Abstract
Purpose
Euphorbia neriifolia Linn. has important medicinal value in the treatment of ulcers, tumors, inflammation, chronic respiratory troubles, and so on. Although many ingredients with anti-inflammatory activity have been discovered and isolated from the Euphorbia neriifolia, the current research still cannot explain its multivariate effects on the immune response. This article aims to introduce two Ingenane-type diterpenoids from Euphorbia neriifolia with macrophage regulatory effects and to investigate the mechanism of their action.
Methods
The stem bark of E. neriifolia was extracted with various separation methods to obtain ingenane-type diterpenoids. The RAW264.7 cells were treated with lipopolysaccharide (LPS, 1 μg/mL) to establish an inflammatory cell model. The cell viability was detected by MTT assay. The secretion of PGE2, TNF-α, IL-1β, and IL-6 was tested with ELISA. The levels of iNOS, COX-2, IκBα, JNK, ERK, p38, p-IκBα, p-JNK, p-ERK, and p-p38 in cells were detected by Western blotting. The translocation of nuclear factor-kappa B (NF-κB)/p65 subunit were evaluated by Immunofluorescence staining.
Results
Ingenane-type diterpenoids, eurifoloid A (Euri A) and a new compound euphorneroid E (Euph E), were isolated from the EtOAc fraction of E. neriifolia stem bark extracts. Euph E and Euri A exhibited significant inhibition on the levels of pro-inflammatory mediators NO, IL-1β, IL-6, and iNOS on LPS-induced macrophage RAW264.7. Cellular signaling pathway studies showed that they prevented the degradation of IκBα and the translocation of NF-κB/p65 subunit. Furthermore, the production of PGE2, TNFα, and COX-2 was dramatically increased under the influence of the compounds, which were closely related to the phosphorylation of protein kinase C δ (PKCδ) and activation of mitogen-activated protein kinase (MAPKs) signaling pathway.
Conclusion
These results demonstrated that Euph E and Euri A exhibited multidirectional regulation on cytokines and immune function of macrophages, in addition to a good anti-inflammatory activity, and which was closely related to the regulation of PKCδ/MAPKs and NF-κB signal pathways.
Keywords: euphorneroid E, eurifoloid A, ingenane-type diterpenoids, Euphorbia neriifolia, M-macrophage, immunoregulation
Graphical abstract
Introduction
Innate Immunity, considered as the first line of defense against infection, can induce the body to produce immune responses to endogenous and exogenous stimuli, meanwhile playing an important role in maintain homeostasis and immune regulation.1,2 Macrophages, derived from monocytes, play an important role in fighting infections, anti-tumors, and participating in the body’s repair process based on their powerful phagocytosis and immune activation effects. With the change of microenvironment, different types of macrophages are precisely regulated and mutually transformed, maintaining homeostasis in the organism. Classically activated macrophages can secrete inflammatory cytokines and chemokines, and have a strong phagocytic function, mainly playing the role of immune defense. However, excessive activation of macrophages result in the production of a large number of inflammatory factors which will aggravate the tissue damage of the body and cause various autoimmune diseases.3,4 Alternatively activated macrophages can alleviate the inflammatory response, and promote wound healing and tissue recovery,5 but tumor growth may take advantage of this feature, leading to the immune escape of tumor cells.6,7 Therefore, an in-depth study on the regulation of macrophage function is of important guiding significance to further elucidate the immune defense, immune homeostasis mechanism, and the treatment of various diseases.
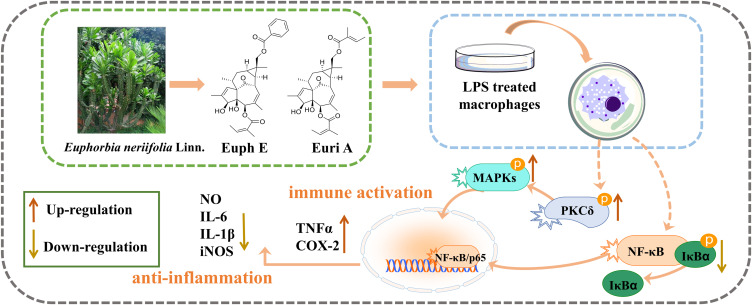
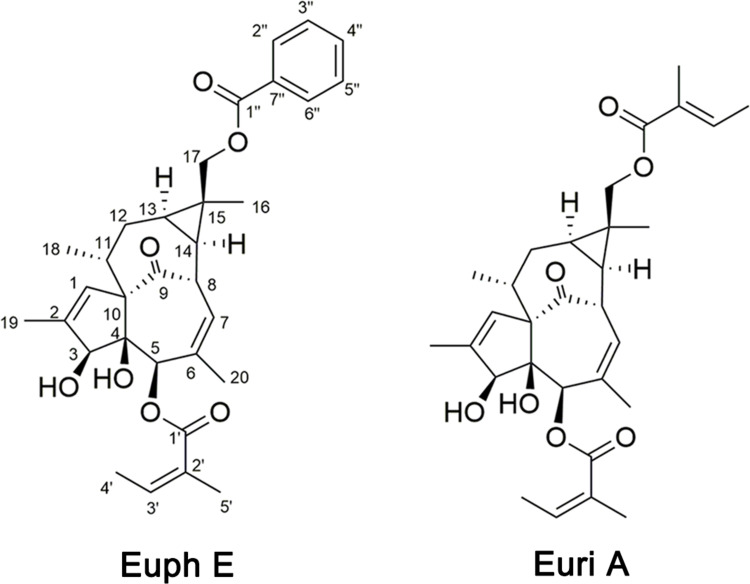
Euphorbia neriifolia Linn, a succulent plant, belongs to the Euphorbiaceae family, and has been recognized as a potential therapist for edema, bronchitis, piles, tuberculosis, anemia, ulcers, tumors, inflammation, and chronic respiratory troubles for centuries.8–11 In recent years, Euphorbia has attracted much attention for its immunomodulatory and antitumor activities. However, in addition to its good anti-inflammatory activity, Euphorbia neriifolia has also been found to cause primary irritation to the skin and mucous membranes, resulting in swelling and inflammation of the skin and mucous membranes. There has been no clear report about these diametrically opposed pharmacological effects so far. Our research group carried out comprehensive studies on the phytochemistry and pharmacology of E. neriifolia and found a series of novel compounds,12,13 and multiple structural types of compounds with significant anti-inflammatory activity have been found. Recently, two ingenane-type diterpenoids, euphorneroid E (Euph E) and eurifoloid A (Euri A), were isolated from the stem bark of E. neriifolia, and Euph E was a new compound (Figure 1). Euri A was first isolated from Euphorbia neriifolia in 2014,14 and was subsequently found to have anti-HIV and reverse tumor resistance effects.15 At present, this compound has been able to achieve total synthesis.16 Ingenane-type diterpenoids mainly existed in Euphorbia species, and the representative compound Ingenol 3-Angelate (PEP005) was found to have good antitumor activity and has been clinically used to treat actinic keratosis.17 We have supplemented some cellular biomolecular information involved in this study in the preface. Here Euph E and Euri A showed the dual effects on macrophages, this is, immune activation and anti-inflammatory immunosuppression, which will help to explain the opposite pharmacological effect of E. neriifolia.
Figure 1 .
Structures of Euph E and Euri A isolated from Euphorbia neriifolia Linn.
In this study, we aimed to reveal the possible mechanism of Euph E and Euri A regulating the immune function of macrophages, with emphasis on the hypothesis, whether NF-κB, MAPKs, and PKC signaling pathways were involved.
Materials and Methods
Plant Extraction and Compound Isolation
The stem bark of Euphorbia neriifolia Linn. was collected from Xishuangbanna, Yunnan Province, China, in May 2015, and identified by Prof. Yumei Zhang, Xishuangbanna Tropical Botanical Garden, CAS. A voucher specimen (JIN 201505) was deposited at the Laboratory of Phytochemistry, Faculty of Life Science and Technology, Kunming University of Science and Technology.
The air-dried and powdered stem bark of E. neriifolia (21.0 kg) was extracted with 75% acetone/H2O (3×30 L, 24 hours, each) at room temperature. After removing the solvent by reduced pressure distillation, the crude extract was suspended in water and extracted with ethyl acetate (EtOAc) to obtain the EtOAc-soluble fraction (550.0 g), which was subjected to column chromatography (MCI, 6.5×120 cm) eluted with gradient Methanol (MeOH)/H2O system (30, 60, 75, 90, and 100%) to afford three fractions (Fr. I–III). Fraction I (1.2 g) was subjected to Sephadex LH-20 or silica gel repeatedly to yield Euph E (2.9 mg) and Euri A (7.0 mg). Their structures were elucidated by NMR techniques, as well as comparison with literature.
Cell Culture Conditions
The RAW264.7 murine macrophages cell line (Conservation Genetics CAS Kunming Cell Bank, Yunan, China, No.KCB200603YJ) was maintained in Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fisher Scientific, Massachusetts, USA) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific) and penicillin (10,000 U/mL)-streptomycin (10,000 μg/mL, Solarbio Life Sciences, Peking, China) at 37°C in a humidified 5% CO2 atmosphere.
Cell Viability Assay
Cell viability was measured via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The RAW264.7 cells were seeded into 96-well culture plates (Corning Incorporated, Corning, USA) at a density of 4×104 cells/well for 24 hours, then pretreated with various concentrations of Euph E or Euri A (2.5–80 μM) for 1 hour, followed by LPS (1 μg/mL, Escherichia coli 055:B5; Sigma-Aldrich, St. Louis, MO, USA) for another 24 hours. After washing with phosphate-buffered saline (PBS) buffer twice, 20 µL of MTT solution (5 mg/mL in PBS, pH 7.4; Solarbio Life Sciences, Peking, China) was added into each culture well and the cells were further incubated at 37°C for 4 hours. The MTT was carefully removed and 150 μL of DMSO (Solarbio Life Sciences) was added into each well to dissolve the formazan crystals. After 15 minutes, the optical density was measured at 490 nm with a Thermo Fisher Scientific microplate reader .
NO Assay
The production of NO was measured by the determination of nitrite in culture supernatant by Griess reaction (Beyotime Institute of Biotechnology, Shanghai, China). RAW264.7 cells were seeded into 96-well cell culture plates at a density of 6×104 cells/well and incubated for 24 hours. Then, the cells were pretreated with Euph E or Euri A at concentrations of 2.5, 5, 10, and 20 µM for 1 hour and activated with LPS (1 μg/mL) for 24 hours in a 37°C, 5% CO2 incubator. The culture supernatant (70 µL) was mixed with Griess reagent A (50 µL) and Griess reagent B (50 µL) and incubated for 10 minutes on the Shaking table at room temperature. Then the absorbance of the mixture was measured at 540 nm with a microplate reader (Thermo Fisher Scientific,).
Enzyme-Linked Immunosorbent Assay
To detect the inflammatory cytokines produced by mouse macrophages RAW264.7, such as IL-1β, IL-6, TNF-α, and PGE2, cells were seeded into 24-well cell culture plates at a density of 4×105 cells/well. After incubation of 24 hours, the cells were pretreated with 2.5–20 μM of Euph E or Euri A for 1 hour and then activated with LPS (1 μg/mL) for 24 hours. Subsequently, cell supernatants were collected from each well. According to the manufacturer’s instructions, ELISA kit (IL-1β and IL-6: R&D Systems, Minneapolis, USA; TNFα: BOSTER, Wuhan, China; PGE2: CUSABIO Biotech CO., Hubei, China) was used to determine the levels of IL-1β, IL-6, TNF-α, and PGE2 in the supernatants.
Immunofluorescence Staining
RAW264.7 cells were incubated on round glass coverslips in 24-well plates overnight, pretreated with compounds for 1 hour, then stimulated with LPS (1 μg/mL) for 30 minutes. After washing with PBSCM three times, they were fixed in 4% paraformaldehyde for 30 minutes, followed by being permeabilized with 0.1% Triton X-100 for 15 minutes, and blocked in fluorescence antibody dilution buffer for 1 hour. After that, the cells were incubated with rabbit anti-NF-κB/p65 polyclonal antibodies (1:100 dilution; Cell Signaling Technology, Danvers, USA) at 4°C overnight. The coverslips were washed with PBSCM and incubated with Cy3-conjugated sheep anti-rabbit IgG at room temperature for 1 hour. Finally, immunofluorescence was observed with laser scanning confocal microscopy (Nikon, Japan).
Western Blot Analysis
RAW264.7 cells were added to 6-well culture plates at a density of 1×106 cells/well and cultured for 24 hours. After pretreating with 2.5–20 μM of Euph E or Euri A for 1 hour, cells were co-incubated with LPS (1 μg/mL) for 30 minutes (for MAPKs, IκBα, and PKCδ; Cell Signaling Technology) or 18 hours (for iNOS and COX-2; Cell Signaling Technology) in a 37°C, 5% CO2 incubator. Then, cells were collected and and lysed with lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) containing 1 mM PMSF (Beyotime Institute of Biotechnology) and 1:25 Complete Mini Protease Inhibitor cocktail (PI; Roche, Boehringer Mannheim, Germany). The cytosolic fractions were prepared by centrifuging and Western blot analysis of supernatants was performed as described previously.18
Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA), and was based on at least three independent experiments. The results were considered to be statistically significant when the p-value was less than 0.05. All statistical tests were conducted by the computer program SPSS (SPSS Inc., Chicago, USA).
Results
Structural Elucidation
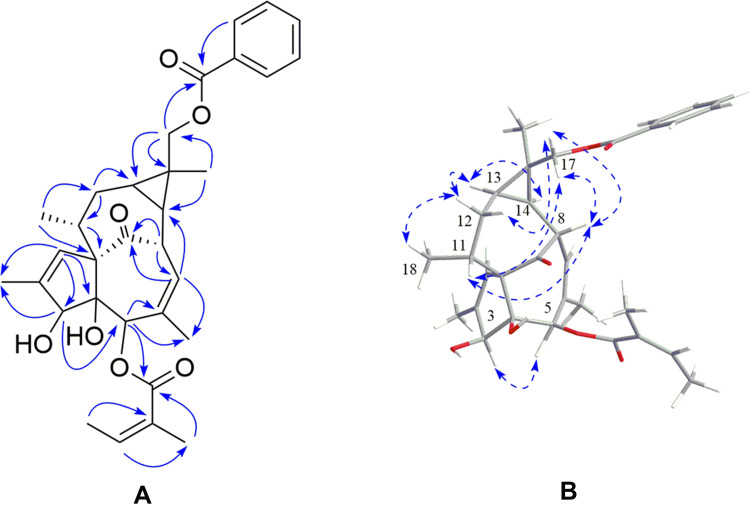
Euph E was obtained as white amorphous powder. Its HR-ESI-MS (Supplementary Figure S1) exhibited a quasi-molecular ion peak at m/z 557.2526 [M+Na]+ (calcd 557.2510), suggesting a molecular formula of C32H38O7 with 14 degrees of unsaturation. Its IR spectrum showed the presence of hydroxy (3,437 cm−1), carbonyl (1,711 cm−1), and olefinic (1,651 cm−1) functional groups. The 1H NMR spectroscopic data (Table 1, Supplementary Figure S2) of Euph E showed a group of protons for a monosubstituted benzene ring at δH 7.45 (2H, t, J=7.8 Hz, H-3’’ 5’’, 7.57 (1H, t, J=7.4 Hz, H-4’’ and 8.06 (2H, d, J=7.0 Hz, H-2’’ 6’’), three olefinic protons at δH 5.85 (1H, m, H-7), 5.97 (1H, d, J=1.1 Hz, H-1), and 6.16 (1H, qd, J=7.2, 1.2 Hz, H-3ʹ), two oxymethine protons at δH 3.74 (1H, br s, H-3) and 5.26 (1H, br s, H-5), a pair of oxymethylene protons at δH 4.49 (1H, d, J=11.9 Hz, H-17a) and 4.59 (1H, d, J=11.9 Hz, H-17b), together with six methyl groups at δH 1.23 (3H, s, Me-16), 1.00 (3H, d, J=7.0 Hz, Me-18), 1.82 (3H, s, Me-19), 1.52 (3H, s, Me-20), 1.99 (3H, dq, J=7.2, 1.2 Hz, Me-4ʹ), and 1.94 (3H, s, Me-5ʹ). The above 1H NMR data combined with the carbon resonances at δC 16.0, 20.7, 126.9, 140.2, 167.1; 128.3, 129.5, 130.3, 132.9, and 166.8 in the 13C NMR spectrum (Table 1), was suggestive of the existence of an angeloyloxy group and a benzoyloxy group.19 The 13C NMR spectrum in combination with the DEPT spectrum (Supplementary Figure S3) showed 20 other signals: four methyls, two methylenes (one oxymethylene at δC 66.3), eight methines (two oxymethines at δC 77.2 and 80.3, and two olefinic methines at δC 125.0 and 129.6), and six quaternary carbons (including one ketone at δC 206.0, two olefinic at δC 135.2 and 139.5, and one oxygenated at δC 85.2). The aforementioned data suggested that Euph E was structurally similar to (3S,4S,5R,8S,10S,11R,13R,14R,15R)-3β-O-angeloyl-17-benzoyloxy-20-deoxyingenol, a known ingenane-type diterpenoid.19 The major difference was that Euph E had an angeloyloxy group located at C-5, rather than C-3, which was supported by the HMBC correlations (Figure 2, Supplementary Figure S4, S5) from H-5, H-3ʹ, and H-5ʹ to the ester carbonyl carbon at δC 167.1 (C-1ʹ). Moreover, the HMBC correlations from H2-17, H-2’’and H-6’’to the other ester carbonyl carbon at δC 166.8 (C-1’’ suggested that the benzoyloxy group was connected to C-17. Thus, the planar structure of Euph E was constructed according to the above spectroscopic data analysis. The relative configurations of C-3, C-4, C-5, and C-10 were found to be identical to those of known analogues through comparing their chemical shifts and coupling constants and from the key ROESY correlations (Figure 2) of H-3/H-5.20,21 The NOE cross-peaks (Supplementary Figure S6) of H-8/H-11, H-8/H2-17, H2-17/H-11, and H2-17/H-12β indicated that these protons were cofacial and assigned as β-orientation, while the ROESY correlations of Me-18/H-12α, H-13/H-12α, and H-13/H-14 revealed that they adopted an α-orientation. Thus, the structure of Euph E was assigned as depicted in Figure 1.
Table 1.
1H-(600 MHz) and 13C-(150 MHz) NMR Data of Euph E in CDCl3 (δ in Ppm, J in Hz)
| Position | Euph E | |
|---|---|---|
| δH | δC | |
| 1 | 5.97 (d, J=1.1 Hz, 1H) | 129.5 |
| 2 | 139.5 | |
| 3 | 3.74 (br s, 1H) | 80.3 |
| 4 | 85.2 | |
| 5 | 5.26 (br s, 1H) | 77.2 |
| 6 | 135.2 | |
| 7 | 5.85 (m, 1H) | 125.0 |
| 8 | 4.42 (d, J=11.4 Hz, 1H) | 43.5 |
| 9 | 206.0 | |
| 10 | 72.7 | |
| 11 | 2.46 (m, 1H) | 39.4 |
| 12α | 1.90 (m, 1H) | 30.9 |
| 12β | 2.24 (m, 1H) | |
| 13 | 0.98 (m, 1H) | 24.0 |
| 14 | 1.20 (dd, J=12.1, 8.3 Hz, 1H) | 23.8 |
| 15 | 27.9 | |
| 16 | 1.23 (s, 1H) | 24.5 |
| 17a | 4.49 (d, J=11.9 Hz, 1H) | 66.3 |
| 17b | 4.59 (d, J=11.9 Hz, 1H) | |
| 18 | 1.00 (d, J=7.0 Hz, 1H) | 17.0 |
| 19 | 1.82 (s, 1H) | 15.5 |
| 20 | 1.52 (s, 1H) | 21.4 |
| 1’ | 167.1 | |
| 2’ | 126.9 | |
| 3’ | 6.16 (qd, J=7.2, 1.2 Hz, 1H) | 140.2 |
| 4’ | 1.99 (dq, J=7.2, 1.2 Hz, 1H) | 16.0 |
| 5’ | 1.94 (s, 1H) | 20.7 |
| 1ʹʹ | 166.8 | |
| 2ʹʹ | 130.3 | |
| 3ʹʹ7ʹʹ | 8.06 (d, J=7.0 Hz, 1H) | 129.5 |
| 4ʹʹ6ʹʹ | 7.45 (t, J=7.8 Hz, 1H) | 128.3 |
| 5ʹʹ | 7.57 (t, J=7.4 Hz, 1H) | 132.9 |
Figure 2 .
(A) Key HMBC correlations and (B) ROESY correlation of Euph E.
The structure of Euri A was determined by comparison of its spectroscopic data with those in the literature.14
Characterization of Euph E
White amorphous powder; [α]20.6 D=+13.6 (c 0.10, MeOH); 1H NMR (600 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Table 1; HRESIMS m/z 557.2526 [M+Na]+ (calcd for C32H38NaO7+, 557.2510).
Effects of Euph E and Euri A on Macrophage Cells Viability
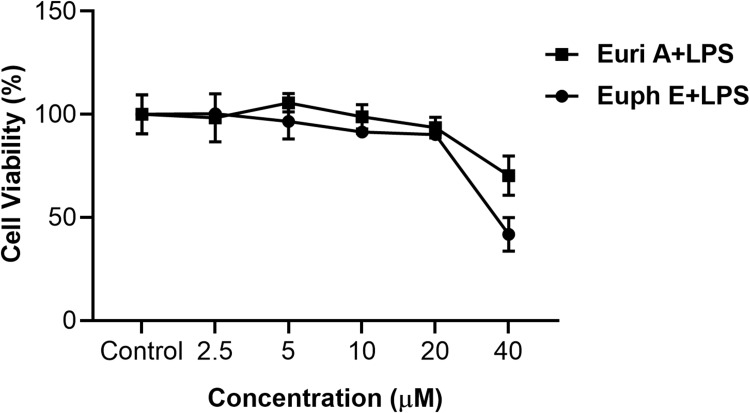
The effect of Euph E and Euri A on macrophage cells viability was studied by a cytotoxicity test using MTT assay. Cells were incubated with Euph E or Euri A at various concentrations (2.5, 5, 10, 20, and 40 µM). The results showed that compounds displayed cytotoxicity when the concentration of them was above 20 µM, but did not significantly affect the viability of RAW264.7 cells compared to untreated controls at 2.5 μM to 20 μM (Figure 3). Thus, the concentration of 20 μM of Euph E and Euri A was used as the maximum dose in subsequent experiments.
Figure 3 .
Effects of compounds on cell viability in RAW264.7 macrophages. Cells were treated with Euph E or Euri A (2.5-40 μM) and LPS (1 µg/mL) for 24 h; Cell viability was measured by MTT assay. Values are expressed as means ± SD of three independent experiments.
Effects of Euph E and Euri A on NO Production and iNOS Expression
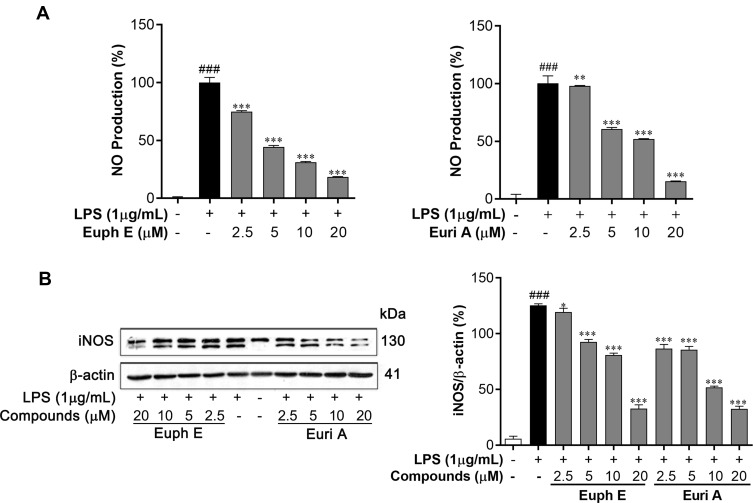
In order to investigate the effects of Euph E and Euri A on macrophage function, we first measured the levels of nitrite in supernatants to investigate the ability of RAW264.7 macrophages to secrete NO in response to LPS stimulation. As shown in Figure 4A, the production of NO was significantly inhibited in a concentration-dependent manner (2.5–20 μM) with the treatment of Euph E or Euri A. The half maximal inhibitory concentration (IC50) values of them were 6.37 μM and 5.78 μM, respectively, compared with the positive control L-NMMA (NG-Monomethyl-L-arginine, an iNOS inhibitor), with an IC50 value of 16.13 μM. Furthermore, iNOS, a key enzyme that promotes the production and circulation of nitric oxide in the body, was investigated in LPS-induced cells by Western blot analyses. Comparing with LPS-stimulation only, Euph E or Euri A treatment significantly suppressed the protein expression of iNOS in macrophages (Figure 4B). In conclusion, Euph E and Euri A could inhibit LPS-induced NO production, probably through down-regulating iNOS protein expression.
Figure 4 .
Euph E and Euri A inhibit NO production and iNOS expression. Cells were pretreated with Euph E or Euri A (2.5-20 μM) and then co-incubated with LPS (1 μg/mL). (A) NO production was determined by measuring the level of nitrite in culture medium. (B) The expression of iNOS protein was determined by Western blot analysis. Actin served as an internal control. The values represent the mean ± SD of three independent experiments (Supplementary Figure S7). Compared with control group, ###P<0.001; Compared with LPS group, *P<0.05, ***P<0.001.
Euph E and Euri A Reduced Secretion of IL-1β and IL-6
Based upon the inhibitory effect of Euph E and Euri A on the levels of NO and iNOS, ELISA assay was performed to evaluate the regulatory effects of compounds on inflammatory cytokines, such as IL-1β and IL-6. As shown in Figure 5, LPS (1 μg/mL) stimulation significantly up-regulated IL-1β and IL-6 production of RAW264.7 cells compared to the control group (p<0.001), whereas pretreating with Euph E or Euri A (2.5–20 μM) markedly inhibited LPS-induced secretion of IL-1β and IL-6 in a dose-dependent manner (p<0.001 or p<0.05). These results showed that Euph E and Euri A exhibited significant down-regulation effects on IL-1β and IL-6.
Figure 5 .
Euph E and Euri A reduced IL-1β and IL-6 secretion. Cells were pretreated with Euph E or Euri A (2.5-20 μM) and then co-incubated with LPS (1 μg/mL) for 24 h. The secretion of (A) IL-1β and (B) IL-6 in culture medium were determined using ELISA kits. The values represent the mean ± SD of three independent experiments. Compared with control group, ###P<0.001; Compared with LPS group, *P<0.05,**P<0.01, ***P<0.001.
Euph E and Euri A Suppressed NF-κB Signaling Pathway in Macrophages
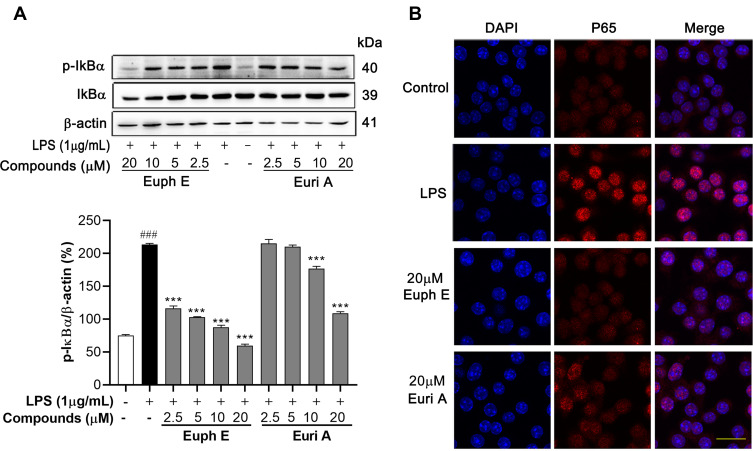
It is widely believed that bacterial products trigger a series of intracellular signal transduction by binding to various cell surface receptors in LPS-induced inflammatory response, resulting in activation of NF-κB.22 Therefore, we examined the effects of Euph E and Euri A on the NF-κB signaling pathway. As shown in Figure 6A, LPS stimulation induced the IκBα phosphorylation in RAW264.7 cells, and when Euph E and Euri A were added prior to LPS treatment, it could be significantly suppressed (when concentrations >10 μM, p<0.001). We next investigated whether compounds also inhibit the translocation of the NF-κB/P65 subunit from cytosol to nucleus, as P65 is a major participant in NF-κB activation in LPS-activated macrophages. Immunofluorescent staining of NF-κB showed that LPS stimulation resulted in a greater proportion of P65 translocated to the nucleus. However, compounds pretreatment effectively suppressed p65 nuclear accumulation (Figure 6B). This suggested that the regulating effect of Euph E and Euri A on LPS-induced inflammatory mediators expression (NO, IL-1β, IL-6, and iNOS) might be closely related to the role of blocking the activation of NF-κB signaling pathways.
Figure 6 .
Effects of compounds on IκBα/NF-κB signaling pathway in macrophages. Cells were pretreated with Euph E or Euri A for 1 h, followed by co-incubated with LPS (1 μg/mL) for 30 min. (A) The protein levels of phospho-IκBα and IκBα were detected by Western blot analysi. Actin was probed as an internal control. The experiments were performed in triplicate (Supplementary Figure S7). Compared with control group, ###P<0.001; Compared with LPS group, ***P<0.001. (B) The nuclear translocation of NF-κB p65 was detected by immunofluorescence. Nuclei were stained with DAPI (blue). Scale bar=20 μm.
Euph E and Euri A Up-Regulated PGE2 and TNF-α Secretion and COX-2 Expression
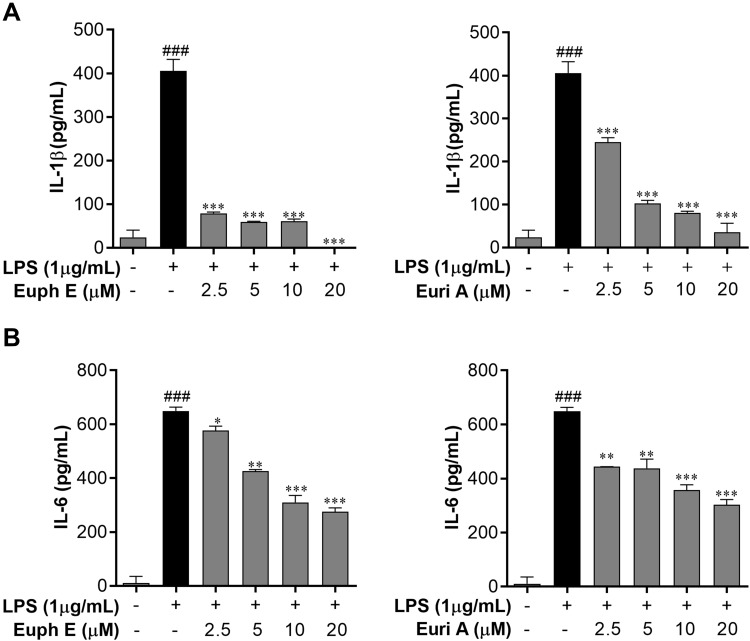
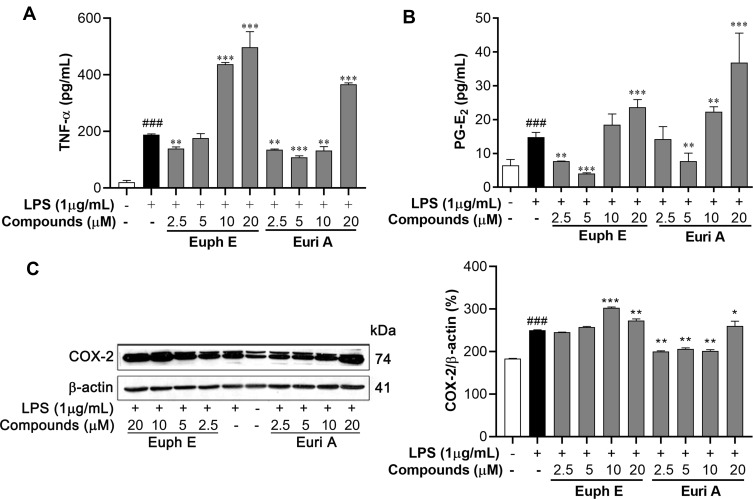
In view of the regulatory effects of the Euph E and Euri A on inflammatory factors in the above results, we further examined the effects of the compounds on PGE2 and TNF-α. Interestingly, as shown in Figure 7A, the expression of TNF-α and PGE2 were up-regulated by treatment of Euph E or Euri A at 10–20 μM (p<0.001) (Figure 7B). Accordingly, the expression of COX-2, a critical rate-limiting enzyme for regulating PGE2,23 was also significantly promoted by Euph E or Euri A on stimulation in macrophages (p<0.05), which was confirmed by Western blotting assay (Figure 7C). These results indicated that compounds at higher concentrations could promote the production of markers associated with the activation of classically activated macrophages, which might contribute to the enhancement of phagocytic function and immune defense function of macrophages.
Figure 7 .
Euph E and Euri A promoted PGE2 and TNF-α secretion and COX-2 expression. Cells were pretreated with Euph E or Euri A (2.5-20 μM) for 1 h and then co-incubated with LPS (1 μg/mL). The production of (A) TNF-α and (B) PGE2 in culture medium were measured using ELISA kits. (C) The expression of COX-2 protein was determined by Western blot analysis. Values are expressed as means ± SD of the three independent experiments (Supplementary Figure S7). Compared with control group, ###P<0.001; Compared with LPS group, *P<0.05, **P<0.01, ***P<0.001.
Euph E and Euri A Promoted LPS-Induced MAPK Pathways Activation
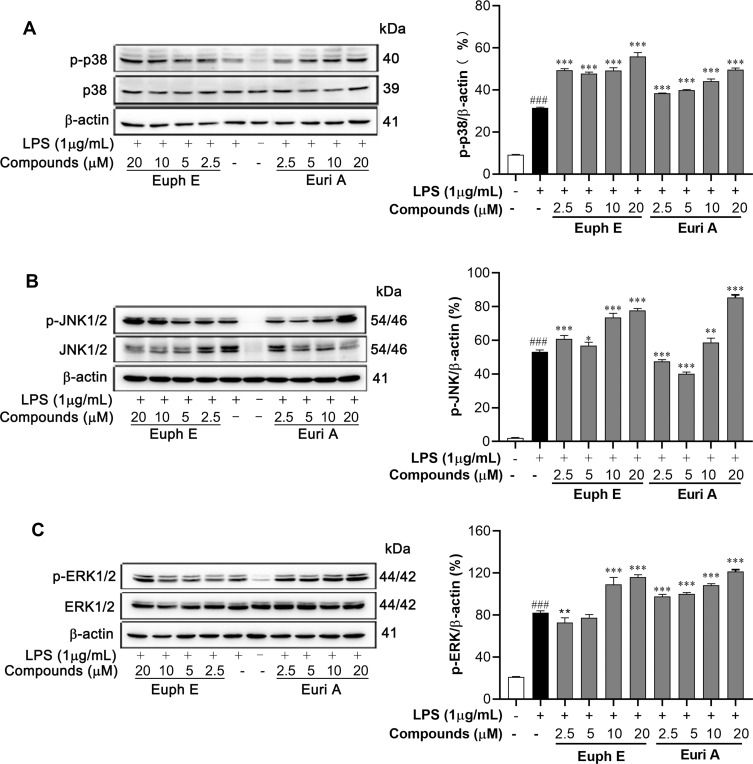
The MAPKs signaling pathways, which are closely related to regulating of the above cytokines,24 were detected by Western blotting. As shown in Figure 8, the levels of phosphorylated ERK, JNK, and p38 MAPK were significantly enhanced by compounds stimulation, compared to that measured in the macrophages treated with LPS alone (p<0.05). Therefore, it provided a reasonable explanation that our compounds might promote the growth and differentiation of macrophages and the secretion of some cytokines by increasing the phosphorylation of MAPK signaling pathway.
Figure 8 .
Euph E and Euri A promoted MAPK pathways activation. Cells were pretreated with compounds (2.5-20 μM) for 1 h, then were stimulated with LPS (1 μg/mL) for 30 min. Protein extracts were analyzed by Western blot analyses using (A) anti-phospho-p38MAPK, anti-p38MAPK, (B) anti-phospho-JNK, anti-JNK, (C) anti-phospho-ERK and anti-ERK antibodies against the activated MAPKs. Actin was assessed as a loading control. The data shown are representative of at least three independent experiments (Supplementary Figure S8). Compared with control group, ###P<0.001; Compared with LPS group, *P<0.05, **P<0.01, ***P<0.001.
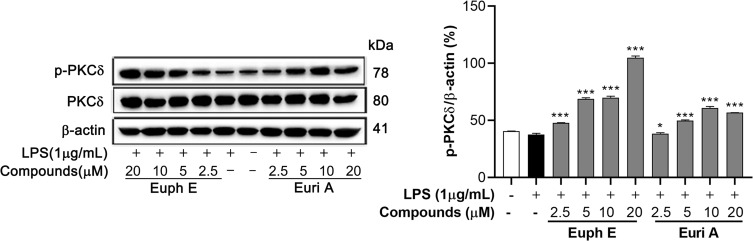
Euph E and Euri A Promoted PKCδ Phosphorylation in Macrophages
Based on the results about MAPKs, we examined the regulatory effects of Euph E and Euri A on upstream kinases – protein kinase C, a family of signaling isoenzymes that played critical roles in regulating cell proliferation, differentiation, and apoptosis.25,26 As shown in Figure 9, compounds significantly induced the phosphorylation of PKCδ in a dose-dependent manner (p<0.05) in RAW264.7 macrophages, suggesting that the compounds might be an agonist of PKC isoenzymes.
Figure 9 .
Euph E and Euri A promoted PKCδ activation in macrophages. Cells were co-incubated with LPS (1 μg/mL) for 30 min after pretreated with Euph E or Euri A for 1 h. Protein extracts were then analysed for PKC-δ phosphorylation by Western blotting. Actin was assessed as a loading control. The experiments were performed in triplicate (Supplementary Figure S9). Compared with LPS group, *P<0.05, ***P<0.001.
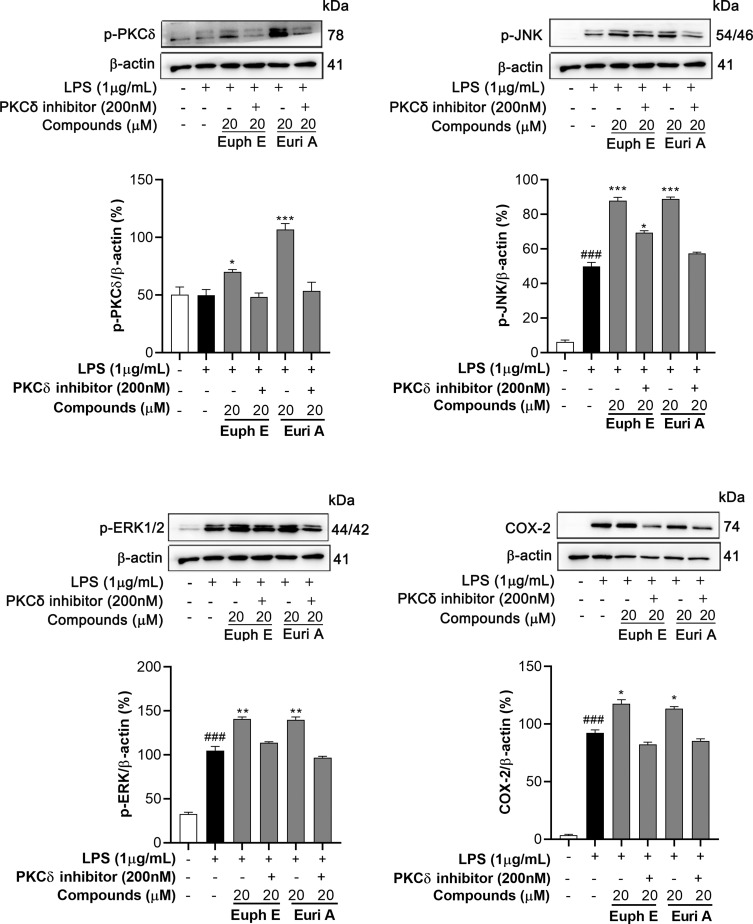
Immune Activation Induced by Euph E and Euri A Could Be Inhibited by PKCδ Inhibitors
To check whether the activation of PKCδ is related to the up-regulation of downstream MAPKs signaling pathway and inflammatory mediators such as COX-2, RAW264.7 cells were preincubated with compounds (20 µM) in the absence or presence of PKCδ inhibitors (200 nM) and then stimulated with 1 µg/mL LPS. We measured the phosphorylation of PKCδ, JNK, and ERK, and the expression of COX-2 using Western blotting assays. The experiment results showed that PKCδ inhibitors significantly inhibited phosphorylation of PKCδ induced by Euph E or Euri A. Correspondingly, the phosphorylation levels of JNK and ERK which were significantly up-regulated by compounds treatment were obviously inhibited in the presence of PKCδ inhibitors, also suppressed was the expression of COX-2 (Figure 10). These results revealed that PKCδ might be a key target of compounds induced immune activation of macrophages.
Figure 10 .
PKCδ inhibitors inhibited compounds inducing immune activation. Cells were preincubated with compounds (20 µM) in the absence or presence of PKCδ inhibitors (200 nM) for 1 h and stimulated with 1 μg/mL LPS for 30 min (for p-PKCδ, p-JNK and p-ERK) or 18 h (for COX-2). Actin was assessed as a loading control. Compared with control group, ###P<0.001; Compared with LPS group, *P<0.05, **P<0.01, ***P<0.001.
Discussion
Macrophages are one of the most important immune cells with strong plasticity and can be activated into different types to perform different functions due to the different microenvironments and activators. Imbalance of macrophage subsets is associated with the occurrence and progression of autoimmune diseases, tumors, and other diseases.27 Therefore, the regulation of macrophage activity plays an important role in maintaining the stability of the body’s internal environment and regulating innate immunity.
Activated macrophages are known to release a variety of cytokines that affect the proliferation, differentiation, and function of immune cells, thereby regulating cellular and humoral immune responses, and they can also act as killers of microorganisms and tumor cells.28 In previous studies, we found that abietane-type, pimarane-type, and atisane-type diterpenoids isolated from the Euphorbia neriifolia have significant anti-inflammatory activity,29 which well confirmed the good anti-inflammatory activity of E. neriifolia in traditional use. Likewise, Euph E and Euri A also showed strongly anti-inflammatory activity. iNOS are considered as the main enzyme catalyzing the production of NO among the three isoforms of nitric oxide synthase (NOS). The release of NO is conducive for activated macrophages to kill invading pathogens and tumor cells,30,31 at the same time, its excessive production is also an important reason for causing damage to adjacent tissues and cells, inducing abnormal dilatation of blood vessels and lymphatic vessels and increasing permeability. IL-1β and IL-6 are the most important cytokines secreted by macrophages, which mediate a variety of biological functions and are involved in the response to infection, injury, and inflammation,32,33 and the overexpression of which always tends to promote tumorigenesis and inflammatory damage. In our study, Euph E and Euri A markedly inhibited the production of NO, IL-1β, and IL-6 and the expression of iNOS in the LPS-induced RAW264.7 cells, these results provided important evidence for the good anti-inflammatory activity of the compounds.
Increasing evidence indicates that NF-κB is a key transcription factor that regulates the expressions of inflammatory mediators, including NO, IL-1β, IL-6, and others.34 NF-κB is usually present in the cytosol before the cells are stimulated. When cells are exposed to inflammatory stimuli, such as LPS, the phosphorylation and degradation of the IκB-α protein promote NF-κB transcription into the nucleus and activate the transcription of inflammatory genes. Our studies found that Euph E and Euri A inhibited the phosphorylation of IκBα and the translocation of the p65 subunit of NF-κB from the cytosol to the nucleus in macrophages, which indicated that NF-κB dependent cascade was involved in the signaling leading to compounds-induced inflammatory mediator downregulation.
Our results also showed that Euph E and Euri A significantly increased the releases of TNF-α, PGE2, and the expression of COX-2 in macrophages. TNF-α and COX-2, as pro-inflammatory cytokines secreted by macrophages, are involved in a variety of immune and inflammatory responses, including chronic inflammation, malignant tumors, and other diseases.35 The expression level of them is an important indicator of immune function of macrophages and monocytes, and also affects the polarization of macrophages and the prognosis of tumor therapy.36,37 Euphorbia neriifolia is usually easy to cause primary irritation, swelling, inflammation, and allergic reactions to the skin and mucous membranes during traditional use, which is probably closely related to the up-regulation of various proinflammatory factors mentioned above. This group of Ingenane-type diterpenoids might play an important role in further activation of macrophages and enhancement of immune response.
Our further results proved that Euph E and Euri A dramatically increased PKCδ phosphorylation, thereby activating the PKC signaling pathway. PKCδ belongs to the novel subtype of PKC isoenzymes family, which widely distributes in mammalian tissues and cells and plays an important biological role in cell growth, metabolism, proliferation, and differentiation.38 Combined with the structural information of the compounds, Euph E and Euri A were structural analogues of PEP005, which is considered as an effective activator of PKCs. It indicated that the signaling mechanism of the compounds may be related to the regulation of PKC isoenzymes. The activation of PKCs regulates downstream cellular kinases, such as MAPKs,39 which plays a principal role in regulating cell growth and differentiation as well as the secretion of cytokines.40 At least three distinct MAPK protein subfamilies have been found in eukaryotic cells, including ERKs, JNKs, and p38 MAPKs. Once activated by upstream specificity kinases, these MAPKs phosphorylate and activate specific substrates on amino acid residues and affect downstream targets.41 In our study, Euph E and Euri A enhanced the phosphorylation of JNK, ERK, and p38 MAPK in LPS-stimulated RAW264.7 cells. Additionally, previous works had reported that MAPKs cascade was one of the pivotal signaling mechanisms that regulated cytokine induced COX-2 protein expression and prostaglandin biosynthesis.42 Our results also showed that under the action of PKCδ inhibitors, the phosphorylation levels of PKCδ, JNK, and ERK and the expression of COX-2 induced by Euph E and Euri A were significantly inhibited, suggesting that PKCδ was a key target of immune-activated macrophages induced by the compounds. Taken together, we concluded that the compounds probably acted on PKCδ and then activated the MAPK signaling pathway and downstream cytokines, which further actively regulate phagocytic and immune functions of macrophages.
Conclusion
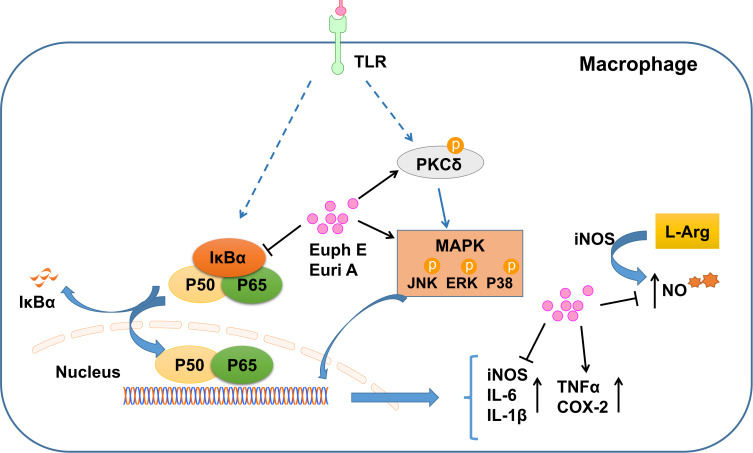
Taken together, our current studies demonstrated that Euph E and Euri A provided a pivotal signaling transduction mechanism that might be involved in the regulation of macrophage function, and were closely related to the regulation of PKCδ/MAPKs and NF-κB signaling pathways (Figure 11). The multidirectional effects of Euph E and Euri A on inflammatory mediators indicated that the compounds complicatedly regulated and balanced the functions of these important inflammatory cytokines and might activate certain negative feedback mechanisms in inflammatory response of macrophages. At the same time, it also reasonably explained the dual effects of immune activation and anti-inflammatory immune suppression in the immunopharmacological activity of Euphorbia neriifolia, and was conducive to the more reasonable development and utilization of it.
Figure 11 .
Schematic of Euph E and Euri A affecting the immnue function of macrophages.
Acknowledgments
This research work was financially supported by a grant from the National Natural Science Foundation of China (No. 81860618, 82073737, 22067012), the Applied Basic Research Projects of Yunnan Province (No. 2018FB031), and the Innovative Team of Yunnan Province (No. 2019HC018).
Disclosure
Dr Dan Liu reports grants from the National Natural Science Foundation of China, grants from the National Natural Science Foundation of China, grants from the National Natural Science Foundation of China, grants from the Applied Basic Research Projects of Yunnan Province, and grants from the Innovative Team of Yunnan Province, during the conduct of the study.
The authors declare that they have no competing interests.
References
- 1.Chen X, Lu J, Zhang Y, et al. Studies of macrophage immuno-modulating activity of polysaccharides isolated from Paecilomyces tenuipes. Int J Biol Macromol. 2008;43(3):252–256. doi: 10.1016/j.ijbiomac.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 2.Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206(1):149–159. doi: 10.1111/j.0105-2896.2005.00288.x [DOI] [PubMed] [Google Scholar]
- 3.Schroder K, Sweet MJ, Hume DA. Signal integration between IFNgamma and TLR signalling pathways in macrophages. Immunobiology. 2006;211(6–8):511–524. doi: 10.1016/j.imbio.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 4.Iwaszkiewicz KS, Schneider JJ, Hua S. Targeting peripheral opioid receptors to promote analgesic and anti-inflammatory actions. Front Pharmacol. 2013;4:132. doi: 10.3389/fphar.2013.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80(6):1298–1307. doi: 10.1189/jlb.0406249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu CY, Jerry Teng CL, Hung PS, et al. Ovatodiolide isolated from anisomeles indica induces cell cycle G2/M arrest and apoptosis via a ROS-dependent ATM/ATR signaling pathways. Eur J Pharmacol. 2018;819:16–29. doi: 10.1016/j.ejphar.2017.09.050 [DOI] [PubMed] [Google Scholar]
- 7.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–550. doi: 10.1038/nrc1388 [DOI] [PubMed] [Google Scholar]
- 8.Palit P, Mandal SC, Bhunia B. Total steroid and terpenoid enriched fraction from Euphorbia neriifolia Linn offers protection against nociceptive-pain, inflammation, and in vitro arthritis model: an insight of mechanistic study. Int Immunopharmacol. 2016;41:106–115. doi: 10.1016/j.intimp.2016.10.024 [DOI] [PubMed] [Google Scholar]
- 9.Qi WY, Gao XM, Ma ZY, Xia CL, Xu HM. Antiangiogenic activity of terpenoids from Euphorbia neriifolia Linn. Bioorg Chem. 2020;96:96. doi: 10.1016/j.bioorg.2019.103536 [DOI] [PubMed] [Google Scholar]
- 10.Benhadji KA, Serova M, Ghoul A, et al. Antiproliferative activity of PEP005, a novel ingenol angelate that modulates PKC functions, alone and in combination with cytotoxic agents in human colon cancer cells. Br J Cancer. 2008;99(11):1808–1815. doi: 10.1038/sj.bjc.6604642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spivak AM, Nell RA, Petersen M, et al. Synthetic ingenols maximize protein kinase C-induced HIV-1 latency reversal. Antimicrob Agents Chemother. 2018;62(11):11. doi: 10.1128/AAC.01361-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li JC, Zhang ZJ, Yang T, et al. Six new ent-abietane-type diterpenoids from the stem bark of Euphorbia neriifolia. Phytochem Lett. 2019;34:13–17. doi: 10.1016/j.phytol.2019.09.003 [DOI] [Google Scholar]
- 13.Li JC, Dai WF, Liu D, et al. Bioactive ent-isopimarane diterpenoids from Euphorbia neriifolia. Phytochemistry. 2020;175:175. doi: 10.1016/j.phytochem.2020.112373 [DOI] [PubMed] [Google Scholar]
- 14.Zhao JX, Liu CP, Qi WY, et al. Eurifoloids A-R, structurally diverse diterpenoids from Euphorbia neriifolia. J Nat Prod. 2014;77(10):2224–2233. [DOI] [PubMed] [Google Scholar]
- 15.Shaker S, Sang J, Yan XL, Fan RZ, Yin SJP. Diterpenoids from Euphorbia royleana reverse P-glycoprotein-mediated multidrug resistance in cancer cells. Phytochemistry. 2020;176:112395. doi: 10.1016/j.phytochem.2020.112395 [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Liu J, Zhao J, Li S, Li CC. Toward the total synthesis of eurifoloid A. Org Lett. 2017;19(10):2742–2745. doi: 10.1021/acs.orglett.7b01102 [DOI] [PubMed] [Google Scholar]
- 17.Rosen R. Clinical development of ingenol mebutate (PEP005) gel for actinic keratosis. 2010.
- 18.Ci XX, Ren R, Xu K, et al. Schisantherin A exhibits anti-inflammatory properties by down-regulating NF-kappa B and MAPK signaling pathways in lipopolysaccharide-treated RAW 264.7 cells. Inflammation. 2010;33(2):126–136. doi: 10.1007/s10753-009-9166-7 [DOI] [PubMed] [Google Scholar]
- 19.Wang P, Xie C, An L, et al. Bioactive diterpenoids from the stems of euphorbia royleana. J Nat Prod. 2019;82(2):183–193. doi: 10.1021/acs.jnatprod.8b00493 [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Li W, Huang L, et al. Identification, structural modification, and dichotomous effects on human immunodeficiency virus type 1 (HIV-1) replication of ingenane esters from Euphorbia kansui. Eur J Med Chem. 2018;156:618–627. doi: 10.1016/j.ejmech.2018.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YS, Lu Y, Chen CH, Lee KH, Chen DF. Potent anti-HIV ingenane diterpenoids from euphorbia ebracteolata. J Nat Prod. 2019;82(6):1587–1592. doi: 10.1021/acs.jnatprod.9b00088 [DOI] [PubMed] [Google Scholar]
- 22.Barré B, Perkins ND. A cell cycle regulatory network controlling NF-kappaB subunit activity and function. EMBO J. 2007;26(23):4841–4855. doi: 10.1038/sj.emboj.7601899 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Zidar N, Odar K, Glavac D, Jerse M, Zupanc T, Stajer D. Cyclooxygenase in normal human tissues–is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J Cell Mol Med. 2009;13(9b):3753–3763. doi: 10.1111/j.1582-4934.2008.00430.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang M, Wang Y, Patel G, et al. In vitro and in vivo anti-inflammatory effects of different extracts from Epigynum auritum through down-regulation of NF-kappa B and MAPK signaling pathways. J Ethnopharmacol. 2020;261. [DOI] [PubMed] [Google Scholar]
- 25.Kanda Y, Satoh R, Takasaki T, et al. Stress granules as a feedback mechanism of MAPK signaling by sequestering PKC/Pck2. J Cell Sci. 2020. [DOI] [PubMed] [Google Scholar]
- 26.Mandal JP, Shiue CN, Chen YC, et al. PKCdelta mediates mitochondrial ROS generation and oxidation of HSP60 to relieve RKIP inhibition on MAPK pathway for HCC progression. Free Radic Biol Med. 2020. [DOI] [PubMed] [Google Scholar]
- 27.Xiong XQ, Geng Z, Zhou B, et al. FNDC5 attenuates adipose tissue inflammation and insulin resistance via AMPK-mediated macrophage polarization in obesity. Metabolism. 2018;83:31–41. doi: 10.1016/j.metabol.2018.01.013 [DOI] [PubMed] [Google Scholar]
- 28.Baron S, Horowitz J, Poast J, et al. Role of interferon-activated macrophages in eradication of human tumor cells by innate immunity. Cytokine. 2009;48(1–2):48. doi: 10.1016/j.cyto.2009.07.134 [DOI] [Google Scholar]
- 29.Xue Y, Yang T, Yan SL, et al. Study on anti-inflammatory activity of diterpenes from Euphorbia neriifolia. J Kunming Univ Sci Tech. 2018;5:84–90. [Google Scholar]
- 30.Mylonas KJ, Nair MG, Prieto-Lafuente L, Paape D, Allen JE. Alternatively activated macrophages elicited by helminth infection can be reprogrammed to enable microbial killing. J Immunol. 2009;182(5):3084–3094. doi: 10.4049/jimmunol.0803463 [DOI] [PubMed] [Google Scholar]
- 31.Thakur A, Schalk D, Tomaszewski E, et al. Microenvironment generated during EGFR targeted killing of pancreatic tumor cells by ATC inhibits myeloid- derived suppressor cells through COX2 and PGE(2) dependent pathway. J Transl Med. 2013;11:11. doi: 10.1186/1479-5876-11-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snoussi K, Strosberg AD, Bouaouina N, Ben Ahmed S, Chouchane L. Genetic variation in pro-inflammatory cytokines (interleukin-1 beta, interleukin-1 alpha and interleukin-6) associated with the aggressive forms, survival, and relapse prediction of breast carcinoma. Eur Cytokine Netw. 2005;16(4):253–260. [PubMed] [Google Scholar]
- 33.Mobasheri A, Shakibaei M. Is tendinitis an inflammatory disease initiated and driven by pro-inflammatory cytokines such as interleukin 1β? Histol Histopathol. 2013;28(8):955–964. doi: 10.14670/HH-28.955 [DOI] [PubMed] [Google Scholar]
- 34.Kim JB, Han AR, Park EY, et al. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappaB inactivation in RAW 264.7 macrophage cells. Biol Pharm Bull. 2007;30(12):2345–2351. doi: 10.1248/bpb.30.2345 [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Ye X, Zhang Y, Ji F. Flurbiprofen suppresses the inflammation, proliferation, invasion and migration of colorectal cancer cells via COX2. Oncol Lett. 2020;20(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W, Wang M, Cheng W, et al. Bioactive antiinflammatory antibacterial hemostatic citrate-based dressing with macrophage polarization regulation for accelerating wound healing and hair follicle neogenesis. Bioact Mater. 2021;6(3):721–728. doi: 10.1016/j.bioactmat.2020.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiainen S, Tumelius R, Rilla K, et al. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology. 2015;66(6):873–883. doi: 10.1111/his.12607 [DOI] [PubMed] [Google Scholar]
- 38.Merida I, Arranz-Nicolas J, Rodriguez-Rodriguez C, Avila-Flores A. Diacylglycerol kinase control of protein kinase C. Biochem J. 2019;476(8):1205–1219. doi: 10.1042/BCJ20180620 [DOI] [PubMed] [Google Scholar]
- 39.Kamboj K, Jana S, Sharma SK. Mechanisms of protein kinase C-induced sustained activation of extracellular signal-regulated kinase in the hippocampus. Biochem Biophys Res Commun. 2019;520(2):453–458. doi: 10.1016/j.bbrc.2019.10.046 [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Jiang JC, Dai XX, Fan HY. Function and molecular mechanism of mitogen-activated protein kinase (MAPK) in regulating oocyte meiotic maturation and ovulation. Sheng Li Xue Bao. 2020;72(1):48–62. [PubMed] [Google Scholar]
- 41.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000 [DOI] [PubMed] [Google Scholar]
- 42.Guan Z, Baier LD, Morrison AR. p38 mitogen-activated protein kinase down-regulates nitric oxide and up-regulates prostaglandin E2 biosynthesis stimulated by interleukin-1beta. J Biol Chem. 1997;272(12):8083–8089. doi: 10.1074/jbc.272.12.8083 [DOI] [PubMed] [Google Scholar]