Abstract
Objective:
We examined relationships between plasma biomarkers and neurodevelopment in children from Sub-Saharan Africa with perinatal HIV (PHIV) with controlled viremia on antiretroviral therapy (ART).
Design:
Longitudinal retrospective cohort study of children with controlled blood HIV replication.
Methods:
Children (N=213; 57% female) started ART at <3 years old, had neurodevelopmental assessments (cognition, attention/impulsivity, motor proficiency, global executive functions) at 5–11 years, and achieved controlled viremia (HIV-1 RNA <400 copies/mL for ≥9 months before initial assessment). Twenty-three plasma biomarkers were measured at onset of controlled viremia, week 0 (first neurodevelopmental assessment), and week 48 (second neurodevelopmental assessment). Factor analysis was conducted at each time point. Multivariable linear regressions assessed associations between factors and neurodevelopmental scores.
Results:
Median age at week 0 was 7.0 years. Nineteen biomarkers loaded on 6 factors: A (L-10, IFNγ, IFNα2, IL-1β, IL-6, IP-10, TNFα); B (sCD163, sICAM-1, sVCAM-1, CRP); C (sE-selectin, sP-selectin); D (MIP-1β, VEGF-A); E (sCD14, CRP); and F (CX3CL1, MCP-1). Higher Factor B scores were consistently associated with worse cognition and attention/impulsivity, and higher Factor D scores with better attention/impulsivity.
Conclusions:
These results suggest a detrimental effect of increased endothelial cell activation (sICAM-1, sVCAM-1) and monocyte/macrophage scavenger function (sCD163) and a beneficial effect of increased CCR5 ligand and HIV entry blocker MIP-1β and angiogenesis stimulant VEGF concentrations on the neurodevelopment of children with PHIV. The model that emerges is of vascular inflammation leading to neurodevelopmental deficits. The role of persistent HIV replication in the central nervous system also needs to be further explored.
INTRODUCTION
Children with perinatal HIV infection (PHIV) have poorer neurodevelopmental (ND) outcomes compared to their uninfected peers, despite initiation of antiretroviral therapy (ART) in early childhood and sustained virologic control.[1] Soluble biomarkers of monocyte/microglial and endothelial activation may have clinical utility in predicting ND outcomes in children with PHIV.[2–5] Expression of CD163, a marker of monocyte/macrophage scavenger activity, has been associated with developmental delays in a Kenyan cohort of children with PHIV and suppressed viremia who had initiated ART in infancy,[2] but not in a cohort of late-diagnosed and treated school-aged children in Thailand and Cambodia[3], where elevated subsets of activated monocytes following ART initiation were associated with higher neurocognitive scores.[3] Biomarkers of pro-coagulant state (fibrinogen and P-selectin) have been individually associated with adverse ND outcomes in youth with PHIV.[4]
While these studies suggests associations between biomarkers and ND outcomes in children, they are limited due to selective and restrictive panels of biomarkers, analyses at a single time point, research participants in different stages of HIV disease, and variations in treatment status and level of virologic control. Furthermore, most published studies fail to capture the known redundancy of inflammatory and immune responses, such that different individuals may respond to illness or injury with different cytokines or chemokines within the same family.[2–4, 6–7]
One way to account for this complexity is to examine a wide range of candidate biomarkers utilizing a statistical approach that captures potential aggregate effects of subsets of biomarkers. Using this approach, we have previously identified a latent factor based on fibrinogen, CRP, and IL-6 that was negatively associated with a measure of processing speed in a cross-sectional US-based study of youth with virologically suppressed PHIV.[5] The generalizability of this finding was limited, however, due to the small number of biomarkers (n=9), lack of longitudinal data, and assessment of ND outcomes that used only one test (WISC-IV).
In the present hypothesis-generating study, we explore possible pathophysiological pathways and their cross-sectional and longitudinal association with four ND outcomes. The study included assays of 23 biomarkers relevant to monocyte/microglial and endothelial activation and was restricted to children with virologically controlled HIV infection to minimize potential confounding effects of uncontrolled viremia. This manuscript summarizes the identified groupings of biomarkers and their longitudinal association with ND outcomes.
METHODS
Study Sample, Eligibility Criteria and Time Points of Interest
This analysis used data from two studies: P1060[8] (NCT00307151) and P1104s[9] (NCT02140255). P1060 was a randomized controlled trial comparing nevirapine versus lopinavir/ritonavir-based ART initiated in children with PHIV at age 2 months to 3 years at six study sites in South Africa, Malawi, Uganda and Zimbabwe.[8] P1104s, a sub-study of P1060, assessed the feasibility, reliability, and validity of administering a ND assessment battery to children aged 5–11 years. All children with PHIV in P1104s participated in P1060.[9–10]
The analysis focused on P1104s children with PHIV who had controlled viremia, defined as HIV-1 plasma RNA (viral load; VL) <400 copies/mL, for ≥9 months prior to P1104s entry. VLs were assessed every 6 months. Eligible children had at least one VL <400 copies/mL between 6–12 months prior to P1104s entry and no VL ≥1000 copies/mL, and no more than one VL of 400–999, for the 9 months prior to P1104s entry. Children were excluded if ND assessment results were not available at both P1104s entry (+/−6 weeks) and week 48 visits (+/−12 weeks). A VL cutoff of 400 copies/mL was chosen as this was the lower limit of detection for the assay in 2005, when P1060 opened for enrollment.
There were three time points of interest: time of onset of controlled viremia prior to P1104s entry, P1104s entry (week 0), and 48 weeks after P1104s entry (week 48). Start of controlled viremia was defined as the date of the earliest VL <400 copies/mL with no subsequent consecutive measurements ≥400 copies/mL and no 12-month periods with more than one VL ≥1000 copies/mL prior to P1104s entry. Participants with subsequent uncontrolled viremia (consecutive VL ≥400 copies/mL or more than one VL ≥1000 copies/mL) between P1104s entry and their week 48 visit were included in week 0 analyses but excluded from week 48 analyses.
Biomarkers
Plasma samples were collected and stored during P1060. The cryopreserved samples closest to and within six months of each of the time-points of interest were identified, shipped and tested in a central laboratory. Plasma levels of 23 biomarkers were assayed, including acute phase reactants (CRP), adhesion factors (ICAM-1, ICAM-5, VCAM-1), anti-inflammatory cytokines (IL-10), chemoattractants (CX3CL1, MCP-1, MIP-1β), inflammatory cytokines (IFNγ, IFNα2, IL-1β, IL-6, IP-10, TNFα), and markers of endothelial activation (sE-selectin, endothelin-1, VEGF-A), matrix digestion (MMP9), monocyte activation (CD14, CD163), neuronal toxicity (neurofilament-light [NFL]), and pro-coagulant state (fibrinogen, P-selectin). Analytes were measured as per manufacturers’ instructions using the following commercial kits: MMP-9, IFNα2, IFNγ, IL-10, IL-1β, IL-6, IP-10, MCP-1, MIP-1β, TNFα, VEGF-A, CRP (lower limit of detection = 1.33 pg/ml), sICAM-1, sVCAM-1, sE-selectin, sP-selectin (Mesoscale Discovery, chemiluminescence microarrays); fibrinogen, CX3CL1 (Millipore; ELISA); sCD14, sCD163, endothelin-1, sICAM-5, NFL (R&D, ELISA).
Neurodevelopmental Outcomes
The ND measures were previously adapted for pediatric HIV research in Africa, validated for school-age children in Sub-Saharan Africa, and described elsewhere.[1,9–10] Briefly, the Kaufman Assessment Battery for Children, 2nd edition (KABC-II) Mental Processing Index is a standardized composite (mean=100, SD=15) of four cognitive domains (sequential and simultaneous processing, learning ability, and planning ability); higher values indicate better performance. The Tests of Variables of Attention (TOVA) D-prime is an unstandardized signal detection measure assessing attention and impulsivity based on correct responses to signal and withholding of responses to non-signal in proportion to incorrect responses; TOVA D-prime can range from 0 to 10 with higher values indicating better performance.[11–12] The Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition (BOT-2) is a standardized measure (mean=50, SD=10) assessing fine-motor precision, fine-motor integration, manual dexterity, bilateral coordination, balance, running speed and agility, upper-limb coordination and strength; higher values indicate better performance. The Behavior Rating Inventory of Executive Function (BRIEF), Parent Form, Global Executive Composite (GEC) is a standardized measure of parental perceptions of their child’s executive functioning (mean=50, SD=10); higher values indicate poorer performance. For this analysis, the BRIEF GEC was subtracted from 100 (reverse scored) so that higher values indicated better performance. Consistent with previous research,[10] the performance on the KABC-II, BOT-2, and BRIEF was age-standardized using American norms, while TOVA D-prime outcomes were adjusted for age and sex.[9–10]
Statistical Methods
ND measures and biomarker levels at each time point and changes between time points were summarized. Changes greater than zero indicated that the measurement at the later time point was larger than the measurement at the earlier time point. Paired t-tests were used to assess changes in ND measurements between week 0 and week 48.
Biomarker results were log10-transformed and standardized (mean=0, SD=1) to put all measures on the same scale prior to conducting factor analyses. Factor loadings were calculated using varimax rotations. The number of factors was selected after evaluating scree plots and cumulative variance plots.
After reviewing the resulting seven factors at each time point, we observed six groupings of biomarkers that remained essentially unchanged across timepoints. Therefore, instead of analyzing seven different factors at each of the three timepoints, we simplified the approach post hoc by focusing our exploratory analysis on these six groupings of biomarkers, which we refer to as “consensus factors”, defined as groupings of biomarkers with loadings >0.4 on the same factor at ≥2 time points. Typically, factor loadings above 0.40 are considered meaningful loadings.[13] The consensus factors were labeled “A” through “F”.
For each consensus factor, we checked for consistency in directionality of the biomarkers. If a factor loading was negative, the corresponding biomarker value was multiplied by −1 to convert it to the same numerical standard. Consensus factor scores for a given participant at each time point were calculated by summing the standardized values of that participant’s biomarker measurements in each grouping and dividing by the number of biomarkers in the grouping.
Multivariable linear regression models were fit to assess associations between the consensus factor scores and ND outcomes. For each consensus factor, separate models were fit using each of the four ND outcomes at week 0, week 48 and the change in ND outcomes between weeks 0 and 48. Each model was adjusted for study site, sex, age at P1104s entry, age at ART initiation, ART regimen at time of biomarker specimen collection (LPV/r-based, NVP-based, or other), and whether the participant switched regimens between P1060 entry and biomarker specimen collection (a proxy for virologic failure). For models involving the change between weeks 0 and 48, we also adjusted for consensus factor scores at week 0. We report regression coefficient estimates and 95% confidence intervals, highlighting estimates whose 95% confidence interval did not contain zero. We used a complete case analysis approach to handle data. Participants that were missing any biomarker measurement at a given time point were excluded from the factor analyses and regression models corresponding to that time point. Given the exploratory nature of this study, no adjustments were made for multiple comparisons.[14–16] Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA, 2013) and graphics generated in R version 3.2.2 (http://www.R-project.org).
RESULTS
Participant Characteristics
Of 246 children with PHIV who were enrolled in both P1060 and P1104s, 213 were included in this analysis (Supplemental Figure 1). Excluded children either had uncontrolled viremia in the 9 months prior to P1104s entry (n=25) or did not have biomarker results at any of the key time points (n=8). The remaining 213 children had at least one ND result at both weeks 0 and 48; two children were removed from week 48 analyses due to loss of virologic control following P1104s entry. Table 1 shows demographic and clinical characteristics of the cohort. Supplemental Table 1 shows ART treatment history across multiple time points.
Table 1:
Characteristics of children with PHIV (N=213) included in this analysis.
| Valuea | |
|---|---|
| Siteb | |
| South Africa: Harriet Shezi | 25 (12%) |
| South Africa: Soweto (PHRU) | 37 (17%) |
| South Africa: Tygerberg | 48 (23%) |
| Malawi: Lilongwe | 27 (13%) |
| Uganda: Kampala | 31 (15%) |
| Zimbabwe: Harare | 45 (21%) |
| Sex | |
| Male | 92 (43%) |
| Female | 121 (57%) |
| Age at initiation of ART (yrs) | 1.2 (0.5, 2.6) |
| P1060 treatment arm | |
| NVP-based regimen | 109 (51%) |
| LPV/r-based regimen | 104 (49%) |
| Age at P1104s entry (yrs) | 7.0 (5.7, 8.7) |
| ART regimen at P1104s entry | |
| NVP-based regimen | 63 (30%) |
| LPV/r-based regimen | 143 (67%) |
| Other | 7 (3%) |
| CD4%, nadir | 14.8 (7.7, 24.6) |
| Weight z-score at P1104s entry | −0.72 (−1.91, 0.47) |
| Height z-score at P1104s entry | −1.01 (−2.24, 0.28) |
| Years aviremic before P1104s entry | 5.0 (0.8, 7.8) |
| Caregiver at entry | |
| Biological mother | 181 (85%) |
| Other | 32 (15%) |
| Socio-economic indexc | 6.1 (2.1, 10.0) |
| Premature birth (< 37 weeks) | 20 (9%) |
| Birthweight < 2000 grams | 11 (5%) |
Value corresponds to median (10th, 90th percentile) for continuous variables and frequency (percentage) for categorical variables.
All participants with PHIV in P1104s were previously enrolled in P1060 from six study sites in : South Africa (Wits RHI Shandukani clinic [Johannesburg], Chris Hani HIV Unit [Soweto], Family Clinical Research Unit [Cape Town]), Malawi (Kamuzu Central Hospital HIV clinic [Lilongwe]), Uganda (Makerere University – Johns Hopkins University Clinic Mulago National Referral Hospital [Kampala]), and Zimbabwe (Parirenyatwa General Hospital [Harare]).
The socioeconomic index is a composite of caregiver education, fuel and water sources, sufficiency of household income for basic needs, caregiver education and work status, major source of information, and whether household has a refrigerator. Values range from 0 (lower SES) to 10 (higher SES).
ND Results over Time
Mean ND scores at week 0 were: 73.1 for KABC-II (95%CI: 71.7, 74.5), 48.4 for BOT-2 (95%CI: 47.2, 49.6), 2.4 for TOVA D-prime (95%CI: 2.3, 2.6), and 47.2 for BRIEF (95%CI: 45.4, 49.0). There were statistically significant changes between weeks 0 and 48 in three of four ND measures: BOT-2 (p = 0.016), TOVA D-prime (p < 0.001), and BRIEF (p < 0.001). Between weeks 0 and 48, mean [95% CI] scores increased 1.0 for the BOT-2 (0.2, 1.9), 0.6 for TOVA D-prime (0.5, 0.8), and 5.2 for the BRIEF (3.5, 6.8) and decreased 0.3 (−1.4, 0.8) for the KABC-II (Figure 1).
Figure 1: Boxplots of ND Test Results by Week.
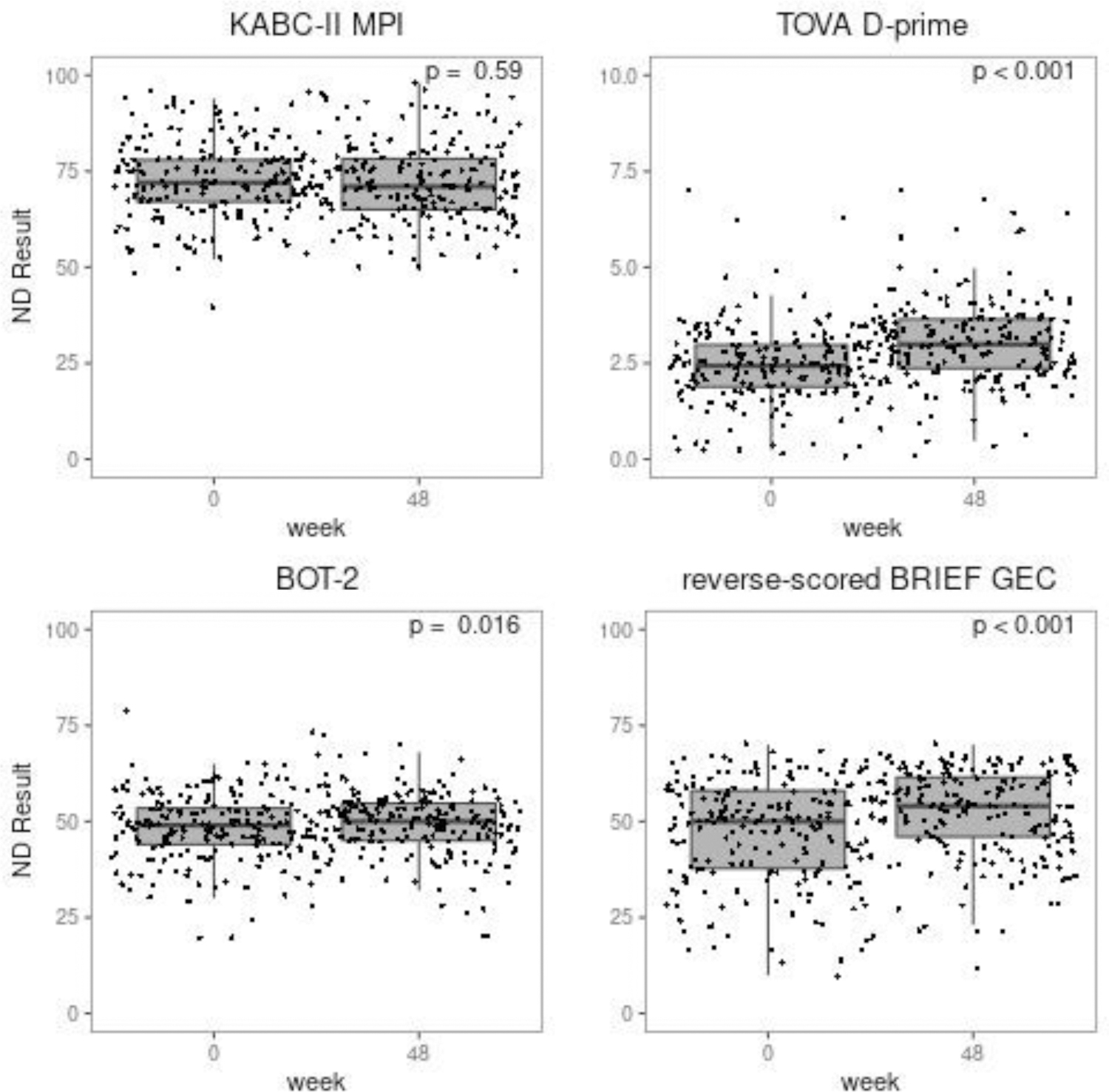
ND test results at weeks 0 and 48.
KABC-II MPI: Kaufman Assessment Battery for Children, 2nd edition; TOVA D-prime = Tests of Variables of Attention D-prime; BOT-2: Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition; BRIEF GEC: Behavior Rating Inventory of Executive Function, Global Executive Composite;
Biomarkers over Time
Boxplots for the 23 biomarkers at the three time points are provided in Figure 2. Ninety-two percent of sICAM-5 values were below the lower limit of detection (LLOD); all other biomarkers had less than 8% of values above or below the corresponding upper or lower detection limits.
Figure 2: Boxplots of biomarkers at three time points (start of controlled viremia, week 0, week 48).
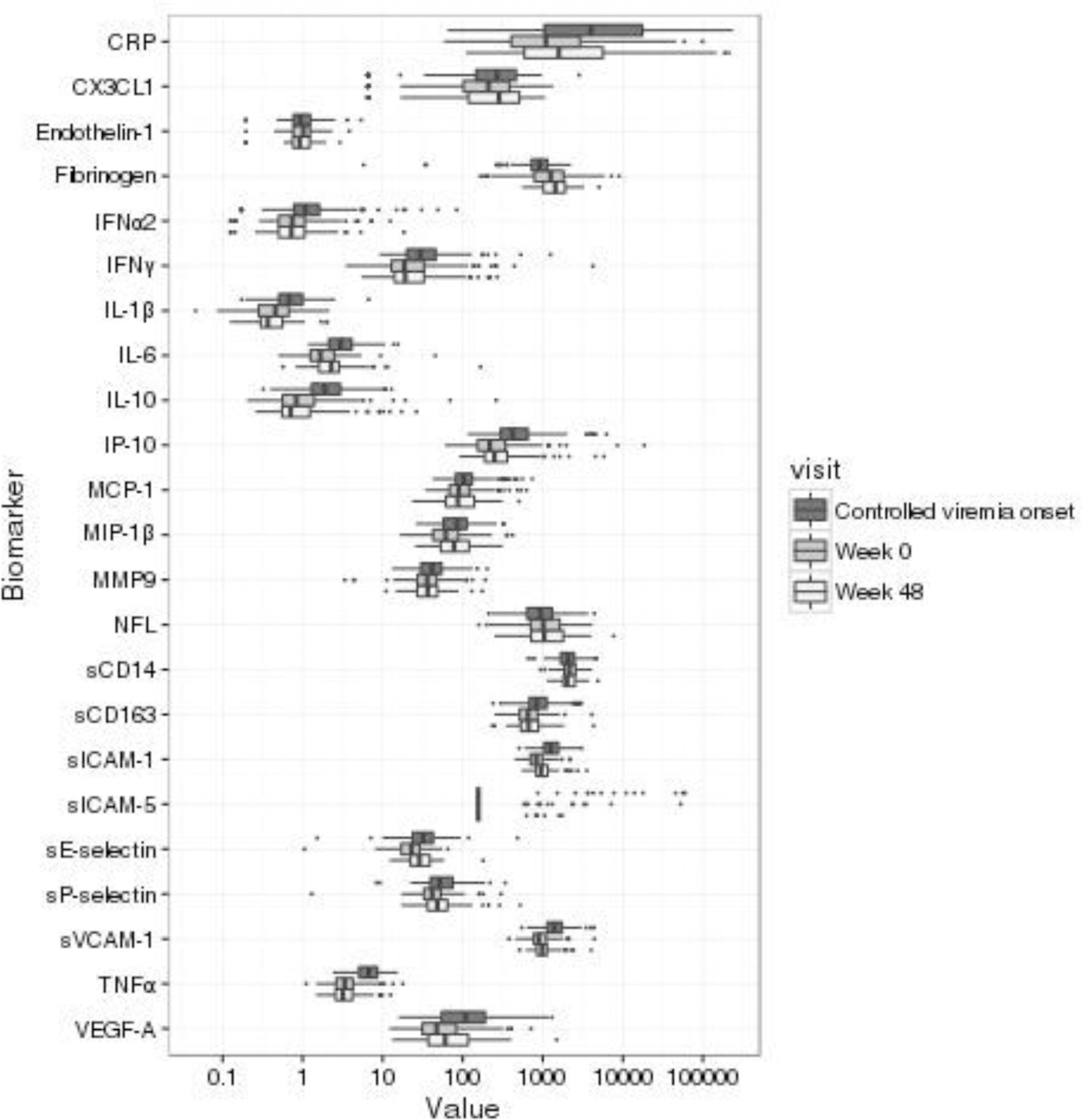
Numbers of participants with available specimens by time point: controlled viremia onset (n = 184), week 0 (n = 185), week 48 (n = 91);
CRP = C-Reactive Protein; CX3CL1 = C-X3-C Motif Chemokine Ligand 1; IL-10 = Interleukin 10; IFNα2 = Interferon α2; IFNγ - Interferon γ; IL-1β = Interleukin 1β; IL-6 = Intereleukin 6; IP-10 = interferon-γ inducible protein 10; MCP-1 = Monocyte Chemoattractant Protein 1; MIP-1β = Macrophage Inflammatory Protein 1β; MMP9 = Matrix Metallopeptidase 9; NFL=neurofilament-light; sCD14 = soluble Cluster of Differentiation 14; soluble Cluster of Differentiation 163; sICAM-1 = soluble Intercellular Adhesion Molecule 1; sICAM-5 = soluble Intercellular Adhesion Molecule 5; sVCAM-1 = soluble Vascular Cell Adhesion Molecule 1; sE-selectin = soluble E-selectin; sP-selectin = soluble P-selectin; TNFα = Tissue Necrosis Factor α; VEGF-A = Vascular endothelial growth factor A;
Biomarker Reduction using Factor Analysis
Two biomarkers (sICAM-5 and endothelin-1) were not included in the factor analyses because of high rates of values below LLOD (92%) or missing observations (30%), respectively, leaving 21 biomarkers for the factor analysis. Additionally, missing data excluded 15 of 184 (8%) children from factor analyses at the start of controlled viremia, 6 of 185 (3%) at week 0, and 8 of 91 (8%) at week 48.
Eigenvalues and scree plots from each factor analysis suggested six or seven factors at each time point. The total variance explained by seven factors was between 68% and 74%. Factor loadings for each biomarker at each time point are shown in Table 2.
Table 2:
Factor loadings from a seven-factor analysis using principal components method and varimax rotation by each time point
| Start of controlled viremia | Week 0 | Week 48 | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker | F1 | F2 | F3 | F4 | F5 | F6 | F7 | Communality | F1 | F2 | F3 | F4 | F5 | F6 | F7 | Communality | F1 | F2 | F3 | F4 | F5 | F6 | F7 | Communality |
| IFNα2 | 0.77 | −0.08 | 0.21 | −0.03 | 0.23 | −0.00 | 0.02 | 0.69 | 0.79 | 0.07 | 0.03 | −0.13 | −0.22 | 0.02 | 0.04 | 0.70 | 0.80 | 0.04 | −0.13 | 0.22 | 0.15 | −0.03 | 0.21 | 0.77 |
| IFNγ | 0.76 | −0.01 | 0.23 | −0.21 | 0.02 | −0.09 | 0.13 | 0.69 | 0.82 | 0.13 | −0.12 | 0.05 | −0.03 | 0.09 | −0.09 | 0.72 | 0.88 | 0.05 | 0.07 | 0.06 | 0.04 | 0.08 | −0.04 | 0.79 |
| IL-10 | 0.74 | 0.22 | 0.00 | 0.00 | 0.13 | 0.03 | −0.08 | 0.62 | 0.81 | −0.01 | −0.09 | 0.04 | 0.13 | 0.04 | 0.12 | 0.70 | 0.72 | 0.24 | 0.17 | −0.18 | 0.35 | −0.17 | −0.08 | 0.80 |
| IL-1β | 0.77 | 0.02 | −0.09 | 0.23 | −0.07 | 0.07 | −0.01 | 0.66 | 0.66 | 0.17 | 0.19 | −0.16 | −0.46 | −0.18 | −0.07 | 0.77 | 0.81 | −0.08 | −0.12 | 0.23 | −0.20 | 0.11 | 0.11 | 0.80 |
| IL-6 | 0.51 | 0.15 | 0.61 | 0.14 | −0.16 | −0.00 | 0.03 | 0.71 | 0.89 | 0.06 | 0.13 | −0.09 | 0.03 | 0.05 | −0.09 | 0.84 | 0.78 | −0.06 | −0.25 | −0.01 | −0.22 | 0.21 | 0.00 | 0.76 |
| IP-10 | 0.89 | 0.14 | 0.15 | 0.04 | −0.04 | 0.07 | −0.04 | 0.84 | 0.90 | 0.13 | −0.02 | 0.01 | −0.01 | 0.07 | −0.08 | 0.84 | 0.85 | 0.15 | 0.12 | 0.04 | −0.03 | 0.22 | −0.01 | 0.81 |
| TNFα | 0.73 | 0.22 | −0.12 | 0.22 | 0.27 | 0.04 | 0.04 | 0.73 | 0.88 | 0.12 | 0.06 | 0.05 | −0.03 | −0.03 | 0.07 | 0.80 | 0.80 | 0.06 | 0.12 | −0.03 | 0.35 | −0.05 | 0.07 | 0.79 |
| sCD163 | −0.13 | 0.70 | 0.15 | 0.09 | 0.05 | 0.08 | 0.06 | 0.55 | 0.05 | 0.44 | 0.16 | 0.01 | 0.62 | −0.26 | −0.03 | 0.67 | 0.21 | 0.69 | 0.07 | −0.18 | 0.28 | −0.08 | 0.08 | 0.65 |
| sICAM-1 | 0.27 | 0.79 | 0.20 | 0.00 | −0.15 | 0.05 | −0.06 | 0.76 | 0.14 | 0.85 | 0.12 | −0.13 | −0.16 | −0.04 | −0.09 | 0.81 | 0.00 | 0.86 | 0.02 | 0.12 | −0.21 | 0.23 | 0.10 | 0.86 |
| sVCAM-1 | 0.39 | 0.75 | −0.10 | −0.03 | 0.00 | 0.00 | −0.04 | 0.74 | 0.10 | 0.89 | 0.11 | −0.08 | 0.06 | −0.04 | −0.04 | 0.84 | −0.05 | 0.90 | −0.10 | 0.06 | −0.01 | 0.05 | −0.03 | 0.84 |
| CRP | 0.16 | 0.11 | 0.74 | 0.04 | −0.15 | 0.12 | 0.08 | 0.63 | 0.17 | 0.63 | −0.12 | 0.22 | −0.02 | 0.42 | 0.13 | 0.68 | 0.35 | 0.48 | 0.16 | 0.04 | −0.52 | 0.04 | 0.08 | 0.65 |
| sCD14 | −0.04 | 0.05 | 0.84 | −0.12 | 0.11 | −0.10 | −0.01 | 0.74 | 0.10 | −0.00 | 0.16 | −0.13 | 0.04 | 0.79 | −0.08 | 0.68 | 0.27 | 0.26 | 0.29 | −0.02 | −0.38 | 0.32 | 0.40 | 0.64 |
| sE-selectin | −0.06 | 0.23 | 0.19 | −0.24 | 0.32 | 0.67 | 0.17 | 0.73 | 0.04 | 0.17 | 0.63 | 0.06 | 0.08 | 0.29 | 0.01 | 0.52 | 0.01 | 0.31 | 0.40 | 0.10 | −0.22 | 0.63 | −0.09 | 0.73 |
| sP-selectin | 0.05 | 0.00 | −0.11 | 0.26 | −0.07 | 0.80 | −0.06 | 0.74 | −0.07 | 0.02 | 0.83 | 0.16 | 0.05 | −0.00 | −0.03 | 0.72 | 0.23 | 0.03 | −0.14 | 0.08 | 0.20 | 0.82 | −0.03 | 0.80 |
| MIP-1β | 0.17 | 0.24 | −0.12 | 0.64 | 0.01 | 0.14 | −0.16 | 0.56 | 0.54 | −0.12 | 0.40 | 0.21 | 0.09 | −0.08 | 0.22 | 0.57 | 0.16 | 0.04 | 0.20 | 0.82 | 0.17 | −0.02 | 0.00 | 0.77 |
| VEGF-A | 0.32 | −0.10 | 0.01 | 0.64 | −0.05 | 0.33 | 0.11 | 0.65 | 0.15 | 0.17 | 0.45 | 0.06 | −0.51 | −0.22 | −0.05 | 0.56 | 0.06 | 0.00 | −0.19 | 0.81 | −0.03 | 0.14 | −0.06 | 0.72 |
| CX3CL1 | 0.09 | −0.31 | −0.02 | 0.27 | 0.66 | −0.02 | −0.10 | 0.62 | 0.02 | −0.06 | 0.14 | 0.73 | −0.20 | 0.02 | 0.07 | 0.60 | −0.12 | −0.01 | 0.27 | 0.22 | 0.04 | 0.26 | −0.69 | 0.68 |
| MCP-1 | 0.20 | 0.12 | −0.07 | −0.01 | 0.71 | 0.09 | 0.09 | 0.58 | 0.61 | 0.06 | −0.01 | 0.44 | 0.12 | 0.14 | 0.09 | 0.62 | 0.31 | 0.06 | −0.12 | 0.23 | 0.74 | 0.15 | 0.03 | 0.74 |
| Fibrinogen | 0.01 | 0.07 | 0.25 | 0.18 | −0.34 | 0.31 | −0.52 | 0.58 | 0.02 | −0.11 | 0.13 | −0.20 | 0.71 | 0.07 | −0.03 | 0.58 | −0.02 | −0.15 | 0.79 | −0.09 | 0.04 | 0.12 | 0.07 | 0.67 |
| MMP9 | −0.23 | −0.05 | 0.10 | 0.70 | 0.21 | −0.18 | 0.11 | 0.64 | −0.04 | −0.03 | 0.13 | 0.74 | −0.01 | −0.16 | −0.18 | 0.62 | 0.01 | 0.13 | 0.69 | 0.07 | −0.18 | −0.13 | −0.21 | 0.59 |
| NFL | 0.03 | 0.02 | 0.17 | 0.09 | −0.06 | 0.13 | 0.87 | 0.81 | 0.01 | −0.05 | −0.00 | −0.08 | −0.02 | −0.06 | 0.95 | 0.92 | −0.01 | 0.07 | 0.03 | 0.08 | 0.03 | 0.06 | 0.83 | 0.71 |
| Eigenvalue | 4.40 | 2.08 | 2.00 | 1.74 | 1.44 | 1.43 | 1.17 | 5.52 | 2.29 | 1.67 | 1.54 | 1.53 | 1.11 | 1.09 | 5.00 | 2.57 | 1.72 | 1.65 | 1.61 | 1.54 | 1.49 | |||
Communality refers to the proportion of variance in the biomarker accounted for by the seven factors at a given time point
Factor loadings (0.4 < |F| ≤ 1.0) denoted by light highlighting.
Color corresponds to the factor loading number at each time point (F1=blue, F2=Red, F3=Green, F4=Orange, F5-F7=Grey).
The six identified consensus factors were Factor A (IL-10, IFNγ, IFNα2, IL-1β, IL-6, IP-10, and TNFα), Factor B (sCD163, sICAM-1, sVCAM-1, and CRP), Factor C (sE-selectin and sP-selectin), Factor D (MIP-1β and VEGF-A); Factor E (CRP and sCD14), and Factor F (CX3CL1 and MCP-1). Two biomarkers (MMP9 and NFL) did not meet the post-hoc criteria and were not assigned to any of the six consensus factors. Supplemental Table 2 presents the six consensus factors and the 19 corresponding biomarkers. Supplemental Table 3 shows the factor loadings at each time point, grouped according to consensus factors.
Association between Biomarker Factors and ND Outcomes
Table 3 presents the estimated association between the consensus factor scores at Week 0 and the four ND outcomes at week 0 and week 48 and the change in ND outcomes between weeks 0 and 48. At week 0, higher Factor B scores were associated with lower (poorer) KABC-II scores at weeks 0 and 48 and poorer TOVA D-prime scores at weeks 0 and 48. Higher Factor D scores at week 0 were associated with higher (better) TOVA D-prime scores at week 48 and a greater increase in TOVA D-prime scores between weeks 0 and 48. Participants with higher Factor E scores at week 0 had a smaller increase, on average, in KABC-II scores between weeks 0 and 48. Factors A, C, and F at Week 0 were not associated with any of the ND outcomes. None of the six consensus factors at week 0 was associated with the BOT-2 or BRIEF at either Week 0 or Week 48.
Table 3:
Estimated association between biomarker factor scores at Week 0 and corresponding ND outcomes at Weeks 0 and 48 and Change Between Weeks 0 and 48
| Consensus Factor* at Week 0 | BOT-2 | Reverse-Scored BRIEF GEC | KABC-II MPI | TOVA D-prime |
|---|---|---|---|---|
| Week 0 ND measurements | ||||
| A | 0·52 (−0·68, 1·71) | 0·78 (−1·11, 2·66) | 0·31 (−1·18, 1·80) | −0·04 (−0·18, 0·10) |
| B | −0·65 (−1·87, 0·57) | −1·38 (−3·28, 0·52) | −2·19 (−3·67, −0·71) | −0·22 (−0·36, −0·07) |
| C | −0·10 (−1·35, 1·14) | −0·36 (−2·32, 1·60) | 0·74 (−0·80, 2·27) | −0·09 (−0·23, 0·06) |
| D | 0·40 (−0·80, 1·60) | 1·25 (−0·63, 3·13) | −0·07 (−1·57, 1·42) | −0·08 (−0·22, 0·06) |
| E | −0·47 (−1·68, 0·74) | −0·06 (−1·97, 1·85) | −0·51 (−2·01, 0·99) | −0·07 (−0·22, 0·07) |
| F | 0·31 (−1·12, 1·73) | 0·68 (−1·56, 2·92) | 1·09 (−0·69, 2·87) | 0·08 (−0·09, 0·25) |
| Week 48 ND measurements | ||||
| A | −0·36 (−1·54, 0·82) | 0·91 (−0·79, 2·61) | −0·24 (−1·96, 1·49) | 0·05 (−0·10, 0·21) |
| B | −1·07 (−2·26, 0·12) | −1·53 (−3·23, 0·18) | −3·04 (−4·73, −1·34) | −0·18 (−0·34, −0·03) |
| C | −0·11 (−1·34, 1·11) | 0·58 (−1·19, 2·35) | 0·27 (−1·52, 2·06) | −0·05 (−0·21, 0·11) |
| D | −0·32 (−1·50, 0·86) | 0·40 (−1·30, 2·11) | −0·04 (−1·77, 1·69) | 0·17 (0·02, 0·33) |
| E | −0·49 (−1·68, 0·69) | −0·75 (−2·47, 0·97) | −1·57 (−3·29, 0·16) | 0·01 (−0·15, 0·16) |
| F | −0·41 (−1·80, 0·98) | 0·00 (−2·02, 2·02) | −0·02 (−2·08, 2·04) | 0·13 (−0·05, 0·32) |
| Week 48 - Week 0 ND measurements | ||||
| A | −0.71 (−1.54, 0.12) | 0.62 (−0.82, 2.06) | −0.43 (−1.44, 0.59) | 0.08 (−0.05, 0.21) |
| B | −0.59 (−1.44, 0.26) | −0.82 (−2.28, 0.63) | −0.91 (−1.97, 0.14) | −0.04 (−0.18, 0.10) |
| C | −0.08 (−0.95, 0.79) | 0.66 (−0.83, 2.16) | −0.45 (−1.51, 0.61) | −0.02 (−0.15, 0.12) |
| D | −0.60 (−1.43, 0.24) | −0.11 (−1.55, 1.34) | 0.03 (−0.99, 1.04) | 0.20 (0.08, 0.33) |
| E | −0.19 (−1.03, 0.65) | −0.69 (−2.14, 0.76) | −1.17 (−2.18, −0.16) | 0.04 (−0.09, 0.18) |
| F | −0.60 (−1.59, 0.39) | −0.35 (−2.06, 1.35) | −0.72 (−1.94, 0.50) | 0.05 (−0.11, 0.21) |
All analyses adjusted for site, sex, age at enrollment, age at ART initiation, ART regimen at time of biomarker specimen collection (LPV/r-based, NVP-based, or other), and whether the participant switched regimens between P1060 entry and biomarker specimen collection. Analyses involving the change between weeks 0 and 48 also adjusted for values at week 0. Estimates correspond to a one standard deviation increase in the factor score.
Significant regression coefficients (95% CI does not contain 0) denoted by grey highlighting.
Only ND measures with statistically significant relationships with the biomarker factors are shown.
KABC-II MPI = Kaufman Assessment Battery for Children, 2nd edition, Mental Processing Index; TOVA D-prime = Tests of Variables of Attention D-prime; BOT-2 = Bruininks-Oseretsky Test of Motor Proficiency, 2nd edition, Total Motor Composite Score
Associations between consensus factor scores at the start of controlled viremia and at week 48 are provided in Supplemental Tables 4 and 5, respectively. Higher Factor D scores assessed at the start of controlled viremia were associated with higher TOVA D-prime values at Week 0; consensus factor scores at the start of controlled viremia were not associated with BOT-2, BRIEF, or KABC-II outcomes. At week 48, higher Factor B scores were associated with poorer KABC-II scores, and higher Factor C scores were associated with poorer BOT-2 scores.
DISCUSSION
We investigated cross-sectional and longitudinal associations between biomarkers reflective of inflammation and immune activation and ND outcomes in a cohort of children with PHIV with controlled viremia. Notably, almost all of the noteworthy associations between biomarker consensus factors and ND outcomes involved consensus factors subsequent to a period of sustained virologic control (i.e., at weeks 0 and 48), rather than at the onset of virologic control. It is possible that the impact of specific inflammatory cascades is not easily discernable proximate to a period of uncontrolled viral replication and may only become apparent after a period of sustained control of viral replication; alternatively, an insult to the CNS that occurred during uncontrolled viremia may only manifest later, despite virological control. One exception, the association between Factor D at the onset of controlled viremia and TOVA D-prime at Week 0, suggests the relatively early prognostic value of this factor. The hypothesis that emerges is that, in addition to suppressing viral replication, modulating inflammation and immune activation may improve ND outcomes.
The consensus factor most consistently associated with ND outcomes was Factor B (sICAM-1, sVCAM-1, sCD163, and CRP). Factor B at week 0 was negatively associated with measures of working memory, learning, visual-spatial analysis, and planning/reasoning (KABC-II) and with measures of attention and impulsivity (TOVA D-prime), at both weeks 0 and 48. This factor was also cross-sectionally negatively associated with the KABC-II at week 48. ICAM-1 and VCAM-1 are adhesion molecules expressed by endothelial and immune system cells. Their expression is induced by inflammatory cytokines and results in leukocyte transmigration across the endothelium into tissues.[17–18] Their expression can be associated with endothelial dysfunction.[17–18] CD163 is a marker of monocyte/macrophage activation and scavenger activity, which can be triggered by endothelial damage and can further contribute to vascular damage.[7] Previous studies have also shown that sCD163 may be a measure of HIV replication.[19] In children with PHIV initiating ART in infancy, CD163 expression has been associated with developmental delays [2] Conversely, in late-diagnosed and treated school-aged children, elevated subsets of activated monocytes following ART initiation were associated with better neurocognitive scores[3], while in adults with acute HIV infection normalization of CD163 expression after ART initiation was associated with lack of neurological sequelae.[20] Combined, these results suggest that timing of infection and ART initiation with respect to individual immune maturation may affect the balance between damaging and protective roles of CD163. CRP, a marker of increased inflammation, was identified in a latent factor (fibrinogen, CRP, and IL-6) negatively associated with a measure of processing speed in youth with virologically suppressed PHIV.[5] Elevated CRP has also been associated with cardiovascular disorder. Adults with HIV are at high risk of cardiovascular disease that may have neurologic complications. The association of elevated CRP, sCD163 and endothelial activation with ND deficits in children with PHIV suggests a potential role of cardiovascular disorder in the genesis of ND deficits that warrants further investigation.
Factor D, consisting of VEGF-A (which promotes angiogenesis in the developing CNS[21–24]) and MIP-1β (a competitive CCR5 receptor ligand[25]) showed favorable longitudinal associations with a measure of attention and impulsivity (TOVA D-prime). CCR5 is a coreceptor of HIV entry into T cells and monocytes. CCR5 and CCR2 (another HIV coreceptor) antagonists in adults with controlled HIV replication in the blood compartment improved their neurocognitive performance.[26] Collectively, these results suggest a combined neuroprotective effect of angiogenesis and CCR5 antagonism in the developing brains of children with PHIV.
Associations between Factors C and E and ND outcomes were less consistent across timepoints and, therefore, should be interpreted with caution. Factor C, which included the adhesion molecules E-selectin (expressed solely on endothelial cells) and P-selectin (expressed on both endothelial cells and platelets),[6,27] was cross-sectionally associated with poorer motor proficiency (BOT-2) at week 48, suggesting a detrimental effect of endothelial dysfunction on motor outcomes. Factor E (CRP and CD14) at week 0 was associated with a decline in KABC-II scores between weeks 0 and 48. Similar to sCD163, sCD14 is a marker of monocyte/macrophage activation. In the HIV literature, sCD14 has been used as a marker of microbial translocation.[7] Low-grade microbial translocation and elevated sCD14 persists in individuals with HIV despite virologic suppression, remaining at levels higher than in non-infected individuals.[28,29] However, the association of sCD14 with microbial translocation is based on data generated in white males with HIV and has not been confirmed in black people with HIV,[30] such as our study population. Further studies are needed to determine if persistent residual microbial translocation plays a role in the development of ND deficits in children with PHIV.
Factor A, comprised of inflammatory biomarkers (IL-10, IFNγ, IFNα, IL-1β, IL-6, IP-10, and TNFα) involved in multiple cascades of systemic inflammation,[6,7] and Factor F (chemoattractants MCP-1 and CX3CL-1) were not associated with any of the ND outcomes. ND vulnerability in virologically controlled children with PHIV might be better understood by examining specific inflammatory or neuroimmune pathways (such as the ones reflected by Factors B, C, D, and E) rather than the generic pro-inflammatory milieu reflected by Factors A and F.
None of the consensus factors were associated with the measure of executive function (BRIEF). This measure may be relatively less sensitive to plasma biomarker levels because it is based on parent report and could be influenced by factors such as parental depression[31] or a change in caregiver during the interval between measurements.[10]
This study has limitations. It was intended to be hypothesis-generating rather than hypothesis-testing. We report signals of potentially detrimental or protective effects without adjusting for multiple comparisons; thus, hypotheses generated by this analysis will require confirmation in future studies.[14–16] Although we used reproducible, quantitative criteria to identify consensus factors (biomarkers with loadings >0.4), the consensus-based approach is subjective by definition. Nevertheless, our approach was based on the observation of multiple biomarker groupings that varied little over time, guiding our choice to simplify the factors by creating consensus factors across time points. The generalizability of the findings is constrained by the standards and practices in place in 2005, when P1060 enrollment began. We used a cutoff of 400 HIV RNA copies/mL to identify participants with controlled viremia because that was the lower limit of detection for the assay used for most participants throughout P1060. This cutoff is higher than contemporary assays with limits of detection of <20 to <40 copies/mL. It is possible that viral loads in the range between 20 and 400 copies could have affected levels of inflammatory biomarkers and/or ND outcomes in some participants. Although our multivariable models controlled for potential effects of ART regimen at time of specimen collection, some newer ART agents developed since the completion of P1060 might have different effects on inflammatory markers or ND outcomes compared to the agents used in P1060.
In summary, we identified several biomarker groupings associated with ND outcomes in children with PHIV with controlled viremia. The factor analysis-based approach may lead to an improved ability to identify children with PHIV at high risk of lowered ND performance, understand the underlying mechanisms of persistent ND deficits, and suggest potential therapeutic interventions. Specifically, our results suggest that there may be continuous HIV replication in the CNS compartment of children with PHIV with lowered ND performance that could be controlled with targeted antiviral therapy. An alternative mechanism for ND impairment that needs to be further explored is cardiovascular disorder, which has not been studied in children with PHIV. Mitigating interventions could be deployed if this mechanism is confirmed. Finally, a potential role of CCR5 inhibitors in ameliorating ND deficits in children with PHIV needs to be studied.
Supplementary Material
Supplemental Figure 1: CONSORT Diagram
Flow diagram showing the eligibility of International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) network protocols P1060 and P1104 S for the present study
ACKNOWLEDGMENTS:
We thank the study participants, parents and caregivers, and study personnel at each of the six sites for their invaluable contribution to this research. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References:
- 1.Boivin MJ, Chernoff M, Fairlie L et al. African Multi-Site 2-Year Neuropsychological Study of School-Age Children Perinatally Infected, Exposed, and Unexposed to Human Immunodeficiency Virus. Clin Infect Dis pii: ciz1088. 10.1093/cid/ciz1088. [DOI] [PMC free article] [PubMed]
- 2.Benki-Nugent SF, Martopullo I, Laboso T et al. High Plasma Soluble CD163 During Infancy Is a Marker for Neurocognitive Outcomes in Early-Treated HIV-Infected Children. J Acquir Immune Defic Syndr 2019; 81:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ananworanich J, Kerr SJ, Jaimulwong T et al. Soluble CD163 and monocyte populations in response to antiretroviral therapy and in relationship with neuropsychological testing among HIV-infected children. J Virus Erad 2015; 1:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapetanovic S, Leister E, Nichols S et al. Relationship between Markers of Vascular Dysfunction and Neurodevelopmental Outcomes in Perinatally HIV-Infected Youth. AIDS 2010; 24:1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapetanovic S, Griner R, Zeldow B et al. Biomarkers and Neurodevelopment in Perinatally HIV-Infected or Exposed Youth: A Structural Equation Model Analysis. AIDS 2014; 28:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neaton JD, Neuhaus J, Emery S. Soluble biomarkers and morbidity and mortality among people infected with HIV: summary of published reports from 1997 to 2010. Curr Opin HIV AIDS 2010; 5: 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leeansyah E, Malone DF, Anthony DD, Sandberg JK. Soluble biomarkers of HIV transmission, disease progression and comorbidities. Curr Opin HIV AIDS 2013; 8:117–124. [DOI] [PubMed] [Google Scholar]
- 8.Violari A, Lindsey JC, Hughes MD et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med 2012; 366:2380–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boivin MJ, Barlow-Mosha L, Chernoff MC et al. Neuropsychological performance in African children with HIV enrolled in a multisite antiretroviral clinical trial. AIDS 2018; 32:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernoff MC, Laughton B, Ratswana M et al. Validity of Neuropsychological Testing in Young African Children Affected by HIV. J Pediatr Infect Dis 2018; 13:185–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boivin MJ, Ruel TD, Boal HE et al. HIV-subtype A is associated with poorer neuropsychological performance compared with subtype D in antiretroviral therapy-naive Ugandan children. AIDS 2010; 24:1163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruel TD, Boivin MJ, Boal HE et al. Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis 2012;54: 1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Rourke Norm, and Hatcher Larry. A Step-by-Step Approach to Using SAS® for Factor Analysis and Structural Equation Modeling, Second Edition. Cary, NC: SAS Institute Inc; 2013. [Google Scholar]
- 14.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1: 43–46. [PubMed] [Google Scholar]
- 15.Blume J, Peipert JF. What your statistician never told you about P-values. The Journal of the American Association of Gynecologic Laparoscopists 2003; 10:439–444. [DOI] [PubMed] [Google Scholar]
- 16.Althouse AD. 2016. Adjust for multiple comparisons? It’s not that simple. The Annals of thoracic surgery 2016; 101:1644–1645. [DOI] [PubMed] [Google Scholar]
- 17.Nagel T, Resnick N, Atkinson WJ, Dewey CF Jr, Gimbrone MA Jr. Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest 1994; 94:885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruyndoncky L, Hoymans VY, Van Craenenbroeck AH et al. Assessment of endothelial dysfunction in childhood obesity and clinical use. Oxidative Med Cell Longev 2013; 10.1155/2013/174782 [DOI] [PMC free article] [PubMed]
- 19.Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 2011; 204:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Antoni ML, Byron MM, Chan P et al. Normalization of Soluble CD163 Levels After Institution of Antiretroviral Therapy During Acute HIV Infection Tracks with Fewer Neurological Abnormalities. J Infect Dis 2018; 218:1453–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrara N, Carver-Moore K, Chen H et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996; 380:439–442. [DOI] [PubMed] [Google Scholar]
- 22.Jin KL, Mao XO, Greenberg DA. Vascular endothelial growth factor: direct neuroprotective effect in in vitro ischemia. Proc Natl Acad Sci USA 2000; 97:10242–10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storkebaum E, Lambrechts D, Dewerchin M et al. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci 2005; 8:85–92. [DOI] [PubMed] [Google Scholar]
- 24.Carmeliet P, Ferreira V, Breier G et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996; 380:435–9. [DOI] [PubMed] [Google Scholar]
- 25.Obuku AE, Bugembe DL, Musinguzi K et al. Macrophage Inflammatory Protein-1 Beta and Interferon Gamma Responses in Ugandans with HIV-1 Acute/Early Infections. AIDS Res Hum Retroviruses 2016; 32:237–246. [DOI] [PubMed] [Google Scholar]
- 26.D’Antoni ML, Paul RH, Mitchell BI et al. Improved Cognitive Performance and Reduced Monocyte Activation in Virally Suppressed Chronic HIV After Dual CCR2 and CCR5 Antagonism. J Acquir Immune Defic Syndr 2018; 79:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Kim YJ, Mantel C, Broxmeyer HE. P-selectin enhances generation of CD14+CD16+ dendritic-like cells and inhibits macrophage maturation from human peripheral blood monocytes. J Immunol 2003; 171:669–77. [DOI] [PubMed] [Google Scholar]
- 28.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol 2013; 21:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Younas M, Psomas C, Reynes C, et al. Microbial Translocation Is Linked to a Specific Immune Activation Profile in HIV-1-Infected Adults With Suppressed Viremia. Front Immunol 2019; 10:2185. 10.3389/fimmu.2019.02185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Voeght A, Maes N, Moutschen M. sCD14 is not a bona-fide biomarker of microbial translocation in HIV-1-infected Africans living in Belgium. AIDS 2016; 30:921–924. 10.1097/QAD.0000000000000996 [DOI] [PubMed] [Google Scholar]
- 31.Familiar I, Chernoff M, Ruisenor-Escudero H et al. Association between caregiver depression symptoms and child executive functioning. Results from an observational study carried out in four sub-Saharan countries. AIDS Care 2020; 32:486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: CONSORT Diagram
Flow diagram showing the eligibility of International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) network protocols P1060 and P1104 S for the present study


