Abstract
Background:
The gut microbiome protects the host from infection by promoting epithelial integrity and providing basal immunologic stimulation. Disruption of this delicate ecosystem is linked to morbidity and mortality among critically ill patients, but the impact of traumatic injury on the gut microbiome is poorly understood. This study sought to identify alterations in gut microbiota following trauma and persistent stress in rodents without confounding antibiotics.
Methods:
Male Sprague-Dawley rats aged 9–11 weeks were randomized to naïve, lung contusion with hemorrhagic shock (LCHS), and LCHS plus either 7 (LCHS/CS 7/7) or 14 days (LCHS/CS 14) of restraint cylinder stress for 2 hours daily. Stool was collected on days 0, 3, 7, and 14 for bacterial whole genome DNA isolation. Alpha diversity, or the number and relative abundance of unique bacterial species within each cohort, was assessed using Chao1 indices. Beta diversity, or the measure of differences in biodiversity across cohorts, was assessed by principle coordinate analysis. False discovery rate correction was applied to all statistical analyses to correct for cohousing effects.
Results:
Rodent groups subject to restraint stress demonstrated a progressive increase in alpha diversity over time. These microbiota changes resolved after cessation of stress (LCHS/CS 7/7) but continued to increase among rats subjected to ongoing stress (LCHS/CS 14). LCHS/CS 7/7 also demonstrated reductions in class Actinobacteria and increased abundance of the genus Bacteroides by day 7, which resolved by day 14. Increased abundance of Bacteroides was also noted in the LCHS/CS 14 cohort, suggesting the role of chronic stress in its destabilization.
Conclusions:
This study points to persistent stress as a potential source of the destabilization of microbial diversity seen after trauma. This lack of microbiota stability could be associated with worse long-term outcomes in critically ill trauma patients. Further studies are warranted to elucidate mechanistic pathways and potential therapeutic modalities.
Keywords: trauma, microbiome, dysbiosis, chronic stress, persistent inflammation
Introduction
Approximately one quarter of all hospitalized trauma patients, and 44% of those with blunt abdominal injuries, spend a portion of their hospital stay in the intensive care unit (ICU).(1, 2) Among those critically injured individuals, over half receive antibiotics, one-third develop infections, 10% develop septic shock, and 6% develop multi-organ dysfunction (MODS).(3) Both critical illness and injury alone bring dramatic loss of microbial diversity, a reduction in beneficial commensal bacteria, alterations in bacterial cross-talk and host-pathogen interactions, and overgrowth of opportunistic or pathogenic organisms.(4, 5) A growing body of evidence links disruption of this microbe-host ecosystem to systemic inflammatory response syndrome, MODS, and mortality.(6–10)
The gut and its microbiome work together to protect the host from infection by supporting intestinal epithelial integrity and providing basal immunologic stimuli.(11) Both hemorrhagic shock and burns compromise the protective epithelial barrier, leading to increased risk of translocation, sepsis, and MODS.(8, 9, 12, 13) Injuries and other acute physiologic insults lead to reductions in Lactobacillus and Bacteroidales and increases in pathogenic Enterococcus and Pseudomonas.(14, 15) Environmental and psychosocial stressors can also alter the gut microbiome.(16–18) A single two-hour social stressor can induce significant changes in patterns of relative gut species abundance in mice and, when the stress is repeated daily, reductions in commensal Lactobacillus.(19) Prolonged stress is associated with decreased alpha diversity (the number and relative abundance of bacterial species within each cohort), alterations in beta diversity (the measure of differences in bacterial biodiversity across cohorts), behavioral changes, and altered immune cell phenotypes and responses.(20) There is limited data demonstrating how quickly post-injury alterations in gut biodiversity resolve, if there is ever a full return to microbial “baseline”, or how the microbiome responds to persistent stressors after an initial injury.
While clinical studies are necessary to understand the gut microbiome after injury, it has proven difficult to control for the use of antibiotics and/or transfusions in trauma patients, both of which can independently alter the gut microbiome.(21, 22) To correct for this, we used an established rat model of trauma and hemorrhagic shock to simulate an acute trauma and post-injury period with the addition of daily chronic stress, continued for either 7 or 14 days. The aim of this study was to characterize the trajectory of changes in the gut microbiome after trauma in the presence or absence of ongoing stress, and to determine the role of daily stress after injury. We hypothesized that following trauma and hemorrhagic shock, rodents would sustain significant changes in gut microbial composition which would be accelerated or worsened by the addition of chronic stress, and that removal of the stressor would prevent further derangements and promote return of the gut microbiome back to “baseline”.
Materials and Methods
Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA) aged 9–11 weeks weighing 280 to 400g were housed in pairs on a single rack in a single room with ad lib access to standard irradiated pelleted diet and water. Prior to initiation of the experiment, rats were acclimated to a 12-hour light-dark cycle for at least 72 hours and rats that were to be assigned to the same cohort were housed in the same cage for at least 10 days. Given the coprophagic nature of rodents, this assured that rodents in the same cage would have similar gut microbial biodiversity in order to ensure microbiota stability prior to study enrollment. Rodents remained with their same-cohort cage mate for the duration of the study. Female animals were excluded due to estrous cycle variability and its impact after hemorrhagic shock. The animal protocol had been approved by the University of Florida Institutional Animal Care and Use Committee.
Experimental Design
Rodents were randomly assigned into the following cohorts: naïve control (n=8); lung contusion and hemorrhagic shock (LCHS, n=8); lung contusion and hemorrhagic shock plus daily restraint stress through postoperative day seven, followed by seven days of routine daily handling (LCHS/CS 7/7, n=8); and lung contusion and hemorrhagic shock plus daily restraint stress through postoperative day 14 (LCHS/CS 14, n=8). These injury models were chosen to recapitulate common clinical scenarios: blunt chest trauma accompanied by hemorrhage with early recovery (LCHS), blunt chest trauma accompanied by hemorrhage followed by daily chronic stress then recovery (LCHS/CS 7/7), blunt chest trauma accompanied by hemorrhage followed by persistent daily restraint stress associated with the ICU environment (LCHS/CS 14).
All rodents were handled daily and sacrificed on day 14. Stool was collected via clean catch under aseptic conditions under a laminar flow hood on the day of surgery and on postoperative days three and seven and harvested from the descending colon at time of sacrifice on day 14. Stool samples were immediately snap frozen and stored at −80°C until processing.
Trauma Model
For this study we used a previously validated model of trauma and hemorrhagic shock.(23) After anesthesia with intraperitoneal pentobarbital, a unilateral lung contusion was made using a manual nail gun (Arrow, Saddle River, NJ) applied directly to a 12mm metal plate placed over the rodent’s right axilla. Next, the right femoral artery and right internal jugular vein were cannulated using polyethylene-10 and polyethylene-50 tubing, respectively. The femoral artery tubing was connected to a BP-2 Digital Blood Pressure Monitor device (Columbus Instruments, Columbus, Ohio) for continuous measurement of mean arterial pressure (MAP). Blood was then withdrawn at a rate of 1 mL/minute from the venous cannula to produce a MAP of 30–35 mmHg for 45 minutes, after which 50% of shed blood was reinfused. Shed blood was stored at 37°C until time of reinfusion.
Chronic restraint stress was performed in designated groups starting the day after surgery. Rats were restrained in clear plastic rodent nose cone cylinders (Kent Scientific Corporation, Torrington, CT). Every 30 minutes for two hours daily the rats were subjected to 2 minutes of loud constant alarms and repositioning. After each two-hour session, rats were returned to their cages and brought back to the vivarium.
DNA Extraction, Targeted Library Preparation, and Sequencing
The ZymoBIOMICS®−96 MagBead DNA Kit (Zymo Research, Irvine, CA) was used to extract total DNA using an automated platform. The samples were processed and analyzed with the Quick Biology® Targeted Sequencing Service for Microbiome Analysis (Quick Biology, Pasadena, CA). Bacterial 16S ribosomal RNA (rRNA) gene sequencing was performed using the Quick-16S™ NGS Library Prep Kit (Zymo Research, Irvine, CA) by amplification of the V3–V4 hypervariable region of the 16S rRNA gene.
The final PCR products were quantified with qPCR fluorescence readings and pooled together based on equal molarity. The final pooled library was cleaned with the Select-a-Size DNA Clean & Concentrator™ (Zymo Research, Irvine, CA), then quantified with TapeStation® (Agilent Technologies, Santa Clara, CA) and Qubit® (Thermo Fisher Scientific, Waltham, WA). The final library was sequenced on Illumina® MiSeq™ with a v3 reagent kit (600 cycles). The sequencing was performed with 10% PhiX spike-in.
The ZymoBIOMICS® Microbial Community Standard (Zymo Research, Irvine, CA) was used as a positive control for each DNA extraction. The ZymoBIOMICS® Microbial Community DNA Standard (Zymo Research, Irvine, CA) was used as a positive control for each targeted library preparation. Negative controls (i.e., blank extraction control, blank library preparation control) were included to assess the level of bioburden carried by the wet-lab process.
Bioinformatics Analysis
Demultiplexed reads were imported into DADA2 (v.1.16) pipeline (24) and quality filtered and trimmed using DADA2 filterAndTrim function with the following options: truncLen=250, maxN=0, maxEE=2, truncQ=2, rm.phix=T. Sequences were then corrected for Illumina amplicon sequence errors, dereplicated, and amplicon sequence variants (ASVs) were generated followed by chimera removal. Taxonomic classification was performed using DADA2 assignTaxonom and addSpecies using silva_nr_v138_train_set.fa.gz and silva_species_assignment_v138.fa.gz, respectively. We then removed any sequence that was classified as non-bacterial and all singleton ASVs. This resulted in a total of 4,171,125 reads (mean=32,587, minimum=17,971 and maximum= 47,874 reads per sample).
We rarefied the read depth in samples to the minimum in all samples (17,971 reads) to remove any effects of varying sequencing depth between samples, then used the rarified counts to generated Principal Coordinate Analysis (PCoA) using the phyloseq (v.1.28) R (v.3.6.3) package (R Foundation for Statistical Computing, Vienna, Austria) from Bray-Curtis dissimilarity matrix.(25) Alpha diversity (Chao1 diversity index) was calculated using the phyloseq R package on the rarefied counts. The assessment of alpha and beta diversity of samples allows for the measurement of microbial variation within a community and between communities, respectively. (26–28)
A linear Mixed-Effects Model (lme function) in the R nlme package (version 3.1–140), with the REML method was used to fit a generalized mixed linear model of the following forms: var ~ group + 1|cage, for the group comparisons, or var ~ time + ~1|cage/rat, for the time comparisons. Var is PCoA axis, Chao1 index, or taxa count (considering only taxa present in at least 25% of the samples). Group is naive, LCHS, LCHS/CS 7/7 and LCHS/CS14. Time is t0, t3, t7, and t14. The term 1|cage indicates that we used the cage as a random effect to account for cohousing effects and ~1|cage/rat indicates rat was nested in cages to account for multiple measure from the same animal and also cohousing effects.(29) When applicable, taxonomy that have significant abundance among different groups were identified by LEfSe using default settings.(30) P-values were obtained from Analysis of Variance (ANOVA) on the above model and were false discovery rate (FDR) corrected using the Benjamini & Hochberg approach (31) using R’s p.adjust function and p<0.05 was considered statistically significant. Unless otherwise specified, all reported comparisons and statistical analyses were performed with FDR correction.
Results
Microbiota diversity increases with exposure to persistent chronic stress after injury but does not discriminate between stress cohorts.
There were no significant differences in intestinal beta diversity between groups at baseline (day 0; data not shown). While there were differences in the alpha diversity of the naïve cohort after 1 week compared to baseline, stability was demonstrated afterwards, between POD 3 vs 7 (p=0.9), POD 3 vs 14 (p=0.1), and POD 7 vs 14 (p=0.2) which may be due to slight differences in the age of naïve mice compared to the other cohorts at POD 0 (Figure 1A). The LCHS cohort demonstrated stability of their microbiota throughout the time course despite trauma (Figure 1B). However, the addition of stress via daily restraint after trauma (LCHS/CS 7/7 and 14) resulted in a progressive increase in alpha diversity over time (Figure 1C). By day 14, these initial changes in alpha diversity resolved after cessation of stress (LCHS/CS 7/7) but continued to increase among those rats subjected to ongoing chronic stress (LCHS/CS 14) (Figure 1D). Furthermore, given our findings that a peak change in alpha diversity occurred 7–14 days after the initiation of daily stress in the LCHS/CS 7/7 and LCHS/CS 14 cohorts (Figure 1C–D), PCoA plots were generated (Figure 2) based on Bray-Curtis dissimilarity indices.(28) Although changes in alpha diversity were apparent within cohorts over time, PCoA plots did not identify significant differences in beta diversity at 7 or 14 days when comparing LCHS vs. LCHS/CS 7/7 (Figure 2A–B) or LCHS vs. LCHS/CS 14 (Figure 2C–D).
Figure 1.

Changes in intestinal alpha diversity, or the number and relative abundance of unique bacterial species within each cohort, based on Chao1 indices at POD 0, 3, 7, and 14 are represented by traditional boxplots in naïve rats (A), LCHS (B), LCHS/CS 7/7 (C), and LCHS/CS 14 (D) cohorts. Data demonstrate a relative stable microbiota alpha diversity within the naïve and LCHS cohorts but with the addition of chronic stress (CS) to the model of trauma, there is a statistically significant change in the alpha diversity by POD 7 in the LCHS/CS 7/7 and LCHS/CS 14 cohorts. POD (post-operative day); LCHS (lung contusion and hemorrhagic shock); LCHS/CS 7/7 (lung contusion, hemorrhagic shock and 7 days of chronic restraint stress followed by 7 days of routine daily handling); LCHS/CS 14 (lung contusion, hemorrhagic shock, plus 14 days of chronic restraint stress); NS (not significant).
Figure 2.
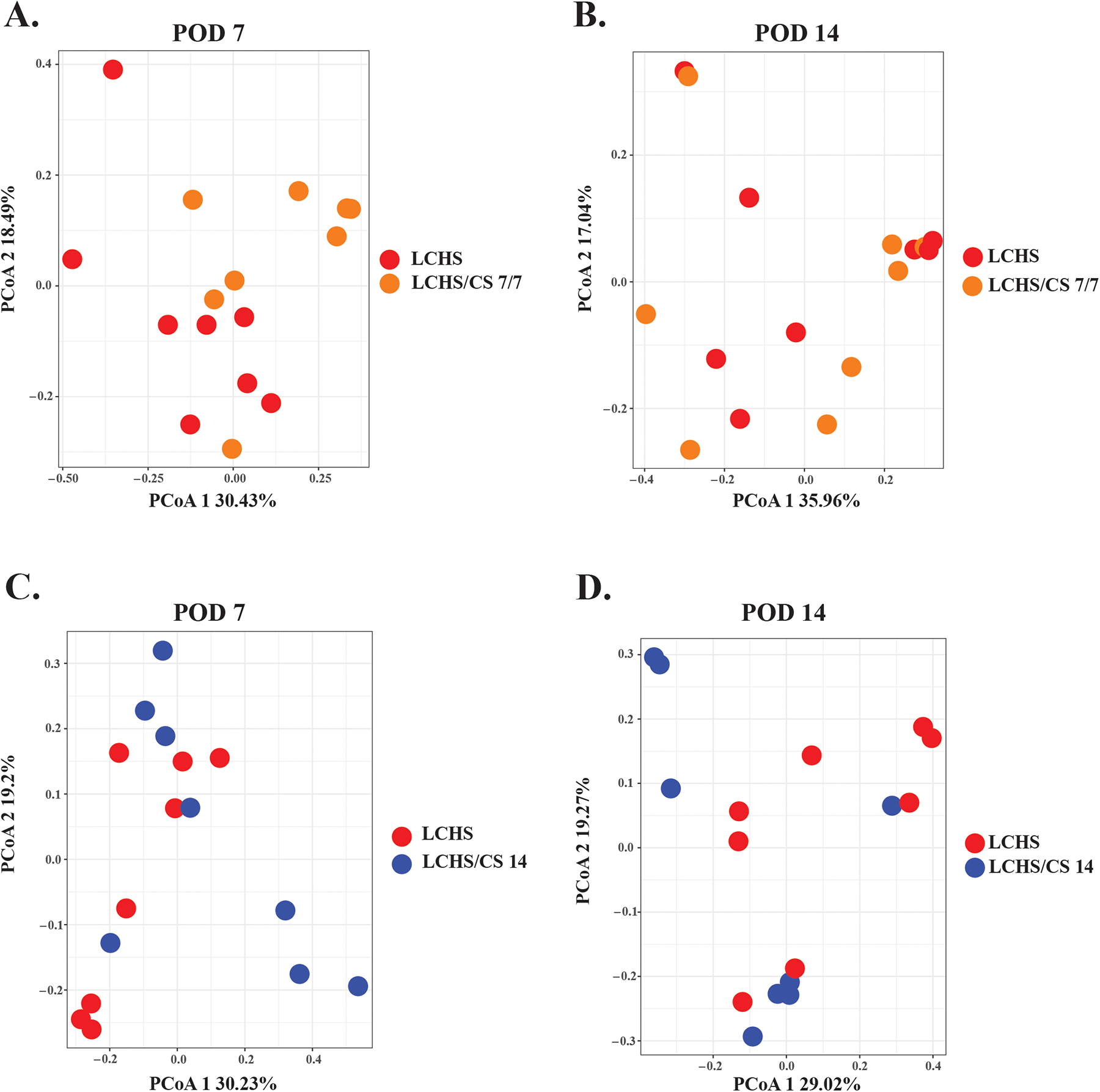
Principle coordinate analysis (PCoA) plots showing changes in beta diversity, or the measure of differences in biodiversity across cohorts, based on Bray-Curtis analysis comparing the LCHS model (red dots) to the LCHS/CS 7/7 (orange dot, A and B) and LCHS/CS 14 (blue dot, C and D) models in order to compare the addition of chronic stress at the time points of POD 7 (A, C) and 14 (B, D) which were noted to be the time at which alpha diversity significantly changed in these cohorts. There was no clustering noted between cohorts at the time point, indicating no differences in overall diversity between cohorts. POD (post-operative day); LCHS (lung contusion and hemorrhagic shock); LCHS/CS 7/7 (lung contusion, hemorrhagic shock and 7 days of chronic restraint stress followed by 7 days of routine daily handling); LCHS/CS 14 (lung contusion, hemorrhagic shock, plus 14 days of chronic restraint stress).
Chronic stress after trauma leads to progressive changes in relative microbial composition.
We next sought to determine the taxonomic changes induced within individual cohorts based on the introduction of chronic stress after trauma (LCHS/CS cohorts) that were responsible for the alpha diversity changes. Linear discriminant Effect Size (LEfSe) was used to determine taxonomic differences between time points based on differences in relative abundances. To increase our discrimination, only taxa with linear discriminant analysis (LDA) scores >3.5 with a p value ≤0.05 were considered (Figure 3). The cohort of LCHS rats subjected to seven days of restraint stress followed by seven days of routine handling (LCHS/CS 7/7) exhibited several unique shifts in gut microbiota that were dominated by the family Ruminococcaceae and seen as early as POD 3, persisted at POD 7, and then disappeared by POD 14 in association with the cessation of chronic stress (Figure 3A). Furthermore, class Actinobacteria was present at POD 0 but lost afterwards and did not regain significant presence in the gut microbiota afterwards or with the cessation of chronic stress. Animals in the LCHS/CS 14 cohort demonstrated a loss of the genus Clostridium, specifically C. celatum after POD 0 with a predominance of of the genera Corynebacterium and Bacteroides (Figure 3B). Most notably, the genus Bacteroides was common between both the LCHS/CS 7/7 and the LCHS/CS 14 cohorts, suggesting that the bloom of species within the genera are most impacted by the addition of chronic stress by POD 7. This is best illustrated by its increase in relative abundance over time postoperatively, with its peak relative abundance on POD 7 (Figure 4).
Figure 3.

Linear discriminant Effect SizE (LEfSe) was performed to determine changes in taxa abundance in the LCHS/CS 7/7 (A) and LCHS/CS 14 (B) cohorts along the postoperative spectrum (POD 0–14). In order to increase the discrimination of taxonomic changes found in these cohorts, only taxa with a linear discriminant analysis (LDA) score >3.5 and p ≤0.05 are listed. This demonstrated the loss of phylum Actinobacteria in the LCHS/CS 7/7 cohort, genus Clostridium in the LCHS/CS 14 cohort, and a significant increase in the genus Bacteroides in both cohorts at POD 7. POD (post-operative day); LCHS/CS 7/7 (lung contusion, hemorrhagic shock and 7 days of chronic restraint stress followed by 7 days of routine daily handling); LCHS/CS 14 (lung contusion, hemorrhagic shock, plus 14 days of chronic restraint stress); LDA (linear discriminate analysis). Taxa are represented as follows: k_Kingdom.p_Phyla.c_Class.o_Order.f_Family.g_Genus.s_species.
Figure 4.
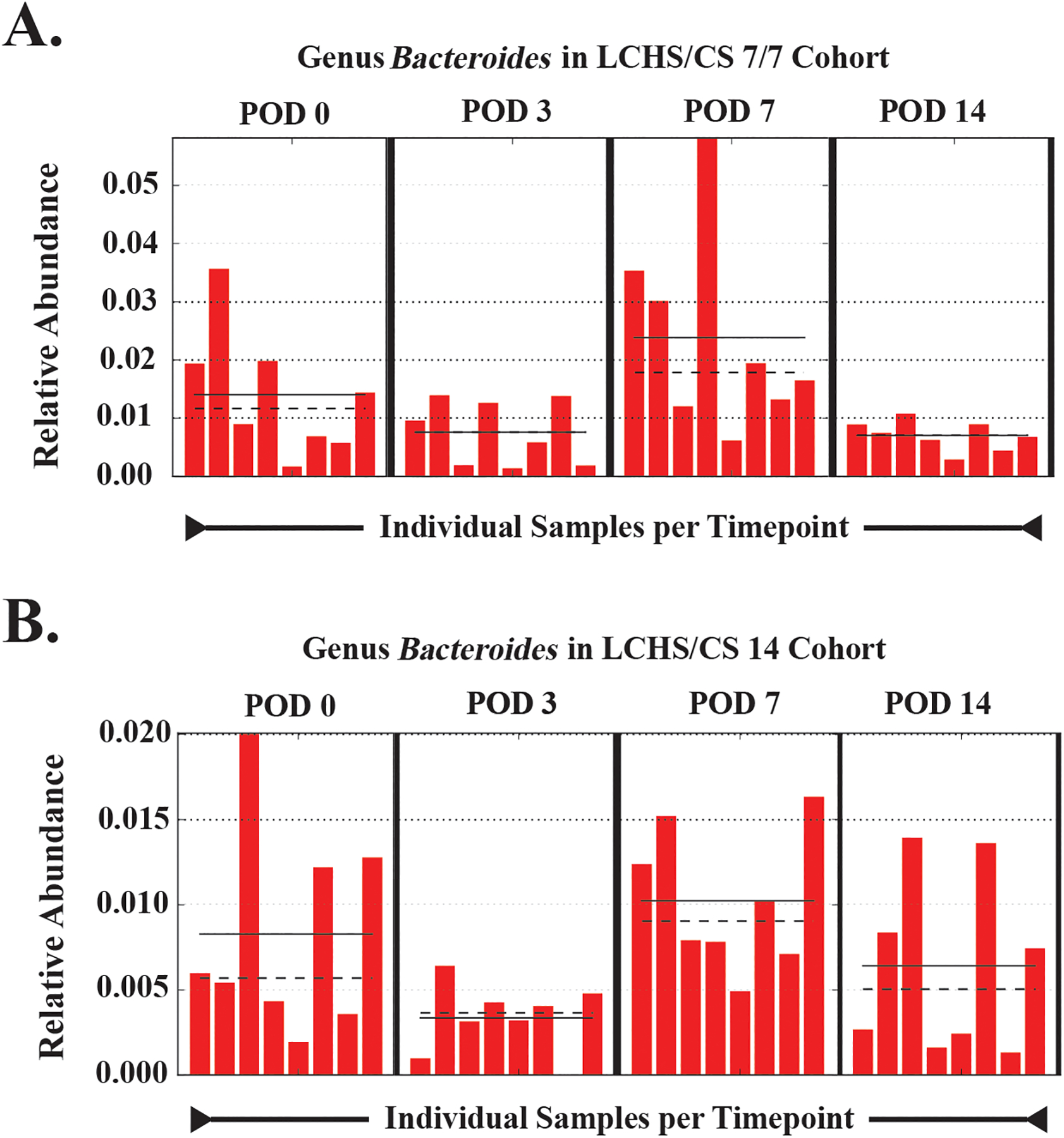
Changes in the relative abundance of the genus Bacteroides are shown based on the increased abundance of this genus at POD 7 in both the LCHS/CS 7/7 (A) and LCHS/CS 14 (B) cohorts. Shown are the changes in the mean (dashed lines) and median (solid lines) relative abundance over time (POD 0–14) for each evaluated specimen that corroborates the increased abundance noted on linear discriminant analysis at POD 7. POD (post-operative day); LCHS/CS 7/7 (lung contusion, hemorrhagic shock and 7 days of chronic restraint stress followed by 7 days of routine daily handling); LCHS/CS 14 (lung contusion, hemorrhagic shock, plus 14 days of chronic restraint stress).
Discussion
By using an established rat model of trauma and hemorrhagic shock coupled with either 0, 7, or 14 days of stress, the results of this study suggest that persistent stress may be a driving factor associated with the continued changes in microbial diversity seen after trauma. Following trauma and hemorrhagic shock alone, there was a relatively stable microbiota diversity. This study is novel as it characterized the trajectory of gut microbial changes after trauma in the presence of ongoing chronic stress in a temporal fashion as long as the chronic stress stimulus remained in place. Gut microbiota began to normalize, and in some cases fully normalized, with resolution of daily stress. On the other hand, changes such as the loss of class Actinobacteria in the LCHS/CS 7/7 cohort persisted even after cessation of chronic stress. This is consistent with a small preliminary study among burn patients, which identified survivors as reaching a peak derangement followed by partial resolution of baseline biodiversity; in contrast, among those who did not survive there was a steady and progressive derangement of the normal microbiome leading up until the time of death.(32) How loss of a baseline taxa without repopulation relates to outcomes after trauma remains to be elucidated. While beta diversity in our study demonstrated lack of segregation by cohort at any time point, this may demonstrate an increased importance of gut microbial changes within an individual host rather than across disease spectrum. This has been previously demonstrated by members of our group.(33) These findings support our hypothesis that the pathologic changes within the gut microbiome are not permanent, and likely can be prevented or reversed to alter post-trauma outcomes.
Yang et al. (34) identified an initial surge in Bacteroidales, Clostridiales, and Ruminococcus growth in rabbits within 24 hours after hemorrhage that continued for seven days postinjury. Our results did not replicate this robust response to trauma-hemorrhage alone, but rather this study identified an overabundance of taxa similar to those identified in their study but only after the addition of chronic stress over the course of three, seven, or fourteen days. This finding may be partly attributable differences across species, housing, and diet, or perhaps due to the fact that the rabbits were not resuscitated following hemorrhage, while our experiment featured resuscitation with 50% of shed blood. Nicholson et al. (35) demonstrated increased Lachnospiraceae and decreased Bacteroidaceae in a rodent polytrauma model two hours postinjury. In contrast, in this study rodents were followed from injury to fourteen days postinjury and demonstrated an increase in members of the family Bacteroidaceae, driven by an increase in species within the genus Bacteroides.
A deeper understanding of the relationship between the gut microbiome and inflammation is critically important following severe injury. A ten-day ICU stay has been associated with an extreme decline in alpha diversity with sharp reductions in Bacteroidetes, Firmicutes and anti-inflammatory commensals while pathogens like Enterobacter flourished. We did not see changes in Enterobacter numbers – while a common finding in human microbiome studies, we are not aware of any rat study to date which has identified changes in Enterobacter; this may be a limitation of our chosen animal model. Another human study also demonstrated decreased Bacteroidales and increased Clostridiales.(15) In contrast to these findings, we identified an increase in the genus Bacteroides corresponding to presence of chronic stress in both stress cohorts. Bacteroides is dually known for serving important commensal functions in human carbohydrate metabolism, immunomodulation, and colonization resistance against other pathogens, as well as for possessing multiple virulence factors that contribute to its own pathogenicity.(36, 37) The capsular polysaccharide complex of Bacteroides is particularly suited to abscess formation.(38) B. fragilis and B. thetaiotamicron are among the most frequently isolated organisms in gangrenous and perforated appendicitis.(39) B. fragilis also accounts for approximately 55% of blood isolates in patients with anaerobic bacteremia, with 19–31% associated mortality.(40) Although the aftereffects of microbiota changes were outside the scope of this study, the “bloom” of Bacteroides seen in our cohorts subjected to daily stress may impart a predisposition towards future pathogenicity and warrants further investigation.
Changes in gut microbiota have been linked to stressful experiences and even development of post-traumatic stress disorder (PTSD).(41–43) Consistent with previous studies, we identified altered microbiota composition among those groups subjected to chronic restraint stress, then went further to identify a temporal effect over the course of two weeks which improved after removal of stressful stimuli.(19, 20) Although our study did not include behavioral assessments or metabolites, murine social stress has been associated with changes in concentrations of microbial metabolites that correlated with cecal IgA levels and reduced social interaction scores.(44) A change in production of neurochemical precursors has also been observed after murine stress.(20) Our finding of decreased Actinobacteria in the setting of persistent stress is echoed by earlier findings in humans linking decreased abundance of this species to higher PTSD scores and opens up opportunities for future research in this area.(41) The relationship between changes in the gut microbiome after trauma and development of PTSD is still unclear.
This study has several limitations. Our results suggest that chronic stress was the driving factor in pathologic changes in gut microbiome and biodiversity after trauma, however, we did not specifically include a cohort subjected to chronic stress in the absence of trauma. This study also does not address potential causal mechanisms. Changes in microbiota are presumed to be linked to a complex array of intestinal barrier changes identified following intestinal ischemia and inflammation, which alters the unique constellation of host pathogen interactions and transform the gut microbiome into a so-called “pathobiome”.(5, 7, 9, 45) While genus Bacteroides was common between the LCHS/CS 7/7 and LCHS/CS 14 groups at POD 7, suggesting that chronic stress contributes to its increased abundance, this increase was not persistent at POD 14 in the LCHS/CS 14 cohort. One would expect this abundance increase to persist with continued chronic stress, especially given that with the removal of chronic stress by POD 14 in the LCHS/CS 7/7 cohort resulted in a concomitant decrease in Bacteroides in this group. Ultimately gnotobiotic experiments will need to be performed to confirm our findings but our model of hemorrhagic shock and resuscitation presents inherent difficulties using germ-free animals.
Our work highlights some of the long-term changes in the gut microbiome seen after trauma. This study also underscores the important role of persistent stress on microbial derangements acquired by the gut after trauma. Further work is needed to determine the role of dietary modifications or probiotics, antibiotic treatments or fecal microbiota transplant, and environmental enrichment on reversing or minimizing changes in the gut microbiome. Altering the gut microbiome changes may lead to improved pathologic clinical outcomes following trauma.
Acknowledgements
The authors would like to thank Quick Biology, Inc. for their assistance with DNA isolation and 16S rRNA gene next-generation genomic sequencing, and portions of the bioinformatics analysis.
Source of funding
The authors declare that they have no relevant conflicts of interests. This research was supported by the National Institutes of Health. AMM was supported by NIH NIGMS R01 GM105893-01A1. LSK, DBD, and BPF were supported by postgraduate training grant T32 GM-008721 in burns, trauma, and perioperative injury by NIGMS. PAE was supported NIH NIGMS R01 GM113945-01. Finally, AMM and PAE were supported by NIH NIGMS P50 GM111152-01.
Footnotes
Conflict of Interest
The authors declare that they have no relevant conflicts of interests.
Disclosure Statement:
The authors report no proprietary, commercial or conflicts of interest in any product mentioned or concept discussed in this article.
These findings were presented virtually at the 34th Annual Scientific Assembly for Eastern Association for the Surgery of Trauma January 13–14, 2021.
References
- 1.Nathens AB, Maier RV, Jurkovich GJ, Monary D, Rivara FP, Mackenzie EJ. The delivery of critical care services in us trauma centers: Is the standard being met? J Trauma. 2006;60(4):773–83; disucssion 83–4. [DOI] [PubMed] [Google Scholar]
- 2.Bowman JA, Jurkovich GJ, Nuno M, Utter GH. Hospital-level intensive care unit admission for patients with isolated blunt abdominal solid organ injury. J Trauma Acute Care Surg. 2020;88(3):408–15.31923050 [Google Scholar]
- 3.Michetti CP, Fakhry SM, Brasel K, Martin ND, Teicher EJ, Newcomb A, group Ts. Trauma icu prevalence project: The diversity of surgical critical care. Trauma Surg Acute Care Open. 2019;4(1):e000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald D, Ackermann G, Khailova L, Baird C, Heyland D, Kozar R, Lemieux M, Derenski K, King J, Vis-Kampen C, et al. Extreme dysbiosis of the microbiome in critical illness. mSphere. 2016;1(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alverdy JC, Laughlin RS, Wu L. Influence of the critically ill state on host-pathogen interactions within the intestine: Gut-derived sepsis redefined. Crit Care Med. 2003;31(2):598–607. [DOI] [PubMed] [Google Scholar]
- 6.Meng M, Klingensmith NJ, Coopersmith CM. New insights into the gut as the driver of critical illness and organ failure. Curr Opin Crit Care. 2017;23(2):143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20(4):214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krezalek MA, DeFazio J, Zaborina O, Zaborin A, Alverdy JC. The shift of an intestinal “microbiome” to a “pathobiome” governs the course and outcome of sepsis following surgical injury. Shock. 2016;45(5):475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klingensmith NJ, Coopersmith CM. The gut as the motor of multiple organ dysfunction in critical illness. Crit Care Clin. 2016;32(2):203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burmeister DM, Johnson TR, Lai Z, Scroggins SR, DeRosa M, Jonas RB, Zhu C, Scherer E, Stewart RM, Schwacha MG, et al. The gut microbiome distinguishes mortality in trauma patients upon admission to the emergency department. J Trauma Acute Care Surg. 2020;88(5):579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivanov II, Honda K Intestinal commensal microbes as immune modulators. Cell Host Microbe. 2012;12(4):496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rupani B, Caputo FJ, Watkins AC, Vega D, Magnotti LJ, Lu Q, Xu DZ, Deitch EA. Relationship between disruption of the unstirred mucus layer and intestinal restitution in loss of gut barrier function after trauma hemorrhagic shock. Surgery. 2007;141(4):481–9. [DOI] [PubMed] [Google Scholar]
- 13.Earley ZM, Akhtar S, Green SJ, Naqib A, Khan O, Cannon AR, Hammer AM, Morris NL, Li X, Eberhardt JM, et al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLoS One. 2015;10(7):e0129996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayakawa M, Asahara T, Henzan N, Murakami H, Yamamoto H, Mukai N, Minami Y, Sugano M, Kubota N, Uegaki S, et al. Dramatic changes of the gut flora immediately after severe and sudden insults. Dig Dis Sci. 2011;56(8):2361–5. [DOI] [PubMed] [Google Scholar]
- 15.Howard BM, Kornblith LZ, Christie SA, Conroy AS, Nelson MF, Campion EM, Callcut RA, Calfee CS, Lamere BJ, Fadrosh DW, et al. Characterizing the gut microbiome in trauma: Significant changes in microbial diversity occur early after severe injury. Trauma Surg Acute Care Open. 2017;2(1):e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karl JP, Hatch AM, Arcidiacono SM, Pearce SC, Pantoja-Feliciano IG, Doherty LA, Soares JW. Effects of psychological, environmental and physical stressors on the gut microbiota. Front Microbiol. 2018;9:2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. 2017;7:124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu RT. The microbiome as a novel paradigm in studying stress and mental health. Am Psychol. 2017;72(7):655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galley JD, Nelson MC, Yu Z, Dowd SE, Walter J, Kumar PS, Lyte M, Bailey MT. Exposure to a social stressor disrupts the community structure of the colonic mucosa-associated microbiota. BMC Microbiol. 2014;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J, Forsythe P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology. 2016;63:217–27. [DOI] [PubMed] [Google Scholar]
- 21.Iapichino G, Callegari ML, Marzorati S, Cigada M, Corbella D, Ferrari S, Morelli L. Impact of antibiotics on the gut microbiota of critically ill patients. J Med Microbiol. 2008;57(Pt 8):1007–14. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson SE, Burmeister DM, Johnson TR, Zou Y, Lai Z, Scroggins S, DeRosa M, Jonas RB, Merrill DR, Zhu C, et al. A prospective study in severely injured patients reveals an altered gut microbiome is associated with transfusion volume. J Trauma Acute Care Surg. 2019;86(4):573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bible LE, Pasupuleti LV, Gore AV, Sifri ZC, Kannan KB, Mohr AM. Chronic restraint stress after injury and shock is associated with persistent anemia despite prolonged elevation in erythropoietin levels. J Trauma Acute Care Surg. 2015;79(1):91–6; discussion 6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. Dada2: High-resolution sample inference from illumina amplicon data. Nat Methods. 2016;13(7):581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMurdie PJ, Holmes S. Phyloseq: An r package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8(4):e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuomisto H A diversity of beta diversities: Straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography. 2010;33(1):2–22. [Google Scholar]
- 27.Whittaker RH. Evolution and measurement of species diversity. TAXON. 1972;21:213–51. [Google Scholar]
- 28.Knight R, Vrbanac A, Taylor BC, Aksenov A, Callewaert C, Debelius J, Gonzalez A, Kosciolek T, McCall LI, McDonald D, et al. Best practices for analysing microbiomes. Nat Rev Microbiol. 2018;16(7):410–22. [DOI] [PubMed] [Google Scholar]
- 29.McCafferty J, Muhlbauer M, Gharaibeh RZ, Arthur JC, Perez-Chanona E, Sha W, Jobin C, Fodor AA. Stochastic changes over time and not founder effects drive cage effects in microbial community assembly in a mouse model. ISME J. 2013;7(11):2116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological). 1995;57:289–300. [Google Scholar]
- 32.Shimizu K, Ogura H, Asahara T, Nomoto K, Matsushima A, Hayakawa K, Ikegawa H, Tasaki O, Kuwagata Y, Shimazu T. Gut microbiota and environment in patients with major burns - a preliminary report. Burns. 2015;41(3):e28–33. [DOI] [PubMed] [Google Scholar]
- 33.Mankowski RT, Thomas RM, Darden DB, Gharaibeh RZ, Hawkins RB, Cox MC, Apple C, Nacionales DC, Ungaro RF, Dirain ML, et al. Septic stability? Gut microbiota in young adult mice maintains overall stability after sepsis compared to old adult mice. Shock. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Zhang J, Zhao C, Gai Z, Mu X, Wang Y, Zhang C, Su Z, Gao L, Zhu D, et al. Blood loss leads to increase in relative abundance of opportunistic pathogens in the gut microbiome of rabbits. Curr Microbiol. 2020;77(3):415–24. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson SE, Merrill D, Zhu C, Burmeister DM, Zou Y, Lai Z, Darlington DN, Lewis AM, Newton L, Scroggins S, et al. Polytrauma independent of therapeutic intervention alters the gastrointestinal microbiome. Am J Surg. 2018;216(4):699–705. [DOI] [PubMed] [Google Scholar]
- 36.Wexler HM. Bacteroides: The good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007;20(4):593–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with clostridium difficile infection. J Med Microbiol. 2002;51(5):448–54. [DOI] [PubMed] [Google Scholar]
- 38.Tzianabos AO L KD, B. OA. Structure and function of bacteroides fragilis capsular polysaccharides: Relationship to induction and prevention of abscesses. Clin Infect Dis. 1995;20:S132–S40. [DOI] [PubMed] [Google Scholar]
- 39.Bennion RS, Baron EJ, Thompson JEJ, Downes J, Summanen P, Talan DA, Finegold SM. The bacteriology of gangrenous and perforated appendicitis--revisited. Ann Surg. 1990;211(2):165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldstein EJC. Anaerobic bacteremia. Clin Infect Dis. 1996;23:S97–S101. [DOI] [PubMed] [Google Scholar]
- 41.Hemmings SMJ, Malan-Muller S, van den Heuvel LL, Demmitt BA, Stanislawski MA, Smith DG, Bohr AD, Stamper CE, Hyde ER, Morton JT, et al. The microbiome in posttraumatic stress disorder and trauma-exposed controls: An exploratory study. Psychosom Med. 2017;79(8):936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leclercq S, Forsythe P, Bienenstock J. Posttraumatic stress disorder: Does the gut microbiome hold the key? Can J Psychiatry. 2016;61(4):204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu M, Wang C, Krolick KN, Shi H, Zhu J. Difference in post-stress recovery of the gut microbiome and its altered metabolism after chronic adolescent stress in rats. Sci Rep. 2020;10(1):3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aoki-Yoshida A, Aoki R, Moriya N, Goto T, Kubota Y, Toyoda A, Takayama Y, Suzuki C. Omics studies of the murine intestinal ecosystem exposed to subchronic and mild social defeat stress. J Proteome Res. 2016;15(9):3126–38. [DOI] [PubMed] [Google Scholar]
- 45.Alverdy JC, Krezalek MA. Collapse of the microbiome, emergence of the pathobiome, and the immunopathology of sepsis. Crit Care Med. 2017;45(2):337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]


