Abstract
Germline POT1 mutations are found in a spectrum of cancers and confer increased risk. Recently, we identified a series of novel germline POT1 mutations that predispose carrier families to the development of glioma. Despite these strong associations, how these glioma-associated POT1 mutations contribute to glioma tumorigenesis remains undefined. Here we show that POT1-G95C increases proliferation in glioma-initiating cells in vitro and in progenitor populations in the developing brain. In a native mouse model of glioma, loss of Pot1a/b resulted in decreased survival in females compared to males. These findings were corroborated in human glioma, where low POT1 expression correlated with decreased survival in females. Transcriptomic and immunohistochemistry profiling of Pot1a/b-deficient glioma revealed that tumors in females exhibited decreased expression of immune markers and increased expression of cell cycle signatures. Similar sex-dependent trends were observed in human gliomas that had low expression of POT1. Together, our studies demonstrate context-dependent functions for POT1 mutation or loss in driving progenitor proliferation in the developing brain and sexual dimorphism in glioma.
Introduction
Diffuse gliomas comprise the majority of malignant primary brain tumors and are often fatal. While most gliomas arise sporadically, familial cases associated with several known genetic syndromes (e.g. Li-Fraumeni syndrome) have been well described.(1) Glioma cases also aggregate among non-syndromic families. Whole exome sequencing in a number of these families led to the discovery of germline POT1 (Protection Of Telomeres 1) mutations in affected individuals,(2) linking POT1 mutations with the risk of glioma development. Mutations in POT1 are present in several other familial cancers such as melanoma, leukemia, colorectal cancer, and angiosarcoma, further strengthening the overall association of POT1 mutations with cancer.(3–9) Despite these strong correlations with cancer predisposition, whether and how germline POT1 mutations promote glioma tumorigenesis remains undefined.
POT1 is a member of the Shelterin complex, which binds telomeres and regulates extension by controlling telomerase access.(10) Cancer-associated POT1 mutations are all heterozygous, and expression of these variants in vitro results in telomeric elongation and dysfunction, suggesting they operate as dominant-negative alleles.(7,11–13) In mice, POT1 has two orthologous genes, Pot1a and Pot1b, and both are required for protection of telomeres.(14) While germline deletion of Pot1a causes early embryonic lethality, mice with germline Pot1b deletion are viable and do not develop spontaneous tumors.(14,15) In the brain, conditional knockout of Pot1a results in telomere dysfunction and genomic instability and has a p53-dependent effect on senescence and neuronal loss without any tumor formation.(16) While telomeric dysfunction and the resultant genomic instability are thought to contribute to tumorigenesis,(7,11) the observation that Pot1a/b loss does not lead to tumorigenesis in the brain (or other organs) suggests the existence of other mechanisms where POT1 loss is permissive or promotes but is not sufficient for tumorigenesis.
To decipher how POT1 mutants influence gliomagenesis, we functionally screened a set of 3 glioma-associated mutants, finding that POT1-G95C promotes proliferation in the post-natal brain in vivo. We assessed Pot1a/b function in a native mouse model of glioma, finding that its loss results in sex-dependent effects on survival, with females exhibiting decreased survival relative to males. These observations are corroborated in human glioma, where sex-dependent effects on survival in glioma patients is correlated with low POT1 expression. Molecular profiling of Pot1a/b deficient mouse tumors revealed sex-dependent changes in expression of cell cycle and immune signatures. Together, these findings demonstrate context-dependent functions for POT1 mutation or loss in driving proliferation and sexual dimorphism in glioma.
Materials and Methods
In utero electroporation
To generate mouse gliomas, we introduced CRISPR/Cas9 constructs into E16.5 embryonic mouse brain via in utero electroporation (IUE) as previously described.(17) The injected DNA was composed of PBCAG-eGFP and CRISPR plasmids (each at 1.0 ug/ul) as well as pGLAST-PBase (2.0 μg/ul). For the non-glioma model, we substituted the CRISPR plasmids with wildtype or mutant PBCAG-eGFP-2a-POT1 plasmid or the PBCAG-eGFP (Control) plasmid. See Supplementary Methods for construct details. All procedures were approved by the Institutional Animal Care and Use Committee and conform to the US Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Limiting dilution assay
The glioma tumor was dissected from adult mouse brain shortly after sacrifice and cultured under stem cell conditions. Mutant POT1 or mCherry control were then introduced via lentiviral transduction and the cells were plated at limiting dilution densities in a 96 well plate and monitored for a week for sphere formation. The sex of all cell lines used in this experiment was determined to be male.
Protein, DNA, and RNA isolation
The glioma tumor was dissected from adult mouse brain shortly after sacrifice under a fluorescence dissection microscope. GFP-positive tissue was then homogenized with lysis buffer for Western blot (WB), subjected to Qiagen DNA extraction protocol for qPCR assay, or to Qiagen RNeasy Mini kit to purify RNA for sequencing.
Immunohistochemistry and immunofluorescence
Adult mouse brains containing glioma were perfused and fixed in 4% paraformaldehyde followed by 70% ethanol and embedded in paraffin. Ten micron vibratome sections were then deparaffinized. For antibody information, see supplementary methods.
Telomere content quantification
For telomere qPCR assay, DNA extracted from glioma tumors was subjected to qPCR analysis as previously described,(18) and T/S ratios for different samples were reported as Telomere Content. For telomere FISH assay, telomere hybridization and quantification were performed as previously described.(19) For each nucleus, the ratio of total PNA signal (telomere content) and Hoechst signal (DAPI channel, nuclear DNA content) was used as Telomere Content. See Supplementary Methods.
Mouse glioma RNA sequencing
We confirmed RNA integrity (RIN) ≥ 8.0 was confirmed on all extracted RNA samples and constructed Illumina sequencing libraries with 6-bp single indices were constructed from 1 μg total RNA using the TruSeq Stranded mRNA LT kit (Illumina). The resulting libraries were validated using the Standard Sensitivity NGS Fragment Analysis Kit (AATI). Equal concentrations (2 nM) of libraries were pooled and then subjected to paired-end (2×75) sequencing of approximately 20 million reads per sample using the Mid Output v2 kit (Illumina) on an Illumina NextSeq500 following the manufacturer’s instructions.
All subsequent RNA-seq data analysis was carried out in R (version 3.5.3, The R Project for Statistical Computing). Data were aligned to mouse transcriptome using mm10 index (GRCm38) provided in Rsubread package and mapped read counts were obtained using featureCounts. All mouse glioma raw RNA-seq counts have been deposited in NCBI’s Gene Expression Omnibus: GSE161823.
Human glioma data
We obtained clinical data, IDH mutation and 1p19q codeletion status from the TCGA collection of diffuse glioma,(20) and excluded PA-like and highly mutated tumors. See Supplementary Methods for details.
RNA-seq data analysis
Raw RNA-seq counts were normalized using TMM method (edgeR) and Log2 transformed using voom (limma). Moderated statistics including P value for linear model fit for gene expression were computed using eBayes (limma). GSEA (clusterProfiler) using fgsea method and 10000 permutations was used to quantify GSEA statistics for Broad MSigDB gene sets provided via msigdbr (version 7.1.1). Immune lineage mMCP scores were computed using mMCP-counter. See Supplementary Methods section for statistical analysis.
Results
POT1-G95C mutant increases self-renewal of mouse glioma cells
Several mutations in POT1 are associated with familial glioma.(2) However, it remains unknown whether these POT1 mutants impact glioma susceptibility by influencing proliferation or frequency of glioma initiating cells. To test this we used lentivirus to overexpress the glioma-associated POT1 mutants (or control) in glioma cells generated from our established native model of glioma.(21) To evaluate how these mutants influence proliferation, we performed a stem cell limiting dilution assay (Figure 1A) and quantified sphere formation. We found that self-renewal frequency was significantly increased in the presence of POT1-G95C, but not the other POT1 mutants (Figures 1B–1D). This finding indicates that POT1-G95C promotes the proliferation and expansion of glioma cell populations.
Figure 1. Proliferative effects of POT1-G95C and Pot1a/b knockout.
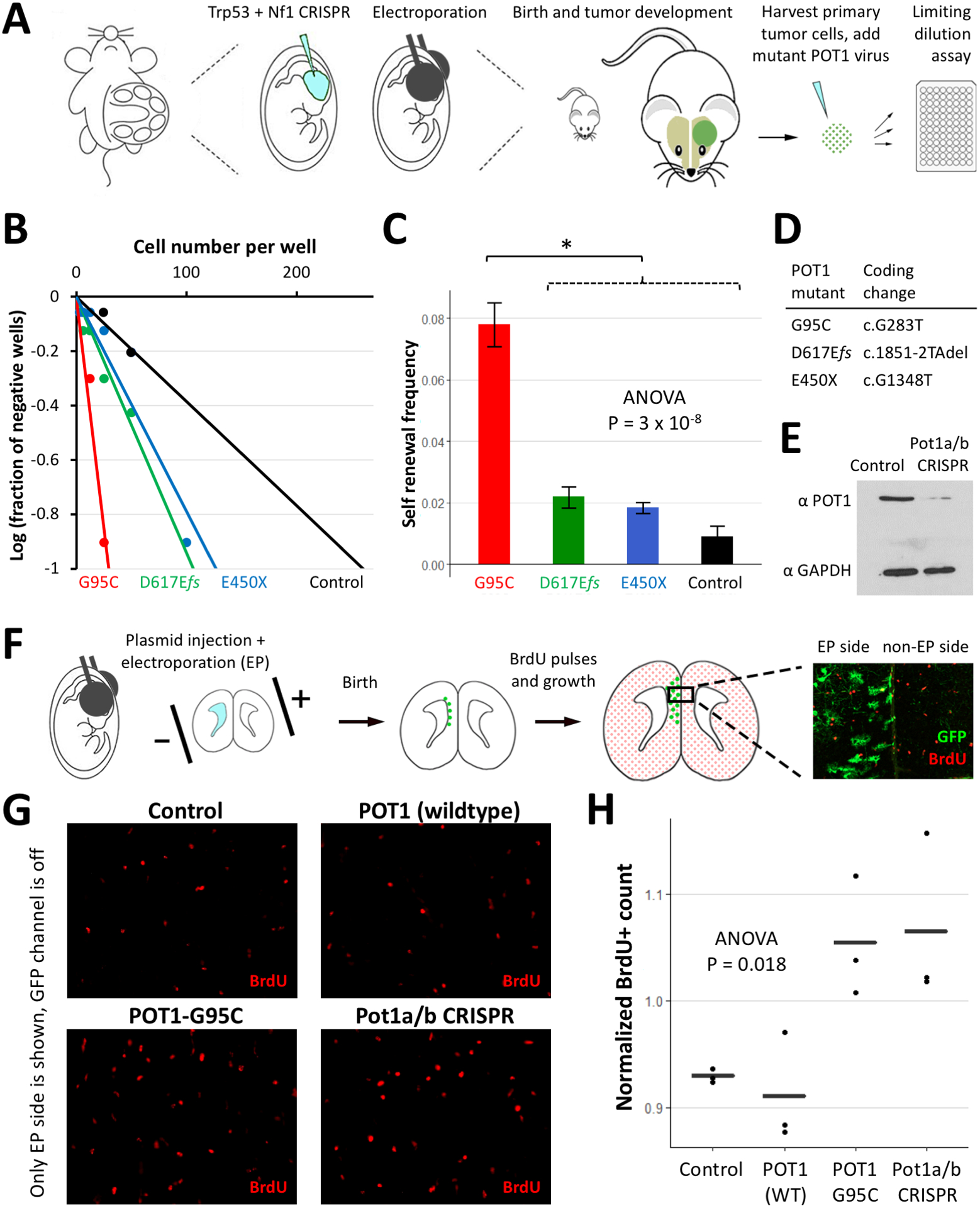
(A) Schematic of the IUE glioma model and generation of glioma cells followed by limiting dilution assay. (B) Expression of POT1 mutants in glioma cells increases sphere formation. (C) Quantification of the self-renewal frequency and standard errors showed statistical significance only in the case of POT1-G95C (* Tukey’s HSD P=7×10−5). (D) Glioma-associated POT1 mutants and their coding mutations. (E) Western blot showing depletion of mouse Pot1a/b protein in electroporated cells using CRISPR/Cas9 technology (F) Schematic of the non-cancer IUE model followed by BrdU pulses and sacrifice at P13. GFP marks the electroporated (EP) side of the prefrontal cortex and the non-EP side is used for normalization. (G) Representative sections of the EP side of prefrontal cortex. (H) Normalized BrdU+ count is higher in POT1-G95C and Pot1a/b CRISPR injected brains as compared to wildtype POT1 or Control. Plot shows mean ± standard error.
Expression of POT1-G95C or loss of Pot1a/b promotes proliferation in vivo
Increased self-renewal in the presence of POT1-G95C suggests that it may play a direct role in proliferation. However, it is unclear whether POT1-G95C can influence proliferation during the formative stages of glioma tumorigenesis, prior to cellular transformation. This question is particularly important given these POT1 mutations are present in the germline and are likely to exert these effects in normal, non-transformed cells. To examine whether POT1-G95C promotes proliferation in a non-cancer setting, in the native brain, we used in utero electroporation (IUE) to overexpress it in glial progenitors in the developing cortex. After IUE at E16.5, we assessed proliferation by administering BrdU pulses postnatally followed by sacrifice at P13; notably a GFP reporter cassette marked the electroporated (EP) side of the brain and the non-EP side was used for normalization (Figure 1E–F). Comparison of the normalized BrdU+ counts revealed increased proliferation upon expression of POT1-G95C (Figure 1H).
Prior studies suggest that other cancer-associated POT1 mutants exert a dominant negative function,(7,11,12) raising the possibility that loss of Pot1a/b expression in the mouse could phenocopy POT1-G95C overexpression. Therefore, we combined CRISPR/Cas9 technology with our IUE approaches to delete both Pot1a and Pot1b in the developing cortex (Figure 1E). Analysis of normalized BrdU+ counts revealed an increase in proliferation (Figure 1H) that was comparable to the increase observed with POT1-G95C overexpression. These results indicate that expression of POT1-G95C can recapitulate the effect of Pot1a/b loss, supporting a dominant negative role for POT1-G95C. Moreover, these findings indicate that POT1-G95C and Pot1a/b loss confer a proliferative advantage to non-transformed progenitor populations in the developing cortex.
Loss of POT1 orthologs in mouse glioma has sex-dependent effects on survival
Given the proliferative effect of Pot1a/b knockout in normal, progenitor populations in the developing cortex, we next examined whether knockout of Pot1a and Pot1b affects development and growth of glioma tumors. Towards this, we employed our CRISPR/Cas9-based model of malignant glioma, where we introduce guideRNAs targeting key tumor suppressors NF1, Trp53, and Pten for deletion via IUE of the E16.5 cortex (Figure 2A). This model (termed C3, Figure 2A) generates glioma tumors in the cortex around P70.(17) To directly test the role of Pot1a/b in this model, we introduced the C3 guideRNAs, along with those used to delete Pot1a/b (see Figure 1E), via IUE of the embryonic cortex and evaluated survival as a surrogate for tumor growth. This model (termed C5, Figure 2A) generates tumors that share the aggressive histological features of C3 glioma tumors including vascular proliferation and necrosis (Supplementary Figure 1A–B). Next, we subjected the survival data to a multivariable Cox proportional hazard analysis using a linear model consisting of tumor type, sex, and an interaction term between the two. Strikingly, this analysis identified a significant interaction between sex and tumor type (Figure 2B), indicating the effects of Pot1a/b loss on glioma survival significantly differ depending on sex.
Figure 2. Sex-dependent effects of loss of POT1 orthologs on mouse glioma survival.
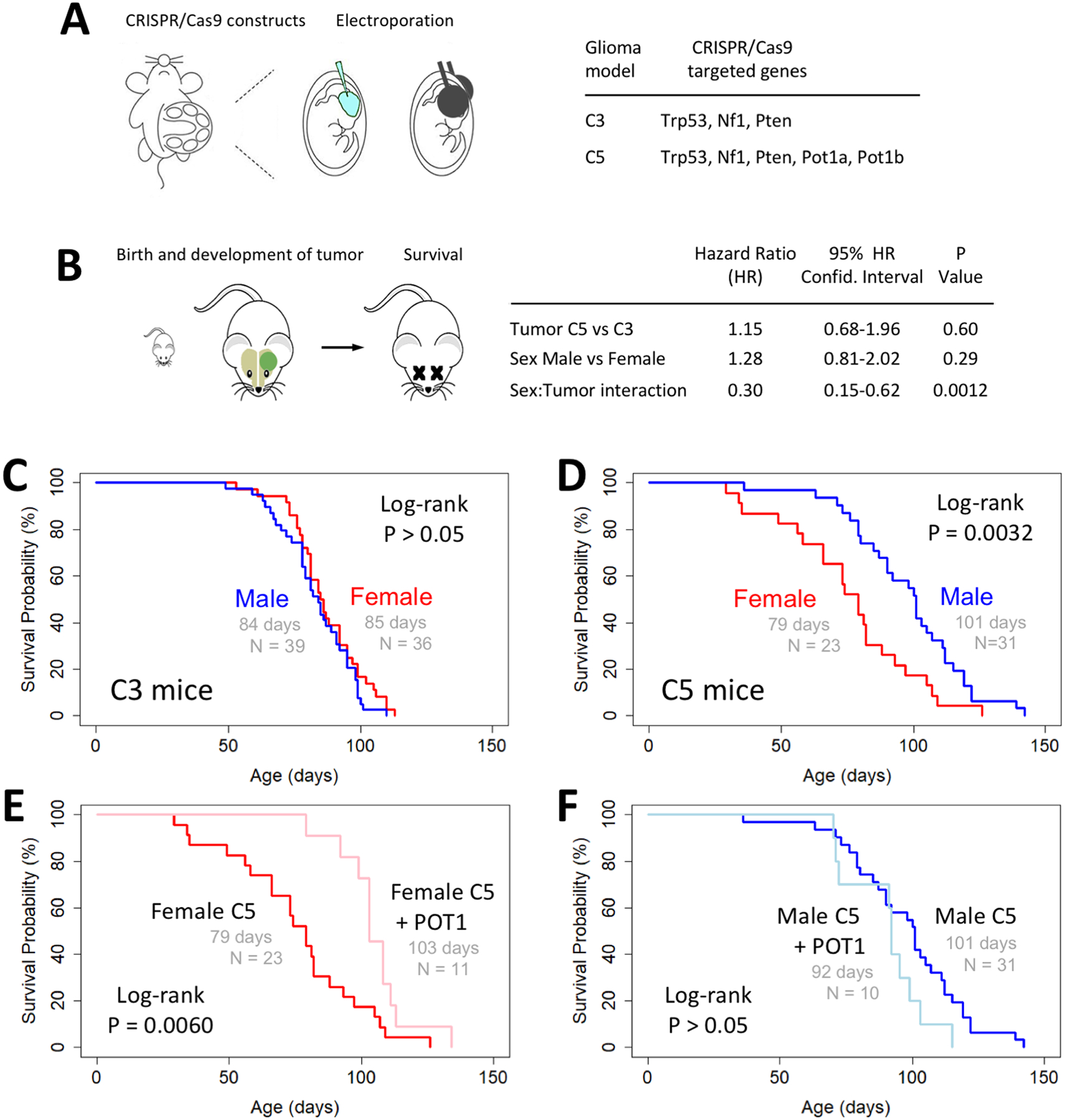
(A) Schematic depicting IUE generation of glioma and list of genes targeted for CRISPR/Cas9 knockout in C3 and C5 glioma models. (B) Schematic of survival analysis and Cox proportional hazard analysis of survival data shows significant interaction between sex and tumor type. (C and D) Kaplan-Meier plots showing similar survival for male and female mice with C3 tumors and a sexual dimorphism of survival with C5 tumors. (E and F) Kaplan-Meier survival analysis shows expression of wildtype human POT1 rescues the shorter survival phenotype in female C5 mice but does not significantly alter the longer survival in male C5 mice.
Kaplan-Meier survival analysis demonstrated similar survival for male and female mice with C3 tumors (Figure 2C) while additional knockout of Pot1a/b in C5 tumors resulted in a sexually dimorphic survival phenotype, with female mice demonstrating decreased survival compared to their male counterparts (Figure 2D). We then performed a rescue experiment by co-introducing a wildtype human POT1 expression vector in the C5 model. This specifically extended the survival of female C5 mice (Figure 2E) and did not alter the survival of male C5 mice (Figure 2F). These findings indicate that the effects of Pot1a/b knockout on tumor growth and survival are more pronounced in females, and that expression of human POT1 can compensate for the loss of mouse Pot1a/b in the same sex-dependent manner.
Low POT1 expression in human glioma is associated with sex-dependent survival
Given our observations in mouse models that loss of Pot1a/b has sex-dependent effects, we next sought to determine if the level of POT1 expression in glioma tumors is associated with sex-dependent survival in human glioma. As our mouse model is an IDH-wildtype model of glioma, we analyzed the TCGA collection of diffuse glioma(20) and assembled a 207-sample cohort of IDH-wildtype tumors with available clinical and RNA-seq expression data. Patient age and tumor grade are the main known predictors of survival for patients diagnosed with IDH-wildtype tumors. In a multivariable Cox proportional hazard model accounting for the effects of patient age and tumor grade, the level of POT1 expression as a continuous variable is directly associated with overall survival (Figure 3A). We defined a cohort of low POT1 expressing tumors using an arbitrary threshold at the bottom quartile of POT1 expression values. The high (N=155) and low (N=52) POT1 expression groups did not differ significantly in the distribution of patient age, sex, or tumor grade (Figure 3A).
Figure 3. Low POT1 expression in human glioma is associated with sex-dependent effects on survival.
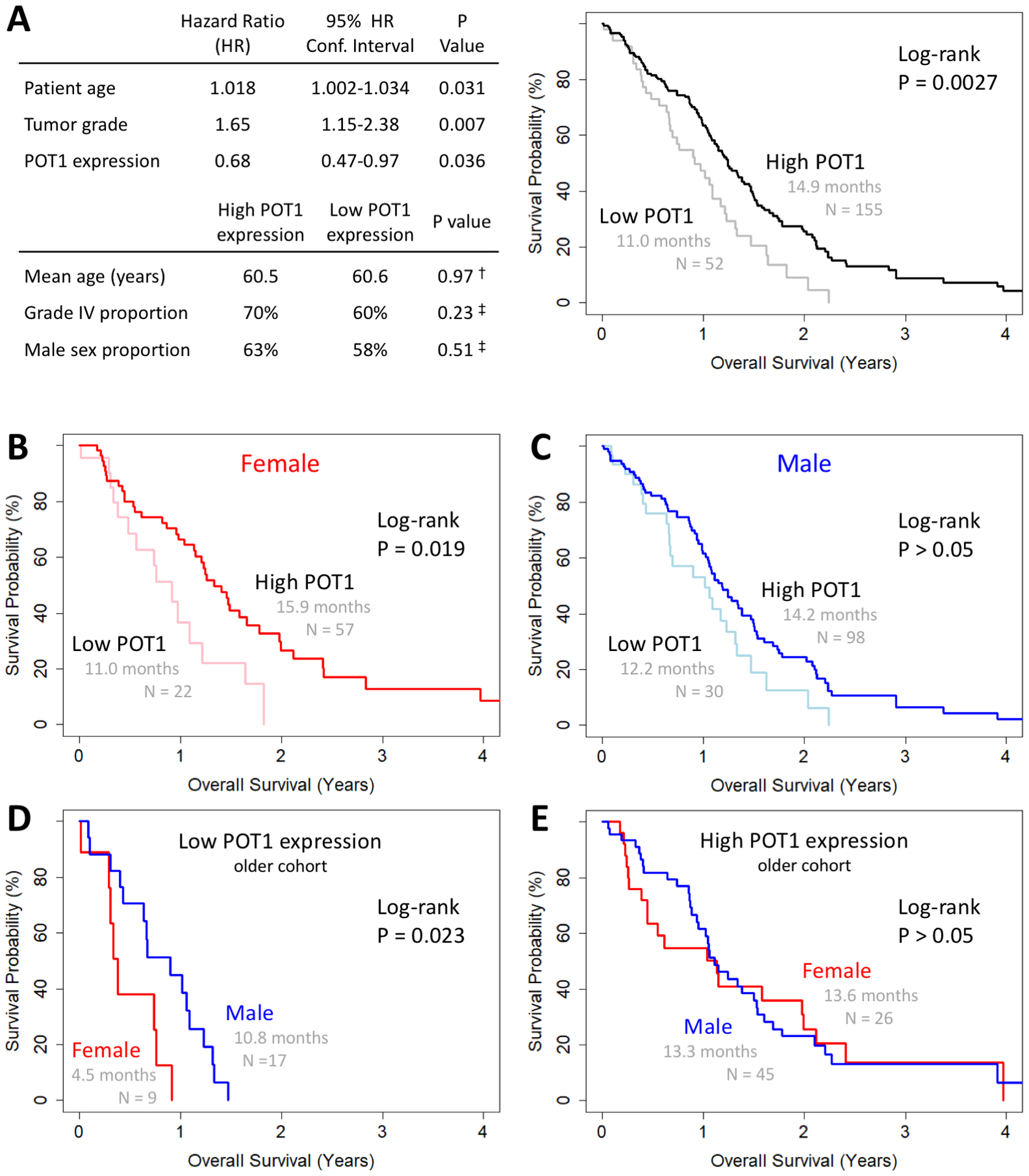
(A) Multivariable Cox proportional hazard analysis shows direct association between tumor POT1 expression and patient survival independently of age and tumor grade. Defining low POT1 expression tumors as the bottom quartile of POT1 expression values, there is no significant difference in the distribution of patient age, sex, or tumor grade between low and high POT1 expression tumors. †Student’s t test. ‡Fisher’s exact test. Kaplan-Meier plot showing a lower median survival in glioma patients with low POT1 expression tumors. (B–C) Female patients with low POT1 expression tumors have a more drastic reduction in survival. The reduction in males is not statistically significant. (D–E) In the older cohort (>61 years old), female patients with low POT1 expressing tumors have a shorter survival than males while the survival of patients with high POT1 expressing tumors is not sex-dependent.
Kaplan-Meier survival analysis demonstrated a lower median survival by about 4 months in glioma patients with low POT1 expression tumors (Figure 3A). Separating by sex, female patients with low POT1 expression tumors showed a more drastic reduction in survival (Figure 3B) compared to the reduction in males, which was not statistically significant (Figure 3C). To reduce the confounding effect of young age on survival in these cohorts, we performed another analysis focusing on the older half of patients (>61 years old, median age for the low POT1 expression tumors). In this older cohort, female patients with low-POT1 expression in their tumors had a shorter survival than males (Figure 3D), while there was no significant sex difference in the survival of patients with high-POT1 expression tumors (Figure 3E). Kaplan-Meier survival analysis in the subset of grade IV tumors (glioblastoma) showed similar results (Supplementary Figure 2A–E), indicating that inclusion of lower grade IDH-wildtype gliomas did not confound the results. These findings show striking parallels to the mouse survival results and further suggest that depletion of POT1 has sex-dependent effects on tumor growth.
Loss of POT1 orthologs in mouse glioma has sex-dependent effects on telomere content
POT1 and its mouse orthologs are thought to play a role in regulation of telomere length. As loss of Pot1a/b has sex-dependent effects on mouse glioma survival, we sought to evaluate the telomere content in these tumors and test for the presence of sex-dependent effects. We performed two independent assays to measure telomere content in mouse C3 and C5 glioma tumors. For the first assay, we extracted DNA from mouse glioma tissue (N=32) and used quantitative PCR to evaluate telomeric DNA content normalized to a single copy gene (schematic in Figure 4A). Linear modeling revealed a significant interaction between sex and tumor type (P = 0.010) and demonstrated that the female-male difference in telomere content is higher in C5 tumors than in C3 tumors (Figure 4B). This analysis indicates that sex differences in glioma telomere content depend on Pot1a/b presence or loss.
Figure 4. Loss of POT1 in mouse glioma has sex-dependent effects on telomere content.
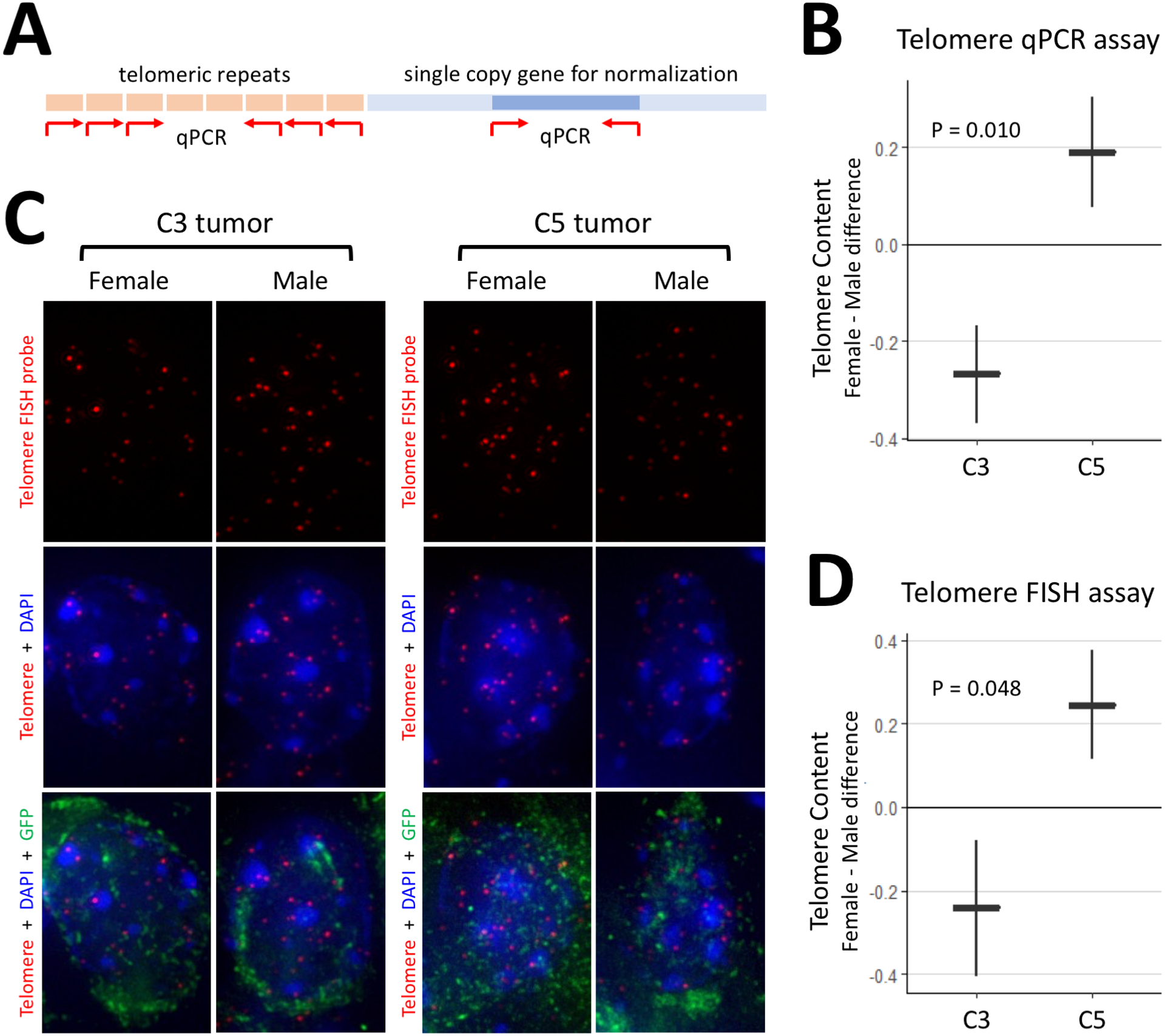
(A) Schematic of the qPCR assay for telomere content. Red arrows denote primers. (B) Results of the qPCR assay show that female-male difference in telomere content is higher in C5 tumors. (C) Representative images of the FISH assay for telomere content. Quantification is performed on GFP+ glioma cells. (D) Results of the FISH assay show that female-male difference in telomere content is higher in C5 tumors. Plots show mean ± standard error, and units are arbitrary. C3 tumors contain knockouts of Trp53, Nf1, and Pten. C5 tumors contain additional knockouts of Pot1a and Pot1b.
The second assay makes use of the fluorescence signal intensity of a telomere-specific FISH probe normalized to total nuclear DNA signal (Figure 4C). Here we performed telomere-FISH on GFP+ tumor cells in sections of glioma tissue from mouse brains (N=15). Linear modeling showed a significant interaction between sex and tumor type (P=0.048) with a similar change in female-male difference in telomere content (Figure 4D). Both assays, performed on different animals, demonstrate strikingly similar results suggesting that Pot1a/b loss in mouse glioma significantly increases the female-male difference in telomere content. These findings raise the possibility that the sex-dependent changes in telomere content contribute to the observed sex-dependent effects of Pot1a/b loss on glioma survival. Since POT1 loss is also associated with telomere stress and DNA damage, we subjected sections from the same mouse brain (N=15) to IHC against TP53BP1, a marker of DNA double-strand break. Results (Supplementary Figure 3) show a sex-dependent increase in DNA damage as a result of Pot1a/b loss in males relative to females.
POT1 depletion has sex-dependent effects on glioma immune infiltration
To determine the mechanisms underlying the sex-dependent effects of Pot1a/b loss on glioma biology, we performed RNA-seq expression analysis of C3 and C5 tumors including both sexes (N=26), yielding 10.5 ± 2.2 million reads per sample (mean ± s.d.) mapped across 23177 mm10 Entrez genes. Principal component analysis showed clear separation of samples based on sex with overlapping tumor clusters (Supplementary Figure 4A–B). To determine sex differences in gene expression that resulted from Pot1a/b knockout, the normalized Log2-transformed expression data were subjected to a linear model that included a sex-tumor interaction term. This analysis enables us to assess the C5-C3 difference in Log2 female/male expression ratios (Log2(F/M)) across all genes. Any significant deviation of C5-C3 Log2(F/M) from zero is indicative of sex-tumor interaction. Genes with absolute C5-C3 Log2(F/M) > 1 and P < 0.05 are termed ‘top genes’ (Supplementary Figure 4C) and are listed in Supplementary Table 1.
We subjected all C5-C3 Log2(F/M) values to Gene Set Enrichment Analysis (GSEA) to quantify the enrichment of KEGG, BIOCARTA, and PID canonical pathways for sex-tumor interaction. For human glioma, low-POT1 and high-POT1 expressing tumors were used in place of mouse C5 and C3 tumors, respectively. The top 10 gene sets in each category are listed in Figure 5A and are dominated by immune-related gene sets in both mouse and human analyses. All of these gene sets showed a lower female/male enrichment in POT1-depleted tumors (ES<0) and several top gene sets in each category were shared between mouse and human results (colored red). Next, we performed GSEA on expression signatures for Macrophages and T-Cell activation and found a similar sex-dependent effect in both mouse and human glioma (ES<0, Figure 5A). Individual genes from these two gene sets in mice are marked in the volcano plot in Figure 5C and the top genes with sex-tumor interaction among these are marked alongside the heatmap in Figure 5B. Similar patterns were observed using RNA-seq estimation of different immune cell lineages (Supplementary Figure 5). Together, these analyses suggest that POT1 depletion reduces the immune response in female tumors relative to males in both mouse and human glioma.
Figure 5. POT1 depletion has sex-dependent effects on glioma immune infiltration.
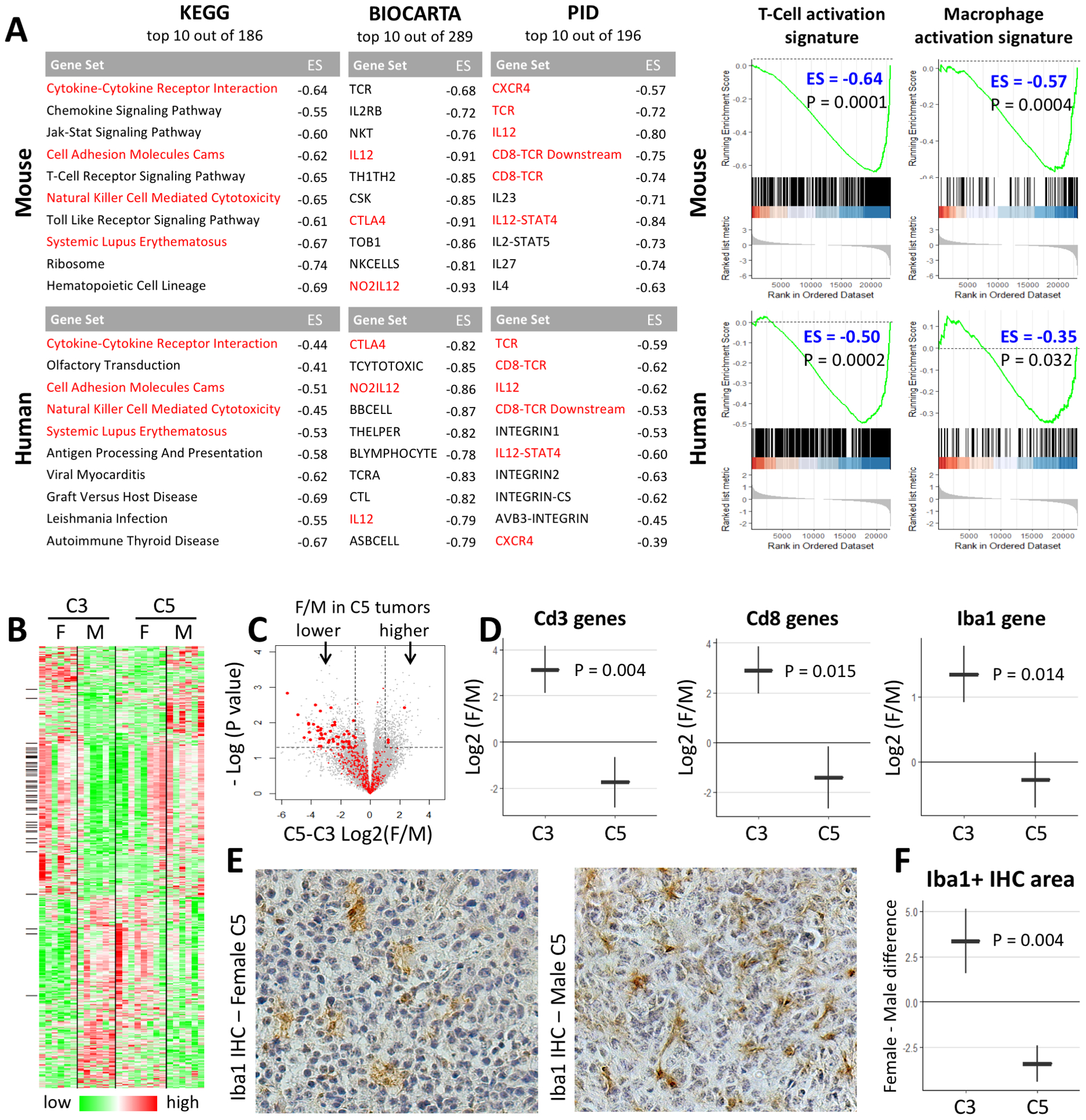
(A) Top 10 GSEA-ranked gene sets with sex-tumor interaction. Negative Enrichment Score (ES<0) signifies a lower female/male enrichment in POT1-depleted tumors. Red font denotes occurrence on both mouse and human lists. All adjusted P values <0.01. GSEA plots demonstrate POT1 depletion in mouse and human glioma results in similar sex-dependent changes (ES<0) in T-Cell and Macrophage activation signatures. (B) Heatmap of Log2(expression) values for top genes with sex-tumor interaction. Black marks on the left side denote genes in T-Cell or Macrophage gene sets. (C) Volcano plot for sex-tumor interaction. Marked in red are genes in T-Cell and Macrophage gene sets which show a clear deviation to the left side of the plot. Larger red dots denote top genes which also have a black mark in C. (D) Markers of T-lymphocytes (Cd3 and Cd8) and macrophages/microglia (Iba1) show similar sex-dependent changes at the RNA level. (E–F) Iba1 IHC demonstrates sex-dependent changes in microglia/macrophage infiltration similar to those seen at the RNA level. Plots in E and G show mean ± standard error. C3 tumors contain knockouts of Trp53, Nf1, and Pten. C5 tumors contain additional knockouts of Pot1a and Pot1b.
Next, we investigated the expression of immune cell marker genes in C3 and C5 tumors. Analysis at the RNA level (Figure 5D) showed that Pot1a/b loss in mouse glioma reduces the expression of pan and cytotoxic T-lymphocytes markers (Cd3, Cd8) and microglia (Iba1) in female tumors relative to males. To verify these RNA-based findings at the protein-level, we used Iba1 immunohistochemistry (IHC) to quantify tumor infiltration by microglia/macrophages. Using automated image quantification, we compared the female-male difference in Iba1+ IHC area in C3 and C5 tumors (N=14) and found a similar sex-dependent change (Figure 5E–F). These results support our informatics data indicating that Pot1a/b loss in mouse glioma confers decreased macrophage/microglial infiltration in female tumors relative to males. Furthermore, these observations raise the possibility that a sex-dependent change in immune response contributes to the observed sex differences in tumor aggressiveness driven by Pot1a/b loss.
POT1 depletion in glioma has sex-dependent effects on cell cycle
Prior studies indicate that sexual dimorphism in glioma is mediated, in part, by sex-dependent differences in the regulation of CDK activity and disruption of the RB pathway.(21,22) Therefore, we performed GSEA on mouse and human glioma expression data to quantify the enrichment of two gene sets associated with these two biological processes. This analysis revealed a higher female/male enrichment in POT1-depleted tumors (ES>0, significant values in bold) in mouse and human glioma (Figure 6A). Given our finding of sex-dependent effects of Pot1a/b loss on telomeres and the known role of telomeres in cancer and senescence,(23–25) we also performed GSEA on a gene set associated with telomere stress induced senescence. This analysis revealed a lower female/male enrichment in POT1-depleted tumors (ES<0) in mouse and human glioma (Figure 6A). Lastly, to verify these RNA-based findings at the protein level using a marker of mitotic activity, we subjected our C3 and C5 tumors (N=14) to Ki67 IHC and automated cell counting. The results confirm that Pot1a/b loss in mouse glioma confers increased mitotic activity in female tumors relative to males (Figure 6B–C). Together, our findings support the role of cell cycle and senescence regulators in mediating the observed sex-dependent effects in glioma survival.
Figure 6. POT1 depletion in glioma has sex-dependent effects on cell cycle.
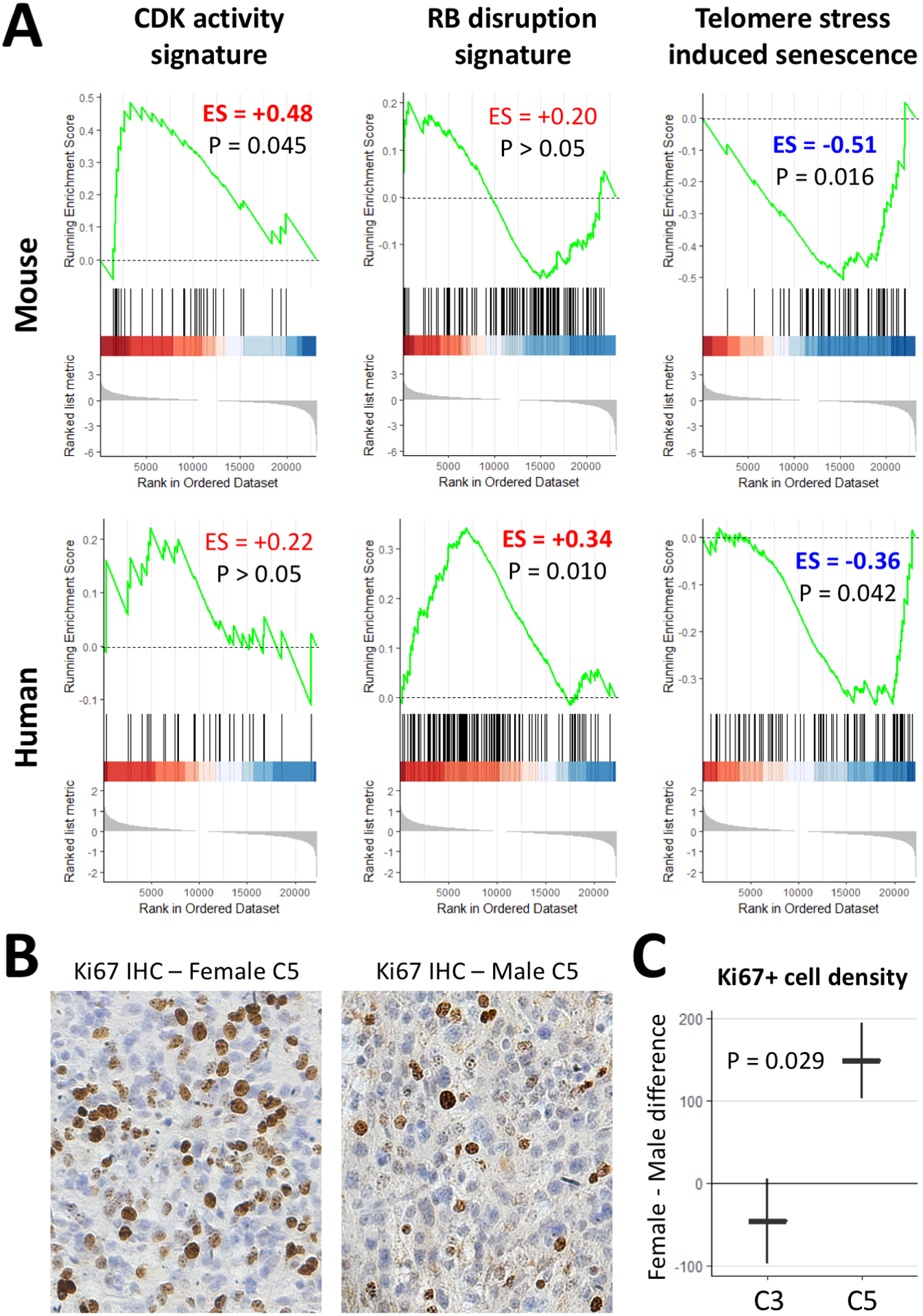
(A) GSEA using mouse and human glioma expression data demonstrates higher female/male enrichment (ES>0, bold if significant) of the CDK activity and RB disruption signatures and lower female/male enrichment (ES<0) of the telomere-stress-induced senescence signature in POT1-depleted tumors. (B–C) Ki67 IHC demonstrates increased female-male difference in mitotic activity as a result of Pot1a/b loss in C5 tumors. Plot in C shows mean ± standard error. C3 tumors contain knockouts of Trp53, Nf1, and Pten. C5 tumors contain additional knockouts of Pot1a and Pot1b.
Discussion
Pathogenic POT1 mutations are highly heterogenous. These include nonsense and frame-shift mutations early and late in the coding sequence as well as missense mutations in all functional domains of POT1 protein.(9,26) Therefore, it is not surprising that different POT1 mutations can elicit different biological effects. While two of the 3 familial glioma-associated POT1 mutations are truncating mutations (E450X and D617Efs) in the TPP1 binding domain of POT1, the third is a missense mutation (G95C) in the OB1 DNA-binding domain.(2) Consistent with this heterogeneity, we found that only POT1-G95C expression significantly increased self-renewal frequency, suggesting a proliferative role for this mutant in glioma initiating cells. Since familial glioma-associated POT1 mutations occur in the germline and are present in all cells during development, it is possible for them to exert effects early in life, prior to the transformation of glial lineage cells into glioma. We tested this possibility in the case of POT1-G95C by expressing it in the brain during the gliogenic period, where many cells of glial lineage retain a proliferative capacity. We observed increased proliferation as a result of POT1-G95C expression, further suggesting that this variant confers a proliferative advantage to prospective, non-transformed, cells of origin of glioma in the native brain. This proliferative advantage could explain how this particular POT1 mutant may increase the risk of glioma development. However, this is not the only potential mechanism as other glioma-associated mutants did not confer this advantage in our experiments and it will be important to decipher additional oncogenic mechanisms that apply to other POT1 mutants.
Sex differences in incidence of various cancers, including glioma, have been well documented,(27–29) and some of these differences have even been linked to germline variants.(30) Previously we found that mice bearing glioma driven by Trp53 and Nf1 loss have sexually dimorphic survival, with female mice living longer.(21) The current study demonstrates that additional knockout of Pten in C3 model shortens and equalizes the survival of both sexes, only to have these sex differences reversed with the additional knockout of Pot1a/b in C5 glioma. These observations collectively highlight a complex interplay between sex differences, tumor suppressor pathways, and telomere regulation in glioma. Nevertheless, our profiling studies revealed that the same cell-intrinsic mechanisms involving CDK activity and its downstream RB pathway appear to underlie sex differences across these glioma models. CDK activation and RB disruption in cancer can arise from a defective p53 response in the setting of oncogenic stresses, two conditions that are provided in the C3 mouse model by simultaneous knockout of Trp53, Nf1, and Pten. The added telomere damage and stress in the setting of Pot1a/b loss(7,11,15) and a lack of proper p53 response mechanism can further exacerbate baseline sex-dependent differences in the activity of these cell-intrinsic pathways and alter cell cycle and senescence dynamics. It remains unclear how telomere length and DNA damage are regulated differentially between the sexes, and we speculate epigenetic makeup and hormonal function may also play a role. Future studies will focus on how sex differences in telomere regulation converge with the core tumor suppressor pathways in glioma.
A somewhat surprising finding in this study was the sex-dependent changes in expression signatures and markers of immune infiltration as a result of Pot1a/b loss in glioma. Immune response has been hypothesized to contribute to sex differences in incidence and survival of various cancers(31) including glioma.(32) Increased tumor infiltration by CD3+ and CD8+ T-lymphocytes has generally been associated with improved glioma survival.(33–35) However, tumor-associated microglia/macrophages are thought to play dual roles in promoting and inhibiting tumor growth.(36) High Iba1 intensity has been associated with improved survival,(37) while high CD163 expression, essential for the pro-tumoral activity of microglia/macrophages,(38) has been associated with decreased survival.(39–41) Consistent with this, we found sex-dependent association of Pot1a/b loss with Cd3, Cd8, and Iba1 expression at the RNA level as well as Iba1 at the protein level. However, there was no sex-dependent association with Cd163 at the RNA levels (sex-tumor interaction P=0.80), suggesting that the observed sex-dependent changes in macrophage/microglial population may be due to the anti-tumor population of these cells. Expression signatures of T-Cell and Macrophage activation also showed similar sex-dependent associations, pointing to immune infiltration and response as a potential contributor to sex-dependent differences in survival. Of note, baseline sex differences in immune composition of glioma tumors are known to exist and do not necessarily lead to a differential growth. This is the case in our C3 tumor model as well as an optic glioma model in mice, where female glioma tumors have a higher density of microglia without a difference in proliferation or tumor growth.(42) On the other hand, recent studies have shown that subsets of myeloid-derived suppressor cells have differential, sex-dependent effects on glioma tumor growth, further supporting the notion that sex-dependent differences in immune populations can contribute to differential tumor growth.(43) Together with our findings, these results suggest that immune modulators and checkpoint blockade may be applied in a sex-dependent manner to treat glioma.
The link between immune response, as a cell-extrinsic mechanism, with cell-intrinsic mechanisms discussed previously can be explained by senescence-associated secretory phenotype (SASP), a phenomenon where cell cycle inhibition and senescence activate a cascade of cytokine production and immune response in tumors.(44) Consistent with this, Pot1a/b loss in male tumors relative to females, is associated with shorter telomeres and more DNA damage, reduced cell cycle and an enrichment of a senescence signature, an elevated level of immune-related signatures and markers, and a longer survival (Figure 7). Finally, it should be stated that modeling human POT1 mutation or loss in mice, which have two different POT1 orthologs and longer telomeres, is challenging. While many groups have used mouse models to gain insight into the function of POT1, there are limitations in extrapolating such findings to humans. Nevertheless, our parallel findings in mice and humans support common biological mechanisms.
Figure 7. Mode of sex-dependent effects of Pot1a/b knockout in mouse glioma.
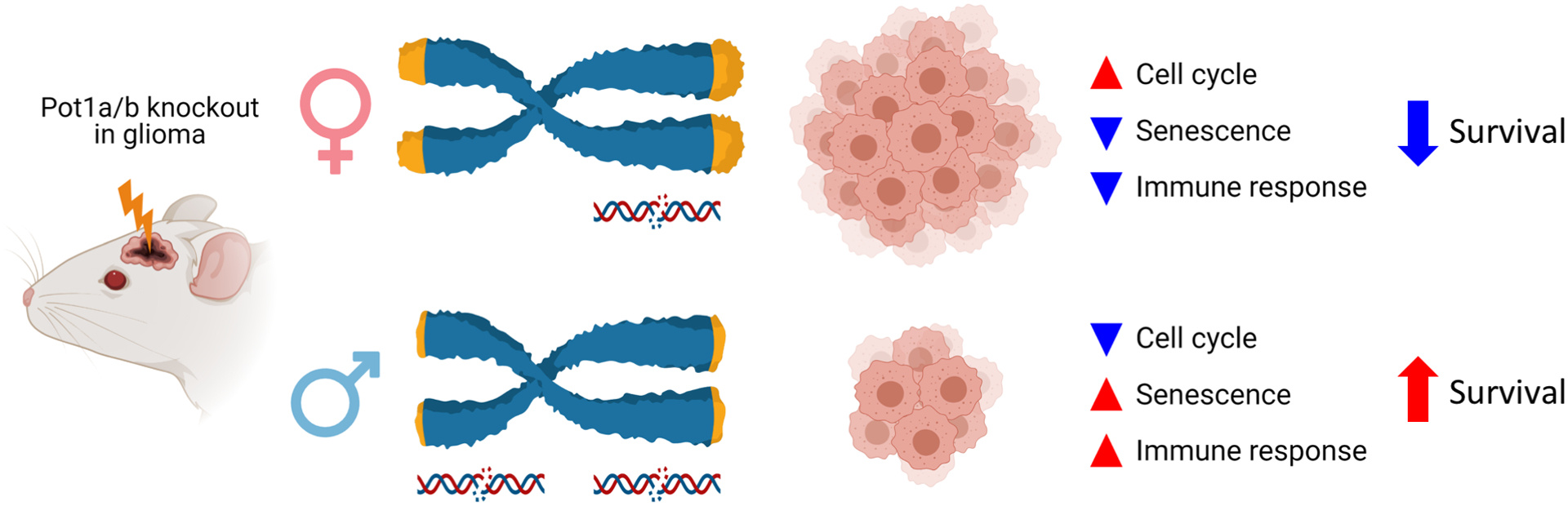
Pot1a/b loss in mouse glioma results in relative elongation of female telomeres and shorter male telomeres with more DNA damage. While longer telomeres may be more conducive to cell cycle and tumor growth in females, shorter telmoeres and increased DNA damage in males leads to increase senescence. Senescence and its associated secretory profile can, in turn, activate the immune respoonse which may modulate further tumor growth and survival.
Supplementary Material
Statement of Significance.
This study shows that manipulation of POT1 expression in glioma has sex-specific effects on tumorigenesis and associated immune signatures
Acknowledgments
This study was supported by NIH grants K08-NS110976 (AJ), R01-CA217105 (MLB, BD, and MNB), R01-NS094615 (GR), National Cancer Institute-Cancer Therapeutic Discovery (U01-CA217842 to BD), National Institute of Health (R50-CA252125 to KY) and the American Cancer Society-Rob Rutherford Glioblastoma Research Postdoctoral Fellowship (PF-15-220-01-TBG to KY). In addition, we would like to acknowledge the support provided by the Integrated Microscopy Core at Baylor College of Medicine and the Center for Advanced Microscopy and Image Informatics (CAMII) with funding from NIH (DK56338, CA125123, ES030285), CPRIT (RP150578, RP170719), the Dan L. Duncan Comprehensive Cancer Center, and the John S. Dunn Gulf Coast Consortium for Chemical Genomics.
Footnotes
The Authors Declare No Potential Conflicts of Interest
References
- 1.Kyritsis AP, Bondy ML, Rao JS, Sioka C. Inherited predisposition to glioma. Neuro-oncology. 2010;12:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bainbridge MN, Armstrong GN, Gramatges MM, Bertuch AA, Jhangiani SN, Doddapaneni H, et al. Germline mutations in shelterin complex genes are associated with familial glioma. J Natl Cancer Inst. 2015;107:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robles-Espinoza CD, Harland M, Ramsay AJ, Aoude LG, Quesada V, Ding Z, et al. POT1 loss-of-function variants predispose to familial melanoma. Nature Genetics. 2014;46:478–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi J, Yang XR, Ballew B, Rotunno M, Calista D, Fargnoli MC, et al. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nat Genet. 2014;46:482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chubb D, Broderick P, Dobbins SE, Frampton M, Kinnersley B, Penegar S, et al. Rare disruptive mutations and their contribution to the heritable risk of colorectal cancer. Nature Communications. 2016;7:11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speedy HE, Kinnersley B, Chubb D, Broderick P, Law PJ, Litchfield K, et al. Germ line mutations in shelterin complex genes are associated with familial chronic lymphocytic leukemia. Blood. 2016;128:2319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsay AJ, Quesada V, Foronda M, Conde L, Martínez-Trillos A, Villamor N, et al. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nature Genetics. 2013;45:526–30. [DOI] [PubMed] [Google Scholar]
- 8.Calvete O, Martinez P, Garcia-Pavia P, Benitez-Buelga C, Paumard-Hernández B, Fernandez V, et al. A mutation in the POT1 gene is responsible for cardiac angiosarcoma in TP53-negative Li-Fraumeni-like families. Nat Commun. 2015;6:8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvete O, Garcia-Pavia P, Domínguez F, Bougeard G, Kunze K, Braeuninger A, et al. The wide spectrum of POT1 gene variants correlates with multiple cancer types. European Journal of Human Genetics. 2017;25:1278–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lange T Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–10. [DOI] [PubMed] [Google Scholar]
- 11.Pinzaru AM, Hom RA, Beal A, Phillips AF, Ni E, Cardozo T, et al. Telomere Replication Stress Induced by POT1 Inactivation Accelerates Tumorigenesis. Cell Reports. 2016;15:2170–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice C, Shastrula PK, Kossenkov AV, Hills R, Baird DM, Showe LC, et al. Structural and functional analysis of the human POT1-TPP1 telomeric complex. Nature Communications. 2017;8:14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, Kiselar J, Whited TL, Hernandez-Sanchez W, Taylor DJ. POT1-TPP1 differentially regulates telomerase via POT1 His266 and as a function of single-stranded telomere DNA length. Proc Natl Acad Sci. 2019;116:23527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hockemeyer D, Daniels J-P, Takai H, de Lange T. Recent Expansion of the Telomeric Complex in Rodents: Two Distinct POT1 Proteins Protect Mouse Telomeres. Cell. 2006;126:63–77. [DOI] [PubMed] [Google Scholar]
- 15.Wu L, Multani AS, He H, Cosme-Blanco W, Deng Y, Deng JM, et al. Pot1 Deficiency Initiates DNA Damage Checkpoint Activation and Aberrant Homologous Recombination at Telomeres. Cell. 2006;126:49–62. [DOI] [PubMed] [Google Scholar]
- 16.Lee Y, Brown EJ, Chang S, McKinnon PJ. Pot1a Prevents Telomere Dysfunction and ATM-Dependent Neuronal Loss. J Neurosci. 2014;34:7836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin C-CJ, Yu K, Hatcher A, Huang T-W, Lee HK, Carlson J, et al. Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci. 2017;20:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amano H, Chaudhury A, Rodriguez-Aguayo C, Lu L, Akhanov V, Catic A, et al. Telomere Dysfunction Induces Sirtuin Repression that Drives Telomere-Dependent Disease. Cell Metabolism. 2019;29:1274–1290.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell. 2016;164:550–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kfoury N, Sun T, Yu K, Rockwell N, Tinkum KL, Qi Z, et al. Cooperative p16 and p21 action protects female astrocytes from transformation. Acta Neuropathologica Communications. 2018;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun T, Warrington NM, Luo J, Brooks MD, Dahiya S, Snyder SC, et al. Sexually dimorphic RB inactivation underlies mesenchymal glioblastoma prevalence in males. J Clin Invest. 2014;124:4123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng Y, Chan SS, Chang S. Telomere dysfunction and tumour suppression: the senescence connection. Nature Reviews Cancer. 2008;8:450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taboski MAS, Sealey DCF, Dorrens J, Tayade C, Betts DH, Harrington L. Long Telomeres Bypass the Requirement for Telomere Maintenance in Human Tumorigenesis. Cell Reports. 2012;1:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shay JW. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016;6:584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen E, Xiu J, Lopez GY, Bentley R, Jalali A, Heimberger AB, et al. POT1 mutation spectrum in tumour types commonly diagnosed among POT1-associated hereditary cancer syndrome families. J Med Genet. 2020;57:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook MB, McGlynn KA, Devesa SS, Freedman ND, Anderson WF. Sex Disparities in Cancer Mortality and Survival. Cancer Epidemiol Biomarkers Prev. 2011;20:1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubrow R, Darefsky AS. Demographic variation in incidence of adult glioma by subtype, United States, 1992–2007. BMC Cancer. 2011;11:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radkiewicz C, Johansson ALV, Dickman PW, Lambe M, Edgren G. Sex differences in cancer risk and survival: A Swedish cohort study. European Journal of Cancer. 2017;84:130–40. [DOI] [PubMed] [Google Scholar]
- 30.Ostrom QT, Kinnersley B, Wrensch MR, Eckel-Passow JE, Armstrong G, Rice T, et al. Sex-specific glioma genome-wide association study identifies new risk locus at 3p21.31 in females, and finds sex-differences in risk at 8q24.21. Scientific Reports. 2018;8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorak MT, Karpuzoglu E. Gender differences in cancer susceptibility: an inadequately addressed issue. Front Genet. 2012;3:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun T, Plutynski A, Ward S, Rubin JB. An integrative view on sex differences in brain tumors. Cell Mol Life Sci. 2015;72:3323–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang I, Tihan T, Han SJ, Wrensch MR, Wiencke J, Sughrue ME, et al. CD8+ T-Cell Infiltrate in Newly Diagnosed Glioblastoma is Associated with Long-Term Survival. J Clin Neurosci. 2010;17:1381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, et al. Effector T-Cell Infiltration Positively Impacts Survival of Glioblastoma Patients and Is Impaired by Tumor-Derived TGF-β. Clin Cancer Res. 2011;17:4296–308. [DOI] [PubMed] [Google Scholar]
- 35.Kmiecik J, Poli A, Brons NHC, Waha A, Eide GE, Enger PØ, et al. Elevated CD3+ and CD8+ tumor-infiltrating immune cells correlate with prolonged survival in glioblastoma patients despite integrated immunosuppressive mechanisms in the tumor microenvironment and at the systemic level. Journal of Neuroimmunology. 2013;264:71–83. [DOI] [PubMed] [Google Scholar]
- 36.Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sørensen MD, Dahlrot RH, Boldt HB, Hansen S, Kristensen BW. Tumour-associated microglia/macrophages predict poor prognosis in high-grade gliomas and correlate with an aggressive tumour subtype. Neuropathology and Applied Neurobiology. 2018;44:185–206. [DOI] [PubMed] [Google Scholar]
- 38.Shiraishi D, Fujiwara Y, Horlad H, Saito Y, Iriki T, Tsuboki J, et al. CD163 Is Required for Protumoral Activation of Macrophages in Human and Murine Sarcoma. Cancer Res. 2018;78:3255–66. [DOI] [PubMed] [Google Scholar]
- 39.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. The Journal of Pathology. 2008;216:15–24. [DOI] [PubMed] [Google Scholar]
- 40.Komohara Y, Horlad H, Ohnishi K, Fujiwara Y, Bai B, Nakagawa T, et al. Importance of direct macrophage - Tumor cell interaction on progression of human glioma. Cancer Science. 2012;103:2165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prosniak M, Harshyne LA, Andrews DW, Kenyon LC, Bedelbaeva K, Apanasovich TV, et al. Glioma Grade Is Associated with the Accumulation and Activity of Cells Bearing M2 Monocyte Markers. Clin Cancer Res. 2013;19:3776–86. [DOI] [PubMed] [Google Scholar]
- 42.Toonen JA, Solga AC, Ma Y, Gutmann DH. Estrogen activation of microglia underlies the sexually dimorphic differences in Nf1 optic glioma–induced retinal pathology. Journal of Experimental Medicine. 2016;214:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bayik D, Zhou Y, Park C, Hong C, Vail D, Silver DJ, et al. Myeloid-Derived Suppressor Cell Subsets Drive Glioblastoma Growth in a Sex-Specific Manner. Cancer Discov. 2020;10:1210–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faget DV, Ren Q, Stewart SA. Unmasking senescence: context-dependent effects of SASP in cancer. Nature Reviews Cancer. 2019;19:439–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


