Abstract
Some immortalized lens epithelial cell lines have been established and are useful for molecular analysis. The establishment of additional cell lines must, however, enable a variety of in-vitro examinations. The objective of this study was to establish a new canine lens epithelial cell line by isolating CLC-1 cells from the lens tissue of a dog with cataracts. In CLC-1 cells, transforming growth factor beta (TGF-β) treatment significantly decreased gene expression of an epithelial marker and elevated that of mesenchymal markers; these characteristics are similar to those of a human lens epithelial cell line. Interestingly, CLC-1 cells exhibited lower expression of an epithelial marker and higher expression of mesenchymal markers than an anterior lens capsule. These results suggest that CLC-1 cells were derived from a cell population that was committed to epithelial-mesenchymal transition in cataract lens tissue. In conclusion, CLC-1 cells could be useful for analyzing molecular pathogenesis in canine cataracts.
Résumé
Certaines lignées de cellules épithéliales du cristallin immortalisées ont été établies et sont utiles pour analyse moléculaire. L’établissement de lignées cellulaires supplémentaires doit cependant permettre une variété d’examens in vitro. L’objectif de cette étude était d’établir une nouvelle lignée cellulaire épithéliale du cristallin canin en isolant les cellules CLC-1 du tissu du cristallin d’un chien atteint de cataracte. Dans les cellules CLC-1, le traitement par le facteur de croissance transformant bêta (TGF-β) a significativement diminué l’expression génique d’un marqueur épithélial et élevé celle des marqueurs mésenchymateux; ces caractéristiques sont similaires à celles d’une lignée cellulaire épithéliale du cristallin humain. Fait intéressant, les cellules CLC-1 présentaient une expression inférieure d’un marqueur épithélial et une expression plus élevée de marqueurs mésenchymateux qu’une capsule antérieure du cristallin. Ces résultats suggèrent que les cellules CLC-1 étaient dérivées d’une population cellulaire qui était impliquée dans la transition épithéliale-mésenchymateuse dans le tissu du cristallin de la cataracte. En conclusion, les cellules CLC-1 pourraient être utiles pour analyser la pathogenèse moléculaire dans les cataractes canines.
(Traduit par Docteur Serge Messier)
Although surgical treatment is the only way to restore visual disturbance caused by cataracts, postoperative complications can lead to a patient’s low vision (1). Posterior capsule opacification (PCO) is one of the most frequent vision-threatening complications after surgical treatment for cataracts in dogs and humans (2,3). Posterior capsule opacification is caused by aberrant migration and growth of lens epithelial cells across the lens capsule, which decrease lens clarity (2).
Many studies have identified that the epithelial-mesenchymal transition (EMT) is a key factor in PCO (2–4). Lens epithelial cells committed to EMT accumulate alpha smooth muscle actin (αSMA), which is normally absent in these cells (2). Additionally, these cells express the upregulation of integrins, such as integrin subunit alpha V (ITGAV), along with the loss of epithelial marker expression, including E-cadherin (CDH) (2). It is widely accepted that transforming growth factor beta (TGF-β) initiates EMT in lens epithelial cells (2,5).
To date, several lens epithelial cell lines, such as HLE-B3, HLE-B4, SRA 01/04, and cdLEC cells, have been established and validated through confirmation of the expression of Crystallin (CRY) αB, a major protein of the eye lens (6–9). The HLE-B3, HLE-B4, and SRA 01/04 cells were derived from infant human lenses and immortalized by transfection of SV40 large T antigen (Simian Vacuolating Virus 40 Tag) (6,7). In dogs, cdLEC cells were derived from a dog with mature cataracts and transfected with SV40 large T antigen (8). While these cell lines have been widely used in cataract investigations (4,10), the establishment of additional cell lines would enable a variety of in-vitro examinations and provide more generalized information about cellular abnormalities in cataracts. The objective of the present study was to establish a new canine lens epithelial cell line.
A dog diagnosed with cataracts at the Okayama University of Science Veterinary Medical Teaching Hospital participated in this study. All clinical examinations and treatments were carried out after the dog’s owner gave informed and written consent. All experiments with animals were approved by the Clinical Research Ethics Committee of the Faculty of Veterinary Medicine of the Okayama University of Science.
Phacoemulsification, aspiration, and implantation of the intraocular lens were conducted with a common surgical procedure for therapeutic purposes. An anterior lens capsule (ALC) was resected using a continuous curvilinear capsulorrhexis technique. Lens epithelial cells and lens fiber cells were collected with an emulsification aspirator (Stellaris PC Vision Enhancement System; Bausch & Lomb Japan, Tokyo, Japan). The collected ALC was halved; one half was used for the extraction of total RNA and the other half was incubated at 37°C in Dulbecco’s Modified Eagle’s Medium (DMEM) (Fujifilm Wako Chemicals, Tokyo, Japan), supplemented with 10% fetal bovine serum (FBS) (Biosera, Nuaille, France), 20 IU/mL penicillin, and 10 μg/mL streptomycin.
Proliferating primary lens epithelial cells (pLECs) were passaged by a dilution of 1:2 every 7 d. The lens epithelial cells and lens fiber cells collected with the emulsification aspirator were also incubated in the culture medium at 37°C. After a week, proliferating epithelial-like cells were observed. Some fiber-like cells were observed, but they did not proliferate. The proliferating epithelial-like cells were passaged at 70 to 80% confluence by a dilution of 1:2. For the passage of cultured cells, the supernatant was removed, and the cells were washed with phosphate-buffered saline (PBS). Subsequently, the cells were detached from the flasks by treatment with 2.5 g/L trypsin and 1 mmol/L ethylenediaminetetraacetic acid (EDTA) (Nacalai Tesque, Kyoto, Japan) for 5 min. The cells were washed and resuspended in culture medium. The number of cells was calculated using the Trypan blue dye exclusion test.
Western blotting was carried out to detect CRYαB protein. Proliferating epithelial-like cells (45th passage) were lysed with Radioimmunoprecipitation Assay (RIPA) Buffer (Nacalai Tesque). Separated proteins with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were transferred onto Immobilon-P membranes (Millipore, Bedford, Massachusetts, USA). The membrane was incubated with rabbit anti-CRYαB antibody (Cat. No. 15808-1-AP; Proteintech, Rosemont, Illinois, USA), followed by horseradish peroxidase (HRP)-conjugated antibody. Molecular weight of the detected protein was inferred with Precision Plus Protein WesternC standards (Bio-Rad, Hercules, California, USA). Additionally, immunocytochemistry was conducted to visualize the distribution of CRYαB protein.
After overnight incubation on glass coverslips in 6-well plates, the cells were fixed with 4% paraformaldehyde for 10 min at room temperature. After permeabilization with 0.1% polyoxyethylene sorbitan monolaurate, the cells were incubated with rabbit anti- CRYαB antibody, followed by Alexa Fluor 594-conjugated antibody (Abcam plc, Cambridge, UK). Nuclear staining was carried out with 4′,6-diamidino-2-phenylindolea (DAPI) (Fluoro-KEEPER Antifade Reagent; Nacalai Tesque).
Total RNA was extracted from the ALC tissue, pLEC (3rd passage), and proliferating epithelial-like cells (3rd, 14th, and 45th passage) using a NucleoSpin Plus Kit (Takara Bio, Shiga, Japan). For the 45th passage proliferating epithelial-like cells, total RNA was extracted from cells incubated either with or without 10 ng/mL of human recombinant TGF-β (Peprotech; Rocky Hill, New Jersey, USA) for 6 h.
The extracted RNA was then reverse-transcribed into cDNA using a PrimeScript II 1st strand cDNA Synthesis Kit (Takara Bio). Real-time polymerase chain reaction (RT-PCR) was carried out with TB Green Premix Ex Taq II (Takara Bio) in the presence of 0.2 μM each of the forward and reverse primers for canine CDH, canine alpha smooth muscle actin (αSMA), canine integrin subunit alpha (ITGAV), or canine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Table I) (11–13). The PCR amplification comprised pre-denaturation (95°C, 10 s), 40 cycles of denaturation (95°C, 10 s), annealing, and extension (60°C, 30 s). Fluorescence intensity was measured in real time during extension steps using the QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific, Waltham, Massachusetts, USA). All mRNA expression levels were normalized to the reference gene (GAPDH). The values were standardized to a value in a sample of the medium alone for detection of changes in gene expression after TGF-β treatment.
Table I.
Primers used in study.
| Gene | Forward primer (5′-3′) | Reverse primer (3′-5′) | Reference |
|---|---|---|---|
| CDH1 | AAAACCCACAGCCTCATGTC | CACCTGGTCCTTGTTCTGGT | Yao et al (11) |
| αSMA | CGCCACATCTCAACTCTGAA | GCTGAAGCCTGTTCTTGGTC | Dong et al (12) |
| ITGAV | GGCGATGGCGTAGATGACTT | GGCGCTCCGATGAACACAT | Agarwal et al (13) |
| GAPDH | AACATCATCCCTGCTTCCAC | GACCACCTGGTCCTCAGTGT | Yao et al (11) |
CDH1 — E-cadherin1; αSMA — alpha smooth muscle action; ITGAV — integrin subunit alpha V; GAPDH — glyceraldehyde-3-phosphate dehydrogenase.
Statistical significance was analyzed by Student’s t-test with the use of EZR, which is a statistical software package for R version 3.5.2. P-values < 0.05 were considered statistically significant.
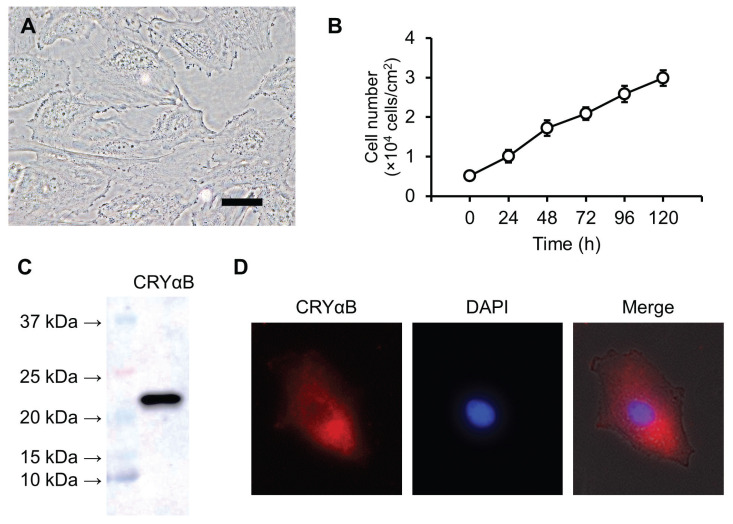
Proliferating epithelial-like cells showed continuous cell growth for over 12 mo. After 40 passages, the cells were named CLC-1 as a novel canine lens epithelial cell line. The CLC-1 cells had a polygonal shape (Figure 1A) and proliferated linearly at a cell concentration of 1 to 3 × 104 cells/cm3; the doubling time was 48.8 ± 3.3 h (Figure 1B).
Figure 1.
Characteristics of CLC-1 cells. CLC-1 cells have a polygonal shape (A) and proliferate linearly (B). Proliferation activity was evaluated by a Trypan blue dye exclusion test. Cells were suspended in culture medium at 1 × 104 cells/mL, then the number of cells was counted every 24 h. The expression of CRYαB protein was detected by western blotting (C) and immunocytochemistry (D). Rabbit anti-CRYαB antibody and Alexa Fluor 594-conjugated secondary antibody were used for the detection. 6-Diamidino-2-phenylindole (DAPI) was used for nuclear counterstaining.
Scale bar = 10 μm.
We confirmed the expression of CRYαB protein in CLC-1 cells by western blotting (Figure 1C). Immunocytochemistry showed a diffuse distribution of CRYαB in the cytoplasm of CLC-1 cells (Figure 1D).
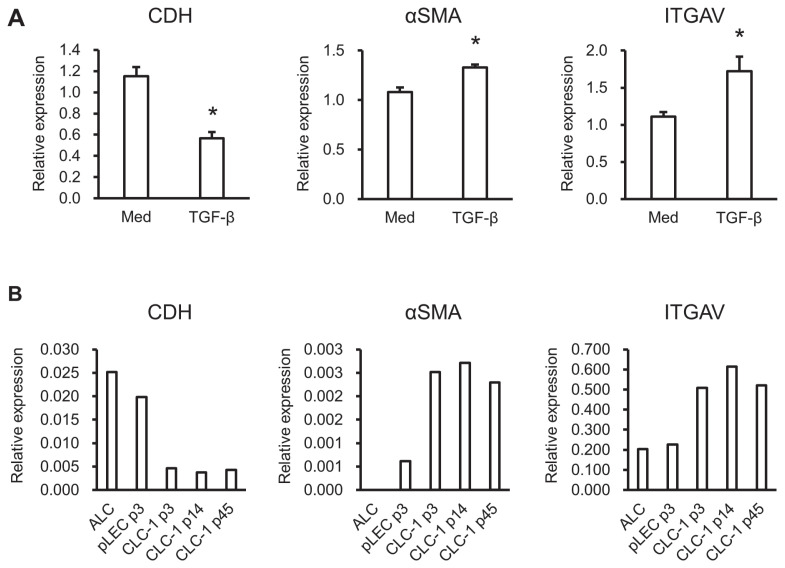
Changes in the expression levels of EMT-related genes in CLC-1 cells after TGF-β treatment were analyzed by RT-PCR. Treatment with TGF-β significantly suppressed the expression of the epithelial marker CDH and elevated the expression of the mesenchymal markers αSMA and ITGAV in CLC-1 cells (Figure 2A).
Figure 2.
Relative expression levels of epithelial or mesenchymal markers. Real-time polymerase chain reaction (RT-PCR) was carried out to determine the expression levels of CDH, αSMA, and ITGAV in ALC, pLEC, and CLA-1 cells (A). The values of target genes were normalized to that of GAPDH. Real-time PCR was carried out to detect the changes in gene expression after TGF-β treatment (10 ng/mL) in CLC-1 cells (B). Three independent experiments were conducted. The values of target genes were normalized to that of GAPDH and then standardized to that of medium alone.
* P < 0.05 versus medium alone.
CDH — E-cadherin; αSMA — alpha smooth muscle action; ITGAV — integrin subunit alpha V; ALC — anterior lens capsule; pLEC — primary lens epithelial cells; GAPDH — glyceraldehyde-3-phosphate dehydrogenase; TGF-β — transforming growth factor beta; p3 — 3rd passage; p14 — 14th passage; p45 — 45th passage.
Real-time PCR was carried out to compare the expression levels of EMT-related genes among ALC, pLEC, and CLC-1 cells. The expression of CDH was relatively higher in ALC and pLEC cells than in CLC-1 cells (Figure 2B). In contrast, the expression of αSMA and ITGAV was relatively lower in ALC and pLEC than in CLC-1 cells (Figure 2B). Although there were some small differences, the expression of EMT-related genes in CLC-1 cells was almost identical among the 3rd, 14th, and 45th passages. The expression of αSMA was undetectable in ALC.
In the present study, we established continuously growing canine lens epithelial cells, named CLC-1. We examined whether CLC-1 cells had similar characteristics to reported human lens epithelial cell lines. We found that CRYαB was diffusely distributed in the cytoplasm of CLC-1 cells, similar to the reported cell lines in previous studies (8,14). Transforming growth factor beta (TGF-β) is a well-investigated factor involved in EMT in lens epithelial cells (2,5); it has been reported to increase the expression levels of mesenchymal markers in human lens epithelial cells (4). We confirmed that incubation with TGF-β significantly increased the expression levels of mesenchymal markers in CLC-1 cells.
The reason CLC-1 cells continuously proliferate is unclear. Recent studies have reported that epithelial-mesenchymal transition (EMT) promotes cell proliferation and survival (2,15). Therefore, we hypothesized that a cell population committed to EMT exists in a cataract lens and that these cells constitutively proliferate in culture medium. Gene analysis revealed lower expression of an epithelial marker and higher expression of mesenchymal markers in CLC-1 cells compared with ALC and pLEC. Additionally, this tendency was observed in CLC-1 cells even at low passage. Although further investigations are necessary, spontaneous commitment to EMT of lens epithelial cells might be involved in the pathogenesis of canine cataracts.
Because of the instability of primary lens epithelial cells, some cell lines have been established by immortalization with the transfection of SV40 large T antigen (6–8). These cell lines have great value in the physiological and pathological analyses of lens epithelial cells. Recent studies have demonstrated that the transfection of SV40 large T antigen promotes telomerase activity (16), however, and that telomerase activation promotes EMT (17). Therefore, CLC-1 cells might be useful for investigating the physiological mechanism of EMT in lens epithelial cells because the more spontaneously derived cell line might provide a more natural source of cells for doing such an investigation.
In conclusion, we have established a new canine lens epithelial cell line representing continuous growth and commitment to EMT. This cell line could be a useful tool for analyzing molecular pathogenesis in canine cataracts.
Acknowledgment
This work was supported by Grants-in-Aid for Scientific Research (KAKENHI) of the Japan Society for the Promotion of Science (JSPS), Grant Numbers JP18KK0191 and JP19K06412.
References
- 1.Brian G, Taylor H. Cataract blindness-challenges for the 21st century. Bull World Health Organ. 2001;79:249–256. [PMC free article] [PubMed] [Google Scholar]
- 2.Lovicu FJ, Shin EH, McAvoy JW. Fibrosis in the lens. Sprouty regulation of TGFβ-signaling prevents lens EMT leading to cataract. Exp Eye Res. 2016;142:92–101. doi: 10.1016/j.exer.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi N-Y, Park S-A, Jeong M-B, et al. Phacoemulsification and acryl foldable intraocular lens implantation in dogs: 32 cases. J Vet Sci. 2006;7:281–285. doi: 10.4142/jvs.2006.7.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wernecke L, Keckeis S, Reichhart N, Strauß O, Salchow DJ. Epithelial-mesenchymal transdifferentiation in pediatric lens epithelial cells. Investig Opthalmology Vis Sci. 2018;59:5785–5794. doi: 10.1167/iovs.18-23789. [DOI] [PubMed] [Google Scholar]
- 5.Chong CCW, Stump RJW, Lovicu FJ, McAvoy JW. TGF-β promotes Wnt expression during cataract development. Exp Eye Res. 2009;88:307–313. doi: 10.1016/j.exer.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibaraki N, Chen SC, Lin LR, Okamoto H, Pipas JM, Reddy VN. Human lens epithelial cell line. Exp Eye Res. 1998;67:577–585. doi: 10.1006/exer.1998.0551. [DOI] [PubMed] [Google Scholar]
- 7.Andley UP, Rhim JS, Chylack LT, Fleming TP. Propagation and immortalization of human lens epithelial cells in culture. Invest Ophthalmol Vis Sci. 1994;35:3094–4102. [PubMed] [Google Scholar]
- 8.Kanemaki N, Saito M, Onda K, et al. Establishment of a lens epithelial cell line from a canine mature cataract. Exp Anim. 2012;61:41–47. doi: 10.1538/expanim.61.41. [DOI] [PubMed] [Google Scholar]
- 9.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochiai H, Moriyama J, Kanemaki N, Sato R, Onda K. Analysis of cationic amino acid transport activity in canine lens epithelial cells. Exp Anim. 2013;62:311–317. doi: 10.1538/expanim.62.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao F, Kausalya JP, Sia YY, et al. Recurrent fusion genes in gastric cancer: CLDN18-ARHGAP26 induces loss of epithelial integrity. Cell Rep. 2015;12:272–285. doi: 10.1016/j.celrep.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Dong JD, Gu YQ, Li CM, et al. Response of mesenchymal stem cells to shear stress in tissue-engineered vascular grafts. Acta Pharmacol Sin. 2009;30:530–536. doi: 10.1038/aps.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal P, Gammon EA, Sajib AM, Sandey M, Smith BF. Cell-surface integrins and CAR are both essential for adenovirus type 5 transduction of canine cells of lymphocytic origin. PLoS ONE. 2017;12:e0169532. doi: 10.1371/journal.pone.0169532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blakely EA, Bjornstad KA, Chang PY, et al. Growth and differentiation of human lens epithelial cells in vitro on matrix. Invest Ophthalmol Vis Sci. 2000;41:3898–4907. [PubMed] [Google Scholar]
- 15.Qin J-H, Wang L, Li Q-L, Liang Y, Ke Z-Y, Wang R-A. Epithelial-mesenchymal transition as strategic microenvironment mimicry for cancer cell survival and immune escape? Genes Dis. 2016;4:16–18. doi: 10.1016/j.gendis.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foddis R, De Rienzo A, Broccoli D, et al. SV40 infection induces telomerase activity in human mesothelial cells. Oncogene. 2002;21:1434–1442. doi: 10.1038/sj.onc.1205203. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Bian C, Zhen C, et al. Telomerase reverse transcriptase mediates EMT through NF-κB signaling in tongue squamous cell carcinoma. Oncotarget. 2017;8:85492–85503. doi: 10.18632/oncotarget.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]