Abstract
Background and Objectives:
Current methods of classifying individuals with Substance Use Disorder (SUD) result in vast heterogeneity among persons within a given diagnosis. These approaches, while clinically allowing for distinctions between patient groups, are less than ideal when attempting to recruit a neurobehaviorally- defined subset of subjects into clinical trials. To address this gap, alternative strategies have been proposed, including behavioral phenotyping. The NIDA Phenotyping Assessment Battery (PhAB) is a modular package of assessments and neurocognitive tasks that was developed for use in clinical trials. The goal of the present study is to assess the feasibility of the NIDA PhAB with regard to ease of administration and time burden.
Methods:
Healthy controls, persons with Cocaine Use Disorder (CocUD), Opioid Use Disorder (OUD), Cannabis Use Disorder (CanUD), and combined Opioid and Cocaine Use Disorder (OCUD) were recruited from various sources (N=595). Participants completed screening and 1–3 assessment visits. Time to complete the measures was recorded and a satisfaction interview was administered.
Results:
Of the participants enrolled, 381 were deemed eligible. The majority of eligible participants (83%) completed all assessments. The average completion time was 3 hours. High participant satisfaction ratings were noted, with over 90% of participants endorsing a willingness to participate in a similar study and recommend the study to others.
Conclusion and Scientific Significance:
These findings corroborate the ease with which the PhAB may be easily incorporated into a study assessment visit without undue participant burden.
The PhAB is an efficient method for behavioral phenotyping in addiction clinical trials.
Introduction
Success in the realm of clinical drug development, besides drug candidate factors, rests on the precision of subject characterization, amid study sample homogeneity, and the mechanistic linkage between physiologic system dysfunctions and overt deficits with specific symptoms and syndromes in any given clinical trial. There is an extreme heterogeneity among subjects in clinical studies of psychiatric disorders, including addiction. When entering a clinical trial, a subject is diagnosed using DSM-5 classification of substance use disorders (SUD). SUD can be diagnosed if the subject meets at least 2 of 11 criteria in the last 12 months.1 The number of symptom permutations that confer an SUD diagnosis is very large (>1000), even when the severity criterion is taken into account. There is no psychometric scale available for SUD that could more precisely place the subject on a continuum of disease expression (eg, akin to the Montgomery–Åsberg or Hamilton Depression Rating Scale in major depressive disorder). To address this, psychiatry research is increasingly exploring “biotypes” within specific diagnoses such as schizophrenia or depression, by applying machine-learning and other analyses to examine how features, probed across the genetic, brain, and behavioral assessments differentially aggregate.2–4 This approach to SUDs is lagging.
To compound the variability of symptom permutations to exceed a threshold for SUD, the primary substance of use is oftentimes the principal way in which participants are characterized within diagnosed SUD. This is despite how preference for different substances of abuse could be driven by the same core neurobiological mechanism or motivation. Not surprisingly, defining SUD clinical trial enrollees by preferred substance, irrespective of mechanistic, comorbidity, or motivational factors, together with heavy reliance on DSM-based diagnosis hampers the development of new drugs and treatments for SUD. Getting beyond the DSM-5 based definitions is necessary to “fingerprint” different addiction phenotypes, to identify the predisposition to addiction elements and addiction endophenotypes using machine-learning analysis for big data.
The NIMH Research Domain Criteria (RDoC) dimensional approach to psychiatric disorder diagnosis has provided an opportunity for evaluation of medication impacts on specific neurofunctional domains and constructs.5 The subsequent application of RDoC principles to addiction has led to the Alcohol and Addictions RDoC (AARDoC) variant, where neurofunctional abnormalities in SUD are indexed by the three domains of Negative Emotionality, Incentive Salience, and Executive Function. These domains are ostensibly probed by the Addictions Neuroclinical Assessments (ANA) Battery, a compendium of assessments that includes measures related to attention, impulsivity, aggression, personality, and substance use, and requires approximately 10 hours to complete.6
More recently, to enable a neurofunctional domain-based “fingerprinting” approach to a range of addictions beyond alcohol and to improve upon the initial approach, NIDA sought to develop a harmonized phenotyping battery of assessments and self-rated psychometric scales, supplemented by optional resting-state functional magnetic resonance imaging (fMRI) that could be easily administered to participants in any extramural clinical trial where SUDs are assessed. The NIDA Phenotyping Assessment Battery (PhAB) is meant to be administered during a Phenotyping visit, which is an extension of a screening visit in any addiction clinical trial protocol, with minimal extra time burden placed on the participant.
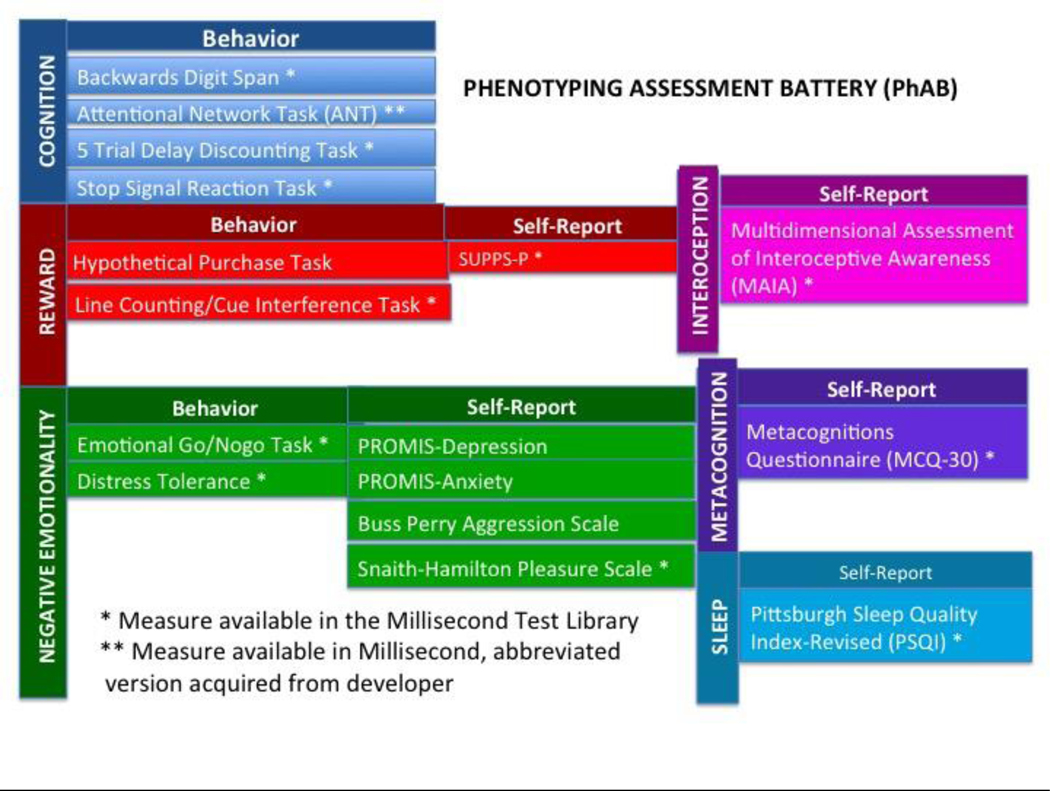
To develop the PhAB, first NIDA assembled an internal workgroup across its institute divisions and NIAAA to build upon the existing 3-domain model.6 Using a Delphi method, an expert panel of addiction researchers was then assembled to participate in a Phenotyping Battery Workshop. The workshop convened in February 2017, to finalize the battery content via consensus of consultants and discussion participants, including NIDA Workgroup members (See “Acknowledgments” section). The resulting NIDA Phenotyping Assessment Battery (PhAB) included six addiction-relevant neurofunctional domains, with Interoception, Metacognition, and Sleep/Circadian Rhythm added to the original 3-domain AARDoC structure (see Fig 1). The specific assessment(s) to capture each phenotyping domain was also determined via consensus from the Workgroup.
Figure 1.
NIDA phenotyping battery domains.
The NIDA PhAB is designed to be administered as a set to characterize “core” addiction-relevant domains in a harmonized way, for example, across NIDA clinical trials This core PhAB can then be supplemented with modular optional “platform” assessments depending on study aims, population sample, etc. Thus, in addition to the core PhAB, the Workgroup also developed an ancillary flexible set of “platform” measures that can be administered in conjunction with the PhAB in any addiction clinical trial during the Phenotyping visit, per their relevance to particular clinical trial. We feasibility-tested both the core-PhAB and the platform assessments. Platform instruments included self-report scales of symptom severity (e.g., Adult Self-Report Scale for ADHD (ASRS-ADHD), Visual Analog Scale for Pain (VAS-Pain), trauma history (Trauma History Questionnaire (THQ)), computer-administered measures of intelligence (e.g., Shipley), and substance use measures (Fagerstrom Test for Nicotine Dependence (FTND), Timeline Follow-back), and so forth.7– 12 Clinical trial investigators would administer these scales and behavioral tasks per their choice in addition to protocol’s nonspecific assessments (e.g., demographics) and medical evaluations (e.g, medical history and physical exams, genotyping, and labs) to more precisely characterize each subject.
Because the expert panel recommended tasks for NIDA PhAB have never been administered in tandem in a contiguous assessment battery, the primary goal of the present study was to determine the feasibility and acceptability of the assessment battery, including the ease of administration and participant burden, participant completion rates, and participant satisfaction. Further, data from the feasibility study would be used to inform any additional modifications to the assessment battery composition and/or administration, in efforts to refine the battery.
METHODS
Acquisition of measures.
Given that the ultimate goal for the NIDA PhAB and its platform assessments is widespread adoption as a common assessment battery across addiction clinical trials, several practical considerations were addressed. Measures utilized for the PhAB needed to be widely available, at low or no cost to investigators, easy to administer (requiring minimal instrument-specific formal training of research staff), and relatively brief. Fortunately, many of the assessments were (and are) available for download through the Millisecond Test Library (www.millisecond.com), and are free to use with an Inquisit software license. Additional measures were able to be added to the Millisecond Library for a nominal fee and are now available to other investigators.
In addition to utilizing the Millisecond Library for many of the tasks, hard copy self-report measures were converted into electronic forms (using REDcap electronic data capture system) which allowed for direct computer-based entry by study participants to enhance the efficiency and accuracy of the data collection (permissions were obtained, when indicated). REDcap also has a function that can be enabled to allow participants the opportunity to click on items to have them read aloud to them (reducing issues associated with literacy and/or visual impairments).
In consultation with NIDA program staff, some of the originally proposed measures were eliminated due to cost considerations, wherein reliance on proprietary assessments may inhibit widespread adoption of the PhAB across clinical trials. The State-Trait Anger Expression Inventory (STAXI-2) was replaced with the widely available Buss Perry Aggression Scale, and the Childhood Trauma Questionnaire (CTQ) was exchanged for the Trauma History Questionnaire.9,13 Other modifications included replacing the originally-recommended Facial Emotion Matching Task (for fMRI) with the Emotional Go-Nogo Task, to more appropriately capture cognitive interference by face-emotion valence that was sensitive to behavioral differences at the desktop.14,15 We also eliminated the recommended Choice Task, as it was cocaine-specific, and its vivid drug stimuli were not considered appropriate for individuals who are seeking treatment or for use outside the magnetic resonance imaging (MRI) scanner.16
Eligibility criteria
Eligibility criteria were relaxed to recruit a heterogeneous sample of persons with SUD along with healthy controls. Inclusion criteria for both groups consisted of age between 18 and 70 years, and ability to complete forms and interviews in English. Individuals enrolled as substance users also had to meet DSM5 criteria for current primary SUD for either opioids, marijuana, and/or stimulants. Conditions considered exclusionary were: current psychosis, mania, suicidal/homicidal ideation, history of seizures (excluding childhood febrile seizures), or loss of consciousness from traumatic injury for more than 30 minutes, or any other illness, or condition, which in the opinion of the PI or study physician would preclude safe and/or successful completion of the study. For substance users, DSM-5 diagnosis of any psychoactive SUD other than opioids, marijuana, stimulants, or nicotine was considered exclusionary (although mild to moderate comorbid Alcohol Use Disorder was allowed), healthy controls could not meet DSM-5 criteria for a substance use disorder or current psychiatric disorder.
Recruitment and Screening
The Institutional Review Board at Virginia Commonwealth University approved the study and written informed consent was obtained from all study participants. Individuals were recruited from an established participant registry (VCU IRB# HM20000294, Keyser-Marcus (PI)), and from local advertising. Participants were informed that the goal of the study was to try out a newly developed battery of tests and to determine how much time (on average) it would take for people to complete the study. Compensation was provided for study participation.
The initial eligibility screening study visit included: the MINI International Neuropsychiatric Interview V 7.0.2 (MINI); to determine DSM-5 diagnoses, the Columbia Suicide Severity Rating Scale, collection of biological measures, including vital signs (oral temperature, sitting blood pressure, pulse, respiratory rate, and weight), and urine specimens collected for pregnancy testing (females only), and urine drug screen for cocaine (benzoylecgonine), opiates and opioids (including fentanyl), benzodiazepines, amphetamine, methamphetamine and THC.1,17 Breath alcohol and carbon monoxide (for recent smoking) levels were also obtained.
Phenotyping Assessment Visit
The Phenotyping Assessment Visit was designed a priori to take approximately 3.5 – 4 hours to complete, including scheduled rest breaks. Upon arrival at the clinic, participants first completed (non-PhAB) biological measures, which included vital signs (oral temperature, sitting blood pressure, pulse, respiratory rate, and weight), urine and breath samples. Urine specimens were also collected for pregnancy testing (which was only exclusionary for the MRI portion of the study, not detailed here), and urine drug, breath alcohol and carbon monoxide collection as per the screening visit. As described above, the assessment battery included the phenotyping measures, as well as the ancillary platform measures.
The supplemental platform instruments were not administered in a fixed sequence. Efforts were made to administer as many of the Platform Instruments at the time of the Screening Visit (as time permitted). Otherwise, they were administered during the Phenotyping Visit, in conjunction with the core PhAB assessments. The Phenotyping Measures were administered in non-fixed order. Subjects completed a satisfaction interview immediately following the conclusion of the core PhAB assessment battery. Participants were asked to rate their level of agreement with questions pertaining to likelihood of participating in a similar study in the future, recommending the study to family and/or friends, accuracy of initial estimate of time required provided to them at the beginning of the study, and accuracy of the study description presented to them by research staff. Ratings were made on a 5-point Likert-type scale ranging from 1(Strongly disagree) to 5 (Strongly agree).
RESULTS
Participant Demographics
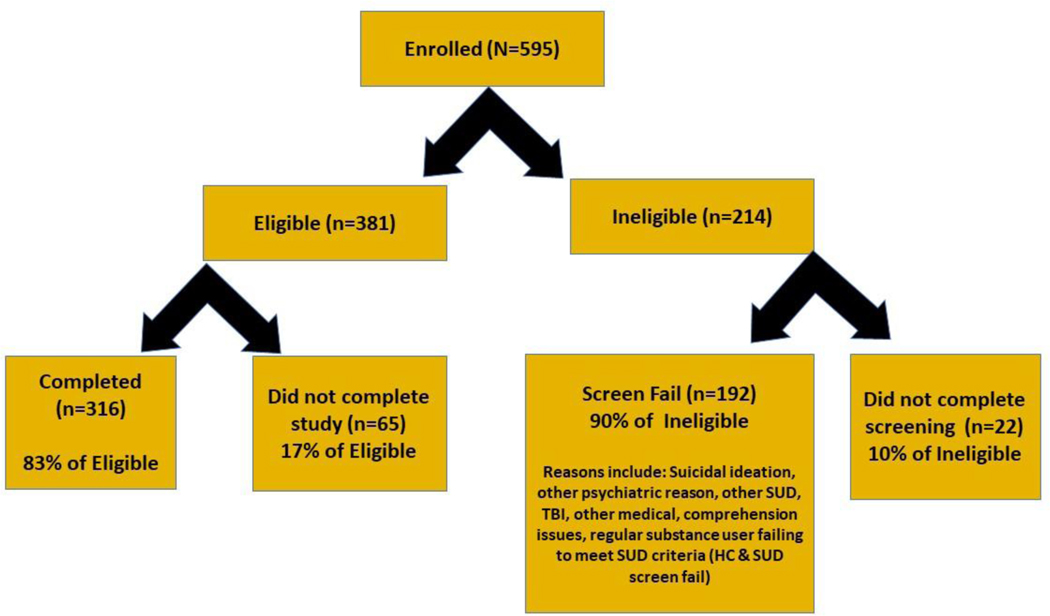
Enrollment started in June 2018, and 595 subjects were enrolled in the study. Of the participants enrolled and screened on-site, 381 (64%) subjects were determined to be eligible, 192 (33%) were deemed ineligible for participation (screen fails), and 22 (3%) did not complete screening and were lost to follow-up. In all, 316 subjects completed the study. Sixty-five subjects were dropped or elected to withdraw from the study post-eligibility screening. This included 56 participants (86%) who did not complete any portion of the assessment visit (due to no-shows/lost to follow-up, time constraints, personal/family issues, and entering residential treatment), 9 (14%) individuals who completed a portion of the assessment visit but were unable to return or lost to follow-up, and (n=5), and (n=4) individuals who were unable to complete the assessment visit due to signs of drug intoxication or withdrawal and were unable to return/lost to follow-up (see Fig 2). Demographically, study completers ranged in age from 18–70, with an average age of 41 years, and were primarily African American (64%), never married (55%), and male (52%) (see Table 1).
Figure 2.
PhAB Feasibility study enrollment
Table 1:
Participant Characteristics (N=316)
| Age (mean, range) | 40.77 (18–70 years) |
|---|---|
| Sex | |
| Male | 166 (52.5%) |
| Female | 150 (47.5%) |
| Race | |
| Black/African American | 203 (64.3%) |
| White/Caucasian | 88 (27.8%) |
| Asian | 10 (3.2%) |
| American Indian/Alaskan Native | 5 (1.6%) |
| Other/Refused | 10 (3.1%) |
| Ethnicity | |
| Hispanic/LatinX | 7 (2.2%) |
| Non Hispantic/LatinX | 289 (91.5%) |
| Refused/Missing | 20 (6.3%) |
| Marital Status | |
| Never married | 175 (55.4%) |
| Married/Living with partner | 82 (25.9%) |
| Separated/Divorced | 50 (15.9%) |
| Widowed | 6 (1.9%) |
| Refused | 3 (0.9%) |
| Employment pattern (past 30 days) | |
| Full-time (35+hours/week) | 101 (32.0%) |
| Part time, regular hours | 28 (8.9%) |
| Part time, irregular hours | 27 (8.5%) |
| Student | 36 (11.4%) |
| Military service | 1 (0.3%) |
| Retired/Disabled | 28 (8.9%) |
| Homemaker | 8 (2.5%) |
| Unemployed | 83 (26.3%) |
| Refused | 4 (1.2%) |
| Primary Substance Use Diagnosis | |
| Healthy Controls | 108 (34.2%) |
| Opioid | 97 (30.7%) |
| Cocaine | 50 (15.8%) |
| Cannabis | 51 (16.1%) |
| Opioid and Cocaine | 10 (3.2%) |
Battery Completion and Assessment Times
The majority (83%) of subjects who were eligible for participation completed the study. Study completion rates by cohort ranged from 75% (opioid use disorder OUD group) to 93% (cocaine use disorder CoCUD group). Assessment completion times are presented in Table 2. The lengthiest assessments included in the Platform portion of the battery were the Shipley-2 (approximately 22 minutes), the Negative Emotional Temperament scale of the Multidimensional Personality Questionnaire (MPQ-NEM, approximately 18 minutes), and the Trauma History Questionnaire (approximately 7 minutes).18 The remainder of the platform assessments each took approximately 5 minutes or less to complete. A significant (albeit small) negative correlation between education and PhAB completion time (r= −.26, n=313, p<.001) was noted, as well as a slightly stronger negative relationship between education and platform battery completion time (r= −.32, n=313, p<.001).
Table 2.
Battery Completion Times
| PLATFORM MEASURES | Domain | AdministrationType | Completion time (mins) |
|---|---|---|---|
| Computerized Shipley-2 | IQ/Achievement | Software | 21.9 |
| Adult Self-Report Symptom (ASRA) Checklist | ADHD | Redcap | 4.3 |
| Recent Life Events Questionnaire (Adults). | Life events | Redcap | 4.7 |
| Family Tree Questionnaire (FTQ) | Family history substance use | Redcap | 2.7 |
| Trauma History Questionnaire (THQ). | Trauma | Redcap | 7.4 |
| PTSD Checklist for DSM5. (PCL5) | Trauma | Millisecond | 3.3 |
| Relationship Scale Questionnaire (RSQ) | Attachment style | Redcap | 5.7 |
| Visual Analog Scale for Pain (VAS-pain) | Pain | Redcap | 1.1 |
| Timeline Follow Back for Drug, Alcohol, Tobacco use | Substance use | Interview | 3.2 |
| Fagerström Test for Nicotine Dependence (FTND) | Substance use | Millisecond | <1 |
| Brief Substance Craving Scale (BSCS) | Substance use | Millisecond | 1.8 |
| WHO QOL Bref | Quality of life | Millisecond | 5.2 |
| Toronto Alexithymia Scale (TAS-20) | Emotional awareness | Millisecond | 3.0 |
| Positive and Negative Affect Schedule (PANAS-20) | Affect | Millisecond | 2.2 |
| Multidimensional Personality Questionnaire- Negative Emotional Temperament (MPQ-NEM). | Affective reactivity | Redcap | 17.7 |
| WHO Disability Assessment Schedule (WHO-DAS) | Health & disability | Redcap | 2.5 |
| Levenson Self-Report Psychopathy Scale (LSRP) | Psychopathy | Millisecond | 4.2 |
| PHENOTYPING MEASURES | |||
| Attentional Network Test (ANT) | Cognition | Millisecond/ Developer | 27.3/ 11.1 |
| Stop Signal Reaction Task (SST) | Millisecond | 10.7 | |
| Visual Digit Span (Backwards) | Millisecond | 7.8 | |
| 5-Trial Adjusting Delay Discounting task | Millisecond | 1.1 | |
| Hypothetical Purchase Task. | Reward | Redcap/ Developer | 4.7 |
| Line counting/cue interference task | Millisecond | 10.3 | |
| SUPPS-P | Millisecond | 3.0 | |
| Multidimensional Assessment of Interoceptive Awareness (MAIA) | Interoception | Millisecond | 4.4 |
| Emotional Go-Nogo Task | Negative Emotionality | Millisecond | 9.3 |
| Distress Tolerance Scale (DTS) | Millisecond | 2.5 | |
| PROMIS-Anxiety | Redcap | 1.1 | |
| PROMIS-Depression | Redcap | 1.1 | |
| Buss-Perry Aggression Scale | Redcap | 5.6 | |
| Snaith-Hamilton Pleasure Scale (SHAPS) | Millisecond | 1.9 | |
| Metacognitions Questionnaire (MCQ-30) | Metacognition | Millisecond | 3.9 |
| Pittsburgh Sleep Quality Index-Revised | Sleep | Millisecond | 4.1 |
Initial feasibility monitoring revealed that the average time to completion for the Millisecond Attentional Network Task (ANT) (for the first 43 participants) was roughly 27 minutes. Subsequently, an alternate version of the ANT task that took 11 minutes on average to complete was made available by the developer and substituted for the remainder of the study.18 The majority of the remaining (core) PhAB assessments were each completed within approximately 5 minutes, except for the Stop Signal Reaction task, Line counting/cue interference task, the Emotional go/nogo task, and the Visual Digit Span, which were completed within approximately 10 minutes each (See Table 2).15, 19–21
The (core) PhAB visit was completed within an average of 3 hours, exclusive of rest breaks, which typically lasted a cumulative 15–20 minutes. The average time to completion for the core PhAB portion of the battery was 83 minutes, and the supplemental platform assessment battery was 92 minutes. Although subjects were highly encouraged to complete the core PhAB within a single visit, to enhance completion rates and participant retention, the option to return for a second assessment visit was made available and utilized by 8% of participants. The individuals who required three study visits took significantly longer (p=.005), to complete the platform assessment battery (100.8 minutes versus 81.8 minutes for participants completing in 1–2 study visits). However, no differences were noted for the core-PhAB times.
Participant Feedback/Satisfaction
Participant feedback was overwhelmingly positive, with 95% of participants endorsing “Agree” or “Strongly Agree” that if given the opportunity they would complete a similar study in the future, and 92% endorsed “Agree” or “Strongly Agree” to the question asking if they would recommend the study to their family or friends. Additionally, 87% of participants reported that they “Agree” or “Strongly agree” with the statement that the estimated amount of time to complete the study was accurately presented to them by the research staff. Finally, 95% of respondents stated that they “Agree” or “Strongly agree” that the staff did a good job of accurately explaining what would be required of them for the study.
Discussion
A better understanding of the diagnostic heterogeneity among individuals with SUD is crucial for understanding the etiologic and functional differences among them to propel tailored pharmacological and behavioral interventions to individuals with specific neurobehavioral traits. Deep phenotyping is one such approach that affords greater sensitivity and specificity in distinguishing among individuals with SUDs and their anticipated responses to various treatment strategies. We found that the NIDA PhAB is a feasible and efficient method to characterize individuals with SUDs along key addiction neurofunctional domains. The PhAB provides additional flexibility in terms of selecting associated supplemental Platform instruments, as indicated for the focus of an investigation. Further, assessments included in the NIDA PhAB are widely available, require minimal staff training, and were not considered burdensome by participants. These findings, together with the ~3 hour completion time, bode well for widespread adoption of this battery, especially relative to the ANA that takes 10 hours to complete.6 The time savings (approximately 7 hours), combined with computer-based administration of the many of the PhAB instruments, serves to keep both participant and staff burden at a minimum, while providing investigators with a wealth of neurobehavioral and psychosocial information.
Limitations of the present study must be noted. Although eligibility criteria were designed to promote the recruitment of a heterogeneous sample of individuals with different SUDs, approximately two-thirds (64.3%) of study participants were African-American, and the study was performed at a single site in an urban-based university setting. Given this, the generalizability of the results to other populations must be interpreted with caution. Similarly, the high participant satisfaction ratings may not generalize to non-compensated assessment settings. Finally, although results from the current study show feasibility and tolerability of a deep phenotyping battery for addiction, additional data and analyses will be needed to validate and demonstrate the utility of the data. For example, corroboration of the theoretical constructs that comprise the six domains through factor analytic techniques is warranted and is currently in progress.
Given the recent onset of the COVID-19 pandemic, remote methods of assessment are highly desirable. Many of the assessments included in the NIDA PhAB can be launched remotely and completed via smartphone, or other electronic devices with internet connections (e.g., tablet, computer). Although self-report assessments lend themselves to remote administration, less is known about the validity of performance measures collected remotely (in an uncontrolled environment). Future research should focus on the feasibility and validity, and test-retest reliability of assessments across modes of delivery.
Acknowledgements:
The authors would like to acknowledge the NIDA Phenotyping Battery Delphi method workgroup participants. Academically affiliated members included: Drs. Warren Bickel (Virginia Tech Carilion School of Medicine), Kathleen Carroll (Yale University School of Medicine), Anna Rose Childress (Perelman School of Medicine, University of Pennsylvania), Dennis Donovan (University of Washington School of Medicine), Jin Fan (Queens College), Rita Goldstein (Icahn School of Medicine at Mt Sinai), Deborah Hasin (Columbia University Medical Center), Michael Milham (Child Mind Institute), Martin Paulus (Laureate Institute for Brain Research), Trevor Robbins (University of Cambridge, UK), John Rotrosen (New York University School of Medicine), Kathleen Merikangas (Yale University School of Medicine), and Susan Tapart (University of California, San Diego). NIDA affiliated workgroup members included Drs. Tatiana Ramey, Meyer Glantz, Udi Ghitza, Geetha Subramaniam, Shelley Su, Kevin Conway, Harold Gordon, and Steven Grant, and Dr Laura Kwako (NIAAA representative). Finally, thanks go to Dr Nora Volkow (NIDA) for her input and feedback during the development of the NIDA Phenotyping Battery. Please contact the corresponding author (Dr Keyser-Marcus) with any inquiries regarding access to the PhAB assessments.
Statement of support:
This research was supported by the National Institute on Drug Abuse (Bethesda, MD) by a supplement awarded through the NIH HEAL Initiative under award number U54DA038999, F Gerard Moeller, PI. Dr Tatiana Ramey was substantially involved in U54DA038999, consistent with her role as Scientific Officer. The views and opinions expressed in this manuscript do not necessarily represent the views, official policy, or position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies.
References
- 1.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22–57 [PubMed] [Google Scholar]
- 2.Mothi SS, Sudarshan M, Tandon N, et al. Machine learning improved classification of psychoses using clinical and biological stratification: Update from the bipolar-schizophrenia network for intermediate phenotypes (B-SNIP). Schizophr Res. 2019;214:60–69. doi: 10.1016/j.schres.2018.04.037 [DOI] [PubMed] [Google Scholar]
- 3.Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9–24. doi: 10.1002/da.22556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latzman RD, DeYoung CG, HiTOP Neurobiological Foundations Workgroup. Using empirically-derived dimensional phenotypes to accelerate clinical neuroscience: The Hierarchical Taxonomy of Psychopathology (HiTOP) framework. Neuropsychopharm. 2020; 45, 1083–1085. doi: 10.1038/s41386-020-0639-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28–35. doi: 10.1002/wps.20087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D. Addictions Neuroclinical Assessment: A neuroscience-based framework for addictive disorders. Biol Psychiatry. 2016;80(3):179–189. doi: 10.1016/j.biopsych.2015.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adler LA, Spencer T, Faraone SV, et al. Validity of pilot Adult ADHD Self- Report Scale (ASRS) to rate Adult ADHD symptoms. Ann Clin Psychiatry. 2006;18(3):145–148. doi: 10.1080/10401230600801077 [DOI] [PubMed] [Google Scholar]
- 8.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13(4):227–236. doi: 10.1002/nur.4770130405 [DOI] [PubMed] [Google Scholar]
- 9.Hooper LM, Stockton P, Krupnick JL, Green BL. (2011) Development, use, and psychometric properties of the Trauma History Questionnaire. J Loss Traum. 16:3, 258–283, DOI: 10.1080/15325024.2011.572035 [DOI] [Google Scholar]
- 10.Shipley WC, Gruber CP, Martin TA, Klein AM. Shipley-2 Manual. Western Psychological Services Los Angeles: CA. 2009. [Google Scholar]
- 11.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- 12.Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav. 2014;28(1):154–162. doi: 10.1037/a0030992 [DOI] [PubMed] [Google Scholar]
- 13.Buss AH, Perry MP. (1992). The aggression questionnaire. J Pers Soc Psychol 63: 52–459. [DOI] [PubMed] [Google Scholar]
- 14.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17(1):317–323. doi: 10.1006/nimg.2002.1179 [DOI] [PubMed] [Google Scholar]
- 15.Tottenham N, Hare TA, Casey BJ. Behavioral assessment of emotion discrimination, emotion regulation, and cognitive control in childhood, adolescence, and adulthood. Front Psychol. 2011;2:39. Published 2011 Mar 16. doi: 10.3389/fpsyg.2011.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moeller SJ, Maloney T, Parvaz MA, et al. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol Psychiatry. 2009;66(2):169–176. doi: 10.1016/j.biopsych.2009.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Posner K, Brent DA, Lucas C, et al. Columbia-Suicide Severity Rating Scale (C-SSRS). Center for Suicide Risk Assessment. Columbia University Medical Center; New York: NY. 2009. [Google Scholar]
- 18.Tellegen A. Manual for the Multidimensional Personality Questionnaire. Minneapolis: University of Minnesota Press. 1995. [Google Scholar]
- 19.Verbruggen F, Logan GD, Stevens MA. STOP-IT: Windows executable software for the stop-signal paradigm. Behav Res Met. 2008; 40(2), 479–483. [DOI] [PubMed] [Google Scholar]
- 20.Woods DL, Kishiyama MM, Yund EW, Herron TJ, Edwards B, Poliva O. Improving digit span assessment of short-term verbal memory. J Clin Exp Neuropsychol. 2011; 33:101–111. 10.1080/13803395.2010.493149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passamonti L, Luijten M, Ziauddeen H, et al. Atomoxetine effects on attentional bias to drug-related cues in cocaine dependent individuals. Psychopharmacology (Berl). 2017;234(15):2289–2297. doi: 10.1007/s00213-017-4643-4 [DOI] [PMC free article] [PubMed] [Google Scholar]