Abstract
This purpose of this study is to correlate a new shear wave elastography (SWE) parameter, mass characteristic frequency (fmass), and other elasticity measure with the prognostic histological factors and immunohistochemical (IHC) biomarkers for the evaluation of heterogeneous breast carcinomas. The new parameter, fmass , first introduced in this paper, is defined as the ratio of the averaged minimum shear wave speed taken spatially within ROIs to the largest mass dimension. 264 biopsy-proven breast cancerous masses were included in this study. Mean (Emean), maximum (Emax), minimum (Emin) shear wave elasticity and standard deviation (Esd) of shear wave elasticity were found significantly correlated with tumor size, axillary lymph node (ALN) status, histological subtypes and IHC subtypes. The areas under the curve (AUCs) for the ALN prediction are 0.73 (0.95 CI 0.67-0.79) and 0.75 (0.95 CI .64-0.81) for the combination of Emean with BI-RADS score and Emax with BI-RADS score, respectively. fmass was significantly correlated with the presence of calcifications, ALN status, histological grade, the expressions of IHC biomarkers and IHC subtypes. To conclude, poor prognostic factors were associated with high shear wave elasticity values and low mass characteristic frequency value. Therefore, SWE provides valuable information that may help with prediction of breast cancer invasiveness.
Keywords: Breast cancer, Prognostic histological features, Immunohistochemical biomarkers, Shear wave elastography, Mass characteristic frequency
Introduction
Breast cancer is a complex and heterogeneous disease at the molecular level (Hanahan and Weinberg 2011). Intra-tumor heterogeneity, diverse molecular and phenotypical profiles within the same tumor are associated with poor prognosis, leading to therapeutic resistance and treatment failure (y Cajal, et al. 2020). Therefore, the advantages of assessing prognostic and predictive factors are becoming increasingly apparent.
Histological grade, lymph node involvement and mass diameter are all important prognostic factors (Carter, et al. 1989, Elston and Ellis 1991, Liu, et al. 2019, Sánchez-Muñoz, et al. 2008, Tang, et al. 2009, Youk, et al. 2013). In addition, statuses of immunohistochemical (IHC) biomarkers, usually obtained from core biopsies, or from the surgical specimen, including estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status and Ki-67 proliferation index, are used for subtype classification (Tamaki, et al. 2013, Tang, et al. 2009). Expressions of ER and PR are used to determine if the patient should be recommended hormone therapy (Harbeck and Wuerstlein 2019). HER2 over-expression indicates a poorer prognosis and these tumors may benefit from targeted anti-HER2 therapy (Cobleigh, et al. 1999). Elevated Ki-67 proliferation index is a negative prognostic factor (Dowsett, et al. 2011). However, results of these biomarker assays from core needle biopsy might be different from the result on surgical excision due to the tumor heterogeneity or sampling errors (Mann, et al. 2005).
Correlation between tumor stiffness and aggressive biology of breast cancer has been studied and findings indicate that stiffness regulates pro-metastatic behaviors of cancer (Acerbi, et al. 2015, Fenner, et al. 2014) and corresponds with tumor progression and metastasis. Accordingly, quantitative estimation of tumor stiffness can potentially add useful information similar to the prognostic features of heterogeneous breast carcinoma. Shear wave elastography (SWE) is a qualitative and quantitative method for measuring tissue stiffness with high reproducibility (Chang, et al. 2011, Evans, et al. 2010). It has been shown that, generally, higher shear wave elasticity values were associated with poorer prognostic factors of invasive breast cancer (Chang, et al. 2013, Evans, et al. 2014, Evans, et al. 2012) and lower survival rate when compared to those with lower shear wave elasticity values (Machida, et al. 2018).
The primary goal of this study was to investigate the relationship between SWE parameters and prognostic factors, including mass diameter, axillary lymph node (ALN) status, presence of calcifications, histologic type, histologic grade, and the statuses of biomarkers (ER, PR, HER2 and Ki-67). In addition, a new shear wave parameter, mass characteristic frequency fmass, has also been introduced in this study. We define fmass as the ratio of the averaged minimum shear wave speed (SWS) within regions of interest (ROIs) to the largest mass dimension. This new parameter can be interpreted as the inverse of the maximum shear wave propagation time in a breast mass. In this study, fmass was successfully correlated with these prognostic histologic features and IHC biomarkers.
Materials and Methods
Patients
The prospective study received institutional review board approval (IRB-Application # 12-003329) and was Health Insurance Portability and Accountability Act (HIPAA) compliant. A signed written informed consent with permission for publication was obtained from each enrolled patient prior to the study. From April 2014 to September 2020, women volunteers with suspicious breast masses and scheduled for breast biopsy were considered for SWE study. During the recruitment, patients with breast implants or mastectomies were excluded. Shear wave acquisition was performed before biopsy. Of total of 678 patients who underwent biopsy and SWE study, 379 patients with benign lesions were excluded since the purpose of this study was to investigate the correlation between the aggressiveness of malignant mass and the SWE parameters.4 patients were excluded because the cancer was not from breast origin. Therefore, 261 patients (age range: 27-89 years, mean age: 61.8±12.6 years, median age: 62 years) with 264 biopsy-proven malignant masses were finally included in this study. The participants were summarized in Fig. 1.
Fig. 1.
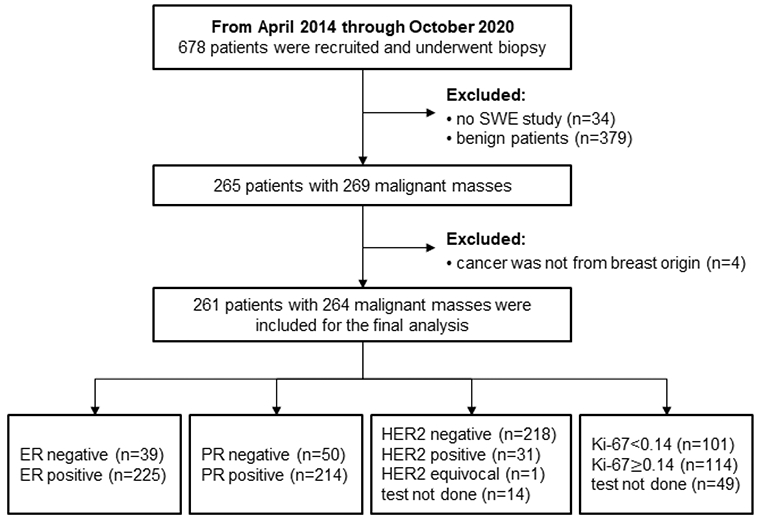
Summary of the immunohistochemical results for the 264 masses included in this study. ER=estrogen receptor status. Abbreviations: PR=progesterone receptor; HER2= human epidermal growth factor receptor 2; SWE=shear wave elastography.
Imaging
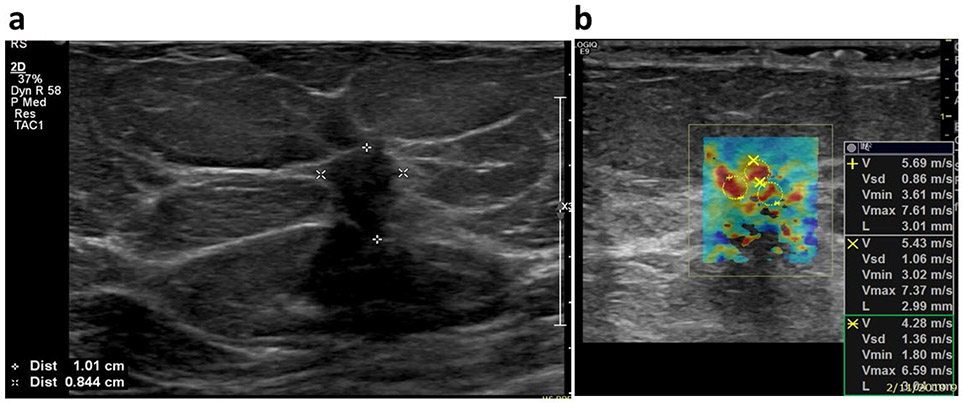
Breast ultrasound examinations were performed by one of our two experienced sonographers (D. D. M and K. K) with more than 30 years of experience in breast ultrasound. We took the following steps to reduces the variability in SWE estimates: Firstly, two highly experienced sonographers were instructed to minimize the pre-compression during the scanning (Barr and Zhang 2012). Secondly, patients were instructed to rest still with no body motion and suspend respiration during the SWE acquisition (for approximately 3 seconds). Thirdly, the scanning was performed by only two sonographers who had more than 30 years of experience and were trained for SWE acquisition. Lastly, we dedicated a single clinical ultrasound scanner with the capability of SWE ( GE LOGIQ E9 ,(GE Healthcare, Wauwatosa, WI), equipped with a 9L-D linear array probe (GE Healthcare, Wauwatosa, WI) with frequency range between 2-8 MHz ) for SWE data acquisition throughout the study. The scanner settings were kept the same for all patients. Six acquisitions were obtained in almost all cases, except for a few cases that we finished only 4 acquisitions due to experimental limitations. Later, one of the members from the investigation team chose one of the most consistent images to draw regions of interest (ROI). Three ROIs, each with 3 mm in diameter were placed at the stiffest positions of the lesion to better represent the heterogeneity of the mass (Bayat, et al. 2017). The number of ROIs was reduced when lesion was less than 9 mm along the greatest dimension. The mean shear wave speed (SWS), maximum SWS, minimum SWS and standard deviation of the SWS inside each ROI were calculated by the ultrasound machine and were averaged across the ROIs for analysis. The measured SWS was also converted to the elasticity in unit of kilopascal (Denis, et al. 2016). Emean, Emax and Emin represented the mass mean, maximum and minimum stiffness, respectively. The elasticity standard deviation Esd represented the mass stiffness heterogeneity. To further correlate the SWE measurement with prognostic factors, a new shear wave parameter, mass characteristic frequency, denoted by fmass, was first introduced in this paper: fmass = Vmin/d, where fmass is with unit Hz, Vmin is the minimum SWS with unit m/s, d is the mass diameter in m. The mass diameter was measured as the largest dimension shown in the B-mode, regardless of the tumor shape. The clinical and research B-mode images were obtained along the longitudinal and transverse or radial and anti-radial orientations. Mass diameter was measured using the B-mode image that provided the best visualization of the breast lesions. Fig. 2 illustrates the measurements for calculating fmass.
Fig. 2.
An example for calculating the fmass for a 47-years-old female patient with luminal A type carcinoma (ER positive, PR positive, HER2 negative, and Ki-67 index is 4%). (a) The mass diameter d was read as the greatest dimension shown in the clinical B-mode image and it is 10.1 mm in this example. (b) The minimum shear wave speed was calculated as the average value of the minimum shear wave speed from the three ROIs shown in the SWE image and is 3.6 m/s. Therefore, the fmass for this patient is 356.4 Hz. Abbreviations: ER=estrogen receptor status; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
Clinical pathologic data
Presence of calcifications was read from the breast mammographic examination. Axillary lymph node (ALN) status, histological type, grade and the expressions of IHC biomarkers were obtained from core biopsies. Reporting criteria for ER and PR status was based on less than 1% reactive cells was negative and greater than or equal to 1% reactive cells was positive. IHC staining for HER2 was scored according to standard criteria as 0, 1+, 2+ or 3+. Score of 0 and 1+ were considered as negative and score of 3+ was considered as positive. Fluorescence in situ hybridization for HER2 amplification was performed when HER2 score was 2+. Positive fluorescence in situ hybridization amplification was considered as HER2 positive. Information about the four IHC biomarkers was summarized in Fig. 1. The histological grade was determined by the method of Elston and Ellis. The IHC results for the 264 lesions included in this study were summarized in Fig. 1. Based on the four IHC biomarkers, the cancers were divided into five molecular subtypes according to the St. Gallen criteria (Goldhirsch, et al. 2011): Luminal A - ER positive, PR positive/negative, HER2 negative, Ki-67<14%; Luminal B with HER2+ - ER positive, PR positive/negative, HER2 negative, and Ki-67≥14%; Luminal B with HER2− - ER positive, PR positive/negative, HER2 positive, any Ki-67; HER2 positive - ER negative and PR negative, HER2 positive; Triple-negative (TN) - ER negative, PR negative and HER2 negative.
Statistical analysis
Statistical analysis was conducted with RStudio (RStudio, PBC, Boston, MA) and it was performed on the SWE parameters measured on the ROIs. The relationship between the SWE parameters and each prognostic factor was evaluated using the Kruskal-Wallis method. Differences were deemed to be statistically significant if p value was less than 0.05. Multivariable logistic regression analysis was performed for studying the combination of multiple parameters for breast cancer invasiveness prediction. ROC curve analysis was used to determine the optimal cutoff values, as well as the corresponding sensitivity and specificity. The optimal cut-point was defined as the point with maximum specificity while keeping sensitivity more than 80%.
Results
Relationship between SWE parameters and histologic factors
Table 1 summarized the relationship between SWE parameters (Emean, Emax, Emin, Esd and fmass) and the histologic features. High shear wave elasticity parameters were all significantly correlated with large mass diameter (p<0.001), positive ALN status, and invasive histological subtypes. Invasive lobular carcinoma showed the highest stiffness among the four types, while ductal carcinomas in situ exhibited the lowest stiffness. Both Emax and Esd were significantly correlated with histological grade with p=0.01. No significant difference was found for shear wave elasticity among the lesions with and without calcifications presence. Moreover, low fmass was significantly associated with the calcifications presence (p=0.004), positive ALN status (p=0.02), and high histological grade (p<0.001).
Table 1.
Summary of the relationship between shear wave elastography parameters and histologic parameters.
| SWE parameters |
Emean±SD (kPa) |
Emax±SD (kPa) |
Emin±SD (kPa) |
Esd±SD (kPa) |
fmass±SD (Hz) |
|---|---|---|---|---|---|
| Mass diameter | |||||
| P value | <0.001* | <0.001* | <0.001* | <0.001* | ND |
| ≤10 mm (84) | 55.2±34.4 | 107.8±65.7 | 22.6±17.4 | 1.7±1.3 | 329.7±138.4 |
| 10-20 mm (103) | 79.1±33.8 | 158.3±53.6 | 30.1±22.3 | 2.7±1.7 | 205.2±84.7 |
| >20 mm (77) | 95.0±34.5 | 184.7±53.8 | 40.3±26.0 | 2.8±1.7 | 122.4±50.0 |
| Calcifications | |||||
| P value | 0.96 | 0.64 | 0.45 | 0.40 | 0.001* |
| None (167) | 76.9±38.0 | 149.0±63.8 | 31.2±22.6 | 2.3±1.5 | 235.3±123.0 |
| Present (97) | 74.8±36.8 | 151.7±67.5 | 29.8±24.0 | 2.6±2.0 | 195.5±131.9 |
| Lymph node | |||||
| P value | <0.001* | <0.001* | 0.01* | <0.001* | 0.01* |
| Negative (186) | 68.4±36.4 | 134.6±62.8 | 28.2±21.8 | 2.1±1.6 | 231.6±127.7 |
| Positive (78) | 94.6±33.7 | 186.6±55.1 | 36.6±25.1 | 3.1±1.8 | 194.5±124.0 |
| Subtype | |||||
| P value | <0.001* | <0.001* | 0.001* | 0.03* | 0.44 |
| IDC (170) | 76.2±37.1 | 151.7±64.9 | 30.3±23.3 | 2.5±1.8 | 223.9±132.7 |
| IMC (45) | 82.5±36.0 | 161.7±55.6 | 33.8±23.0 | 2.4±1.4 | 225.2±115.9 |
| ILC (33) | 85.7±35.3 | 161.7±61.4 | 36.7±23.1 | 2.4±1.5 | 222.5±128.0 |
| DCIS (16) | 37.8±29.4 | 74.8±54.8 | 14.1±12.1 | 1.3±1.3 | 169.6±97.7 |
| Histological grade | |||||
| P value | 0.11 | 0.01* | 0.68 | 0.01* | <0.001* |
| I/II (182) | 75.6±35.8 | 147.4±62.5 | 31.5±22.3 | 2.3±1.6 | 243.7±132.8 |
| III (64) | 84.8±38.9 | 171.0±61.9 | 31.7±25.4 | 2.9±1.8 | 172.9±98.7 |
The numbers in parentheses are mass numbers.
Abbreviations: IDC=invasive ductal carcinoma; IMC=invasive mammary carcinoma with ductal and lobular features; ILC=invasive lobular carcinoma; DCIS=ductal carcinoma in situ; SD=standard deviation; ND=not done.
p<0.05, difference is statistically significant.
Relationship between SWE parameters and the expressions of IHC biomarkers
Table 2 summarized the correlation between shear wave parameters and the expressions of IHC biomarkers. Positive ER, positive PR, high Ki-67 (≥14%) tended to have high stiffness and strong stiffness heterogeneity, though the correlations were not statistically significant. Significant difference was found for Emax and Esd among different HER2 statuses. Significant differences were also found for SWE elasticity among different IHC subtypes. Since there were only 6 HER2+ cases, p values among the IHC subtypes were calculated for both the conditions with and without the HER2+ group included in the analysis. p values were similar for all the parameters for both conditions.
Table 2.
Summary of the relationship between shear wave elastography parameters and IHC parameters.
| SWE parameters |
Emean±SD (kPa) |
Emax±SD (kPa) |
Emin±SD (kPa) |
Esd±SD (kPa) |
fmass±SD (Hz) |
|---|---|---|---|---|---|
| ER | |||||
| P value | 0.63 | 0.59 | 0.08 | 0.20 | 0.004* |
| Negative (39) | 73.4±36.6 | 153.3±64.9 | 26.6±23.6 | 2.7±1.9 | 166.0±78.2 |
| Positive (225) | 76.6±37.7 | 149.4±65.2 | 31.4±23.0 | 2.4±1.7 | 230.1±132.1 |
| PR | |||||
| P value | 0.48 | 0.77 | 0.10 | 0.24 | 0.001* |
| Negative (50) | 72.5±34.3 | 152.8±64.4 | 26.6±21.7 | 2.7±2.0 | 160.4±76.7 |
| Positive (214) | 77.0±38.3 | 149.3±65.4 | 31.6±23.4 | 2.3±1.6 | 234.7±133.0 |
| HER2 | |||||
| P value | 0.67 | 0.004* | 0.45 | <0.001* | <0.001* |
| Negative (218) | 79.0±37.9 | 151.2±62.9 | 32.5±24.2 | 2.3±1.6 | 235.1±129.4 |
| Positive (31) | 79.1±22.8 | 184.6±45.6 | 25.7±13.1 | 3.6±2.1 | 130.4±66.4 |
| Ki-67 | |||||
| P value | 0.05 | 0.03* | 0.21 | 0.12 | <0.001* |
| <14% (101) | 74.5±38.0 | 144.5±62.9 | 30.5±22.4 | 2.2±1.5 | 262.4±128.9 |
| ≥14% (114) | 83.6±36.0 | 162.5±62.3 | 34.8±24.8 | 2.6±1.9 | 206.6±128.0 |
| IHC subtype | |||||
| P value | 0.01* | <0.001* | 0.01* | 0.01* | <0.001* |
| Luminal A (99) | 75.6±37.5 | 145.6±61.8 | 31.0±22.3 | 2.2±1.5 | 265.5±128.2 |
| Luminal B with HER2− (82) | 84.1±38.8 | 156.1±65.7 | 36.4±25.8 | 2.4±1.7 | 227.8±138.4 |
| Luminal B with HER2+ (23) | 83.5±21.8 | 192.9±39.4 | 27.6±13.1 | 3.6±2.0 | 127.6±59.6 |
| HER2+ (6) | 64.6±24.8 | 162.2±65.4 | 18.8±13.7 | 3.9±2.8 | 125.8±87.9 |
| TN (23) | 75.6±37.5 | 153.1±66.5 | 26.6±21.7 | 2.5±1.7 | 175.8±77.0 |
The numbers in parenthesis are the mass numbers. SD indicates standard deviation.
Abbreviations: IHC= immunohistochemical; ER=estrogen receptor status; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; TN=triple negative.
p<0.05, difference is statistically significant.
Besides, as shown in Table 2, negative ER, negative PR, positive HER2 status and higher Ki-67 (<14%) were significantly associated with lower fmass value. Significant difference was found for fmass among different IHC subtypes, and luminal B with HER2+ and HER2+ groups showed lower fmass values.
ROC curves for predicting the ALN status and IHC expressions with SWE parameters
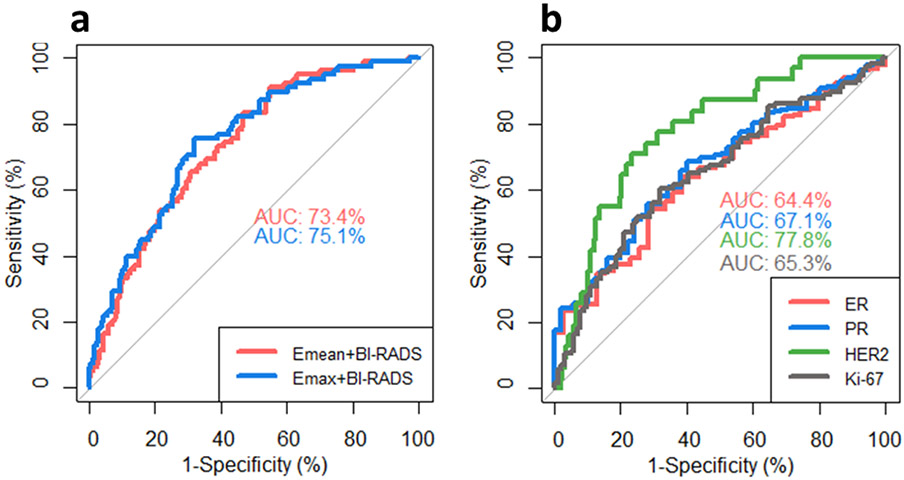
The areas under the curve (AUCs) for the prediction of ALN status with the combination of BI-RADS score and Emean, Emax and Emin were 0.73 (0.95 CI: 0.67-0.80), 0.75 (0.95 CI: 0.69-0.81) and 0.69 (0.95 CI: 0.62-0.76), respectively. Among them, the combination of BI-RADS score with Emean and Emax show better performances, and the corresponding ROCs are plotted in Fig. 3(a). For the combination of Emean and BI-RADS score, with an optimal cutoff of the probability of being positive ALN at 0.23, the sensitivity is 0.81 and the specificity is 0.54. For the combination of Emax and BI-RADS score, with an optimal cutoff of the probability of being positive ALN at 0.24, the sensitivity is 0.81 and the specificity is 0.57. No significant difference was found for the AUCs among the two combinations (p=0.28).
Fig. 3.
(a) ROC curves of combining mean elasticity (Emean) with BI-RADS score and maximum elasticity (Emax) with BI-RADS score for diagnosing ALN status. (b) ROC curves of combining mass characteristic frequency (fmass) with BI-RADS score for diagnosing immunohistochemical biomarker (ER, PR, HER2 and Ki-67) status. AUC=area under the curve. Abbreviations: ROC=receiver operating characteristic; ER=estrogen receptor status; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
Figure 3(b) shows the ROC curves for predicting the expressions of IHC biomarkers fmass. The AUCs for combing the BI-RADS score with ER, PR, HER2 and Ki-67 were 0.65 (0.95 CI: 0.56-0.73), 0.65 (0.95 CI: 0.57-0.73), 0.78 (0.95 CI: 0.70-0.86), and 0.65 (0.95 CI: 0.57-0.72), respectively. The ROC curve analysis yielded cutoff values of the fmass at: 125 Hz for ER, with a sensitivity of 0.80 and a specificity of 0.33; 129 Hz for PR, with a sensitivity of 0.80 and a specificity of 0.37; 169 Hz for HER2, with a sensitivity of 0.81 and a specificity of 0.65; 293 Hz for Ki-67, with a sensitivity of 0.81 and a specificity of 0.36.
Discussion and Conclusion
In this study, the relationships between SWE parameters and breast cancer prognostic factors were investigated. Our results showed that large malignant mass diameter, positive ALN, and invasive histological subtypes were significantly correlated with high elasticity values (Emean and Emax) and strong stiffness heterogeneity (Esd). The fmass parameter was significantly correlated with the presence of calcifications, ALN status and histological grade. For the IHC biomarkers, positive ER, positive PR, negative HER2 status and lower Ki-67 (<14%) were significantly correlated with higher fmass values. Emax and Esd were significantly correlated with HER2 status. All SWE parameters were significantly correlated with IHC subtypes.
Multiple studies have shown that larger mass diameter was the dominant determinant of larger mean elasticity, and was associated with poor prognostic factors of invasive breast cancer (Berg, et al. 2015, Chang, et al. 2013, Evans, et al. 2016, Evans, et al. 2012, Kang, et al. 2018, Son, et al. 2020). Similarly, our study also showed that the tumor size was significantly correlated with shear wave elasticity (Emean, Emax, Emin and Esd). Our study also showed that breast cancer patients with positive ALN exhibited higher stiffness in their invasive breast masses. Emean and Emax gave similar performance in predicating the ALN status. Moreover, our study found that the SWE parameters (Emax and Esd) exhibit significant differences between the Grades I/II and Grade III cancers, with the Grade III cancers showing higher stiffness and stronger stiffness heterogeneity values. This finding is also in agreement with the results reported in (Chang, et al. 2013, Evans, et al. 2012). The cause of tumor stiffness has been investigated extensively. Some studies suggested that the combination of cellularity, microvascular density, necrosis and fibrosis, contributes to the stiffness in high grade cancers (Baker, et al. 2010, Chang, et al. 2013). Moreover, the abnormal extracellular matrix, which derails stromal cells and leads to tumor-associated angiogenesis and inflammation, is also the factor for increased stiffness during tumor progression (Lu, et al. 2012). Whereas, some studies reported that the cancer stiffness could be due to desmoplastic reaction which associated with dense fibrous, and it is more marked in low grade cancerous mass than high grade cancers (Barr 2012). Although the presence of cluster, coarse or large calcifications in benign breast masses can induce apparent high stiffness in benign lesions and misdiagnosed as malignant (Gregory, et al. 2015), no statistically significant relationship between the elasticity of invasive mass and the presence of calcifications has been found in our study. This is in agreement with the study reported in (Gemici, et al. 2020). Interestingly, fmass was found significantly correlated with the presence of calcifications. One explanation is: fmass is inversely proportional to the mass diameter. Though Emean, Emax and Emin were not significantly correlated with the presence of microcalcifications, large mass diameter was found to be highly correlated with the presence of microcalcifications (p=0.014). Therefore, fmass is inversely correlated with the microcalcifications. Moreover, fmass was found significantly correlated IHC biomarker statuses (ER, PR, HER2 and Ki-67), and could be used for predicting the expressions of IHC biomarkers.
In our study, the breast cancers were divided into different histological types according to the pathology report. Significant difference was found for the elasticity among different subtypes. We also found that the lobular carcinoma exhibited the highest stiffness among the histological types, which is consistent with the results of studies reported in (Evans, et al. 2012, Ganau, et al. 2015, Youk, et al. 2013). Using IHC biomarkers, breast cancers can also be divided into subtypes with strong prognostic effects (Hugh, et al. 2009). Distinguishing tumor subtypes is useful for therapeutic management. In our study, all SWE parameters were found to be significantly correlated with the IHC subtypes, while no statistically significant correlation with IHC biomarkers or molecular subtypes was reported in (Cho, et al. 2019). Other studies have reported a correlation between high stiffness values and aggressive subtypes of breast cancer (Chang, et al. 2013).
In general, luminal-type tumors have a relatively good prognosis, whereas patients with TN tumors have shorter and poor response to the typical endocrine therapies. In young women with genetic mutation, TN tumors often manifest as circumscribed, benign-looking masses (Uematsu, et al. 2009). In this study, we found the luminal subtypes were associated with higher fmass. Lower fmass was seen in more aggressive types, including luminal B with HER2+, HER2+ and TN. Thus, our study showed that lower fmass indicated a poor prognostic factor.
The fmass parameter represents the SWS weighted by the inverse of the mass diameter (fmass = Vmin/d, Vmin is in m/s and d is in m). Our study showed that adding fmass to the statistical analysis improved the overall diagnostic performance. It has been shown in previous study that SWS will be underestimated in small lesions. This is partly due to shear wavelength being too large for accurate estimation of speed in relatively small masses. However, very weak correlation (r<0.2) was found between the SWS and mass diameter for both the small tumors and large tumors when tumors of all types are considered. Therefore, the correlation between SWS and mass diameter will not affect the fmass values directly.
We explored using Vmin, Vmean, and Vmax for fmass estimation, however, only Vmin was found to show significant differences among the IHC biomarker statuses or IHC subtypes; thus, only Vmin was used for fmass calculation. Moreover, fmass is obtained by dividing Vmin by the largest dimension of the tumor regardless of the shear wave propagation direction. Alternatively, one may use the mass dimension along the shear wave propagation direction. However, shear wave propagation path could vary for different cases and mass positions; hence, the propagation direction is not always known. Therefore, the largest tumor dimension is used in this work. A potential source of error in this method is measuring mass diameter when the mass shape is irregular. In such cases, it may be helpful to approximate the mass shape with an ellipse encompassing the mass. Also, a common metric for depicting object shape is to measure mass perimeter and calculate equivalent circular diameter. In the future, fmass could be further studied by using different approaches to define mass diameter. Moreover, more studies are needed to better understand the role of fmass in SWE accuracy. The physical meaning of the new parameter, fmass, can be explained as the inverse of the maximum shear wave propagation time in a breast mass. With that in mind, one may define “mass transit time” as the shear wave transit time across the largest dimension of the mass and use this new parameter as an alternative to fmass for the analysis described in this paper.
There are some limitations in our study. Firstly, although the sample size of invasive masses was adequate, the numbers for some subtypes were limited. For example, we only had 6 HER2+ type and 22 TN type cancers. Likewise, the histological types were not adequate as most of the cancers were invasive ductal carcinomas. For these reasons, our results for the TN and HER2+ type cancers should be considered as “preliminary results” that warrant further studies with a larger population and adequate number of subtypes to obtain statistically solid conclusions regarding these two subtypes. Secondly, anisotropy of the elasticity in breast masses was not considered in our study. Previous studies showed that anisotropy was significant in malignant masses (Chen, et al. 2018, Skerl, et al. 2016). Further prospective comparative studies are necessary to compare the SWE parameters along the two orthogonal orientations.
In conclusion, this study introduced a new SWE parameter fmass for breast cancer characterization. The relationships between SWE parameters, including the elasticity and fmass, and breast cancer prognostic factors, including mass diameter, presence of calcifications, ALN status, histological type and grade, IHC biomarkers (ER, PR, HER2 and Ki-67), as well as the IHC subtypes were investigated. Briefly, poor prognostic factors were associated with high shear wave elasticity values and low mass characteristic frequency values. The combination of Emean or Emax with BI-RADS score could be used for ALN status prediction. fmass could be used for predicting the expressions of IHC biomarkers. Therefore, SWE provides helpful information in breast cancer treatment planning.
Acknowledgments
The authors would like to thank Ms. Cindy Andrist for her valuable help in patient recruitment, Dr. Max Denis, PhD; Dr. Mahdi Bayat, PhD; Dr. Viksit Kumar, PhD; Dr. Bae-Hyung Kim, PhD; Dr. Rohit Nayak, PhD; Dr. Saba Adabi, PhD; Mr. Jeremy Webb, PhD; Dr. Redouane Ternifi, PhD; Ms. Adriana Gregory, and Ms. Yinong Wang for their assistance in SWE data acquisition at different time periods during the course of the study. Also, we thank Mr. Duane Meixner, R.V.T., R.D.M.S., and Ms. Kate Knoll, R.V.T., R.D.M.S for patient scanning.
Funding
The study was supported by the grant from National Cancer Institute, National Institutes of Health; R01CA148994. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The NIH did not have any additional role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Conflict of Interest Statement
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article and the authors affirm that they do not have any potential financial interest related to the technology referenced in this paper.
References
- Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, Chen Y, Liphardt J, Hwang E, Weaver V. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integrative Biology 2015; 7:1120–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EL, Lu J, Yu D, Bonnecaze RT, Zaman MH. Cancer cell stiffness: integrated roles of three-dimensional matrix stiffness and transforming potential. Biophysical journal 2010; 99:2048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RG. Sonographic breast elastography: a primer. J Ultrasound Med 2012; 31:773–83. [DOI] [PubMed] [Google Scholar]
- Barr RG, Zhang Z. Effects of precompression on elasticity imaging of the breast: development of a clinically useful semiquantitative method of precompression assessment. J Ultrasound Med 2012; 31:895–902. [DOI] [PubMed] [Google Scholar]
- Bayat M, Denis M, Gregory A, Mehrmohammadi M, Kumar V, Meixner D, Fazzio RT, Fatemi M, Alizad A. Diagnostic features of quantitative comb-push shear elastography for breast lesion differentiation. PLoS One 2017; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg WA, Mendelson EB, Cosgrove DO, Doré CJ, Gay J, Henry J-P, Cohen-Bacrie C. Quantitative maximum shear-wave stiffness of breast masses as a predictor of histopathologic severity. Am J Roentgenol 2015; 205:448–55. [DOI] [PubMed] [Google Scholar]
- Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989; 63:181–87. [DOI] [PubMed] [Google Scholar]
- Chang JM, Moon WK, Cho N, Yi A, Koo HR, Han W, Noh D-Y, Moon H-G, Kim SJ. Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res Treat 2011; 129:89–97. [DOI] [PubMed] [Google Scholar]
- Chang JM, Park IA, Lee SH, Kim WH, Bae MS, Koo HR, Yi A, Kim SJ, Cho N, Moon WK. Stiffness of tumours measured by shear-wave elastography correlated with subtypes of breast cancer. Eur Radiol 2013; 23:2450–58. [DOI] [PubMed] [Google Scholar]
- Chen Y-l, Gao Y, Chang C, Wang F, Zeng W, Chen J-j. Ultrasound shear wave elastography of breast lesions: correlation of anisotropy with clinical and histopathological findings. Cancer Imaging 2018; 18:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, Park CS, Kim SH, Kim HS, Kim K, Lee JW, Shin YR, Jun S-Y, Oh S-J. Correlation of the Strain Elastography-Derived Elasticity Scores with Prognostic Histologic Features, Immunohistochemical Markers, and Molecular Subtypes of Invasive Ductal Carcinoma. Journal of the Korean Society of Radiology 2019; 80:717–27. [Google Scholar]
- Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999; 17:2639–39. [DOI] [PubMed] [Google Scholar]
- Denis M, Gregory A, Bayat M, Fazzio RT, Whaley DH, Ghosh K, Shah S, Fatemi M, Alizad A. Correlating tumor stiffness with immunohistochemical subtypes of breast cancers: prognostic value of comb-push ultrasound shear elastography for differentiating luminal subtypes. PLoS One 2016; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. Journal of the National cancer Institute 2011; 103:1656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991; 19:403–10. [DOI] [PubMed] [Google Scholar]
- Evans A, Rauchhaus P, Whelehan P, Thomson K, Purdie CA, Jordan LB, Michie CO, Thompson A, Vinnicombe S. Does shear wave ultrasound independently predict axillary lymph node metastasis in women with invasive breast cancer? Breast Cancer Res Treat 2014; 143:153–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A, Sim YT, Thomson K, Jordan L, Purdie C, Vinnicombe SJ. Shear wave elastography of breast cancer: sensitivity according to histological type in a large cohort. The Breast 2016; 26:115–18. [DOI] [PubMed] [Google Scholar]
- Evans A, Whelehan P, Thomson K, McLean D, Brauer K, Purdie C, Baker L, Jordan L, Rauchhaus P, Thompson A. Invasive breast cancer: relationship between shear-wave elastographic findings and histologic prognostic factors. Radiology 2012; 263:673–77. [DOI] [PubMed] [Google Scholar]
- Evans A, Whelehan P, Thomson K, McLean D, Brauer K, Purdie C, Jordan L, Baker L, Thompson A. Quantitative shear wave ultrasound elastography: initial experience in solid breast masses. Breast Cancer Res 2010; 12:R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner J, Stacer AC, Winterroth F, Johnson TD, Luker KE, Luker GD. Macroscopic stiffness of breast tumors predicts metastasis. Scientific reports 2014; 4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganau S, Andreu FJ, Escribano F, Martín A, Tortajada L, Villajos M, Baré M, Teixidó M, Ribé J, Sentís M. Shear-wave elastography and immunohistochemical profiles in invasive breast cancer: evaluation of maximum and mean elasticity values. Eur J Radiol 2015; 84:617–22. [DOI] [PubMed] [Google Scholar]
- Gemici AA, Ozal ST, Hocaoglu E, Inci E. Relationship Between Shear Wave Elastography Findings and Histologic Prognostic Factors of Invasive Breast Cancer. Ultrasound Q 2020; 36:79–83. [DOI] [PubMed] [Google Scholar]
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn H-J, Members P. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011; 22:1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory A, Mehrmohammadi M, Denis M, Bayat M, Stan DL, Fatemi M, Alizad A. Effect of calcifications on breast ultrasound shear wave elastography: an investigational study. PLoS One 2015; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. cell 2011; 144:646–74. [DOI] [PubMed] [Google Scholar]
- Harbeck N, Wuerstlein R. Truly personalized therapy—an end to the era of one size fits all. Nature Reviews Clinical Oncology 2019; 16:77–78. [DOI] [PubMed] [Google Scholar]
- Hugh J, Hanson J, Cheang MCU, Nielsen TO, Perou CM, Dumontet C, Reed J, Krajewska M, Treilleux I, Rupin M. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol 2009; 27:1168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Kim JY, Lee NK, Lee JW, Song YS, Park SY, Shin JK. Three-dimensional versus two-dimensional shear-wave elastography: Associations of mean elasticity values with prognostic factors and tumor subtypes of breast cancer. Clin Imag 2018; 48:79–85. [DOI] [PubMed] [Google Scholar]
- Liu H, Wan J, Xu G, Xiang L-H, Fang Y, Ding S-S, Jiang X, Sun L-P, Zhang Y-F. Conventional US and 2-D Shear Wave Elastography of Virtual Touch Tissue Imaging Quantification: Correlation with Immunohistochemical Subtypes of Breast Cancer. Ul Trasound Med Biol 2019; 45:2612–22. [DOI] [PubMed] [Google Scholar]
- Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. Journal of Cell Biology 2012; 196:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida Y, Shimauchi A, Okuma H, Tozaki M, Isobe S, Fukuma E. Shear Wave Speed of the Lesion in Preoperative Breast Ultrasonography: Association with Disease-free Survival of Patients with Primary Operable Invasive Breast Cancer. Acda Radiol 2018; 25:1003–09. [DOI] [PubMed] [Google Scholar]
- Mann GB, Fahey VD, Feleppa F, Buchanan MR. Reliance on hormone receptor assays of surgical specimens may compromise outcome in patients with breast cancer. J Clin Oncol 2005; 23:5148–54. [DOI] [PubMed] [Google Scholar]
- Sánchez-Muñoz A, García-Tapiador AM, Martínez-Ortega E, Dueñas-García R, Jaén-Morago A, Ortega-Granados AL, Fernández-Navarro M, de la Torre-Cabrera C, Dueñas B, Rueda AI. Tumour molecular subtyping according to hormone receptors and HER2 status defines different pathological complete response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Clin Transl Oncol 2008; 10:646–53. [DOI] [PubMed] [Google Scholar]
- Skerl K, Vinnicombe S, Thomson K, McLean D, Giannotti E, Evans A. Anisotropy of solid breast lesions in 2D shear wave elastography is an indicator of malignancy. Acda Radiol 2016; 23:53–61. [DOI] [PubMed] [Google Scholar]
- Son MJ, Kim S, Jung HK, Ko KH, Koh JE, Park AY. Can Ultrasonographic Vascular and Elastographic Features of Invasive Ductal Breast Carcinoma Predict Histologic Aggressiveness? Acda Radiol 2020; 27:487–96. [DOI] [PubMed] [Google Scholar]
- Tamaki K, Tamaki N, Kamada Y, Uehara K, Miyashita M, Ishida T, Sasano H. A non-invasive modality: the US virtual touch tissue quantification (VTTQ) for evaluation of breast cancer. Japanese journal of clinical oncology 2013; 43:889–95. [DOI] [PubMed] [Google Scholar]
- Tang P, Skinner KA, Hicks DG. Molecular classification of breast carcinomas by immunohistochemical analysis: are we ready? Diagn Mol Pathol 2009; 18:125–32. [DOI] [PubMed] [Google Scholar]
- Uematsu T, Kasami M, Yuen S. Triple-negative breast cancer: correlation between MR imaging and pathologic findings. Radiology 2009; 250:638–47. [DOI] [PubMed] [Google Scholar]
- y Cajal SR, Sesé M, Capdevila C, Aasen T, De Mattos-Arruda L, Diaz-Cano SJ, Hernández-Losa J, Castellví J. Clinical implications of intratumor heterogeneity: challenges and opportunities. Journal of Molecular Medicine 2020:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youk JH, Gweon HM, Son EJ, Kim J-A, Jeong J. Shear-wave elastography of invasive breast cancer: correlation between quantitative mean elasticity value and immunohistochemical profile. Breast Cancer Res Treat 2013; 138:119–26. [DOI] [PubMed] [Google Scholar]