Abstract
Background
This an update of a Cochrane Review.
Paraquat is a widely used herbicide, but is also a lethal poison. In some low‐ and middle‐income countries (LMICs) paraquat is commonly available and inexpensive, making poisoning prevention difficult. Most of the people poisoned by paraquat have taken it as a means of self‐poisoning.
Standard treatment for paraquat poisoning prevents further absorption and reduces the load of paraquat in the blood through haemoperfusion or haemodialysis. The effectiveness of standard treatments is extremely limited.
The immune system plays an important role in exacerbating paraquat‐induced lung fibrosis. Immunosuppressive treatment using glucocorticoid and cyclophosphamide in combination has been developed and studied as an intervention for paraquat poisoning.
Objectives
To assess the effects of glucocorticoid with cyclophosphamide for moderate to severe oral paraquat poisoning.
Search methods
The most recent searches were run in September 2020. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Injuries Trials Register), Ovid MEDLINE(R), Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid OLDMEDLINE, Embase Classic + Embase (Ovid), ISI WOS (SCI‐EXPANDED, SSCI, CPCI‐S, and CPSI‐SSH), and trials registries. We also searched the following three resources: China National Knowledge Infrastructure database (CNKI 数据库); Wanfang Data (万方数据库); and VIP (维普数据库) on 12 November 2020. We examined the reference lists of included studies and review papers.
Selection criteria
We included randomised controlled trials (RCTs). For this update, in accordance with Cochrane Injuries' Group policy (2015), we included only prospectively registered RCTs for trials published after 2010. We included trials which assessed the effects of glucocorticoid with cyclophosphamide delivered in combination. Eligible comparators were standard care (with or without a placebo), or any other therapy in addition to standard care. Outcomes of interest included mortality and infections.
Data collection and analysis
We calculated the mortality risk ratio (RR) and 95% confidence interval (CI). Where possible, we summarised data for all‐cause mortality at relevant time periods (from hospital discharge to three months after discharge) in meta‐analysis, using a fixed‐effect model. We conducted sensitivity analyses based on factors including whether participants were assessed at baseline for plasma paraquat levels. We also reported data on infections within one week after initiation of treatment.
Main results
We included four trials with a total of 463 participants. The included studies were conducted in Taiwan (Republic of China), Iran, and Sri Lanka. Most participants were male. The mean age of participants was 28 years.
We judged two of the four included studies, including the largest and most recently conducted study (n = 299), to be at low risk of bias for key domains including sequence generation. We assessed one study to be at high risk of selection bias and another at unclear risk, since allocation concealment was either not mentioned in the trial report or explicitly not undertaken. We assessed three of the four studies to be at unclear risk of selective reporting, as no protocols could be identified. An important source of heterogeneity amongst the included studies was the method of assessment of participants' baseline severity using analysis of plasma levels (two studies employed this method, whilst the other two did not).
No studies assessed the outcome of mortality at 30 days following ingestion of paraquat.
Low‐certainty evidence from two studies indicates that glucocorticoids with cyclophosphamide in addition to standard care may slightly reduce the risk of death in hospital compared to standard care alone ((RR 0.82, 95% CI 0.68 to 0.99; participants = 322); results come from sensitivity analysis excluding studies not assessing plasma at baseline). However, we have limited confidence in this finding as heterogeneity was high (I2 = 77%) and studies varied in terms of size and comparators. A single large study provided data showing that there may be little or no effect of treatment at three months post discharge from hospital (RR 0.98, 95% CI 0.85 to 1.13; 1 study, 293 participants; low‐certainty evidence); however, analysis of long‐term results amongst participants whose injuries arose from self‐poisoning must be interpreted with caution.
We remain uncertain of the effect of glucocorticoids with cyclophosphamide on infection within one week after initiation of the treatment; this outcome was assessed by two small studies only (31 participants, very low‐certainty evidence) that considered leukopenia as a proxy or risk factor for infection. Neither study reported infections in any participants.
Authors' conclusions
Low‐certainly evidence suggests that glucocorticoids with cyclophosphamide in addition to standard care may slightly reduce mortality in hospitalised people with oral paraquat poisoning. However, we have limited confidence in this finding because of substantial heterogeneity and concerns about imprecision. Glucocorticoids with cyclophosphamide in addition to standard care may have little or no effect on mortality at three months after hospital discharge. We are uncertain whether glucocorticoid with cyclophosphamide puts patients at an increased risk of infection due to the limited evidence available for this outcome. Future research should be prospectively registered and CONSORT‐compliant. Investigators should attempt to ensure an adequate sample size, screen participants for inclusion rigorously, and seek long‐term follow‐up of participants. Investigators may wish to research the effects of glucocorticoid in combination with other treatments.
Plain language summary
What are the benefits and risks of treating paraquat poisoning with a combination of steroids and cyclophosphamide (an anti‐cancer medicine)?
Key messages
‐ Steroids given with cyclophosphamide (an anti‐cancer medicine) are unlikely to reduce the risk of death after paraquat poisoning in the short term, or at three months after hospital discharge.
‐ We are uncertain whether these medicines increase the risk of infection.
‐ Future studies need to be larger, measure the level of paraquat poisoning of patients accurately, and monitor patients in the long term. Research into steroids combined with other treatments could be useful.
What happens in people with paraquat poisoning?
Paraquat is used as a herbicide, but is also a deadly poison. Most people who are poisoned by paraquat have taken it as a means of self‐poisoning.
Treatment for paraquat poisoning focuses on the physical removal (via stomach pumping and other methods) of as much paraquat as possible from the person's digestive system (stomach) and blood. Any paraquat that remains inside the body causes inflammation that can damage the lungs severely and lead to death.
Steroids and cyclophosphamide (a medicine normally used in cancer treatment) are medicines that fight inflammation and so are also used to treat paraquat poisoning.
What did we want to find out?
We wanted to find out if a combination of steroids and cyclophosphamide (plus usual care) works better than usual care alone to reduce the number of people who die from paraquat poisoning.
We also wanted to find out if treatment with steroids plus cyclophosphamide causes an increased number of infections in patients.
What did we do?
We searched for studies that investigated the use of steroids and cyclophosphamide (plus usual care) compared with usual care alone in people poisoned with paraquat.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found four studies that involved 463 people with confirmed paraquat poisoning. Two studies were conducted in Taiwan (Republic of China), one in Iran, and one in Sri Lanka.
All participants were given either:
‐ usual care only, or
‐ steroids (methylprednisolone alone, or with dexamethasone) plus cyclophosphamide, as well as usual care. Cyclophosphamide was given before the steroid(s) or at the same time as them.
Two of the studies measured the severity of poisoning by testing patients’ plasma (a component of blood) at the start of the study. Plasma tests provide the best assessment of how seriously a person is affected by paraquat poisoning.
One study used a placebo (sham) treatment in addition to usual care. Two studies gave patients a steroid (dexamethasone) as part of the usual care.
Death while in hospital
The combined results of two studies showed that steroids plus cyclophosphamide (plus usual care) may slightly reduce the risk of death compared to usual care alone (with, or without, placebo) in people with paraquat poisoning.
Death 3 months after hospital discharge
One large study showed that at 3 months after discharge from hospital there may be no difference in the number of deaths between the people treated with steroids plus cyclophosphamide (plus usual care) and those treated with usual care alone.
Infection
Two small studies checked levels of white blood cells in patients (low levels can increase risk of infection). Neither study reported any infections in the week following treatment with steroids and cyclophosphamide. Due to the small size of the studies, we are very uncertain about whether the treatment affects the risk of infection within one week of treatment.
What are the limitations of the evidence?
The four studies differed in terms of the number of people in them, assessment of level of paraquat poisoning, and types of treatment. This limited our ability to draw firm conclusions from the evidence.
Overall, the studies we found were too small to provide answers to our questions.
How up to date is this evidence?
This review updates our previous review on this subject. The evidence is up to date to September 2020.
Summary of findings
Summary of findings 1. Glucocorticoid with cyclophosphamide plus usual care compared to usual care (with or without placebo) for oral paraquat poisoning.
| Glucocorticoid with cyclophosphamide plus usual care compared to usual care, with or without placebo, for oral paraquat poisoning | |||||
| Patient or population: people with moderate to severe oral paraquat poisoning Setting: hospital Intervention: glucocorticoid(s) with cyclophosphamide plus usual care Comparison: usual care (with or without placebo) | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with standard care | Risk with glucocorticoid plus cyclophosphamide | ||||
| Mortality at 30 days following the ingestion of paraquat | No included studies reported this outcome. | ||||
| All‐cause mortality at final follow‐up: at hospital discharge | 322 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 | (RR 0.82, 95% CI 0.68 to 0.99) (results of a sensitivity analysis) |
63 per 100 | 52 per 100 (43 to 62) |
| All‐cause mortality at final follow‐up: 3 months |
293 (1 RCT) | ⊕⊕⊝⊝ LOW 2,3 | RR 0.98 (0.85 to 1.13) | 72 per 100 | 71 per 100 (61 to 81) |
| New infection within 1 week after initiation of treatment Assessed through clinical diagnosis |
0 (0 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 | No clinical infections were diagnosed within 1 week after initiation of methylprednisolone and cyclophosphamide in the included studies. Lin 1999 reported the related outcome of leukopenia in 8 of 22 participants (36.4%) in the intervention arm. These participants spontaneously recovered 1 week later, with no mortality. Lin 2006 reported leukopenia in 6 of 16 participants (37.4%) in the intervention arm, all of whom recovered within 1 to 2 weeks. Neither Gawarammana 2017, the largest included study, nor Afzali 2008, measured infection or leukopenia. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1The sample size fell short of the required information size (439 participants required in each arm of the study), therefore we downgraded one level for imprecision, and a further level because of the high heterogeneity (I2 = 77%). 2We downgraded one level for imprecision, as the sample fell short of the required information size (as above). 3We downgraded once for indirectness: we consider that the reasons for participant mortality whilst being treated after self‐poisoning in hospital may differ substantially from the reasons for participant mortality at longer‐term follow‐up, in the absence of any other information on cause of death. 4The largest RCT, Gawarammana 2017, did not assess infection as an outcome, therefore this outcome was assessed by only two small trials. We therefore downgraded twice for serious imprecision, and once for indirectness (i.e. the outcome of leukopenia is a precursor of, but not identical to, infection).
Background
Paraquat is one of the most widely used herbicides worldwide, and is highly toxic to humans when ingested. It is commercially produced and has been sold in around 130 countries since 1961 (Tomlin 1994). Because it is inexpensive and widely available, it is a common cause of poisoning, through accidental or voluntary ingestion. Paraquat poisoning is most prevalent in lower‐ and middle‐income countries (LMICs), where its use is less stringently regulated.
Based on prevalence studies, Karunarathne 2020 estimates the global death toll from pesticide poisoning between 1960 and 2018 to be between 9,859,667 and 17,303,333, and notes that this is likely to be an underestimate. Paraquat is a principal cause of this fatal poisoning in many part of Asia and South America (Dawson 2010; Mew 2017).
The incidence of self‐poisoning increases with age, and is higher in males, although in some LMICs the incidence of self‐poisoning for young adult females exceeds that of males (Gunnell 2003).
The number of deaths due to poisoning, including those by paraquat, is now falling worldwide. This is likely to be a combination of industrialisation and the consequent movement of people out of rural areas, and the fact that many of the most dangerous pesticides, including paraquat, are being banned in an increasing number of countries (Karunarathne 2020). For example, paraquat was phased out and eventually banned in 2010 in Sri Lanka, where it had been the leading cause of death from pesticide poisoning (Eddleston 2003; Knipe 2014). In addition, the overall suicide rate by any means has also decreased markedly in China, which has driven down global numbers (Mew 2017). However, this global trend is unfortunately not reflected in all parts of the world, and the incidence of pesticide poisoning is increasing in some countries, such as Malaysia (Kamaruzaman 2019). Worldwide, pesticide poisoning still represents a serious challenge to public health and accounts for an estimated 150,000 to 168,000 annual deaths, representing around 20% of global suicides (Karunarathne 2020; Mew 2017).
Description of the condition
The mortality rate for paraquat poisoning is estimated to be as high as 60% to 90%, but the exact mechanism of this poisoning is still poorly understood (Gao 2020; Xu 2019). The damage done by paraquat is thought to be primarily due to redox cycling and the subsequent generation of highly reactive oxygen and nitrite species. The resultant oxidative stress results in mitochondrial toxicity, induced apoptosis, lipid peroxidation, and severe secondary inflammation. These mechanisms are thought to act synergistically to cause organ damage (Gao 2020; Gawarammana 2011).
Lung injury is one of the main features of paraquat poisoning: paraquat molecules selectively accumulate in the lungs, and severe inflammation and irreversible pulmonary fibrosis follows, which is known as 'paraquat lung' (Fukuda 1985; Smith 1975). This fibrosis leads to reduced ventilatory and lung diffusion capacity, resulting in hypoxaemia, which is often lethal. A large proportion of patients appear asymptomatic until signs of breathing difficulty emerge; it is difficult to predict the outcome of a patient who appears normal but is actually suffering lung fibrosis (Eddleston 2003).
Although the lung is the primary target organ, multiple organs are affected by paraquat poisoning. Renal function is impaired as the body attempts to excrete paraquat, and the hepatobiliary system, nervous system, and heart are also affected, often resulting in multiple organ failure (Gao 2020).
Diagnosis of paraquat poisoning has evolved over time. Currently the only reliable biomarker for the condition is time‐adjusted paraquat concentration.
The prognosis in paraquat poisoning is associated with the amount of toxin ingested.
In low‐dose poisoning (< 20 mg of paraquat ion per kilogram of body weight), patients are often asymptomatic, or may develop vomiting or diarrhoea, but have a good chance of recovery.
In moderate‐dose poisoning (20 mg to 40 mg of paraquat ion per kilogram of body weight), initial renal and hepatic dysfunction is common. Mucosal damage may become apparent with sloughing of the mucous membranes in the mouth. Difficulty in breathing may develop after a few days in more severe cases. After about 10 days, although renal function often returns to normal, radiological signs of lung damage usually develop. Lung damage is usually followed by irreversible massive pulmonary fibrosis manifested by the progressive loss of the lungs' ability to breathe, and deterioration continues until the patient eventually dies, between two and four weeks after ingestion.
In high‐dose poisoning (> 40 mg paraquat ion per kilogram of body weight), toxicity is much more severe, and death occurs early (within 24 h to 48 h) from multiple organ failure. Vomiting and diarrhoea are severe, with considerable fluid loss. Renal failure, cardiac arrhythmias, coma, convulsions, and oesophageal perforation lead to death (WHO IPCS 2009). This is known as fulminant poisoning (Vale 1987).
Description of the intervention
There is no specific antidote for paraquat poisoning or standard guidelines for treatment (Gawarammana 2011). Treatment often involves decontamination using absorbents such as activated charcoal or Fuller's earth; or elimination methods such as haemodialysis, haemofiltration, or haemoperfusion; antioxidant therapy; or immunosuppressive therapy (Lavergne 2018).
Immunosuppressive therapy
Glucocorticoid drugs are usually used to reduce inflammation and to suppress the immune response. Glucocorticoids are a class of corticosteroid hormones that bind to glucocorticoid receptors. The activated glucocorticoid receptor‐glucocorticoid complex leads to the up‐regulation of the expression of anti‐inflammatory proteins. The glucocorticoids commonly used to treat paraquat poisoning are methylprednisolone and dexamethasone.
Cyclophosphamide is a broad‐spectrum immunomodulatory drug, usually used in the treatment of cancer and autoimmune disease.
Since the 1970s, glucocorticoids and cyclophosphamide have been used in combination as a means of suppressing the inflammation responsible for pulmonary fibrosis (Eddleston 2003), and were endorsed as a successful treatment by Addo and Poon‐King (Addo 1986). The effectiveness of this treatment combination for paraquat poisoning remains unclear.
How the intervention might work
In animal studies, glucocorticoids such as dexamethasone have been shown to reduce lipid peroxidation and paraquat accumulation in the lungs (Dinis‐Oliveira 2006). Cyclophosphamide has a broad immunomodulatory effect.
Given the profound secondary inflammation seen in paraquat poisoning, particularly in the lungs where paraquat molecules actively accumulate, immunosuppression would appear to be a logical therapy for paraquat poisoning.
Why it is important to do this review
Though it has been inferred from experimental, Lee 1984, and clinical experience, Agarwal 2006, that immunosuppressive therapy might reduce deaths among paraquat‐poisoned patients, there is no consensus on the effectiveness of this treatment. Considering the potential hazards associated with immunosuppressive drugs (Winsett 2004) ‐ such as making patients more prone to infection ‐ an update of the previous Cochrane Review on this topic was due in order to revisit assessments of effectiveness and risk, support decision‐making and inform further research.
Objectives
To assess the effects of glucocorticoid with cyclophosphamide for moderate to severe oral paraquat poisoning.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), including cluster‐RCTs. We excluded any trials of a cross‐over design as this is incompatible with our review question. For RCTs published after 2010, we considered only prospectively registered RCTs to be eligible for this 2021 update, in accordance with Cochrane Injuries Group policy.
Types of participants
Any person of any age, diagnosed with oral paraquat poisoning (although poisoning via inhalation or contact with skin is possible, these were not foci of the review). The review focuses on participants with moderate to severe poisoning, given that generally in trials (as in clinical practice) individuals with mild cases of paraquat poisoning (which tend to resolve on their own) and those with extremely severe intoxication (see definition of 'fulminant' in both Description of the condition and Included studies) may not be treated; or, if they are treated, may not be analysed with other groups of participants, due to prognosis.
Types of interventions
Intervention: glucocorticoid with cyclophosphamide, in combination.
Eligible comparators: placebo; standard care alone; or any alternative therapy in addition to standard care.
We excluded studies that focused on any single immunosuppressant (either a glucocorticoid drug or cyclophosphamide by themselves) or other combinations of therapies.
Types of outcome measures
Mortality at 30 days following the ingestion of paraquat.
All‐cause mortality at the end of the maximum follow‐up period recorded by investigators, as long as they are clinically compatible.
Otherwise we reported findings in the short term (typically at hospital discharge, which of necessity varied between participants), and longer term (three months after discharge in the one trial that reported any follow‐up), as appropriate.
New infections diagnosed within one week after initiation of treatment.
Search methods for identification of studies
In order to reduce publication and retrieval bias we did not restrict our search by language, date, or publication status.
Search strategies with notes for this update are listed in Appendix 1. Search methods and strategies for previous versions of the review can be found in Appendix 2 and Appendix 3.
Electronic searches
The Cochrane Injuries Group's Information Specialists searched:
the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Injuries Trials Register) in the Cochrane Library (searched 21 September 2020);
Ovid MEDLINE(R), Ovid MEDLINE In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid OLDMEDLINE (1946 to 21 September 2020);
Embase Classic + Embase (OvidSP) (1947 to 21 September 2020);
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to 21 September 2020);
ISI Web of Science: SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI (2017 to 22 September 2020)
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (21 September 2020);
Current Controlled Trials (www.controlled-trials.com/) (20 September 2020);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/) (21 September 2020).
The following Chinese databases were searched by one review author (LL):
China National Knowledge Infrastructure (CNKI 数据库) (12 November 2020);
Wanfang Data (万方数据库) (12 November 2020);
VIP (维普数据库) (12 November 2020).
Searching other resources
We searched the internet through search engines google.com and baidu.com on 12 November 2020 using the term 'clinical trial & paraquat'. We also checked the reference lists of reports and systematic/literature reviews on paraquat poisoning for potentially relevant published or unpublished trials. We contacted the authors of the included trials for further information.
Data collection and analysis
Selection of studies
For the present version of the review, three review authors (LL, JD and HW) independently screened the search results from the English language databases. LL, CY and BC independently screened the results from the Chinese databases. We obtained and assessed the full‐text versions of potentially relevant trials. Duplicate reports were identified and noted.
Review authors LL and BC disagreed about the inclusion of the Afzali 2008 study due to the use of alternate allocation as the method of sequence generation. Review author CY moderated the discussion on inclusion of this trial, and in the last published version of this review (Li 2014), it was agreed that the trial would be included, but classified as being at high risk of bias. Following statistical advice in 2021, the author team as a whole decided once again to retain the trial, whilst highlighting issues to do with its interpretation, on the basis not only of design, but also due to investigator‐acknowledged failure to assess participants via plasma concentration.
We identified one new study that met our eligibility criteria (Gawarammana 2017). A flow chart of the study selection process is shown in Figure 1.
1.
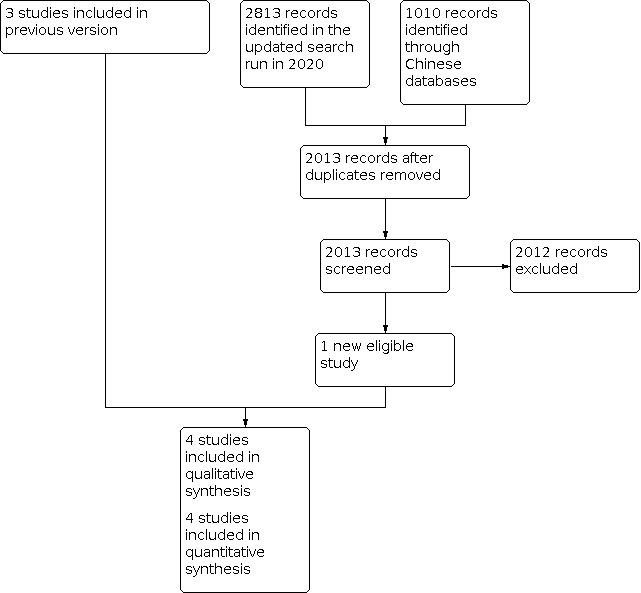
Study flow diagram for 2021 update
Data extraction and management
Three review authors (LL, BC, and JD) independently extracted the following data from the four included trials:
study design;
study setting (country, city, number of centres);
inclusion and exclusion criteria for studies;
number, gender, and severity of intoxication of participants in the intervention and control groups;
outcome data including number of deaths and infection cases assessed at each data collection point;
loss to follow‐up/withdrawals from analysis;
information on study conduct sufficient to assess risk of bias, including evidence of prospective trial registration for all studies published from 2010 onwards.
Assessment of risk of bias in included studies
Four review authors (LL, BC, JD, and HW) independently evaluated the risk of bias for each included trial based on the following domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and any other sources of bias. We based our judgements on the criteria outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed each domain as at low or high risk, or unclear risk if the information provided was insufficient to permit a judgement on a particular area of bias, or if it was unclear in which direction a bias might lead. Risk of bias judgements are provided in the risk of bias tables in Characteristics of included studies, and summaries of the judgements are given in Figure 2 and Figure 3.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study
Measures of treatment effect
Where feasible, we calculated the risk ratio (RR) and 95% confidence interval (CI). We summarised data for all‐cause mortality at final follow‐up in a meta‐analysis using a fixed‐effect model. When assuming a 75% and 65% mortality rate for control and treatment group respectively, with a level of significance of 5% and a power of 90%, we calculated that 439 participants would be required in each arm of a study to detect the effect sufficiently.
Unit of analysis issues
No unit of analysis issues arose within the current version of this review. We excluded cross‐over studies as inappropriate for our review question (given our focus on mortality). We identified no cluster‐RCTs and no trials with multiple eligible intervention (or comparator) arms.
Should the issue of clustering arise in future updates, we plan to use the methods detailed in the Cochrane Handbook for Systematic Reviews of Interventions as applicable (Higgins 2021), based on information provided by investigators, including: the number of clusters (or groups) randomised or the average (mean) size of each cluster; and an estimate of the intracluster (or intraclass) correlation coefficient (ICC).
Should the issue of multiple eligible arms or comparator groups arise, we will consider appropriate methods including pooling data from eligible arms, or conversely treating a multiple‐arm study as two or more studies. In the latter case, we will take care to reduce the number of participants in the arm(s) left intact, in order to preserve the independence of findings.
Dealing with missing data
The amount of missing data in each trial was assessed, outcome by outcome, and any potential effects on results informed our risk of bias assessment (see Risk of bias in included studies) and GRADE ratings.
Assessment of heterogeneity
We considered possible causes of clinical heterogeneity (e.g. how studies measured severity of poisoning, treatment regimen, variation in standard care, length of follow‐up) when making decisions about whether to pool data.
Where possible and appropriate, we examined statistical heterogeneity using the Chi2 test and I2 statistic. For the outcome of mortality, we considered an I2 value of more than 50% as indicative of substantial heterogeneity, and acknowledged this in our interpretation of the data.
Assessment of reporting biases
Had we identified 10 or more eligible trials for this update, we would have investigated the possibility of reporting biases, including publication bias, by assessing funnel plots for asymmetry where 10 or more studies reported on the same outcome (Egger 1997). We acknowledge that asymmetry could be due to publication bias or to a genuine relationship between trial size and effect size. We also searched for evidence of prospective trial registration and trial protocols for all studies included in the review.
Data synthesis
Where feasible, we analysed data using Review Manager 5 (Review Manager 2020). Where this was not feasible, we reported results narratively.
Subgroup analysis and investigation of heterogeneity
Had there been sufficient studies, we would have performed subgroup analysis based on the treatment regimen used to investigate the impact on both mortality and adverse effects.
Sensitivity analysis
We performed post hoc sensitivity analyses to investigate the effect of reporting bias in the Lin 1999 study, as well as on the effect of possible selection bias in the Afzali 2008 study (see Effects of interventions). These two studies also merited exclusion from sensitivity analyses because they were subject to bias based on lack of appropriate assessment of plasma paraquat levels on entry to the study, leading ultimately to our decision to present a sensitivity analysis for the outcome 'all‐cause mortality at final follow‐up: at hospital discharge' in Table 1.
Summary of findings and assessment of the certainty of the evidence
We summarised our findings for all outcomes in Table 1.
We assessed the certainty of the evidence for each outcome measure using the GRADE approach (Yan 2016). GRADE is used to assess the certainty of evidence according to the following five domains: imprecision, indirectness, inconsistency, risk of bias, and publication bias. The certainty of evidence assessments are provided in Table 1 and were generated within the online tool GRADEpro GDT (GRADEpro GDT 2020).
Results
Description of studies
Results of the search
The study selection process is outlined in Figure 1.
The English language electronic search retrieved a total of 2813 records across all years. We identified eight potentially relevant trials, four of which were eligible for inclusion in this update.
Review author LL identified a total of 1010 reports through a Chinese language search on Chinese language databases; none of these met our inclusion criteria.
Review author LL identified one report whilst searching google.com using the term 'clinical trial & paraquat', which was later excluded (Tsai 2009). We identified no additional eligible RCTs through screening reference lists or literature reviews.
Included studies
In this update, we included one new study (Gawarammana 2017), which resulted in a total of four included studies, with a combined total of 463 participants for analysis (Afzali 2008; Gawarammana 2017; Lin 1999; Lin 2006). See also Characteristics of included studies.
Design and setting
All of the included studies were reported as parallel RCTs; however, following contact with one trialist, it became clear that one study used quasi‐randomised methods to generate the sequence (Afzali 2008).
Two studies were conducted at a single site (a hospital in Taiwan, which featured a dedicated poison control centre) (Lin 1999; Lin 2006); one study took place at single site in Hamadan, Iran; and one study was a multisite RCT conducted at six district hospitals in Sri Lanka (Gawarammana 2017).
Sample sizes
No sample size calculation is mentioned in the text of Lin 1999, which reports data for 121 participants but retrospectively excluded those with fulminant poisoning who died within one week of intoxication (n = 71), leaving 50 participants. The same trialists reported for their later, smaller study (n = 23), which assessed a different treatment regimen, that an "a priori estimate of sample size for this study could not be performed because only case reports using the new treatment method were noted in previous literature" (Lin 2006). No sample size calculation is mentioned in the text of Afzali 2008 (n = 20). Investigators in Gawarammana 2017 (n = 299) did provide a sample size calculation, reporting: "In order to be able to detect whether either regimen increases survival from 18% to 28%, with a significance level (alpha) of 5% and a power of 80%, a minimum of 295 patients must be recruited to each arm of the trial (i.e. 590 patients in total)" (p 634). This recruitment objective was manifestly unmet due to the trial stopping early, which was sanctioned by the Data Monitoring and Ethics Committee because of a "collapse" in recruitment due firstly to restrictions on, and subsequently the banning of, paraquat use in Sri Lanka.
Participants
Inclusion criteria
All four included trials involved paraquat‐intoxicated individuals who had had their paraquat poisoning confirmed by sodium dithionite test. The included trials varied in the time period during which participants could be recruited. In both Lin 1999 and Lin 2006, participants had to present within 24 hours of ingestion of paraquat. No explicit criteria were given for presentation in the trial reported in Afzali 2008, but figures provided for mean time between poisoning and admission to hospital never exceeded seven hours. The largest and most recent trial reported inclusion criteria permitting recruitment up to 48 hours after ingestion (Gawarammana 2017).
As mentioned in Description of the condition and Types of participants, symptoms of paraquat ingestion are dose‐dependent, and intoxication is usually categorised as mild or 'low dose', moderate, or severe or 'high dose'. Mild cases tend to resolve without further sequelae and therefore trialists typically exclude such individuals when conducting trials of therapeutics. Similarly, where they can be identified, 'fulminant' participants who have ingested a dose likely to kill within 48 to 72 hours due to multiple organ failure are also typically excluded, if possible. Palliative care tends to be the recommended course for people in this category. Methods for assessing degree of intoxication evolved between the conduct of the oldest study in this review, Lin 1999, and the latest study, Gawarammana 2017. We considered these differential criteria for defining severely poisoned patients to be a potential source of clinical heterogeneity, and this was reflected in sensitivity analysis for mortality and in our GRADE assessments.
All of the included studies attempted to exclude severely poisoned patients who were expected to die imminently with little chance of responding to therapy: Gawarammana 2017 excluded severely poisoned patients with a Glasgow Coma Score of less than 8/15 or a systolic blood pressure less than 70 mmHg that did not respond to 1 L of intravenous fluid; Afzali 2008 only provided analysis for 20 of 45 participants screened for the trial, apparently not randomising 15 participants whose sodium dithionite reactions tests indicated their cases were too mild, and 10 participants considered fulminant; they specifically listed their failure to conduct plasma assessments as a study limitation.
The Lin 2006 trial only included participants with a predicted mortality of > 50% and ≤ 90% according to the Hart 1984 formula. Lin 1999 excluded all participants who died within one week of poisoning in what appears to be a post hoc decision (see Risk of bias in included studies), but which in fact leads to a population broadly in line with other studies included in this review. The authors of the Lin 1999 paper ‐ the oldest in the review ‐ defended their choice to analyse as they did by explaining that they were constrained at the time of recruitment "because no paper suggested an index to accurately predict the clinical severity of PQ [paraquat]‐poisoned patients up till now" (Buckley 2001).
Demographics
Although inclusion criteria, where specified, admitted the recruitment of adolescents from the age of 14, Gawarammana 2017, or 15, Lin 2006, the included participants tended to be adults, with means of 33 to 37 years of age in Lin 2006, 26 in Afzali 2008, 27 in Gawarammana 2017, and 27 to 37 in the two groups reported within Lin 1999. The majority of participants across trials (69%) were male.
Diagnostic certainty/severity
Two of the four included studies used the Severity Index of Paraquat Poisoning (SIPP), defined as plasma paraquat poisoning in mg/L multiplied by the number of hours since paraquat ingestion, as a means of assessing severity on arrival to hospital (Sawada 1988). By this measure, participants were comparable at baseline within Lin 2006 (mean values between 14 and 16), but investigators in Gawarammana 2017 reported a small but significant baseline difference in participants allocated to intervention (mean 18.4) compared to control (mean 13.1). Lin 1999, Lin 2006, and Afzali 2008 also reported urine colour (whether "dark blue" or "navy blue") and found groups comparable, although there is consensus in the field that only plasma tests are truly reliable. The authors of Afzali 2008 state lack of such testing in the "limitations" section of their paper; the authors of Lin 1999 did not, but they included such testing in their subsequent study (Lin 2006).
Interventions
All studies compared the use of standard care alone versus standard care and glucocorticoid (invariably methylprednisolone or dexamethasone, or both) with cyclophosphamide for individuals with paraquat poisoning. Gawarammana 2017 also provided normal saline as a placebo within the control group, which no other trial did.
Standard care
Standard care given to both groups was defined in Lin 1999 and Lin 2006 as including gastric lavage with normal saline followed by active charcoal added in magnesium citrate given through a nasogastric tube, courses of 8‐hour active charcoal haemoperfusion therapy and administration of intravenous dexamethasone. "Conventional treatment" within the small Afzali 2008 study was similar, including gastric lavage with normal saline, "charcoal‐sorbitol lavage every two to four hours for three days, forced alkalinised diuresis in the first day of admission to the hospital, and haemodialysis of four hours duration". This differed from Gawarammana 2017, where standard care was limited to intravenous fluid, activated charcoal, and pain relief.
Treatment regimens
The combinations of cyclophosphamide and glucocorticoid treatments in the four studies used pulse therapy administration (high doses daily over a short period of time, to maximise therapeutic effect whilst minimising toxicity).
Following gastric lavage, active charcoal and charcoal haemoperfusion therapy, the treatment arm in Lin 1999 received infusions of cyclophosphamide for two hours per day for two days, and methylprednisolone for two hours per day for three days; 10 mg dexamethasone was then administered every eight hours for 14 days. The treatment arm in Gawarammana 2017 was similar, except that the methylprednisolone was infused over one hour rather than two, and participants were given 8 mg dexamethasone daily for 14 days. The authors of Afzali 2008 reported a similar regimen (but without administration of dexamethasone), with cyclophosphamide infused in two hours for two days with methylprednisolone also infused for four hours, repeated over three consecutive days. Participants in the Afzali 2008 and Gawarammana 2017 trials also received MESNA (2‐mercaptoethane sulfonate sodium (Na)) to mitigate the effects of cyclophosphamide.
Lin 2006 had a more complex treatment regimen. Following the same initial pulse therapy of cyclophosphamide and methylprednisolone described in Lin 1999, 5 mg dexamethasone was administered every six hours until the participant's partial pressure of oxygen (PaO2) reached a certain threshold. Another pulse of methylprednisolone and cyclophosphamide (the latter only for one day, and only if it was more than two weeks since the previous cyclophosphamide dose) was repeated if physiological parameters fell below a certain threshold, indicating a poor outcome. Dexamethasone was then continued until the desired PaO2 was reached.
Outcomes
All of the included studies reported the primary outcome, mortality, at hospital discharge (Afzali 2008; Gawarammana 2017; Lin 1999; Lin 2006), and in one case, at three months after hospital discharge (Gawarammana 2017). No study reported on the outcome of infections (a known sequela of immunosuppression therapy), although two studies considered the related outcome of leukopenia (Lin 1999; Lin 2006). Afzali 2008 did not report on infections or on adverse events per se, but did confirm that no complications of treatment appeared by the time participants had been discharged. The fourth trial assessed participants for two specific potential adverse effects of treatment (haematuria, bladder pain) attributable to cyclophosphamide (Gawarammana 2017).
Study funding sources
Two studies did not report any source of funding (Afzali 2008; Lin 1999); one reported funding from a governmental body in Taiwan (Lin 2006). One study reported mixed sources of funding including charitable and research grants from the UK and Australia as well as support from Syngenta, a manufacturer of herbicides including paraquat (Gawarammana 2017).
Excluded studies
We excluded four trials: one trial with uncertain sequence generation that assessed the effects of methylprednisolone only (Tsai 2009); one trial that used a historical control (Perriens 1992); and two potentially eligible studies that were not preregistered as required by the Cochrane Injuries Group for studies published after the year 2010 (Chen 2014; Ghorbani 2015).
Risk of bias in included studies
We assessed Lin 2006 to be at low risk of bias across most domains. Lin 1999 randomised all urine‐positive patients, but presented the outcomes for those who died within one week of poisoning separately from those who survived longer. Presenting the data separately to exclude very severely poisoned people is reasonable given the specific clinical features of paraquat poisoning, but this post hoc decision introduces the risk of selective reporting. The small trial conducted by Afzali 2008 was poorly reported, but personal communication with the authors permitted some judgements with respect to risk of bias, which was frequently assessed as high risk because of the method of sequence generation. Gawarammana 2017 was a well‐conducted, preregistered multicentre RCT that was underpowered due to stopping early. Our risk of bias judgements are recorded in the risk of bias tables in Characteristics of included studies and displayed in Figure 2 and Figure 3.
Allocation
Lin 2006 reported an appropriate method of sequence generation and allocation concealment using a sequence of labelled cards in sealed envelopes that were prepared by a statistical advisor. Gawarammana 2017 used computer programs to generate the random sequence by an IT consultant, and the allocations were conducted by the pharmacist and concealed from other members of the team. Lin 1999 generated the randomisation sequence using a random numbers table, but there was no mention of allocation concealment, so we judged this to be at unclear risk of bias.
Afzali 2008 used alternate allocation as a method of sequence generation, which obviates concealment of allocation and under normal circumstances would call for an assessment of high risk of bias and exclusion of the study in sensitivity analysis, if the trial were to be included in the review at all. After discussion with the Cochrane Injuries statistician, we revised our assessment to unclear risk, given that the pace of recruitment (just 20 eligible participants across 25 months) reduced the risk of manipulation of the allocation of any given participant to a particular group.
Blinding
In Gawarammana 2017, the pharmacist allocated participants and prepared identical treatment packs of both active treatment and placebo, protecting team members and participants from awareness of allocation.
In Lin 1999 and Lin 2006, the statistician who contributed to the trial report was blinded to the allocation. Treating physicians and participants were not blinded, nor could they be, as there was no placebo. Given that the primary outcome is death, this may not constitute a significant risk of bias. We therefore judged the risk of bias for blinding as low in this version of the review, instead of high as in previous versions, but considered the lack of blinding to be an issue when performing the GRADE assessment for the outcome of infection.
Investigators involved in the Afzali 2008 trial confirmed by personal correspondence that no blinding was attempted of any of the staff involved in the trial (nor could they be, with no placebo in use). As with Lin 1999 and Lin 2006, we believe this does not constitute a high risk of bias for the objective outcome used in our review (mortality at discharge).
Incomplete outcome data
Our main outcome of interest was mortality, which was reported in full in all studies either at hospital discharge or at six weeks. In Gawarammana 2017, the primary result, in‐hospital death, was reported for all 299 randomised participants, as well as follow‐up at three months after hospital discharge, by which time only six participants had been lost to follow‐up, two out of 152 (1.3%) from the control group and four out of 147 (2.7%) from the treatment group. Given the high risk of death (65% in treatment group and 75% in control group), we judged the potential impact of the loss of outcome data on the risk of attrition bias to be small.
Selective reporting
We identified no suggestion of selective reporting in Gawarammana 2017, as it was prospectively registered and appears to have reported methods and data in full as planned, up to the time of stopping prematurely (see below).
Lin 2006 appears to have reported on all relevant outcomes, but in the absence of a prospective registration or a trial protocol, and in compliance with the Cochrane Injuries Group policy (2015), we must assess the risk of selective outcome reporting as (at best) unclear whenever no prospective plans are made public.
Lin 1999 randomised 121 participants, but made what appears to be a post hoc decision to exclude 71 of these participants following treatment, based on the very severe nature of their poisoning. Although the clinical decision to exclude these fulminant patients seems sensible given the predictable course of very severe paraquat poisoning ‐ which was done in all four included studies ‐ the criteria by which these patients were defined (death within a week) and the post hoc nature of the decision leaves the study open to reporting bias. Nevertheless, we believe we have obviated the risk of bias from selective reporting by presenting data for both groups and by using sensitivity analysis. However, as there is no evidence of prospective reporting and no identifiable protocol, we are again obliged to report the risk of bias for this domain to be unclear.
Afzali 2008 was a small study reported in a very short paper, with virtually no information on study conduct and a lack of clarity on the screening and allocation process. Some relevant information was acquired by personal correspondence to which an author on a previous version of this review has lost access. In view of this, and in the absence of a prospective registration or a trial protocol, and in compliance with the Cochrane Injuries Group policy, we must assess the risk of selective outcome reporting as at best unclear whenever no prospective plans are made public.
Other potential sources of bias
The Gawarammana 2017 trial was stopped early "after consultation with the data monitoring and ethics committee due to a collapse in recruitment" following the phasing out and eventual banning of paraquat in Sri Lanka, where the trial was being conducted. However, since the reason for stopping early was unrelated to the observed intervention effect, we do not deem this to be a source of bias. The same trialists reported a small baseline difference in the median SIPP score of participants allocated to intervention (median 18.4) compared to control (median 13.1). As the method of randomisation was unlikely to have been compromised (it was done using purpose‐designed software), this difference is likely to have been caused by chance alone, and is therefore not considered to be a source of bias.
Effects of interventions
See: Table 1
Mortality at 30 days following the ingestion of paraquat
None of the included studies assessed this outcome.
All‐cause mortality at the end of the follow‐up period
See Figure 4; Figure 5; Figure 6
4.

Forest plot of comparison: 1 All‐cause mortality, outcome: 1.1 All‐cause mortality
5.

Forest plot of comparison: 1.2 Sensitivity analysis: all‐cause mortality at final follow‐up: including fulminant (and excluding Afzali 2008 due to risk of selection bias)
6.

Forest plot of comparison: 1 All‐cause mortality, outcome: 1.3 Sensitivity analysis: all‐cause mortality: excluding studies without plasma paraquat assessment at baseline
All‐cause mortality (in hospital)
The primary analysis suggests that participants who receive glucocorticoids with cyclophosphamide in addition to standard care may have lower mortality in hospital than those who received standard care alone (risk ratio (RR) 0.73, 95% CI 0.61 to 0.88; 4 studies; 392 participants). Statistical heterogeneity for this result was substantial (I2 = 70%) (Analysis 1.1). There was also some clinical heterogeneity relating to participant inclusion criteria: Lin 1999 randomised 121 participants, but made what appears to be a post hoc decision to exclude 71 following treatment, given that they died within a week due to the very severe nature of their poisoning.
1.1. Analysis.

Comparison 1: All‐cause mortality, Outcome 1: All‐cause mortality
We thus had serious concerns about the possibility of selection bias, and to investigate, we performed an exploratory post hoc sensitivity analysis to re‐include the 71 fulminant participants from Lin 1999. With the fulminant participants included, the estimated effect of glucocorticoid with cyclophosphamide in addition to standard care was reduced to a less clear effect (RR 0.80, 95% CI 0.69 to 0.93; 463 participants). (Analysis 1.2). This was expected, given that fulminant patients will almost inevitably die irrespective of treatment (WHO IPCS 2009; Gawarammana 2017). Heterogeneity remained substantial (I2 = 53%).
1.2. Analysis.

Comparison 1: All‐cause mortality, Outcome 2: Sensitivity analysis: all‐cause mortality re‐including fulminant participants
We also elected to perform a second previously unplanned sensitivity analysis, excluding all participants from both the Lin 1999 and Afzali 2008 trials, on the grounds that they stood out amongst the included trials by not using SIPP methods at baseline. The results left two studies with combined results suggesting that there may be a small benefit of the intervention on the outcome of mortality (RR 0.82, 95% CI 0.68 to 0.99; participants = 322) (Analysis 1.3). The I2 of 77% indicates that only the smaller study (Lin 2006, n = 23) with just seven participants in the control group showed benefit, whilst the larger trial (Gawarammana 2017) found none. We chose to present the latter results as our main analysis for the purposes of GRADE, and judged the certainty of the evidence to be low, downgrading twice for imprecision and inconsistency (Analysis 1.3; Table 1).
1.3. Analysis.

Comparison 1: All‐cause mortality, Outcome 3: Sensitivity analysis: all‐cause mortality: excluding studies without plasma paraquat assessment at baseline
All‐cause mortality (long term ‐ three months after discharge from hospital)
Low‐certainty evidence suggests that there may be little or no difference in mortality at three months after discharge from hospital between participants who received glucocorticoids with cyclophosphamide in addition to standard care and those who received standard care alone (RR 0.98, 95% CI 0.85 to 1.13) (Analysis 1.1). We downgraded the certainty of the evidence for imprecision (the total number of participants across the included studies fell short of the optimal information size) and for indirectness (which we defined as uncertainty with regard to the interpretation of long‐term mortality data in a population where injury is sustained as a result of self‐harm).
New infection diagnosed within one week after initiation of treatment
Two of the four included trials assessed the outcome of infection (Lin 1999; Lin 2006). Neither study reported any new infections diagnosed within one week after initiation of methylprednisolone and cyclophosphamide. Lin 1999 reported the related outcome of leukopenia in 8 of 22 participants (36.4%) in the intervention arm. These participants spontaneously recovered one week later, with no mortality. Lin 2006 reported leukopenia in 6 of 16 participants (37.4%) in the intervention arm, all of whom recovered within one to two weeks. There were no reports of leukopenia in the control groups.
Afzali 2008 and Gawarammana 2017 measured neither infection nor leukopenia. As this outcome was reported by only two small trials, and since we have concerns about reporting bias in the larger of the two trials, we downgraded for serious imprecision and risk of bias for this outcome, assessing the evidence to be of very low certainty.
Discussion
Summary of main results
This systematic review update includes four trials with a combined total of 463 participants who had moderate to severe paraquat poisoning. Low‐certainty evidence from two trials indicates that participants who received glucocorticoids with cyclophosphamide in addition to standard care may have a slightly lower risk of death than those receiving standard care alone in the short term (at hospital discharge). We chose the results of a sensitivity analysis as the main analysis for this outcome, conceding that the lack of plasma testing at baseline rendered the results of two studies unreliable. Heterogeneity was high, as one small study found a large benefit whilst the other (the largest and best conducted within the review) found none. The same single, large study provided low‐certainty evidence of little or no effect of treatment on mortality at three‐month follow‐up after discharge from hospital.
We are uncertain about the effects of glucocorticoids with cyclophosphamide in addition to standard care on infection risk within one week of treatment. Two trials recorded leukopenia in just over one‐third of participants in the treatment arm, all of whom recovered within two weeks and none progressed to a diagnosed infection. We assessed the evidence for this outcome to be of very low certainty.
Overall completeness and applicability of evidence
The completeness of the evidence for the effects of glucocorticoid with cyclophosphamide for paraquat‐poisoned patients is limited by the fact that we were only able to include four RCTs, three of which were small, and the largest of which was still underpowered due to trial termination. Differing inclusion criteria regarding severity of paraquat poisoning, leading to concerns about clinical heterogeneity, was also an issue (see Quality of the evidence).
With regard to representation of populations most affected by this condition, trials are relatively restricted, having only been conducted in China (two studies), Iran (one study), and Sri Lanka (one study), and the context of recruitment may well have changed since the time when these studies were conducted (range 1997 to 2010). The mean age of participants was in the upper 20s, and the vast majority of participants were male. Since poisoning as a means of attempting suicide is most prevalent in older age groups in many countries (e.g. China and Korea), this review is limited in its applicability to this population (Wang 2019). This is particularly important since age can increase physiological susceptibility to the effects of pesticides (Ginsberg 2005).
One consideration of our results is that this effect estimate applies only to moderate to severe cases of poisoning: mild cases with a negative urine dithionite test were excluded, as were very severe cases. This makes clinical sense, as mild cases tend to make a complete recovery with only mild symptoms, whilst very severe cases of fulminant poisoning are expected to die imminently and have little chance of responding to therapy (WHO IPCS 2009).
Quality of the evidence
We judged two of the four included studies to be at low risk of bias for key domains, including the largest of the three trials (Gawarammana 2017, n = 299). Although three trials, Afzali 2008, Lin 1999 and Lin 2006, did not use a placebo, we did not consider this to be a major source of bias since our main outcome of interest was mortality. We assessed Lin 1999 to be at unclear risk of bias for allocation concealment, as this was not mentioned in the trial report. This study randomised 121 participants regardless of the severity of poisoning, and data on mortality were presented in full. However, the trialists made what appears to be a post hoc decision to exclude very severely poisoned participants ("fulminant", n = 71) from the final analysis. We performed a sensitivity analysis to investigate the effect of including these participants in the final analysis. The effect estimate was reduced, as would be expected given that people with fulminant paraquat poisoning are likely to die imminently irrespective of treatment. The Afzali 2008 trial was small and poorly reported, but considering that its use of alternate allocation was unlikely to seriously bias results given the slow pace of recruitment, we included data from this study. We further conducted sensitivity analysis to consider the impact of diagnostic uncertainty, as noted below.
Our meta‐analyses revealed substantial statistical heterogeneity, therefore we downgraded the certainty of evidence accordingly. There were several possible sources for this heterogeneity, as follows.
Differing inclusion criteria regarding severity: Lin 1999 did not employ plasma testing, and also retrospectively excluded participants who died within a week. Lin 2006 only included participants with a predicted mortality of > 50% and ≤ 90% according to the Hart 1984 formula. Afzali 2008 did not use SIPP values and provided limited detail on inclusion criteria other than that "mild" and "fulminant" cases were excluded. Gawarammana 2017 excluded severely poisoned people with a Glasgow Coma Score of less than 8/15 or a systolic blood pressure less than 70 mmHg.
Variation between treatment arms across trials: dosages and infusion times varied; the Afzali 2008 regimen did not include dexamethasone, unlike the other three studies.
Variation between standard care arms across trials: Gawarammana 2017 limited standard care to intravenous fluid, activated charcoal, and pain relief, whilst Lin 1999 and Lin 2006 included gastric lavage with normal saline followed by active charcoal added in magnesium citrate given through a nasogastric tube, courses of 8‐hour active charcoal haemoperfusion therapy and administration of intravenous dexamethasone. It is possible that there was a synergistic interaction between one of these elements of standard care in the Lin trials, when combined with glucocorticoids and cyclophosphamide, as suggested by Xu 2019.
The sample size for this review fell short of the 429 participants required in each arm of the study, therefore we downgraded the certainty of evidence to reflect this imprecision.
Potential biases in the review process
This review was conducted according to predefined inclusion criteria and methodology to select and appraise eligible studies. The search for trials was extensive, and was conducted on both English and Chinese language databases. Publication bias is a consideration in any systematic review. Although only four trials were included in the review, we believe that given the extent of the search for trials, these were the only eligible RCTs addressing this research question at the time of the search. We excluded two studies because they were not prospectively registered and therefore did not meet the Cochrane Injuries Group inclusion criteria for studies conducted after the year 2010.
We differed from the authors of previous updates and used the data as analysed in Lin 1999, in which the trialists, following completion of the trial, excluded participants with very severe fulminant poisoning from their analysis. Whilst we acknowledge that this introduces a high risk of selection bias, we considered that it made better sense clinically to exclude these participants, since people with fulminant poisoning are very unlikely to respond to any therapy, and the other studies also excluded these participants. We accounted for this high risk of selection bias in our GRADE assessment, and undertook sensitivity analysis. If there are sufficient data in future updates of this review, we will perform subgroup analysis according to severity of poisoning.
Agreements and disagreements with other studies or reviews
The authors of the largest and best‐conducted study included in this review, Gawarammana 2017, appear to be sceptical of any real benefit of the treatment regimen they studied. Based on their experience and their assessment of other literature in the field, they concluded that it is likely that "any possible benefit" of the glucocortoid/cyclophosphamide combined treatment "is due to the dexamethasone component rather than high‐dose immunosuppression. We believe clinical research efforts would be best spent on exploring the optimal dose of dexamethasone and other inexpensive and low toxicity antidotes with favourable effects in animal studies (for example acetylcysteine)" (Gawarammana 2017, p 639, emphasis added).
This is in considerable contrast to the findings of two recently published systematic reviews, whose authors conclude that "immunosuppressive pulse therapy can efficiently reduce the mortality of PQ [paraquat] poisoning and is relatively safe" (Xu 2019, p 588), and that immunosuppressive drugs generally "may reduce the mortality and incidence rate of MODS [multiple organ dysfunction syndrome] in moderate to severe PQ poisoning patients, and severe PQ poisoning patients might benefit more from ISDs [immunosuppressant drugs]" (Gao 2020). However, both systematic reviews had markedly different inclusion criteria to our review, including results from many non‐randomised studies, as well as one recent RCT that we excluded because there was no evidence of preregistration. Furthermore, Xu 2019 unaccountably omits results from the largest study included within this review (Gawarammana 2017).
The findings of our review align more closely with those of Gawarammana 2017 itself, for obvious reasons. We believe their trial, and our review, provide low‐certainty evidence that participants who received glucocorticoid with cyclophosphamide in addition to standard care may have little reduction in mortality compared to those receiving standard care alone at hospital discharge, and little or no effect at three months after discharge from hospital. We considered longer‐term data as more equivocal than shorter‐term data, believing it to be possible that the special circumstances of this type of injury (paraquat is normally ingested in quantities seen in the included studies as a method of self‐poisoning) render any interpretation of longer‐term data difficult, as factors other than the intervention may have a greater impact on the long‐term well‐being and survival of patients.
Authors' conclusions
Implications for practice.
Low‐certaintly evidence indicates that glucocorticoid with cyclophosphamide in addition to standard care may have a small effect on mortality in people with moderate to severe paraquat poisoning during hospitalisation, however, there appears to be little or no effect at three months post hospital discharge. Our confidence in the evidence is limited by the small number of participants contributing reliable data and the heterogeneity of results across studies. We are uncertain about the potential of glucocorticoid with cyclophosphamide to increase the risk of infection due to limited evidence.
Implications for research.
To enable further study of the effects of glucocorticoid with cyclophosphamide for individuals with moderate to severe paraquat poisoning, hospitals may provide this treatment as part of a randomised controlled trial with appropriate allocation concealment. All future research should be prospectively registered, CONSORT‐compliant, and investigators should make rigorous efforts to ensure an adequate sample size as well as seek long‐term follow‐up of participants, including on adverse events. In research contexts, no participant should be recruited without an assessment of plasma for time‐adjusted paraquat concentration being conducted.
Since the drafting of the protocol for this review in 2009, other trials have been conducted to assess the effects of glucocorticoids in combination with other treatments, such as haemoperfusion. Use of other treatment options such as therapy with high‐dose, long‐term antioxidant free radicals is also increasing. We aim in future updates to conduct a network meta‐analysis in an effort to determine the most effective therapy ‐ or combination of therapies ‐ for paraquat poisoning.
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2021 | New citation required and conclusions have changed | Review updated with a search date of 21 September 2020 and one new study added. |
| 12 November 2020 | New search has been performed | The search has been updated to 21 September 2020. This update includes four studies. One study has been added since the previous published version of this review (Gawarammana 2017). |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 6, 2010
| Date | Event | Description |
|---|---|---|
| 12 November 2020 | Amended | Jane Dennis has been added as an author for this update. |
| 12 December 2019 | Amended | Helen Wakeford was added as an author for this update. |
| 12 December 2019 | Amended | Helen Wakeford was added as an author for this update. |
| 20 July 2019 | Amended | We added the outcome 'new infections within one week after initiation of the treatment' (adverse effect of treatment). |
| 20 July 2019 | Amended | Emma Sydenham is no longer an author for this update. |
| 20 July 2019 | Amended | Deirdre Beecher is no longer an author for this update. |
| 25 May 2012 | New search has been performed | The search has been updated to 1 February 2012. No new studies were identified. The results and conclusions remain the same. |
| 24 May 2012 | New citation required but conclusions have not changed | The search has been updated, but no new studies were identified. The results and conclusions remain the same. |
Acknowledgements
We thank Karen Blackhall for providing the search strategy and the search of English language databases for previous versions of this review. We thank Xi Lv for searching Chinese language databases in 2012. We thank Iris Gordon for help with search processing with English language databases. We thank Marialena Trivella for statistical peer review, and Elizabeth Royle for managing the editorial process. We thank peer reviewers Dr James Coulson (Cardiff University/Cardiff & Vale University Health Board, UK), Prof Narcisse Elenga (University of French West Indies/Cayenne General Hospital, French Guiana), Harrison Davies, and Danial Sayyad for their constructive comments, which we believe improved this manuscript profoundly.
This project was supported by the UK National Institute for Health Research, through Cochrane Infrastructure funding to the Cochrane Injuries Group. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies for update searches run in 2017 and 2020
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) 1946 to September 21, 2020 1 exp Herbicides/ 2 exp Paraquat/ 3 (Paraquat or (methyl adj3 viologen) or Dimethyl* or gramoxone or grammoxone or paragreen or Herbicide* or Pyridinium compound* or pathclear or weedol).mp. 4 1 or 2 or 3 5 exp Glucocorticoids/ 6 glucocorticoid*.ab,ti. 7 exp Cyclophosphamide/ 8 (cyclophosphamid* or carloxan or clafen or cycloblastin or cycloblastine or cyclofos amide or cyclofosfamid or cyclofosfamide or cyclophosphan*or cycloxan or cyphos or cytophosphan* or cytoxan or endocyclo phosphate or endoxan* or enduxan or genoxalor mitoxan or neosan or neosar or noristan or nsc 26271 or nsc 2671 or b‐518 or procytox* or semdoxan or sendoxan).ab,ti. 9 5 or 6 or 7 or 8 10 4 and 9 11 randomi?ed.ab,ti. 12 randomized controlled trial.pt. 13 controlled clinical trial.pt. 14 placebo.ab. 15 clinical trials as topic.sh. 16 randomly.ab. 17 trial.ti. 18 Comparative Study/ 19 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 20 (animals not (humans and animals)).sh. 21 19 not 20 22 10 and 21
EMBASE 1980 to 2020 Week 38 1 exp Herbicide/ 2 exp Paraquat/ 3 exp pyridinium derivative/ 4 (Paraquat or (methyl adj3 viologen) or Dimethyl* or gramoxone or grammoxone or paragreen or Herbicide* or Pyridinium compound* or pathclear or weedol).mp. 5 1 or 2 or 3 or 4 6 exp Glucocorticoids/ 7 glucocorticoid*.ab,ti. 8 exp Cyclophosphamide/ 9 exp cyclophosphamide derivative/ 10 (cyclophosphamid* or carloxan or clafen or cycloblastin or cycloblastine or cyclofos amide or cyclofosfamid or cyclofosfamide or cyclophosphan*or cycloxan or cyphos or cytophosphan* or cytoxan or endocyclo phosphate or endoxan* or enduxan or genoxalor mitoxan or neosan or neosar or noristan or nsc 26271 or nsc 2671 or b‐518 or procytox* or semdoxan or sendoxan).ab,ti. 11 6 or 7 or 8 or 9 or 10 12 5 and 11 13 exp Randomized Controlled Trial/ 14 exp controlled clinical trial/ 15 exp controlled study/ 16 comparative study/ 17 randomi?ed.ab,ti. 18 placebo.ab. 19 *Clinical Trial/ 20 exp major clinical study/ 21 randomly.ab. 22 (trial or study).ti. 23 13 or 14 or 15 or 17 or 18 or 19 or 20 or 21 or 22 24 exp animal/ not (exp human/ and exp animal/) 25 23 not 24 26 5 and 11 and 25 27 limit 26 to embase 28 limit 27 to yr="2017 ‐ 2021"
WEB OF SCIENCE. SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI (2017 to 22 September 2020)SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, ESCI (all years to 21 Sept 2020) # 3#2 AND #1 Indexes=SCI‐EXPANDED, CPCI‐S Timespan=2017‐2020 # 2TS=(cyclophosphamid* or carloxan or clafen or cycloblastin or cycloblastine or cyclofos amide or cyclofosfamid or cyclofosfamide or cyclophosphan*or cycloxan or cyphos or cytophosphan* or cytoxan or endocyclo phosphate or endoxan* or enduxan or genoxalor mitoxan or neosan or neosar or noristan or nsc 26271 or nsc 2671 or b‐518 or procytox* or semdoxan or sendoxan) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=2017‐2020 # 1TS=(Paraquat or Dimethyl* or gramoxone or grammoxone or paragreen or Herbicide* or pathclear or weedol or Pyridinium compound*) or TS=(methyl same viologen) Indexes=SCI‐EXPANDED, CPCI‐S Timespan=2017‐2020
CENTRAL (searched 21 September 2020) #1 MeSH descriptor: [Herbicides] explode all trees #2 MeSH descriptor: [Paraquat] explode all trees #3 (Paraquat or (methyl near/3 viologen) or Dimethyl* or gramoxone or grammoxone or paragreen or Herbicide* or Pyridinium compound* or pathclear or weedol):ti,ab,kw (Word variations have been searched) #4 #1 or #2 or #3 #5 MeSH descriptor: [Glucocorticoids] explode all trees #6 (cyclophosphamid* or carloxan or clafen or cycloblastin or cycloblastine or cyclofos amide or cyclofosfamid or cyclofosfamide or cyclophosphan*or cycloxan or cyphos or cytophosphan* or cytoxan or endocyclo phosphate or endoxan* or enduxan or genoxalor mitoxan or neosan or neosar or noristan or nsc 26271 or nsc 2671 or b‐518 or procytox* or semdoxan or sendoxan):ti,ab,kw (Word variations have been searched) #7 MeSH descriptor: [Cyclophosphamide] explode all trees #8 #5 or #6 or #7 #9 #4 and #8
Chinese databases were searched using the general strategy: Paraquat AND cyclophosphamide AND lung in abstracts, with synonym expansion, and publication time from 2014 to 2020.
CNKI: 检索范围: (摘要%百草枯) AND (摘要%环磷酰胺) AND (摘要%肺) 资源范围: 总库;同义词扩展;时间范围:发表时间:2014‐04‐01到2020‐11‐12;
WAN FANG DATA: 更新时间: 不限万方:检索表达式:百草枯 * 环磷酰胺 * 肺 * Date:2014‐2020
VIP: 文摘=百草枯AND文摘=环磷酰胺AND文摘=肺 AND 年份:2014‐2020
Translation:
China National Knowledge Infrastructure (CNKI): (Abstract % paraquat ) AND (Abstract% cyclophosphamide ) AND (Abstract % lung). All databases; synonym expand; Time: from 2014‐04‐01 to 2020‐11‐12.
WAN FANG DATA: paraquat*cyclophosphamide*lung*Date: 2014‐2020.
VIP: Abstract=paraquat AND Abstract=cyclophosphamide AND Abstract=lung AND year: 2014‐2020.
Appendix 2. Search strategies 2014 update
For this update the search strategies were modified: the terms relating to lung were removed and the RCT filters were added. The strategies, as they were, did not retrieve the included studies even though they were indexed in MEDLINE and /or Embase. The included studies may have been retrieved either by screening reference lists.The added study filter is a modified version of the Ovid MEDLINE Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011); for Embase we added to the search strategy study design terms as used by the UK Cochrane Centre (Lefebvre 2011).
Other strategies for databases in the English language were not modified.
Cochrane Injuries Group's Specialised Register
#1 ((Paraquat or (methyl and viologen) or Dimethyl* or gramoxone or grammoxone or paragreen or Herbicide* or "Pyridinium compound" or pathclear or weedol)) AND ((cyclophosphamid* or carloxan or clafen or cycloblastin or cycloblastine or "cyclofos amide" or cyclofosfamid or cyclofosfamide or cyclophosphan* or cycloxan or cyphos or cytophosphan* or cytoxan or "endocyclo phosphate" or endoxan* or enduxan or "genoxalor mitoxan" or neosan or neosar or noristan or "nsc 26271" or "nsc 2671" or b‐51)) [REFERENCE] [STANDARD]
MEDLINE (OvidSP) 1. exp Herbicides/ 2. exp Paraquat/ 3.(Paraquat or (methyl adj3 viologen) or Dimethyl* or gramoxone or grammoxone or paragreen or Herbicide* or Pyridinium compound* or pathclear or weedol).mp. 4. 1 or 2 or 3 5. exp Lung Diseases/ 6. exp Pulmonary Fibrosis/ 7. ((Pulmonary or lung) adj3 (fibrosis or fibroses)).ab,ti. 8. ((Alveolitis or alveolitides) adj3 fibrosing).ab,ti. 9. 5 or 6 or 7 or 8 10. exp Glucocorticoids/ 11. glucocorticoid*.ab,ti. 12. exp Cyclophosphamide/ 13. (cyclophosphamid* or carloxan or clafen or cycloblastin or cycloblastine or cyclofos amide or cyclofosfamid or cyclofosfamide or cyclophosphan*or cycloxan or cyphos or cytophosphan* or cytoxan or endocyclo phosphate or endoxan* or enduxan or genoxalor mitoxan or neosan or neosar or noristan or nsc 26271 or nsc 2671 or b‐518 or procytox* or semdoxan or sendoxan).ab,ti. 14. 10 or 11 or 12 or 13 15. 4 and 9 and 14 16. (animals not (humans and animals)).sh. 17. 15 not 16
Embase (Ovid) 1. exp Herbicide/ 2. exp Paraquat/ 3. exp pyridinium derivative/ 4. (Paraquat or (methyl adj3 viologen) or Dimethyl* or gramoxone or grammoxone or paragreen or Herbicide* or Pyridinium compound* or pathclear or weedol).mp. 5. 1 or 2 or 3 or 4 6. exp Lung Disease/ 7. exp lung fibrosis/ 8. ((Pulmonary or lung) adj3 (fibrosis or fibroses)).ab,ti. 9. ((Alveolitis or alveolitides) adj3 fibrosing).ab,ti. 10. 6 or 7 or 8 or 9 11. exp Glucocorticoids/ 12. glucocorticoid*.ab,ti. 13. exp Cyclophosphamide/ 14. exp cyclophosphamide derivative/ 15. (cyclophosphamid* or carloxan or clafen or cycloblastin or cycloblastine or cyclofos amide or cyclofosfamid or cyclofosfamide or cyclophosphan*or cycloxan or cyphos or cytophosphan* or cytoxan or endocyclo phosphate or endoxan* or enduxan or genoxalor mitoxan or neosan or neosar or noristan or nsc 26271 or nsc 2671 or b‐518 or procytox* or semdoxan or sendoxan).ab,ti. 16. 11 or 12 or 13 or 14 or 15 17. 5 and 10 and 16 18. exp animal/ not (exp human/ and exp animal/) 19. 17 not 18
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) and ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S)
1. (Paraquat or Dimethyl* or gramoxone or grammoxone or paragreen or Herbicide* or pathclear or weedol or Pyridinium compound*) or (methyl same viologen) 2. (cyclophosphamid* or carloxan or clafen or cycloblastin or cycloblastine or cyclofos amide or cyclofosfamid or cyclofosfamide or cyclophosphan*or cycloxan or cyphos or cytophosphan* or cytoxan or endocyclo phosphate or endoxan* or enduxan or genoxalor mitoxan or neosan or neosar or noristan or nsc 26271 or nsc 2671 or b‐518 or procytox* or semdoxan or sendoxan) 3. ((Pulmonary or lung) SAME (fibrosis or fibroses)) OR ((Alveolitis or alveolitides) SAME fibrosing) OR (lung* SAME disease*) 4. 1 and 2 and 3
Clinical trials registries Search terms: paraquat Study results: All studies
Chinese databases
The databases orginially searched in 2012 are now incorporated in a new Government sponsored database: China National Knowledge Infrastructure (CNKI 数据库) The authors also searched the following which were not included in the 2012 search: WAN FANG DATA(万方数据库)(维普数据库) The search string was limited to, Paraquat AND lung AND cyclophosphomide, due to the difficulties in using the search interfaces.
Appendix 3. Search methods for previous versions of the review
Cochrane Injuries Group's Specialised Register 1. (Paraquat or (methyl and viologen) or Dimethyl* or gramoxone or grammoxone or paragreen or Herbicide* or "Pyridinium compound" or pathclear or weedol) 2. (cyclophosphamid* or carloxan or clafen or cycloblastin or cycloblastine or "cyclofos amide" or cyclofosfamid or cyclofosfamide or cyclophosphan* or cycloxan or cyphos or cytophosphan* or cytoxan or "endocyclo phosphate" or endoxan* or enduxan or "genoxalor mitoxan" or neosan or neosar or noristan or "nsc 26271" or "nsc 2671" or b‐51 3. 1 and 2
CENTRAL (The Cochrane Library) #1 MeSH descriptor Herbicides explode all trees #2 MeSH descriptor Paraquat explode all trees #3 (Paraquat or (methyl near3 viologen) or Dimethyl* or gramoxone or grammoxone or paragreen or Herbicide* or Pyridinium compound* or pathclear or weedol) #4 (#1 OR #2 OR #3) #5 MeSH descriptor Glucocorticoids explode all trees #6 MeSH descriptor Cyclophosphamide explode all trees #7 (cyclophosphamid* or carloxan or clafen or cycloblastin or cycloblastine or cyclofos amide or cyclofosfamid or cyclofosfamide or cyclophosphan*or cycloxan or cyphos or cytophosphan* or cytoxan or endocyclo phosphate or endoxan* or enduxan or genoxalor mitoxan or neosan or neosar or noristan or nsc 26271 or nsc 2671 or b‐518 or procytox* or semdoxan or sendoxan) #8 (#5 OR #6 OR #7) #9 (#4 AND #8)
Ovid MEDLINE 1. exp Herbicides/ 2. exp Paraquat/ 3. (Paraquat or (methyl adj3 viologen) or gramoxone or paragreen or Herbicide* or Pyridinium Compound*).mp. 4. 1 or 2 or 3 5. exp Lung Diseases/ 6. exp Pulmonary Fibrosis/ 7. ((Pulmonary or lung) adj3 (fibrosis or fibroses)).ab,ti. 8. ((Alveolitis or alveolitides) adj3 fibrosing).ab,ti. 9. 5 or 6 or 7 or 8 10. exp Glucocorticoids/ 11. glucocorticoid*.ab,ti. 12. exp Cyclophosphamide/ 13. (Cyclophosphamide* or cytophosphan or cyclophosphane or procytox or sendoxan or b‐518 or neosar or cytoxan or endoxan or nsc‐26271).ti,ab. 14. 10 or 11 or 12 or 13 15. randomi?ed.ab,ti. 16. randomized controlled trial.pt. 17. controlled clinical trial.pt. 18. placebo.ab. 19. clinical trials as topic.sh. 20. randomly.ab. 21. trial.ti. 22. 15 or 16 or 17 or 18 or 19 or 20 or 21 23. (animals not (humans and animals)).sh. 24. 22 not 23 25. 4 and 9 and 14 and 24
CBM (1978 to April 2012), CMCC (1995 to April 2012), CMAC (1994 to April 2012) 1. exp herbicide/ 2. exp paraquat/ 3. 1 or 2 4. exp lung disease/ 5. exp pulmonary fibrosis/ 6. 4 or 5 7. exp corticosteroids/ 8. steroids*.ab,ti. 9. exp cyclophosphamide/ 10. 7 or 8 or 9 11.(randomised).ab,ti. 12. randomized controlled study.pt. 13. clinical controlled study.pt. 14. randomly.ab. 15. trial.ti. 16. 11 or 12 or 13 or 14 or 15. 17. 3 and 6 and 10 and 16.
Data and analyses
Comparison 1. All‐cause mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Mortality at hospital discharge | 4 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.88] |
| 1.1.2 Mortality at 3 months | 1 | 293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.13] |
| 1.2 Sensitivity analysis: all‐cause mortality re‐including fulminant participants | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Mortality at hospital discharge | 4 | 463 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.69, 0.93] |
| 1.3 Sensitivity analysis: all‐cause mortality: excluding studies without plasma paraquat assessment at baseline | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Mortality at hospital discharge | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.68, 0.99] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Afzali 2008.
| Study characteristics | ||
| Methods |
Study design: quasi‐randomised controlled trial (alternate allocation) Duration of study: 3 months Number of centres: 1 Location: Iran Study setting: recruitment in ED of Sina Hospital, Hamadan Date of study: September 2003 to October 2005 |
|
| Participants |
Study inclusion criteria: no formal selection criteria were given; investigators apparently intended to limit recruitment to patients with moderate to severe (but not fulminant) poisoning by the following means (emphasis added): "Sodium dithionite reaction test was done on the urine samples of all patients as soon as possible. ... Navy blue (NB) or dark blue (DB) colors usually indicate significant PQ poisoning. The studied patients were divided into three groups on the basis of clinical manifestations and test results: 1) Fulminant poisoning: The urine test was NB color. All of these patients died during the first few days (three to four days) after poisoning because of multiorgan involvement, such as acute tubular necrosis, myocarditis, hepatic necrosis, and pulmonary bleeding. 2) Moderate to severe poisoning: The urine test color was NB or DB and the clinical manifestations were oropharyngeal burns, pharyngeal pseudomembranes, vomiting, severe diarrhoea, and acute renal and hepatic failure 3) Mild poisoning: The urine test was colourless or light blue and the clinical manifestations included transient diarrhoea, vomiting, and buccal hyperemia, which mostly resolved without further sequelae. Of the 45 patients assessed in this study, 15 patients with mild and 10 patients with fulminant poisoning were excluded. So, 20 patients remained. Of them 11 patients received 'conventional treatment' (group 1) and nine patients received 'conventional' treatment plus 'new treatment' (group 2) randomly" (Afzali 2008, p 388). Study exclusion criteria: no formal criteria given bar those implied above Number of participants: 45 screened; 15 excluded due to mild and 10 due to fulminant status. 20 were randomised (9 to intervention group, 11 to conventional treatment). Age in years, mean (∓): intervention: 27 years (∓ 10); control: 25 years (∓ 10) Gender, n (%): intervention: female 1 (11.1%), male 8 (88.9%); control: female 3 (27.3%), male 8 (72.7%) Severity (urine colour): intervention group: 3 NB, 6 DB; control group: 4 NB, 7 DB "In our study, all patients used PQ in order to commit suicide and there were no cases of homicide or accidental use" (Afzali 2008, p 390) |
|
| Interventions |
Intervention: "Conventional treatment included fixation of a nasogastric tube, gastric lavage with normal saline, charcoal‐sorbitol gavage [sic] every two to four hours for three days, forced alkalinized diuresis in the first day of admission to the hospital, and haemodialysis of four hours duration for both groups. In addition, 15 mg/kg of CP [cyclophosphamide] in dextrose saline (200 mL) was infused in two hours for two days in [the intervention group] ... MP (methylprednisolone), one gram in 200 mL dextrose saline was also infused for four hours and was repeated for three consecutive days ... as well. Meanwhile, 15 mg/kg of MESNA was prescribed (for four days) in order to avoid the side effects of CP" (Afzali 2008, p 388). Control: "Conventional treatment" (as above) alone |
|
| Outcomes |
Primary outcome: not stated. Would appear to be mortality in‐hospital, prior to discharge (referred to in document as "during the admission") Secondary outcomes: none specifically stated. Investigators reported that creatinine > 1.4 mg/dL, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) > 70 IU/L, and PaO2 < 70 mmHg (in the room air) were considered asacute renal failure (ARF), hepatitis, and hypoxia, respectively (emphasis added). Information was provided concerning daily in‐hospital assessments: "laboratory tests included liver function tests (LFT), complete blood count (CBC), arterial blood gas (ABG), blood urea nitrogen (BUN), and creatinine"; chest radiography was repeated every 2 days. Finally, autopsies were conducted for "all expired patients". Data on adverse effects do not appear to have been rigorously sought, but investigators mention that there were "no severe complications ... in the new treatment group" (Afzali 2008, p 390). |
|
| Notes |
Funding: none reported. Registration: none reported (though it is noted that "this survey [sic] was approved by the Ethics Committee of Hamadan University of Medical Sciences") (Afzali 2008, p 388). Interests: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "11 patients received 'conventional treatment' ... and nine patients received 'conventional' treatment plus 'new treatment' ... randomly" (Afzali 2008, p 388) We contacted the first author of the study, who informed us that alternate allocation was used (Afzali 2010 ‐ personal correspondence between lead investigator and previous review author Emma Sydenham. Correspondence details are no longer available). Whilst we initially considered this domain as at high risk of bias, given the long period of the study (25 months, in which only 45 patients were screened), we reconsidered that the danger of manipulation of the allocation of successive participants was not serious. |
| Allocation concealment (selection bias) | High risk | Concealment of allocation is not mentioned in the paper, and is not possible with use of alternate allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding was not reported, and according to the author was not done (Afzali 2010 ‐ personal correspondence ‐ see above). Given that the main outcome of interest (mortality) is objective, we did not consider it likely that the lack of blinding introduced bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The main outcome was death at discharge from hospital, which appears to be reported in full. |
| Selective reporting (reporting bias) | Unclear risk | The study reported the main outcome for this review (mortality) in full; however, in the absence of a trial protocol or evidence of prospective registration, we are obliged to report the risk of bias as unclear. |
| Other bias | High risk | Investigators acknowledged the "limitation" that they "did not check plasma PQ" in participants on entry to the study (or at any other point). |
Gawarammana 2017.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Duration of study: 3 months Number of centres: 6 Location: Sri Lanka Study setting: district hospitals Date of study: March 2007 to November 2010 |
|
| Participants |
Study inclusion criteria: individuals aged 14 years and over with positive urine dithionite test who presented within 48 h of paraquat ingestion and received no other specific treatments such as haemodialysis or haemoperfusion Study exclusion criteria: people "with a systolic blood pressure < 70mmHg that did not respond to 1 L of intravenous fluid or a Glasgow Coma score less than 8/15 were excluded" (Gawarammana 2017, p 2) Number of participants: 604 screened; 305 excluded due to negative urine test, refusing consent, death before randomisation, GCS < 8, pregnant, aged under 14; late admission. 299 participants were randomised (147 to intervention group, 152 to placebo). 143 received intervention as allocated; 147 received placebo as allocated. Age in years, median (IQR): intervention: 27 (21 to 38); control: 27 (21 to 36) Gender, n (%): intervention: female 47 (32%), male 100 (68%); control: female 39 (26%), male 113 (74%) Median (IQR) and range of SIPP: intervention group (data for 125): 18.4 (2.7 to 56.2); control group (data for 127): 13.1 (3.1 to 58.4). Investigators reported that the SIPP had a small but significant difference at baseline. |
|
| Interventions |
Intervention: on day 0 and 1 (i.e. shortly following randomisation and 24 h later):
On day 2: 1 g methylprednisolone given over 1 hour by infusion From day 3 onwards, participants were given IV or oral dexamethasone 8 mg (State Pharmaceutical Manufacturing Corporation, Colombo, Sri Lanka) 3 times daily for 2 weeks or placebo. Comparison: normal saline (State Pharmaceutical Manufacturing Corporation, Colombo, Sri Lanka) was used as placebo for all IV drugs. * "The protocol suggests a possibility of using major rounding of doses (to 750 mg or 1000 mg) if an actual weight could not be measured but this might have meant doses of up to 20 mg/kg for some individuals. This was not done and 15 mg/kg based on estimated weight was used." (Supplemental Box 1) |
|
| Outcomes |
Primary outcome: mortality in‐hospital Secondary outcomes: mortality at 3 months; lung function (formal lung functions and a high resolution CT scan (HRCT) at long‐term follow‐up); however, this was not possible for "a meaningful number of patients" (Gawarammana 2017, p 634) Data on adverse effects, specifically haematuria and bladder pain, were sought in hospital. |
|
| Notes |
Funding: Syngenta Crop Protection AG; Wellcome Trust/National Health and Medical Research Council International Collaborative Research Grant (ICRG 071669); Australian Leadership Award (ALA00379) Registration: ISRCTN85372848 Interests: "MW [10th author] was an employee of Syngenta at the time of trial inception and until 2009. AHD [12th author] received funding to attend DMEC meeting from Syngenta. ME [11th author] have [sic] received travel expenses from Syngenta to attend meetings of a scientific advisory group in relation to studies of new paraquat formulations. No other conflicts declared" (Gawarammana 2017, p 639) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using purpose‐designed computer software. |
| Allocation concealment (selection bias) | Low risk | "The random sequence and allocation were concealed prior to randomization ... The allocation sequences were generated and encrypted independently by an IT consultant who had no role in patient recruitment, treatment and assessment." (Gawarammana 2017, p 634) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The allocation was only known to the pharmacists who had no other role in patient management and data collection." (Gawarammana 2017, p 634) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "We were able to follow up all but six patients post discharge." 4 losses to follow‐up were in the intervention group and 2 in the control group. |
| Selective reporting (reporting bias) | Low risk | Study was prospectively registered (ISRCTN85372848). Ethics were granted a year before recruitment began. Methods and results seem to be in concert with planned analyses; reasons for lack of presentation of planned lung function results are reported, as are grounds for early stopping. |
| Other bias | Low risk | This RCT was well‐conducted; however, it was stopped early "after consultation with the data monitoring and ethics committee due to a collapse in recruitment". The collapse followed regulatory decisions which saw the phasing out of paraquat use "from Sri Lankan agricultural practice through 2008 and 2009"; the herbicide was "finally banned in August 2010" (Gawarammana 2017, p 635). Gawarammana 2017 reported a small difference in the median SIPP score of participants allocated to intervention (median 18.4) compared to control (median 13.1). As the method of randomisation was unlikely to have been compromised (it was done using purpose‐designed software), this difference is likely to have been caused by chance alone, therefore we do not consider this to be a source of bias. |
Lin 1999.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Duration of study: hospital admission to discharge (maximum length not stated) Number of centres: 1 Location: Taipei, Taiwan, Republic of China Study setting: recruitment in ED; treatment in specialised poison centre Date of study: January 1999 to December 2003 |
|
| Participants |
Study inclusion/exclusion criteria: people who had ingested paraquat within the previous 24 h, and had a urine sample that resulted in a navy blue or dark blue reaction to a sodium dithionite test, were screened. Number of participants: 142 screened. Investigators reported that they excluded 71 people with "fulminant" cases and 21 with "mild" cases, and "recruited" only 50 participants; however, they reported data for the "fulminant" and the "recruited" cases and excluded only "mild" cases, for which data were not presented. N at baseline: "Fulminant": intervention group (n = 34); control group (n = 37) "Recruited": intervention group (n = 22); control group (n = 28) Age in years: "Fulminant" group (n = 71): intervention group: mean 35.7 years (SD 13.6); control group: mean 38.2 years (SD 16.3) "Recruited" group (n = 50): intervention group: mean 28.2 years (SD 11.1); control group: mean 26.8 years (SD 10.1) Gender, n: "Fulminant" group' (n = 71): intervention group: 14 F, 20 M; control group: 10 F, 27 M "Recruited" group (n = 50): intervention group: 9 F, 13 M; control group: 10 F, 18 M Severity (urine colour): "Fulminant" group (n = 71): intervention group: 2 NB, 35 DB; control group: 2 NB, 32 DB "Recruited" group (n = 50): intervention group: 8 NB, 14 DB; control group: 9 NB, 19 DB |
|
| Interventions |
All participants received: "... active charcoal added in magnesium citrate ... given through a nasogastric tube after gastric lavage with normal saline. All patients received two courses of 8‐h active charcoal haemoperfusion therapy in the emergency room (ER), and dexamethasone 10 mg intravenous injection every 8 h was given for 14 d after admission" (Lin 1999, p 357) Intervention: the intervention group also received: "pulse therapy after haemoperfusion at ER. Pulse therapy included 15 g/kg of CP in 5% glucose saline 200 ml and 1 g MP in the other 200 ml 5% glucose saline intravenously infused for 2 h/d. CP was infused for 2 d and MP for 3 d" (Lin 1999, p 357). Control: no pulse therapy |
|
| Outcomes |
|
|
| Notes |
Funding: this trial was "supported by the National Science Council Foundation, Taiwan, ROC (NSC‐88‐2314‐B‐182A‐049)" (Lin 1999, p 358). Registration: not identified Interests: no interests declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... according to random digit methods" (Lin 1999, p 357) |
| Allocation concealment (selection bias) | Unclear risk | There was no mention of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Since no placebo was used, blinding of the treating physician and participants was not possible. However: "At the end of this study, to avoid bias, the data were collected and analyzed by other doctors who were not aware of the study" (Lin 1999, p 357). Given that the main outcome of interest (mortality) is objective, we did not consider it likely that the lack of blinding introduced bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The main outcome was death at final follow‐up, which was reported in full. |
| Selective reporting (reporting bias) | Unclear risk | In the absence of a trial protocol or evidence of prospective registration, we are obliged to report the risk of bias as unclear. Furthermore, 121 participants were randomised into the study regardless of the severity of poisoning, and information on the primary outcome (mortality) was presented in full. However, the trialists made what appears to be a post hoc decision to exclude very severely poisoned patients ("fulminant"; n = 71) from the final analysis. We have chosen to report mortality data both in aggregate and separately in order to consider the impact of all data provided and to minimise the potential for selective reporting. |
| Other bias | High risk | Methods of diagnosing poisoning severity did not include plasma assessment (likely leading to the post protocol decision to exclude participants later known to have been fulminant on admission). |
Lin 2006.
| Study characteristics | ||
| Methods |
Study design: randomised controlled trial Duration of study: 42 days Number of centres: 1 Location: Taipei, Taiwan, Republic of China Study setting: recruitment in ED; treatment in specialised poison centre Date of study: January 1992 to December 1997 |
|
| Participants |
Study inclusion criteria: people who:
Study exclusion criteria: "exposure to paraquat via skin or intravascular [sic] injection; absence of paraquat levels in biological fluids; or had ingested paraquat due to major systemic diseases including cancer and heart, lung, renal, and liver diseases; or did not give informed consent" (Lin 2006, p 369) Number of participants: 96 screened. Investigators excluded 13 for arriving > 24 h after ingestion; 6 for exposure to paraquat other than that stated in the inclusion criteria; 21 for excessive predicted mortality (> 90%); 18 for insufficient predicted mortality (< 50%); 15 for having no paraquat detectable in urine. 23 participants remained, of whom 16 were assigned to the intervention and 7 to control. Age in years: intervention group: mean 33.6 years (SD 14.3); control group: mean 37.0 years (SD 13.8) Gender, n: intervention group: 5 F, 11 M; control group: 2 F, 5 M Severity (urine colour): intervention group: 5 NB, 11 DB; control group: 1 NB, 6 DB Mean SIPP: intervention group: 14.6 (SD 9.1); control group: 15.5 (SD 7.0) |
|
| Interventions |
All participants received: "To prevent absorption of paraquat from the gastrointestinal tract, activated charcoal 1 g/kg added to 250 mL of magnesium citrate was given through a nasogastric tube after gastric lavage with normal saline. In addition, all patients received two doses of 8‐hr active charcoal‐containing haemoperfusion therapy in the emergency room. After hemoperfusion therapy, the control group received dexamethasone 5 mg in an intravenous injection every 6 hrs until their arterial blood gas showed PaO2 11.5 kPa (80mm Hg) or they died" (p 369) Intervention: "... pulse therapy with 15 mg/kg cyclophosphamide in 5% glucose saline 200 mL and 1 g of methylprednisolone in the other 200 mL of 5% glucose saline intravenously infused for 2 hrs per day. Cyclophosphamide was infused for 2 days and methylprednisolone for 3 days simultaneously. Preceding dexamethasone, a 5‐mg intravenous injection every 6 hrs was given until the arterial blood gas showed PaO2 11.5 kPa (80 mm Hg). Repeated pulse therapy with 1 g of methylprednisolone in the other 200 mL of 5% glucose saline intravenously infused for 2 hrs per day for 3 days was given again if PaO2 was 8.64 kPa (60 mm Hg). In addition, 15mg/kg/day cyclophosphamide was infused for 1 day again if patients’ white cell counts were 3000/m3 and the duration was 2 wks after initial cyclophosphamide pulse therapy to avoid a severe leukopenia episode" (Lin 2006, p 369) Control: no pulse therapy, but as in intervention group: "... dexamethasone 5 mg in an intravenous injection every 6 hrs until their arterial blood gas showed PaO2 11.5 kPa (80mm Hg) or they died" (Lin 2006, p 369) |
|
| Outcomes |
|
|
| Notes |
Funding: not specified Registration: not identified Interests: "None of the authors have any financial interests to disclose" (Lin 2006, p 368) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All study patients were randomly allocated to control and study groups in the proportion of 1:2 by means of a sequence of labelled cards contained in sealed numbered envelopes that were prepared by a statistical adviser." (Lin 2006, p 369) |
| Allocation concealment (selection bias) | Low risk | "... [the envelope was] opened by the researcher in the presence of patients." (Lin 2006, p 369) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Neither stratification nor blinding was made [sic] in this study." (Lin 2006, p 369) "The data were collected and analyzed by other doctors not familiar with the study." (Lin 2006, p 369) Given that the main outcome of interest (mortality) is objective, we did not consider it likely that the lack of blinding introduced bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The main outcome was death at final follow‐up, which was reported in full. |
| Selective reporting (reporting bias) | Unclear risk | The study reported the main outcome (death) at final follow‐up in full; however, in the absence of a trial protocol or evidence of prospective registration, we are obliged to report the risk of bias as unclear. |
| Other bias | Low risk | We did not identify any other areas of bias. |
Abbreviations
CP = cyclophosphamide; CT = computed tomography; DB = dark blue; DMEC = Data Monitoring and Ethics Committee; ED = Emergency Department; F = female; GCS = Glasgow Coma Score; HRCT = high resolution CT scan; IQR = interquartile range; M = male; MESNA = 2‐mercaptoethane sulfonate sodium (Na); MP = methylprednisolone; NB = navy blue; PaO2 = partial pressure of oxygen; PQ = paraquat; SD = standard deviation; SIPP = Severity Index of Paraquat Poisoning
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 2014 | This study was characterised as an RCT within Gao 2020; however, the paper is very brief, has 1 author, no detail on methods (including randomisation), and lacked prospective registration. |
| Ghorbani 2015 | This RCT lacked evidence of prospective registration, which is required for the inclusion of studies conducted after the year of 2010, according to Cochrane Injuries Group policy (2015). |
| Perriens 1992 | This comparative study used an historic control group. "Patients admitted before October 10, 1986 received the standard treatment only, because IV cyclophosphamide and IV dexamethasone were not available in Suriname until that time. Patients presenting after October 10, 1986 received high‐dose cyclophosphamide and dexamethasone treatment, in addition to standard treatment" (Perriens 1992, p 130) |
| Tsai 2009 | This study focused on methylprednisolone (an eligible glucocorticoid) only, without cyclophosphamide; moreover, there was no mention of randomisation. |
IV = intravenous; RCT = randomised controlled trial
Differences between protocol and review
We adjusted the title for the 2021 update from the original 'Glucocorticoid with cyclophosphamide for paraquat‐induced lung fibrosis' to 'Glucocorticoid with cyclophosphamide for oral paraquat poisoning' to reflect the scope of the review more accurately.
In the protocol for this review, we specified that we would search the Chinese language databases Chinese Bio‐Medical Literature & Retrieval System (CBM), Chinese Medical Current Contents (CMCC), and Chinese Medical Academic Conference (CMAC). These databases have now been included in the China National Knowledge Infrastructure database. For the 2014 and 2021 updates, we searched the databases China National Knowledge Infrastructure (数据库), Wanfang Data (万方数据库), and VIP (维普数据库).
The protocol for this review (2009) contained no plans for dealing with issues concerning unit of analysis or subgroup or sensitivity analyses. These have been added to the 2021 update to meet current Cochrane methodological standards.
For the 2021 version, we added infection within one week after initiation of the treatment as an outcome for side effects of the treatment. We also mentioned leukopenia, as related to infection risk.
We used the GRADE approach to assess the certainty of evidence for each important outcome.
For the 2021 version, a peer reviewer suggested that we exclude any study not assessing plasma for time‐adjusted paraquat concentration. Whilst we did not agree to the extent of excluding studies as a whole, we did perform sensitivity analysis to exclude these studies for the most important outcome (mortality).
Contributions of authors
For the 2021 version of this review: Iris Gordon and Jane Dennis (JD) re‐ran the searches in English language databases (in 2017 and 2020 respectively). LL, Helen Wakeford (HW) and JD screened the search results. HW updated the Background section. JD extracted data and rewrote the Characteristics of included studies and Description of studies. All authors contributed to the Results and Discussion. LL, JD, and HW performed the GRADE assessment of the certainty of evidence for each outcome. LL searched Chinese language databases and screened the search results. CY and BC also screened the Chinese language search results.
For the 2014 version: Deirdre Beecher (DB) developed the search strategy, searched English language databases, and screened the search results. ES screened some of the search results. LL developed the search strategy, searched Chinese language databases, and screened the search results. CY and BC also screened the Chinese language search results. DB, LL, and ES updated the manuscript. All authors contributed to the updated review.
For the 2010 and 2012 versions: Luying Ryan Li (LL) and Chao You (CY) were responsible for writing the protocol. Bhuwan Chaudhary (BC) and LL selected the trials from English language databases. LL and CY selected trials from Chinese language databases. CY offered interpretation of the clinical features of paraquat poisoning and arbitrated on the inclusion of one trial (Afzali 2008). Emma Sydenham (ES), LL, and BC independently assessed the risk of bias of the included trials and extracted data. LL and ES interpreted the data and wrote the manuscript. All authors agreed on the final manuscript.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institute for Health Research (NIHR), UK
Cochrane Infrastructure Funding to the Cochrane Injuries Group
Declarations of interest
Luying Ryan Li: none known.
Bhuwan Chaudhary: none known.
Chao You: none known.
Jane Dennis: none known. JD was employed by Cochrane Injuries during her involvement in the development of the review.
Helen Wakeford: none known. HW was employed by Cochrane Injuries and the Cochrane Central Executive Team during her involvement in the development of the review.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Afzali 2008 {published data only}
- Afzali S, Gholyaf M. The effectiveness of combined treatment with methylprednisolone and cyclophosphamide in oral paraquat poisoning. Archives of Iranian Medicine 2008;11(4):387-91. [PubMed] [Google Scholar]
Gawarammana 2017 {published and unpublished data}
- Gawarammana I, Buckley NA, Mohamed F, Naser K, Jeganathan K, Ariyananada PL, et al. High-dose immunosuppression to prevent death after paraquat self-poisoning - a randomised controlled trial. Clinical Toxicology 2017;56(7):633-9. [CTG: ] [DOI: 10.1080/15563650.2017.1394465] [DOI] [PubMed] [Google Scholar]
Lin 1999 {published data only}
- Lin JL, Leu ML, Liu YC, Chen GH. A prospective clinical trial of pulse therapy with glucocorticoid and cyclophosphamide in moderate to severe paraquat-poisoned patients. American Journal of Respiratory and Critical Care Medicine 1999;159(2):357-60. [DOI] [PubMed] [Google Scholar]
Lin 2006 {published data only}
- Lin JL, Lin-Tan DT, Chen KH, Huang WH. Repeated pulse of methylprednisolone and cyclophosphamide with continuous dexamethasone therapy for patients with severe paraquat poisoning. Critical Care Medicine 2006;34(2):368-73. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chen 2014 {published data only}
- Chen YP. Effect of early intensive immunosuppressive therapy on moderate to severe paraquat poisoning [早期强化免疫抑制治疗对中重度 百草枯中毒的疗效 [Chinese]]. Chinese Journal of Primary Medicine and Pharmacology 2014;21:3305-6. [Google Scholar]
Ghorbani 2015 {published data only}
- Ghorbani A, Masoumi K, Forouzan A, Rahmani AH, Rahim F, Taeybi B, et al. Effect of pulse therapy with glucocorticoids and cyclophosphamide in patients with paraquat poisoning. Hong Kong Journal of Emergency Medicine 2015;22(4):235-40. [Google Scholar]
Perriens 1992 {published data only}
- Perriens JH, Benimadho S, Kiauw IL, Wisse J, Chee H. High-dose cyclophosphamide and dexamethasone in paraquat poisoning: a prospective study. Human and Experimental Toxicology 1992;11(2):129-34. [DOI] [PubMed] [Google Scholar]
Tsai 2009 {published data only}
- Tsai J, Lee R, Wang C, Fang T, Hsu B. A clinical study of prognosis and glucocorticoid pulse treatment in patients with acute paraquat intoxication. Tzu Chi Medical Journal 2009;21(2):156-60. [Google Scholar]
Additional references
Addo 1986
- Addo E, Poon-King T. Leucocyte suppression in treatment of 72 patients with paraquat poisoning. Lancet 1986;1:1117–20. [DOI] [PubMed] [Google Scholar]
Agarwal 2006
- Agarwal R, Srinivas R, Aggarwal AN, Gupta D. Experience with paraquat poisoning in a respiratory intensive care unit in North India. Singapore Medical Journal 2006;47(12):1037. [PubMed] [Google Scholar]
Buckley 2001
- Buckley N. Letter to Editor: Pulse corticosteroids and cyclophosphamide in paraquat poisoning (includes response by authors Lin 1999). American Journal of Respiratory and Critical Care Medicine 2001;163:585. [DOI] [PubMed] [Google Scholar]
Dawson 2010
- Dawson H, Eddleston M, Senarathna L, Mohamed F, Gawarammana I, Bowe SJ, et al. Acute human lethal toxicity of agricultural pesticides: a prospective cohort study. PLOS Medicine 2010;7(10):e1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dinis‐Oliveira 2006
- Dinis-Oliveira RJ, Duarte JA, Remião F, Sánchez-Navarro A, Bastos ML, Carvalho F. Single high dose dexamethasone treatment decreases the pathological score and increases the survival rate of paraquat-intoxicated rats. Toxicology 2006;227(1-2):73-85. [DOI] [PubMed] [Google Scholar]
Eddleston 2003
- Eddleston M, Wilks MF, Buckley NA. Prospects for treatment of paraquat-induced lung fibrosis with immunosuppressive drugs and the need for better prediction of outcome: a systematic review. QJM: An International Journal of Medicine 2003;96:809-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI: 10.1136/bmj.315.7109.629] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fukuda 1985
- Fukuda Y, Ferrans VJ, Schoenberger CI, Rennard SI, Crystal RG. Patterns of pulmonary structural remodeling after experimental paraquat toxicity. The morphogenesis of intraalveolar fibrosis. American Journal of Medicine 1985;118(3):452-75. [PMC free article] [PubMed] [Google Scholar]
Gao 2020
- Gao L, Yuan H, Xu E, Junting L. Toxicology of paraquat and pharmacology of the protective effect of 5-hydroxy-1-methylhydantoin on lung injury caused by paraquat based on metabolomics. Nature Scientific Reports 2020;10:1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gawarammana 2011
- Gawarammana I, Buckley NA. Medical management of paraquat ingestion. British Journal of Clinical Pharmacology 2011;72(5):1365-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ginsberg 2005
- Ginsberg G, Hattis D, Russ A, Sonawane B. Pharmacokinetic and pharmacodynamic factors that can affect sensitivity to neurotoxic sequelae in elderly individuals. Environmental Health Perspectives 2005;113(9):1243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2020 [Computer program]
- GRADEpro Guideline Development Tool. Evidence Prime Inc, Version Available from gradepro.org. McMaster University, 2020.
Gunnell 2003
- Gunnell D, Eddleston M. Suicide by intentional ingestion of pesticides: a continuing tragedy in developing countries. International Journal of Epidemiology 2003;32(6):902-9. [DOI: 10.1093/ije/dyg307] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hart 1984
- Hart TB, Nevitt A, Whitehead A. A new statistical approach to the prognostic significance of plasma concentrations. Lancet 1984;324(8413):1222-3. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Kamaruzaman 2019
- Kamaruzaman NA, Leong Y, Jaafar MH, Khan HR, Rani NA, Razali MF, et al. Epidemiology and risk factors of pesticide poisoning in Malaysia: a retrospective analysis by the National Poison Centre (NPC) from 2006 to 2015. BMJ Open 2020;10:e036048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karunarathne 2020
- Karunarathne A, Gunnell D, Konradsen F, Eddleston M. How many premature deaths from pesticide suicide have occurred since the agricultural Green Revolution? Clinical Toxicology 2020;58(4):227-32. [DOI: 10.1080/15563650.2019.1662433] [DOI] [PubMed] [Google Scholar]
Knipe 2014
- Knipe DW, Metcalfe C, Fernando R, Pearson M, Konradsen F, Eddleston M, et al. Suicide in Sri Lanka 1975–2012: age, period and cohort analysis of police and hospital data. BMC Public Health 2014;14:839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavergne 2018
- Lavergne V, Gosselin S, Ghannoum M, Hoegberg LC, Roberts DM. Extracorporeal blood purification for treating acute paraquat poisoning. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD013112. [DOI: 10.1002/14651858.CD013112] [DOI] [Google Scholar]
Lee 1984
- Lee SS, Kawanami O, Aihara K, Chung CH, Hirohata Y. Paraquat intoxication on rat lung. Korean Journal of Pathology 1984;18(4):333. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Mew 2017
- Mew EJ, Padmanathan P, Konradsen F, Eddleston M, Chang SS, Phillips MR, et al. The global burden of fatal self-poisoning with pesticides 2006-15: systematic review. Journal of Affective Disorders 2017;219:93–104. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Sawada 1988
- Sawada Y, Yamamoto I, Hirokane T, Nagai Y, Satoh Y, Ueyama M. Severity index of paraquat poisoning. Lancet 1988;331(8598):1333. [DOI] [PubMed] [Google Scholar]
Smith 1975
- Smith P, Heath D. The pathology of the lung in paraquat poisoning. Journal of Clinical Pathology 1975;9:81-93. [PMC free article] [PubMed] [Google Scholar]
Tomlin 1994
- Tomlin C. The Pesticide Manual. 10th edition. Farnham (UK): British Crop Protection Council and the Royal Society of Chemistry, 1994. [Google Scholar]
Vale 1987
- Vale JA, Meredith TJ, Buckley BM. Paraquat poisoning: clinical features and immediate general management. Human Toxicology 1987;6(1):41-7. [DOI] [PubMed] [Google Scholar]
Wang 2019
- Wang N, Jiang Q, Han L, Zhang H, Zhu B, Liu X. Epidemiological characteristics of pesticide poisoning in Jiangsu Province, China, from 2007 to 2016. Nature Scientific Reports 2019;9:8604. [DOI: 10.1038/s41598-019-44986-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO IPCS 2009
- World Health Organization - International Programme for Chemical Safety. Poison information monograph 399. Paraquat. www.inchem.org/documents/pims/chemical/pim399.htm (accessed 28 June 2021).
Winsett 2004
- Winsett RP, Arheart K, Stratta RJ, Alloway R, Wicks MN, Gaber AO, et al. Evaluation of an immunosuppressant side effect instrument. Progress in Transplantation 2004;14(3):210-6. [DOI] [PubMed] [Google Scholar]
Xu 2019
- Xu YG, Lu YQ. Systematic review and meta-analysis of the efficacy and safety of immunosuppressive pulse therapy in the treatment of paraquat poisoning. Journal of Zhejiang University-Science B 2019;20(7):588-97. [DOI: 10.1631/jzus.B1800640] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2016
- Yan R, Hill S. How to GRADE the quality of the evidence. Cochrane Consumers and Communication Group. Version 3.0. Available at cccrg.cochrane.org/author-resources (accessed prior to 22 June 2021).
References to other published versions of this review
Li 2014
- Li LR, Sydenham E, Chaudhary B, Beecher D, You C. Glucocorticoid with cyclophosphamide for paraquat‐induced lung fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 8. Art. No: CD008084. [DOI: 10.1002/14651858.CD008084.pub4] [DOI] [PubMed] [Google Scholar]


