Abstract
Purpose:
Understand how the clinical history has been used to risk stratify patients reporting a beta-lactam allergy, both in clinical care pathways and predictive models.
Recent Findings:
Drug allergy clinical care pathways have emerged as a safe and effective method of stratifying patients with a reported beta-lactam allergy into risk categories, with “low-risk” patients able to proceed straight to direct challenges or test doses. These methods have streamlined antibiotic stewardship policies and penicillin allergy de-labeling. However, how to define “low-risk” has been subject to much debate. New research has developed predictive models that utilize the clinical history to assess a patient’s true-risk of beta-lactam allergy.
Summary:
The clinical history has long been an essential part of drug allergy evaluation, and has proven invaluable within the past decade in the development of drug allergy clinical pathways. Evidence-based predictive models that use the clinical history to assess a patient’s true risk of beta-lactam allergy offer tremendous promise, but differ in crucial areas such as the populations they study, the predictor variables they use, and the ultimate accuracy they attain. These models highlight key aspects of the drug allergy history and pave the way for future large-scale research.
Keywords: beta-lactam, allergy, clinical history, pathway, predictive model, delabeling, penicillin, skin test, drug challenge, test dose, antibiotic stewardship
Introduction:
An estimated 8% of the general population reports a beta-lactam allergy, with rates up to 15% in hospitalized patients [1, 2]. Penicillin allergy comprises the vast majority of these beta-lactam allergies; only about 1–2% of individuals using healthcare in the United States (U.S.) report a cephalosporin allergy [3, 4]. However, just 5% of patients with a reported penicillin allergy would prove to be truly allergic after formal evaluation [5, 6]. Because a penicillin allergy conveys important individual and public health consequences, recent research has focused on how allergists and non-allergists alike should evaluate and treat patients with a reported, unconfirmed beta-lactam allergy. While historically international guidelines have recommended that all patients with a penicillin allergy undergo a formal assessment including skin testing with oral drug challenges reserved for skin-test negative cases, recent studies have demonstrated the safety of direct beta-lactam antibiotic challenges and test doses in defined “low-risk” inpatients and outpatients [7–16]. Although these risk stratification pathways have proven safe and effective, they have differed in their definitions of a “low-risk” clinical history [17]. Five recent studies, reviewed in this article, describe evidence-based prediction models for estimating a patient’s true risk of penicillin or beta-lactam allergy. While these models differ substantially, they demonstrate the value of the clinical history and argue for the creation and distribution of large international datasets that can then be used to devise accurate point-of-care prediction rules for optimal clinical care.
Text of Review:
Penicillins are first-line therapy for a wide variety of common bacterial infections; however, their use is limited by the fact that they are also the most commonly reported drug allergy [18]. This problem is illustrated by the fact that an estimated 10–15% of U.S. patients report a penicillin allergy but more than 95% of them are actually found to be penicillin tolerant in formal drug allergy assessment [19, 20]. This discrepancy increases morbidity and mortality; when clinicians eschew beta-lactam antibiotics in favor of broad-spectrum antibiotics, they risk inferior infection outcomes, antibiotic-related complications such as Clostridiodes difficile infection and surgical site infection, antimicrobial resistance, and increased healthcare spending [18, 21–23]. In order to confront this public health threat, international efforts have focused on developing safe, efficient, and cost-effective ways to remove invalid penicillin allergies (“de-labeling”) [11].
The Reference Standard
The current reference standard for penicillin allergy de-labeling consists of penicillin skin testing (ST) followed by an oral challenge (OC) with amoxicillin (if ST is negative) [7, 11]. While this model certainly offers advantages in its accuracy and safety profile, and henceforth is advisable in patients with tenuous cardiopulmonary status, pregnancy, or high-risk allergy histories, it also poses distinct disadvantages [18, 24]. First, ST is performed to detect true IgE-mediated allergy, but true sensitization rates are low and the rate of severe acute anaphylactic reactions with penicillin is exceedingly rare (less than 1 in 1,000 penicillin allergies are anaphylaxis) [18, 25, 26]. Penicillin ST, based on a recent meta-analysis, has a sensitivity of 31% and a specificity of 97% [27]. Despite this high specificity, the positive predictive value of ST decreases in patients with a low pre-test probability for true penicillin allergy, thereby increasing the rate of false positives [14, 18, 28, 29]. Among decidedly low-risk patients, we know from one randomized controlled trial that 8.8% more patients were de-labeled if the penicillin allergy evaluation was a direct oral challenge (DOC) rather than ST first, although this 8.8% difference was not statistically significant [30]. Finally, ST followed by OC is more cumbersome, challenging to learn for the non-allergist, and resource-intensive; although performing penicillin allergy evaluations is potentially a cost-saving intervention, with an estimated 25 to 30 million US residents harboring a penicillin allergy label and only 6,000 US allergists and immunologists, the need for penicillin allergy evaluation outstrips available testing resources and requires a “rejuvenated look” at our current model of drug allergy testing that is reliant on a specialist workforce [5, 18, 31–33].
Risk Stratification Clinical Pathways
Rather than having all patients with a reported beta-lactam allergy undergo ST followed by OC, risk stratification clinical pathways have emerged as a method of identifying “low-risk” patients who can skip the labor- and resource-intensive step of ST and proceed straight to DOC. Such pathways, whether developed within a single clinic or instituted throughout an entire healthcare system, have proven to be safe, effective, and well-received by patients [17–19]. Moreover, these clinical pathways have transformed both outpatient and inpatient allergy care. In the allergy clinic, such pathways have streamlined outpatient beta-lactam allergy evaluations, increasing access to care with a lower overall associated cost [16, 29, 34]. For inpatients, drug allergy pathways have proven revolutionary by simply bringing beta-lactam allergy evaluation to the hospital bedside – a place where beta-lactams may be critically needed, yet previously unreached. Whether performing a DOC to penicillin or amoxicillin (thereby “de-labeling” the penicillin allergy) or alternatively, performing a challenge to the indicated therapeutic beta-lactam (often a cephalosporin, which facilitates timely, appropriate antibiotic treatment for acutely infected patients), drug allergy pathways have emerged as an inpatient standard of care and integral part of antibiotic stewardship programs [35].
Despite the widespread adoption of drug allergy clinical pathways, some which use DOC, experts continue to disagree over what constitutes a “low-risk” patient, or “low-risk” index reaction. For instance, whereas some researchers define a low-risk reaction as an isolated cutaneous reaction and exposure more than 1 year prior to evaluation, others define it as a mild cutaneous reaction (within any timeframe) or an unknown reaction more than 5 years prior to evaluation [20, 24]. In 2019, the American Academy of Allergy, Asthma and Immunology (AAAAI) endorsed Shenoy et al.’s low-risk definition: patients having isolated non-allergic symptoms (e.g. gastrointestinal symptoms) or patients solely with a family history of a penicillin allergy, symptoms of pruritis without rash, or remote (>10 years) unknown reactions without features suggestive of an IgE-mediated reaction [34]. The AAAAI agreed that these patients could proceed to DOC. Further complicating what constitutes as “low-risk,” there may be a low-risk index reaction in a patient that is not low-risk; some clinical pathways incorporate the patient’s clinical stability and comorbidities into their framework, whereas others exclude patients with clinical instability from their pathway entirely and others simply reclassify them as high-risk regardless of their index reaction [36–39].
Adopting a universal low-risk definition may be impractical because of epidemiological, cultural, and institutional differences. Indeed, as Chiriac et al. illustrates, drug allergy clinical pathways often differ on the type of health care provider who collects the history and/or performs risk stratification, local prevalence of confirmed beta-lactam allergy, the antibiotic pharmacy formulary, local interpretations of beta-lactam cross-reactivity, and hospital resources including access to ST and allergy consultation [39]. These distinctions in provider type, practice setting, and patient population make it challenging to create a “one-size-fits-all” low-risk definition. Indeed, the greatest successes in standardizing such definitions have been at a national level. For instance, the Australian Therapeutic Guidelines include a drug allergy pathway for beta-lactam prescribing [39, 40]. Similarly, the European Network of Drug Allergy and the Drug Allergy Interest Group of the European Academy of Allergy and Clinical Immunology are developing a risk stratification pathway [39].
Predictive Models
While drug allergy risk stratification clinical pathways have provided major advancements in the field of beta-lactam allergy evaluation and antibiotic stewardship, they have also highlighted the need for evidence-based predictive models to develop data-driven point-of-care clinical decision rules that define true risk of penicillin or beta-lactam allergy [6]. The following section will review articles in the field of beta-lactam predictive models and synthesize the collective findings from these studies into recommendations for clinical allergy evaluation as well as areas for future research [6, 11, 41–43]. The collective findings are summarized in table 1 and figure 1 [2, 4].
Table 1:
Summary of Predictive Models for B-Lactam Allergy
| Author, Year | Patient type | Allergy type | Method | Int valid | Ext valid | Predictor Variables | AUC (ROC) | NPV |
|---|---|---|---|---|---|---|---|---|
| Chiriac, 2017 | Unspecified (outpatient – allergy unit) | B-lactam | Logistic Regression + Decision Tree | France, 1991 [retro] | France, 200, adult ≥18yo [pro] | -Anaphylaxis with or without shock (Y/N) -Multiple reactions (Y/N) -Time since reaction -LR: Continuous -DT: Y/N <62.43 months -Penicillin as culprit beta-lactam (Y/N) -Reaction onset time -LR: Continuous -DT: Y/N <1hour |
LR - 0.67 (Int valid); 0.73 (Ext valid) |
DT - 81.6% (Int valid); 81.3% (Ext valid) |
| Siew, 2019 | Unspecified (outpatient) | B-lactam | Logistic regression | UK, 1092, adult ≥15yo [retro] | N/A | -Anaphylaxis with or without shock (Y/N) -Time since reaction (Y/N, <1 year) -Patient recall of culprit beta-lactam (Y/N) -Penicillin as culprit beta-lactam drug [1, 2] |
98.4% (Low risk); 80.6% (Not low risk) |
|
| Stevenson, 2020 | Outpatient | Penicillin | Logistic regression | Australia, 447, adult ≥16yo [retro] | N/A | -Reaction severity (Y/N, Grade 1 – Rash-only or non-allergic) -Reaction onset time (Y/N, <1 year) |
94.7% | |
| Trubiano, 2020 | Both | Penicillin | Logistic regression | Australia, 622, adult ≥16yo [pro] | Australia + Nashville TN, 945, adult ≥16yo [retro] | -Reaction <5 years previous (Y/N) -Anaphylaxis (Y/N) -Angioedema (Y/N) -SCAR (Y/N) -Treatment required for reaction (Y/N) |
0.81 (Int valid); 0.81, 0.73, 0.85 (Ext valid) |
96.3% (Int valid); 95.0%, 84.9%, 98.4% (Ext valid) |
| Moreno, 2020 | Unspecified (outpatient) | B-lactam | Machine learning | Spain*, 656, adult [retro] | Spain*, 615, adult [pro] | Categorical input neurons -Age -Time between reaction and allergic study -Suspected antibiotic -Day of treatment -Latency between last dose and reaction -Type of reaction -Duration of time to recovery -Total IgE Binary input neurons -Sex -History of atopy -Life-threatening reactions |
0.939 (ML); 0.844 (LR) |
ML - 92.1% (Int valid); 95.2% (Ext valid) |
Same allergy unit
LR – Logistic Regression
DT – Decision Tree
ML – Machine Learning
Pro – Prospective
Retro – Retrospective
Int valid – Internal validation cohort(s)
Ext valid – External validation cohort(s)
Y/N – Binary model input
Figure 1: Variability in predictive factors of B-lactam allergy models due to varied modelling approaches, differing sets of considered variables, and limitations of dataset sizes.
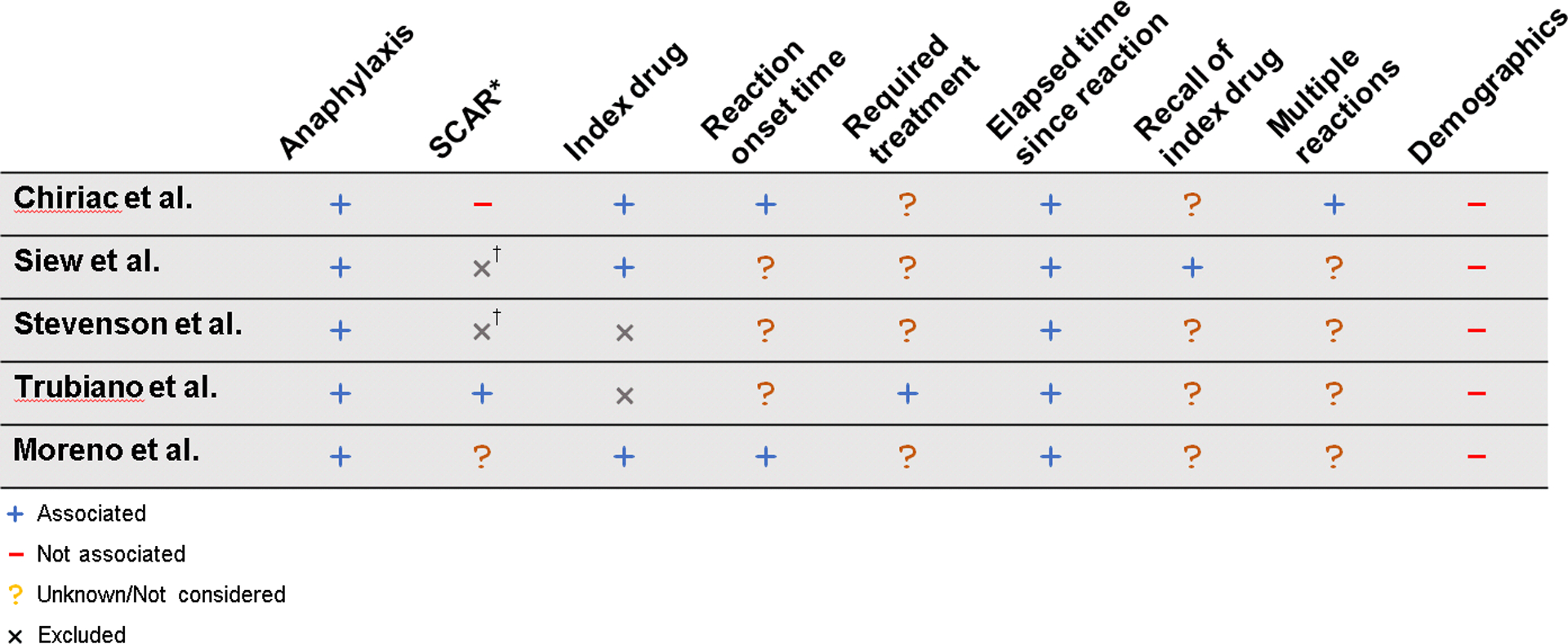
† Excluded cephalosporins; index penicillin was not significant
* SCAR is difficult to denote because studies differ in how it is used or grouped with other symptoms. Angioedema likewise difficult, but not excluded by any studies
Stevenson et al. published a retrospective multicenter analysis of 447 penicillin-allergic adult patients across 7 outpatient clinics in Australia [11]. Applying statistical modeling, the authors determined that the optimal low-risk definition was a history of non-SCAR (Severe Cutaneous Adverse Reaction) penicillin-associated rash (without angioedema, mucosal ulceration, or systemic symptoms) more than 1 year previous. In their retrospective analysis, 54.6% of patients met this low-risk definition, of which 97.1% tolerated a DOC with no anaphylaxis noted among those who reacted [11]. While their study was limited by its lack of internal and external validation as well as heterogeneity of testing in the retrospective cohort, it is key to future investigations and can be used to guide clinical allergy practice.
Chiriac et al. designed predictive models for beta-lactam allergy using retrospective cohort analysis, but their study went further by subsequently performing external validation on a prospective data set [41]. First, they utilized multivariate logistic regression (LR) and decision tree methods (a type of machine learning method) to build a predictive model based off a retrospective cohort of 1,991 allergy-referred patients to a single-center in France. Then, they performed external validation of select models in 200 prospectively enrolled patients with a history of beta-lactam allergy in 3 allergy centers across France. Through LR analysis, they discovered that a history of anaphylactic shock was associated with 4-fold increased odds of having a true beta-lactam allergy (adjusted odds ratio [aOR] 4.15) with other significant predictors: anaphylaxis without shock (aOR 2.23), history of multiple reactions (aOR 2.22), symptoms occurring within 1 hour (aOR 2.08), and penicillin as the culprit beta-lactam drug (aOR 1.53). Despite these important findings, they found that the overall performance of both predictive models was poor; the negative predictive value (NPV) for the LR and decision tree models were 82% and 81% respectively. While the authors concluded that their prediction models were not accurate enough to replace the work of the consultant allergist, it is important to realize that if their models were applied to a U.S. population with a lower true prevalence of penicillin allergy, they would achieve over a 95% NPV and therefore be highly effective at identifying patients who could undergo DOC [44]. Indeed, despite its findings not being strong enough for France and a study design limited by its prediction of all beta-lactam allergies (rather than penicillin allergy alone) and a small validation cohort, the study highlighted the importance of anaphylaxis and timing as clear candidate predictors for future prediction tools. The study was also the first to use non-traditional prediction methods through the use of decision tree/classification and regression trees.
Siew et al. derived a simple prediction model which could accurately identify low-risk beta-lactam allergies based on only 3 predictors [42]. Using audit data of 1,092 patients in the United Kingdom who underwent penicillin allergy evaluation, the authors found that the absence of anaphylaxis, unknown name of the index drug, and reaction occurring over 1 year prior had an NPV of 98.4%, which the authors noted is similar to the NPV of penicillin ST. While this model offered tremendous appeal, its generalizability was limited by its medical records review (certain variables such as demographics, index reaction, etc. were not available for every patient), inability to weigh the risks of a wider array of allergy histories (e.g. angioedema), and absence of external validation procedures.
Trubiano et al. published a clinical decision tool for penicillin allergy prediction that is also appealing because its simplicity, but also had rigorous validation methods [6]. First, they performed a multicenter prospective cohort study of 622 Australian patients to derive and internally validate their penicillin allergy prediction model. Next, they performed external validation in retrospective penicillin allergy-tested cohorts largely in Australia, but with one U.S. site. Based on LR analysis, they made a simplified version of their model with a point system for 4 predictive features associated with a positive penicillin allergy test which was summarized by the catchy mnemonic PEN-FAST: penicillin allergy, five or fewer years ago (2 points), anaphylaxis or angioedema or SCAR (2 points), and treatment required for reaction (1 point). Using <3 as their cut off for low-risk, they found that this definition resulted in an NPV of 96.3% and was thus an effective tool to identify low-risk patients who could proceed directly to DOC. Limitations of this study include its derivation cohort, which was comprised of inpatients with over half being immunocompromised, and terminology that required advanced specialized drug allergy training (e.g., anaphylaxis, angioedema, SCAR knowledge). However, Trubiano’s study offered hope that a simple prediction model could safely and accurately risk-stratify penicillin allergic patients.
While the prior studies used LR prediction methods (Chiriac also used decision tree), Moreno at al.’s article was the first to use artificial neural networks (ANNs) to develop a predictive model for beta-lactam allergy [43]. Unlike LR models, ANNs can recognize nonlinear relationships and complex interactions among different variables and in some cases have been proven to have greater prognostic ability compared to LR [43]. To demonstrate an ANN’s applicability and superiority in beta-lactam allergy prediction, Moreno first constructed an ANN using a retrospective data set of patients with a reported beta-lactam allergy in Spain. The parameters of the ANN included: age, sex, past medical history of atopy, elapsed time between reaction and allergic study, suspected antibiotic, day of treatment, latency between last dose and reaction, type of reaction, life-threatening reactions, and duration of time to recovery. After constructing the ANN, they subsequently composed a LR model based on the prospective evaluation of 615 patients with a reported beta-lactam allergy. Moreno found that the NPV of the ANN in the retrospective and prospective study was 92.1% and 92.5% respectively and was superior to LR. While there were notable limitations – mainly, that it was a single center study over a long time period with differences between the retrospective and prospective cohorts – Moreno’s study demonstrated that ANNs may prove to be superior to LR in predicting reaction risk, thereby catapulting future study into ANNs in a broader patient base.
While these studies differ in methodology and population, they all help allergy specialists and nonspecialists alike use the clinical history to risk stratify patients with unverified beta-lactam allergies. Clearly, the simple and intuitive models proposed by Trubiano and Siew offer a risk classification model that could be easily implemented by various providers after some dedicated training. However, simplicity comes at a trade-off for accuracy; machine learning models such as ANN may prove superior to LR in terms of accuracy but are certainly less intuitive and harder to export to diverse healthcare systems. Ultimately, all prediction studies to date were limited in terms of generalizability – models were developed and validated in select geographic locations (Spain, Australia, France, etc.), among select patients (adults versus children) with select allergies (penicillin versus beta-lactam) in select healthcare settings (inpatient versus outpatient). In all cases, predictors, even when from the patient’s reported history, required a specialist’s phenotype assessment, although methods to use patient-reported data directly may be critical to enhance prediction model use to populations without allergy specialist access. In order to formulate prediction models that can accurately tease out these factors and be safely applied more universally, large international data sets with identical data definitions and architecture are needed.
Taking a Drug Allergy History
Despite the lack of a universally applicable penicillin or beta-lactam allergy prediction model, these data can still guide history-based risk assessments by allergists or general practitioners. In evaluating allergic risk, the index reaction must be determined as anaphylactic or not, as all prediction model studies to date found that anaphylaxis or an otherwise life-threatening reaction indicated patients were at greater risk for true allergy. Additionally, recency of the index reaction at the time of allergy evaluation was unanimously determined to signify higher risk of allergy, be that within the past year (Chiriac, Siew, and Stevenson), past 4 years (Moreno), or past 5 years (Trubiano).
Many factors were denoted as significant to increase true allergy risk only by select studies. Chiriac’s and Moreno’s models identified that reported reactions to penicillins, as well as immediate reactions (defined as 45–60 minutes after final dose), were more likely to represent true allergies. Some factors were found by only a single study to increase risk of true allergy while not considered by the others such as Trubiano’s model which considered if the index reaction required medical treatment, Siew’s model if the patient could recall their index antibiotic, and Chiriac’s model if the patient had multiple allergic episodes to the index antibiotic. Although demographics such as age and sex were considered as potential predictors by all five studies, none of the models found these significant predictors.
Conclusion:
While the clinical history has long been an essential part of drug allergy evaluation, it was also foundational for the development of clinical drug allergy pathways. Currently, researchers are using the clinical history to devise predictive models for beta-lactam allergy. While these models differ substantially, they have achieved considerable diagnostic accuracy within their selected populations. Moving forward, predictive models will need to draw on larger, more diverse cohorts in order to formulate precise point-of-care decision tools that could safely and accurately risk stratify diverse patients with reported beta-lactam allergies presenting across clinical settings.
Key Points:
Clinical pathways for beta-lactam allergy risk stratification have adapted the allergy history for inpatient and outpatient use, demonstrating effectiveness as an antibiotic stewardship tool.
Beta-lactam allergy prediction models using logistic regression and machine learning methods on clinical allergy history data have demonstrated high negative predictive values: 81.3% (Chiriac et al), 98.4% (Siew), 94.7% (Stevenson), 98.4% (Trubiano), and 95.2% (Moreno).
The core aspects of the allergy specialist history which have been validated in predictive models include: history of anaphylaxis, elapsed time since reaction, index drug, reaction onset time, required treatment, and history of multiple reactions.
Future research directions must consider predictive models that draw on larger, more diverse data sets and use uniform data definitions in order to devise accurate point-of-care clinical decision rules that can precisely define true risk of penicillin or beta-lactam allergy.
Acknowledgements:
We would like to thank Sajeewani Upeka Samarakoon, PhD, MS, MPH for her research assistance.
Financial Support and Sponsorship:
There was no direct funding associated with this manuscript. Dr. Blumenthal receives funding from the National Institutes of Health (NIH) K01AI125631, the American Academy of Allergy Asthma and Immunology (AAAAI) Foundation, and the Massachusetts General Hospital (MGH) Department of Medicine Transformative Scholar Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, AAAAI Foundation, nor the MGH.
Abbreviations used:
- U.S.
United States
- ST
skin testing
- OC
oral challenge
- DOC
direct oral challenge
- LR
logistic regression
- aOR
adjusted odds ratio
- NPV
negative predictive value
- ANN
artificial neural network
Footnotes
Conflicts of Interest: Dr. Blumenthal reports a licensed decision support tool to Persistent Systems.
References:
- 1.Abrams EM, Wakeman A, Gerstner TV, Warrington RJ, & Singer AG (2016). Prevalence of beta-lactam allergy: a retrospective chart review of drug allergy assessment in a predominantly pediatric population. Allergy Asthma Clin Immunol, 12, 59. doi: 10.1186/s13223-016-0165-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Dijk SM, et al. , The High Impact of Penicillin Allergy Registration in Hospitalized Patients. J Allergy Clin Immunol Pract, 2016. 4(5): p. 926–31. [DOI] [PubMed] [Google Scholar]
- 3.Macy E (2014). Penicillin and beta-lactam allergy: epidemiology and diagnosis. Curr Allergy Asthma Rep, 14(11), 476. doi: 10.1007/s11882-014-0476-y [DOI] [PubMed] [Google Scholar]
- 4.Macy E, Roppe LB, and Schatz M, Routine Penicillin Skin Testing in Hospitalized Patients with a History of Penicillin Allergy. Perm J, 2004. 8(3): p. 20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trubiano JA, Adkinson NF, and Phillips EJ, Penicillin Allergy Is Not Necessarily Forever. JAMA, 2017. 318(1): p. 82–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trubiano JA, et al. , Development and Validation of a Penicillin Allergy Clinical Decision Rule. JAMA Intern Med, 2020. 180(5): p. 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This study demonstrates that the PEN-FAST rule can accurately identify low-risk penicillin allergies that do not require formal allergy testing.
- 7.Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol, 2010. 105(4): p. 259–273. [DOI] [PubMed] [Google Scholar]
- 8.Mirakian R, et al. , Management of allergy to penicillins and other beta-lactams. Clin Exp Allergy, 2015. 45(2): p. 300–27. [DOI] [PubMed] [Google Scholar]
- 9.Penicillin Allergy in Antibiotic Resistance Workgroup. Penicillin Allergy Testing Should Be Performed Routinely in Patients with Self-Reported Penicillin Allergy. (2017). J Allergy Clin Immunol Pract, 5(2), 333–334. doi: 10.1016/j.jaip.2016.12.010 [DOI] [PubMed] [Google Scholar]
- 10.Demoly P, et al. , International Consensus on drug allergy. Allergy, 2014. 69(4): p. 420–37. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson B, et al. , Multicenter Australian Study to Determine Criteria for Low- and High-Risk Penicillin Testing in Outpatients. J Allergy Clin Immunol Pract, 2020. 8(2): p. 681–689 e3. [DOI] [PubMed] [Google Scholar]; **This study demonstrated that history of a penicillin-associated rash (without angioedema, mucosal ulceration, or systemic involvement) more than 1 year prior was sufficient to identify patients with low-risk penicillin allergies that could proceed directly to oral penicillin challenge.
- 12.Mill C, et al. , Assessing the Diagnostic Properties of a Graded Oral Provocation Challenge for the Diagnosis of Immediate and Nonimmediate Reactions to Amoxicillin in Children. JAMA Pediatrics, 2016. 170(6): p. e160033. [DOI] [PubMed] [Google Scholar]
- 13.Confino-Cohen R, et al. , Oral Challenge without Skin Testing Safely Excludes Clinically Significant Delayed-Onset Penicillin Hypersensitivity. J Allergy Clin Immunol Pract, 2017. 5(3): p. 669–675. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg A and Confino-Cohen R, Skin testing and oral penicillin challenge in patients with a history of remote penicillin allergy. Ann Allergy Asthma Immunol, 2008. 100(1): p. 37–43. [DOI] [PubMed] [Google Scholar]
- 15.Iammatteo M, et al. , Safety and Outcomes of Oral Graded Challenges to Amoxicillin without Prior Skin Testing. J Allergy Clin Immunol Pract, 2019. 7(1): p. 236–243. [DOI] [PubMed] [Google Scholar]
- 16.Tucker MH, et al. , Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract, 2017. 5(3): p. 813–815. [DOI] [PubMed] [Google Scholar]
- 17.Ramsey A, Caubet JC, and Blumenthal K, Risk Stratification and Prediction in Beta-Lactam Allergic Patients. J Allergy Clin Immunol Pract, 2019. 7(7): p. 2182–2184. [DOI] [PubMed] [Google Scholar]
- 18.Banks TA, Tucker M, & Macy E (2019). Evaluating Penicillin Allergies Without Skin Testing. Curr Allergy Asthma Rep, 19(5), 27. doi: 10.1007/s11882-019-0854-6 [DOI] [PubMed] [Google Scholar]
- 19.Conway EL, et al. , Impact of Penicillin Allergy on Time to First Dose of Antimicrobial Therapy and Clinical Outcomes. Clin Ther, 2017. 39(11): p. 2276–2283. [DOI] [PubMed] [Google Scholar]
- 20.Macy E and Vyles D, Who needs penicillin allergy testing? Ann Allergy Asthma Immunol, 2018. 121(5): p. 523–529. [DOI] [PubMed] [Google Scholar]
- 21.Blumenthal KG, et al. , The Impact of a Reported Penicillin Allergy on Surgical Site Infection Risk. Clin Infect Dis, 2018. 66(3): p. 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDanel JS, et al. , Comparative Effectiveness of Beta-Lactams Versus Vancomycin for Treatment of Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections Among 122 Hospitals. Clinical Infectious Diseases, 2015. 61(3): p. 361–367. [DOI] [PubMed] [Google Scholar]
- 23.Macy E and Shu Y-H, The Effect of Penicillin Allergy Testing on Future Health Care Utilization: A Matched Cohort Study. The Journal of Allergy and Clinical Immunology: In Practice, 2017. 5(3): p. 705–710. [DOI] [PubMed] [Google Scholar]
- 24.Blumenthal KG, et al. , Risk-based pathway for outpatient penicillin allergy evaluations. J Allergy Clin Immunol Pract, 2019. 7(7): p. 2411–2414 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanca M, et al. , Clinical evaluation of Pharmacia CAP System RAST FEIA amoxicilloyl and benzylpenicilloyl in patients with penicillin allergy. Allergy, 2001. 56(9): p. 862–70. [DOI] [PubMed] [Google Scholar]
- 26.Blumenthal KG, Park MA, and Macy EM, Redesigning the allergy module of the electronic health record. Ann Allergy Asthma Immunol, 2016. 117(2): p. 126–31. [DOI] [PubMed] [Google Scholar]
- 27.Sousa-Pinto B, Tarrio I, Blumenthal KG, Araújo L, Azevedo LF, Delgado L, & Fonseca JA (2021). Accuracy of penicillin allergy diagnostic tests: A systematic review and meta-analysis. Journal of Allergy and Clinical Immunology, 147(1), 296–308. doi: 10.1016/j.jaci.2020.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knezevic B, et al. , The revolving door: antibiotic allergy labelling in a tertiary care centre. Intern Med J, 2016. 46(11): p. 1276–1283. [DOI] [PubMed] [Google Scholar]
- 29.Mill C, et al. , Assessing the Diagnostic Properties of a Graded Oral Provocation Challenge for the Diagnosis of Immediate and Nonimmediate Reactions to Amoxicillin in Children. JAMA Pediatr, 2016. 170(6): p. e160033. [DOI] [PubMed] [Google Scholar]
- 30.Mustafa SS, Conn K, and Ramsey A, Comparing Direct Challenge to Penicillin Skin Testing for the Outpatient Evaluation of Penicillin Allergy: A Randomized Controlled Trial. J Allergy Clin Immunol Pract, 2019. 7(7): p. 2163–2170. [DOI] [PubMed] [Google Scholar]
- 31.Marshall GD, The status of US allergy/immunology physicians in the 21st century: a report from the American Academy of Allergy, Asthma & Immunology Workforce Committee. J Allergy Clin Immunol, 2007. 119(4): p. 802–7. [DOI] [PubMed] [Google Scholar]
- 32.Blumenthal KG, et al. , The Cost of Penicillin Allergy Evaluation. The Journal of Allergy and Clinical Immunology: In Practice, 2018. 6(3): p. 1019–1027.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sousa-Pinto B, et al. , Penicillin allergy testing is cost-saving: An economic evaluation study. Clin Infect Dis, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This economic evaluation study showed that penicillin allergy testing was projected to be cost-saving across different scenarios with allergy testing resulting in average savings of $657 for inpatients and $2,746 for outpatients.
- 34.Shenoy ES, et al. , Evaluation and Management of Penicillin Allergy: A Review. JAMA, 2019. 321(2): p. 188–199. [DOI] [PubMed] [Google Scholar]
- 35.Wolfson AR, Huebner EM, and Blumenthal KG, Acute care beta-lactam allergy pathways: approaches and outcomes. Ann Allergy Asthma Immunol, 2019. 123(1): p. 16–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blumenthal KG, et al. , Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. Journal of Allergy and Clinical Immunology, 2017. 140(1): p. 154–161.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blumenthal KG, et al. , Addressing Inpatient Beta-Lactam Allergies: A Multihospital Implementation. J Allergy Clin Immunol Pract, 2017. 5(3): p. 616–625 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishna MT, Huissoon AP, Li M, Richter A, Pillay DG, Sambanthan D, … Misbah SA (2017). Enhancing antibiotic stewardship by tackling "spurious" penicillin allergy. Clin Exp Allergy, 47(11), 1362–1373. doi: 10.1111/cea.13044 [DOI] [PubMed] [Google Scholar]
- 39.Chiriac AM, et al. , Controversies in Drug Allergy: Drug Allergy Pathways. J Allergy Clin Immunol Pract, 2019. 7(1): p. 46–60 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alam M, & Bastakoti B (2015). Therapeutic Guidelines: Antibiotic. Version 15. Australian Prescriber, 38(4), 137. 10.18773/austprescr.2015.049 [DOI] [Google Scholar]
- 41.Chiriac AM, et al. , Designing Predictive Models for Beta-Lactam Allergy Using the Drug Allergy and Hypersensitivity Database. The Journal of Allergy and Clinical Immunology: In Practice, 2018. 6(1): p. 139–148.e2. [DOI] [PubMed] [Google Scholar]
- 42.Siew LQC, et al. , Identifying Low-Risk Beta-Lactam Allergy Patients in a UK Tertiary Centre. J Allergy Clin Immunol Pract, 2019. 7(7): p. 2173–2181 e1. [DOI] [PubMed] [Google Scholar]
- 43.Moreno EM, Moreno V, Laffond E, Gracia-Bara MT et al. (2020). Usefulness of an Artificial Neural Network in the Prediction of beta-Lactam Allergy. J Allergy Clin Immunol Pract. doi: 10.1016/j.jaip.2020.07.010 [DOI] [PubMed] [Google Scholar]
- 44.Blumenthal KG, The Role of the Clinical History in Drug Allergy Prediction. J Allergy Clin Immunol Pract, 2018. 6(1): p. 149–150. [DOI] [PubMed] [Google Scholar]


