Abstract
Purpose of the Review:
Nonfasting lipid testing has been introduced into several guidelines over the past decade or so however, the uptake into clinical practice has not been universal. This review highlights some of the prevalent reasons for provider reluctance to use nonfasting testing and the evidence to support nonfasting testing for routine screening in most patients.
Recent Findings:
Several studies have found nonfasting lipids to be as, or more, strongly associated with CVD risk prediction. In particular, nonfasting tests improve system efficiency, are safe for patients with diabetes, the elderly, children, and in the vast majority of patients, do not need to be followed up with fasting studies due to severe hypertriglyceridemia.
Summary:
Nonfasting lipids are a convenient first test for screening that offers equivalent, if not improved CVD risk prediction. Common misconceptions about nonfasting tests are not supported by the evidence.
Keywords: Nonfasting lipids, cholesterol screening, cardiovascular risk, prevention, cholesterol guidelines
Introduction:
It is well known that cholesterol levels measured early in life influence long-term cardiovascular risk. (1–3) Recent guidelines suggest that cholesterol screening for children with a nonfasting sample should start early (age 10 years) and repeated every 5 years. (1) In higher risk populations, if there is a family history of hypercholesterolemia or premature cardiovascular disease, cholesterol screening should start even earlier at age 2 and repeated every 3 to 5 years for early identification of familial hypercholesterolemia and hereditary dyslipoproteinemias, even if the initial profile is normal. Guidelines also suggest that in adults, lipid panel and risk factor screening should start at age 20 and continue at least every 5 years depending on the individual. (2, 4, 5)
Nonfasting Testing in the Guidelines
While routine lipid panel screening has been part of clinical practice for decades, obtaining these tests without the prerequisite 8 to 12 hour fast has evolved only over the past decade. The first recommendations adopting nonfasting lipid testing on a national level came from the 2009 Danish Society for Clinical Biochemistry, which recommended nonfasting testing for all their national laboratories. Since then several international societies have approved of nonfasting lipid testing for routine screening.(5, 6–8) In 2014 the US Department of Veterans Affairs clinical practice guidelines became the first US guidelines to approve use of nonfasting lipid tests. These were followed in 2017 by the American Association of Clinical Endocrinologists and American Association of Endocrinology recommendations. (9) In 2018, the American College of Cardiology/American Heart Association (ACC/AHA) cholesterol guidelines modified previous 2013 recommendations for fasting and allowed nonfasting testing for routine screening, re-iterated again in the 2019 ACC/AHA prevention guidelines. (2, 4) Furthermore, these guidelines also considered fasting or nonfasting triglycerides ≥175 mg/dL as a risk enhancing factor that could prompt consideration for initiating or intensifying statin therapy. (2,4) In the 2020 American Diabetes Association guidelines, nonfasting or fasting elevation in triglycerides ≥175 mg/dL serves as an indication for physicians to address lifestyle factors (obesity, metabolic syndrome) and also search for secondary causes such as medications that can raise triglycerides or other medical conditions such as undiagnosed diabetes, liver and kidney disease. (10) Key clinical guidelines and statements related to nonfasting panels are summarized in the Table. Despite these guidelines, the adoption of nonfasting tests as the screening method of choice for routine screening has not been embraced comprehensively. About two thirds of surveyed laboratories in Europe use nonfasting panels routinely, leaving one third still using fasting tests as first line studies. (11) In addition, while the prevalence of nonfasting testing in the US is currently unknown, it is certainly not universal. In this review, we will discuss the advantages of nonfasting studies in daily practice and highlight the evidence in support of nonfasting tests for routine screening.
Table.
Key guideline and consensus recommendations on nonfasting lipid testing
| Guideline or statement | Year | CVD risk assessment or before starting lipid lowering therapy | During lipid lowering therapy | Nonfasting triglycerides |
|---|---|---|---|---|
| American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines (2) (4) | 2018 2019 | Nonfasting lipids are acceptable alternative for fasting lipids for risk assessment in primary prevention and for assessment of baseline lipids in patients not yet on statin therapy. | LDL-C is the primary target. | Nonfasting triglycerides ≥175 mg/dL (≥2mmol/L) are abnormal and considered a risk enhancing factor. Fasting required for triglycerides ≥400 mg/dL (≥4.5 mmol/L). |
| European Atherosclerosis Society/European Federation for Laboratory Medicine (5)(6)(7) | 2016 2018 2019 | Fasting lipids are not routinely required | Fasting is not required if patients are on stable drug therapy | For triglycerides > 440 mg/dL (>5mmol/L), fasting may be considered; refer to a specialist Nonfasting triglycerides ≥175 mg/dL (≥2 mmol/L) is elevated |
| American Association of Clinical Endocrinologists and American Association of Endocrinology (9) | 2017 | Nonfasting lipids are an acceptable alternative if fasting lipids are impractical | LDL-C is the primary target | Nonfasting triglycerides ≥150 mg/dL(≥1.7 mmol/L) are abnormal. Fasting required for management and treatment of hypertriglyceridemia |
| Canadian Hypertension Education Program Guidelines | 2016 | A fasting sample is no longer required, nonfasting is equally appropriate | ||
| Canadian Cardiovascular Society Dyslipidemia Guidelines (8) | 2016 | A nonfasting sample is considered acceptable alternative to fasting | LDL-C is primary target of therapy Non-HDL cholesterol or apoB are alternate treatment targets to LDL-C |
For triglycerides >400 mg/dL (>4.5 mmol/l), repeat fasting triglyceride level |
| European Society of Cardiology Dyslipidemia Guidelines | 2016 | A nonfasting sample can be used in patients without severe hypertriglyceridemia or very low LDL-C | LDL-C is the primary treatment target Non-HDL cholesterol or apolipoprotein B are secondary targets |
For general screening of hypertriglyceridemia, nonfasting triglycerides can be used in patients without severe hypertriglyceridemia |
| National Clinical Guideline Center (NICE) and Joint British Societies Guidelines | 2014 | A fasting sample is not needed | Consider an annual nonfasting non-HDL cholesterol | For triglycerides > 880 mg/dL (>20 mmol/L), refer to a specialist For triglycerides between 10 and 20 mmol/L (880 to 1770 mg/dL), repeat fasting |
| Veterans Affairs /Department of Defense Clinical Practice Guideline for the Management of Dyslipidemia for Cardiovascular Risk Reduction | 2014 | A nonfasting sample is recommended | Do not advocate treating to a target LDL-C or non-HDL-C for secondary prevention | For triglycerides >400 mg/dL (5.0 mmol/L), repeat fasting |
| American College of Cardiology/American Heart Association Guidelines | 2013 | A fasting sample is preferred (but not mandatory) | Fasting lipids to assess per cent reduction in LDL cholesterol and adequate response to statin therapy | Elevated nonfasting triglycerides ≥200 mg/dL (2.3 mmol/L) should be repeated fasting If ≥500 mg/dL (5.6 mmol/L), screen for secondary causes |
| Danish Society for Clinical Biochemistry | 2009 | A nonfasting sample is recommended. | NA | For triglycerides >350 mg/dL (>4 mmol/L), repeat fasting |
LDL-C: LDL cholesterol
Evidence for Nonfasting Testing
Changing established practice is difficult, however there are several valid arguments for more widespread acceptance of nonfasting testing. On a physiological level, the rationale behind using nonfasting testing is appealing since, except for a few hours in the morning, most of us spend the majority of our lives in a nonfasting state. Capturing lipids and identifying risk while nonfasting therefore provides a more accurate representation of our normal physiological state. In fact, the adequacy of nonfasting lipids for general screening of CVD risk has been verified by numerous large prospective studies, over the past several decades. (12) An evidence-based review of the published literature from >300,000 individuals found no diminution of lipid relationships with predicting incident events for nonfasting lipids (6) and at least three large statin clinical trials have used nonfasting lipids (involving nearly 43,000 patients). (6) More recently, data from the UK Biobank study add to the growing evidence base that fasting is not necessary when assessing lipid-related cardiovascular risk. In a study involving 346,686 participants with non-fasting blood samples, risk associations with CVD were similar to those previously found from other studies for fasting or nonfasting lipids. (13)
In addition, nonfasting lipid panels can provide clinicians with incremental knowledge when assessing patients’ CVD risk with studies reporting similar or even stronger risk associations of nonfasting lipids with CVD, particularly for triglycerides. (14) Specifically, genetic studies using Mendelian randomization have linked nonfasting triglycerides and remnant cholesterol to increased risk of CVD and mortality. (15) In certain patients, including those with metabolic syndrome, diabetes mellitus, or specific genetic abnormalities, fasting can mask abnormalities in triglyceride metabolism which is captured by nonfasting measurements. In a large primary prevention study, nonfasting lipids were associated with higher levels of triglycerides as well as large very low density lipoprotein (VLDL) cholesterol and particles, inclusive of chylomicrons, and medium sized VLDL cholesterol and particles, compared with fasting samples. (16) Nonfasting panels therefore may help to identify residual lipid-related CVD risk in patients with a proatherogenic milieu, despite optimal guideline-based treatment. (17, 18)
Resistance to Nonfasting Testing
One of the major concerns regarding nonfasting testing has been the argument that population level risk associations would not capture individual variability based on fasting status. Recent data published on 8,270 participants from the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA) trial with prospective follow-up provided robust evidence addressing this concern. (18) Both fasting and nonfasting lipids were measured in the same individuals four weeks apart with no intervention or advice given between the two visits. The association of baseline lipids with CVD events was similar irrespective of fasting status, and importantly, results were similar by randomized allocation to statin versus placebo (18).
A somewhat related concern has been regarding misclassification of individuals into a lower CVD risk category based on variability of low density lipoprotein (LDL) cholesterol levels in nonfasting panels. This variability is directly related to known variability in triglycerides in the hours following food intake and the relationship of triglycerides to LDL cholesterol calculation via the Friedewald equation. However, nonfasting vs. fasting differences in routine lipids are small: high-density lipoprotein [HDL] cholesterol change is negligible; slightly lower levels are seen [up to −8 mg/dL(0.21 mmol/L)] for nonfasting total cholesterol, LDL cholesterol, and non-HDL cholesterol compared with fasting; and modest changes [up to 26 mg/dL(0.3 mmol/L) higher] for triglycerides (4, 16). Importantly the widely used atherosclerotic CVD risk calculator utilizes pooled cohort equations for risk calculation which use total cholesterol and HDL cholesterol (not triglycerides or LDL cholesterol), hence there is little impact of nonfasting on risk estimates using these methods. Addressing the possibility of misclassification in particular, the ASCOT-LLA study found no significant misclassification that would adversely affect the decision for initiation of statin therapy, with high concordance (94.8%) between fasting and nonfasting lipids measured from the same individuals for classification into CVD risk categories. (18) Recently investigators assessed the use of nonfasting TC and HDL cholesterol alone and found no benefit to adding nonfasting LDL cholesterol into risk prediction models (13) suggesting that the current risk assessment methods are adequate when using nonfasting samples.
For clinicians concerned about using nonfasting LDL cholesterol when titrating therapies for patients close to an absolute LDL cholesterol target, there is data to promote the use of the more accurate Martin-Hopkins equation, a modification of the Friedewald equation, when LDL cholesterol is low and for nonfasting samples (4,19). In fact, this is now the standard calculation method for LDL cholesterol reporting by a large global clinical laboratory provider and would therefore not require additional computation time on the part of health care providers in busy office settings. More recently, a novel equation to calculate LDL cholesterol has been proposed as being more accurate than current methods, including the Martin-Hopkins equation, especially in the setting of elevated triglycerides. (20) Authors of this study assessed accuracy of their equation by sex and fasting state (fasting vs. nonfasting) and found its accuracy to be unaffected by either. Authors concluded that their method should be used to provide a more accurate LDL cholesterol measure in all patients, but especially those with elevated triglycerides > 400 mg/dL. (20)
Several societal guidelines also allow for nonfasting non-HDL cholesterol or apolipoprotein B to be used to guide therapy as it is well-known that both of these are better risk markers than LDL cholesterol, in particular in the setting of low LDL cholesterol or when triglycerides are ≥200 mg/dL(2.3 mmol/L) (21). Non-HDL cholesterol may represent a more complete view of risk related to circulating plasma cholesterol content, as shown in an international study of more than 500,000 individuals with more than 40 year follow-up. (22) Established treatment goals for non-HDL cholesterol are 30 mg/dL higher than for LDL cholesterol (fasting or nonfasting) and these values are automatically reported by most laboratories regardless of fasting status. Beyond cholesterol, it has been demonstrated that the concentration (number of particles per unit volume] of atherogenic lipoprotein particles (the lipid-protein assemblies which transport cholesterol and triglycerides in the circulation) might better reflect the potential for these cholesterol transporters to be taken up into the neointima of atheromatous lesions, depositing cholesterol which becomes modified, inciting and then propagating atherosclerotic coronary disease. (7) Most studies, but not all, found that apoB was more closely associated with cardiovascular risk than LDL cholesterol. (7) Recently studies examining genetic variants that mimic discordance between apoB and LDL cholesterol suggest that genetically determined lipid risk also tracked more closely with apoB than LDL cholesterol. (23) Hence, although in most patients’ standard lipids will suffice for risk assessment and management, in the subset of patients with multiple cardiometabolic risk factors or low LDL cholesterol, testing for apoB captures lipoprotein cardiovascular risk information that may not be captured by cholesterol alone. The 2018 and 2019 ACC/AHA cholesterol and prevention guidelines included apoB>130 mg/dL (>80th population percentile) as a risk-enhancing factor that could inform patient-clinician risk discussions and guide the need for initiating or intensifying statin therapy among borderline or intermediate risk patients for the primary prevention of cardiovascular disease. Notably, apoB and non-HDL cholesterol testing, similar to LDL or total cholesterol, also show minimal variation with fasting status.
Nonfasting and Postprandial Triglycerides
Triglyceride levels peak about 4–6 hours after a meal, however individual plateau levels vary depending on multiple factors. (16) Genetic studies suggest that the association between plasma triglycerides and cardiovascular risk is causal, but many genetic variants are pleotropic and are often also associated with differences in VLDL/remnant cholesterol, apoB, or HDL cholesterol, making it challenging to identify the causal atherogenic component. In a meta-analysis of randomized statin and non-statin trials (N=374,358 participants), triglyceride lowering was associated with lower cardiovascular risk (~ 15% lower risk per 1 mmol/L reduction in triglycerides), which was somewhat lower than for LDL cholesterol (~ 20% lower risk per 1 mmol/L reduction in LDL cholesterol) and attenuated when the REDUCE-IT trial was excluded. (24)
Given the independent risk conferred by elevated triglycerides and triglyceride-rich lipoproteins, the cut-point associated with higher risk in terms of nonfasting triglyceride levels has also been investigated. Most guidelines, including the latest US and European guidelines, define elevated nonfasting triglycerides as ≥175 mg/dL (≥2 mmol/L), a cut point that has been validated prospectively in a large study of US women. (2, 14, 25) Similarly, the 2018 and 2019 ACC/AHA guidelines also consider fasting or nonfasting triglycerides greater than 175 mg/dL (2 mmol/L) as a risk enhancing factor that could prompt consideration for initiating or intensifying statin therapy. (2, 4)
There are some differences in the guideline cut-points for severe hypertriglyceridemia, defined as fasting triglycerides ≥500 mg/dL (5.7 mmol/L) in US guidelines (b) and >10 mmol/L (885 mg/dL) in European guidelines. (5) At high triglyceride levels (>4.5 mmol/l, 400 mg/dL), the Friedewald calculation for LDL cholesterol becomes inaccurate as it assumes a fixed triglyceride to cholesterol ratio and underestimates the true LDL cholesterol. Instead, guidelines recommend using non-HDL cholesterol or apoB instead of calculated LDL cholesterol in patients with hypertriglyceridemia, as direct LDL cholesterol assays may also be inaccurate. (7)
Nonfasting Lipids Suitable for All?
In a general US study population enriched with African American participants, there were no significant race specific effects on lipoprotein levels and nonfasting status. (16) More data is needed to address the role of specific ethnicities on nonfasting lipoprotein cut-points. In addition, for some patients, concerns that consumption of a fatty meal prior to testing will result in markedly increased triglycerides and reduced test validity with respect to accurate LDL cholesterol levels has been cited as a potential disadvantage for nonfasting samples. However, numerous studies have found that the increase in plasma triglycerides, observed after habitual food intake, is much less than that observed during a fat tolerance test, making this less of a concern for most patients. (18, 26) Simply advising patients to have a lighter meal or avoid fast food prior to their nonfasting blood draw would be sufficient counsel for patients prior to testing.
Special Populations
In patients being screened for lipoprotein (a) (Lp(a)), fasting status has minimal effect on Lp(a) levels, therefore nonfasting tests for this biomarker can be performed with accuracy. (6,16) Indeed, when assessing the risk of incident type 2 diabetes, a prospective study from the Women’s Health Study (N=26,746 participants) that examined fasting and nonfasting Lp(a) levels found that nonfasting Lp(a) was significantly more robust for prediction of incident type 2 diabetes in this population during a 13 year period. (27) For patients with suspected familial hypercholesterolemia, recent recommendations suggest that nonfasting screening can be performed as the initial routine screening evaluation, with subsequent referral to specialty lipid clinics upon diagnosis. (28) Finally, it is well known that triglycerides and lipoprotein cholesterol levels increase markedly with pregnancy, as part of the normal physiology of pregnancy. In general, it is recommended that routine lipid screening for cardiovascular risk assessment occurs prior to pregnancy. (29)
Fasting-Evoked En-Route Hypoglycemia in Diabetes (FEEHD)
Recently, an important safety issue has been raised by investigators exploring the association of fasting for laboratory testing and the incidence of hypoglycemic episodes in patients with diabetes. In this population, the potential for fasting induced hypoglycemia has been highlighted as an under-appreciated concern with as many as 1 in 4 patients with diabetes reporting a fasting-evoked en-route hypoglycemic event (FEEHD) due to fasting for routine blood work. (30–33) These hypoglycemic episodes add unnecessarily to patient morbidity that could easily be avoided by adopting nonfasting screening.
The (Limited) Role of Fasting Lipids
Robust and high-quality evidence supports the use of nonfasting lipid testing for the majority of patients (Figure). Fasting panels may be useful in selected patients prior to starting treatment that may itself result in or modify significant hypertriglyceridemia and in patients with genetic lipid disorders being followed for hypertriglyceridemia in lipid clinics. There is no consensus between the various guidelines as to the triglyceride cut-point that may prompt providers to order a repeat fasting panel at this time, although based on the Danish experience, about 10% of routine panels were repeated fasting due to hypertriglyceridemia. For those with known primary chylomicronemia, which results in significantly elevated TGs usually beyond cut-points suggested for nonfasting studies, in accordance with published guidelines, we suggest that fasting studies would be preferred for management. (34)
Figure.
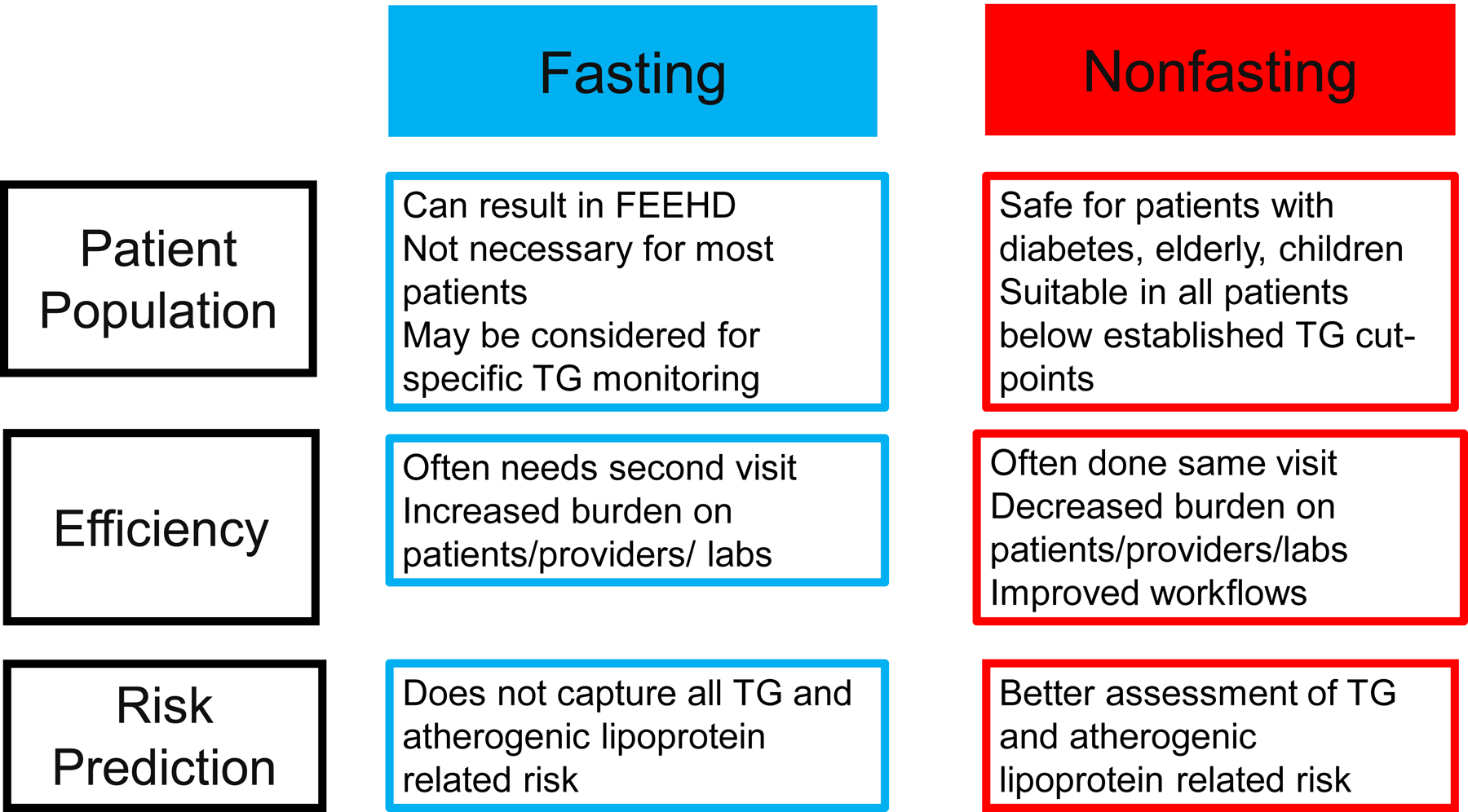
Summary of the Advantages and Disadvantages of Nonfasting and Fasting Lipid Screening
TG= Triglycerides, FEEHD= Fasting-evoked en-route hypoglycemia in diabetes
Improved Healthcare Delivery
From a systems perspective, nonfasting screening allows for more efficient healthcare delivery and resultant patient and provider satisfaction. Even though there are no studies to date assessing the cost-effectiveness of fasting versus nonfasting lipid testing, clinicians can relate to the frequency of patients presenting for follow up appointments without having performed tests due to either forgetting to fast or not scheduling time for repeat laboratory visits. Management decisions are then deferred until follow up tests and visits can be scheduled, with resultant increased outpatient waiting times and potential decreased access to care for other patients. Laboratory workflow can also suffer from an influx of early morning visits for fasting tests, decreasing system efficiency. It is not hard to surmise that decreased efficiency in multiple levels of the health care system leads to increased costs, burden on healthcare providers, and decreased patient satisfaction.
Conclusion
The weight of the evidence suggests that nonfasting lipid screening is suitable in routine settings and may in fact provide more a tailored approach to CVD risk management in certain members of the population (Figure). While it may take some more time for universal acceptance of nonfasting screening, the advantages afforded by nonfasting tests such as improved risk prediction especially related to triglyceride-related risk identification, improved safety considerations in patients with diabetes, more streamlined healthcare delivery and patient satisfaction should be easily identified as benefitting us all.
Key Points.
We spend the majority of our lives in a nonfasting state thus nonfasting lipid screening is more reflective of our physiological state.
Nonfasting lipids have been accepted as suitable alternatives to fasting lipid panels for routine screening by numerous guidelines over the past decade.
Nonfasting lipids and lipoproteins have similar or even stronger risk associations for CVD risk prediction.
Nonfasting studies are safer for patients with diabetes, elderly, children, and may improve healthcare systems’ efficiency, costs and stakeholder satisfaction.
Acknowledgements:
Financial Support and Sponsorship:
Dr. Farukhi was supported by the National Heart, Lung, and Blood Institute (T32 HL007575).
Dr. Mora has received institutional research grant support from the National Heart, Lung, And Blood Institute of the National Institutes of Health (K24 HL136852).
Footnotes
Conflicts of Interest:
Dr. Farukhi has no disclosures. Dr. Mora has served as a scientific consultant (modest) to Pfizer and Quest Diagnostics, and received institutional research support from Atherotech Diagnostics for work unrelated to the current manuscript.
References
- 1.de Ferranti SD, Steinberger J, Ameduri R, et al. Cardiovascular Risk Reduction in High-Risk Pediatric Patients: A Scientific Statement From the American Heart Association. Circulation. 2019. March 26;139(13):e603–e634. doi: 10.1161/CIR.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019. June 25;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. Epub 2018 Nov 10. Erratum in: J Am Coll Cardiol. 2019 Jun 25;73(24):3237–3241. [DOI] [PubMed] [Google Scholar]
- 3.Borén J, Chapman MJ, Krauss RM, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020. June 21;41(24):2313–2330. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019. September 10;74(10):e177–e232. doi: 10.1016/j.jacc.2019.03.010. Epub 2019 Mar 17. Erratum in: J Am Coll Cardiol. 2019 Sep 10;74(10):1429–1430. Erratum in: J Am Coll Cardiol. 2020 Feb 25;75(7):840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mach F, Baigent C, Catapano AL, et al. (ESC Scientific Document Group). 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020. January 1;41(1):111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 6.Nordestgaard BG, Langsted A, Mora S, et al. Fasting is not routinely required for determination of a lipid profile: Clinical and laboratory implications including flagging at desirable concentration cut-points. A joint consensus statement from the EAS and EFLM. Eur Heart J. 2016; Jul 2016, 37 (25) 1944–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langlois MR, Chapman MJ, Cobbaert C, et al. Quantifying Atherogenic Lipoproteins: Current and Future Challenges in the Era of Personalized Medicine and Very Low Concentrations of LDL Cholesterol. A Consensus Statement from EAS and EFLM. Clin Chem. 2018;64(7):1006–1033. doi: 10.1373/clinchem.2018.287037 [DOI] [PubMed] [Google Scholar]
- 8.Anderson TJ, Gregoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Canadian Journal of Cardiology 32 (2016) 1263e1282 [DOI] [PubMed] [Google Scholar]
- 9.Jellinger PS, Handelsman Y, Rosenblit PD et al. AACE/ACE Guidelines for Management of Dyslipidemia and Cardiovascular Disease Endocr Pract. 2017. April;23(Suppl 2):1–87. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020. January;43(Suppl 1):S111–S134. doi: 10.2337/dc20-S010. [DOI] [PubMed] [Google Scholar]
- 11.De Wolf HA, Langlois MR, Suvisaari J, et al. How well do laboratories adhere to recommended guidelines for dyslipidaemia management in Europe? The CArdiac MARker Guideline Uptake in Europe (CAMARGUE) study. Clin Chim Acta. 2020;508:267–272. doi: 10.1016/j.cca.2020.05.038 [DOI] [PubMed] [Google Scholar]
- 12.Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.*.Welsh C, Celis-Morales CA, Brown R, et al. Comparison of Conventional Lipoprotein Tests and Apolipoproteins in the Prediction of Cardiovascular Disease. Circulation. 2019. August 13;140(7):542–552. doi: 10.1161/CIRCULATIONAHA.119.041149. Epub 2019 Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]; This UK Biobank study used nonfasting lipid and lipoprotein data from 346 686 individuals to examine lipoprotein associations with CVD risk. The authors determined that there was no incremental benefit to adding LDL cholesterol to models containing nonfasting total cholesterol and HDL cholesterol levels.
- 14.*.Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial Hypertriglyceridaemia Revisited in the Era of Non-fasting Lipid Profiles: Executive Summary of a 2019 Expert Panel Statement. Curr Vasc Pharmacol. 2019;17(5):538‐540. doi: 10.2174/1570161117999190517115432 [DOI] [PubMed] [Google Scholar]; This expert summary discusses postprandial triglyceride cut-points and appropriate use of fat tolerance tests.
- 15.Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014. July 3;371(1):32–41. doi: 10.1056/NEJMoa1308027. Epub 2014 Jun 18. [DOI] [PubMed] [Google Scholar]
- 16.*.Farukhi ZM, Demler OV, Caulfield MP, et al. Comparison of nonfasting and fasting lipoprotein subfractions and size in 15,397 apparently healthy individuals: An analysis from the VITamin D and OmegA-3 TriaL. J Clin Lipidol. 2020;14(2):241–251. doi: 10.1016/j.jacl.2020.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study to analyze the association of lipoprotein subfraction cholesterol levels and particle concentrations with fasting status in a large primary prevention population enriched with African Americans.
- 17.Nordestgaard BG. A Test in Context: Lipid Profile, Fasting Versus Nonfasting. J Am Coll Cardiol. 2017. September 26;70(13):1637–1646. doi: 10.1016/j.jacc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 18.*.Mora S, Chang CL, Moorthy MV, Sever PS. Association of Nonfasting vs Fasting Lipid Levels With Risk of Major Coronary Events in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm. JAMA Intern Med. 2019;179(7):898–905. doi: 10.1001/jamainternmed.2019.0392 [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated similar CVD risk associations for fasting and nonfasting lipids when plasma levels were taken from the same individuals, 4 weeks apart. Results were similar for statin versus placebo treatment.
- 19.Sathiyakumar V, Park J, Golozar A, et al. Fasting Versus Nonfasting and Low-Density Lipoprotein Cholesterol Accuracy. Circulation. 2018;137(1):10–19. doi: 10.1161/CIRCULATIONAHA.117.030677 [DOI] [PubMed] [Google Scholar]
- 20.*.Sampson M, Ling C, Sun Q, et al. A New Equation for Calculation of Low-Density Lipoprotein Cholesterol in Patients With Normolipidemia and/or Hypertriglyceridemia. JAMA Cardiol. 2020. May 1;5(5):540–548. doi: 10.1001/jamacardio.2020.0013. . [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes a novel method to calculate LDL cholesterol and compares its accuracy to existing methods, highlighting its superiority especially in the setting of hypertriglyceridemia and nonfasting.
- 21.Martin SS, Blaha MJ, Elshazly MB, et al. Friedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implications. J Am Coll Cardiol. 2013. August 20;62(8):732–9 [DOI] [PubMed] [Google Scholar]
- 22.*.Brunner FJ, Waldeyer C, Ojeda F, et al. Multinational Cardiovascular Risk Consortium. Application of non-HDL cholesterol for population-based cardiovascular risk stratification: results from the Multinational Cardiovascular Risk Consortium. Lancet. 2019. December 14;394(10215):2173–2183. doi: 10.1016/S0140-6736(19)32519-X. Epub 2019 Dec 3. Erratum in: Lancet. 2019 Dec 6;: Erratum in: Lancet. 2020 Jan 4;395(10217):32. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examines the importance of non-HDL cholesterol and cardiovascular risk prediction over 40 years of follow-up.
- 23.Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017. August 21;38(32):2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.*.Marston NA, Giugliano RP, Im K, et al. Association Between Triglyceride Lowering and Reduction of Cardiovascular Risk Across Multiple Lipid-Lowering Therapeutic Classes: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Circulation. 2019. October 15;140(16):1308–1317. doi: 10.1161/CIRCULATIONAHA.119.041998. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review addresses the association between lowering of triglyceride levels and CVD risk.
- 25.White KT, Moorthy MV, Akinkuolie AO, et al. Identifying an Optimal Cutpoint for the Diagnosis of Hypertriglyceridemia in the Nonfasting State. Clin Chem. 2015;61(9):1156‐1163. doi: 10.1373/clinchem.2015.241752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation 2008;118:2047 – 2056. [DOI] [PubMed] [Google Scholar]
- 27.Mora S, Kamstrup PR, Rifai N, et al. Lipoprotein(a) and risk of type 2 diabetes. Clin Chem. 2010. August;56(8):1252–60. doi: 10.1373/clinchem.2010.146779. Epub 2010 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGowan MP, Hosseini Dehkordi SH, et al. Diagnosis and Treatment of Heterozygous Familial Hypercholesterolemia. J Am Heart Assoc. 2019. December 17;8(24):e013225. doi: 10.1161/JAHA.119.013225. Epub 2019 Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta LS, Warnes CA, Bradley E, et al. American Heart Association Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; and Stroke Council. Cardiovascular Considerations in Caring for Pregnant Patients: A Scientific Statement From the American Heart Association. Circulation. 2020. June 9;141(23):e884–e903. doi: 10.1161/CIR.0000000000000772. Epub 2020 May 4. Erratum in: Circulation. 2020 Jun 9;141(23):e904. [DOI] [PubMed] [Google Scholar]
- 30.Aldasouqi S, Mora S, Bhalla G, et al. Fasting-Evoked En Route Hypoglycemia in Diabetes (FEEHD): An Overlooked Form of Hypoglycemia in Clinical Practice. Int J Endocrinol. 2018. October 24;2018:1528437. doi: 10.1155/2018/1528437. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aldasouqi S, Sheikh A, Klosterman P, et al. Hypoglycemia in patients with diabetes on antidiabetic medications who fast for laboratory tests. Diabetes Care. 2011. May;34(5):e52. doi: 10.2337/dc10-2402. Erratum in: Diabetes Care. 2011 Aug;34(8):1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aldasouqi S, Corser WS, Abela G, et al. Fasting for Laboratory Tests Poses a High Risk of Hypoglycemia in Patients with Diabetes: A Pilot Prevalence Study in Clinical Practice. International Journal of Clinical Medicine, 7, 653–667. doi: 10.4236/ijcm.2016.710071. [DOI] [Google Scholar]
- 33.*.Abdelfattah OM, Aldasouqi SA, Hassanein MH, et al. Fasting- Evoked En route Hypoglycemia in Diabetes (FEEHD): From guidelines to clinical practice. Curr Diabetes Rev. 2020. January 6. [DOI] [PubMed] [Google Scholar]; This literature review examines the prevalence of hypoglycemia induced by fasting for laboratory tests in patients with diabetes.
- 34.Hegele RA, Borén J, Ginsberg HN, et al. Rare dyslipidaemias, from phenotype to genotype to management: a European Atherosclerosis Society task force consensus statement. Lancet Diabetes Endocrinol. 2020. January;8(1):50–67. doi: 10.1016/S2213-8587(19)30264-5. Epub 2019 Sep 30. [DOI] [PubMed] [Google Scholar]


