L., Jardé E., Jaffrézic A., Liotaud M., Goyat Q., Mercier F., Le Bot B. Veterinary pharmaceutical residues in water resources and tap water in an intensive husbandry area in France. Sci. Total Environ. 2019;664:605–615. doi: 10.1016/j.scitotenv.2019.01.303. [DOI] [PubMed] [Google Scholar]
- Chen Y.S., Yu S., Hong Y.W., Lin Q.Y., Li H.B. Pharmaceutical residues in tidal surface sediments of three rivers in southeastern China at detectable and measurable levels. Environ. Sci. Pollut. Res. 2013;20:8391–8403. doi: 10.1007/s11356-013-1871-y. [DOI] [PubMed] [Google Scholar]
- Chen W.Y., Lin C.J., Liao C.M. Assessing exposure risks for aquatic organisms posed by Tamiflu use under seasonal influenza and pandemic conditions. Environ. Pollut. 2014;184:377–384. doi: 10.1016/j.envpol.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Chen W.Y., Wu Y.T., Lin H.C., Ieong M.I., Lee B.H. Impact of long-term parental exposure to Tamiflu metabolites on the development medaka offspring (Oryzias latipes) Environ. Pollut. 2020;261 doi: 10.1016/j.envpol.2020.114146. [DOI] [PubMed] [Google Scholar]
- Chen X., Lei L., Liu S., Han J., Li R., Men J., Li L., Wei L., Sheng Y., Yang L., Zhou B., Zhu L. Occurrence and risk assessment of pharmaceuticals and personal care products (PPCPs) against COVID-19 in lakes and WWTP-river-estuary system in Wuhan, China. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian T., Schneider R.J., Färber H.A., Skutlarek D., Meyer M.T., Goldbach H.E. Determination of antibiotic residues in manure, soil, and surface waters. Acta Hydrochim. Hydrobiol. 2003;31:36–44. doi: 10.1002/aheh.200390014. [DOI] [Google Scholar]
- Chung C.P., Avalos I., Raggi P., Stein C.M. Atherosclerosis and inflammation: insights from rheumatoid arthritis. Clin. Rheumatol. 2007;26:1228–1233. doi: 10.1007/s10067-007-0548-7. [DOI] [PubMed] [Google Scholar]
- Coelho A.S., Chagas C.E.P., de Pádua R.M., Pianetti G.A., Fernandes C. A comprehensive stability-indicating HPLC method for determination of chloroquine in active pharmaceutical ingredient and tablets: identification of oxidation impurities. J. Pharm. Biomed. Anal. 2017;145:248–254. doi: 10.1016/j.jpba.2017.06.023. [DOI] [PubMed] [Google Scholar]
- Collado N., Rodriguez-Mozaz S., Gros M., Rubirola A., Barceló D., Comas J., Rodriguez-Roda I., Buttiglieri G. Pharmaceuticals occurrence in a WWTP with significant industrial contribution and its input into the river system. Environ. Pollut. 2014;185:202–212. doi: 10.1016/j.envpol.2013.10.040. [DOI] [PubMed] [Google Scholar]
- da Luz T.M., Araújo A.P., da C., Estrela F.N., Braz H.L.B., Jorge R.J.B., Charlie-Silva I., Malafaia G. Can use of hydroxychloroquine and azithromycin as a treatment of COVID-19 affect aquatic wildlife? A study conducted with neotropical tadpole. Sci. Total Environ. 2021;780 doi: 10.1016/j.scitotenv.2021.146553. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dabić D., Babić S., Škorić I. The role of photodegradation in the environmental fate of hydroxychloroquine. Chemosphere. 2019;230:268–277. doi: 10.1016/j.chemosphere.2019.05.032. [DOI] [PubMed] [Google Scholar]
- Daughton C.G. The Matthew Effect and widely prescribed pharmaceuticals lacking environmental monitoring: case study of an exposure-assessment vulnerability. Sci. Total Environ. 2014;466–467:315–325. doi: 10.1016/j.scitotenv.2013.06.111. [DOI] [PubMed] [Google Scholar]
- Davoodi S., Dahrazma B., Goudarzi N., Gorji H.G. Adsorptive removal of azithromycin from aqueous solutions using raw and saponin-modified nano diatomite. Water Sci. Technol. 2019;80:939–949. doi: 10.2166/wst.2019.337. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Antiviral drugs in current clinical use. J. Clin. Virol. 2004;30:115–133. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- de Sousa D.N.R., Insa S., Mozeto A.A., Petrovic M., Chaves T.F., Fadini P.S. Equilibrium and kinetic studies of the adsorption of antibiotics from aqueous solutions onto powdered zeolites. Chemosphere. 2018;205:137–146. doi: 10.1016/j.chemosphere.2018.04.085. [DOI] [PubMed] [Google Scholar]
- De Vrieze E., Van Kessel M.A.H.J., Peters H.M., Spanings F.A.T., Flik G., Metz J.R. Prednisolone induces osteoporosis-like phenotype in regenerating zebrafish scales. Osteoporos. Int. 2014;25:567–578. doi: 10.1007/s00198-013-2441-3. [DOI] [PubMed] [Google Scholar]
- DellaGreca M., Fiorentino A., Isidori M., Lavorgna M., Previtera L., Rubino M., Temussi F. Toxicity of prednisolone, dexamethasone and their photochemical derivatives on aquatic organisms. Chemosphere. 2004;54:629–637. doi: 10.1016/j.chemosphere.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Dubernet A., Larsen K., Masse L., Allyn J., Foch E., Bruneau L., Maillot A., Lagrange-Xelot M., Thomas V., Jaffar-Bandjee M.C., Gauzere L., Raffray L., Borsu K., Dibernardo S., Renaud S., André M., Moreau D., Jabot J., Coolen-Allou N., Allou N. A comprehensive strategy for the early treatment of COVID-19 with azithromycin/hydroxychloroquine and/or corticosteroids: results of a retrospective observational study in the French overseas department of Réunion Island. J. Glob. Antimicrob. Resist. 2020;23:1–3. doi: 10.1016/j.jgar.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunge A., Chakraborti A.K., Singh S. Mechanistic explanation to the variable degradation behaviour of stavudine and zidovudine under hydrolytic, oxidative and photolytic conditions. J. Pharm. Biomed. Anal. 2004;35:965–970. doi: 10.1016/j.jpba.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Eastman R.T., Roth J.S., Brimacombe K.R., Simeonov A., Shen M., Patnaik S., Hall M.D. Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent. Sci. 2020;6:672–683. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C.A., Atiyeh R.M., Römbke J. Environmental impact of avermectins. Rev. Environ. Contam. Toxicol. 2001;171:111–137. doi: 10.1007/978-1-4613-0161-5_3. [DOI] [Google Scholar]
- Eggen R.I.L., Hollender J., Joss A., Schärer M., Stamm C. Reducing the discharge of micropollutants in the aquatic environment: the benefits of upgrading wastewater treatment plants. Environ. Sci. Technol. 2014;48:7683–7689. doi: 10.1021/es500907n. [DOI] [PubMed] [Google Scholar]
- EMEA . 2020. Assessment Report - Veklury International Non-proprietary Name: Remdesivir. European Medicines Health - Science Medicines Health.https://www.ema.europa.eu/en/documents/assessment-report/veklury-epar-public-assessment-report_en.pdf [Google Scholar]
- Essid N., Allouche M., Lazzem M., Harrath A.H., Mansour L., Alwasel S., Mahmoudi E., Beyrem H., Boufahja F. Ecotoxic response of nematodes to ivermectin, a potential anti-COVID-19 drug treatment. Mar. Pollut. Bull. 2020;157 doi: 10.1016/j.marpolbul.2020.111375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyer L., Nencka R., de Clercq E., Seley-Radtke K., Růžek D. Nucleoside analogs as a rich source of antiviral agents active against arthropod-borne flaviviruses. Antivir. Chem. Chemother. 2018;26:1–28. doi: 10.1177/2040206618761299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z., Wu S., Chang H., Hu J. Behaviors of glucocorticoids, androgens and progestogens in a municipal sewage treatment plant: comparison to estrogens. Environ. Sci. Technol. 2011;45:2725–2733. doi: 10.1021/es103429c. [DOI] [PubMed] [Google Scholar]
- Ferrando-Climent L., Reid M.J., Rodriguez-Mozaz S., Barceló D., Thomas K.V. Identification of markers of cancer in urban sewage through the use of a suspect screening approach. J. Pharm. Biomed. Anal. 2016;129:571–580. doi: 10.1016/j.jpba.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Fiolet T., Guihur A., Rebeaud M.E., Mulot M., Peiffer-Smadja N., Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin. Microbiol. Infect. 2020;2019 doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frediansyah A., Tiwari R., Sharun K., Dhama K., Harapan H. Antivirals for COVID-19: a critical review. Clin. Epidemiol. Glob. Heal. 2020:0–1. doi: 10.1016/j.cegh.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke J., Prasse C., Ternes T.A. Identification of transformation products of antiviral drugs formed during biological wastewater treatment and their occurrence in the urban water cycle. Water Res. 2016;98:75–83. doi: 10.1016/j.watres.2016.03.045. [DOI] [PubMed] [Google Scholar]
- Funke J., Prasse C., Dietrich C., Ternes T.A. Ozonation products of zidovudine and thymidine in oxidative water treatment. Water Res. X. 2021;11 doi: 10.1016/j.wroa.2021.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado R.H.M., Berwanger O., Fonseca H.A., Corrêa T.D., Ferraz L.R., Lapa M.G., Zampieri F.G., Veiga V.C., Azevedo L.C.P., Rosa R.G., Lopes R.D., Avezum A., Manoel A.L.O., Piza F.M.T., Martins P.A., Lisboa T.C., Pereira A.J., Olivato G.B., Dantas V.C.S., Milan E.P., Gebara O.C.E., Amazonas R.B., Oliveira M.B., Soares R.V.P., Moia D.D.F., Piano L.P.A., Castilho K., Momesso R.G.R.A.P., Schettino G.P.P., Rizzo L.V., Neto A.S., Machado F.R., Cavalcanti A.B. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago-Ferrero P., Bletsou A.A., Damalas D.E., Aalizadeh R., Alygizakis N.A., Singer H.P., Hollender J., Thomaidis N.S. Wide-scope target screening of &2000 emerging contaminants in wastewater samples with UPLC-Q-ToF-HRMS/MS and smart evaluation of its performance through the validation of 195 selected representative analytes. J. Hazard. Mater. 2020;387 doi: 10.1016/j.jhazmat.2019.121712. [DOI] [PubMed] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/BST.2020.01047. [DOI] [PubMed] [Google Scholar]
- Garric J., Vollat B., Duis K., Péry A., Junker T., Ramil M., Fink G., Ternes T.A. Effects of the parasiticide ivermectin on the cladoceran Daphnia magna and the green alga Pseudokirchneriella subcapitata. Chemosphere. 2007;69:903–910. doi: 10.1016/j.chemosphere.2007.05.070. [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Tissot Dupont H., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Sevestre J., Mailhe M., Doudier B., Aubry C., Amrane S., Seng P., Hocquart M., Eldin C., Finance J., Vieira V.E., Tissot-Dupont H.T., Honoré S., Stein A., Million M., Colson P., La Scola B., Veit V., Jacquier A., Deharo J.C., Drancourt M., Fournier P.E., Rolain J.M., Brouqui P., Raoult D. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med. Infect. Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh G.C., Okuda T., Yamashita N., Tanaka H. Occurrence and elimination of antibiotics at four sewage treatment plants in Japan and their effects on bacterial ammonia oxidation. Water Sci. Technol. 2009;59:779–786. doi: 10.2166/wst.2009.067. [DOI] [PubMed] [Google Scholar]
- Göbel A., McArdell C.S., Joss A., Siegrist H., Giger W. Fate of sulfonamides, macrolides, and trimethoprim in different wastewater treatment technologies. Sci. Total Environ. 2007;372:361–371. doi: 10.1016/j.scitotenv.2006.07.039. [DOI] [PubMed] [Google Scholar]
- Gong J., Lin C., Xiong X., Chen D., Chen Y., Zhou Y., Wu C., Du Y. Occurrence, distribution, and potential risks of environmental corticosteroids in surface waters from the Pearl River Delta, south China. Environ. Pollut. 2019;251:102–109. doi: 10.1016/j.envpol.2019.04.110. [DOI] [PubMed] [Google Scholar]
- González-Pleiter M., Pedrouzo-Rodríguez A., Verdú I., Leganés F., Marco E., Rosal R., Fernández-Piñas F. Microplastics as vectors of the antibiotics azithromycin and clarithromycin: Effects towards freshwater microalgae. Chemosphere. 2021;268 doi: 10.1016/j.chemosphere.2020.128824. [DOI] [PubMed] [Google Scholar]
- Goodarzi P., Mahdavi F., Mirzaei R., Hasanvand H., Sholeh M., Zamani F., Sohrabi M., Tabibzadeh A., Jeda A.S., Niya M.H.K., Keyvani H., Karampoor S. Coronavirus disease 2019 (COVID-19): immunological approaches and emerging pharmacologic treatments. Int. Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosu V., Gurjar B.R., Zhang T.C., Surampalli R.Y. Oxidative degradation of quinoline using nanoscale zero-valent iron supported by granular activated Carbon. J. Environ. Eng. (United States) 2016;142:1–11. doi: 10.1061/(ASCE)EE.1943-7870.0000981. [DOI] [Google Scholar]
- Grabicova K., Grabic R., Blaha M., Kumar V., Cerveny D., Fedorova G., Randak T. Presence of pharmaceuticals in benthic fauna living in a small stream affected by effluent from a municipal sewage treatment plant. Water Res. 2015;72:145–153. doi: 10.1016/j.watres.2014.09.018. [DOI] [PubMed] [Google Scholar]
- Grein J., Myers R.P., Brainard D. Compassionate use of remdesivir in Covid-19. Reply. N. Engl. J. Med. 2020;382:e101. doi: 10.1056/NEJMc2015312. [DOI] [PubMed] [Google Scholar]
- Gruchlik Y., Linge K., Joll C. Removal of organic micropollutants in waste stabilisation ponds: a review. J. Environ. Manage. 2018;206:202–214. doi: 10.1016/j.jenvman.2017.10.020. [DOI] [PubMed] [Google Scholar]
- Halley B.A., Jacob T.A., Lu A.Y.H. The environmental impact of the use of ivermectin: environmental effects and fate. Chemosphere. 1989;18:1543–1563. doi: 10.1016/0045-6535(89)90045-3. [DOI] [Google Scholar]
- Haque S., Mirjafari H., Bruce I.N. Atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Wolters Kluwer Heal. 2008;19:338–343. doi: 10.1097/MOL.0b013e328304b65f. [DOI] [PubMed] [Google Scholar]
- Hassan M.E., Hasan H.M., Sridharan K., Elkady A., ElSeirafi M.M. Dexamethasone in severe COVID-19 infection: a case series. Respir. Med. Case Reports. 2020;31 doi: 10.1016/j.rmcr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlíková L., Šatínský D., Solich P. Aspects of decontamination of ivermectin and praziquantel from environmental waters using advanced oxidation technology. Chemosphere. 2016;144:21–28. doi: 10.1016/j.chemosphere.2015.08.039. [DOI] [PubMed] [Google Scholar]
- He H. Vaccines and antiviral agents. Curr. Issues Mol. Virol. - Viral Genet. Biotechnol. Appl. 2013 doi: 10.5772/56866. [DOI] [Google Scholar]
- Heberer T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol. Lett. 2002;131:5–17. doi: 10.1111/j.1439-0388.1936.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Herrero P., Borrull F., Pocurull E., Marcé R.M. Determination of glucocorticoids in sewage and river waters by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2012;1224:19–26. doi: 10.1016/j.chroma.2011.12.054. [DOI] [PubMed] [Google Scholar]
- Hill A., Khoo S., Fortunak J., Simmons B., Ford N. Minimum costs for producing hepatitis c direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin. Infect. Dis. 2014;58:928–936. doi: 10.1093/cid/ciu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossen M.S., Barek M.A., Jahan N., Safiqul Islam M. A review on current repurposing drugs for the treatment of COVID-19: reality and challenges. SN Compr. Clin. Med. 2020 doi: 10.1007/s42399-020-00485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard P.H., Muir D.C.G. Identifying new persistent and bioaccumulative organics among chemicals in commerce II: pharmaceuticals. Environ. Sci. Technol. 2011;45:6938–6946. doi: 10.1021/es201196x. [DOI] [PubMed] [Google Scholar]
- Ibáñez M., Borova V., Boix C., Aalizadeh R., Bade R., Thomaidis N.S., Hernández F. UHPLC-QTOF MS screening of pharmaceuticals and their metabolites in treated wastewater samples from Athens. J. Hazard. Mater. 2017;323:26–35. doi: 10.1016/j.jhazmat.2016.03.078. [DOI] [PubMed] [Google Scholar]
- Iglesias L.E., Saumell C.A., Fernández A.S., Fusé L.A., Lifschitz A.L., Rodríguez E.M., Steffan P.E., Fiel C.A. Environmental impact of ivermectin excreted by cattle treated in autumn on dung fauna and degradation of faeces on pasture. Parasitol. Res. 2006;100:93–102. doi: 10.1007/s00436-006-0240-x. [DOI] [PubMed] [Google Scholar]
- Jain S., Kumar P., Vyas R.K., Pandit P., Dalai A.K. Occurrence and removal of antiviral drugs in environment: a review. Water Air Soil Pollut. 2013;224 doi: 10.1007/s11270-012-1410-3. [DOI] [Google Scholar]
- Jank L., Hoff R.B., da Costa F.J., Pizzolato T.M. Simultaneous determination of eight antibiotics from distinct classes in surface and wastewater samples by solid-phase extraction and high-performance liquid chromatography-electrospray ionisation mass spectrometry. Int. J. Environ. Anal. Chem. 2014;94:1013–1037. doi: 10.1080/03067319.2014.914184. [DOI] [Google Scholar]
- Jin Y.-H., Cai L., Cheng Z.-S., Cheng H., Deng T., Fan Y.-P., Fang C., Huang D., Huang L.-Q., Huang Q., Han Y., Hu B., Hu F., Li B.-H., Li Y.-R., Liang K., Lin L.-K., Luo L.-S., Ma J., Ma L.-L., Peng Z.-Y., Pan Y.-B., Pan Z.-Y., Ren X.-Q., Sun H.-M., Wang Y., Wang Yun-Yun, Weng H., Wei C.-J., Wu D.-F., Xia J., Xiong Y., Xu H.-B., Yao X.-M., Yuan Y.-F., Ye T.-S., Zhang X.-C., Zhang Y.-W., Zhang Y.-G., Zhang H.-M., Zhao Y., Zhao M.-J., Zi H., Zeng X.-T., Wang Yong-Yan, Wang X.-H. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil. Med. Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jjemba P.K. The effect of chloroquine, quinacrine, and metronidazole on both soybean plants and soil microbiota. Chemosphere. 2002;46:1019–1025. doi: 10.1016/S0045-6535(01)00139-4. [DOI] [PubMed] [Google Scholar]
- Johnson R.M., Vinetz J.M. Dexamethasone in the management of covid -19. BMJ. 2020;370:1–2. doi: 10.1136/bmj.m2648. [DOI] [PubMed] [Google Scholar]
- Kanoh S., Rubin B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010;23:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan K.G., Meyer M.T. Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA. Sci. Total Environ. 2006;361:196–207. doi: 10.1016/j.scitotenv.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Knopp G., Prasse C., Ternes T.A., Cornel P. Elimination of micropollutants and transformation products from a wastewater treatment plant effluent through pilot scale ozonation followed by various activated carbon and biological filters. Water Res. 2016;100:580–592. doi: 10.1016/j.watres.2016.04.069. [DOI] [PubMed] [Google Scholar]
- Koch D.E., Bhandari A., Close L., Hunter R.P. Azithromycin extraction from municipal wastewater and quantitation using liquid chromatography/mass spectrometry. J. Chromatogr. A. 2005;1074:17–22. doi: 10.1016/j.chroma.2005.03.052. [DOI] [PubMed] [Google Scholar]
- Kolpin D.W., Furlong E.T., Meyer M.T., Thurman E.M., Zaugg S.D., Barber L.B., Buxton H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance. Environ. Sci. Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kumar M., Kuroda K., Dhangar K., Mazumder P., Sonne C., Rinklebe J., Kitajima M. Potential Emergence of Antiviral-Resistant Pandemic Viruses via Environmental Drug Exposure of Animal Reservoirs. Environ. Sci. Technol. 2020;54:8503–8505. doi: 10.1021/acs.est.0c03105. [DOI] [PubMed] [Google Scholar]
- Kumar V., Singh S.B., Singh S. COVID-19: environment concern and impact of Indian medicinal system. J. Environ. Chem. Eng. 2020;8 doi: 10.1016/j.jece.2020.104144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Rana A., Guo C., Sharma G., M Katubi K.M., Alzahrani F.M., Naushad M., Sillanpää M., Dhiman P., Stadler F.J. Acceleration of photo-reduction and oxidation capabilities of Bi4O5I2/SPION@calcium alginate by metallic Ag: wide spectral removal of nitrate and azithromycin. Chem. Eng. J. 2021 doi: 10.1016/j.cej.2021.130173. 130173. [DOI] [Google Scholar]
- Kumar M., Mazumder P., Mohapatra S., Kumar Thakur A., Dhangar K., Taki K., Mukherjee S., Kumar Patel A., Bhattacharya P., Mohapatra P., Rinklebe J., Kitajima M., Hai F.I., Khursheed A., Furumai H., Sonne C., Kuroda K. A chronicle of SARS-CoV-2: seasonality, environmental fate, transport, inactivation, and antiviral drug resistance. J. Hazard. Mater. 2021;405 doi: 10.1016/j.jhazmat.2020.124043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmerer K. The presence of pharmaceuticals in the environment due to human use - present knowledge and future challenges. J. Environ. Manage. 2009;90:2354–2366. doi: 10.1016/j.jenvman.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Lalone C.A., Villeneuve D.L., Olmstead A.W., Medlock E.K., Kahl M.D., Jensen K.M., Durhan E.J., Makynen E.A., Blanksma C.A., Cavallin J.E., Thomas L.M., Seidl S.M., Skolness S.Y., Wehmas L.C., Johnson R.D., Ankley G.T. Effects of a glucocorticoid receptor agonist, dexamethasone, on fathead minnow reproduction, growth, and development. Environ. Toxicol. Chem. 2012;31:611–622. doi: 10.1002/etc.1729. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Silverman E., Bargman J.M. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat. Rev. Nephrol. 2011;7:718–729. doi: 10.1038/nrneph.2011.150. [DOI] [PubMed] [Google Scholar]
- Lester M., Sahin A., Pasyar A. The use of dexamethasone in the treatment of COVID-19. Ann. Med. Surg. 2020;56:218–219. doi: 10.1016/j.amsu.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Zhou H., Huang L., Zhang J., Cui J., Li X. Role of adsorption during nanofiltration of sulfamethoxazole and azithromycin solution. Sep. Sci. Technol. 2020 doi: 10.1080/01496395.2020.1806326. [DOI] [Google Scholar]
- Lindroos M., Hörnström D., Larsson G., Gustavsson M., van Maris A.J.A. Continuous removal of the model pharmaceutical chloroquine from water using melanin-covered Escherichia coli in a membrane bioreactor. J. Hazard. Mater. 2019;365:74–80. doi: 10.1016/j.jhazmat.2018.10.081. [DOI] [PubMed] [Google Scholar]
- Liu X., Wang X.J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J. Genet. Genomics. 2020;47:119–121. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löffler D., Römbke J., Meller M., Ternes T.A. Environmental fate of pharmaceuticals in water/sediment systems. Environ. Sci. Technol. 2005;39:5209–5218. doi: 10.1021/es0484146. [DOI] [PubMed] [Google Scholar]
- Lopes C., Charles S., Vollat B., Garric J. Toxicity of ivermectin on cladocerans: comparison of toxic effects on Daphnia and Ceriodaphnia species. Environ. Toxicol. Chem. 2009;28:2160–2166. doi: 10.1897/08-607.1. [DOI] [PubMed] [Google Scholar]
- Lorenz C., Opitz R., Lutz I., Kloas W. Corticosteroids disrupt amphibian metamorphosis by complex modes of action including increased prolactin expression. Comp. Biochem. Physiol. - C Toxicol. Pharmacol. 2009;150:314–321. doi: 10.1016/j.cbpc.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Loureiro R.J., Roque F., Teixeira Rodrigues A., Herdeiro M.T., Ramalheira E. Use of antibiotics and bacterial resistances: brief notes on its evolution. Rev. Port. Saude Publica. 2016;34:77–84. doi: 10.1016/j.rpsp.2015.11.003. [DOI] [Google Scholar]
- Madelain V., Nguyen T.H.T., Olivo A., de Lamballerie X., Guedj J., Taburet A.M., Mentré F. Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin. Pharmacokinet. 2016;55:907–923. doi: 10.1007/s40262-015-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magagnoli J., Narendran S., Pereira F., Cummings T.H., Hardin J.W., Sutton S.S., Ambati J. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. Med. 2020:1–14. doi: 10.1016/j.medj.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margiotta-Casaluci L., Owen S.F., Huerta B., Rodríguez-Mozaz S., Kugathas S., Barceló D., Rand-Weaver M., Sumpter J.P. Internal exposure dynamics drive the Adverse Outcome Pathways of synthetic glucocorticoids in fish. Sci. Rep. 2016;6:1–13. doi: 10.1038/srep21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins L.D., da Silva I., Batista W.V., de Fátima Andrade M., Dias de Freitas E., Martins J.A. How socio-economic and atmospheric variables impact COVID-19 and Influenza outbreaks in tropical and subtropical regions of Brazil. Environ. Res. 2020;191 doi: 10.1016/j.envres.2020.110184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa L., Gutiérrez M.F., Montalto L., Perez V., Lifschitz A. Concentration and environmental fate of ivermectin in floodplain wetlands: an ecosystem approach. Sci. Total Environ. 2020;706 doi: 10.1016/j.scitotenv.2019.135692. [DOI] [PubMed] [Google Scholar]
- Mhuka V., Dube S., Nindi M.M. Occurrence of pharmaceutical and personal care products (PPCPs) in wastewater and receiving waters in South Africa using LC-OrbitrapTM MS. Emerg. Contam. 2020;6:250–258. doi: 10.1016/j.emcon.2020.07.002. [DOI] [Google Scholar]
- Midassi S., Bedoui A., Bensalah N. Efficient degradation of chloroquine drug by electro-Fenton oxidation: effects of operating conditions and degradation mechanism. Chemosphere. 2020;260 doi: 10.1016/j.chemosphere.2020.127558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miège C., Choubert J.M., Ribeiro L., Eusèbe M., Coquery M. Fate of pharmaceuticals and personal care products in wastewater treatment plants - Conception of a database and first results. Environ. Pollut. 2009;157:1721–1726. doi: 10.1016/j.envpol.2008.11.045. [DOI] [PubMed] [Google Scholar]
- Ministry of Health Malaysia Malaysian Statistics on Medicines 2009 & 2010. Pharm. Serv. Div. Clin. Res. Cent. Minist. Heal. 2014:1–204. [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Altman D., Antes G., Atkins D., Barbour V., Barrowman N., Berlin J.A., Clark J., Clarke M., Cook D., D’Amico R., Deeks J.J., Devereaux P.J., Dickersin K., Egger M., Ernst E., Gøtzsche P.C., Grimshaw J., Guyatt G., Higgins J., Ioannidis J.P.A., Kleijnen J., Lang T., Magrini N., McNamee D., Moja L., Mulrow C., Napoli M., Oxman A., Pham B., Rennie D., Sampson M., Schulz K.F., Shekelle P.G., Tovey D., Tugwell P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni S.N., Amooey A.A., Tashakkorian H., Amouei A.I. Removal of dexamethasone from aqueous solutions using modified clinoptilolite zeolite (equilibrium and kinetic) Int. J. Environ. Sci. Technol. 2016;13:2261–2268. doi: 10.1007/s13762-016-1045-9. [DOI] [Google Scholar]
- Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., de Castro N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori N., Katayama M., Nukaga S. Triple therapy with hydroxychloroquine, azithromycin, and ciclesonide for COVID-19 pneumonia. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosekiemang T.T., Stander M.A., de Villiers A. Simultaneous quantification of commonly prescribed antiretroviral drugs and their selected metabolites in aqueous environmental samples by direct injection and solid phase extraction liquid chromatography - tandem mass spectrometry. Chemosphere. 2019;220:983–992. doi: 10.1016/j.chemosphere.2018.12.205. [DOI] [PubMed] [Google Scholar]
- Nakada N., Shinohara H., Murata A., Kiri K., Managaki S., Sato N., Takada H. Removal of selected pharmaceuticals and personal care products (PPCPs) and endocrine-disrupting chemicals (EDCs) during sand filtration and ozonation at a municipal sewage treatment plant. Water Res. 2007;41:4373–4382. doi: 10.1016/j.watres.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Nannou C., Ofrydopoulou A., Evgenidou E., Heath D., Heath E., Lambropoulou D. Antiviral drugs in aquatic environment and wastewater treatment plants: a review on occurrence, fate, removal and ecotoxicity. Sci. Total Environ. 2020;699 doi: 10.1016/j.scitotenv.2019.134322. [DOI] [PubMed] [Google Scholar]
- Naraginti S., Yu Y.-Y., Fang Z., Yong Y.-C. Visible light degradation of macrolide antibiotic azithromycin by novel ZrO2/Ag@TiO2 nanorod composite: transformation pathways and toxicity evaluation. Process Saf. Environ. Prot. 2019;125:39–49. doi: 10.1016/j.psep.2019.02.031. [DOI] [Google Scholar]
- Nippes R.P., Macruz P.D., Neves Olsen Scaliante M.H. Toxicity reduction of persistent pollutants through the photo-fenton process and radiation/H2O2 using different sources of radiation and neutral pH. J. Environ. Manage. 2021;289 doi: 10.1016/j.jenvman.2021.112500. [DOI] [PubMed] [Google Scholar]
- Olatunde J.O., Chimezie A., Tolulope B., Aminat T.T. Determination of pharmaceutical compounds in surface and underground water by solid phase extraction-liquid chromatography. J. Environ. Chem. Ecotoxicol. 2014;6:20–26. doi: 10.5897/jece2013.0312. [DOI] [Google Scholar]
- Olu-Owolabi B.I., Diagboya P.N., Mtunzi F.M., Düring R.A. Utilizing eco-friendly kaolinite-biochar composite adsorbent for removal of ivermectin in aqueous media. J. Environ. Manage. 2021;279 doi: 10.1016/j.jenvman.2020.111619. [DOI] [PubMed] [Google Scholar]
- Oppel J., Broll G., Löffler D., Meller M., Römbke J., Ternes T. Leaching behaviour of pharmaceuticals in soil-testing-systems: A part of an environmental risk assessment for groundwater protection. Sci. Total Environ. 2004;328:265–273. doi: 10.1016/j.scitotenv.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Paital B., Das K., Parida S.K. Inter nation social lockdown versus medical care against COVID-19, a mild environmental insight with special reference to India. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani A., Lauriola M., Romandini A., Scaglione F. Macrolides and viral infections: focus on azithromycin in COVID-19 pathology. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patibandla S., Jiang J.Q., Shu X. Toxicity assessment of four pharmaceuticals in aquatic environment before and after ferrate(VI) treatment. J. Environ. Chem. Eng. 2018;6:3787–3797. doi: 10.1016/j.jece.2018.05.024. [DOI] [Google Scholar]
- Pazoki M., Parsa M., Farhadpour R. Removal of the hormones dexamethasone (DXM) by Ag doped on TiO2 photocatalysis. J. Environ. Chem. Eng. 2016;4:4426–4434. doi: 10.1016/j.jece.2016.09.034. [DOI] [Google Scholar]
- Pereira A.M.P.T., Silva L.J.G., Meisel L.M., Lino C.M., Pena A. Environmental impact of pharmaceuticals from Portuguese wastewaters: geographical and seasonal occurrence, removal and risk assessment. Environ. Res. 2015;136:108–119. doi: 10.1016/j.envres.2014.09.041. [DOI] [PubMed] [Google Scholar]
- Petrovic M., Gros M., Barcelo D. Multi-residue analysis of pharmaceuticals in wastewater by ultra-performance liquid chromatography-quadrupole-time-of-flight mass spectrometry. J. Chromatogr. A. 2006;1124:68–81. doi: 10.1016/j.chroma.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Prasse C., Löffler D., Ternes T.A. Environmental fate of the anthelmintic ivermectin in an aerobic sediment/water system. Chemosphere. 2009;77:1321–1325. doi: 10.1016/j.chemosphere.2009.09.045. [DOI] [PubMed] [Google Scholar]
- Prasse C., Schlüsener M.P., Schulz R., Ternes T.A. Antiviral drugs in wastewater and surface waters: a new pharmaceutical class of environmental relevance? Environ. Sci. Technol. 2010;44:1728–1735. doi: 10.1021/es903216p. [DOI] [PubMed] [Google Scholar]
- Praveena S.M., Shaifuddin S.N.M., Sukiman S., Nasir F.A.M., Hanafi Z., Kamarudin N., Ismail T.H.T., Aris A.Z. Pharmaceuticals residues in selected tropical surface water bodies from Selangor (Malaysia): occurrence and potential risk assessments. Sci. Total Environ. 2018;642:230–240. doi: 10.1016/j.scitotenv.2018.06.058. [DOI] [PubMed] [Google Scholar]
- Price R.N., von Seidlein L., Valecha N., Nosten F., Baird J.K., White N.J. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect. Dis. 2014;14:982–991. doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh M., Anitha S., Poopal R.K., Shobana C. Evaluation of acute and sublethal effects of chloroquine (C18H26CIN3) on certain enzymological and histopathological biomarker responses of a freshwater fish Cyprinus carpio. Toxicol. Reports. 2018;5:18–27. doi: 10.1016/j.toxrep.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECOVERY (Randomised Evaluation of COVid-19 thERapY) 2020. Trial Low-cost Dexamethasone Reduces Death by up to One Third in Hospitalised Patients With Severe Respiratory Complications of COVID-19.https://www.recoverytrial.net/files/recovery_dexamethasone_statement_160620_final.pdf [Google Scholar]
- Rendal C., Kusk K.O., Trapp S. The effect of pH on the uptake and toxicity of the bivalent weak base chloroquine tested on Salix viminalis and Daphnia magna. Environ. Toxicol. Chem. 2011;30:354–359. doi: 10.1002/etc.391. [DOI] [PubMed] [Google Scholar]
- Rezazadeh T., Dalali N., Sehati N. Investigation of adsorption performance of graphene oxide/polyaniline reinforced hollow fiber membrane for preconcentration of Ivermectin in some environmental samples. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2018;204:409–415. doi: 10.1016/j.saa.2018.06.040. [DOI] [PubMed] [Google Scholar]
- Rhen T., Cidlowski J.A. Antiinflammatory action of glucocorticoids - New mechanisms for old drugs. N. Engl. J. Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- Rivera-Utrilla J., Sánchez-Polo M., Ferro-García M.Á., Prados-Joya G., Ocampo-Pérez R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere. 2013;93:1268–1287. doi: 10.1016/j.chemosphere.2013.07.059. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gil J.L., San Sebastián Sauto J., González-Alonso S., Sánchez Sanchez P., Valcarcel Y., Catalá M. Development of cost-effective strategies for environmental monitoring of irrigated areas in Mediterranean regions: traditional and new approaches in a changing world. Agric. Ecosyst. Environ. 2013;181:41–49. doi: 10.1016/j.agee.2013.09.007. [DOI] [Google Scholar]
- Russo D., Siciliano A., Guida M., Galdiero E., Amoresano A., Andreozzi R., Reis N.M., Li Puma G., Marotta R. Photodegradation and ecotoxicology of acyclovir in water under UV254 and UV254/H2O2 processes. Water Res. 2017;122:591–602. doi: 10.1016/j.watres.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Russo D., Siciliano A., Guida M., Andreozzi R., Reis N.M., Li Puma G., Marotta R. Removal of antiretroviral drugs stavudine and zidovudine in water under UV254 and UV254/H2O2 processes: Quantum yields, kinetics and ecotoxicology assessment. J. Hazard. Mater. 2018;349:195–204. doi: 10.1016/j.jhazmat.2018.01.052. [DOI] [PubMed] [Google Scholar]
- Sanderson H., Johnson D.J., Reitsma T., Brain R.A., Wilson C.J., Solomon K.R. Ranking and prioritization of environmental risks of pharmaceuticals in surface waters. Regul. Toxicol. Pharmacol. 2004;39:158–183. doi: 10.1016/j.yrtph.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Sanderson H., Laird B., Pope L., Brain R., Wilson C., Johnson D., Bryning G., Peregrine A.S., Boxall A., Solomon K. Assessment of the environmental fate and effects of ivermectin in aquatic mesocosms. Aquat. Toxicol. 2007;85:229–240. doi: 10.1016/j.aquatox.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Santos L.H.M.L.M., Gros M., Rodriguez-Mozaz S., Delerue-Matos C., Pena A., Barceló D., Montenegro M.C.B.S.M. Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: Identification of ecologically relevant pharmaceuticals. Sci. Total Environ. 2013;461–462:302–316. doi: 10.1016/j.scitotenv.2013.04.077. [DOI] [PubMed] [Google Scholar]
- Sartor G., Del Riccio M., Dal Poz I., Bonanni P., Bonaccorsi G. COVID-19 in Italy: considerations on official data. Int. J. Infect. Dis. 2020;98:188–190. doi: 10.1016/j.ijid.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayadi M.H., Sobhani S., Shekari H. Photocatalytic degradation of azithromycin using GO@Fe3O4/ ZnO/ SnO2 nanocomposites. J. Clean. Prod. 2019;232:127–136. doi: 10.1016/j.jclepro.2019.05.338. [DOI] [Google Scholar]
- Schweitzer N., Fink G., Ternes T.A., Duis K. Effects of ivermectin-spiked cattle dung on a water-sediment system with the aquatic invertebrates Daphnia magna and Chironomus riparius. Aquat. Toxicol. 2010;97:304–313. doi: 10.1016/j.aquatox.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Selvaraj V. 2020. Short-Term Dexamethasone in Sars-CoV-2 Patients Short-Term Dexamethasone in Sars-CoV-2 Patients. [PubMed] [Google Scholar]
- Sharun K., Tiwari R., Dhama J., Dhama K. Dexamethasone to combat cytokine storm in COVID-19: clinical trials and preliminary evidence. Int. J. Surg. 2020;82:179–181. doi: 10.1016/j.ijsu.2020.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., MacKman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Chang H., Sun Y., Wan Y. Determination and occurrence of natural and synthetic glucocorticoids in surface waters. Environ. Int. 2020;134 doi: 10.1016/j.envint.2019.105278. [DOI] [PubMed] [Google Scholar]
- Singh R., Adhikari D.R., Patil B.P., Talathi N.R., Hanamshetti S.R., Joshi R.M. Amoebic liver abscess: an appraisal. Int. Surg. 2011;96:305–309. doi: 10.9738/CC9.1. [DOI] [PubMed] [Google Scholar]
- Singh A.K., Singh A., Singh R., Misra A. Remdesivir in COVID-19: a critical review of pharmacology, pre-clinical and clinical studies. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:641–648. doi: 10.1016/j.dsx.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitre S., Dhadse S., Satyanarayan S. Toxicity of herbal pharmaceutical wastewater to a freshwater crustacean Ceriodaphnia dubia. Bull. Environ. Contam. Toxicol. 2009;82:275–279. doi: 10.1007/s00128-008-9578-3. [DOI] [PubMed] [Google Scholar]
- Sousa M.A., Gonçalves C., Cunha E., Hajšlová J., Alpendurada M.F. Cleanup strategies and advantages in the determination of several therapeutic classes of pharmaceuticals in wastewater samples by SPE-LC-MS/MS. Anal. Bioanal. Chem. 2011;399:807–822. doi: 10.1007/s00216-010-4297-0. [DOI] [PubMed] [Google Scholar]
- Syslová E., Landa P., Navrátilová M., Stuchlíková L.R., Matoušková P., Skálová L., Szotáková B., Vaněk T., Podlipná R. Ivermectin biotransformation and impact on transcriptome in Arabidopsis thaliana. Chemosphere. 2019;234:528–535. doi: 10.1016/j.chemosphere.2019.06.102. [DOI] [PubMed] [Google Scholar]
- Tanenbaum L., Tuffanelli D.L. Antimalarial agents. Arch. Dermatol. 1980;116:587–591. doi: 10.1007/978-1-61779-213-7_16. [DOI] [PubMed] [Google Scholar]
- Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W., Wu Y., Xiao W., Liu S., Chen E., Chen W., Wang X., Yang J., Lin J., Zhao Q., Yan Y., Xie Z., Li D., Yang Y., Liu L., Qu J., Ning G., Shi G., Xie Q. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:1–11. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tett S., Cutler D., Day R., Brown K. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br. J. Clin. Pharmacol. 1989;27:771–779. doi: 10.1111/j.1365-2125.1989.tb03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tišler T., Kožuh Eržen N. Abamectin in the aquatic environment. Ecotoxicology. 2006;15:495–502. doi: 10.1007/s10646-006-0085-1. [DOI] [PubMed] [Google Scholar]
- Tölgyesi Á., Verebey Z., Sharma V.K., Kovacsics L., Fekete J. Simultaneous determination of corticosteroids, androgens, and progesterone in river water by liquid chromatography-tandem mass spectrometry. Chemosphere. 2010;78:972–979. doi: 10.1016/j.chemosphere.2009.12.025. [DOI] [PubMed] [Google Scholar]
- Usman M., Farooq M., Hanna K. Environmental side effects of the injudicious use of antimicrobials in the era of COVID-19. Sci. Total Environ. 2020;745 doi: 10.1016/j.scitotenv.2020.141053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadi M., Hossinie N., Shekari Z. Comparative study of adsorption isotherms steroidal anti-inflammatory drug dexamethasone on carbon nanotube and activated carbon. Orient. J. Chem. 2013;29:491–496. doi: 10.13005/ojc/290213. [DOI] [Google Scholar]
- Van Wezel A.P., Jager T. Comparison of two screening level risk assessment approaches for six disinfectants and pharmaceuticals. Chemosphere. 2002;47:1113–1128. doi: 10.1016/S0045-6535(02)00048-6. [DOI] [PubMed] [Google Scholar]
- Verlicchi P., Al Aukidy M., Zambello E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment-A review. Sci. Total Environ. 2012;429:123–155. doi: 10.1016/j.scitotenv.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Vermillion Maier M.L., Tjeerdema R.S. Azithromycin sorption and biodegradation in a simulated California river system. Chemosphere. 2018;190:471–480. doi: 10.1016/j.chemosphere.2017.10.008. [DOI] [PubMed] [Google Scholar]
- W.H.O . 2020. WHO Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/ (retrieved on 11.07.2020) [Google Scholar]
- Wang W.L., Wu Q.Y., Wang Z.M., Niu L.X., Wang C., Sun M.C., Hu H.Y. Adsorption removal of antiviral drug oseltamivir and its metabolite oseltamivir carboxylate by carbon nanotubes: effects of carbon nanotube properties and media. J. Environ. Manage. 2015;162:326–333. doi: 10.1016/j.jenvman.2015.07.043. [DOI] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Xi H., Xu L., Jin M., Zhao W., Liu H. Ecotoxicological effects, environmental fate and risks of pharmaceutical and personal care products in the water environment: a review. Sci. Total Environ. 2021;788 doi: 10.1016/j.scitotenv.2021.147819. [DOI] [PubMed] [Google Scholar]
- Weizel A., Schlüsener M.P., Dierkes G., Ternes T.A. Occurrence of glucocorticoids, mineralocorticoids, and progestogens in various treated wastewater, Rivers, and streams. Environ. Sci. Technol. 2018;52:5296–5307. doi: 10.1021/acs.est.7b06147. [DOI] [PubMed] [Google Scholar]
- Wirtz V.J., Dreser A., Gonzales R. Trends in antibiotic utilization in eight Latin American countries, 1997-2007. Rev. Panam. Salud Publica/Pan Am. J. Public Heal. 2010;27:219–225. doi: 10.1590/S1020-49892010000300009. [DOI] [PubMed] [Google Scholar]
- Woche M., Scheibe N., von Tümpling W., Schwidder M. Degradation of the antiviral drug zanamivir in wastewater - the potential of a photocatalytic treatment process. Chem. Eng. J. 2016;287:674–679. doi: 10.1016/j.cej.2015.11.047. [DOI] [Google Scholar]
- Wood T.P., Duvenage C.S.J., Rohwer E. The occurrence of anti-retroviral compounds used for HIV treatment in South African surface water. Environ. Pollut. 2015;199:235–243. doi: 10.1016/j.envpol.2015.01.030. [DOI] [PubMed] [Google Scholar]
- Xu D., Chen M., Pan Xliang, Xia Lping, Wang H. Dexamethasone induces fetal developmental toxicity through affecting the placental glucocorticoid barrier and depressing fetal adrenal function. Environ. Toxicol. Pharmacol. 2011;32:356–363. doi: 10.1016/j.etap.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Yang S.N.Y., Atkinson S.C., Wang C., Lee A., Bogoyevitch M.A., Borg N.A., Jans D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020;177 doi: 10.1016/j.antiviral.2020.104760. [DOI] [PubMed] [Google Scholar]
- Yasojima M., Nakada N., Komori K., Suzuki Y., Tanaka H. Occurrence of levofloxacin, clarithromycin and azithromycin in wastewater treatment plant in Japan. Water Sci. Technol. 2006;53:227–233. doi: 10.2166/wst.2006.357. [DOI] [PubMed] [Google Scholar]
- Yi X.-H., Ji H., Wang C.-C., Li Y., Li Y.-H., Zhao C., Wang A., Fu H., Wang P., Zhao X., Liu W. Photocatalysis-activated SR-AOP over PDINH/MIL-88A(Fe) composites for boosted chloroquine phosphate degradation: performance, mechanism, pathway and DFT calculations. Appl. Catal. B Environ. 2021;293 doi: 10.1016/j.apcatb.2021.120229. [DOI] [Google Scholar]
- Zhou C., Wang Y., Chen J., Xu L., Huang H., Niu J. High-efficiency electrochemical degradation of antiviral drug abacavir using a penetration flux porous Ti/SnO2–Sb anode. Chemosphere. 2019;225:304–310. doi: 10.1016/j.chemosphere.2019.03.036. [DOI] [PubMed] [Google Scholar]
- Zurita J.L., Jos Á., Del Peso A., Salguero M., López-Artíguez M., Repetto G. Ecotoxicological evaluation of the antimalarial drug chloroquine. Aquat. Toxicol. 2005;75:97–107. doi: 10.1016/j.aquatox.2005.07.009. [DOI] [PubMed] [Google Scholar]