Abstract
Background
The diagnosis of nonocclusive mesenteric ischemia (NOMI) is always challenging in critically ill patients. Herein, we aimed to report a case of NOMI associated with a hyperosmolar hyperglycemic state (HHS). A small amount of hepatic portal venous gas (HPVG) triggered the diagnosis of NOMI.
Case Presentation
A 77‐year‐old man was transferred due to shock and disorder of consciousness. He was diagnosed with an HHS. We suspected intestinal ischemia due to a small amount of HPVG revealed by computed tomography (CT). Peritoneal signs were revealed after treatment for the HHS. Computed tomography was carried out again 5 h after admission, which showed a large amount of HPVG, remarkable bowel dilatation, and pneumatosis intestinalis. We performed an emergency laparotomy and resected the small bowel necrosis resulting from NOMI.
Conclusion
An HHS can cause NOMI, and the presence of HPVG on CT is an important finding that suggests mesenteric ischemia, even in small amounts.
Keywords: Computed tomography, diabetes, hepatic portal venous gas, hyperosmolar hyperglycemic state, nonocclusive mesenteric ischemia
The diagnosis of nonocclusive mesenteric ischemia (NOMI) is always challenging in critically ill patients. We reported a case of NOMI associated with a hyperosmolar hyperglycemic state. A small amount of hepatic portal venous gas triggered the diagnosis of NOMI.
![]()
Background
Nonocclusive mesenteric ischemia (NOMI) is a lethal condition with an alarmingly high mortality rate (50%–80%) caused by microvascular hypoperfusion and constriction. 1 Diagnosing NOMI is always challenging in critically ill patients, as physical examination is often limited by disorder of consciousness and anesthesia. 2 Computed tomography (CT) findings support the identification of NOMI, 2 , 3 as the presence of hepatic portal venous gas (HPVG) is a sign of intestinal necrosis in NOMI. 2 , 3
A hyperosmolar hyperglycemic state (HHS) and diabetic ketoacidosis (DKA) are life‐threatening emergencies that occur in some patients with diabetes. 4 The differences in the pathogenesis of DKA and HHS are the varying severity of dehydration resulting from osmotic diuresis and the absence of ketosis. 4 An HHS is characterized by severe hyperglycemia, high serum osmolality, and dehydration. 4
In this case report, describe a patient who was diagnosed after admission with NOMI associated with HHS who subsequently underwent emergency surgery. The presence of a small amount of HPVG in CT imaging triggered the diagnosis of NOMI.
Case
A 77‐year‐old man was transferred to our emergency department by ambulance due to shock; he had a mild disorder of consciousness (Glasgow Coma Scale, E3V4M6) and restlessness on arrival. He presented with comorbid hyperglycemia and hypertension; however, he had not been medicated for approximately 10 years. He had experienced a low‐grade fever and malaise for 5 days before admission, and his oral intake had decreased. He had a blood pressure of 82/60 mmHg, a pulse rate of 116 b.p.m., a respiratory rate of 32 breaths/min, and a body temperature of 36.0°C. The abdominal findings were obscure due to the disorder of consciousness; although he complained of mild pain in the left lower quadrant, no peritoneal sign was evident. The laboratory data on admission showed a plasma glucose concentration of 1,407 mg/dL (normal range, 70–109 mg/dL) and a hemoglobin A1c level of 13.4% (normal range, <6.0%). His sodium concentration was 123 mmol/L (normal range, 138–145 mmol/L), blood urea nitrogen level was 88.2 mg/dL (normal range, 8.0–20.0 mg/dL), creatine level was 3.44 mg/dL (normal range, 0.46–0.79 mg/dL), and white blood cell count was 14,400 cells/μL (normal range, 3,300–8,600 cells/μL). The C‐reactive protein concentration was 8.39 mg/dL (normal range, <0.14 mg/dL), and the concentration of procalcitonin was 23.0 ng/mL (normal range, <0.05 ng/mL). The serum osmolality was 356 mOsm/L (normal range, 270–295 mOsm/L). Arterial blood gas data (O2 10 L/min) showed a pH of 7.38, partial pressure of oxygen (PaO2) of 188, partial pressure of carbon dioxide (PaCO2) of 28.5 mmHg, an HCO3− concentration of 16.6 mmol/L, a base excess of −6.4 mmol/L, and a lactate concentration of 41 mg/dL. The analysis was negative for urinary ketones.
Computed tomography without contrast was carried out due to renal insufficiency; it revealed a small amount of HPVG in the left lobe (Fig. 1A). No remarkable acute mesenteric ischemia finding, such as bowel dilatation or pneumatosis intestinalis, was observed (Fig. 2B). Hepatic portal venous gas was classified using the classification from a retrospective analysis. 5 In addition, we suspected intestinal ischemia/necrosis due to the high lactate concentration and small amount of HPVG on the CT. We initiated a continuous intravenous infusion of regular human insulin and Ringer’s acetate solution to treat the HHS; however, the pulse increased to 128 b.p.m. The noradrenaline dose was increased from 0 to 0.33 μg/kg/min to maintain the mean blood pressure of 60 mmHg, and the elevated lactate concentration persisted (46 mg/dL) for 5 h postadmission. We administered extracellular fluid at a rate of 2,500 mL/4 h before noradrenaline was started. The pain in the left lower quadrant had worsened and peritoneal signs became evident. Thus, we carried out contrast‐enhanced abdominal CT, as intestinal necrosis was suspected. The HPVG had spread within the left lobe compared with the initial CT scan (Fig. 2A). The CT also revealed remarkable bowel dilatation and pneumatosis intestinalis in the absence of contrast‑induced bowel wall enhancement (Fig. 2B). No occlusion of the superior mesenteric artery or superior mesenteric vein due to thrombus or embolism was observed. Based on the physical examination, laboratory data, and CT findings, we suspected NOMI, requiring emergency laparotomy. At the initial operation, necrosis was observed within the small bowel, with segmental and skip lesions (Fig. 3). The necrotic portion of the small bowel, 170 cm in length, was resected using an automatic suture instrument; the remaining small bowel was 20 cm from the ligament of Treitz and 210 cm from the terminal ileum. No anastomosis was carried out. The initial surgery ended with open abdominal management to allow for a second‐look operation on the third hospitalization day, at which time no bowel necrosis was observed; an anastomosis was performed, followed by abdominal closure. We initiated treatment with a vasodilator with continuous intravenous prostaglandin E1 infusion after terminating vasopressor treatment from the third to the seventh hospitalization day, after which the patient was extubated. On the 10th hospitalization day, the patient initiated oral intake. Insulin therapy was substituted by an oral diabetes medication (a dipeptidyl peptidase IV inhibitor), as his plasma glucose concentration was reduced. He was discharged on the 21st day of hospitalization.
Fig. 1.
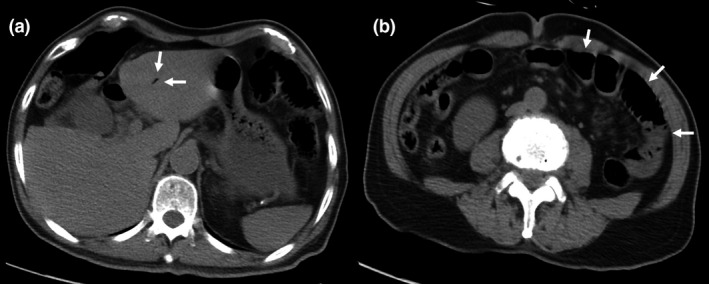
Computed tomography without contrast undertaken on admission of a 77‐year‐old man with nonocclusive mesenteric ischemia. A, A small amount of hepatic portal venous gas in the left lobe (white arrows). B, Computed tomography did not reveal remarkable acute mesenteric ischemia findings, such as bowel wall thickening, a hyperattenuating bowel wall, and paper‐thin bowel wall (white arrows).
Fig. 2.
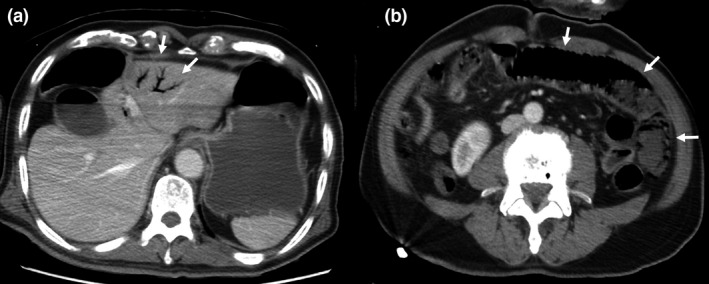
Computed tomography of a 77‐year‐old man with nonocclusive mesenteric ischemia, carried out at 5 h postadmission with contrast enhancement. A, Spreading of hepatic portal venous gas in the left lobe (white arrows) compared with the initial scan. B, Remarkable bowel dilatation and pneumatosis intestinalis (white arrows) in the absence of contrast‐induced bowel wall enhancement.
Fig. 3.

Emergency laparotomy carried out in a 77‐year‐old man with nonocclusive mesenteric ischemia. The small bowel appears necrotic, with segmental and skip lesions visible at the initial operation.
Discussion
Nonocclusive mesenteric ischemia is a lethal condition with a high mortality rate caused by microvascular hypoperfusion and constriction. 1 As HHS is characterized by severe hyperglycemia, high serum osmolality, and dehydration, 4 it might be easy to think that HHS is one of the causes of NOMI. However, we found no report on NOMI associated with HHS published in English after a thorough search of PubMed using the following keywords: hyperosmotic hyperglycemic nonketotic state, hyperosmolar hyperglycemic state, diabetic ketoacidosis, diabetes, and nonocclusive mesenteric ischemia. In this search, NOMI associated with DKA was also rare, as we only found four case reports. 6 , 7 , 8 , 9 The rarity of published works on NOMI with HHS could be explained as follows. First, according to the National Diabetes Statistics Report (2020) by the Centers for Disease Control and Prevention, it was reported that the frequency of HHS patients admitted to the emergency department was much lower than in DKA patients (HHS, 0.9/1,000 adults with diabetes versus DKA, 8.8/1,000 adults with diabetes). 10 Second, there was a possibility that NOMI diagnosis with HHS and DKA might be underdiagnosed as most of the patients in a hyperglycemic state might be initiated by physicians, rather than surgeons. For example, abdominal compartment syndrome (ACS) is a condition that requires surgical management, such as NOMI. A multicenter epidemiological study has shown that the prevalence of intra‐abdominal hypertension (50.5%) and ACS (8.2%) in the intensive care unit was quite high, and that intra‐abdominal hypertension prevalence was higher in medical admissions than in surgical admissions (59% versus 41%). 11 Another previous study showed that surgically trained intensivists experienced a higher number of ACS cases than medically trained and pediatric‐trained intensivists. 12 As with ACS, it is possible that NOMI diagnosis with HHS might not have been fully recognized by the physicians who initiated treatment.
In addition to the mechanism of dehydration due to the hyperglycemic state, as the endothelial glycocalyx (EG) was shown to be damaged during a hyperglycemic crisis in the recent study, 13 we considered that one of the mechanisms of NOMI onset with HHS or DKA might be based on EG dysfunction. Specifically, the EG is a layer of proteoglycans that covers the vascular endothelium, consisting of a core protein that carries one or more covalently attached glycosaminoglycan chains. 14 Diseases such as diabetes, atherosclerosis, sepsis, and ischemia/reperfusion injury display high degrees of vascular inflammation leading to EG degradation. 14 As the EG regulates vascular tonus and permeability, inflammation, and coagulation, it is possible that EG damage during hyperglycemic conditions could cause vasospasm, hypoperfusion, development of inflammation, and microthrombi formation due to hypercoagulability, resulting in NOMI.
In such cases, the patient should be monitored for intestinal necrosis, even if only a small amount of HPVG is observed, as HPVG is associated with intestinal necrosis, digestive tract dilatation, mucosal disruption, gastric ulcer, ulcerative colitis, Crohn’s disease, and complications following endoscopic procedures. 15 , 16 Although intestinal necrosis is the most critical condition associated with HPVG in these conditions, a proper diagnosis is sometimes difficult. 15 , 16 In addition, HPVG has been shown to be associated with various pathological conditions and the clinical significance ranges from benign findings to bowel necrosis. 15 , 16 A review article suggested that emergency laparotomy is recommended if CT findings reveal HPVG with concurrent signs of bowel necrosis or ischemia and a concomitant increase in lactate concentration. 15 Here, we suspected bowel ischemia/necrosis early after admission because of the small amount of HPVG detected in the initial CT and the persistently increased lactate concentrations after fluid resuscitation.
Conclusion
An HHS can cause NOMI, and the presence of even a small amount of HPVG is an important finding that suggests mesenteric ischemia/necrosis.
Disclosure
Approval of the research protocol: N/A.
Informed consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Registry and registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
Funding Information
No funding information provided.
References
- 1. Trompeter M, Brazda T, Remy CT, Vestring T, Reimer P. Non‐occlusive mesenteric ischemia: etiology, diagnosis, and interventional therapy. Eur. Radiol. 2002; 12: 1179–87. [DOI] [PubMed] [Google Scholar]
- 2. Bourcier S, Oudjit A, Goudard G, et al. Diagnosis of non‐occlusive acute mesenteric ischemia in the intensive care unit. Ann. Intensive Care. 2016; 6: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pérez‐García C, de Miguel CE , Gonzalo AF, et al. Non‐occlusive mesenteric ischaemia: CT findings, clinical outcomes and assessment of the diameter of the superior mesenteric artery. Br. J. Radiol. 2018; 91: 20170492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. French EK, Donihi AC, Korytkowski MT. Diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome: review of acute decompensated diabetes in adult patients. BMJ 2019; 365: l1114. [DOI] [PubMed] [Google Scholar]
- 5. Schindera ST, Triller J, Vock P, et al. Detection of hepatic portal venous gas: its clinical impact and outcome. Emerg. Radiol. 2006; 12: 164–70. [DOI] [PubMed] [Google Scholar]
- 6. Hohmann C, Teuteberg S, Aschenbrenner I, et al. Non‐occlusive Mesenteric Ischemia caused by Diabetic Ketoacidosis ‐ Pneumatosis intestinalis and Portal Venous Gas as an Indication of Mesenteric Ischemia. Dtsch. Med. Wochenschr. 2019; 23: 1638–41. [DOI] [PubMed] [Google Scholar]
- 7. Itoh Y, Sagawa R, Kinoshita H, et al. Small‐intestinal necrosis due to non‐occlusive mesenteric ischemia with diabetic ketoacidosis after quetiapine treatment. Diabetol. Int. 2019; 10: 225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gocho N, Aoki E, Okada C, et al. Non‐occlusive mesenteric ischemia with diabetic ketoacidosis and lactic acidosis following the administration of a sodium glucose co‐transporter 2 inhibitor. Intern. Med. 2016; 55: 1755–60. [DOI] [PubMed] [Google Scholar]
- 9. Soravia C, Höhn L, Mentha G, et al. Non occlusive mesenteric ischemia: a late complication of cardiogenic shock. Ann. Chir. 1994; 11: 1029–31. [PubMed] [Google Scholar]
- 10. National Diabetes Statistics Report . CDC [homepage on the internet]. Centers for Disease Control and Prevention Online Resources, 2020. [updated August 28, 2020]. Available from: https://www.cdc.gov/diabetes/data/statistics‐report/index.html
- 11. Malbrain ML, Chiumello D, Pelosi P, et al. Prevalence of intra‐abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive. Care. Med. 2004; 30: 822–9. [DOI] [PubMed] [Google Scholar]
- 12. Kimball EJ, Rollins MD, Mone MC, et al. Survey of intensive care physicians on the recognition and management of intra‐abdominal hypertension and abdominal compartment syndrome. Crit. Care. Med. 2006; 9: 2340–8. [DOI] [PubMed] [Google Scholar]
- 13. Nieuwdorp M, van Haeften TW, Gouverneur MC, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 2006; 55: 480–6. [DOI] [PubMed] [Google Scholar]
- 14. Sieve I, Münster‐Kühnel AK, Hilfiker‐Kleiner D. Regulation and function of endothelial glycocalyx layer in vascular diseases. Vascul. Pharmacol. 2018; 100: 26–33. [DOI] [PubMed] [Google Scholar]
- 15. Nelson AL, Millington TM, Sahani D, et al. Hepatic portal venous gas: the ABCs of management. Arch. Surg. 2009; 144: 575–81. [DOI] [PubMed] [Google Scholar]
- 16. Abboud B, El Hachem J, Yazbeck T, et al. Hepatic portal venous gas: physiopathology, etiology, prognosis and treatment. World J. Gastroenterol. 2009; 15: 3585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


