Abstract
Epidermal squamous cell carcinoma develops in response to ultraviolet light exposure and is among the most common cancers. The transglutaminase 2 cancer cell survival protein stimulates activity of the YAP1/TEAD transcription complex to drive expression of genes that promote aggressive epidermal squamous cell carcinoma cell invasion, migration and tumor formation. Therefore, we are interested in mechanisms that may inhibit these events. Vestigial-like protien-4 (VGLL4) is a transcription cofactor/tumor suppressor that inhibits several pro-cancer pathways including YAP1 signaling. Our present studies show that VGLL4 inhibits YAP1/TEAD-dependent transcription to reduce expression of YAP1 target genes (CCND1, CYR61 and CTGF) and pro-cancer collagen genes (COL1A2, COL3A1). We further show that loss of these YAP1 regulated genes is required for VGLL4 suppression of the cancer cell phenotype, as forced CCND1 or COL1A2 expression partially restores the aggressive cancer phenotype in VGLL4 expressing cells. Consistent with these findings, VGLL4 expression reduces tumor formation, and this is associated with reduced CCND1, CYR61, CTGF, COL1A2 and COL1A3 mRNA and protein level, and reduced EMT marker expression. These findings indicate that VGLL4 suppresses the malignant epidermal squamous cell carcinoma cancer phenotype by inhibiting YAP1/TEAD-dependent pro-cancer signaling.
Keywords: YAP, TAZ, TEAD, Hippo signaling, transglutaminase 2, CTGF, CYR61, CCND1, COL1A2, COL3A1, cancer stem cell, epidermal squamous cell carcinoma
Introduction
Epidermal squamous cell carcinoma (SCC) is an extremely common cancer that is caused by exposure to ultraviolet light 1. Treatment is by surgical removal of the primary tumor, but recurrence is common, and the recurring tumors are aggressive and therapy resistant 1. Cancer stem cells play a significant role in tumor formation, recurrence and metastasis in a host of cancers 2. We have characterized epidermal squamous cell carcinoma cancer stem cells (ECS cells) which express stem cell markers, form aggressive and highly vascularized tumors, and display enhanced migratory and invasive potential 3.
Transglutaminase 2 (TG2) is a multifunctional protein that is enriched in ECS cells and regulates a range of signaling pathways that maintain ECS cell and non-stem cancer cell survival 4–7. We are intersted in the role of TG2 in maintaining the cancer phenotype and have shown that it stimulates VEGF 8, NRP1 9, GIPC1/SYX/RhoA/p38 9, α6/β4-integrin 10,11 and YAP1/TAZ 10 signaling. We have shown that TG2 is constitutively expressed in SCC cells where it interacts with α6/β4 integrin to stimulate FAK/Src signaling leading to PI3K activation of phosphoinositide-dependent kinase 1 (PDK1). PDK1, in turn, inhibits Hippo signaling to enhance nuclear YAP1 accumulation which stimulates epidermal squamous cell carcinoma spheroid formation, invasion, and migration 10. YAP1 complexes with TEAD transcription factors in the nucleus to activate transcription of pro-cancer survival genes 12. In the present study, we describe a mechanism that attenuates TG2-dependent YAP1 signaling 10.
Vestigial-like protein-4 is an important tumor suppressor in a host of cancers that is regulated by various signaling cascades 13. Recent studies show that vestigial like protein-4 (VGLL4) suppresses YAP1 function by competitive inhibition of YAP1/TEAD interaction 14. VGLL4 binds to TEAD transcription factor via two TDU domains that interact with TEAD factors via the YAP binding domain. VGLL4 forms a VGLL4/TEAD complex that prohibits YAP1/TEAD complex formation thereby reducing YAP1/TEAD-dependent transcription 13. Reduced VGLL4 expression predicts poor survival in many cancers including lung, gastric, breast, colorectal, bladder, pancreatic and esophageal cancer 14. VGLL4 also negatively regulates Wnt/β-catenin signaling, suppresses epithelial-mesenchymal transition and activates apoptosis 13,14.
Since VGLL4 suppresses YAP1/TEAD associated signaling in esophageal squamous cell carcinoma 15, we tested if VGLL4 regulates YAP1/TEAD signaling in epidermal squamous cell carcinoma. Our previous studies show that YAP1 stimulates SCC cell spheroid formation, invasion, migration and tumor formation 10. We show that these responses are attenuated by VGLL4 and that this is associated with reduced YAP1/TEAD-dependent transcription and reduced expression of YAP1 downstream target genes. Moreover, reduced target gene expression is required for VGLL4 action, as forced expression of these genes partially reverses VGLL4 suppression of the cancer phenotype. We further show that VGLL4 expression reduces tumor YAP1 levels, YAP1 target gene mRNA and protein expression, and EMT and that this is associated with reduced tumor formation. These findings suggest that VGLL4 suppresses the malignant cancer phenotype by inhibiting YAP1-dependent signaling.
Materials and Methods
Reagents
Rabbit anti-VGLL4 (HPA038225), mouse anti-FLAG (F3165), mouse anti-TG2 (MAB3839) and mouse anti-β-actin (A5441) were obtained from Millipore Sigma (St. Louis, MO). Rabbit antibodies against YAP1 (4912S), YAP1-P (13008S), pan-TEAD (13295S), CTGF (10095S), CYR61 (39382S), Cyclin D1 (2922S), Cyclin D1-P (3300S), E-Cadherin (3195S), vimentin (5741S) and mouse anti-Snail (3895S) were obtained from Cell Signaling Technology (Danvers, MA). Rabbit anti-COL1A2 (ab96723), anti-Slug (ab27568) and anti-Twist (ab49254) were from Abcam (Cambridge, MA). Mouse anti-Ezh2 (612667), anti-fibronectin (610077) and anti-N-Cadherin (610920) were obtained from BD Transduction Laboratories (San Jose, CA). Rabbit anti-COL3A1 (GTX102997) was from GeneTex (Irvine, CA). pGL3-B-8xGTIIC plasmid (34615) was from Addgene (Watertown, MA). pCMV6-VGLL4-FLAG (RC200886), pCMV6-CCND1 (RC204957) and pCMV6-COL1A2 (RC208484) were obtained from Origene (Rockville, MD). Control siRNA (sc-37007) was obtained from Santa Cruz Biotechnology (Dallas, TX) and siRNA targeting YAP1 (S10662954, S10443867, S104438644, S104438367) was obtained from Qiagen (Germantown, MD). DAPI (D9542) was obtained from Sigma Aldrich (Milwaukee, WI). Millicell chambers (353097) and Matrigel (354234) were purchased from BD Biosciences (San Diego, CA). Fugene 6 (E2691) was from Promega (Madison, WI). G418 (11811) was from Invitrogen (Waltham, MA). The YAP1 signaling inhibitor, CA3 (CIL56) 16, was purchased from SelleckChem (Houston, TX). Peroxidase-conjugated sheep anti-mouse IgG (NA931V) and peroxidase-conjugated donkey anti-rabbit IgG (NA934V) secondary antibodies were purchased from GE Healthcare Systems (Chicago, IL). Statistical analysis used the student’s t-test of a minimum of triplicate repeats. Single asterisks indicate a significant reduction, p > 0.001 and double astersks indicate a significant increase, p > 0.001.
Electroporation
Cells (1.2 million) were suspended in 100 μl of keratinocyte nucleofector reagent (VPD-1002, Lonza) containing 3 μg of plasmid. The cells were electroporated using the AMAXA nucleofector device on the T-018 setting 17 and the cells were permitted to recover in medium for 48 h before they were plated for use in biological assays (cell proliferation, spheroid formation, invasion, migration). For transient knockdown of targets, 1.2 million cells were electroporated with 3 μg of siRNA as above, and after recovery the electroporation was repeated a second time before the cells were plated for use in biological assays 17.
Cell culture
SCC-13 are human epidermis derived, tumor forming, squamous cell carcinoma cells 18. HaCaT are immortalized cells derived from human epidermis that do not form tumors 19. Growth medium contained DMEM supplemented with 4.5 mg/ml D-glucose, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 U/ml streptomycin and 5 % fetal calf serum. Cell proliferation, spheroid formation, invasion and migration assays were performed as previously described 20. Spheroids were grown in spheroid medium [DMEM/F12 (1:1) containing 2% B27 serum-free supplement, 20 ng/ml EGF, 0.4% bovine serum albumin and 4 mg/ml insulin on ultra-low attachment plates] 20. To create VGLL4 overexpressing stable cell lines, SCC-13 cells were electroporated with 1 μg of pCMV6-VGLL4-FLAG plasmid and VGLL4-FLAG positive cells were selected by treatment with 2 μg/ml G418 for 2 weeks before deriving clonal cell lines. The resulting clonal SCC13-VGLL4-FLAG1–8 and SCC13-VGLL4-FLAG1–9 cell lines were maintained in growth medium.
TEAD luciferase reporter and qRT-PCR assays
To monitor TEAD responsive transcription activity, cells were transfected with 0.5 μg of pGL3-B-Luc or pGL3-B-8xGTIIC-Luc using Fugene 6 and at 24 h the cells were harvested for luciferase assay. For qRT-PCR measurement of target gene RNA level, RNA was isolated using the Illustra RNAspin Mini Kit (25050070, GE Healthcare Life Sciences), reverse transcribed, and quantified using LightCycler 480 SYBR Green I and gene-specific primers. Cyclophilin A forward (5’-CAT CTG CAC TGC CAA GAC TGA-3’) and reverse (5’-TTC ATG CCT TCT TTC ACT TTG C-3’) primers 21 were used as a normalizing control gene. CTGF forward (5′-GGA AAT GCT GCG AGG AGT GG-3’) and reverse (5′-GAA CAG GCG CTC CAC TCT GTG-3’) primers, CYR61 forward (5′-CAC ACC AAG GGG CTG GAA TG-3’) and reverse (5′-CCC GTT TTG GTA GAT TCT GG-3’) primers and CCND1 forward (5′-TGA AGG AGA CCA TCC CCC TG-3’) and reverse (5′-TGT TCA ATG AAA TCG TGC GG-3’) primers were as described 22. Transglutaminase 2 primers were forward (5′-TAA GAG ATG CTG TGG AGG AG-3’) and reverse (5′-CGA GCC CTG GTA GAT AAA-3’). The COL3A1 forward (5’-TGG TCT GCA AGG AAT GCC TGG A-3’) and reverse (5’-TCT TTC CCT GGG ACA CCA TCA G-3’) primers (HP200076) and the COL1A2 forward (5’-CCT GGT GCT AAA GGA GAA AGA GG-3’) and reverse (5’-ATC ACC ACG ACT TCC AGC AGG A-3’) primers (HP200075) were purchased from Origene (Rockville, MD).
Immunoblot Analysis
Equal protein equivalents of cell extract were boiled in Laemmli sample buffer, loaded onto 10–12% denaturing polyacrylamide gels and electrophoresed. The proteins were transferred to a nitrocellulose membrane and the membrane was blocked with 5% nonfat dry milk, incubated in primary antibodies overnight at 4°C and secondary antibody at room temperature for 1 h. The proteins were visualized using chemiluminescent detection reagents.
Tumor Xenograft
Wild-type SCC-13 and SCC13-VGLL4-FLAG1–9 cells were grown in monolayer culture and 100,000 cells were resuspended in 100 μl PBS containing 30% Matrigel and injected into each front flank of 5 female NOD Scid IL2 receptor gamma chain knockout mice (NSG) per treatment group. Tumor volume was measured as volume = 4/3π x (diameter/2)3. The tumor samples were harvested, stored frozen in liquid nitrogen and used to prepare extracts for immunoblot and qRT-PCR analysis.
Results
VGLL4 suppresses YAP1-dependent transcription and YAP1/TEAD target gene expression
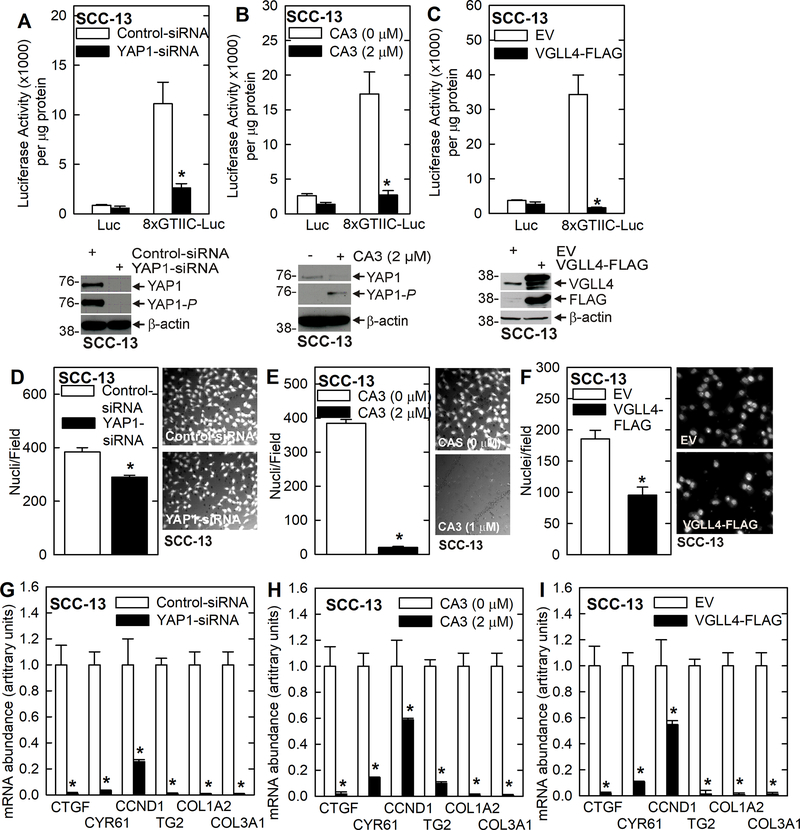
VGLL4 has been reported to suppress YAP1/TEAD-associated transcription and expression of downstream target genes 15,23–25. We first determined if VGLL4 regulates YAP1-dependent transcription in SCC-13 cells. Fig. 1A/B show that YAP1 knockdown or treatment with CA3, which inhibits YAP1 activity 16,26, reduced YAP1 transcription as shown by reduced activity of a TEAD transcription factor reporter construct, 8xGTIIC-Luc. Fig. 1C shows that VGLL4-FLAG overexpression produced a similar reduction in YAP1/TEAD-dependent transcription. We next monitored the impact of these treatments on SCC-13 cell invasion. Cells were seeded atop a Matrigel layer in medium containing 1% FCS and the ability of cells to move through the Matrigel to the lower chamber, which contains 10% FCS, was monitored. Fig. 1D/E/F shows that YAP1 knockdown, CA3 treatment and VGLL4-FLAG expression reduced cell invasion. We next monitored the impact of these treatments on downstream markers of YAP1 action. Fig. 1G/H/I shows that YAP1 knockdown, CA3 treatment and VGLL4-FLAG overexpression reduce YAP1 target gene (CTGF, CCND1, CYR61) and pro-cancer collagen gene (COL1A2 and COL3A1) mRNA levels.
Fig. 1.
VGLL4 inhibits SCC-13 cell YAP1/TEAD-dependent transcription and gene expression and suppresses the cancer phenotype. A/B/C YAP1 knockdown, YAP1 inhibitor (CA3) treatment, and transient VGLL4 expression suppress 8xGTIIC-Luc activity signifying suppression of YAP1/TEAD dependent transcription. These treatments also reduce cancer cell invasion (D/E/F) and YAP1-target gene (CTGF, CYR61, CCND1), pro-cancer collagen gene (COL1A2 and COL3A1) and TG2 mRNA levels (G/H/I). The asterisks indicate a significant reduction, n ≥ 3, p > 0.001.
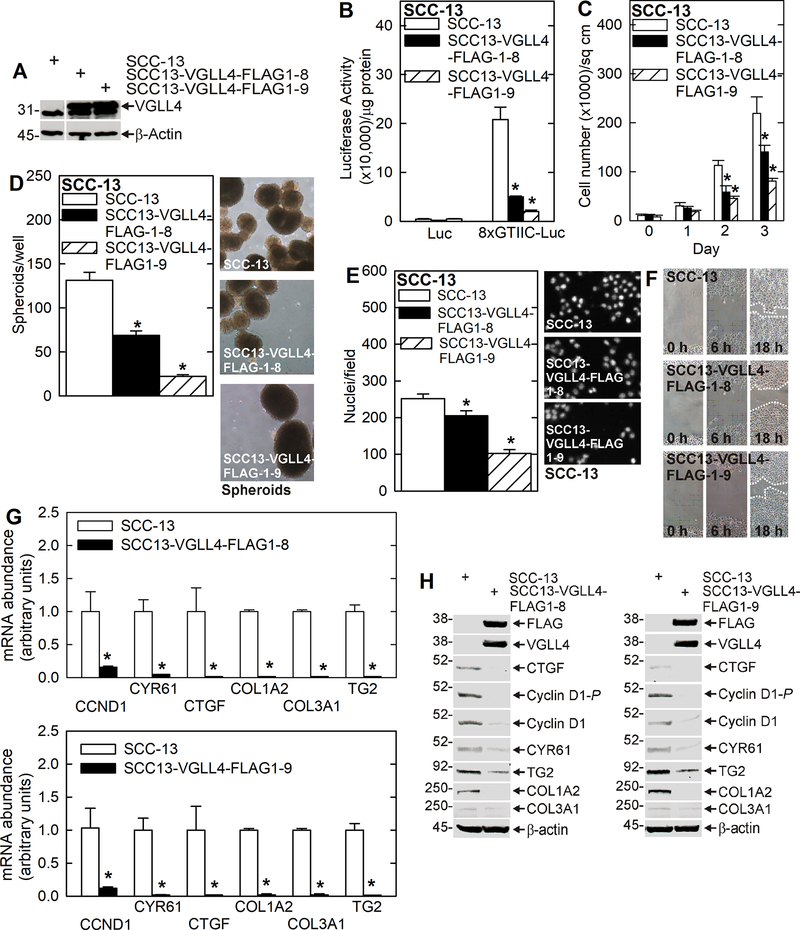
We next monitored the impact of stable VGLL4-FLAG overexpression on YAP1/TEAD transcription and spheroid formation, invasion and migration. We show that vector-mediated VGLL4-FLAG stable expression in two SCC-13 cell clones (Fig. 2A) reduced YAP1/TEAD transcriptional activity, cell proliferation, spheroid formation, invasion and migration (Fig. 2B/C/D/E/F). In addition, CCND1, CYR61, CTGF, COL1A2, COL3A1 and TG2 mRNA and protein levels are reduced in the VGLL4-FLAG overexpressing lines (Fig. 2G/H).
Fig. 2.
VGLL4 attenuates the cancer cell phenotype and YAP1/TEAD-dependent gene expression. A Stable VGLL4-FLAG overexpression cell lines as compared to wild-type SCC-13 cells. B TEAD-dependent transcription is suppressed in VGLL4 overexpressing cells. C/D/E/F VGLL4 overexpressing cells line display reduced cell proliferation, spheroid formation, invasion and migration. G/H Stable VGLL4 overexpression suppresses YAP1-target gene, pro-cancer collagen gene and TG2 gene mRNA and protein levels. The asterisks indicate a significant reduction, n ≥ 3, p > 0.001.
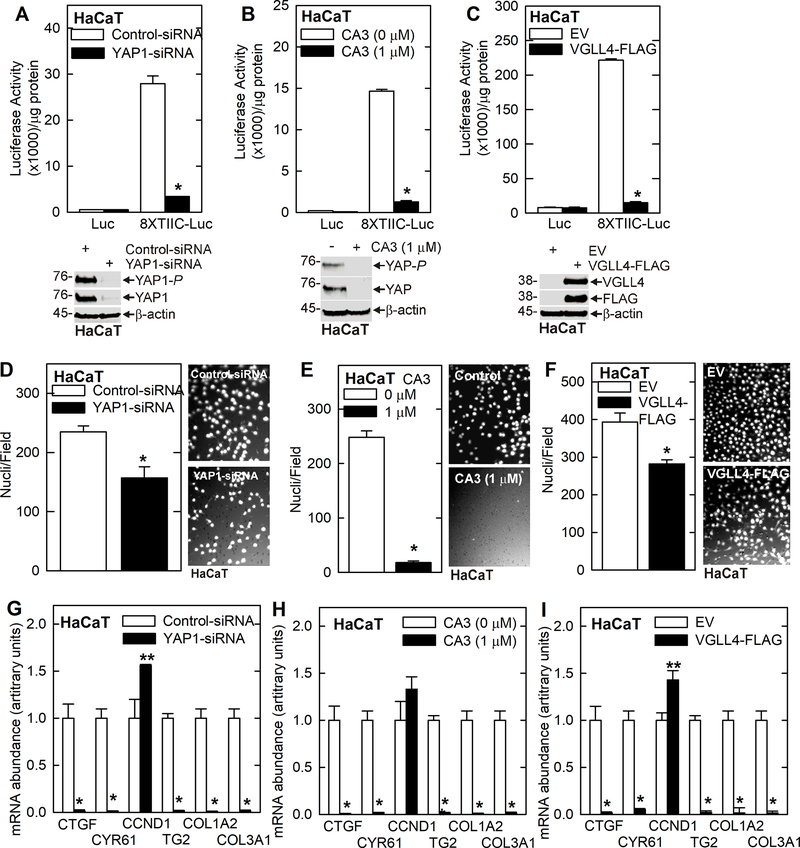
To assure that these findings can be generalized, we monitored the impact of modulating YAP1 and VGLL4 function in epidermis-derived HaCaT cells 19. Treating HaCaT cells with YAP1-siRNA, YAP1 inhibitor or VGLL4-FLAG overexpression suppressed YAP1/TEAD-related transcription (Fig. 3A/B/C) and reduced invasion (Fig. 3D/E/F). Moreover, these treatments reduced YAP1/TEAD responsive gene mRNA levels (Fig. 3G/H/I). The one exception is CCND1, which is slightly elevated or not changed by these treatments.
Fig. 3.
VGLL4 inhibits HaCaT cell YAP1/TEAD-dependent transcription and gene expression, and suppresses the cancer phenotype. A/B/C YAP1 knockdown, YAP1 inhibitor (CA3) treatment, and transient VGLL4 expression suppress 8xGTIIC-Luc activity signifying suppression of YAP1/TEAD-dependent transcription. These treatments also reduce cancer cell invasion (D/E/F) and YAP1-target gene (CTGF, CYR61), pro-cancer collagen gene (COL1A2 and COL3A1) and TG2 mRNA levels (G/H/I).
Role of YAP1 and VGLL4 responsive downstream target genes
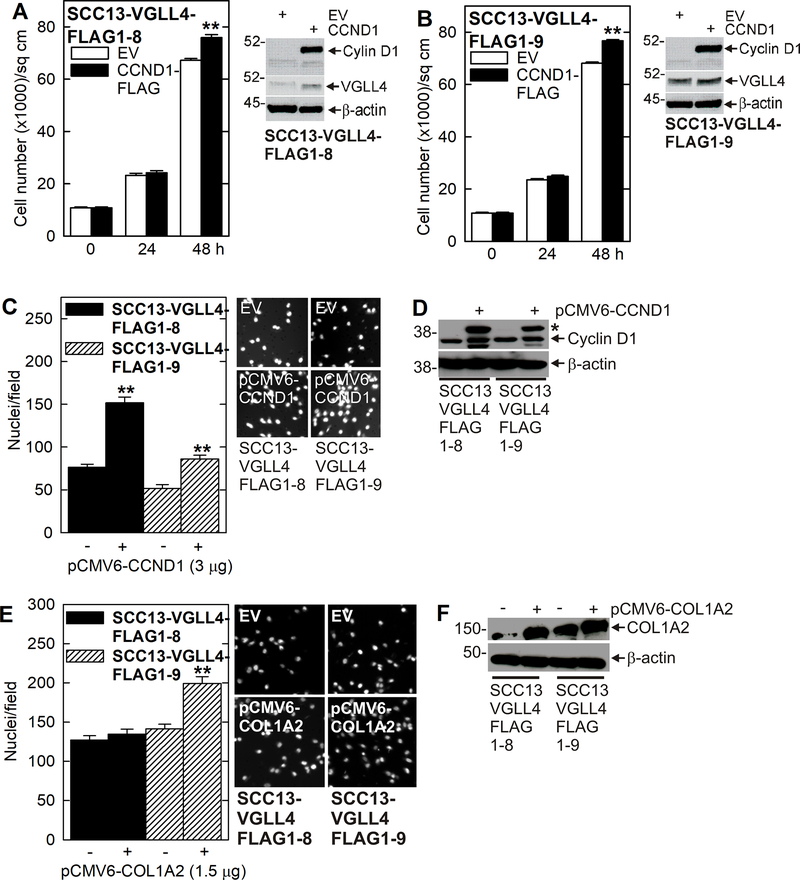
If VGLL4 suppression of YAP1 responsive genes is important for the reduction in aggressive pro-cancer phenotype observed in Figs. 1, 2 and 3, overexpression of these genes should partially restore the aggressive phenotype. To test this, we monitored the ability of selected target genes to restore the aggressive phenotype in VGLL4-FLAG expressing cells. Fig. 4A/B show that forced CCND1-FLAG (cyclin D1) expression in SCC13-VGLL4-FLAG1–8 and FLAG1–9 cells slightly increased cell proliferation and partially restored invasion (Fig. 4C/D). Fig. 4E/F shows that forced COL1A2 expression increased SCC13-VGLL4-FLAG1–9 invasion but has modest effects on SCC13-VGLL4-FLAG1–8 cells. These findings suggest that reduced YAP1 target gene expression is important for VGLL4 attenuation of the cancer phenotype and that forced YAP1/TEAD target gene expression can partially restore the aggressive phenotype.
Fig. 4.
CCND1 and COL1A2 partially restore the cancer phenotype in VGLL4 overexpressing cells. A/B Forced cyclin D1 (CCND1-FLAG) expression stimulates a modest increase in cell proliferation in VGLL4 overexpressing cells. C/D Cell invasion is partially increased by forced CCND1-FLAG expression in VGLL4 overexpressing cells. The asterisk in panel D indicate migration of the CCND1-FLAG fusion protein. E/F COL1A2 expression partially restores invasion in SCC13-VGLL4-FLAG1–9 cells. The double asterisks indicate a significant increase, n ≥ 3, p > 0.001.
Impact of VGLL4 on tumor formation
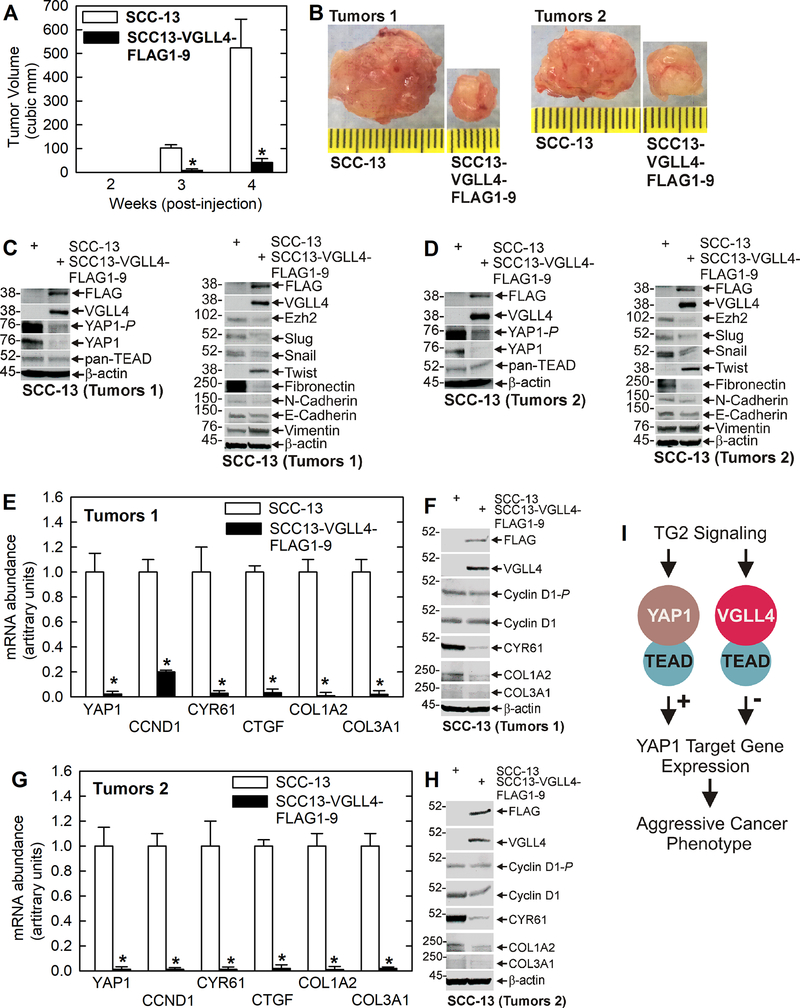
We next compared tumor formation in wild-type and VGLL4-FLAG overexpressing cells. Wild-type and VGLL4-FLAG overexpressing cells, derived from monolayer cultures, were injected into each front flank in NSG mice and tumor formation was monitored. Fig. 5A/B shows a marked reduction in tumor growth of VGLL4-FLAG expressing cells. Consistent with reduced YAP1 signaling activity, YAP1 and YAP1-P levels are reduced in these tumors (Fig. 5C/D). Moreover, a survey of malignancy markers revealed a decrease in Ezh2 level, an ECS cell-enriched survival marker 3,20,27, and reduced expression of many (Slug, Snail, fibronectin, N-cadherin), but not all (Twist, vimentin), EMT markers 28 in VGLL4-FLAG expressing tumors. In addition, E-cadherin, which is associated with an epithelial phenotype, is not altered in level in wild-type versus VGLL4-FLAG overexpressing tumors. We also monitored expression of YAP1-responsive target genes and show that VGLL4-FLAG expressing tumors display reduced levels of YAP1, CCND1, CYR61, CTGF, COL1A2 and COL3A1 mRNA and protein (Fig. 5E/F/G/H), which is consistent with the expression changes observed in cultured cells (Fig. 2G/H).
Fig. 5.
VGLL4 overexpression suppresses tumor formation. Wild-type SCC-13 and SCC13-VGLL4-FLAG1–9, derived from cells grown as monolayer cultures, were injected into each front flank of NSG mice and tumor formation and growth was monitored over the course of 4 weeks. A/B VGLL4-FLAG overexpression in SCC13-VGLL4-FLAG1–9 markedly reduces tumor growth. Representative size tumors were selected for analysis. The asterisks indicate a significant reduction in tumor size, n = 10 tumors (5 mice x 2 tumor/mouse), p > 0.001. C/D The VGLL4-dependent reduction in tumor growth is associated with decreased YAP1, YAP1-P and TEAD levels, loss of the Ezh2 marker of aggressive cancer 20, and a reduction in the level of many EMT (Slug, Snail, Fibronectin and N-Cadherin) markers. E/F/G/H VGLL4 overexpressing tumors display reduced YAP1, YAP1/TEAD target gene (CCND1, CYR61, CTGF) and pro-cancer collagen gene (COL1A2, COL3A1) mRNA and protein levels. The asterisks indicate a significant reduction compared to control, n = 3, p > 0.001. Note that for some genes we observe a dramatic reduction in mRNA level and a more modest reduction in protein in VGLL4 overexpressing tumors. This is likely due to slower protein turnover and/or a compensatory increase in protein translation. I Schematic model of proposed signaling cascade. Previous reports show that TG2 interaction with ɑ6/β4 integrins results in a signaling cascade that activates YAP1/TEAD transcription and signaling, and ultimately enhances the cancer phenotype 10. Our present studies suggest that a VGLL4/TEAD inhibitory complex can suppress TG2-dependent YAP1/TEAD signaling to attenuate the cancer phenotype.
Discussion
Epidermal squamous cell carcinoma is a common cancer that is caused by mutagens. The most common cause is exposure to UVB irradiation 29. Therapy is via surgical removal, which is successful in many cases. However, approximately 10 – 30% of these tumors recur as aggressive and therapy-resistant cancer. Thus, studies are ongoing to identify agents that suppress recurrent cancer. TG2 is a multifunctional regulator that has two mutually exclusive activities associated with distinct protein conformations. In the closed/folded conformation TG2 functions as a GTP binding protein while the open/extended TG2 conformation functions as a transamidase enzyme 4,30–32. The closed conformation predominates in the intracellular environment in the presence of high GTP levels, while the open conformation is induced by calcium binding and is often active in dying cells 4,30. The closed and open conformations can both drive cancer cell survival. However, the closed GTP binding form drives malignancy in the vast majority of cancers 6,28,33 and we have shown that the closed TG2 GTP binding conformation drives cancer cell spheroid formation, invasion, migration and tumor formation and that this is attenuated by mutation of the TG2 GTP binding site 28.
Although TG2 is an important survival factor in squamous cell carcinoma, the mechanism of TG2 action is not yet well understood in this or any other cancer. However, some information is available. For example, we have shown that TG2 activates a host of cascades in SCC cells including VEGF 8, NRP1 9, GIPC1/SYX/RhoA/p38 9, α6/β4-integrin 10,11 and YAP1 10 signaling. In the present study, we focus on TG2 regulation YAP1 signalling. Our previous studies showed that TG2 associates with a6/b4-integrin and that this complex activates FAK/Src signaling which activates PDK1 to inhibit Hippo signaling leading to accumulation of active YAP1 in the nucleus where it interacts with TEAD transcription factors to activate transcription to drive the aggressive cancer phenotype 10. We also identified a novel signaling cascade that involves NRP-1, and showed that NRP-1 forms a complex with GIPC1 and α6/β4-integrin to activate FAK/Src signaling, which stabilizes nuclear YAP1 to enhance cancer cell survival, invasion, and angiogenesis 9. We also showed that sulforaphane, a diet-derived anti-cancer agent, reduces YAP1 signaling to attenuate the SCC cell phenotype 34.
Because of the central role of YAP1 signaling in these cascades, we wanted to examine the role of VGLL4, an important suppressor of YAP1-dependent transcription 35. VGLL4 was initially reported to bind TEAD1 to suppress TEAD1-dependent a1-adrenergic activation in cardiac myocytes 36. Later it was demonstrated to inhibit nuclear YAP1/TEAD interaction by competing with YAP1/TEAD complex formation to suppress TEAD-dependent tumorigenesis 37,38. Moreover, a super-TDU peptide, designed based on the structure of the VGLL4/TEAD4 complex, can inhibit YAP1/TEAD interaction to reduce YAP1 signaling and tumor formation 35,39.
VGLL4 suppresses TEAD-dependent transcription and cancer cell invasion
Our studies confirm that YAP1/TEAD signaling is active in squamous cell carcinoma and is associated with an aggressive SCC cell phenotype as measured by enhanced spheroid formation, collagen invasion and migration. Moreover, YAP1/TEAD-dependent transcription and cancer cell spheroid formation, invasion and migration are reduced in VGLL4 overexpressing cells. This is observed in the highly tumorigenic and aggressive SCC-13 cells and in the immortalized but non-tumorigenic HaCaT cells. In addition, stable overexpression of VGLL4-FLAG in SCC-13 cells confirmed that VGLL4 suppresses the aggressive YAP1/TEAD dependent cancer phenotype.
YAP1 signaling maintains stable expression of YAP1 pro-cancer target genes, including CCND1, CYR61 and CTGF 40–44, which are markedly reduced in VGLL4-FLAG expressing cells. VGLL4-FLAG expression also reduced COL1A2 and COL3A1, which are collagen genes associated with an aggressive cancer phenotype 45–49. To confirm that loss of expression of these targets is required for VGLL4 suppression of the cancer phenotype, we forced CCND1 (cyclin D1) and COL1A2 (collagen type I alpha 2) expression in VGLL4 overexpressing cells and were able to partially antagonize VGLL4 suppression of cancer cell invasion. These experiments also identify a VGLL4-dependent reduction in TG2 encoding mRNA, suggesting that YAP1 signaling may maintain TG2 level. This is an interesting finding, considering that our previous studies show that TG2 activates YAP1 signaling to drive the aggressive cancer cell cancer phenotype 10. Further studies will be necessary to assess if a TG2/YAP1 positive feedback loop helps drive the cancer phenotype.
VGLL4 suppresses tumor formation
Tumor studies reveal that VGLL4-FLAG overexpressing cells produce 10-fold smaller tumors than wild-type cells. Moreover, this is associated with reduced YAP1 mRNA and protein levels. It is interesting that VGLL4 overexpression is associated with a modest reduction in TEAD level, suggesting that YAP1 stability may be more sensitive to the action of VGLL4. We also observe a substantial reduction in EMT markers including Slug, Snail, fibronectin, and N-cadherin. However, not all EMT markers are reduced as vimentin levels are not changed and Twist level is increased in VGLL4 overexpressing cells. Moreover, the level of the epithelial marker, E-cadherin, is not changed in VGLL4 expressing cells. These findings indicate that VGLL4 reduces YAP1/TEAD signaling and suppresses EMT as part of the process that reduces tumor growth. It is important that YAP1 regulated target genes, including CCND1, CYR61, CTGF, COL1A2 and COL3A1, are reduced in VGLL4 expressing tumors which agrees with the observations in the cultured cells.
Sulforaphane is an important diet-derived cancer preventive the treatment agent 50. Our studies show that sulforaphane treatment reduces YAP1 level to inhibit skin cancer cell YAP1 signaling which attenuates the aggressive cancer phenotype 34. We had thought that sulforaphane may increase VGLL4 level as part of the mechanism to attenuate YAP1 activity, but no increase in VGLL4 level was observed in sulforaphane treated cells (not shown).
VGLL4 mechanism of action in skin cancer
Our previous studies 10,51 show that a TG2/a4/b6-integrin and TG2/NRP-1 activated signaling cascades trigger YAP1 nuclear localization to maintain cancer cell spheroid formation, invasion, migration and tumor formation. Our present studies suggest that the VGLL4 tumor suppressor forms a VGLL4/TEAD complex that inhibits YAP1/TEAD interaction to reduce TG2-stimulated YAP1/TEAD transcription and target gene expression to attenuate the cancer phenotype (Fig. 5I).
Acknowledgements:
This work was supported by NIH CA211909 (RLE) and utilized the facilities of the Greenebaum Comprehensive Cancer Center (P30 CA134274).
Footnotes
Conflict of Interest: The authors declare no competing conflicts of interest.
Data Sharing: The authors elect to not share data.
Literature Cited
- 1.Lansbury L, Bath-Hextall F, Perkins W, Stanton W, Leonardi-Bee J. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ. 2013;347:f6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adhikary G, Grun D, Kerr C, et al. Identification of a population of epidermal squamous cell carcinoma cells with enhanced potential for tumor formation. PLoS One. 2013;8(12):e84324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckert RL. Transglutaminase 2 takes center stage as a cancer cell survival factor and therapy target. Mol Carcinog. 2019;58(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckert RL, Fisher ML, Grun D, Adhikary G, Xu W, Kerr C. Transglutaminase is a tumor cell and cancer stem cell survival factor. Mol Carcinog. 2015;54(10):947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckert RL, Kaartinen MT, Nurminskaya M, et al. Transglutaminase regulation of cell function. Physiol Rev. 2014;94(2):383–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta K, Kumar A, Kim HI. Transglutaminase 2: a multi-tasking protein in the complex circuitry of inflammation and cancer. Biochem Pharmacol. 2010;80(12):1921–1929. [DOI] [PubMed] [Google Scholar]
- 8.Grun D, Adhikary G, Eckert RL. VEGF-A acts via neuropilin-1 to enhance epidermal cancer stem cell survival and formation of aggressive and highly vascularized tumors. Oncogene. 2016;35:4379–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grun D, Adhikary G, Eckert RL. NRP-1 interacts with GIPC1 and SYX to activate p38 MAPK signaling and cancer stem cell survival. Mol Carcinog. 2019;58(4):488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher ML, Kerr C, Adhikary G, et al. Transglutaminase Interaction with alpha6/beta4-Integrin Stimulates YAP1-Dependent DeltaNp63alpha Stabilization and Leads to Enhanced Cancer Stem Cell Survival and Tumor Formation. Cancer Res. 2016;76(24):7265–7276. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 11.Fisher ML, Keillor JW, Xu W, Eckert RL, Kerr C. Transglutaminase is required for epidermal squamous cell carcinoma stem cell survival. Mol Cancer Res. 2015;13:1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Huang T, Cheng AS, Yu J, Kang W, To KF. The TEAD Family and Its Oncogenic Role in Promoting Tumorigenesis. Int J Mol Sci. 2016;17(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaguchi N Multiple Roles of Vestigial-Like Family Members in Tumor Development. Front Oncol. 2020;10:1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng X, Fang L. VGLL4 is a transcriptional cofactor acting as a novel tumor suppressor via interacting with TEADs. Am J Cancer Res. 2018;8(6):932–943. [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang W, Yao F, He J, et al. Downregulation of VGLL4 in the progression of esophageal squamous cell carcinoma. Tumour Biol. 2015;36(2):1289–1297. [DOI] [PubMed] [Google Scholar]
- 16.Song S, Xie M, Scott AW, et al. A Novel YAP1 Inhibitor Targets CSC-Enriched Radiation-Resistant Cells and Exerts Strong Antitumor Activity in Esophageal Adenocarcinoma. Mol Cancer Ther. 2018;17(2):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adhikary G, Chew YC, Reece EA, Eckert RL. PKC-delta and -eta, MEKK-1, MEK-6, MEK-3, and p38-delta Are Essential Mediators of the Response of Normal Human Epidermal Keratinocytes to Differentiating Agents. J Invest Dermatol. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 18.Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res. 1981;41(5):1657–1663. [PubMed] [Google Scholar]
- 19.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106(3):761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adhikary G, Grun D, Balasubramanian S, Kerr C, Huang JM, Eckert RL. Survival of skin cancer stem cells requires the Ezh2 polycomb group protein. Carcinogenesis. 2015;36(7):800–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen AE, Cameron-Smith D, Crowe TC. Conjugated linoleic acid suppresses myogenic gene expression in a model of human muscle cell inflammation. J Nutr. 2008;138(1):12–16. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Shen H, Withers HG, et al. VGLL4 Selectively Represses YAP-Dependent Gene Induction and Tumorigenic Phenotypes in Breast Cancer. Sci Rep. 2017;7(1):6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiao S, Li C, Hao Q, et al. VGLL4 targets a TCF4-TEAD4 complex to coregulate Wnt and Hippo signalling in colorectal cancer. Nat Commun. 2017;8:14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng YZ, Chen PP, Wang Y, et al. Connective tissue growth factor is overexpressed in esophageal squamous cell carcinoma and promotes tumorigenicity through beta-catenin-T-cell factor/Lef signaling. J Biol Chem. 2007;282(50):36571–36581. [DOI] [PubMed] [Google Scholar]
- 25.Sekido Y Targeting the Hippo Pathway Is a New Potential Therapeutic Modality for Malignant Mesothelioma. Cancers (Basel). 2018;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kandasamy S, Adhikary G, Rorke EA, et al. The YAP1 Signaling Inhibitors, Verteporfin and CA3, Suppress the Mesothelioma Cancer Stem Cell Phenotype. Mol Cancer Res. 2020;18(3):343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adhikary G, Grun D, Alexander HR, et al. Transglutaminase is a mesothelioma cancer stem cell survival protein that is required for tumor formation. Oncotarget. 2018;9(77):34495–34505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher ML, Adhikary G, Xu W, Kerr C, Keillor JW, Eckert RL. Type II transglutaminase stimulates epidermal cancer stem cell epithelial-mesenchymal transition. Oncotarget. 2015;6(24):20525–20539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344(13):975–983. [DOI] [PubMed] [Google Scholar]
- 30.Kerr C, Szmacinski H, Fisher ML, et al. Transamidase site-targeted agents alter the conformation of the transglutaminase cancer stem cell survival protein to reduce TG2 binding activity and cancer stem cell survival. Oncogene. 2017;36:2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 31.Caron NS, Munsie LN, Keillor JW, Truant R. Using FLIM-FRET to measure conformational changes of transglutaminase type 2 in live cells. PLoS One. 2012;7(8):e44159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gundemir S, Colak G, Tucholski J, Johnson GV. Transglutaminase 2: a molecular Swiss army knife. Biochim Biophys Acta. 2012;1823(2):406–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar A, Xu J, Sung B, et al. Evidence that GTP-binding domain but not catalytic domain of transglutaminase 2 is essential for epithelial-to-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2012;14(1):R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher ML, Ciavattone N, Grun D, Adhikary G, Eckert RL. Sulforaphane reduces YAP/Np63alpha signaling to reduce cancer stem cell survival and tumor formation. Oncotarget. 2017;8(43):73407–73418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JH, Shin JE, Park HW. The Role of Hippo Pathway in Cancer Stem Cell Biology. Mol Cells. 2018;41(2):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen HH, Mullett SJ, Stewart AF. Vgl-4, a novel member of the vestigial-like family of transcription cofactors, regulates alpha1-adrenergic activation of gene expression in cardiac myocytes. J Biol Chem. 2004;279(29):30800–30806. [DOI] [PubMed] [Google Scholar]
- 37.Koontz LM, Liu-Chittenden Y, Yin F, et al. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev Cell. 2013;25(4):388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Gao Y, Li P, et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014;24(3):331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiao S, Wang H, Shi Z, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25(2):166–180. [DOI] [PubMed] [Google Scholar]
- 40.Xie JJ, Xu LY, Xie YM, et al. Involvement of Cyr61 in the growth, invasiveness and adhesion of esophageal squamous cell carcinoma cells. Int J Mol Med. 2011;27(3):429–434. [DOI] [PubMed] [Google Scholar]
- 41.Chuang JY, Yu NY, Chiang IP, Lai CH, Lin CD, Tang CH. Cyr61 increases matrix metalloproteinase-3 expression and cell motility in human oral squamous cell carcinoma cells. J Cell Biochem. 2012;113(6):1977–1986. [DOI] [PubMed] [Google Scholar]
- 42.Shimo T, Nakanishi T, Nishida T, et al. Involvement of CTGF, a hypertrophic chondrocyte-specific gene product, in tumor angiogenesis. Oncology. 2001;61(4):315–322. [DOI] [PubMed] [Google Scholar]
- 43.Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71(7):2728–2738. [DOI] [PubMed] [Google Scholar]
- 44.Mizuno T, Murakami H, Fujii M, et al. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene. 2012;31(49):5117–5122. [DOI] [PubMed] [Google Scholar]
- 45.Ao R, Guan L, Wang Y, Wang JN. Silencing of COL1A2, COL6A3, and THBS2 inhibits gastric cancer cell proliferation, migration, and invasion while promoting apoptosis through the PI3k-Akt signaling pathway. J Cell Biochem. 2018;119(6):4420–4434. [DOI] [PubMed] [Google Scholar]
- 46.Januchowski R, Swierczewska M, Sterzynska K, Wojtowicz K, Nowicki M, Zabel M. Increased Expression of Several Collagen Genes is Associated with Drug Resistance in Ovarian Cancer Cell Lines. J Cancer. 2016;7(10):1295–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, Ding Y, Li A. Identification of COL1A1 and COL1A2 as candidate prognostic factors in gastric cancer. World J Surg Oncol. 2016;14(1):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su B, Zhao W, Shi B, et al. Let-7d suppresses growth, metastasis, and tumor macrophage infiltration in renal cell carcinoma by targeting COL3A1 and CCL7. Mol Cancer. 2014;13:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang XQ, Tang ZX, Yu D, et al. Epithelial but not stromal expression of collagen alpha-1(III) is a diagnostic and prognostic indicator of colorectal carcinoma. Oncotarget. 2016;7(8):8823–8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y The molecular basis that unifies the metabolism, cellular uptake and chemopreventive activities of dietary isothiocyanates. Carcinogenesis. 2012;33(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grun D, Adhikary G, Eckert RL. NRP-1 interacts with GIPC1 and alpha6/beta4-integrins to increase YAP1/ΔNp63α-dependent epidermal cancer stem cell survival. Oncogene. 2018;37:4711–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]