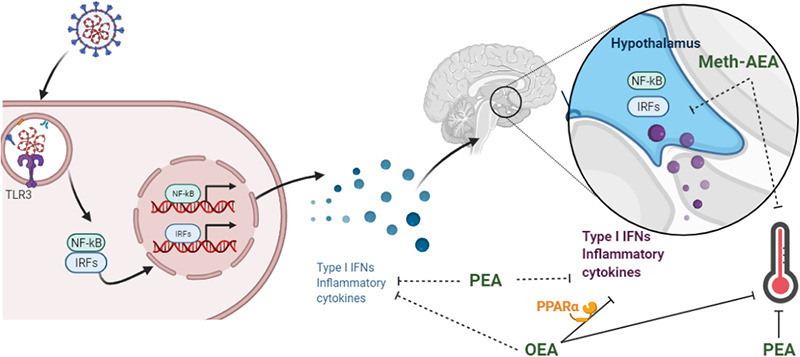
Abstract
Increasing evidence suggests that SARS-CoV-2, the virus responsible for the COVID-19 pandemic, is associated with increased risk of developing neurological or psychiatric conditions such as depression, anxiety or dementia. While the precise mechanism underlying this association is unknown, aberrant activation of toll-like receptor (TLR)3, a viral recognizing pattern recognition receptor, may play a key role. Synthetic cannabinoids and enhancing cannabinoid tone via inhibition of fatty acid amide hydrolase (FAAH) has been demonstrated to modulate TLR3-induced neuroimmune responses and associated sickness behaviour. However, the role of individual FAAH substrates, and the receptor mechanisms mediating these effects, are unknown. The present study examined the effects of intracerebral or systemic administration of the FAAH substrates N-oleoylethanolamide (OEA), N-palmitoylethanolamide (PEA) or the anandamide (AEA) analogue meth-AEA on hyperthermia and hypothalamic inflammatory gene expression following administration of the TLR3 agonist, and viral mimetic, poly I:C. The data demonstrate that meth-AEA does not alter TLR3-induced hyperthermia or hypothalamic inflammatory gene expression. In comparison, OEA and PEA attenuated the TLR3-induced hyperthermia, although only OEA attenuated the expression of hyperthermia-related genes (IL-1β, iNOS, COX2 and m-PGES) in the hypothalamus. OEA, but not PEA, attenuated TLR3-induced increases in the expression of all IRF- and NFκB-related genes examined in the hypothalamus, but not in the spleen. Antagonism of PPARα prevented the OEA-induced attenuation of IRF- and NFκB-related genes in the hypothalamus following TLR3 activation but did not significantly alter temperature. PPARα agonism did not alter TLR3-induced hyperthermia or hypothalamic inflammatory gene expression. These data indicate that OEA may be the primary FAAH substrate that modulates TLR3-induced neuroinflammation and hyperthermia, effects partially mediated by PPARα.
Keywords: Viral infection, Cannabinoid, PPAR, PEA, OEA, Anandamide, Temperature, Hypothalamus, Neuroinflammation
Abbreviations: AEA, anandamide; CB1, cannabinoid receptor 1; CB2, cannabinoid receptor 2; CNS, central nervous system; COX-2, cyclooxygenase 2; FAAH, Fatty acid amide hydrolase; IFN, interferon; IL, interleukin; i.p, intraperitoneal; IP-10, Interferon gamma-induced protein 10; IRF, interferon regulatory factor; NFκB, Nuclear factor kappa B; OEA, N-oleoylethanolamide; OFT, Open field test; PEA, N-palmitoylethanolamide; PGE2, prostaglandin E2; Poly I:C, Polyinosinic: polycytidylic acid; SPT, Sucrose preference test; TLR, Toll-like receptor; TNF, tumour necrosis factor
Graphical abstract
1. Introduction
Uncontrolled immune responses to viral infection have been proposed to underlie the pathophysiology and exacerbation of a host of neurological and psychiatric conditions. Thus, unsurprisingly, recent evidence indicates this is also the case following SARS-CoV-2 infection (Harapan and Yoo, 2021; Mahalakshmi et al., 2021; Taquet et al., 2021), which is responsible for coronavirus disease 2019 (COVID-19), a pandemic that has overtaken the world during the past year. Viral antigens mediate immune responses by activating pattern recognition receptors such as toll-like receptor (TLR)3, resulting in induction of type 1 interferon (IFN-α and IFN-β) and NFĸB-inducible (e.g. IL-1β, IL-6 and TNF-α) inflammatory cascades responsible for host defences, homeostasis and response to injury. However, uncontrolled and aberrant activation of TLR3 has been shown to impair contextual and working memory (Baghel et al., 2018; Galic et al., 2009), elicit anxiety- and depressive-like behaviour (Gibney et al., 2013), increase neuronal excitability and seizure susceptibility (Costello and Lynch, 2013; Galic et al., 2009) and exacerbate underlying neurodegenerative processes (Deleidi et al., 2010; Field et al., 2010). Furthermore, TLR3 expression has been demonstrated to be increased in the brain of patients with neurodegenerative (Walker et al., 2018) and psychiatric (Pandey et al., 2014) disorders. Thus, modulating the neuroinflammatory, and consequently neurological, effects of TLR3 activation is of critical physiological and therapeutic importance.
The cannabinoid system exhibits well recognised immune-modulatory properties (Henry et al., 2016; Russo et al., 2018; Tahamtan et al., 2016). Accordingly, cannabinoids and related N-acylethanolamines such as N-palmitoylethanolamide (PEA) have been proposed as potential therapeutics limiting mast cell activation and inflammatory response to SARS-CoV-2 (Gigante et al., 2020; Lucaciu et al., 2021). Recent data have indicated that the plant-derived cannabinoid cannabidiol inhibits SARS-CoV-2 replication and viral gene expression, induces interferon (IFN) expression and up-regulates its antiviral signalling pathways (Nguyen et al., 2021). Similarly, the synthetic cannabinoid agonist WIN55,212 has been shown to increase TLR3-induced IFN-β levels while attenuating pro-inflammatory NFκB-related immune responses in astrocytes (Downer et al., 2011). Increasing endogenous cannabinoid tone by inhibiting the catabolism enzymes for anandamide and 2-AG, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) respectively, has shown that FAAH, but not MAGL, inhibition attenuates TLR3-induced neuroinflammatory, but not peripheral, immune responses (Flannery et al., 2018a; Henry et al., 2014). Furthermore, inhibition of TLR3-induced neuroinflammation following FAAH inhibition is associated with an attenuation of TLR3-associated hyperthermia, anxiety-like behaviour and enhanced nociceptive responding (Flannery et al., 2018b), indicating that FAAH substrates are important modulators of TLR3-induced neuroinflammation and associated behavioural responding. In addition to the endocannabinoid anandamide, FAAH also metabolises the related fatty acid amides, N-oleoylethanolamide (OEA) and PEA (Cravatt et al., 1996), and thus, inhibition of FAAH results in increases in all three substrates (Fegley et al., 2005; Flannery et al., 2018a). It is unknown if one or all of these substrates is responsible for modulating the TLR3-induced neuroinflammatory and associated behavioural responding following FAAH inhibition.
The effects of AEA, OEA and PEA on TLR4-induced inflammatory responses have been well documented. AEA attenuates TLR4-induced production of pro-inflammatory cytokines and mediators such as TNF-α, IL-1β, prostaglandins (PG) and nitric oxide (NO) (Facchinetti et al., 2003; Molina-Holgado et al., 1997; Puffenbarger et al., 2000), while concurrently increasing anti-inflammatory mediators such as IL-10 (Correa et al., 2010; Krishnan and Chatterjee, 2012). N-acylethanolamine acid amidase (NAAA) is a further metabolic pathway for OEA and PEA, inhibition of which elicits potent immunosuppressive effects (Alhouayek et al., 2015; Piomelli et al., 2020; Skaper et al., 2015; Solorzano et al., 2009). PEA reduces TLR4-induced increases in TNF-α production and IL-6 and iNOS expression in macrophages (Li et al., 2012; Solorzano et al., 2009) and inhibits TLR4-induced pro-inflammatory M1 microglia while augmenting anti-inflammatory M2a microglia (D'Aloia et al., 2021). OEA decreased TLR4-induced increases in expression of pro-inflammatory cytokines iNOS and COX-2 in macrophages (Fan et al., 2014; Yang et al., 2016). OEA and PEA induce anti-inflammatory effects in a mouse model of colitis, directly via inhibition of TLR4-mediated immune responses (Esposito et al., 2014; Lama et al., 2020). Within the brain, AEA modulates TLR4-induced inflammatory responses, temperature changes and hypophagia (Hollis et al., 2011; Steiner et al., 2011). OEA and PEA attenuated TLR4-induced NFκB activity, IL-1β, COX-2, mPGES-1 expression and PGE2 levels in the hypothalamus, an effect associated with potentiation of TLR4-induced hypothermia (Sayd et al., 2015). OEA blocks the TLR4-mediated increases in pro-inflammatory cytokines and chemokines, oxidative and nitrosative stress, and neurodegenerative cascades in frontal cortex of a rodent model of alcohol abuse (Orio et al., 2018; Rivera et al., 2019) and neuropsychiatric conditions (Moya et al., 2021). Collectively, this demonstrates that AEA, OEA and PEA modulate TLR4-induced inflammatory responses; however, there is a paucity of studies investigating the effects of individual N-acylethanolamines on TLR3-induced inflammatory responses. PEA has been shown to inhibit TLR3-induced increase in the expression and release of the chemokine MCP-1 in keratinocytes (Petrosino et al., 2010). TLR3 plays a key role in the induction of the TMEV-model of multiple sclerosis, and FAAH inhibition, AEA and PEA has been shown to attenuate microglial activation, the expression of pro-inflammatory cytokines and ameliorates motor symptoms in this model (Mestre et al., 2005; Ortega-Gutierrez et al., 2005; Loria et al., 2008; Loria et al., 2010; Correa et al., 2011; Hernangomez et al., 2012). However, effects of individual FAAH substrates on the acute TLR3-mediated neuroimmune responses and associated sickness behaviour has not been examined. Enhancing FAAH substrate levels inhibits TLR3-induced hyperthermia without altering other aspects of the acute sickness response (Flannery et al., 2018b). As such, this study examined the effects of intracerebral or systemic administration of meth-AEA, OEA and PEA on TLR3-induced hyperthermia and expression of neuroinflammatory genes. OEA and PEA elicit their anti-inflammatory and neuroprotective effects mainly through the activation of nuclear peroxisome proliferator-activated receptor-alpha (PPAR-α) (Di Cesare Mannelli et al., 2013; Gonzalez-Aparicio et al., 2014; Lo Verme et al., 2005; Rankin and Fowler, 2020; Zhou et al., 2012). As such, the role of PPARα on OEA-mediated modulation of TLR3-induced hyperthermia and inflammatory gene expression was also examined.
2. Methods
2.1. Animals
Experiments were carried out on female Sprague-Dawley rats (weight, 200-350 g; In house bred), housed singly in transparent plastic bottomed cages on a constant temperature (21 ± 2 °C) under standard light-dark cycle conditions (12: 12 h light-dark, lights on from 0800 to 2000 h). All experiments were carried out during the light phase between 0800 h and 1800 h. Food and water were available ad libitum. Animals were habituated to handling and received an intraperitoneal (i.p.) injection of sterile saline (0.89% NaCl) for 3–4 days before experimentation in order to minimise the influence of the injection procedure on behaviour and biological endpoints. The experimental protocol was carried out in accordance with the guidelines of the Animal Care and Research Ethics Committee, National University of Ireland Galway under licence from the Irish Health Products regulatory Authority and in compliance with the European Communities Council directive 2010/63/EU.
2.2. Experimental design
2.2.1. Experiment 1: the effect of methanandamide on TLR3-induced hyperthermia and neuroinflammatory gene expression
Rats were randomly assigned to one of three treatment groups: Vehicle-Saline (n = 6), Vehicle-Poly I:C (n = 9), Methanandamide (meth-AEA)-Poly I:C (n = 10). Meth-AEA (20μg, Abcam, UK) or Vehicle (100% DMSO) were administered in a single acute i.c.v. injection, in an injection volume of 4 μl. This was followed 10 min later by an i.p, injection of poly I:C (3 mg/kg) or sterile saline (0.89% NaCl) administered in an injection volume of 1.5 mg/kg. Due to the rapid metabolism of AEA in vivo, the stable AEA analogue meth-AEA was administered directly to the brain (i.c.v). The concentration of meth-AEA used was chosen based on previous literature demonstrating antinociceptive and gastroprotective effect when administered centrally (Garzon et al., 2009; Shujaa et al., 2009). Core body temperature was measured using a rectal probe prior to any experimental manipulation and 4 h post poly I:C/saline injection. Animals were sacrificed by decapitation at 4 h post-poly I:C/saline administration, the hypothalamus excised, snap-frozen on dry ice and stored at -80 °C until assayed for expression of inflammatory mediators.
2.2.2. Experiment 2: the effects of OEA and PEA on TLR3-induced hyperthermia and neuroinflammatory gene expression
Rats were randomly assigned to one of four treatment groups: Vehicle-saline (n = 8), Vehicle-poly I:C (n = 9), OEA-poly I:C (n = 9) and PEA-poly I:C (n = 9). OEA and PEA (20 mg/kg, Abcam, UK) or Vehicle (ethanol: Cremophor: saline; 1:1:18) were administered i.p. in an injection volume of 2 ml/kg followed 10 min later by an i.p, injection of poly I:C (3 mg/kg) or sterile saline (0.89% NaCl) administered in an injection volume of 1.5 ml/kg. The dose of OEA was chosen as this has been shown to increase striatal levels of OEA from 15 min to 2 h post administration (Gonzalez-Aparicio et al., 2014; Plaza-Zabala et al., 2010) and pilot data in the lab demonstrated increased OEA concentration in the hypothalamus 1 h post administration. The dose of PEA was chosen based on published data demonstrating efficacy in reducing nociceptive behaviour (Pessina et al., 2015). Temperature was recorded prior to injection and 4 h post poly I:C/saline administration. Animals were sacrificed by decapitation at 4 h post-poly I:C/saline administration, the spleen and hypothalamus excised, snap-frozen on dry ice and stored at -80 °C until assayed for inflammatory gene expression.
2.2.3. Experiment 3: the effects of PPARα antagonism, in the presence and absence of OEA, on TLR3-induced hyperthermia and neuroinflammatory gene expression
Rats were randomly assigned to one of four treatment groups: Vehicle-Vehicle-saline (n = 8), Vehicle-Vehicle-poly I:C (n = 9), Vehicle-OEA-poly I:C (n = 8), GW6471-OEA-poly I:C (n = 8) and GW6471-Vehicle-PEA-poly I:C (n = 7). OEA (20 mg/kg, Abcam, UK) and GW6471 (2 mg/kg) were dissolved in Vehicle (ethanol: cremophor: saline; 1:1:18) were administered i.p. in an injection volume of 2 ml/kg. GW6471 or Vehicle was administered 20 min prior to administration of OEA or vehicle followed 10 min later by an i.p, injection of poly I:C (3 mg/kg) or sterile saline (0.89% NaCl) in an injection volume of 1.5 ml/kg. The dose of GW6471 was chosen based on efficacy in reversing PEA-induced protective effects (Pessina et al., 2015; Scuderi et al., 2014), without affecting nociceptive responding (Gaspar et al., 2020) or anxiety-like behaviour (unpublished in-house data). Temperature was recorded prior to injection and 4 h post poly I:C/saline administration, after which animals were sacrificed hypothalamus excised, snap-frozen on dry ice and stored at -80 °C until assayed for gene expression.
2.2.4. Experiment 4: the effects of PPARα agonism on TLR3-induced hyperthermia and neuroinflammatory gene expression
Rats were randomly assigned to one of three treatment groups: Vehicle-saline (n = 6), Vehicle-poly I:C (n = 8), Vehicle-WY14643 (n = 6). WY14643 (20 mg/kg, Abcam, UK) was dissolved in Vehicle (10% DMSO) were administered i.p. in an injection volume of 2 ml/kg, followed 30 min later by an i.p, injection of poly I:C (3 mg/kg) or sterile saline (0.89% NaCl) in an injection volume of 1.5 ml/kg. The dose of WY14643 was chosen based on in vivo efficacy in several models (Lysne et al., 2019; Okine et al., 2015; Song et al., 2016). Temperature was recorded prior to injection and 4 h post poly I:C/saline administration, after which animals were sacrificed hypothalamus excised, snap-frozen on dry ice and stored at -80 °C until assayed for gene expression.
2.3. Intracerebroventricular (i.c.v.) guide cannula implantation
Intracerebroventricular (i.c.v.) guide cannulae were implanted into the rat brain as previously described (Henry et al., 2014). In brief, under isoflurane anaesthesia (1–3% in O2; 0.5 L/min), a guide cannula (5 mm, Plastics One Inc., Roanoke, Virginia, USA) was stereotaxically implanted into the right lateral ventricle (coordinates: AP: −0.07 mm; ML: −0.15 mm, DV: −0.30 mm; (Paxinos, 2006)). The cannula was permanently fixed to the skull using stainless steel screws and dental acrylic cement and the guide remained patent by the insertion of a stainless steel stylet (Plastics One Inc., USA). Animals received the broad spectrum antibiotic enrofloxacin (2.5 mg/kg s.c.; Baytril, Bayer Ltd., Ireland) on the day of and for 3 days post surgery. Correct cannula placement was verified by the Angiotensin (Ang) II drinking test 3 days prior to the experiment. Animals were considered non-responders if they drank <3mls over 20 min post AngII infusion and were not included in the experiment. Over all experiments, the average number of non-responders was <5%. Animals were allowed to recover from surgery for at least 6 days prior to experimentation.
2.4. Expression of inflammatory mediators using quantitative real-time PCR
RT-qPCR was performed as previously described (Flannery et al., 2018a; Flannery et al., 2018b; Henry et al., 2014). In brief, mRNA was isolated from hypothlamic tissue using NucleoSpin RNA II total RNA isolation kit (Macherey-Nagel, Germany) and reverse transcribed into cDNA using a High Capacity cDNA Archive kit (Applied Biosystems, UK). Taqman gene expression assays (Applied Biosystems, UK) were used to quantify the gene of interest and real-time PCR was performed using an ABI Prism 7500 instrument (Applied Biosystems, UK. Assay IDs for the genes were as follows: IP-10 (Rn00594648_m1), IRF7 (Rn01450778_g1), TNFα (Rn99999017_m1), IL-1β (Rn00580432_m1), IL-10 (Rn00563409_m1), iNOS (NOS2) (Rn00561646_m1), COX-2 (Rn01483828_m1), m-PGE-s (Rn00572047_m1), SOCS1 (Rn00595838_s1) and SOCS3 (Rn00585674_s1). β-actin was used as an endogenous control to normalise gene expression data. Relative gene expression was calculated using the ∆∆CT method.
2.5. Statistical analysis
Data were analysed and graphs using Graph Pad Prizm v9. Normality and homogeneity of variance were assessed using Shapiro-Wilk and Levene's test, respectively. Data were analysed by One-Way ANOVA's followed by Student Newman Keules (SNK) post hoc analysis where appropriate. The level of significance was set at p < 0.05. Data are expressed as group means ± standard error of the mean (SEM).
3. Results
3.1. Meth-AEA does not alter TLR3-induced hyperthermia or inflammatory gene expression in the hypothalamus
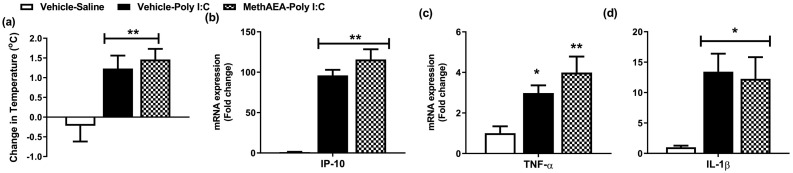
The data revealed that poly I:C-induced an increase in temperature [F(2,19) = 6.35, p = 0.007] and IP-10 [F(2,19) = 29.97, p < 0.001], TNF-α [F(2,19) = 4.89, p = 0.019] and IL-1β [F(2,19) = 3.73, p = 0.044] expression in the hypothalamus, 4 h post administration (Fig. 1a-d). Meth-AEA (i.c.v.) did not alter poly I:C-induced hyperthermia or inflammatory gene expression in the hypothalamus (Fig. 1a-d).
Fig. 1.
The effect of meth-AEA (i.c.v.) on poly I:C induced (a) hyperthermia and increases in (b) IP-10, (c) TNF-α and (d) IL-1β expression in the hypothalamus, 4 h post poly I:C administration. Data expressed as mean ± SEM (n = 5–9 per group). *p < 0.05; ** p < 0.01 vs vehicle-saline-treated counterparts.
3.2. OEA and PEA attenuates TLR3-induced hyperthermia, but only OEA attenuates TLR3- induced inflammatory gene expression in the hypothalamus
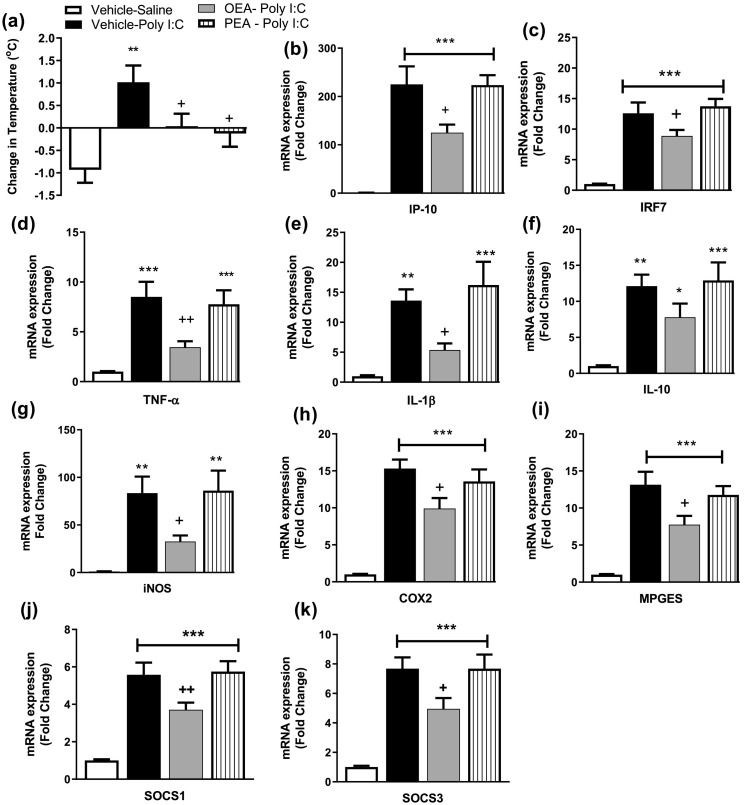
The data revealed that poly I:C significantly increased temperature at 4 h post administration [F(3,24) = 5.842, p = 0.004]. Systemic administration of either OEA or PEA prevented poly I:C-induced hyperthermia (Fig. 2a).
Fig. 2.
The effect of OEA or PEA on poly I:C induced (a) hyperthermia and increases in inflammatory gene expression of (b) IP-10, (c) IRF7, (d) TNF-α, (e) IL-1β, (f) IL-10, (g) iNOS, (h) COX2, (i) MPGES, (j) SOCS1 and (k) SOCS3 in the hypothalamus, 4 h post poly I:C administration. Data expressed as mean ± SEM (n = 6–8 per group). ***p < 0.001; **p < 0.01; * p < 0.05 vs vehicle-saline-treated counterparts. ++p < 0.01; +p < 0.05 vs vehicle-poly I:C-treated counterparts.
Analysis revealed a significant effect of treatment on the hypothalamic expression of IFN-inducible genes IP-10 [F(3,25) = 24.32, p < 0.001] and IRF7 [F(3,25) = 25.6, p < 0.001], and the NFκB-inducible genes TNF-α [F(3,25) = 11.03, p < 0.001], IL-1β [F(3,25) = 9.02, p < 0.001], IL-10 [F(3,25) = 8.328, p < 0.01]. Post hoc analysis revealed that poly I:C-induced a significant increase in the expression of all inflammatory genes examined in the hypothalamus compared to vehicle-saline-treated counterparts, 4 h post administration (Fig. 2b-f). OEA significantly attenuated the poly-I:C-induced increase in IP-10, IRF7, TNF-α and IL-1β, but not IL-10, expression in the hypothalamus. In contrast, systemic administration of PEA did not alter the poly I:C-induced increase in neuroinflammatory gene expression in the hypothalamus (Fig. 2b-f).
In order to determine if the effects of OEA on poly I:C-induced hyperthermia are accompanied by an attenuation of COX2-PEG2 activity, the expression of genes regulating this pathway was also examined. Analysis revealed a significant effect of treatment on expression iNOS [F(3,25) = 8.506, p < 0.01], COX2 [F(3,24) = 20.06, p < 0.01] and MPEGS [F(3,25) = 20.47, p < 0.01]. Post hoc analysis revealed that poly I:C induced an increase in expression of iNOS, COX2 and MPGES, an effect attenuated by OEA, but not PEA (Fig. 2g-i). Furthermore, analysis revealed a significant effect of treatment the expression of the regulatory genes SOCS1 [F(3,25) = 22.51, p < 0.01] and SOCS3 [F(3,25) = 17.57, p < 0.01] and confirmed that poly I:C-induced an increase in expression of SOCS1 and SOCS3, an effect attenuated by OEA, but not PEA (Fig. 2j-k).
3.3. OEA or PEA do not alter TLR3-induced inflammatory gene expression in the spleen
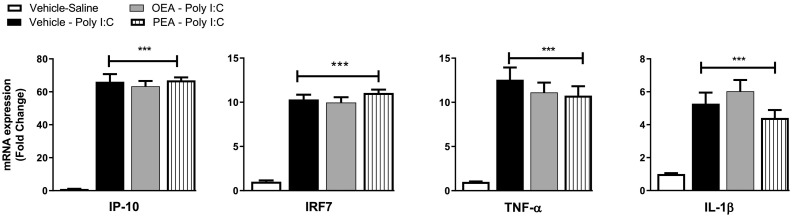
In order to determine if the effect of OEA on inflammatory gene expression in the hypothalamus are due to modulation of peripheral immune responses following TLR3 activation, inflammatory gene expression was also examined in the spleen. Poly I:C-induced a significant increase in IP-10 [F(3,25) = 129.8, p < 0.001], IRF7 [F(3,25) = 104.1, p < 0.001], TNF-α [F(3,25) = 25.46, p < 0.001] and IL-1β expression [F(3,25) = 16.59, p < 0.001] in the spleen, an effect not altered by OEA or PEA (Fig. 3 ).
Fig. 3.
The effect of OEA or PEA on poly I:C induced increases in inflammatory gene expression of (a) IP-10, (b) IRF7, (c) TNF-α and (d) IL-1β in the spleen, 4 h post poly I:C administration. Data expressed as mean ± SEM (n = 6–8 per group). ***p < 0.001 vs vehicle-saline-treated counterparts.
3.4. PPARα antagonism blocks the OEA-induced attenuation of inflammatory gene expression in the hypothalamus following TLR3 activation
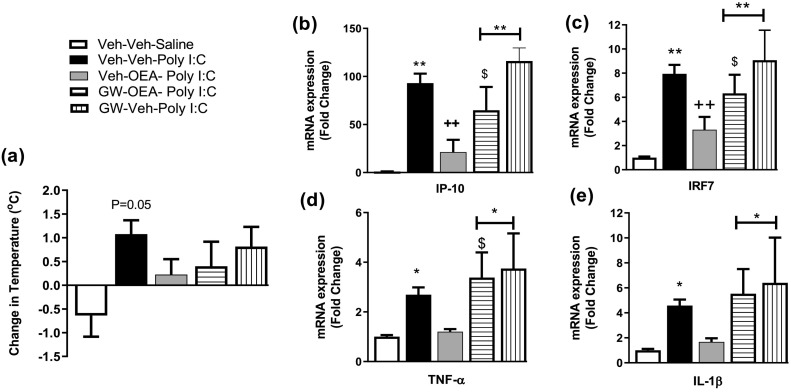
Several studies have demonstrated that anti-inflammatory effects of OEA have been attributed to activation of PPARα. Thus, the role of PPARα in mediating the effects of OEA on TLR3-induced hyperthermia and neuroinflammatory gene expression in the hypothalamus were examined in the current study. Analysis revealed that poly I:C- induced an increase in body temperature 4 h post administration (P = 0.05), which was not observed in rats that received OEA and/or the PPARα antagonist GW6471 (Fig. 4a). Poly I:C significantly increased the expression of IP-10 [F(4,33) = 10.86, p < 0.001], IRF7 [F(4,33) = 11.31, p < 0.001], TNF-α [F(4,33) = 5.13, p = 0.002] and IL-1β [F(4,33) = 4.52, p = 0.005] in the hypothalamus, an effect not observed in rats pre-treated with OEA (Fig. 4b-e). Administration of GW6471 blocked the effects of OEA on inflammatory gene expression following poly I:C administration. There was no significant effect of GW6471 alone on poly I:C-induced inflammatory gene expression in the hypothalamus (Fig. 4b-e).
Fig. 4.
The effect of GW6471 on OEA-induced changes in (a) temperature and f (b) IP-10, (c) IRF7, (d) TNF-α and (e) IL-1β expression in the hypothalamus. Data expressed as mean ± SEM (n = 7–8 per group). *p < 0.05; **p < 0.01 vs Veh-Veh-Saline. ++p < 0.01 vs Veh-Veh-poly I:C. $p < 0.05 vs Veh-OEA-poly I:C.
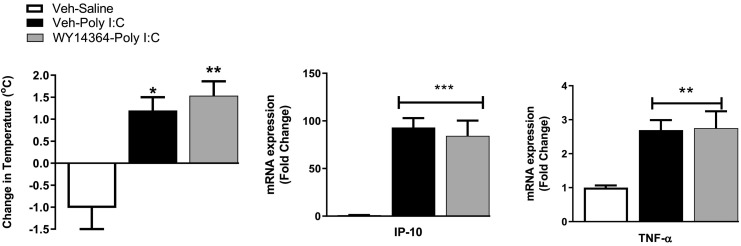
In order to determine if the effects of OEA on TLR3-induced responses could be mimicked by PPARα agonism, the effects of systemic administration of the PPARα agonist WY14643 were examined. WY14643 did not alter TLR3-induced hyperthermia, IP-10 or TNFα expression in the hypothalamus (Fig. 5 ).
Fig. 5.
The effect of systemic administration of WY14643 on poly I:C induced (a) hyperthermia and (b) increases in (b) IP-10 and (c) TNF-α expression in the hypothalamus. Data expressed as mean ± SEM (n = 6–8 per group). ***p < 0.001 **p < 0.01 *p < 0.05 vs vehicle-saline.
4. Discussion
N-acylethanolamines exhibit potent anti-inflammatory effects, however, effects on viral-mediated immune responses within the brain have not been extensively examined. The present study demonstrated that OEA and PEA, but not AEA, attenuate TLR3-induced hyperthermia and OEA attenuates the expression of IRF- and NFκB-related genes in the hypothalamus, including hyperthermic related genes (IL-1β, iNOS, COX2 and m-PGES). Antagonism of PPARα prevented the OEA-induced attenuation of IRF- and NFκB-related genes in the hypothalamus following TLR3 activation, without altering temperature. However, PPARα agonism did not alter TLR3-induced hyperthermia or hypothalamic inflammatory gene expression. While the mechanisms mediating the effects of PEA on TLR3-mediated hyperthermia remain to be determined, the data indicate that OEA attenuates TLR3-induced neuroinflammation and hyperthermia, an effect partially mediated by PPARα.
In line with previous data, (Cunningham et al., 2007; Flannery et al., 2018a; Henry et al., 2014; Murray et al., 2015), the present study confirmed that poly I:C-induced activation of TLR3 elicits a robust induction of IFN- and NFκB-mediated immune responses both peripherally and centrally, accompanied by hyperthermia. Increasing FAAH substrate levels has been demonstrated to attenuate TLR3-induced hyperthermic and neuroinflammatory responses, effects specifically mediated at the level of the central nervous system (Flannery et al., 2018a; Flannery et al., 2018b; Henry et al., 2014). The current data demonstrate that OEA and PEA, but not meth-AEA, attenuate TLR3-induced hyperthermia. In comparison, AEA, OEA and PEA have been shown to modulate TLR4-induced hypo- (Sayd et al., 2015; Steiner et al., 2011) or hyper-thermia (Hollis et al., 2011), although no effect was observed when all 3 substrates are enhanced following FAAH inhibition (Henry et al., 2017). It is possible that competitive inhibition exists when all three FAAH substrates are enhanced which overrides the effects of individual N-acylethanolamines on TLR4-induced changes in core body temperature. Such competitive inhibition between FAAH substrates may not take place in response to TLR3 activation, as AEA does not play a significant role in TLR3-mediated thermoregulation. Accordingly, AEA-induced activation of CB1 receptors plays a key role in the thermoregulatory response following TLR4 activation (Duncan et al., 2013; Fraga et al., 2009; Steiner et al., 2011). In comparison, TLR3-mediated hyperthermia is maintained in CB1 − / − mice (Duncan et al., 2013), a finding further supported by unpublished data from our lab demonstrating a lack of effect of central CB1 or CB2 receptor agonism on TLR3-induced hyperthermia. Thus, taken together, these data suggest that AEA-CB1 receptor activation plays a key role in TLR4-, but not TLR3-, induced thermoregulatory and neuroinflammatory responses.
OEA and PEA modulate the TLR4-induced hypothermic response, an effect associated with an attenuation in hypothalamic IL-1β, COX-2, mPGES-1 expression and PGE2 levels (Sayd et al., 2015). Similarly, the TLR3-induced hyperthermic response has been shown to be primarily mediated by the IL-1β-COX2 pathway (Fortier et al., 2004). The current study demonstrated that OEA and PEA attenuates TLR3-induced hyperthermia; however, only OEA attenuates the hypothalamic expression of hyperthermic related genes (IL-1β, COX2, iNOS and m-PGES-1). Published and pilot data have demonstrated that OEA crosses the blood brain barrier and increases OEA levels in the brain 20 mins following i.p. administration (Gonzalez-Aparicio et al., 2014) and can remain elevated up to 2 h post injection (Plaza-Zabala et al., 2010). Furthermore, OEA did not alter the expression of TLR3-induced inflammatory genes in the spleen. Thus, it is likely that OEA acts directly at the level of the hypothalamus to attenuate the TLR3-induced activation of the IL1β-COX2-PGE2 pathway and consequently, the associated hyperthermia. The neuro-immuno-modulatory effects of FAAH inhibition following TLR3 activation have been demonstrated to be mediated directly at the level of the brain (Flannery et al., 2018a; Henry et al., 2014). Thus, given the lack of effect of meth-AEA or PEA on TLR3-induced neuroimmune mediators, it is likely that OEA is the primary FAAH substrate modulating TLR3-induced neuroinflammation and associated hyperthermia. The anti-inflammatory effects of OEA are primarily mediated by PPARα (Russo et al., 2018; Xu et al., 2016) and accordingly, the current study demonstrated that PPARα antagonism blocked the inhibitory effect of OEA on TLR3-induced inflammatory gene expression in the hypothalamus. However, PPARα antagonism failed to alter the inhibitory effect of OEA on TLR3-induced hyperthermia, and PPARα agonism failed to modulate TLR3-induced hyperthermia or hypothalamic gene expression, indicating additional receptor (TRPV1, GPR55) or molecular targets and/or thermoregulatory mechanisms are likely to be also involved in mediating the effects of OEA.
Although PEA attenuated TLR3-induced hyperthermia, no effect was observed on the expression of inflammatory genes in the hypothalamus, suggesting differential mechanisms underlie the effects of OEA and PEA on TLR3-induced hyperthermia. We cannot rule out that PEA may have induced effects on hypothalamic inflammatory gene expression at an earlier timepoint than examined in this study. PEA has been reported to cross the blood brain barrier after an oral administration, although at very low concentrations (<1%) (Artamonov et al., 2005) and unpublished pilot data from our lab suggest that PEA levels were not elevated in the hypothalamus 1 h following administration. It should be noted that in addition to FAAH, OEA and PEA are also hydrolysed by NAAA, the inhibition of which has been shown to elicit potent immunosuppressive activity (Alhouayek et al., 2015; Piomelli et al., 2020; Skaper et al., 2015; Solorzano et al., 2009). NAAA is highly expressed in cells of the immune system and thus, the lack of effect of PEA on hypothalamic gene expression may be due to low central tissue distribution due to its rapid metabolism by NAAA under inflammatory conditions. The effects of PEA on TLR3-induced hyperthermia is most likely mediated peripherally rather than at the level of the hypothalamus. However, the current study demonstrated that PEA did not alter the TLR3-induced increase in IFN- or NFκB-related gene expression in the spleen, indicating that thermoregulatory effects are not merely due to global inhibition of peripheral immune responses to TLR3 activation. However, PEA may have altered the transcription or translation of these genes, the release of immune mediators or elicited effects in other tissues or organs. For example, it is possible that PEA may modulate peripheral, rather than central, PGE2 levels. LPS and poly I:C dramatically increase the plasma level of PGE2 60–90 min following administration, which in critical in the initiation of the hyperthermic response (Davidson et al., 2001; Rotondo et al., 1988). In comparison, later phases of the hyperthermic response are mediated by PGE2 produced by COX-2 and mPGES-1 in perivascular macrophages and endothelial cells in the brain (Steiner et al., 2006). Therefore, by inhibiting poly I:C-induced increases in plasma levels of PGE2, PEA may prevent the TLR3-induced hyperthermic response. It was not possible to assess this directly in the current study due to the time at which samples were taken (4 h post poly I:C administration). Alternatively, PEA may mediate its effects by modulating cardiovascular or metabolic pathways (Karimian Azari et al., 2020; Mattace Raso et al., 2014) involved in thermoregulation. Further studies will be required to examine the mechanisms by which PEA is mediating its inhibitory effects on the poly I:C-induced fever response.
Overall, the data herein demonstrate that the FAAH substrate OEA elicits potent neuro-immuno-regulatory effects partially mediated by PPARα, which limit the hyperthermic response to TLR3 activation following viral infection. These effects mimic those observed following FAAH inhibition, and as such, OEA may be the primary FAAH substrate mediating protective effects on TLR3-induced neuroinflammation, sickness behaviour and long-term psychiatric and neurological changes.
Declaration of Competing Interest
None.
Acknowledgements
The authors would like to gratefully acknowledge funding received from the Science Foundation Ireland Research Frontiers Programme (Grant no. 11/RFP/NES/3175), the National University of Ireland Galway Hardiman Postgraduate Research Scholarship Programme, the discipline of Physiology and The College of Medicine, Nursing and Health Sciences. The funders had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report or in the decision to submit the article for publication.
References
- Alhouayek M., Bottemanne P., Subramanian K.V., Lambert D.M., Makriyannis A., Cani P.D., Muccioli G.G. N-Acylethanolamine-hydrolyzing acid amidase inhibition increases colon N-palmitoylethanolamine levels and counteracts murine colitis. FASEB J. 2015;29:650–661. doi: 10.1096/fj.14-255208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artamonov M., Zhukov O., Shuba I., Storozhuk L., Khmel T., Klimashevsky V., Mikosha A., Gula N. Incorporation of labelled N-acylethanolamine (NAE) into rat brain regions in vivo and adaptive properties of saturated NAE under x-ray irradiation. Ukr. Biokhim. Zh. 2005;77:51–62. (1999) [PubMed] [Google Scholar]
- Baghel M.S., Singh B., Dhuriya Y.K., Shukla R.K., Patro N., Khanna V.K., Patro I.K., Thakur M.K. Postnatal exposure to poly (I:C) impairs learning and memory through changes in synaptic plasticity gene expression in developing rat brain. Neurobiol. Learn. Mem. 2018;155:379–389. doi: 10.1016/j.nlm.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Correa F., Hernangomez M., Mestre L., Loria F., Spagnolo A., Docagne F., Di Marzo V., Guaza C. Anandamide enhances IL-10 production in activated microglia by targeting CB(2) receptors: roles of ERK1/2, JNK, and NF-kappaB. Glia. 2010;58:135–147. doi: 10.1002/glia.20907. [DOI] [PubMed] [Google Scholar]
- Costello D.A., Lynch M.A. Toll-like receptor 3 activation modulates hippocampal network excitability, via glial production of interferon-beta. Hippocampus. 2013;23:696–707. doi: 10.1002/hipo.22129. [DOI] [PubMed] [Google Scholar]
- Cravatt B.F., Giang D.K., Mayfield S.P., Boger D.L., Lerner R.A., Gilula N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cunningham C., Campion S., Teeling J., Felton L., Perry V.H. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C) Brain Behav. Immun. 2007;21:490–502. doi: 10.1016/j.bbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- D’Aloia A., Molteni L., Gullo F., Bresciani E., Artusa V., Rizzi L., Ceriani M., Meanti R., Lecchi M., Coco S., Costa B., Torsello A. Palmitoylethanolamide modulation of microglia activation: characterization of mechanisms of action and implication for its Neuroprotective effects. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22063054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J., Abul H.T., Milton A.S., Rotondo D. Cytokines and cytokine inducers stimulate prostaglandin E2 entry into the brain. Pflugers Arch. 2001;442:526–533. doi: 10.1007/s004240100572. [DOI] [PubMed] [Google Scholar]
- Deleidi M., Hallett P.J., Koprich J.B., Chung C.-Y., Isacson O. The toll-like receptor-3 agonist poly(I:C) triggers nigrostriatal dopaminergic degeneration. J. Neurosci. 2010;30:16091–16101. doi: 10.1523/JNEUROSCI.2400-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare Mannelli L., D’Agostino G., Pacini A., Russo R., Zanardelli M., Ghelardini C., Calignano A. Palmitoylethanolamide is a disease-modifying agent in peripheral neuropathy: pain relief and neuroprotection share a PPAR-alpha-mediated mechanism. Mediat. Inflamm. 2013;2013:328797. doi: 10.1155/2013/328797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer E.J., Clifford E., Gran B., Nel H.J., Fallon P.G., Moynagh P.N. Identification of the synthetic cannabinoid R(+)WIN55,212-2 as a novel regulator of IFN regulatory factor 3 activation and IFN-beta expression: relevance to therapeutic effects in models of multiple sclerosis. J. Biol. Chem. 2011;286:10316–10328. doi: 10.1074/jbc.M110.188599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M., Galic M.A., Wang A., Chambers A.P., McCafferty D.M., McKay D.M., Sharkey K.A., Pittman Q.J. Cannabinoid 1 receptors are critical for the innate immune response to TLR4 stimulation. Am. J. Phys. Regul. Integr. Comp. Phys. 2013;305:R224–R231. doi: 10.1152/ajpregu.00104.2013. [DOI] [PubMed] [Google Scholar]
- Esposito G., Capoccia E., Turco F., Palumbo I., Lu J., Steardo A., Cuomo R., Sarnelli G., Steardo L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-alpha activation. Gut. 2014;63:1300–1312. doi: 10.1136/gutjnl-2013-305005. [DOI] [PubMed] [Google Scholar]
- Facchinetti F., Del Giudice E., Furegato S., Passarotto M., Leon A. Cannabinoids ablate release of TNFalpha in rat microglial cells stimulated with lypopolysaccharide. Glia. 2003;41:161–168. doi: 10.1002/glia.10177. [DOI] [PubMed] [Google Scholar]
- Fan A., Wu X., Wu H., Li L., Huang R., Zhu Y., Qiu Y., Fu J., Ren J., Zhu C. Atheroprotective effect of Oleoylethanolamide (OEA) targeting oxidized LDL. PLoS One. 2014;9 doi: 10.1371/journal.pone.0085337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley D., Gaetani S., Duranti A., Tontini A., Mor M., Tarzia G., Piomelli D. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J. Pharmacol. Exp. Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Field R., Campion S., Warren C., Murray C., Cunningham C. Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration. Brain Behav. Immun. 2010;24:996–1007. doi: 10.1016/j.bbi.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery L.E., Henry R.J., Kerr D.M., Finn D.P., Roche M. FAAH, but not MAGL, inhibition modulates acute TLR3-induced neuroimmune signaling in the rat, independent of sex. J. Neurosci. Res. 2018;96:989–1001. doi: 10.1002/jnr.24120. [DOI] [PubMed] [Google Scholar]
- Flannery L.E., Kerr D.M., Finn D.P., Roche M. FAAH inhibition attenuates TLR3-mediated hyperthermia, nociceptive- and anxiety-like behaviour in female rats. Behav. Brain Res. 2018;353:11–20. doi: 10.1016/j.bbr.2018.06.030. [DOI] [PubMed] [Google Scholar]
- Fortier M.E., Kent S., Ashdown H., Poole S., Boksa P., Luheshi G.N. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am. J. Phys. Regul. Integr. Comp. Phys. 2004;287:R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- Fraga D., Zanoni C.I., Rae G.A., Parada C.A., Souza G.E. Endogenous cannabinoids induce fever through the activation of CB1 receptors. Br. J. Pharmacol. 2009;157:1494–1501. doi: 10.1111/j.1476-5381.2009.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic M.A., Riazi K., Henderson A.K., Tsutsui S., Pittman Q.J. Viral-like brain inflammation during development causes increased seizure susceptibility in adult rats. Neurobiol. Dis. 2009;36:343–351. doi: 10.1016/j.nbd.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon J., de la Torre-Madrid E., Rodriguez-Munoz M., Vicente-Sanchez A., Sanchez-Blazquez P. Gz mediates the long-lasting desensitization of brain CB1 receptors and is essential for cross-tolerance with morphine. Mol. Pain. 2009;5:11. doi: 10.1186/1744-8069-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar J.C., Okine B.N., Llorente-Berzal A., Roche M., Finn D.P. Pharmacological blockade of PPAR isoforms increases conditioned fear responding in the presence of nociceptive tone. Molecules. 2020:25. doi: 10.3390/molecules25041007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney S.M., McGuinness B., Prendergast C., Harkin A., Connor T.J. Poly I:C-induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain Behav. Immun. 2013;28:170–181. doi: 10.1016/j.bbi.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Gigante A., Aquili A., Farinelli L., Caraffa A., Ronconi G., Enrica Gallenga C., Tete G., Kritas S.K., Conti P. Sodium chromo-glycate and palmitoylethanolamide: a possible strategy to treat mast cell-induced lung inflammation in COVID-19. Med. Hypotheses. 2020;143:109856. doi: 10.1016/j.mehy.2020.109856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Aparicio R., Blanco E., Serrano A., Pavon F.J., Parsons L.H., Maldonado R., Robledo P., Fernandez-Espejo E., de Fonseca F.R. The systemic administration of oleoylethanolamide exerts neuroprotection of the nigrostriatal system in experimental parkinsonism. Int. J. Neuropsychopharmacol. 2014;17:455–468. doi: 10.1017/S1461145713001259. [DOI] [PubMed] [Google Scholar]
- Harapan B.N., Yoo H.J. Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19) J. Neurol. 2021 doi: 10.1007/s00415-021-10406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R.J., Kerr D.M., Finn D.P., Roche M. FAAH-mediated modulation of TLR3-induced neuroinflammation in the rat hippocampus. J. Neuroimmunol. 2014;276:126–134. doi: 10.1016/j.jneuroim.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Henry R.J., Kerr D.M., Finn D.P., Roche M. For whom the endocannabinoid tolls: modulation of innate immune function and implications for psychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2016;64:167–180. doi: 10.1016/j.pnpbp.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Henry R.J., Kerr D.M., Flannery L.E., Killilea M., Hughes E.M., Corcoran L., Finn D.P., Roche M. Pharmacological inhibition of FAAH modulates TLR-induced neuroinflammation, but not sickness behaviour: an effect partially mediated by central TRPV1. Brain Behav. Immun. 2017;62:318–331. doi: 10.1016/j.bbi.2017.02.016. [DOI] [PubMed] [Google Scholar]
- Hernangomez M., Mestre L., Correa F.G., Loria F., Mecha M., Inigo P.M., et al. CD200-CD200R1 interaction contributes to neuroprotective effects of anandamide on experimentally induced inflammation. Glia. 2012;60(9):1437–1450. doi: 10.1002/glia.22366. [DOI] [PubMed] [Google Scholar]
- Hollis J.H., Jonaidi H., Lemus M., Oldfield B.J. The endocannabinoid arachidonylethanolamide attenuates aspects of lipopolysaccharide-induced changes in energy intake, energy expenditure and hypothalamic Fos expression. J. Neuroimmunol. 2011;233:127–134. doi: 10.1016/j.jneuroim.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Karimian Azari E., Kerrigan A., O’Connor A. Naturally occurring cannabinoids and their role in modulation of cardiovascular health. J. Diet. Suppl. 2020;17:625–650. doi: 10.1080/19390211.2020.1790708. [DOI] [PubMed] [Google Scholar]
- Krishnan G., Chatterjee N. Endocannabinoids alleviate proinflammatory conditions by modulating innate immune response in muller glia during inflammation. Glia. 2012;60:1629–1645. doi: 10.1002/glia.22380. [DOI] [PubMed] [Google Scholar]
- Lama A., Provensi G., Amoriello R., Pirozzi C., Rani B., Mollica M.P., Raso G.M., Ballerini C., Meli R., Passani M.B. The anti-inflammatory and immune-modulatory effects of OEA limit DSS-induced colitis in mice. Biomed. Pharmacother. 2020;129:110368. doi: 10.1016/j.biopha.2020.110368. [DOI] [PubMed] [Google Scholar]
- Li Y., Yang L., Chen L., Zhu C., Huang R., Zheng X., Qiu Y., Fu J. Design and synthesis of potent N-acylethanolamine-hydrolyzing acid amidase (NAAA) inhibitor as anti-inflammatory compounds. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria F., Petrosino S., Mestre L., Spagnolo A., Correa F., Hernangomez M., et al. Study of the regulation of the endocannabinoid system in a virus model of multiple sclerosis reveals a therapeutic effect of palmitoylethanolamide. Eur. J. Neurosci. 2008;28(4):633–641. doi: 10.1111/j.1460-9568.2008.06377.x. [DOI] [PubMed] [Google Scholar]
- Loria F., Petrosino S., Hernangomez M., Mestre L., Spagnolo A., Correa F., et al. An endocannabinoid tone limits excitotoxicity in vitro and in a model of multiple sclerosis. Neurobiol. Dis. 2010;37(1):166–176. doi: 10.1016/j.nbd.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Lo Verme J., Fu J., Astarita G., La Rana G., Russo R., Calignano A., Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- Lucaciu O., Aghiorghiesei O., Petrescu N.B., Mirica I.C., Benea H.R.C., Apostu D. In quest of a new therapeutic approach in COVID-19: the endocannabinoid system. Drug Metab. Rev. 2021:1–13. doi: 10.1080/03602532.2021.1895204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysne V., Bjorndal B., Grinna M.L., Midttun O., Ueland P.M., Berge R.K., Dierkes J., Nygard O., Strand E. Short-term treatment with a peroxisome proliferator-activated receptor alpha agonist influences plasma one-carbon metabolites and B-vitamin status in rats. PLoS One. 2019;14 doi: 10.1371/journal.pone.0226069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalakshmi A.M., Ray B., Tuladhar S., Bhat A., Paneyala S., Patteswari D., Sakharkar M.K., Hamdan H., Ojcius D.M., Bolla S.R., Essa M.M., Chidambaram S.B., Qoronfleh M.W. Does COVID-19 contribute to development of neurological disease? Immun. Inflamm. Dis. 2021;9:48–58. doi: 10.1002/iid3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace Raso G., Russo R., Calignano A., Meli R. Palmitoylethanolamide in CNS health and disease. Pharmacol. Res. 2014;86:32–41. doi: 10.1016/j.phrs.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Mestre L., Correa F., Arevalo-Martin A., Molina-Holgado E., Valenti M., Ortar G., et al. Pharmacological modulation of the endocannabinoid system in a viral model of multiple sclerosis. J. Neurochem. 2005;92(6):1327–1339. doi: 10.1111/j.1471-4159.2004.02979.x. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F., Lledo A., Guaza C. Anandamide suppresses nitric oxide and TNF-alpha responses to Theiler's virus or endotoxin in astrocytes. Neuroreport. 1997;8:1929–1933. doi: 10.1097/00001756-199705260-00027. [DOI] [PubMed] [Google Scholar]
- Moya M., San Felipe D., Ballesta A., Alen F., Rodriguez de Fonseca F., Garcia-Bueno B., Marco E.M., Orio L. Cerebellar and cortical TLR4 activation and behavioral impairments in Wernicke-Korsakoff syndrome: pharmacological effects of oleoylethanolamide. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;108:110190. doi: 10.1016/j.pnpbp.2020.110190. [DOI] [PubMed] [Google Scholar]
- Murray C., Griffin E.W., O’Loughlin E., Lyons A., Sherwin E., Ahmed S., Stevenson N.J., Harkin A., Cunningham C. Interdependent and independent roles of type I interferons and IL-6 in innate immune, neuroinflammatory and sickness behaviour responses to systemic poly I:C. Brain Behav. Immun. 2015;48:274–286. doi: 10.1016/j.bbi.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L.C., Yang D., Nicolaescu V., Best T.J., Ohtsuki T., Chen S.N., Friesen J.B., Drayman N., Mohamed A., Dann C., Silva D., Gula H., Jones K.A., Millis J.M., Dickinson B.C., Tay S., Oakes S.A., Pauli G.F., Meltzer D.O., Randall G., Rosner M.R. Cannabidiol inhibits SARS-CoV-2 replication and promotes the host innate immune response. bioRxiv. 2021 doi: 10.1101/2021.03.10.432967. [DOI] [Google Scholar]
- Okine B.N., Spicer C., Millns P., Bennett A., Chapman V. Systemic administration of WY-14643, a selective synthetic agonist of peroxisome proliferator activator receptor-alpha, alters spinal neuronal firing in a rodent model of neuropathic pain. Scand J Pain. 2015;9:42–48. doi: 10.1016/j.sjpain.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Orio L., Alen F., Pavon F.J., Serrano A., Garcia-Bueno B. Oleoylethanolamide, neuroinflammation, and alcohol abuse. Front. Mol. Neurosci. 2018;11:490. doi: 10.3389/fnmol.2018.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Gutierrez S., Molina-Holgado E., Guaza C. Effect of anandamide uptake inhibition in the production of nitric oxide and in the release of cytokines in astrocyte cultures. Glia. 2005;52(2):163–168. doi: 10.1002/glia.20229. [DOI] [PubMed] [Google Scholar]
- Pandey G.N., Rizavi H.S., Ren X., Bhaumik R., Dwivedi Y. Toll-like receptors in the depressed and suicide brain. J. Psychiatr. Res. 2014;53:62–68. doi: 10.1016/j.jpsychires.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G.W. 2006. The rat Brain in Stereotaxic Coordinates. [DOI] [PubMed] [Google Scholar]
- Pessina F., Capasso R., Borrelli F., Aveta T., Buono L., Valacchi G., Fiorenzani P., Di Marzo V., Orlando P., Izzo A.A. Protective effect of palmitoylethanolamide in a rat model of cystitis. J. Urol. 2015;193:1401–1408. doi: 10.1016/j.juro.2014.11.083. [DOI] [PubMed] [Google Scholar]
- Petrosino S., Cristino L., Karsak M., Gaffal E., Ueda N., Tuting T., Bisogno T., De Filippis D., D'Amico A., Saturnino C., Orlando P., Zimmer A., Iuvone T., Di Marzo V. Protective role of palmitoylethanolamide in contact allergic dermatitis. Allergy. 2010;65:698–711. doi: 10.1111/j.1398-9995.2009.02254.x. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Scalvini L., Fotio Y., Lodola A., Spadoni G., Tarzia G., Mor M. N-Acylethanolamine acid Amidase (NAAA): structure, function, and inhibition. J. Med. Chem. 2020;63:7475–7490. doi: 10.1021/acs.jmedchem.0c00191. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A., Berrendero F., Suarez J., Bermudez-Silva F.J., Fernandez-Espejo E., Serrano A., Pavon F.-J., Parsons L.H., De Fonseca F.R., Maldonado R., Robledo P. Effects of the endogenous PPAR-α agonist, oleoylethanolamide on MDMA-induced cognitive deficits in mice. Synapse. 2010;64:379–389. doi: 10.1002/syn.20733. [DOI] [PubMed] [Google Scholar]
- Puffenbarger R.A., Boothe A.C., Cabral G.A. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia. 2000;29:58–69. [PubMed] [Google Scholar]
- Rankin L., Fowler C.J. The basal pharmacology of palmitoylethanolamide. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21217942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera P., Silva-Pena D., Blanco E., Vargas A., Arrabal S., Serrano A., Pavon F.J., Bindila L., Lutz B., Rodriguez de Fonseca F., Suarez J. Oleoylethanolamide restores alcohol-induced inhibition of neuronal proliferation and microglial activity in striatum. Neuropharmacology. 2019;146:184–197. doi: 10.1016/j.neuropharm.2018.11.037. [DOI] [PubMed] [Google Scholar]
- Rotondo D., Abul H.T., Milton A.S., Davidson J. Pyrogenic immunomodulators increase the level of prostaglandin E2 in the blood simultaneously with the onset of fever. Eur. J. Pharmacol. 1988;154:145–152. doi: 10.1016/0014-2999(88)90091-x. [DOI] [PubMed] [Google Scholar]
- Russo R., Cristiano C., Avagliano C., De Caro C., La Rana G., Raso G.M., Canani R.B., Meli R., Calignano A. Gut-brain Axis: role of lipids in the regulation of inflammation, pain and CNS diseases. Curr. Med. Chem. 2018;25:3930–3952. doi: 10.2174/0929867324666170216113756. [DOI] [PubMed] [Google Scholar]
- Sayd A., Antón M., Alén F., Caso J.R., Pavón J., Leza J.C., Rodríguez de Fonseca F., García-Bueno B., Orio L. Systemic administration of oleoylethanolamide protects from neuroinflammation and anhedonia induced by LPS in rats. Int. J. Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi C., Stecca C., Valenza M., Ratano P., Bronzuoli M.R., Bartoli S., Steardo L., Pompili E., Fumagalli L., Campolongo P., Steardo L. Palmitoylethanolamide controls reactive gliosis and exerts neuroprotective functions in a rat model of Alzheimer’s disease. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shujaa N., Zadori Z.S., Ronai A.Z., Barna I., Mergl Z., Mozes M.M., Gyires K. Analysis of the effect of neuropeptides and cannabinoids in gastric mucosal defense initiated centrally in the rat. J. Physiol. Pharmacol. 2009;60(Suppl. 7):93–100. [PubMed] [Google Scholar]
- Skaper S.D., Facci L., Barbierato M., Zusso M., Bruschetta G., Impellizzeri D., Cuzzocrea S., Giusti P. N-Palmitoylethanolamine and Neuroinflammation: a novel therapeutic strategy of resolution. Mol. Neurobiol. 2015;52:1034–1042. doi: 10.1007/s12035-015-9253-8. [DOI] [PubMed] [Google Scholar]
- Solorzano C., Zhu C., Battista N., Astarita G., Lodola A., Rivara S., Mor M., Russo R., Maccarrone M., Antonietti F., Duranti A., Tontini A., Cuzzocrea S., Tarzia G., Piomelli D. Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:20966–20971. doi: 10.1073/pnas.0907417106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.W., Kim H.J., Lee H., Kim J.W., Kwak Y.L. Protective effect of peroxisome proliferator-activated receptor alpha activation against cardiac ischemia-reperfusion injury is related to Upregulation of uncoupling Protein-3. Oxidative Med. Cell. Longev. 2016;2016:3539649. doi: 10.1155/2016/3539649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A.A., Ivanov A.I., Serrats J., Hosokawa H., Phayre A.N., Robbins J.R., Roberts J.L., Kobayashi S., Matsumura K., Sawchenko P.E., Romanovsky A.A. Cellular and molecular bases of the initiation of fever. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A.A., Molchanova A.Y., Dogan M.D., Patel S., Pétervári E., Balaskó M., Wanner S.P., Eales J., Oliveira D.L., Gavva N.R., Almeida M.C., Székely M., Romanovsky A.A. The hypothermic response to bacterial lipopolysaccharide critically depends on brain CB1, but not CB2 or TRPV1, receptors. J. Physiol. 2011;589:2415–2431. doi: 10.1113/jphysiol.2010.202465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahamtan A., Tavakoli-Yaraki M., Rygiel T.P., Mokhtari-Azad T., Salimi V. Effects of cannabinoids and their receptors on viral infections. J. Med. Virol. 2016;88:1–12. doi: 10.1002/jmv.24292. [DOI] [PubMed] [Google Scholar]
- Taquet M., Sierra Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.G., Tang T.M., Lue L.F. Increased expression of toll-like receptor 3, an anti-viral signaling molecule, and related genes in Alzheimer’s disease brains. Exp. Neurol. 2018;309:91–106. doi: 10.1016/j.expneurol.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Guo H., Jing Z., Yang L., Chen C., Peng L., Wang X., Yan L., Ye R., Jin X., Wang Y. N-Oleoylethanolamine reduces inflammatory cytokines and adhesion molecules in TNF-alpha-induced human umbilical vein endothelial cells by activating CB2 and PPAR-alpha. J. Cardiovasc. Pharmacol. 2016;68:280–291. doi: 10.1097/FJC.0000000000000413. [DOI] [PubMed] [Google Scholar]
- Yang L., Guo H., Li Y., Meng X., Yan L., Dan Z., Wu S., Zhou H., Peng L., Xie Q., Jin X. Oleoylethanolamide exerts anti-inflammatory effects on LPS-induced THP-1 cells by enhancing PPARalpha signaling and inhibiting the NF-kappaB and ERK1/2/AP-1/STAT3 pathways. Sci. Rep. 2016;6:34611. doi: 10.1038/srep34611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Yang L., Ma A., Zhang X., Li W., Yang W., Chen C., Jin X. Orally administered oleoylethanolamide protects mice from focal cerebral ischemic injury by activating peroxisome proliferator-activated receptor alpha. Neuropharmacology. 2012;63:242–249. doi: 10.1016/j.neuropharm.2012.03.008. [DOI] [PubMed] [Google Scholar]