Abstract
The growing number of honey bee colonies and beekeepers in Canada has led to a great diversity of beekeeping practices. All beekeeping operations, however, need to implement consistent management measures for the control of diseases. The objective of this study was to document the actual disease management practices of beekeeping productions in southwestern Quebec, Canada. A survey was conducted to describe management practices used by 15 beekeepers who own 1824 colonies in that area. Data were obtained by telephone interviews. When infectious diseases were suspected, beekeepers generally avoided using potentially toxic acaricides and chemical treatments associated with antimicrobial resistance and instead used preventive, physical or management methods, although laboratory diagnosis was rarely used. This study highlights the wide variety of operation sizes, activities, and disease management strategies among beekeepers in southwestern Quebec. It identifies the need to encourage the use of services available to them and to propose a standardized preventive medical approach for field veterinarians to avoid the spread of infectious diseases.
Résumé
Le nombre croissant de colonies d’abeilles mellifères et d’apiculteurs au Canada a conduit à une grande diversité de pratiques apicoles. Cependant, toutes les opérations apicoles doivent mettre en oeuvre des mesures de gestion cohérentes pour lutter contre les maladies. L’objectif de cette étude était de documenter les pratiques actuelles de gestion des maladies dans les exploitations apicoles situées au sud-ouest du Québec, Canada. Une enquête a été menée pour décrire les pratiques de régie utilisées par 15 apiculteurs possédant 1824 colonies dans cette région. Les données ont été obtenues par des entretiens téléphoniques. Lorsque des maladies infectieuses étaient suspectées, les apiculteurs évitaient généralement d’utiliser des acaricides potentiellement toxiques et des traitements chimiques associés à la résistance aux antimicrobiens et utilisaient à la place des méthodes préventives, physiques ou de gestion, bien que les diagnostics en laboratoire étaient rarement utilisés. Cette étude met en évidence la grande variété de tailles d’entreprises, d’activités et de stratégies de gestion des maladies de l’abeille par les apiculteurs du sud-ouest du Québec. Il identifie la nécessité d’encourager l’utilisation des services offerts aux apiculteurs et de proposer une approche médicale préventive standardisée aux vétérinaires pour éviter la propagation de maladies infectieuses.
(Traduit par Gabrielle Claing)
Beekeeping has been expanding in Quebec and in all of Canada over the last 10 y, with a growing number of honey bee colonies and beekeepers bringing a great diversity of beekeeping practices (1). Disease management remains a central part of any beekeeping operation, as honey bees can be affected by various bacteria, fungi, viruses, and parasites. Many strategies are used to control bee pathogens, including antibiotics and pesticides, which should be used judiciously to avoid deleterious effects of treatment on the microbiota and survival of honey bees (2,3), to preserve drug effectiveness (4), and to avoid drug residues in bee products (5,6).
This reality is acknowledged in Quebec, where since 1998, anti-microbials, such as the oxytetracycline, tylosin, and fumagillin used in apiaries, require a veterinary prescription. Since December 2018, similar legislation has applied throughout Canada. These regulations support a sound approach to disease control that must be based on an integrated strategy, including preventive screening, disinfection and replacement of material, genetic breeding, supplementary feeding, and mechanical reduction of pathogen loads.
Passive surveillance data suggest that the use of integrated pest management practices and natural organic acids is already well-established in Quebec (1), but this has not been confirmed by any study. The objective of this study was to document the actual disease management practices of beekeeping productions in southwestern Quebec, Canada.
In 2017, a cross-sectional study was conducted in the surveillance zone and as an extension of the active monitoring program for the small hive beetle (SHB) of the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ). This area included 126 provincially registered apiaries that belonged to 38 beekeepers and were located within 15 km of the boundary of the United States or the province of Ontario. A total of 42 apiaries in the adjacent regions of Montérégie-Ouest and Vaudreuil-Soulanges and 34 apiaries in the Pontiac region were randomly selected for mandatory inspection as part of the provincial SHB monitoring program. All beekeepers inspected were invited to participate in our project on a voluntary basis.
For the purpose of the study, the 3 following definitions were used to describe beekeeping activities: i) professional beekeepers live only from beekeeping; ii) semi-professional beekeepers derive part of their income from their beekeeping activities; and iii) hobbyists do not earn income from their hives. “Comb replacement” was defined as systematically eliminating combs in circulation in the brood chambers after a predefined number of years.
Two questionnaires (7) were developed and pre-tested by a commercial beekeeper and a professional apiculturist for clarity and time of completion. The first survey was conducted through telephone interviews with the participating beekeepers from November 21 to December 15, 2017. All questions dealt with the type of activities and the management strategies applied throughout the entire year from January to December of 2017. The second survey was conducted by telephone from May 15 to May 28, 2018 and dealt with wintering practices during the winter of 2017 to 2018. All data management and descriptive statistics were done in SAS version 9.4 (SAS Institute, Cary, North Carolina, USA).
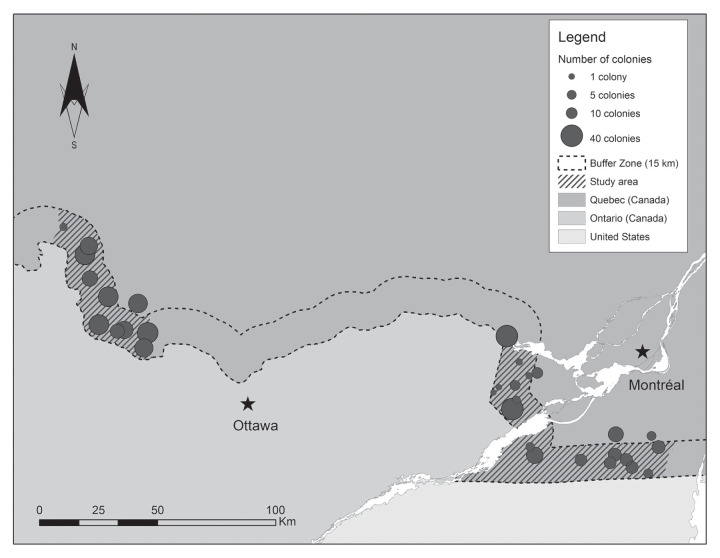
Among the 26 beekeepers who owned the 76 apiaries randomly selected for SHB inspections, 15 agreed to participate in this study, for a 58% participation rate. The geospatial distribution of the 31 participating apiaries is shown in Figure 1. For their total operation, including outside the study area, 6 beekeepers (40%) owned less than 5 colonies, 5 beekeepers (33%) owned from 6 to 80 colonies, and 4 beekeepers (27%) owned more than 80 colonies. Most of these beekeepers (7/15; 47%) operated 1 or 2 apiaries, whereas 4 beekeepers (27%) operated from 3 to 6 apiaries, and 4 beekeepers (27%) operated more than 6 apiaries. The number of colonies in an apiary ranged from 1 to 40, with a median of 14 colonies in each apiary. During the 2017 season, 3 of these 15 beekeepers (20%) produced less than 100 kg of honey, 5 beekeepers (33%) produced 100 to 1000 kg, 3 beekeepers (20%) produced 1000 to 3999 kg, and 4 beekeepers (27%) produced ≥ 4000 kg.
Figure 1.
Spatial distribution of selected apiaries owned by beekeepers participating in a study on beekeeping management practices in southwestern Quebec, 2017. The size of dot representing each apiary is proportional to their number of colonies at time of selection.
Management practices reported by beekeepers are summarized in Tables I to III. These tables depict the number of colonies in the study area owned by beekeepers that reported the use of each specific practice. The tables do not necessarily represent the number of colonies in which the practice was applied. For example, the number of colonies reported as using diagnostic tests is the number of colonies in the study area belonging to a beekeeper that used a test at least once in the season, not the number of colonies tested. The only exception is chemical control of diseases, for which the number of colonies treated is presented.
Table I.
Descriptive statistics on operation management of 15 beekeepers owning 1824 colonies in southwestern Quebec, Canada in 2017.
| Type of activities | Beekeepers | Colonies owned by beekeepersa | ||
|---|---|---|---|---|
|
|
|
|||
| Number | Percentage | Number | Percentage | |
| Occupation | ||||
| Professional | 3 | 20.0 | 1490 | 81.7 |
| Semi-professional | 8 | 53.3 | 302 | 16.6 |
| Hobbyist | 4 | 26.7 | 32 | 1.7 |
| Marketing activitiesb,c | ||||
| Pollination services | 4 | 26.7 | 1520 | 83.3 |
| Sale of honey | 11 | 73.3 | 1792 | 98.3 |
| Sale of wax | 6 | 40.0 | 1492 | 81.8 |
| Otherd | 1 | 6.7 | 1115 | 61.1 |
| No marketing activities | 4 | 26.7 | 32 | 1.7 |
| Apiary inspectione | ||||
| Internal inspection of all hives | 13 | 86.6 | 1721 | 94.3 |
| Internal inspection of some hives | 1 | 6.7 | 80 | 4.4 |
| External inspection only | 1 | 6.7 | 23 | 1.3 |
| Services offered to other beekeepersb,f | ||||
| Extraction of honey in their facilities | 2 | 13.3 | 106 | 5.8 |
| Practical training | 5 | 33.3 | 259 | 14.2 |
| Transport of hives | 2 | 13.3 | 1204 | 66.0 |
| At least one of the above | 6 | 40.0 | 1374 | 75.3 |
| Record of purchases of honey bee colonies | ||||
| Presence of purchases, with records | 1 | 6.6 | 98 | 5.4 |
| Presence of purchases, without records | 4 | 26.6 | 5 | 0.3 |
| No purchases | 10 | 66.6 | 1721 | 94.3 |
| Record of bee movements | ||||
| Presence of movements, with records | 4 | 26.7 | 1435 | 78.7 |
| Presence of movements, without records | 2 | 13.3 | 214 | 11.7 |
| No movement | 9 | 60.0 | 175 | 9.6 |
| Mortality insurance for 2017 to 2018 | ||||
| Yes | 1 | 6.7 | 35 | 1.9 |
| No | 14 | 93.3 | 1789 | 98.1 |
| Autumn feeding | ||||
| Individual | 9 | 60.0 | 1228 | 67.3 |
| Barrels | 5 | 33.3 | 561 | 30.8 |
| Mixed (both methods) | 1 | 6.7 | 35 | 1.9 |
| Honey left in the hive for wintering | ||||
| Yes | 4 | 26.7 | 102 | 5.6 |
| No | 11 | 73.3 | 1722 | 94.4 |
| Wintering method | ||||
| Indoors | 3 | 20.0 | 1227 | 67.3 |
| Outdoors | 12 | 80.0 | 597 | 32.7 |
Total number of colonies in the study area owned by beekeepers who reported the use of each specific practice. This does not necessarily represent the number of colonies in which the practice was applied.
Non-mutually exclusive categories.
Questions about marketing activities were only asked to the 11 professional and semi-professional beekeepers.
One beekeeper also produced pollen, royal jelly, and propolis. No beekeeper sold bees (nuclei, hive of bee package) or queens.
The delay between inspections ranged from 2 to 30 d (median = 7 d).
No respondent reported melting wax for other beekeepers or lending them equipment.
Table II.
Descriptive statistics on Varroa management practices of 15 beekeepers owning 1824 colonies in southwestern Quebec, Canada in 2017.
| Varroa management practices | Beekeepers | Colonies owned by beekeepersa | ||
|---|---|---|---|---|
|
|
|
|||
| Number | Percentage | Number | Percentage | |
| Screening for Varroab | ||||
| Natural mite fall | 4 | 28.6 | 115 | 6.3 |
| Total mite fall (after application of acaricide) | 3 | 20.0 | 26 | 1.4 |
| Alcohol wash | 3 | 20.0 | 1306 | 71.6 |
| Uncapping of drone cells | 6 | 40.0 | 513 | 28.1 |
| Visual identification on adult bees | 10 | 66.7 | 553 | 30.3 |
| At least one of the above | 14 | 93.3 | 1819 | 99.7 |
| Biomechanical control of Varroab | ||||
| Mesh floor in hive | 8 | 53.3 | 321 | 17.6 |
| Destruction of drone cells naturally built in the hive | 3 | 20.0 | 27 | 1.5 |
| Brood trapping (green comb frame) | 1 | 6.7 | 17 | 0.9 |
| Other method | 2 | 13.3 | 142 | 7.8 |
| At least one of the above | 10 | 66.7 | 331 | 18.2 |
| Chemical control of Varroa in springb,c | ||||
| Amitraz (Apivar) | 2 | 13.3 | 1032 | 56.6 |
| Flash treatment (formic acid) | 2 | 13.3 | 95 | 5.2 |
| At least one of the above | 3 | 20.0 | 1087 | 59.6 |
| Chemical control of Varroa in fallb,c | ||||
| Amitraz (Apivar) | 5 | 33.3 | 442 | 24.2 |
| Thymol (Thymovar) | 3 | 20.0 | 1207 | 66.2 |
| Flash treatment (formic acid) | 6 | 40.0 | 433 | 23.7 |
| Mite Away/MAQS (formic acid) | 3 | 20.0 | 30 | 1.6 |
| Oxalic acid | 2 | 13.3 | 135 | 7.4 |
| At least one of the above | 15 | 100.0 | 1850 | 101.4d |
Only the exact number of colonies concerned was asked about chemical control of Varroa. For the other questions, this table depicts the total number of colonies in the study area owned by beekeepers who reported the use of each specific practice. This does not necessarily represent the number of colonies in which the practice was applied.
Non-mutually exclusive categories.
No beekeepers used tau-fluvalinate (Apistan), coumaphos (CheckMite +), or pads for absorbing formic acid (Mite Wipes).
Some of the treated colonies were lost before the count of total colonies in fall, hence the smaller denominator.
Table III.
Descriptive statistics on other disease management practices of 15 beekeepers owning 1824 colonies in southwestern Quebec in Canada, 2017.
| Disease management | Beekeepers | Colonies owned by beekeepersa | ||
|---|---|---|---|---|
|
|
|
|||
| Number | Percentage | Number | Percentage | |
| Systematic visual inspection of brood | ||||
| Yes | 13 | 86.7 | 1814 | 99.5 |
| No | 2 | 13.3 | 10 | 0.5 |
| Use of laboratory diagnosis services for foulbrood | ||||
| Yes | 1 | 6.7 | 66 | 3.6 |
| No | 14 | 93.3 | 1758 | 96.4 |
| Preventive measures against foulbroodb | ||||
| Comb replacement | 9 | 60.0 | 1647 | 90.3 |
| Preventive disinfection of material | 1 | 6.7 | 80 | 4.4 |
| Genetic selection | 2 | 13.3 | 169 | 9.3 |
| Chemical control for foulbrood | ||||
| Oxytetracycline (prophylactic) | 1 | 6.7 | 35 | 1.9 |
| No treatment | 14 | 93.3 | 1789 | 98.1 |
| Use of laboratory diagnosis services for Nosema spp. | ||||
| Yes | 1 | 6.7 | 66 | 3.6 |
| No | 14 | 93.3 | 1758 | 96.4 |
| Chemical control for Nosema spp. | ||||
| Fumagillin | 1 | 6.7 | 5 | 0.3 |
| Nozevit | 2 | 13.3 | 316 | 17.3 |
| Apple cider vinegar | 1 | 6.7 | 17 | 0.9 |
| None | 11 | 73.3 | 1486 | 81.5 |
Only the exact number of colonies concerned was asked about chemical control of Nosema spp. For the other questions, this table depicts the total number of colonies in the study area owned by beekeepers that reported the use of each specific practice. This does not necessarily represent the number of colonies in which the practice was applied.
Non-mutually exclusive categories.
Management practices for dealing with varroosis are described in Table II. Of the 10 beekeepers applying at least 1 method of biomechanical control, most used only 1 of the following methods: mesh floors in hives (n = 6) or the systematic destruction of drone cells under the brood frames (n = 2). Two other beekeepers combined the use of mesh floors with another biomechanical control method, such as colony division to reduce the infestation load or to interrupt egg-laying. It is worth noting that 7 beekeepers, who owned approximately 80% of the colonies in this study, used amitraz. Two of these beekeepers who owned half the colonies in this study used amitraz in the spring.
Management practices for foulbrood and nosemosis are described in Table III. Among the 5 beekeepers who believed they have had European foulbrood (n = 4) and/or American foulbrood (n = 3) in their hives in 2017, only 1 beekeeper had lab samples analyzed for foulbrood, which came back negative. The diagnostic suspicion of the beekeepers was based on visual inspection of the colonies. For the 13 beekeepers carrying out regular brood inspections, the period between 2 inspections averaged 2 wk, ranging from 1 wk to 1 mo. All beekeepers who carried out regular inspections did so at least during the month of May. Almost all of these beekeepers (11/13) inspected throughout the summer. Of the 2 remaining beekeepers (who owned 10 colonies in total), 1 carried out a regular brood inspection in the spring and another in the fall and the second carried out only 1 complete brood inspection in the spring. All beekeepers except 1 (12/13) inspected the brood of all colonies, whereas 1 beekeeper inspected the brood nest only when the colony showed signs of weakness. Beekeepers inspected an average of 6 combs per brood box (from 2 to 10). For the beekeeper who reported carrying out a preventive disinfection, bleach was used in a 5-year rotation, i.e., 20% of material disinfected each year.
The 4 beekeepers who suspected European foulbrood in their hives in 2017 all indicated that they removed positive brood combs and disinfected contaminated equipment, such as hive tools. Three beekeepers reported the destruction of positive brood combs. One of these 3 beekeepers used this method for some combs and disinfection for others and withdrew positive brood boxes for 2 y. Another of these 3 beekeepers destroyed positive colonies, as well as disinfecting or destroying boxes that contained positive brood. In the case of European foulbrood, 2 beekeepers reported changing queens. All 3 beekeepers who suspected American foulbrood in their hives in 2017 reported disinfecting contaminated equipment, such as hive tools, and destroying positive brood combs. Two of these beekeepers disinfected boxes containing suspected positive brood and destroyed the suspected positive colony.
When beekeepers had severe problems that they suspected were nosemosis, 1 reported eliminating the colonies and disinfecting the equipment. When beekeepers considered the problem as mild, they changed the queen. Another beekeeper reported applying a preventive rotation of the combs, i.e., eliminating the older combs, to prevent nosemosis. Only 1 beekeeper confirmed the diagnosis with a laboratory test, which came back positive.
To our knowledge, this is the first study to describe beekeeping practices in Quebec based on a random sample of apiaries. The 38 beekeepers located in the southwestern area owned approximately 11 000 colonies spread throughout the entire province, which represented 17% of the total honey bee colonies (63 500) in Quebec in 2017. The 15 participating beekeepers owned 1824 colonies located in the studied area or 2% of the total colonies in Quebec. Therefore, although the results presented here should be viewed as preliminary, they could serve as guidelines on which to base future research.
Although about 50 large companies owned 80% of Quebec’s bee colonies in 2018, more than 1000 individual beekeepers owned the remaining 20% of colonies (8). Beekeepers owning larger enterprises had a better chance of being selected for this study considering that apiaries were selected first before they were asked to volunteer. Taking the good participation rate (58%) into account, our results probably represent the management practices of most apiaries in the study area. It should be noted that the number of colonies owned by a beekeeper varies greatly throughout the year, as colonies are often divided, united, or eliminated to deal with overcrowding or weakness. With this in mind, the numbers of colonies presented in the tables are estimations.
Apiaries should be inspected within a timeframe that allows problems in the hive to be resolved, such as absence of a queen, swarming, etc., while remaining time-efficient for the beekeeper. In an effort to standardize biosecurity practices country-wide for both small- and large-scale operations, the Canadian Food Inspection Agency (CFIA) recommends that the brood be inspected every 9 or 10 d to assess the well-being of the colony and prevent swarming (9). Eleven beekeepers (73%) reported visiting their hives at the recommended frequency or more often, whether they removed swarm cells or not. While an external inspection of the hive provides additional information, it does not allow detection of problems inside the hive, such as brood and queen problems. Almost all beekeepers (13/15) carried out routine internal inspections of all their colonies in at least 1 box of the brood chamber per hive. More than 100 hives were poorly inspected or not inspected at all during the season, however, which represents about 5% of the studied colonies.
Many beekeeping practices are known to increase the risk of horizontal transmission within a hive and among hives of the same apiary (10). Pathogens could also be transmitted between beekeeping operations by sharing services among beekeepers, although the associated risks have not been quantified to our knowledge. For pathogens that can survive away from their hosts, such as foulbrood agents, Nosema spp., the small hive beetle, and wax moths, loaning equipment, extracting hive products in shared facilities, and shared transportation services are possible indirect transmission paths (9). Most beekeepers in this study did not lend equipment and only 2/15 offered to share extraction or transportation services. This suggests that risky activities for disease transmission among beekeeping operations are not common in southwestern Quebec and that physical proximity of apiaries may play a bigger role in disease transmission between operations, if it should occur.
Purchases and movements, reported by 5 and 6 beekeepers respectively, represent a health risk, which is why record-keeping is required in the province of Quebec in order to facilitate tracing if an exotic disease is introduced. Records are not always kept, however, despite this legal obligation. It should be ensured that beekeepers are made aware of the importance of these records.
Adequate food supply is essential for the colony’s survival throughout winter (9), whether it is provided by the colony’s own honey reserve or replacement feeding. All beekeepers surveyed provided their colonies with supplementary feeding during autumn, fed individually for most of them. As diseases can be transmitted through feeders when bees defecate in the food, it can be hypothesized that barrel feeding, which was used for a third of the hives, may enhance disease transmission between colonies (10).
It is important to note that all diseases reported by beekeepers are based on their own knowledge of clinical signs. As some diseases may be very difficult to detect or differentiate based solely on clinical signs, diseases reported do not necessarily reflect the actual sanitary status of the hives. The importance of sending samples to a laboratory to be correctly identified therefore needs to be emphasized. When infectious diseases are suspected, however, beekeepers from southwestern Quebec instead apply preventive methods or destroy and disinfect combs or hives.
With regard to Varroa destructor, pesticide use is necessary, but should be reduced to a minimum in order to avoid attendant hazards to bees, honey, and the environment. Regular screening and biomechanical control measures can help to delay chemical treatment (11). The only beekeeper who did not carry out screening for V. destructor nevertheless declared that varroosis was suspected in their apiaries in 2017. This beekeeper, who owned 5 hives, felt it unnecessary to monitor V. destructor levels as they were the same each year and didn’t influence the treatment strategy. Installing mesh hive floors, acquiring SMR queens, i.e., selected for suppressed mite reproduction, removing drone brood, and brood trapping, reduce the level of infestation within a colony (11,12). However, these methods can be difficult to obtain in Quebec, time-consuming, or unpractical for medium- or large-scale beekeepers. A mesh floor in the hive was the only biomechanical control commonly used in this study (by 8/15 beekeepers).
Many synthetic pesticides, such as amitraz, tau-fluvalinate, coumaphos, and flumethrin, and natural organic compounds of varying efficiency, such as thymol and oxalic and formic acids, are used to control V. destructor and resistance has been reported for all synthetic pesticides (13). Moreover, it has been reported that coumaphos and tau-fluvalinate are toxic to bees (14) and, in the case of coumaphos, to beekeepers as well if wrongly applied (15). In the fall, most beekeepers (10/15) reported that they avoided using synthetic pesticides, using only natural organic compounds that were not reported to lead to resistance. However, many hives were treated with thymol in the fall and this treatment was not followed with the recommended application of oxalic acid. Thymol may not effectively control Varroa when used alone (16).
The brood should be examined regularly to detect signs of foulbrood, which was done by most beekeepers. Despite the common suspicion of foulbrood (17), only 1 beekeeper reported sending a sample for laboratory analysis. There are 2 distinct foulbrood diseases: European foulbrood caused by Melissococcus plutonius and American foulbrood caused by Paenibacillus larvae, both of which are difficult to differentiate clinically. American foulbrood is a highly virulent disease that poses a significant threat to beekeeping operations if not managed properly. As laboratory diagnosis is provided free-of-charge to registered beekeepers in Quebec and registration is mandatory, we conclude that there is some reason other than monetary that they are not using this service. It could be that beekeepers do not know about this service, a lack of time, a lack of trust in authorities, too long a wait to receive results, or fear of the consequences, e.g., destruction of their hives.
According to the national standard, at least 20% (ideally a third) of brood combs should be replaced every year to reduce the level of spores and acaricide residues in the hive (9). A significant proportion of beekeepers (40%) did not follow those recommendations. The massive use of antibiotics, almost exclusively oxytetracycline, for prevention purposes over 4 decades has led to antimicrobial resistance in P. larvae in the United States (4). We found that the practice of preventing or treating foulbrood diseases by using antibiotics is uncommon in southwestern Quebec.
The antibiotic fumagilin B is used to reduce spore loads in bees infected with Nosema spp. (18). The impact of N. ceranae infection is controversial, however, and poorly understood and the use of medicinal treatment is questionable due to possible inefficiency and human health risks (18,19). The beekeepers in this study rarely used this antibiotic and most beekeepers put no particular control measure in place for this disease.
This study clearly shows the wide variety of beekeeping operation sizes and activities, as well as the discrepancy in confirmation and lack of accurate diagnosis by laboratory analysis of the suspected diseases affecting bees in southwestern Quebec. Some operations do not keep records of the purchase and movement of bees, as required by the MAPAQ to improve response actions in case of disease outbreak. The participating beekeepers generally avoided use of potentially toxic acaricides, and chemical treatments associated with antimicrobial resistance in the fall, although 2 large operations used amitraz in the spring. The free laboratory support was not used, although beekeepers did use preventive, physical or management methods when infectious diseases were suspected. Efforts should focus on encouraging beekeepers to use the medical services available to them and tthe alternative strategies for controlling diseases. This should be part of educational services to large, medium, and small beekeeping operations to standardize a preventive medical approach and to avoid the spread of infectious diseases.
Acknowledgments
The authors thank the participating beekeepers for their time and the Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ) for its in-kind contribution. This study was made possible by a research grant from the Fond du centenaire of the Faculty of Veterinary Medicine of the Université de Montréal and scholarships provided to Gabrielle Claing by the Fonds de recherche du Québec — Nature et Technologies (FRQNT) and by the J. A. DeSève and Bank of Montreal Foundations through the Faculty of Graduate Studies of the Université de Montréal. This work was also made possible by the assistance of the Centre de recherche en Sciences animales de Deschambault.
References
- 1.Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec. Enquête sur la mortalité hivernale des colonies d’abeilles au Québec en 2016–2017. Bulletin zoosanitaire. 2018 [Google Scholar]
- 2.Wu JY, Anelli CM, Sheppard WS. Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PloS ONE. 2011;6:e14720. doi: 10.1371/journal.pone.0014720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu JY, Smart MD, Anelli CM, Sheppard WS. Honey bees (Apis mellifera) reared in brood combs containing high levels of pesticide residues exhibit increased susceptibility to Nosema (Microsporidia) infection. J Invertebr Pathol. 2012;109:326–329. doi: 10.1016/j.jip.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Miyagi T, Peng CY, Chuang RY, Mussen EC, Spivak MS, Doi RH. Verification of oxytetracycline-resistant American foulbrood pathogen Paenibacillus larvae in the United States. J Invertebr Pathol. 2000;75:95–96. doi: 10.1006/jipa.1999.4888. [DOI] [PubMed] [Google Scholar]
- 5.Martel A-C, Zeggane S, Drajnudel P, Faucon J-P, Aubert M. Tetracycline residues in honey after hive treatment. Food Addit Contam. 2006;23:265–273. doi: 10.1080/02652030500469048. [DOI] [PubMed] [Google Scholar]
- 6.Al-Waili N, Salom K, Al-Ghamdi A, Ansari MJ. Antibiotic, pesticide, and microbial contaminants of honey: Human health hazards. Sci World J. 2012;7:930849. doi: 10.1100/2012/930849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claing G, Dubreuil P, Ferland J, Bernier M, Arsenault J. Survey on beekeeping management practices in Southwestern Quebec. Saint-Hyacinthe, Québec: Université de Montréal; 2019. [PMC free article] [PubMed] [Google Scholar]
- 8.Ferland J. Les différents enjeux sanitaires apicoles au Québec et les activités du MAPAQ en apiculture. Bécancour: Journée d’information apicole; Nov 24, 2018. [Google Scholar]
- 9.Government of Canada, Canadian Food Inspection Agency. Section 1: Bee health management. Honey bee producer guide to the national bee farm-level biosecurity standard. Government of Canada; 2013. [Last accessed April 22, 2021]. Available from: http://inspection.canada.ca/biosecurity/honey-bee-producer-guide. [Google Scholar]
- 10.Fries I, Camazine S. Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie. 2001;32:199–214. [Google Scholar]
- 11.Delaplane KS, Berry JA, Skinner JA, Parkman JP, Hood WM. Integrated pest management against Varroa destructor reduces colony mite levels and delays treatment threshold. J Apic Res. 2005;44:157–162. [Google Scholar]
- 12.Wantuch HA, Tarpy DR. Removal of drone brood from Apis mellifera (Hymenoptera: Apidae) colonies to control Varroa destructor (Acari: Varroidae) and retain adult drones. J Econ Entomol. 2009;102:2033–2040. doi: 10.1603/029.102.0603. [DOI] [PubMed] [Google Scholar]
- 13.Milani N. The resistance of Varroa jacobsoni Oud. to acaricides. Apidologie. 1999;30:229–234. [Google Scholar]
- 14.Johnson RM, Pollock HS, Berenbaum MR. Synergistic interactions between in-hive miticides in Apis mellifera. J Econ Entomol. 2009;102:474–479. doi: 10.1603/029.102.0202. [DOI] [PubMed] [Google Scholar]
- 15.Moretto A, Lotti M. Poisoning by organophosphorus insecticides and sensory neuropathy. J Neurol Neurosurg Psychiatry. 1998;64:463–468. doi: 10.1136/jnnp.64.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregorc A, Planinc I. The control of Varroa destructor in honey bee colonies using the thymol-based acaricide — Apiguard. Am Bee J. 2005;145:672–675. [Google Scholar]
- 17.Claing G. [MSc thesis] Saint-Hyacinthe, Québec: Université de Montréal; 2019. Prévalence d’agents pathogènes de l’abeille domestique (Apis mellifera) au Québec et leur impact sur la mortalité hivernale. [Google Scholar]
- 18.Williams GR, Shutler D, Little CM, Burgher-Maclellan KL, Rogers REL. The microsporidian Nosema ceranae, the antibiotic Fumagilin-B®, and western honey bee (Apis mellifera) colony strength. Apidologie. 2011;42:15–22. [Google Scholar]
- 19.Huang W-F, Solter LF, Yau PM, Imai BS. Nosema ceranae escapes fumagillin control in honey bees. PLoS Pathog. 2013;9:e1003185. doi: 10.1371/journal.ppat.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]