Abstract
Background:
Despite the widespread institution of modern massive transfusion protocols with balanced blood product ratios, survival for patients with traumatic hemorrhage receiving ultramassive transfusion (defined as ≥20 units[u] packed red blood cells [RBCs]) in 24 hours[h]) remains low and resource consumption remains high. Therefore, we aimed to identify factors associated with mortality in trauma patients receiving ultramassive transfusion in the modern resuscitation era.
Methods:
An EAST multicenter retrospective study of 461 trauma patients from 17 trauma centers who received ≥20u RBCs in 24h was performed (2014–2019). Multivariable logistic regression and Classification and Regression Tree analysis (CART) were used to identify clinical characteristics associated with mortality.
Results:
The 461 patients were young (median age 35 years[y]), male (82%), severely injured (median injury severity score 33), in shock (median shock index 1.2, base excess −9), and were transfused a median of 29u RBCs, 22u of plasma (FFP), and 24u of platelets (PLT). Mortality was 46% at 24h and 65% at discharge. Transfusion of RBC:FFP≥1.5:1 or RBC:PLT≥1.5:1 was significantly associated with mortality, most pronounced for the 18% of patients who received both RBC:PLT and RBC:FFP ≥1.5:1 (odds ratios 3.11 and 2.81 for mortality at 24h and discharge, both p<0.01). CART identified that age>50y, low initial Glasgow Coma Scale, thrombocytopenia, and resuscitative thoracotomy were associated with low likelihood of survival (14–26%), while absence of these factors was associated with the highest survival (71%).
Conclusions:
Despite modern massive transfusion protocols, one half of trauma patients receiving ultramassive transfusion are transfused either RBC:FFP or RBC:PLT in unbalanced ratios ≥1.5:1, with increased associated mortality. Maintaining focus on balanced ratios during ultramassive transfusion is critical, and consideration of advanced age, poor initial mental status, thrombocytopenia, and resuscitative thoracotomy can aid in prognostication.
Keywords: trauma, hemorrhagic shock, transfusion medicine
Background
Traumatic hemorrhage remains a leading cause of morbidity and mortality in injured patients (1). The implementation of damage control resuscitation practices with initial empiric balanced ratios of packed red blood cells (RBCs), plasma (FFP), and platelets (PLT) followed by goal directed resuscitation in massive transfusion protocols (MTP) has led to improved outcomes and been increasingly adopted in trauma patients (2–9). However, it is not known whether these advances in transfusion strategies are associated with improved outcomes specifically in trauma patients receiving ultramassive transfusion (UMT), defined as transfusion of greater than 20 units (u) of RBCs in 24 hours (10, 11). This is highlighted by the finding that the majority of patients enrolled in the paradigm shifting Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) and Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) trials were transfused less than 10u RBCs in 24 hours (6, 7).
Patients receiving UMT present unique decision-making challenges given their massive use of blood products and hospital resources in the setting of severe injuries, frequent physiologic deterioration requiring emergent, lifesaving interventions, and their very high mortality rates (10, 12). Further, UMT events have the potential to overwhelm trauma care systems in resource limited settings including during mass casualty incidents, in austere environments, or even during large health crises such as the current COVID-19 pandemic (10–13). Due to these issues, understanding the prognosis of patients receiving UMT may assist clinicians in resource allocation during shortages. Because of the rarity of UMT events, only a few studies have examined outcomes for trauma patients specifically meeting UMT criteria, reporting very high mortality rates ranging from 60 to 70% (10–12). These studies could not definitively establish physiologic parameters or transfusion thresholds beyond which survival was very unlikely, but they did identify that patients with higher injury severity, lower Glasgow Coma Scale score (GCS), male gender, and undergoing resuscitative thoracotomy were more likely to die (10–12). Importantly though, none of these studies were performed since the widespread implementation of damage control resuscitation practices, nor with a large cohort of trauma patients or representing practices across multiple trauma institutions.
Therefore, to further advance our understanding of the care of trauma patients who receive UMT for traumatic hemorrhage, we performed a large, multi-center, retrospective study of patients receiving UMT during the modern era of balanced blood product resuscitation (post PROMMT, 2014–2019) (7). We aimed to further characterize outcomes for trauma patients receiving UMT and to identify clinical, physiologic, and transfusion factors predictive of mortality, hypothesizing that balanced transfusion ratios would be associated with improved mortality based on landmark studies of transfusion in trauma (6, 7).
Methods
Study design, inclusion criteria, and participating centers
Seventeen adult level 1 and 2 trauma centers participated in this Eastern Association for the Surgery of Trauma (EAST) multicenter, retrospective study (see Supplemental Table 1 for site accreditation level and massive transfusion protocol characteristics). The cohort included 461 adult trauma patients (≥18 years of age) who received ≥20u of RBCs within 24 hours of presentation to the emergency department (2014–2019). We collected patient demographics, injury and physiologic characteristics on arrival, operative procedures performed, transfusions and hemostatic adjunct use within 24 hours, in-hospital complications, and mortality at 24h and during hospitalization. To standardize transfusion variables across sites for analysis, we defined 1 RBC unit as 300ml, one FFP unit as 250ml, and one PLT unit as 50ml. Whole blood was reported to be available at three of the participating centers, but only two patients in the study received whole blood (two units each). Power calculations revealed that a sample size of 450 patients would yield 80% power to detect a 15% difference in mortality between groups with balanced (<1.5:1) versus unbalanced (≥1.5:1) transfusion ratios, with alpha set at 0.05. The study was approved by the Institutional Review Board at each of the participating centers.
Statistical Analyses
Patient demographics, characteristics, transfusions, and outcomes were described using means and standard deviations for normally distributed continuous variables, median and interquartile ranges for skewed continuous variables, and as percentages for binary and categorical variables. Univariate analyses were performed to test for differences in demographics and clinical characteristics by mortality at discharge using Student’s t-tests for continuous and normally distributed variables, Wilcoxon rank-sum tests for continuous and non-normally distributed variables, and Fisher’s Exact tests for binary and categorical variables. A two-sided p-value < 0.05 was considered statistically significant.
Multivariable logistic regression was used to test the association of three primary predictors (transfusion ratios, cryoprecipitate use, and tranexamic acid use) with mortality at 24 hours and at discharge. These models controlled for known potential confounders specified a priori for each primary predictor and outcome. Transfusion ratios were defined as the ratio of RBCs to FFP and the ratio of RBCs to PLT in the first 24 hours. Transfusion ratios were classified as <1.5:1 or ≥1.5:1 to mitigate the effect of outliers in the data and create clinically meaningful categories. Because RBC:FFP and RBC:PLT ratios are interrelated, both terms were included in a single variable and categorized as both <1.5:1 (reference group), RBC:FFP≥1.5:1, RBC:PLT ≥1.5:1, or both ≥1.5:1. All multivariable regression models included random effects on site to account for differences by participating center (14).
Lastly, Classification and Regression Trees (CART) recursive partitioning was used to build models predictive of survival to discharge based on available patient characteristics on arrival, including demographics, injury mechanisms, physiologic characteristics, and laboratory parameters. CART is a form of machine learning that uses a given set of variables to create validated hierarchical decision trees which has been applied in a range of disciplines, including in studies of trauma patients (15–17). This method was chosen given its utility in determining both variable importance as well as the optimal cutoff values for each variable for predicting outcome (15). To determine optimal splits in our predictor variables, the Gini Coefficient was used, and cost complexity pruning was performed to prevent overfitting and build the most efficient decision tree (15). We identified several plausible sets of predictor variables with varying levels of specificity and used cross-validation to select the final predictor set. In the cross-validation, we fit the models using 90% of the patients (randomly selected) and evaluated model accuracy using the remaining 10%. This process was iterated 100 times to determine the mean proportion correct across the 100 test sets. We additionally summarized the final model fit using the area under the receiver operator characteristic curve (AUC).
To improve model discrimination, a second CART model was developed using a subset of the patients (n=353) with less than 10% missingness on key admission predictor variables identified in the unpruned classification trees from the whole cohort. Descriptive statistics and multivariable modeling were performed in Stata version 15 (StataCorp, Texas). CART analyses were performed using the rpart package in R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria (R Foundation for Statistical Computing, Vienna, Austria) (15, 16).
Results
Patient Characteristics and Univariate Analyses
The 461 patients were young with a median age of 35 years, predominantly male, and had few reported comorbidities or antiplatelet/anticoagulant medication use (Table 1). The patients were severely injured (median injury severity score 33), had a median GCS on arrival of 7, 17% had severe head injury (defined by abbreviated injury scale head≥5), and had evidence of significant shock and impaired tissue perfusion by several parameters (Table 1). Resuscitative thoracotomy was performed in 32% of patients, while 10% had Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) performed (Table 1). Patients were transfused a mean of 29u RBCs, 22u FFP, and 24u PLTs within 24 hours of arrival (Table 1). Thirty five percent of patients had RBC:FFP≥1.5:1, 36% RBC:PLT ratios of ≥1.5:1, 52% had either ratio ≥1.5:1, and 18% had had both ratios ≥1.5:1 (Table 1 and Figure 1). Tranexamic acid (TXA) and cryoprecipitate were administered in the majority of patients, while only a small proportion received recombinant factor VII or prothrombin complex concentrate (Table 1). Mortality was high at 43% at 24 hours and 65% by discharge, and complications were common among those who survived including acute kidney injury (44%), sepsis (41%), and venous thromboembolism (28%, Table 1).
Table 1.
Characteristics and Outcomes Compared by Mortality at Discharge
| Variable | Survived 35% (N=160) |
Expired 65% (N=301) |
All Patients (N=461) | P-value |
|---|---|---|---|---|
| Demographics and Past History | ||||
| Age | 33 (26, 44) | 38 (25, 52) | 35 (25, 50) | 0.06 |
| Male | 81% | 83% | 82% | 0.53 |
| BMI | 27.6±5.6 | 28±6.0 | 28.0±6.0 | 0.23 |
| History of MI or CHF | 1% | 2% | 2% | 0.73 |
| History of diabetes | 6% | 7% | 7% | 0.83 |
| History of cirrhosis | 0% | 5% | 3% | <0.01 |
| History of cancer | 1% | 2% | 2% | 0.65 |
| Antiplatelet agent | 5% | 6% | 6% | 0.81 |
| Anticoagulant agent | 6% | 2% | 4% | 0.19 |
| Injury Characteristics | ||||
| Injury Severity Score | 29 (22, 4) | 34.0 (25, 43) | 33 (22, 43) | <0.01 |
| Severe Head Injury* | 6% | 23% | 17% | <0.01 |
| Blunt | 52% | 61% | 58% | 0.06 |
| Mechanism Subtype | 0.40 | |||
| GSW | 38% | 33% | 35% | |
| SW | 6% | 4% | 4% | |
| PVA/BVA | 9% | 12% | 11% | |
| MVC/MCC | 34% | 39% | 38% | |
| Fall | 3% | 2% | 2% | |
| Physiologic and Laboratory Characteristics on Arrival | ||||
| Glasgow Coma Scale | 14 (4, 15) | 3 (3, 13) | 7 (3, 14) | <0.01 |
| Systolic blood pressure (SBP), mmHg | 90 (76, 113) | 90 (69, 109) | 90 (70, 110) | 0.30 |
| SBP <60mmhg | 9% | 15% | 13% | 0.08 |
| Heart rate**, bpm | 117 (96, 133) | 106 (85, 131) | 110 (90, 132) | <0.01 |
| Pulseless | 2% | 7% | 5% | 0.13 |
| Shock Index | 1.2 (0.9, 1.6) | 1.2 (0.9, 1.6) | 1.2 (0.9, 1.6) | 0.64 |
| Base Excess, mmol/L | −11.7±7.1 | −14.5±8.0 | −10.8±6.7 | <0.01 |
| Lactate, mmol/L | 7.3 (4.5, 11.7) | 10.5 (6.5, 14.3) | 9.1 (5.8, 13.3) | <0.01 |
| Hemoglobin, g/dL | 11.3±2.6 | 10.4±2.6 | 10.9±2.6 | <0.01 |
| Platelet count (x109/L) | 192 (127, 245) | 152 (91, 214) | 172 (107, 224) | <0.01 |
| INR | 1.3 (1.1–1.6) | 1.4 (1.2–1.9) | 1.4 (1.2–1.8) | <0.01 |
| Procedures | ||||
| Exploratory Laparotomy | 61% | 62% | 62% | 0.76 |
| Resuscitative Thoracotomy | 14% | 42% | 32% | <0.01 |
| REBOA | 8% | 11% | 10% | 0.32 |
| Aorta Cross Clamped*** | 14% | 31% | 25% | <0.01 |
| Transfusion Characteristics (24hrs) | ||||
| RBC:PLT Continuous, median (IQR) | 1.1 (0.9, 1.4) | 1.3 (1.0, 1.9) | 1.2 (1.0, 1.8) | <0.01 |
| RBC:FFP Continuous, median (IQR) | 1.2 (1.1, 1.5) | 1.3 (1.1, 1.7) | 1.3 (1.1, 1.7) | <0.01 |
| RBCs, median units (IQR) | 29 (23, 39) | 30 (24, 41) | 29 (24, 40) | 0.33 |
| Platelets, median units (IQR) | 26 (18, 36) | 18 (12, 30) | 24 (18, 36) | <0.01 |
| FFP, median units (IQR) | 22 (18, 31) | 21 (16, 31) | 22 (17, 31) | 0.13 |
| RBC: FFP Ratio ≥ 1.5:1 | 28% | 39% | 35% | 0.02 |
| RBC:PLT Ratio ≥ 1.5:1 | 23% | 43% | 36% | <0.01 |
| RBC:PLT or RBC:FFP Ratio ≥1.5:1 | 40% | 59% | 52% | <0.01 |
| RBC:FFP & RBC:PLT Ratio ≥1.5:1 | 10% | 22% | 18% | <0.01 |
| Hemostatic Adjunct Use (24hrs) | ||||
| Tranexamic acid | 63% | 63% | 63% | 1.00 |
| Factor VII | 3% | 5% | 4% | 0.28 |
| Prothrombin complex concentrate | 4% | 2% | 2% | 0.24 |
| Cryoprecipitate | 81% | 66% | 71% | <0.01 |
| Viscoelastic Testing Performed (TEG/ROTEM) | 41% | 36% | 38% | 0.39 |
| Outcomes | ||||
| Sepsis | 41% | 8% | 19% | <0.01 |
| Acute Kidney injury | 44% | 20% | 28% | <0.01 |
| Myocardial infarction | 1% | 3% | 2% | 0.27 |
| Stroke | 5% | 2% | 3% | 0.17 |
| Venous thromboembolism | 28% | 5% | 13% | <0.01 |
| Mortality at 6 hours | 0% | 41% | 27% | <0.01 |
| Mortality at 24 hours | 0% | 66% | 43% | <0.01 |
| Mortality at discharge | 0% | 100% | 65% | <0.01 |
Severe head injury defined as AIS-head ≥5
Patients who presented in arrest or pulseless included with heart rate of 0
Aortic cross clamping does not include patients who underwent REBOA, includes patients who had cross clamping of the aorta during resuscitative thoracotomy or exploratory laparotomy
AIS- abbreviated injury score, BMI- body mass index, MI-myocardial infarction, CHF- congestive heart failure, GSW- gunshot wound, SW- stab wound, PVA/BVA-pedestrian vs auto or bike vs auto, MVC/MCC- motor vehicle collision or motorcycle collection, Shock Index- Heart rate/systolic blood pressure, max shock index 2.0 and patients pulseless or without measurable SBP assigned shock index of 2.0, INR-international normalized ratio, REBOA- resuscitative endovascular occlusion of the aorta, RBC- packed red blood cells, FFP- fresh frozen plasma, PLT-platelets. TEG-thromboelastography, ROTEM-rotational thromboelastometry
Figure 1. Distribution of RBC:FFP and RBC:PLT Ratios.
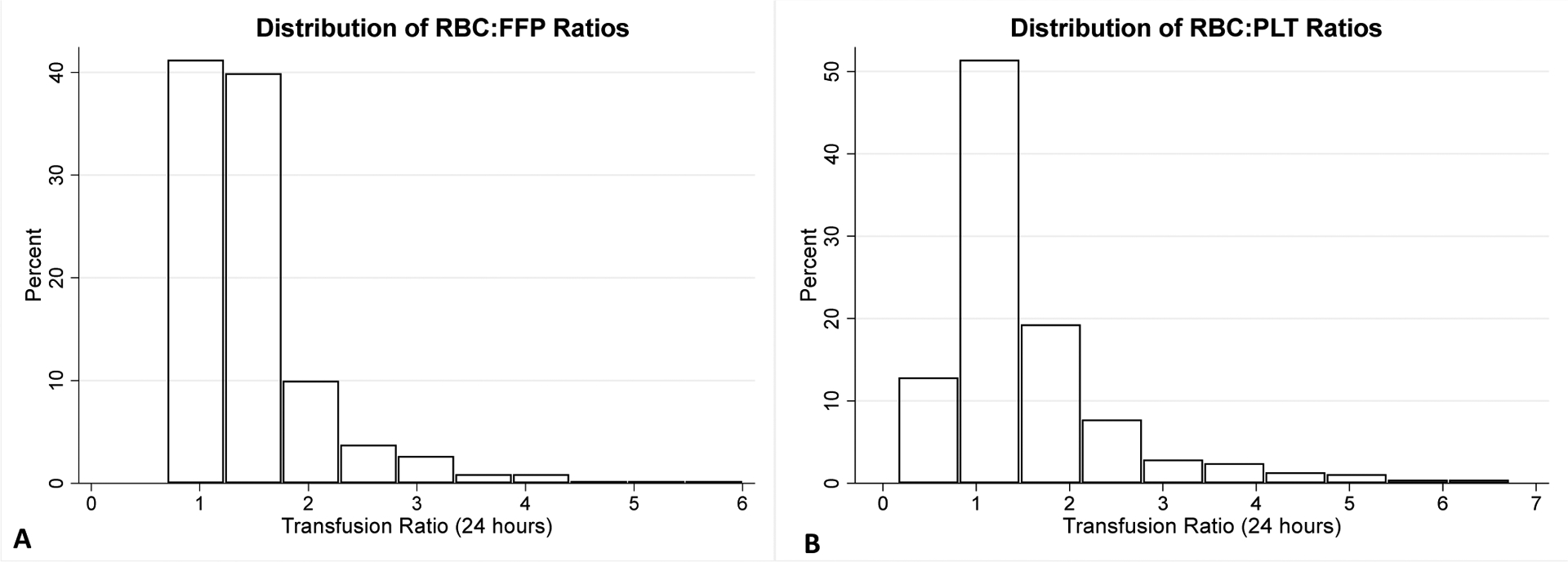
RBC- packed red blood cells, PLT- platelets, FFP- plasma.
1 patient with RBC:PLT ratio of 19 and 4 patients with RBC:FFP ratio of >10 not displayed. N=461.
In univariate analysis, patients who died prior to hospital discharge were older, more severely and bluntly injured, and had lower GCS, hemoglobin, and platelet counts on arrival (Table 1). Those who died also had more evidence of tissue hypoperfusion with higher base deficit and lactate, but systolic blood pressure (SBP) was not different between the groups. However, those who died were more likely to present hypotensive (SBP<60mmHg) or pulseless (Table 1). Forty-two percent of non-survivors underwent a resuscitative thoracotomy compared to 14% of those who survived to discharge (p<0.01), while rates of exploratory laparotomy and REBOA were not different (Table 1). With respect to transfusion ratios, there were significant trends for increased proportion of patients with unbalanced RBC:FFP and RBC:PLT ratios in association with mortality at discharge (Table 1). TXA, Factor VII, and prothrombin complex concentrate use were not different, but cryoprecipitate use was significantly higher among those who survived (p<0.01, Table 1).
Association of Transfusion Ratios, Cryoprecipitate, and TXA with Outcomes
Multivariable, logistic regression was used to test the association of transfusion ratios and hemostatic adjunct use with mortality at 24 hours and at discharge. RBC:PLT ratios ≥1.5:1 were significantly and independently associated with more than twofold increased odds of mortality at 24 hours and discharge (p<0.05, Figure 2 and Supplemental Table 2). RBC:FFP ratios ≥1.5:1 showed a similar trend, statistically significant for mortality at 24 hours (p=0.05, Figure 2 and Supplemental Table 2). The combination of both RBC:PLT and RBC:FFP transfused in ratios ≥1.5 was associated with 3.1 and 2.8 fold increased odds of death at 24 hours and discharge respectively (p<0.01, Figure 2, Supplemental Table 2).
Figure 2. Association of Transfusion Ratios with Mortality at 24 hours and Discharge.
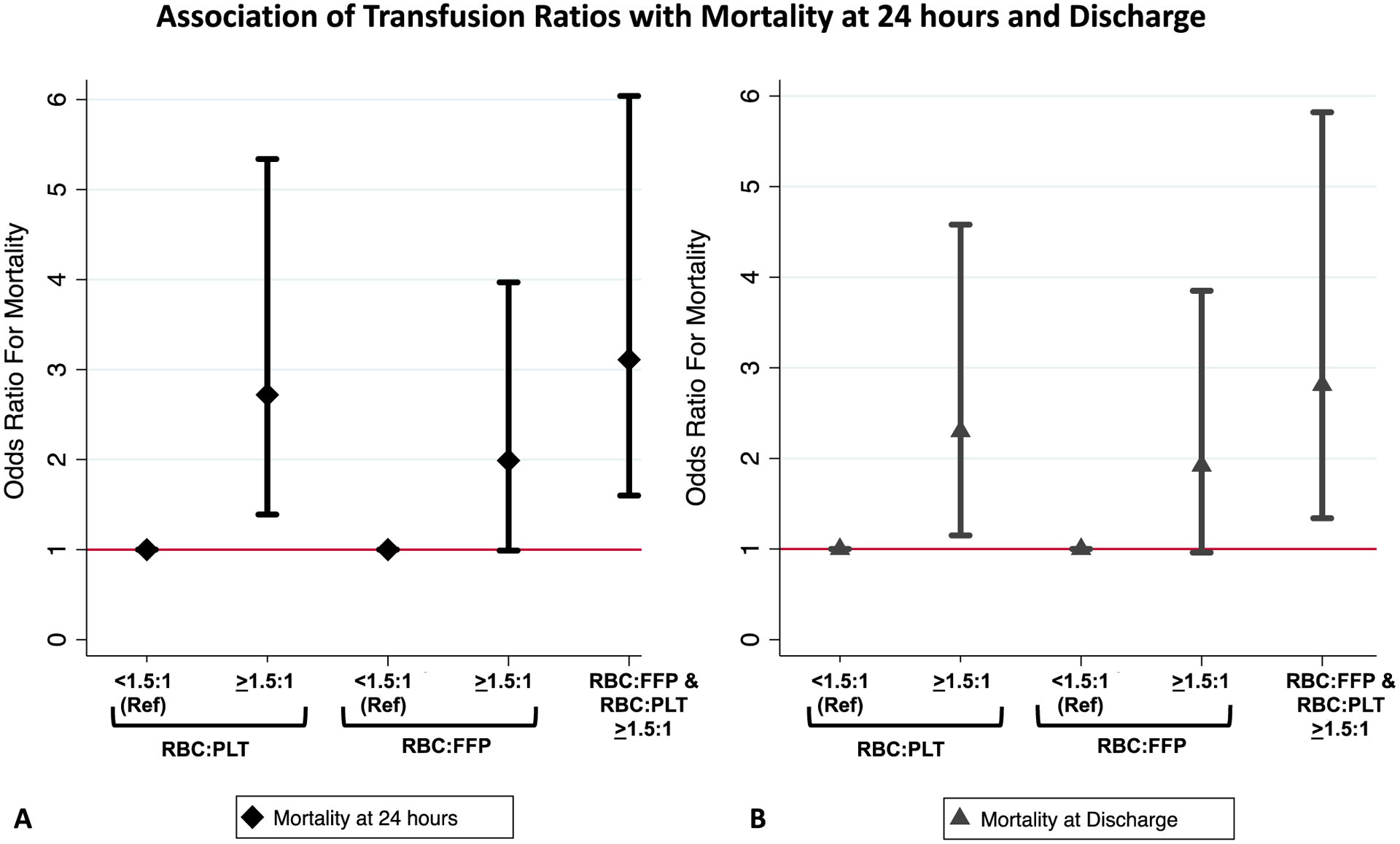
Plots display the point estimates (diamonds and triangles) with surrounding 95% confidence intervals for the odds ratios for mortality at 24 hours (black diamonds, A) and at discharge (gray triangles, B) for RBC:PLT and RBC:FFP ratios categorized as <1.5:1 or ≥1.5:1 or both ≥1.5:1. Odds ratios based on multivariable logistic regression controlling for age, injury severity, blunt mechanism, shock index and resuscitative thoracotomy with a random effect variance parameter to control for inter-site differences. See Supplemental Table 2 for the complete regression model output.
While cryoprecipitate use was significantly associated with reduced mortality at 24 hours and discharge on univariate analysis, on multivariable regression and when excluding early deaths (within 6 hours) to account for survival bias (given that cryoprecipitate is administered later in participating centers’ MTP protocols), these trends towards reduced mortality in patients receiving cryoprecipitate were not statistically significant (Table 2a). There was a non-significant trend towards decreased mortality at 24 hours in those who received TXA, and no difference in mortality at discharge (Table 2b). We also examined whether cryoprecipitate and TXA were associated with venous thromboembolism in patients surviving beyond 24 hours, but there were no significant associations of either cryoprecipitate or TXA use with thromboembolism (Supplemental Tables 3 and 4).
Table 2A.
Association of Cryoprecipitate with Mortality at 24 hours and Discharge*
| Mortality at 24 hours | Mortality at discharge | |||||
|---|---|---|---|---|---|---|
| Covariate | Odds Ratio | 95% CI | P-value | Odds Ratio | 95% CI | P-Value |
| Cryoprecipitate transfused | 0.72 | 0.38–1.37 | 0.32 | 0.79 | 0.43–1.44 | 0.43 |
| Age (per 10 years) | 1.17 | 1.00–1.37 | 0.05 | 1.33 | 1.14–1.55 | <0.01 |
| ISS (per 10 points) | 0.88 | 0.73–1.08 | 0.22 | 1.28 | 1.06–1.54 | 0.01 |
| Blunt Mechanism | 1.76 | 0.92–3.34 | 0.09 | 1.70 | 0.97–2.99 | 0.06 |
| Shock Index | 1.55 | 0.83–2.90 | 0.17 | 0.92 | 0.52–1.63 | 0.78 |
| Resuscitative Thoracotomy | 4.21 | 2.26–7.84 | <0.01 | 7.19 | 3.84–13.47 | <0.01 |
Multivariable logistic regression output with random effect variance parameter by site. Shock Index- HR/SBP, max shock index 2.0, patients pulseless or without measurable blood pressure on arrival assigned shock index of 2.0. ISS- injury severity score. CI- confidence interval.
Patients expiring within 6 hours excluded to control for selection bias in administration of cryoprecipitate
N=312 in A and N=334 in B
Table 2B.
Association of Tranexamic Acid with Mortality at 24 hours and Discharge
| Mortality at 24 hours | Mortality at discharge | |||||
|---|---|---|---|---|---|---|
| Covariate | Odds Ratio | 95% CI | P-value | Odds Ratio | 95% CI | P-Value |
| Tranexamic acid given | 0.71 | 0.43–1.20 | 0.20 | 1.00 | 0.59–1.67 | 0.99 |
| Age (per 10 years) | 1.01 | 0.99–1.03 | 0.25 | 1.02 | 1.01–1.04 | 0.01 |
| ISS (per 10 points) | 1.01 | 0.99–1.02 | 0.57 | 1.02 | 1.00–1.04 | 0.02 |
| Blunt Mechanism | 0.96 | 0.55–1.67 | 0.89 | 1.21 | 0.69–2.10 | 0.51 |
| Shock Index | 1.08 | 0.62–1.88 | 0.79 | 0.84 | 0.47–1.50 | 0.56 |
| Resuscitative Thoracotomy | 6.08 | 3.51–10.53 | 0.00 | 7.35 | 3.91–13.81 | 0.00 |
Multivariable logistic regression output with random effect variance parameter by site. Shock Index- HR/SBP, max shock index 2.0, patients pulseless or without measurable blood pressure on arrival assigned shock index of 2.0. ISS- injury severity score. CI- confidence interval.
Classification and Regression Tree Models (CART)
Lastly, CART models were created using physiologic and patient characteristics on arrival that were predictive of survival to discharge. The final pruned classification tree included arrival GCS, age, resuscitative thoracotomy, and platelet count as key discriminatory variables in predicting outcome (Figure 3). Patients with the best survival (71%) were those who had an initial GCS above 3, were under 50 years of age, did not undergo a resuscitative thoracotomy, and were not thrombocytopenic on arrival (Figure 3). On the other hand, patients with any combination of these factors had significantly poorer survival ranging from 14–26% (Figure 3). CART models with a subset of the cohort (n=353) with more complete data on these key predictors (<10%) were developed to improve model discrimination and performance. With this subset of the cohort, we additionally identified that for patients not undergoing resuscitative thoracotomy, but with a GCS of 3 on arrival, hemoglobin and injury mechanism provided additional stratification of mortality risk: patients with a hemoglobin on arrival <11.0 g/dL had a poor predicted survival of just 11%, while patients with hemoglobin of ≥11.0g/dL and a penetrating mechanism of injury had high survival rates (75%), despite their initial GCS of 3 (Figure 4).
Figure 3. CART Model predictive for survival to discharge for patients receiving ultramassive transfusion.
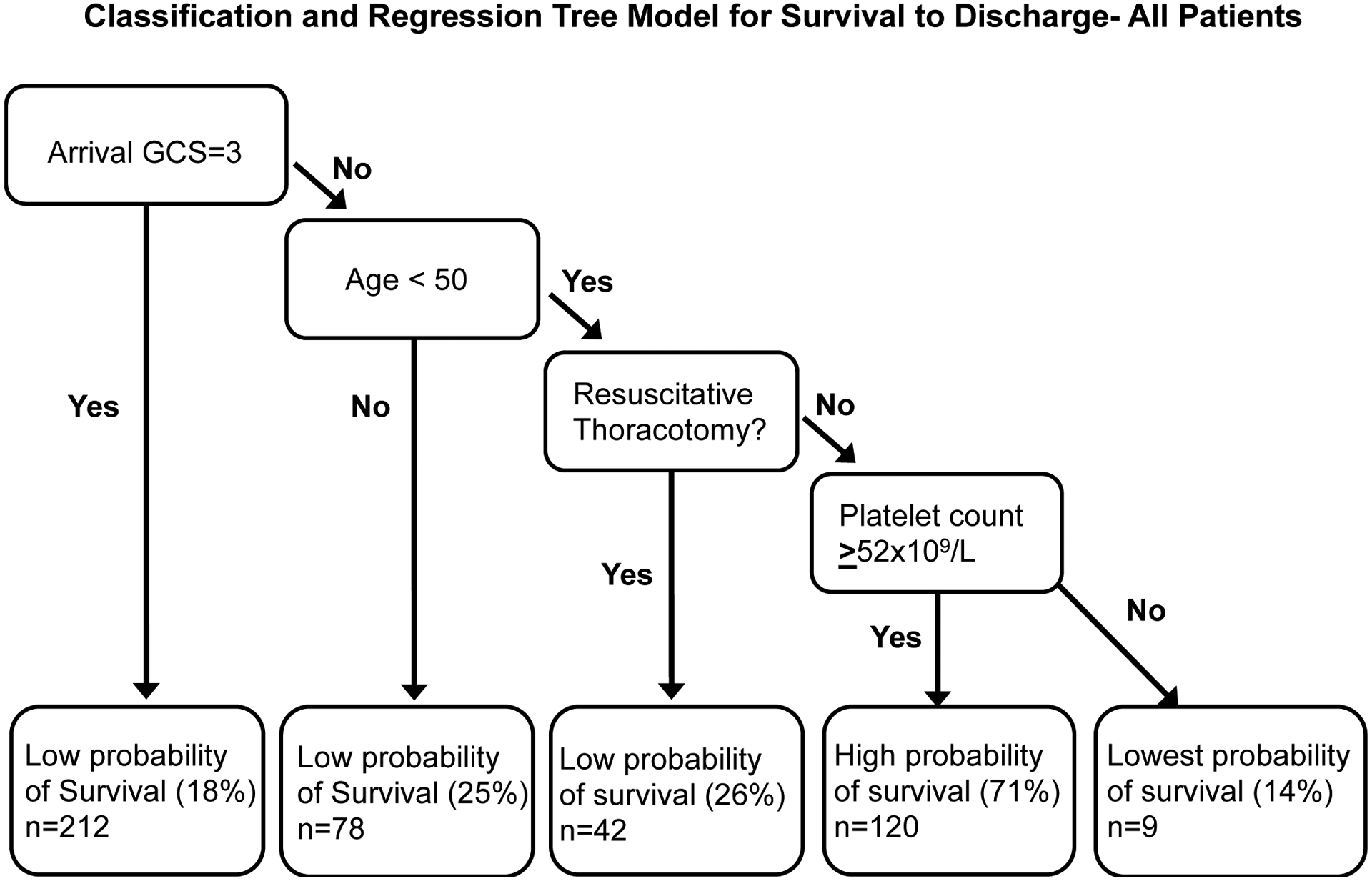
Gini coefficient used to determine splits with 10 fold cost complexity pruning to develop the trimmed tree. Data divided into a training (90%) and a test set (10%) for validation. Area under the receiver operator characteristic curve=79%, mean fraction correct in 100 iterations of test set 76%. GCS= Glasgow coma scale. Includes all 461 patients
Figure 4. CART Model predictive for survival to discharge for patients receiving ultramassive transfusion.
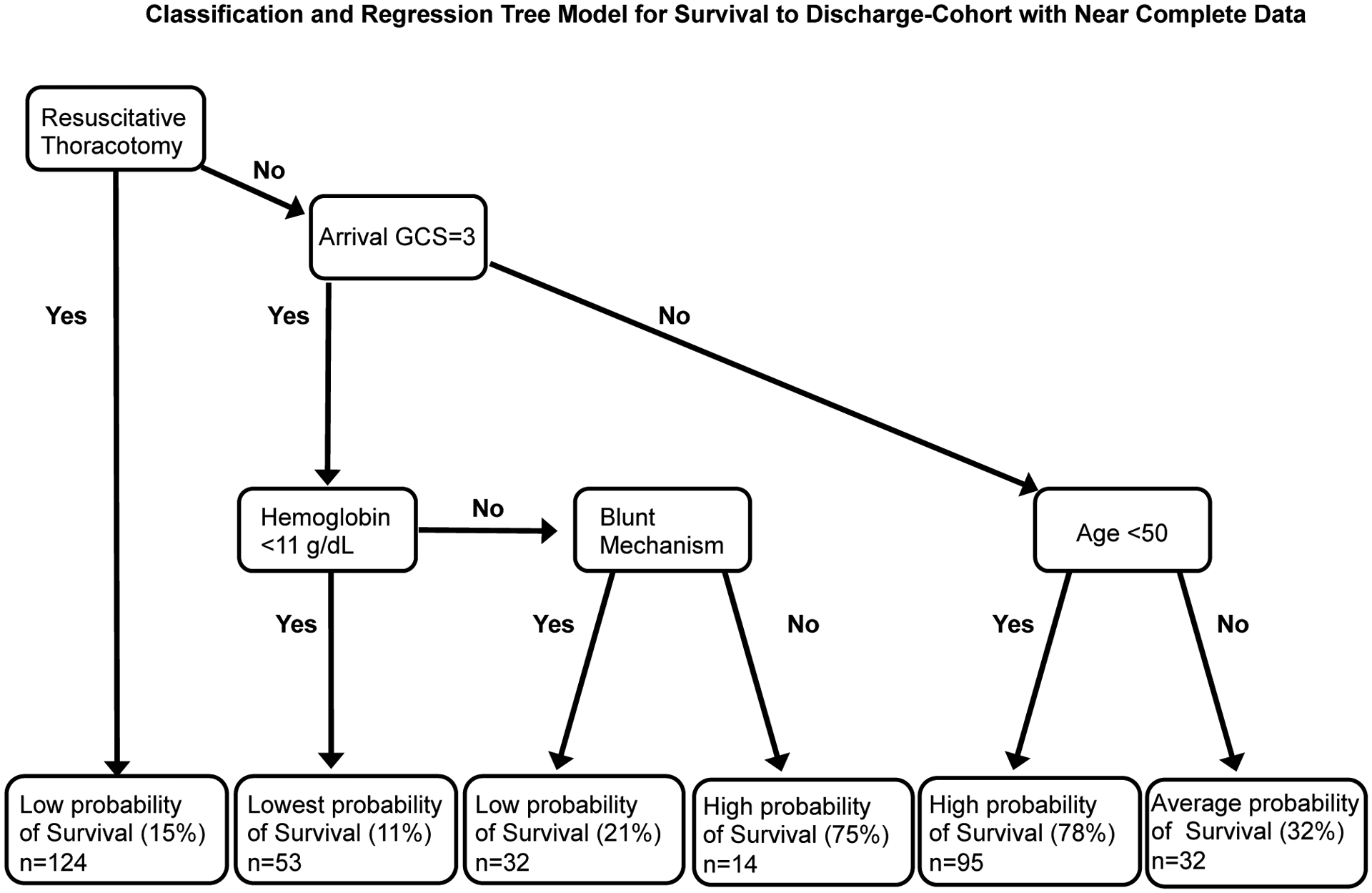
Gini coefficient used to determine splits with 10 fold cost complexity pruning to develop the trimmed tree. Data divided into a training (90%) and a test set (10%) for validation. Area under the receiver operator characteristic curve=77% mean fraction correct in 100 iterations of test set = 76%. GCS= Glasgow coma scale. Includes subset of patients (n=353) with near complete data (<10%) for admission physiologic and injury characteristics.
Discussion
In this study, we investigated predictors of mortality in a modern cohort of injured patients receiving an ultramassive transfusion, defined as ≥20u RBCs during the initial 24 hours of their resuscitation. Mortality rate at discharge was high at 65%, and similar to mortality rates of 60–70% in the only three published prior studies of ultramassive transfusion spanning the 1990s to early 2010s (10–12). This lack of improved mortality despite advances in trauma and critical care may be attributable to several factors. Patients in this study had higher injury severity scores (ISS of 34 versus 29 in studies by Yu et al (12) and Velmahos et al (11)) and lower median GCS on arrival (7 versus 12). Furthermore, unbalanced ratios in large proportion of patients may have also contributed to the persistently high mortality rate, though it is not known what transfusion ratios were achieved in older studies of ultramassive transfusion.
The benefit of damage control resuscitation practices with initial empiric balanced transfusion ratios is well-established for traumatic hemorrhage (6, 7, 9), but to our knowledge this the first study to assess how transfusion ratios may impact mortality in the unique subset of trauma patients receiving ultramassive transfusion. We demonstrate that unbalanced transfusion ratios are associated with more than two-fold increased mortality compared to balanced transfusion ratios. This finding was stronger for RBC:PLT ratios than for RBC:FFP ratios, consistent with prior observational studies of the effects of transfusion ratios in trauma patients (9, 18), and patients with both ratios ≥1.5 had the highest associated increased mortality. These relationships persisted even when controlling for age, injury mechanism and severity, degree of shock, and resuscitative thoracotomy. Despite all participating sites having massive transfusion protocols with balanced blood product allocation algorithms in place prior to the start of our study period, 52% of patients still received either RBC:PLT or RBC:FFP ≥1.5:1, though the transfusion ratios are significantly improved compared to historical observational studies of massive transfusion in trauma (9, 18). Unbalanced transfusion ratios during UMT events may be due to lack of availability of specific blood products as local blood bank resources are depleted, due to rapid rates of hemorrhage and hemodynamic compromise hindering providers’ ability to track and adjust transfusion ratios in real time, due to transition to goal-directed resuscitation, due to death prior to the balancing of ratios, or due to a combination of these factors. Increasing the number of immediately available blood products has been shown to improve transfusion ratios for massively and ultramassively transfused patients, and may represent one important area for improving the implementation of balanced resuscitation during UMT (19).
There was a strong association in univariate analysis between cryoprecipitate use and mortality, but this was mostly explained by differences in rates of cryoprecipitate administration based on survival beyond 6 hours. This likely reflects a survival bias rather than a treatment effect because cryoprecipitate tends to be administered at significantly later timepoints in our center’s MTP protocols, precluding patients who die early from receiving it. Cryoprecipitate use has been shown to be beneficial in severe trauma (20, 21), although further studies are needed to determine if early “off-label” empiric administration in the absence of documented hypofibrinogenemia is feasible and effective in massively bleeding trauma patients (22). The majority of patients also received TXA in our study, with modest and non-significant trends for improved 24 hour mortality, although our sample size was not powered to detect the known small effect sizes observed in large studies of TXA in trauma patients (23, 24).
We identified several factors on CART analysis that were predictive of mortality with the initial and most important split on admission GCS of 3, followed by age ≥50, resuscitative thoracotomy, and the presence of thrombocytopenia. Although none of the resultant combinations of risk could definitively predict mortality or survival, patients with none of these risk factors had good survival (71%) irrespective of other baseline physiologic characteristics, while patients with one or more of these predictors experienced poor to below average survival (14–26%). In the subset of patients with more complete data on admission physiologic characteristics, additional factors identified included an initial hemoglobin of less than 11g/dL and blunt mechanisms of injury as predictors of mortality in patients not undergoing resuscitative thoracotomy, but with arrival GCS of 3. While these models should not replace clinical judgement for any given patient, they are of prognostic value and may assist decision making, particularly in the setting of constrained resources and blood bank shortages (such as during the current COVID-19 pandemic) (25).
Notably, total volume of blood transfused was a weak predictor of mortality in CART models and it was not a significant predictor in our multivariable regression models. This finding is consistent with prior studies of UMT- for example, Yu et al found that total RBCs over 35u had an AUC of just 0.54 for predicting mortality (12). In our study, 15 patients received over 75u RBCs, and 4 survived (27%). This further highlights that patient demographic and clinical characteristics such as age, GCS, hemoglobin and platelet counts, and whether a resuscitative thoracotomy is performed should be considered for prognostication purposes, rather than total units transfused specifically for patients who have already received 20u RBCs.
Limitations
We recognize some important limitations of this study, including its retrospective nature and that our cohort includes patients from a broad array of trauma centers, which may serve different patient populations. We did account for inter-site variation in our analysis, and the multicenter nature of this study may increase external validity and applicability to a wider population of civilian trauma patients receiving UMT. It is also important to note that all patients in the study had to survive long enough to receive at least 20u RBCs and therefore our findings may not apply to patients with catastrophic traumatic hemorrhage who exsanguinate and die prior to being transfused 20u RBCs. The observed mortality differences in association with transfusion ratios could be due to other factors rather than due to transfusion ratios themselves. Patients receiving balanced ratios may also have been more likely to have been transfused at times when more resources and personnel were available to care for them, and in which the trauma team was more adequately able to adhere to other aspects of their MTP, which may have also driven the improved outcomes for these patients. Further, it is possible that survival bias plays some role in the association of transfusion ratios with mortality in this study, however this is partially mitigated because all patients had to survive long enough to receive 20u RBCs. Additionally, sensitivity analyses excluding very early (within 3 hours) and early (within 6 hours) deaths did not suggest the presence of a large degree of survival bias in our multivariable models of transfusion ratios and mortality. The use of viscoelastic testing to guide resuscitation could also impact our results, though we did not find an association between patients who had viscoelastic testing performed and mortality. Use of viscoelastic testing did not modify the relationship between transfusion ratios and mortality when tested in our logistic regression models, but we did not have sufficient data on specific viscoelastic testing results to perform a more detailed analysis. Lastly, several patients had significant missing data for at least some variables such as temperature and classical coagulation assays, which may have limited model discrimination in CART. Similarly, physiologic and laboratory data was not sufficiently available at serial timepoints to further enhance this analysis and investigate how transfusion ratios might impact these parameters over time.
Conclusions
In conclusion, while mortality remains high for patients receiving ultramassive transfusion, we demonstrate strong independent associations of balanced transfusion ratios with improved mortality in this large multicenter study, which is particularly important given the high rate of unbalanced transfusion ratios in this modern cohort of patients. While prospective, randomized studies would be needed to definitively establish causality in these relationships, this may not be practical given the rare occurrence of UMT and challenges in implementing such trials in these patients. Lastly, while no single variable or set of physiologic findings can perfectly predict survival, we identified a limited set of readily available patient characteristics that can aid in prognostication in the setting of resource limitations and when difficult decisions regarding blood allocation need to be considered.
Supplementary Material
Acknowledgements:
The Eastern Association for the Surgery of Trauma, Rachel Dixon, and Michael Matthews.
Disclosure information: Dr. L Kornblith is supported by NIH 1K23GM130892-01
Footnotes
This work was presented virtually at the 34th EAST Scientific Assembly on January 13th, 2021.
Conflicts of interest statement: the authors declare no conflicts of interest
EAST Multicenter Study Group on Ultramassive Transfusion: Erin E. Ross BS1, John J. Park BS1, Brittany Robinson BA, Mary Kathryn Abel AB1, Alexander T. Fields PhD1, Jonathan H. Esensten MD PhD1, Ashok Nambiar MD1, Joanne Moore 1, Claire Hardman5, Pranaya Terse6, Xian Luo-Owen PhD7, Anquonette Stiles DC MPH10, Brenden Pearce BS10, Kimberly Tann BS10, Khaled Abdul Jawad MD19, Gabriel Ruiz MD19.
References
- 1.Callcut RAM, Kornblith LZM, Conroy ASB, Robles AJM, Meizoso JPM, Namias NM, Meyer DEM, Haymaker AB, Truitt MSM, Agrawal VP, et al. The why and how our trauma patients die: A prospective Multicenter Western Trauma Association study. J Trauma Acute Care Surg. 2019;86(5):864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dente CJ, Shaz BH, Nicholas JM, Harris RS, Wyrzykowski AD, Patel S, Shah A, Vercruysse GA, Feliciano DV, Rozycki GS, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma. 2009;66(6):1616–24. [DOI] [PubMed] [Google Scholar]
- 3.Callcut RA, Johannigman JA, Kadon KS, Hanseman DJ, Robinson BR. All massive transfusion criteria are not created equal: defining the predictive value of individual transfusion triggers to better determine who benefits from blood. J Trauma. 2011;70(4):794–801. [DOI] [PubMed] [Google Scholar]
- 4.Callcut RA, Cotton BA, Muskat P, Fox EE, Wade CE, Holcomb JB, Schreiber MA, Rahbar MH, Cohen MJ, Knudson MM, et al. Defining when to initiate massive transfusion: a validation study of individual massive transfusion triggers in PROMMTT patients. J Trauma Acute Care Surg. 2013;74(1):59–65, 7–8; discussion 6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bawazeer M, Ahmed N, Izadi H, McFarlan A, Nathens A, Pavenski K. Compliance with a massive transfusion protocol (MTP) impacts patient outcome. Injury. 2015;46(1):21–8. [DOI] [PubMed] [Google Scholar]
- 6.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesar T, Larentzakis A, Dzik W, Chang Y, Velmahos G, Yeh DD. Association Between Ratio of Fresh Frozen Plasma to Red Blood Cells During Massive Transfusion and Survival Among Patients Without Traumatic Injury. JAMA Surg. 2017;152(6):574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holcomb JB, Zarzabal LA, Michalek JE, Kozar RA, Spinella PC, Perkins JG, Matijevic N, Dong JF, Pati S, Wade CE, et al. Increased platelet:RBC ratios are associated with improved survival after massive transfusion. J Trauma. 2011;71(2 Suppl 3):S318–28. [DOI] [PubMed] [Google Scholar]
- 10.Dzik WS, Ziman A, Cohn C, Pai M, Lozano M, Kaufman RM, Delaney M, Selleng K, Murphy MF, Hervig T, et al. Survival after ultramassive transfusion: a review of 1360 cases. Transfusion. 2016;56(3):558–63. [DOI] [PubMed] [Google Scholar]
- 11.Velmahos GC, Chan L, Chan M, Tatevossian R, Cornwell EE, 3rd, Asensio JA, Berne TV, Demetriades D. Is there a limit to massive blood transfusion after severe trauma? Arch Surg. 1998;133(9):947–52. [DOI] [PubMed] [Google Scholar]
- 12.Yu AJ, Inaba K, Biswas S, de Leon LA, Wong M, Benjamin E, Lam L, Demetriades D. Supermassive Transfusion: A 15-Year Single Center Experience and Outcomes. Am Surg. 2018;84(10):1617–21. [PubMed] [Google Scholar]
- 13.Ngo A, Masel D, Cahill C, Blumberg N, Refaai MA. Blood Banking and Transfusion Medicine Challenges During the COVID-19 Pandemic. Clin Lab Med. 2020;40(4):587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feaster DJ, Mikulich-Gilbertson S, Brincks AM. Modeling site effects in the design and analysis of multi-site trials. Am J Drug Alcohol Abuse. 2011;37(5):383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therneau T, Atkinson B, Ripley B. Recursive Partitioning and Regression Trees 2019. Available from: https://CRAN.R-project.org/package=rpart.
- 16.Servia L, Montserrat N, Badia M, Llompart-Pou JA, Barea-Mendoza JA, Chico-Fernandez M, Sanchez-Casado M, Jimenez JM, Mayor DM, Trujillano J. Machine learning techniques for mortality prediction in critical traumatic patients: anatomic and physiologic variables from the RETRAUCI study. BMC Med Res Methodol. 2020;20(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu CH, Haac BE, Drake M, Bernard AC, Aiolfi A, Inaba K, Hinson HE, Agarwal C, Galante J, Tibbits EM, et al. EAST Multicenter Trial on targeted temperature management for hanging-induced cardiac arrest. J Trauma Acute Care Surg. 2018;85(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–58. [DOI] [PubMed] [Google Scholar]
- 19.Allen CJ, Shariatmadar S, Meizoso JP, Hanna MM, Mora JL, Ray JJ, Namias N, Dudaryk R, Proctor KG. Liquid plasma use during “super” massive transfusion protocol. J Surg Res. 2015;199(2):622–8. [DOI] [PubMed] [Google Scholar]
- 20.Sugiyama K, Fujita H, Nishimura S. Effects of in-house cryoprecipitate on transfusion usage and mortality in patients with multiple trauma with severe traumatic brain injury: a retrospective cohort study. Blood Transfus. 2020;18(1):6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inokuchi K, Sawano M, Yamamoto K, Yamaguchi A, Sugiyama S. Early administration of fibrinogen concentrates improves the short-term outcomes of severe pelvic fracture patients. Acute Med Surg. 2017;4(3):271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curry N, Foley C, Wong H, Mora A, Curnow E, Zarankaite A, Hodge R, Hopkins V, Deary A, Ray J, et al. Early fibrinogen concentrate therapy for major haemorrhage in trauma (E-FIT 1): results from a UK multi-centre, randomised, double blind, placebo-controlled pilot trial. Crit Care. 2018;22(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CRASH-2 trial collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H, Gogichaishvili T, Gupta S, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32. [DOI] [PubMed] [Google Scholar]
- 24.Ramirez RJ, Spinella PC, Bochicchio GV. Tranexamic Acid Update in Trauma. Crit Care Clin. 2017;33(1):85–99. [DOI] [PubMed] [Google Scholar]
- 25.Stanworth SJ, New HV, Apelseth TO, Brunskill S, Cardigan R, Doree C, Germain M, Goldman M, Massey E, Prati D, et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol . 2020;7(10):e756–e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


