Abstract
We describe the case of a 44-year-old female patient on rituximab for the treatment of multiple sclerosis with undetectable severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG specific antibodies 18 days after the second dose of SARS-CoV-2 vaccine. Interferon-gamma release assay testing for SARS-CoV-2 was positive on day 19, demonstrating a robust T cell-mediated response despite the lack of an antibody-mediated response.
Keywords: SARS-CoV-2, SARS-CoV-2 vaccination, T cell respone, Impaired humoral immunity
1. Introduction
Although immunocompromised patients were excluded from the mRNA coronavirus disease 2019 (COVID-19) vaccine clinical trials and made up less than 0.5% of participants in the Janssen/Johnson & Johnson trials, many physician groups are recommending vaccination of these groups. (Jackson et al., 2020, Polack et al., 2020, Sadoff et al., 2021) Vaccine efficacy in immunocompromised patients is thought to be impaired, with limited data specifically in patients with B cell impairment. (Boyarsky et al., 2021)
Neutralizing antibodies are thought to play a protective role against infection, with a decrease in disease severity in patients with early severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection treated with monoclonal antibodies or high-titer convalescent plasma. (Weinreich et al., 2020, Libster et al., 2021) Spike-specific, nucleocapsid-specific, and neutralizing antibodies are associated with protection against SARS-CoV-2 infection after natural infection. (Lumley et al., 2021) All three vaccines currently available in the United States elicit high levels of neutralizing antibodies. (Sadoff et al., 2021, Walsh et al., 2020) The US Centers for Disease Control and Prevention (CDC) recommend against routinely assessing antibody levels after vaccination. Data are accumulating that patients on immunosuppressive medications exhibit impaired antibody production after COVID-19 vaccination, with concerning implications for vaccine efficacy in this group. (Boyarsky et al., 2021) Rituximab is one example: it is an anti-CD20 monoclonal antibody that depletes B cells in the treatment of hematologic malignancies and rheumatologic diseases, and it potently impairs antibody production after vaccination.
SARS-CoV-2-specific antibodies are associated with disease severity, with lower titers noted after asymptomatic and mild disease. (Chen et al., 2020) However, multiple reports of COVID-19 infections in individuals with humoral deficiencies have demonstrated that uneventful recovery can occur in the absence of antibody production. (Gupta et al., 2021) For individuals with impaired antibody production, finding an immunologic correlate of protection is of great interest.
CD4+ and CD8+ T cell responses may contribute to protection against SARS-CoV-2, even in convalescent individuals after asymptomatic or mild infection and in seronegative exposed family members. (Sekine et al., 2020, Sette and Crotty, 2021) All three available vaccines elicit Th1-skewed T cell responses. (Sadoff et al., 2021, Sahin et al., 2020)
A key component of the Th1 response is the production of interferon-gamma (IFN-γ) by T cells. IFN-γ release assays (IGRAs) are in vitro blood diagnostics used to measure IFN-γ released by antigen-specific T cells after overnight stimulation with pathogen peptides. Several studies have reported on the performance of IGRAs for the detection of the cellular immune response to SARS-CoV-2. (Murugesan et al., 2020) We have demonstrated high IGRA sensitivity in convalescent patients in the weeks following COVID-19, which remains sensitive up to 7 months post infection. (Murugesan et al., 2020) The SARS-CoV-2 IGRA is offered as a laboratory-developed test at Stanford Health Care clinical laboratories. This test uses a combination of four peptide pools, which includes the corresponding peptides to spike protein, to stimulate CD4+ and CD8+ T cells and assess IFN-γ concentration by automated enzyme-linked immunosorbent assay (ELISA) with a cutoff value of 0.35 IU/ml. (Murugesan et al., 2020) In addition to using the SARS-CoV-2 IGRA to assess for past infection, the SARS-CoV-2 IGRA can also be used to assess the cellular immune response post vaccination.
2. Case description
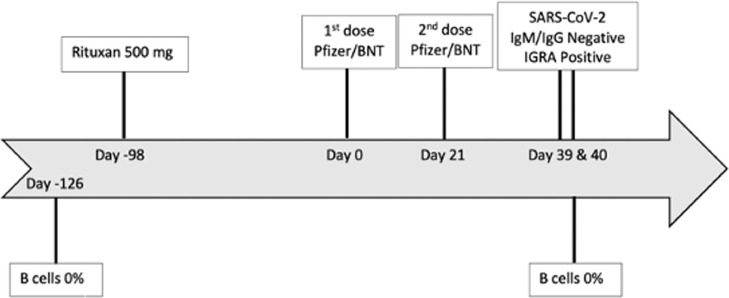
We present the case of a 44-year-old female patient whose medical history was notable only for multiple sclerosis that was well controlled on rituximab administered at a dose of 500 mg every 6 months for the past 3 years, with no prior multiple sclerosis therapies. She had had no symptoms of respiratory infection between April 2020 and January 2021 and no known exposures to SARS-CoV-2. Rituximab was last administered as an intravenous infusion 14 weeks prior to her first dose of Pfizer/BNT vaccine, with two doses given 21 days apart. Lymphocyte subsets 18 weeks prior to and 19 days after the second vaccine dose demonstrated zero B cells. SARS-CoV-2-specific antibodies were not evaluated prior to vaccination. Eighteen days after the second vaccine dose, SARS-CoV-2-specific IgG levels were undetectable, identified by Siemens and Diasorin spike protein assays. Nineteen days after second vaccine dose, the SARS-CoV-2 IGRA was strongly positive: antigen minus nil = 8.39 IU/ml, with reference range <0.35 IU/ml (Figure 1 ). The mitogen nil result was 9.98 IU/ml.
Figure 1.
Timeline of events.
3. Discussion
It is expected that patients receiving B cell depleting agents such as rituximab will have an impaired antibody response to COVID-19 vaccine, as in this case. Despite delaying vaccination until 14 weeks after her last rituximab dose, the case patient did not produce a detectable antibody response. More notably, this patient readily produced an antigen-specific T cell response detectable 19 days after completed vaccination despite having no B cells. The SARS-CoV-2 IGRA provided evidence that this patient mounted a robust cellular immune response after vaccination, similar to findings by others. (Bonelli et al., 2021) Currently, although humoral and cellular immune responses may together reduce disease severity, we do not yet know whether a vaccine-generated T cell response in the absence of antibody production provides protection against COVID-19. (Sette and Crotty, 2021)
Although current guidance recommends temporarily pausing rituximab and other immunosuppression pending COVID-19 vaccination, it is not known whether this practice improves the immunological response. This is an important factor to discuss with patients if delaying therapy could incur risks of disease reactivation and need for corticosteroids, which impair both humoral and cellular immunity. In weighing the risks and benefits of reducing or delaying disease-modifying agents, the benefits are not yet defined.
There are several limitations in interpreting this case. Without pre-vaccination testing, we cannot rule out that the patient had had a prior undetected SARS-CoV-2 infection producing the positive IGRA. This specific IGRA showed a 10% background positive rate in healthy individuals, reflecting either false-positive results, asymptomatic SARS-CoV-2 exposure, or cross-reactivity with seasonal coronaviruses.
The findings of positive SARS-CoV-2 IGRA post-vaccination with negative antibodies in this patient on rituximab with zero B cells, suggests that the IGRA should be studied as a possible correlate of protection after COVID-19 mRNA vaccines in patients on immunosuppressive medications.
Acknowledgments
Funding
No funding sources obtained.
Ethical approval
This work did not require approval by the institutional review board.
Conflict of interest
All authors have no conflict of interest to report.
References
- Sadoff J, Le Gars M, Shukarev G, et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med. 2021;0(0) doi: 10.1056/NEJMoa2034201. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N Engl J Med. 2020;0(0) doi: 10.1056/NEJMoa2022483. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. JAMA. 2021 doi: 10.1001/jama.2021.4385. Published online March 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2035002. Published online December 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libster R, Marc GP, Wappner D, et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N Engl J Med. 2021 doi: 10.1056/NEJMoa2033700. Published online January 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley SF, O'Donnell D, Stoesser NE, et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N Engl J Med. 2021;384(6):533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh EE, Frenck RW, Falsey AR, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Pan Z, Yue S, et al. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Signal Transduct Target Ther. 2020;5(1):1–6. doi: 10.1038/s41392-020-00301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Su H, Narsai T, Agrawal S. SARS-CoV-2-Associated T-Cell Responses in the Presence of Humoral Immunodeficiency. Int Arch Allergy Immunol. 2021;182(3):195–209. doi: 10.1159/000514193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine T, Perez-Potti A, Rivera-Ballesteros O. al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell. 2020;183(1):158–168. doi: 10.1016/j.cell.2020.08.017. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and T H 1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- Murugesan K, Jagannathan P, Pham TD, et al. Interferon-gamma release assay for accurate detection of SARS-CoV-2 T cell response. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 doi: 10.1093/cid/ciaa1537. Published online October 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli MM, Mrak D, Perkmann T, Haslacher H, Aletaha D. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-220764. Published online May 5. [DOI] [PubMed] [Google Scholar]