Abstract
Background
Arterial tortuosity is linked to a higher risk of adverse clinical events after transfemoral transcatheter aortic valve replacement (TF‐TAVR). Currently, there are no assessment tools that can quantify this variable in three‐dimensional space. This study investigated the impact of novel scoring methods of iliofemoral tortuosity on access and bleeding complications after TF‐TAVR.
Methods
The main access vessel was assessed between the aortoiliacal and femoral bifurcation in preoperative multislice computed tomography scans of 240 consecutive patients undergoing TF‐TAVR. Tortuosity was assessed by three methods: largest single angle, sum of all angles, and iliofemoral tortuosity (IFT) score [((true vessel length/ideal vessel length)‐1)*100]. The primary study endpoint was a composite of access and bleeding complications. The secondary study endpoints were 30‐day mortality and long‐term survival.
Results
Among 240 patients, only the IFT score demonstrated a good positive correlation with the composite primary endpoint of access and bleeding complications (P = 0.031). A higher incidence of access and bleeding complications was found in patients with a higher IFT score (56 [36.8%] vs 17 [19.3%]; P = 0.003). In a multivariate logistic regression analysis, only the IFT score was a significant predictor of the primary endpoint (OR: 2.11; 95% CI: 1.09‐4.05; P = 0.026).
Conclusion
Vascular tortuosity is an underestimated risk factor during TF‐TAVR. The IFT score is a valuable tool in risk stratification before TF‐TAVR, predicting periprocedural access and bleeding complications.
Keywords: access, bleeding, TAVI, TAVR, tortuosity, transcatheter
1. INTRODUCTION
Vessel tortuosity is an underexplored area that may be central to reducing vascular complications in transcatheter aortic valve replacement (TAVR). While tortuous arteries are virtually absent in children, the prevalence of vascular tortuosity triples in an elderly cohort (>60 years), quadruples in elderly patients with hypertension, and is linked to an elevated risk for cardiovascular adverse events. 1 , 2 , 3 As the average age for transcatheter aortic valve replacements (TAVR) procedures reported in studies is approximately 80, and TAVR are predominantly performed via a transfemoral approach, procedure‐related complications need to be incorporated in preprocedural risk assessment and special attention should be paid to preventive measures. 4 , 5 , 6 Moreover, there is evidence that increased vascular tortuosity is present in about one third of patients with increased surgical risk, whereas the prevalence of tortuosity is only 10% in patients with lower surgical risk (logistic EUROSCORE < 15). 7 Tortuous arteries and their associated risks are thus more likely manifest in a high‐risk elderly cohort mirroring the one eligible for TAVR and represent a crucial variable to stratify against to improve procedural outcomes.
Despite the correlation between higher tortuosity of the iliofemoral vessels and a higher risk for access complications during transfemoral‐TAVR (TF‐TAVR) seeming implicit, no three‐dimensional assessment method of vascular tortuosity is currently available for preprocedural risk stratification. 8 Qualifying the degree of tortuosity based on the largest angle present within the arteries used for access in TF‐TAVR has had limited success at both predicting or delineating patients who subsequently suffer from major vascular complications. 9 , 10 , 11 , 12 An alternative approach to expressing tortuosity has been to add up the different angulations at various points in a vessel and express this as a ratio relative to the access vessel diameter. While this method has shown promise as an indicator for patients more likely to suffer from major vascular complications, the data fuelling these initial findings were primarily based on single plane angiography, rather than more accurate computed tomography scans. 8
The purpose of this trial was therefore to develop a measurement tool for vascular tortuosity from the preprocedural computed tomography data of patients evaluated for TAVR, and to examine its predictive value for procedure‐related access and bleeding complications.
2. MATERIALS AND METHODS
2.1. Study design, patients and intervention
The present analysis retrospectively investigated 266 consecutive patients from the VIenna CardioThOracic Aortic Valve RegistrY (VICTORY‐Registry) who underwent a TF‐TAVR between June 2009 and January 2017 at the Heart Center Hietzing in Vienna (Austria). The preprocedural assessment, as well as the intervention, were performed in a standard fashion by the institution's heart team and have been described in detail before. 13 Patients were considered for alternative access primarily when the heart team reached a consensus (rather than using a threshold value) that severe calcification or a small iliofemoral lumen diameter would not favour TF‐TAVR, yet no patient was referred for alternative access and excluded from TF‐TAVR based on high iliofemoral tortuosity alone. Patients were transferred to an intermediate care station after the procedure, and were followed up with an echocardiogram and an ultrasound scan of their access route on the first day after the TAVR. In patients where these investigations were clinically of no concern, they were discharged where possible.
Different generations of transcatheter valves developed by Medtronic (Medtronic, Minneapolis, Minnesota, USA), Edwards Lifesciences (Edwards Lifesciences, Irvine, California, USA), Symetis (Symetis SA, a Boston Scientific company, Ecublens, Switzerland) and JenaValve (JenaValve Technology GmbH, Munich, Germany) were used. The valve size was chosen based on preprocedural multislice computed tomography measurements. Vascular closure was primarily performed using Prostar XL during the initial study period, and in later years predominantly with two ProGlide devices (Abbott Vascular, Santa Clara, CA, USA).
Twenty‐six patients had to be excluded from the analysis as the preprocedural multislice computed tomography (MS‐CT) image acquisition, and the corresponding measurements were performed in an extramural setting.
Following approval of the study by the Ethics Committee of the City of Vienna (EK18‐028‐VK), a retrospective analysis of the patients’ iliofemoral access vessels was carried out. Informed consent was waived. Long‐term mortality data were obtained by inquiry to the Federal Institute for Statistics Austria.
The analytic methods, materials and data that support the findings of this study are available from the corresponding author on reasonable request.
2.2. Imaging protocol
Preprocedural electrocardiogram‐gated and contrast‐enhanced MS‐CT scans were performed following an established institutional protocol with a 2x128‐slice Somatom Drive Dual Source CT scanner (Siemens Healthcare, Forchheim, Germany). Two experienced readers independently evaluated all datasets for quantitative measurements using the 3mensio Valves™ software (Version 7.2; 3mensio Medical Imaging BV; Maastricht; Netherlands). The iliofemoral assessment protocol consisted of 3 main steps:
-
1
Determination of two reference points at each side along the iliofemoral centreline. The first reference point is set at the level of the aortoiliac bifurcation as soon as the vessel could be defined as either the left or right common iliac artery. The second reference point was set on the centreline of the common femoral artery, at the last frame before the femoral bifurcation (Figure 1).
-
2
Measurement of the length of the curved vascular centreline (true vessel length) as well as the direct distance between the two reference points (ideal vessel length) at each side. These measurements are exemplified by the purple and white line traces in Figure 1, whose initiation and termination points for measurement were the red markers at each bifurcation. Based on these two values, the iliofemoral tortuosity (IFT) score was calculated using the following formula:
FIGURE 1.
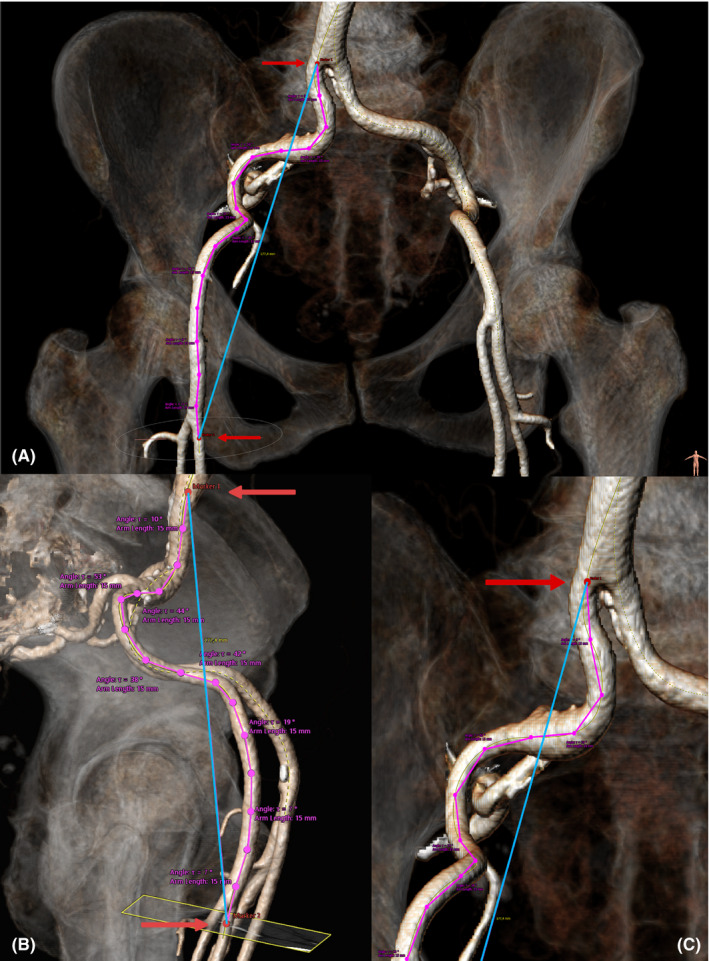
Reconstructed three‐dimensional model of the iliofemoral vasculature based on multislice computed tomography images, viewed from the frontal (A and C) and sagittal plane (B). Superimposed markers show the vessel bifurcation points (red markers), ideal vessel length (blue line trace), true vessel length (purple line trace) and angles of the vessel at each 15‐mm interval (three‐point segments along the purple line trace) used to characterize vascular tortuosity
-
3
The software automatically measures the angle between two points on the centreline within the three‐dimensional plane at 15‐mm intervals, for the entire predefined distance. From these measurements, the largest single angle (LSA), as well as the sum of all measured angles (SAA), was documented.
The average value of the two measurements for the IFT score, as well as the largest single angle and the sum of all angles, was used for analysis. Examining the ratio between the true vessel length and ideal vessel length for the IFT score enabled comparison between individuals, despite anatomical differences in the second reference point (femoral artery bifurcation) among patients.
Skin‐to‐femoral‐artery‐distance was measured in sagittal view at the mid‐level of the femoral head at a 45° angulation. Furthermore, the ratio between the sheath size and the minimal iliofemoral lumen diameter and area were calculated.
The iliofemoral calcification burden was measured using the same lesion definition as for the aortic valve in non‐contrast enhanced CT images. A CT threshold of 130 Hounsfield units and 4 pixels was used for the identification of a calcified lesion. Analogous to aortic valve measurements, the total calcification burden was assessed by summation of all calcified lesions within the predefined segment.
The clinical outcome and the occurrence of peri‐ and postprocedural complications were classified according to the updated Valve Academic Research Consortium (VARC)‐II criteria. 14 The primary study endpoint was defined as a composite of the occurrence of: any life‐threatening, major or minor bleeding complication, or any major or minor vascular access complication (Table 1). The secondary endpoints were mortality at 30‐days and overall long‐term survival.
TABLE 1.
Criteria for bleeding and vascular and access‐related complications based on the VARC‐2 guidelines (adapted from Kappetein et al 14 )
| Type of complication | Classification | Criteria |
|---|---|---|
| Bleeding | Life‐threatening or disabling bleeding |
Fatal bleeding (BARC type 5) OR Bleeding in a critical organ (BARC type 3b and 3c) OR Bleeding causing hypovolaemic shock or severe hypotension requiring vasopressors or surgery (BARC type 3b) OR Overt source of bleeding with drop in haemoglobin ≥ 5 g/dL or whole blood or packed red blood cells (RBCs) transfusion ≥ 4 unitsa (BARC type 3b) |
| Major |
Overt bleeding either with haemoglobin drop of > 3.0 g/dl or requiring transfusion of two or three units of whole blood/RBC, or causing hospitalization or permanent injury, or requiring surgery AND Does not meet criteria of life‐threatening or disabling bleeding |
|
| Minor | Any bleeding worthy of clinical mention (eg access site haematoma) that does not qualify as life‐threatening, disabling, or major | |
| Vascular and access‐related | Major |
Any aortic dissection, aortic rupture, annulus rupture, left ventricle perforation, or new apical aneurysm/pseudoaneurysm OR Access site or access‐related vascular injury leading to death, life‐threatening or major bleeding, visceral ischaemia, or neurological impairment OR Distal embolization (non‐cerebral) from a vascular source requiring surgery or resulting in amputation or irreversible end‐organ damage OR The use of unplanned endovascular or surgical intervention associated with death, major bleeding, visceral ischaemia or neurological impairment OR Any new ipsilateral lower extremity ischaemia documented by patient symptoms, physical exam, and/or decreased or absent blood flow on lower extremity angiogram OR Surgery for access site‐related nerve injury OR Permanent access site‐related nerve injury |
| Minor |
Access site or access‐related vascular injury not leading to death, life‐threatening or major bleeding, visceral ischaemia, or neurological impairment OR Distal embolization treated with embolectomy and/or thrombectomy and not resulting in amputation or irreversible end‐organ damage OR Any unplanned endovascular stenting or unplanned surgical intervention not meeting the criteria for a major vascular complication OR Vascular repair or the need for vascular repair |
Abbreviations: BARC, bleeding academic research consortium; RBC, red blood cell units.
2.3. Statistical analysis
Based on their distribution, continuous variables were expressed either as a median and interquartile range (IQR) or as mean and standard deviation (±SD). For further comparison, Student's t test or the Mann‐Whitney U test was used, respectively. Categorical variables were compared with a chi‐square test or Fisher's exact test and expressed as absolute numbers and percentages.
Receiver‐operating characteristic curves predicting the main study endpoint were constructed for the largest single angle, the sum of all angles and the IFT score. The discriminatory ability was assessed via the area under curve (AUC). Threshold values were calculated with the Youden Index. Only the IFT score (cut‐off > 21.2; sensitivity 80.8%, 1‐specificity 68.9%) provided significant differentiating ability (AUC of 0.59; 95% CI 0.51‐0.67, P = 0.031; Table 2). Consequently, patients were diagnosed with increased vascular tortuosity when their IFT score exceeded the threshold of 21.2. Risk factors for the composite primary endpoint were assessed using a univariate and multivariate logistic regression model to estimate the odds ratios and their associated 95% confidence intervals (CIs). Only factors found to have p‐values of less than 0.1 in the univariate model were included in the multivariate analysis and assessed in a stepwise fashion.
TABLE 2.
Receiver‐operating analysis for composite access and bleeding complications
| AUC | 95% CI | P value | |
|---|---|---|---|
| Largest single angle | 0.549 | 0.510‐0.666 | 0.224 |
| Sum of all angles | 0.501 | 0.423‐0.578 | 0.990 |
| Iliofemoral tortuosity score | 0.588 |
0.469‐0.629 |
0.031 |
Abbreviations: AUC, area under curve; CI, confidence interval.
To examine the association between vascular tortuosity and overall long‐term mortality, a Cox proportional hazards model was used to estimate hazard ratios and 95% CIs. Person‐time was calculated from the date of the replacement to either death or the last available follow‐up. The hazard ratio was stratified by the IFT score and adjusted for baseline characteristics, including age, sex, insulin‐dependent diabetes, hypertension, renal impairment, chronic obstructive pulmonary disease and congestive heart failure in a stepwise fashion.
All reported p‐values are two‐sided, and results were categorized as statistically significant with an alpha level set at < 0.05; the analyses were performed using spss, version 24.0 (IBM Corp, Armonk, NY, USA).
3. RESULTS
3.1. Demographics
Baseline characteristics, including the patients' risk profile, are summarized in Table 3. With respect to the demographic and clinical baseline characteristics, patients with high iliofemoral tortuosity defined by an IFT score above 21.2, were significantly older (High IFT: 84.0 ± 7.0 vs Low IFT: 81.5 ± 10.0 years; P < 0.001). This finding reflects the well‐known increase of vascular tortuosity with increasing age. 2 Furthermore, patients with a higher IFT score also presented with a higher mean pressure across the aortic valve (47.0 ± 20.5 mm Hg vs 42.0 ± 18.0 mm Hg; P = 0.044) and with a lower systolic pulmonary pressure (38.0 ± 49.5 mm Hg vs 44.0 ± 24.5 mm Hg; P = 0.031). No other significant difference in major risk factors or comorbidities evaluated prior to the procedure was detected.
TABLE 3.
Baseline clinical characteristics
|
Overall cohort n = 240 |
Low tortuosity n = 88 |
High tortuosity n = 152 |
P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (±IQR) | 83.0 (7.0) | 81.5 (10.0) | 84.0 (7.0) | <0.001 |
| Female gender, n (%) | 152 (63.3) | 59 (67.0) | 93 (61.2) | 0.221 |
| Body mass index kg/m2, median (±IQR) | 25.8 (6.3) | 26.6 (7.1) | 25.3 (5.6) | 0.095 |
| Risk profile | ||||
| EuroSCORE II, median (±IQR) | 4.4 (5.4) | 4.7 (7.1) | 4.4 (4.2) | 0.548 |
| Logistic EuroSCORE, median (±IQR) | 15.9 (15.0) | 18.4 (17.9) | 14.6 (12.9) | 0.109 |
| STS score, median (±IQR) | 4.5 (3.5) | 4.5 (3.5) | 4.5 (3.4) | 0.809 |
| Incremental risk score, median (±IQR) | 8.0 (15.0) | 8.1 (16.1) | 6.2 (12.1) | 0.583 |
| HAS‐BLED score, median (±IQR) | 2.0 (1.0) | 2.0 (0.0) | 2.0 (1.0) | 0.243 |
| Chronic health conditions and risk factors | ||||
| Dyslipidaemia, n (%) | 126 (52.5) | 52 (59.1) | 74 (48.7) | 0.077 |
| Diabetes mellitus (IDDM), n (%) | 40 (16.7) | 14 (15.9) | 26 (17.1) | 0.480 |
| Hypertension, n (%) | 210 (87.5) | 80 (90.9) | 130 (85.5) | 0.156 |
| COPD, n (%) | 17 (7.1) | 9 (10.2) | 8 (5.3) | 0.115 |
| Peripheral vascular disease, n (%) | 24 (10.0) | 11 (12.5) | 13 (8.6) | 0.223 |
| Renal impairment eGFR < 60 mL/min/1.73 m2, n (%) | 129 (53.8) | 50 (56.8) | 79 (52.0) | 0.409 |
| Cerebrovascular accident, n (%) | 36 (15.0) | 12 (13.6) | 24 (15.8) | 0.415 |
| Atrial fibrillation, n (%) | 70 (29.2) | 20 (22.7) | 50 (32.9) | 0.061 |
| Home oxygen dependence, n (%) | 5 (2.1) | 2 (2.3) | 3 (2.0) | 0.605 |
| Obstructive sleep apnoea, n (%) | 5 (2.1) | 3 (3.4) | 2 (1.3) | 0.260 |
| NYHA class III/IV, n (%) | 212 (88.3) | 80 (90.9) | 132 (86.8) | 0.233 |
| Creatinine mg/dL, median (IQR) | 1.1 (0.6) | 1.1 (0.6) | 1.0 (0.6) | 0.537 |
| Coronary vascular disease, n (%) | 28 (11.7) | 11 (12.5) | 17 (11.2) | 0.456 |
| Prior myocardial infarction, n (%) | 29 (12.1) | 12 (13.6) | 17 (11.2) | 0.357 |
| Coronary artery disease, n (%) | 115 (47.9) | 44 (50.0) | 71 (46.7) | 0.360 |
| Previous PCI, n (%) | 72 (30.0) | 27 (30.7) | 45 (29.6) | 0.486 |
| Previous pacemaker implantation, n (%) | 48 (20.0) | 19 (21.6) | 29 (19.1) | 0.379 |
| Previous CABG, n (%) | 32 (13.3) | 13 (14.8) | 19 (12.5) | 0.377 |
| Previous valve surgery, n (%) | 22 (9.2) | 8 (9.1) | 14 (9.2) | 0.586 |
| Preoperative echocardiographic data | ||||
| Aortic valve area, median (±IQR) | 0.7 (0.3) | 0.7 (0.3) | 0.7 (0.2) | 0.927 |
| Indexed aortic valve area, median (±IQR) | 0.4 (0.1) | 0.4 (0.1) | 0.4 (0.1) | 0.891 |
| Mean pressure gradient, median (±IQR) | 45.0 (20.0) | 42.0 (18.0) | 47.0 (20.5) | 0.044 |
| Max. pressure gradient, median (±IQR) | 70.0 (27.0) | 67.0 (28.0) | 71.0 (26.0) | 0.070 |
| Peak velocity m/sec, median (±IQR) | 4.2 (0.9) | 4.2 (0.9) | 4.3 (0.8) | 0.192 |
| sPAP, median (±IQR) | 40.0 (25.0) | 44.0 (24.5) | 38.0 (49.5) | 0.031 |
| LVEF %, median (±IQR) | 60.0 (15.0) | 60.0 (20.0) | 60.0 (12.0) | 0.138 |
| LFLG‐aortic stenosis, n (%) | 47 (19.6) | 19 (21.6) | 28 (18.4) | 0.332 |
| Computed tomography measurements of iliofemoral‐access segment | ||||
| Femoral artery diameter in mm, median (±IQR) | 6.9 (1.5) | 6.7 (1.7) | 7.0 (1.4) | 0.131 |
| Femoral artery area in mm2, median (±IQR) | 34.0 (17.0) | 35.0 (17.5) | 34.0 (18.0) | 0.447 |
| External iliac artery diam. in mm, median (±IQR) | 8.5 (1.5) | 8.3 (1.9) | 8.5 (1.1) | 0.295 |
| Common iliac artery diam. in mm, median (±IQR) | 10.1 (2.4) | 9.9 (2.6) | 10.2 (2.2) | 0.221 |
| Skin‐to‐FMA distance at 45° in mm, median (±IQR) | 46.6 (20.4) | 44.2 (18.7) | 46.9 (20.8) | 0.146 |
| Sheath‐to‐MLD‐ratio, median (±IQR) | 0.99 (0.3) | 0.99 (0.29) | 0.97 (0.3) | 0.874 |
| Calcification load mm3, median (±IQR) | 1245 (2548) | 1084 (2513) | 1230 (2574) | 0.533 |
| Maximum perpendicular calcification | 0.428 | |||
| 1 Quadrant affected, n (%) | 56 (23.3) | 16 (18.2) | 40 (26.3) | |
| 2 Quadrants affected, n (%) (semicircular), n (%) | 51 (21.3) | 21 (23.7) | 30 (19.7) | |
| 3 Quadrants affected, n (%) | 21 (8.8) | 9 (10.2) | 12 (7.9) | |
| Circular calcification, n (%) | 89 (37.0) | 36 (40.9) | 53 (34.9) | |
Data presented as median (Interquartile range) or number of patients (percent).
Abbreviations: CABG, Coronary Artery Bypass Graft; COPD, chronic obstructive pulmonary disease; diam, diameter; eGFR, estimated glomerular filtration rate; EuroSCORE, European System for Cardiac Operative Risk Evaluation; FMA, femoral artery; HAS‐BLED, Hypertension, Abnormal renal or liver function, Elderly, Stroke, prior major Bleeding or predisposition, Labile INR, Drugs; IDDM, Insulin‐dependent diabetes mellitus; LFLG, low flow low gradient; LVEF, left ventricular ejection fraction; Max., maximum; MLD, minimal lumen diameter; NYHA, New York Heart Association; other abbreviations as in Table 1; PCI, percutaneous coronary intervention; sPAP, systolic Pulmonary Artery Pressure; STS, Society of Thoracic Surgeons Predictive Risk of Mortality.
3.2. Periprocedural data
Similarly, no differences between the two groups could be found regarding the procedural data displayed in Table 4. Neither the vascular closure device employed nor the treatment period tertials were significantly different between the two cohorts (P = 0.441; P = 0.866).
TABLE 4.
Procedural clinical characteristics
|
Overall cohort n = 240 |
Low tortuosity n = 88 |
High tortuosity n = 152 |
p Value | |
|---|---|---|---|---|
| Procedural variables | ||||
| Treatment period tertials, n (%) | 0.866 | |||
| 2009‐2011 | 39 (16.3) | 13 (14.8) | 26 (17.1) | |
| 2012‐2014 | 80 (33.3) | 29 (33.0) | 51 (33.6) | |
| 2015‐2017 | 121 (50.4) | 46 (52.3) | 75 (49.3) | |
| Procedure time (min), median (±IQR) | 85.0 (33.0) | 85.0 (34.0) | 88.5 (35.0) | 0.935 |
| Balloon expanding valve used, n (%) | 69 (28.8) | 26 (29.5) | 43 (28.3) | 0.474 |
| Prosthesis size (mm), median (±IQR) | 29.0 (3.0) | 29.0 (3.0) | 29.0 (3.0) | 0.732 |
| Predilatation necessary, n (%) | 198 (82.5) | 71 (80.7) | 127 (83.6) | 0.346 |
| Delivery system/sheath diameter, median (±IQR) | 16.3 (1.2) | 16.2 (1.1) | 16.3 (1.2) | 0.576 |
| Postdilatation necessary, n (%) | 42 (17.5) | 13 (14.8) | 29 (19.1) | 0.254 |
| Paravalvular leak more than trace, n (%) | 28 (11.7) | 9 (10.2) | 19 (12.5) | 0.391 |
| Closure device strategy, n (%) | 0.441 | |||
| Prostar | 116 (48.3) | 43 (48.9) | 73 (48.0) | |
| Dual Proglide closure | 124 (51.7) | 45 (51.1) | 79 (52.0) | |
| Total hours in ICU (hours), median (±IQR) | 72.0 (48.0) | 48.0 (80.0) | 72.0 (48.0) | 0.755 |
| Max. creatinine within 72h mg/dL, median (±IQR) | 0.95 (0.56) | 0.89 (0.43) | 0.97 (0.60) | 0.105 |
| Mean gradient post‐implant (mm Hg), median (±IQR) | 10.0 (7.0) | 11.0 (7.0) | 10.0 (7.0) | 0.608 |
| Max. gradient post‐implant (mm Hg), median (±IQR) | 18.0 (14.0) | 18.0 (16.0) | 16.0 (11.0) | 0.223 |
| Max. flow post‐implant (m/s), median (±IQR) | 2.1 (1.0) | 2.1 (1.0) | 2.1 (1.0) | 0.645 |
| Length of stay after TAVR (days), median (±IQR) | 8.0 (7.0) | 8.0 (7.0) | 8.0 (8.0) | 0.880 |
Abbreviations: ICU, intensive care unit; other abbreviations as in Tables 1 and 2.
3.3. Post‐operative outcomes and primary composite endpoint
Adverse events are shown in Table 5. Patients with greater iliofemoral tortuosity had a significantly higher risk for access and bleeding complications, represented by the primary composite endpoint of the study (56 [36.8%] vs 17 [19.3%]; P = 0.003). This finding was mainly driven by the increased rate of minor vascular complications (34 [22.4%] vs 11 [12.5%]; P = 0.043) and minor bleeding complications (39 [25.6%] vs 11 [12.5%]; P = 0.007) in the higher IFT scoring cohort. Access‐site haematomas were the most frequent cause of these minor complications and—as per the VARC‐2 standard endpoint definition document—reported in both categories (bleeding and access site complications). 14 There were 15 instances where surgical repair was performed and 4 cases of interventional repair, primarily among patients who presented with major bleeding complications. No difference was observed with regard to 30‐day all‐cause mortality (11 [7.2%] vs 3 [3.4%]; P = 0.176).
TABLE 5.
Adverse events data
|
Overall cohort n = 240 |
Low tortuosity n = 88 |
High tortuosity n = 152 |
P value | |
|---|---|---|---|---|
| Myocardial infarction | 1 (0.4) | 1 (1.1) | 0 (0.0) | 0.371 |
| Vascular access complication | 52 (21.7) | 14 (15.9) | 38 (25.0) | 0.067 |
| Minor access complication | 45 (18.8) | 11 (12.5) | 34 (22.4) | 0.043 |
| Major access complication | 7 (2.9) | 3 (3.4) | 4 (2.6) | 0.507 |
| Bleeding complication | 64 (26.7) | 14 (15.9) | 50 (32.9) | 0.003 |
| Minor bleeding complication | 50 (20.8) | 11 (12.5) | 39 (25.6) | 0.007 |
| Major bleeding complication | 14 (5.8) | 3 (3.4) | 11 (7.2) | 0.176 |
| Any access or bleeding complication | 73 (30.4) | 17 (19.3) | 56 (36.8) | 0.003 |
| Neurological adverse event | 6 (2.5) | 3 (3.4) | 3 (2.0) | 0.386 |
| Acute Kidney Injury | 38 (15.8) | 10 (11.4) | 28 (18.4) | 0.102 |
| Postoperative renal replacement therapy | 6 (2.5) | 2 (2.3) | 4 (2.6) | 0.614 |
| New atrial fibrillation | 17 (7.1) | 4 (4.5) | 13 (8.6) | 0.173 |
| Conversion to open surgery | 1 (0.4) | 0 (0.0) | 1 (0.7) | 0.629 |
| Reoperation for valvular dysfunction | 1 (0.4) | 0 (0.0) | 1 (0.7) | 0.629 |
| Reoperation for bleeding/tamponade | 12 (5.0) | 2 (2.3) | 10 (6.6) | 0.115 |
| Reoperation for non‐cardiac problems | 9 (3.8) | 5 (5.7) | 4 (2.6) | 0.205 |
| Valve in valve bailout | 6 (2.5) | 3 (3.4) | 3 (2.0) | 0.395 |
| 30‐day combined safety endpoint | 208 (86.7) | 76 (86.4) | 132 (86.8) | 0.531 |
| 30‐day all‐cause mortality | 14 (5.8) | 3 (3.4) | 11 (7.2) | 0.176 |
AV, atrioventricular; other abbreviations as in Tables 1‐3.
Further analysis shows that patients satisfying the primary composite endpoint were less likely to reach the early safety endpoint (58 [79.5%] vs 150 [89.8%]; P = 0.027) but did not have different mortality rates 30 days (P = 0.429), 1 year (P = 0.275) and 5 years (P = 0.549). The trend is likely driven by the occurrence of major vascular complications whose analysis showed similar difference with respect to the probability of reaching the 30‐day safety endpoint (7 [0%] vs 208 [89.3%]; P = <0.001) and rates of 30 day, 1 year and 5 year mortality (P = 0.653, P = 0.388, P = 0.610). Increased risk of mortality at these time‐points was only greater in patients with major bleeding complications (30 days: 10 [4.4%] vs 4 [28.6%]; P = 0.005, 1 year: 23[10.2%] vs 7 [50%]; P = <0.001, 5 years: 68 [30.1%] vs 8 [57.1%]; P = 0.038).
3.4. Predictive factors and long‐term survival
In a multivariate logistic regression analysis of predictive factors for the primary endpoint, the IFT score (OR: 2.11; 95% CI: 1.09‐4.05; P = 0.026) was the only significant predictor (Table 6). After adjusting the Cox proportionate hazards model for age, sex, insulin‐dependent diabetes, hypertension, renal impairment, chronic obstructive lung disease, and congestive heart failure, a strong trend for lower long‐term survival of patients with a higher IFT score was demonstrated (adjusted hazard ratio: 0.6; 95% CI: 0.4‐1.0; P = 0.054; Figure 2).
TABLE 6.
Univariate and multivariate logistic regression model of predictive factors for bleeding or access complications after transcatheter aortic valve replacement
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Demographics | |||||||
| Age | 1.015 | 0.973‐1.059 | 0.486 | ||||
| Gender | 0.644 | 0.376‐1.105 | 0.110 | ||||
| Body mass index | 1.003 | 0.953‐1.056 | 0.913 | ||||
| Diabetes mellitus | 0.767 | 0.365‐1.611 | 0.484 | ||||
| Hypertension | 0.878 | 0.393‐1.962 | 0.751 | ||||
| Peripheral vascular disease | 0.378 | 0.126‐1.133 | 0.082 | 0.324 | 0.092‐1.135 | 0.078 | |
| Porcelain aorta | 2.321 | 0.321‐16.771 | 0.404 | ||||
| Procedural variables | |||||||
| Treatment period | 0.883 | 0.399‐1.956 | 0.759 | ||||
| Vascular closure device | 0.662 | 0.374‐1.174 | 0.158 | ||||
| Radiological features | |||||||
| Minimum lumen diameter | 1.078 | 0.854‐1.361 | 0.527 | ||||
| Minimum lumen area | 1.009 | 0.988‐1.029 | 0.412 | ||||
| Skin‐to‐FMA distance at 45° | 1.001 | 0.983‐1.019 | 0.895 | ||||
| Iliofemoral calcification load | 1 | 1‐1 | 0.784 | ||||
| Sheath‐to‐femoral‐artery ratio (MLD) | 0.37 | 0.081‐1.694 | 0.2 | ||||
| Measures of tortuosity | |||||||
| Iliofemoral tortuosity score | 2.436 | 1.306‐4.544 | 0.005 | 2.105 | 1.094‐4.053 | 0.026 | |
| Sum of all angles | 1.163 | 0.654‐2.069 | 0.607 | ||||
| Largest single angle | 2.324 | 1.109‐4.867 | 0.025 | 1.587 | 0.720‐3.496 | 0.252 | |
Abbreviations: OR, odds ratio; other abbreviations as in Tables 1‐4.
FIGURE 2.
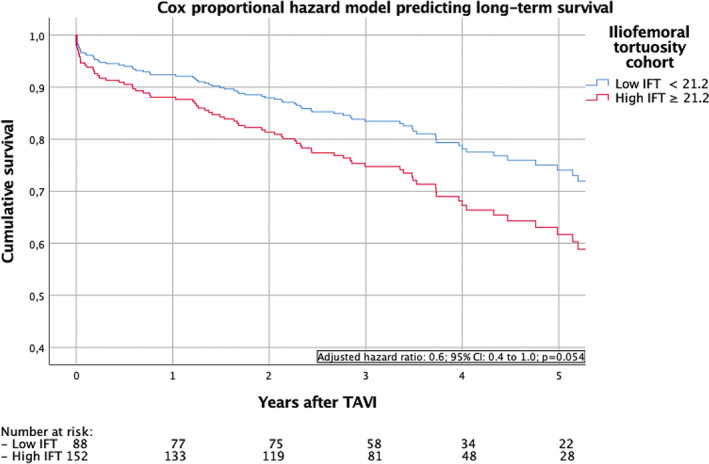
Cox proportional hazard model predicting long‐term survival of patients undergoing transfemoral transcatheter aortic valve replacement (TAVR) with low and high iliofemoral tortuosity (IFT) score
4. DISCUSSION
We present a systematic screening method for vascular tortuosity which is predictive of access related complications after TAVR. Notably, we show that an IFT score greater than 21.2 effectively identifies patients at risk of periprocedural access and bleeding complications.
Among the models used to define tortuosity in TAVR patients, the IFT score distinguishes itself by its ability to predict vascular or bleeding complications using a clear cut‐off value. Other tools such as the LSA did not have any significant association with post‐procedurals outcomes in our cohort, in line with a report by Toggweiler et al which shows that patients who had vascular complications following TAVR did not have significantly higher rates of LSAs equal to or exceeding 45°. 9 Similarly, Kinnel et al found that even when tortuosity was classified as an LSA > 90°, its prevalence was not significantly different between patients who reached an early safety composite endpoint and those who did not. 10 Although it has been suggested that moderate‐severe tortuosity is predictive of vascular complications, when patients have been classified according to their maximum LSA in a study by Hammer et al, no significant difference in vascular complications was found between them. 11 , 12 One of the pitfalls of the LSA is that its value can be high in vasculature with short yet deep indentations, which may be easily traversed. Moreover, the LSA does not consider the frequency of kinks in the iliofemoral system, which may cause the procedure to become technically challenging.
The utility of the SAA in determining patients at higher risk of vascular complications is more contested though. We found no relationship between the SAA and our primary endpoint among our patients; this stands in contrast to Vavuranakis et al, who reported fewer major vascular complications in individuals that had a lower ratio of the SAA to the minimum femoral arterial diameter at the access site. 8 However, it is worth noting that these ratios were based on two‐dimensional measurements, which provide limited information with respect to the true tortuosity of patients. 8 , 15 The limitation in the accuracy of the measurements is acknowledged by the authors and may be the source of the discrepancy between our findings. 8 SAA as a marker of tortuosity has also been shown to poorly detect instances where vessels had long broad curvatures as a prominent feature of their tortuosity thereby ignoring the potentially increased probability of error in prolonged arterial paths during TAVR procedures. Models resembling the IFT excel at identifying such cases, and thus arguably represent a superior tool to evaluate tortuosity in the iliofemoral system. Although other tortuosity classifications exist, the IFT is the only one whose clear cut‐off value is based on precise data from three‐dimensional renderings and predicts vascular complications. 16
As major complications after TAVR are rarely seen in current practice, these findings are mainly driven by minor complications. The clinical value of these minor complications and their subsequent impact on long‐term outcome may be subject to debate. However, stenoses, dissections, perforation, rupture, embolization, endovascular interventions and surgical procedures of essential significance for the treating physicians and most importantly for the patients, even though they do not, by definition of the VARC‐2 criteria, lead directly to death, life‐threatening bleeding, end‐organ damage or neurological impairment. These findings are relevant not only for the elderly high‐risk population, but also for younger, low‐risk patients who have to cope with the clinical consequences for considerably longer periods of time and may be subject to significant restrictions in their physical activity.
The notion that more tortuous vessels worsen outcomes is not surprising. As previously outlined, higher tortuosity may require increased catheter handling and push, thus increasing the probability that damage is caused which post‐operatively may manifest as a bleed or haematoma. A similar line of thought was proposed by Chen et al who suggested that increased manipulation may lead to higher rates of embolization and hence explain the elevated stroke incidence present among patients with higher vessel tortuosity undergoing thoracic endovascular aortic repair. 17 It has also been speculated that tortuosity is the product of arterial wall fragility, which in turn may cause the vasculature to be more susceptible to damage following manipulation by the catheter. 18 The contributory role of iliac artery tortuosity to vessel rupture, may simultaneously promote the formation of haematomas when gaining access to the femoral artery and during closure, as well as escalate forceful intravascular catheter manipulation from negligible vascular injuries into bleeding complications. 19 Vessel tortuosity would thus both raise the probability of vessel damage, and exacerbate any injury past the subclinical threshold thereby increasing bleeding and vascular complications. The intuitive risk potential posed by tortuous vessels may therefore be explained through multiple and potentially synergistic mechanisms, with further research required to verify them.
Advances in understanding vessel tortuosity and quantifying them through tools such as the IFT score, is pivotal to reducing vascular complications and by extension improving TF‐TAVR outcomes. Vascular complications, along with paravalvular leaks and pacemaker requirement, constitute the final barriers in optimizing post‐operative TAVR outcomes as other types of complications seldomly occur. 20 Established risk factors for vascular complications including vessel calcifications and sheath:vessel diameter ratio are already screened and accounted for during the planning stages of TF‐TAVR, and in instances where they present significant challenges, can be circumvented by using alternative access strategies. 21 Our centre's experience was that most cases we considered for alternative access during the study period were predominantly when the Heart Team concluded that a small lumen diameter or severe calcifications would preclude a patient from TF‐TAVR, but no patients were referred for alternative access solely on the basis of highly tortuous vessels despite our clinical experience increasingly suggesting otherwise. The additive risk presented by calcifications are also already mitigated against during the planning stages; selecting the inherently less calcified vessels limits their effects on the access site, and ensuring that the vessel has sufficient lumen diameter after accounting for calcified obstructions reduces their impact further upstream. 22
In contrast, the effects of tortuosity are underestimated as it is believed that even in severe cases, tortuous vessels can be straightened with a sheath insertion to the extent that the safe passage of the delivery system and the valve can be assured. 21 , 23 However, this approach may further exacerbate the aforementioned issues of increased friction, resistance, manipulation and exertion of force on the vessels which could contribute to the formation of (micro)lesions at the access site. As highlighted by our study, in patients with significant tortuosity this may lead to higher rates of haematomas and minor complications at the access site. Insufficient consideration of these factors combined with a scarcity of validated measurement tools has meant that the importance of quantifying vessel tortuosity has been neglected despite its comparable importance to measuring calcifications and vessel diameter. The path towards ultimately integrating all three factors into a single risk assessment score, is also predicated on first establishing the extent to which vessel tortuosity alone predicts post‐operative outcomes and designing an objective method to measure it. In TF‐TAVR patients in which vascular access is currently deemed suitable using established risk factors (low calcifications, sufficient vessel diameter), the IFT score is the only predictor of post‐operative access and bleeding complications; it therefore serves as a crucial tool to address vascular complications and improve TAVR outcomes.
The IFT score, based on the increase in vascular tortuosity with age, also appears to be an inherently valuable radiological marker to discriminate biologically older patients from younger ones. Although not directly associated with mortality, a high IFT score may thus serve as a surrogate measure for reduced life expectancy during preprocedural risk stratification. Applying the IFT score to larger cohorts with extended follow‐up periods is needed to both validate the model and its predictive power as well as verify the weighting that iliofemoral tortuosity needs to be given when evaluating the appropriateness of TF‐TAVR for patients. Further work is required to devise a screening tool that incorporates patients’ vessel tortuosity, calcification and diameter to assess their individual risk of post‐operative vascular complications.
Several limitations to this analysis exist including the retrospective nature of the investigation and the limited cohort size in this pilot study. The ability to infer conclusions based on group comparisons is somewhat limited due to the natural selection bias concerning the choice of the access site. Nevertheless, this study aimed to develop an objective method assessing the iliofemoral tortuosity that can provide crucial support for the heart team in the preprocedural evaluation of complex clinical situations. The use of different devices with diverging sizes and catheter flexibility may be an additional source of bias as well. However, the findings that both the treatment period tertials and the sheath‐to‐femoral artery ratio were not significant predictors of the composite endpoint in both the uni‐ and multivariate analysis are reassuring in this context, and suggest that the effects of heterogenous device usage on our results may be negligible.
Even though the data presented in this analysis are from a tertiary referral centre with considerable experience, the effects of an initial learning curve during the early study phase must also be considered. Moreover, the current absence of an unambiguous definition of clinically relevant access‐site haematomas offers both a limitation to the interpretation of the study and a possibility to take it into account in subsequent VARC endpoint definition documents. The quantitative thresholds of the iliofemoral tortuosity measurements in this pilot study have also not been validated and further investigation is needed.
5. CONCLUSIONS
Iliofemoral tortuosity is a relevant risk factor affecting the outcomes of patients undergoing TF‐TAVR. Preprocedural assessment of vessel tortuosity using the IFT scoring system can help identify patients at risk of developing vascular and bleeding complications who warrant further risk stratification. Additional work is needed to verify the scoring system and its ability to predict long‐term outcome in patients.
CONFLICT OF INTEREST
MM has received a research grant from Edwards Lifesciences, JenaValve and Symetis. MA is proctor (Abbott, Edwards) and advisor (Medtronic). All other authors have reported that they have no relationships relevant to the content.
AUTHOR CONTRIBUTIONS
MM, GD‐K and MG conceptualized the study. MM designed the methodology and conducted the formal analysis. MM, WH, TP and PS conducted the clinical investigation. AS, CA, GD‐K and MG provided the respective resources. MA, BW, SG, DG, PNR and VW were responsible for the data curation. MM, WH and TP wrote the original draft of the manuscript. AS, MA, BW, SG, DG, PNR, VW, GD‐K and MG wrote, reviewed and edited the manuscript. MM and WH were responsible for the visualisation of the study results. GD‐K and MG supervised the study and were responsible for the project administration. All authors have read the manuscript and agreed to its publication.
Supporting information
Video S1
Mach M, Poschner T, Hasan W, et al. The Iliofemoral tortuosity score predicts access and bleeding complications during transfemoral transcatheter aortic valve replacement: Data from the VIenna Cardio Thoracic aOrtic valve registrY (VICTORY). Eur J Clin Invest. 2021;51:e13491. 10.1111/eci.13491
Funding information
This research received no external funding.
REFERENCES
- 1. Han HC. Twisted blood vessels: symptoms, etiology and biomechanical mechanisms. J Vasc Res. 2012;49(3):185‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pancera P, Ribul M, Presciuttini B, Lechi A. Prevalence of carotid artery kinking in 590 consecutive subjects evaluated by Echocolordoppler. Is there a correlation with arterial hypertension? J Intern Med. 2000;248(1):7‐12. [DOI] [PubMed] [Google Scholar]
- 3. Morris SA. Arterial tortuosity in genetic arteriopathies. Curr Opin Cardiol. 2015;30(6):587‐593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chakos A, Wilson‐Smith A, Arora S, et al. Long term outcomes of transcatheter aortic valve implantation (TAVI): a systematic review of 5‐year survival and beyond. Ann Cardiothorac Surg. 2017;6(5):432‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pascual I, Carro A, Avanza P, et al. Vascular approaches for transcatheter aortic valve implantation. J Thorac Dis. 2017;9(Suppl 6):S478‐s487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayashida K, Lefèvre T, Chevalier B, et al. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc Interv. 2011;4(8):851‐858. [DOI] [PubMed] [Google Scholar]
- 7. Chiam PTL, Koh AS, Ewe SH, et al. Iliofemoral anatomy among Asians: implications for transcatheter aortic valve implantation. Int J Cardiol. 2013;167(4):1373‐1379. [DOI] [PubMed] [Google Scholar]
- 8. Vavuranakis M, Kariori M, Voudris V, et al. Predictive factors of vascular complications after transcatheter aortic valve implantation in patients treated with a default percutaneous strategy. Cardiovasc Ther. 2013;31(5):e46‐54. [DOI] [PubMed] [Google Scholar]
- 9. Toggweiler S, Gurvitch R, Leipsic J, et al. Percutaneous aortic valve replacement: vascular outcomes with a fully percutaneous procedure. J Am Coll Cardiol. 2012;59(2):113‐118. [DOI] [PubMed] [Google Scholar]
- 10. Kinnel M, Faroux L, Villecourt A, et al. Abdominal aorta tortuosity on computed tomography identifies patients at risk of complications during transfemoral transcatheter aortic valve replacement. Arch Cardiovasc Dis. 2020;113(3):159‐167. [DOI] [PubMed] [Google Scholar]
- 11. Langouet Q, Martinez R, Saint‐Etienne C, et al. Incidence, predictors, impact, and treatment of vascular complications after transcatheter aortic valve implantation in a modern prospective cohort under real conditions. J Vasc Surg. 2020;72(6):2120‐2129. [DOI] [PubMed] [Google Scholar]
- 12. Hammer Y, Landes U, Zusman O, et al. Iliofemoral artery lumen volume assessment with three dimensional multi‐detector computed tomography and vascular complication risk in transfemoral transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr. 2019;13(1):68‐74. [DOI] [PubMed] [Google Scholar]
- 13. Mach M, Wilbring M, Winkler B, et al. Cut‐down outperforms complete percutaneous transcatheter valve implantation. Asian Cardiovasc Thorac Ann. 2018;26(2):107‐113. [DOI] [PubMed] [Google Scholar]
- 14. Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. Eur Heart J. 2012;33(19):2403‐2418. [DOI] [PubMed] [Google Scholar]
- 15. Marwan M, Achenbach S. Role of cardiac ct before transcatheter aortic valve implantation (TAVI). Curr Cardiol Rep. 2016;18(2):21. [DOI] [PubMed] [Google Scholar]
- 16. Bullitt E, Gerig G, Pizer SM, Lin W, Aylward SR. Measuring tortuosity of the intracerebral vasculature from MRA images. IEEE Trans Med Imaging. 2003;22(9):1163‐1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen C‐K, Liang I‐P, Chang H‐T, et al. Impact on outcomes by measuring tortuosity with reporting standards for thoracic endovascular aortic repair. J Vasc Surg. 2014;60(4):937‐944. [DOI] [PubMed] [Google Scholar]
- 18. Morris SA, Orbach DB, Geva T, Singh MN, Gauvreau K, Lacro RV. Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation. 2011;124(4):388‐396. [DOI] [PubMed] [Google Scholar]
- 19. Murray D, Ghosh J, Khwaja N, Murphy MO, Baguneid MS, Walker MG. Access for endovascular aneurysm repair. J Endovasc Ther. 2006;13(6):754‐761. [DOI] [PubMed] [Google Scholar]
- 20. Lüscher TF. TAVI is on the move! How it compares with surgery and what complications we still have to consider. Eur Heart J. 2019;40(38):3129‐3133. [DOI] [PubMed] [Google Scholar]
- 21. Blanke P, Weir‐McCall JR, Achenbach S, et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR): An Expert Consensus Document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc Imag. 2019;12(1):1‐24. [DOI] [PubMed] [Google Scholar]
- 22. Möllmann H, Kim WK, Kempfert J, Walther T, Hamm C. Complications of transcatheter aortic valve implantation (TAVI): how to avoid and treat them. Heart. 2015;101(11):900‐908. [DOI] [PubMed] [Google Scholar]
- 23. Caruso D, Rosenberg RD, De Cecco CN, et al. Vascular imaging before transcatheter aortic valve replacement (TAVR): why and how? Curr Cardiol Rep. 2016;18(2):14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1


