Abstract
Social contact reduces stress responses in social animals. Mice have been shown to show allogrooming behaviour toward distressed conspecifics. However, the precise neuronal mechanisms underlying allogrooming behaviour remain unclear. In the present study, we examined whether mice show allogrooming behaviour towards distressed conspecifics in a social defeat model and we also determined whether oxytocin receptor‐expressing neurons were activated during allogrooming by examining the expression of c‐Fos protein, a marker of neurone activation. Mice showed allogrooming behaviour toward socially defeated conspecifics. After allogrooming behaviour, the percentages of oxytocin receptor‐expressing neurones expressing c‐Fos protein were significantly increased in the anterior olfactory nucleus, cingulate cortex, insular cortex, lateral septum and medial amygdala of female mice, suggesting that oxytocin receptor‐expressing neurones in these areas were activated during allogrooming behaviour toward distressed conspecifics. The duration of allogrooming was correlated with the percentages of oxytocin receptor‐expressing neurones expressing c‐Fos protein in the anterior olfactory nucleus, insular cortex, lateral septum and medial amygdala. In oxytocin receptor‐deficient mice, allogrooming behaviour toward socially defeated cage mates was markedly reduced in female mice but not in male mice, indicating the importance of the oxytocin receptor for allogrooming behaviour in female mice toward distressed conspecifics. The results suggest that the oxytocin receptor, possibly in the anterior olfactory nucleus, insular cortex, lateral septum and/or medial amygdala, facilitates allogrooming behaviour toward socially distressed familiar conspecifics in female mice.
Keywords: affiliative behaviour, allogrooming, social defeat stress, oxytocin
Mice showed allogrooming behaviour towards distressed conspecifics in a social defeat model. Allogrooming behaviour was correlated with activation of oxytocin receptor‐expressing neurones in the anterior olfactory nucleus, insular cortex, lateral septum and medial amygdala. Oxytocin receptor‐deficient females, but not males, showed impairment of allogrooming behaviour, indicating a sexual difference in neural mechanisms of allogrooming.
1. INTRODUCTION
Affiliative contact behaviours such as allogrooming and side‐by‐side contact have been observed in social animals and have been shown to play an important role in the formation and maintenance of social groups. 1 , 2 , 3 Social animals spend time in grooming their conspecifics far longer than that required to keep the fur clean. Monogamous voles spend over 50% of their time in side‐by‐side contact with conspecifics. 3 Social contact induces anxiolytic actions in social animals. 4
Monogamous male voles show prosocial allogrooming behaviour toward their distressed female partners. 5 Allogrooming behaviour is blocked by an oxytocin receptor antagonist, suggesting that monogamous animals show consolation‐related behaviour in an oxytocin receptor‐dependent manner. 5 Female mice but not male mice 6 and female rats but not male rats 7 have been reported to show approach behaviour toward distressed same‐sex familiar conspecifics. It has also been shown that rats and mice perform allogrooming for distressed conspecifics. 8 However, the precise roles of the oxytocin receptor in allogrooming towards same‐sex distressed conspecifics in commonly used laboratory animals remain to be determined, although oxytocin has been shown to facilitate social interactions 9 , 10 and self‐grooming. 11
On the other hand, the meadow vole, which is a promiscuous rodent, does not show allogrooming behaviour toward conspecifics that are distressed with conditioned fear. 5 However, allogrooming behaviour has been observed mainly after social fighting between conspecifics in natural situations in chimpanzees, 12 and social stress has been shown to activate different brain areas from those activated after noxious or conditioned fear stimuli. 13 Thus, the present study aimed to determine (i) whether mice show allogrooming toward cage mates that have received social defeat stress; (ii) which oxytocin receptor‐expressing neurones are activated during social allogrooming; and (iii) whether oxytocin receptor deficiency impairs allogrooming toward distressed cage mates. We mainly investigated female mice because females have been shown to demonstrate approach behaviour toward distressed conspecifics 6 and females show affiliative behaviours more frequently than males do. 14 We also examined side‐by‐side contact because side‐by‐side contact has been considered to be an affiliative behaviour. 3
2. MATERIALS AND METHODS
2.1. Animals
Mice of the C57BL/6J strain (Charles River Laboratories), retired CD‐1 mice (Charles River Laboratories), oxytocin receptor‐Venus knock‐in heterozygous mice 15 (oxytocin receptor heterozygous knockout mice, backcrossed with C57BL/6J mice for over 18 generations), oxytocin receptor‐Venus knock‐in homozygous mice (oxytocin receptor‐deficient mice), c‐Fos‐deficient mice with a chimeric background (CD‐1 × C57BL6/J) 16 and their wild‐type siblings were used in the present study. In oxytocin receptor‐Venus knock‐in mice, a variant of yellow fluorescent protein (Venus) is expressed under the control of the endogenous regulatory region of the oxytocin receptor gene.
Mice were housed under a 12:12‐hour light/dark photocycle (lights on 7.30 am) at 20‐24°C and 40%‐70% relative humidity. Food and water were available ad lib. All animal procedures were approved by the Institutional Animal Experiment Committee of Jichi Medical University and were conducted in accordance with the Institutional Regulations for Animal Experiments and Fundamental Guidelines for Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology.
2.2. Pair housing
Pairs of oxytocin receptor‐Venus knock‐in heterozygous and wild‐type female mice were housed in cages for 4 weeks from 6 weeks of age. At the age of 10 weeks, wild‐type females were removed from their home cages, kept isolated overnight, given social defeat stress and then returned to their previous home cages where their counterparts, oxytocin receptor‐Venus knock‐in heterozygous females, were staying. In the control group, wild‐type females were removed from their home cages, kept isolated overnight and returned to their previous home cages. Behaviours of the resident oxytocin receptor‐Venus knock‐in heterozygous females toward socially defeated cage mates or toward non‐defeated cage mates were recorded for 30 minutes. The time spent allogrooming, time spent in side‐by‐side contact and time spent sniffing were recorded manually by an experimenter who was blind to treatments. Allogrooming was defined as grooming fur of any body parts of the partner with the mouth or forepaws. Side‐by‐side contact was defined as sitting in contact with the partner. The total duration of side‐by‐side contact, either contact initiated by the resident or contact initiated by the partner, was recorded. Sniffing was defined as olfactory investigation to any body parts of the partner. One hour and 50 minutes after the initiation of re‐pairing with their cage mates, the resident oxytocin receptor‐Venus knock‐in heterozygous mice were anaesthetised with Avertin (tribromoethanol, 250 mg kg‐1, i.p., WAKO Pure Chemical Industries Ltd, Osaka, Japan) and perfused with 4% paraformaldehyde.
In experiments with oxytocin receptor‐deficient female mice, oxytocin receptor‐deficient female mice or their wild‐type female littermates were housed with wild‐type female mice in pairs for 4 weeks from 6 weeks of age. After cohabitation for 4 weeks, wild‐type mice were removed from their home cages, kept isolated overnight, given social defeat stress and returned to their previous home cages where their cage mates were staying. Behaviours of resident oxytocin receptor‐deficient mice or wild‐type female mice toward defeated cage mates that had been returned to their cages were recorded for 30 minutes.
In experiments for observation of behaviours toward unfamiliar conspecifics, female mice produced in our laboratory by mating of C57BL/6J mice that had been obtained from a supplier (Charles River Laboratories) were housed in pairs for 4 weeks from 6 weeks of age. At the age of 10 weeks, one female was removed from each home cage and each female’s counterpart was kept in the home cage. The female mice that had been removed were kept isolated overnight and were given social defeat stress or just kept in isolation. These animals were then placed in cages where unfamiliar female counterparts were staying. Behaviours of the resident females toward socially defeated unfamiliar conspecifics or non‐defeated unfamiliar conspecifics were recorded for 30 minutes.
In experiments with oxytocin receptor‐deficient male mice, wild‐type male mice were castrated under isoflurane anaesthesia at 4 weeks of age. Oxytocin receptor‐deficient mice or their wild‐type male littermates were housed in pairs with wild‐type castrated mice for 4 weeks from 6 weeks of age. Wild‐type castrated mice were removed from their home cages, kept isolated overnight, given social defeat stress and returned to their previous home cages. Behaviours of resident oxytocin receptor‐deficient mice or wild‐type male mice toward socially defeated cage mates were recorded for 30 minutes.
2.3. Social defeat stress
Aggressive CD1 male mice were selected from retired CD1 males as reported previously. 17 For selection of aggressive CD1 males, a C57BL/6J male was placed in the home cage of a retired CD1 mouse. CD1 males that showed aggressive behaviours to the intruder mice within 60 seconds in two consecutive session out of three sessions per day for two consecutive days out of three test days were selected. These aggressive CD1 males were kept singly in individual home cages. Within 2 days before social defeat experiments, a C57BL/6J male mouse was placed again into the home cage of the CD1 mouse to enhance aggression of the CD1 mouse. CD1 mice that showed aggressive behaviours toward the intruder mice immediately after intrusion in the enhancement trial were used in the experiments.
To determine a protocol for social defeat stress of female mice, an adult female C57BL/6J mouse was introduced into a home cage of the selected male CD1 mouse for 10 minutes. Six female and six CD1 mice that had been selected by a criterion of short latency of aggressive behaviours toward C57BL/6J male mice were used. CD1 male mice showed aggressive behaviours towards intruder female mice and the intruder female mice showed species‐specific defeat posture during the first 3 minutes. However, five of the six male mice also started to exhibit anogenital sniffing and mounting behaviour toward the intruder female mice 4‐7 minutes after the initiation of exposure to female mice. Thus, a 3‐minute duration of exposure was used for social defeat stress of female mice.
In the experiment with oxytocin receptor‐Venus knock‐in heterozygous female mice, six mice out of 40 CD1 mice were used for aggressors. In the experiment with oxytocin receptor‐deficient mice, five CD1 mice out of a different series of 40 CD1 mice were used. In the experiment with examination of behaviours toward unfamiliar females, one CD1 mouse from the above mentioned series of aggressors and two CD1 mice out of another series of 40 CD1 mice were used. These 13 mice out of 120 retired CD1 mice consistently showed aggressive behaviours towards female mice or castrated mice.
C57BL/6J cage mates or wild‐type cage mates of the test mice were introduced into cages of aggressive CD1 males. The intruder mice received attacks from CD1 males at least once during the stay. The maximum duration of stay for female mice was 3 minutes. The duration of stay for male mice was 10 minutes according to the standard procedure of social defeat stress. 17 Immediately after exposure to aggressor males, the defeated mice were placed back into their previous home cages where their cage mates were kept or into cages where unfamiliar mice were staying. Behaviours of the intruder C57BL/6J or wild‐type mice were recorded during the test period, and the time during which mice received aggression and the time during which mice showed a social defeat posture were recorded manually in experiments with oxytocin receptor‐Venus knock‐in heterozygous females.
2.4. Immunocytochemical detection of c‐Fos protein and oxytocin receptor‐expressing or oxytocin neurones
For confirmation of the specificity of the c‐Fos antibody used in the present study, male and female c‐Fos‐deficient mice 16 with a chimeric background (CD‐1 × C57BL6/J, 4‐6 weeks old) and their wild‐type siblings were used. Mice were singly placed into plastic cages (115 × 213 × 120 mm) without bedding materials to induce the expression of c‐Fos protein as a result of novel environmental stress. At 110‐210 minutes after the initiation of exposure to novel environmental stimuli, mice were deeply anaesthetised with avertin (250 mg/kg, i.p.) and perfused transcardially with heparinised saline followed by 4% paraformaldehyde. Brains were removed from the skulls, post‐fixed in 4% paraformaldehyde solution overnight and transferred to 30% sucrose solution in 0.1 mol L‐1 phosphate buffer until they sank. The brains were then frozen on dry ice and stored at −80°C until processing for immunocytochemical examination. Coronal brain sections at 30 μm were processed for immunohistochemical detection of c‐Fos protein as described previously. 18 , 19 In brief, sections were incubated with a rabbit polyclonal antibody against the N‐terminal region (amino acid residues 4‐17 of human c‐Fos) of the c‐Fos peptide sequence (diluted 1:10 000 with 0.1 mol L‐1 phosphate buffer containing 0.3% Triton X‐100 and 5% normal goat serum; Ab‐5; Oncogene Science, Cambridge, MA USA; RRID:AB_2314042) at 4°C for 2 days and with peroxidase‐labelled goat anti‐rabbit IgG (diluted 1:500; PI‐1000; Vector Laboratories, Burlingame, CA, USA) at 4°C overnight. c‐Fos immunoreactivity was visualised as a black nuclear profile by incubation with 3,3′‐diaminobenzidine tetrahydrochloride (DAB), nickel sulphate, glucose and glucose oxidase in 0.1 mol L‐1 acetate buffer. More than 12 sections of the forebrain and midbrain areas of each rat were examined.
For detection of neurones activated after exposure to socially defeated cage mates, mice were exposed to socially defeated cage mates and expression of c‐Fos protein was examined. Because expression of c‐Fos protein is known to peak between 90 and 120 minutes after stimuli, mice were deeply anaesthetised with avertin (250 mg kg‐1, i.p.) 110 minutes after the initiation of exposure to socially defeated cage mates and perfused transcardially with heparinised saline (20 U mL‐1) followed by 4% paraformaldehyde in 0.1 mol L‐1 phosphate buffer (pH 7.4).
Brain sections were cut coronally at 30 μm with a freezing sledge microtome and then processed for immunohistochemical detection of c‐Fos protein and Venus or c‐Fos protein and oxytocin at an interval of 90 μm, as described previously. 18 , 19 In brief, sections were processed for immunocytochemical detection of c‐Fos protein as described above and then processed for immunocytochemical detection of Venus to identify oxytocin receptor‐expressing cells. Sections were incubated with rat monoclonal anti‐green fluorescent protein (GFP) antibody (diluted 1:2000 with 0.1 mol L‐1 phosphate buffer containing 0.3% Triton X‐100 and 5% normal goat serum; GF090R; Nacalai Tesque, Kyoto, Japan; RRID:AB_2314545) at 4°C for 2 days and a peroxidase‐labelled polymer solution containing an anti‐rat Fab′ fragment, amino acid polymer and peroxidase [Histofine® mouse MAX PO (Rat); Nichirei Biosciences Inc., Tokyo, Japan] at room temperature for 2 hours. GFP immunoreactivity was visualised as a pink cytoplasmic precipitate with 0.01% Tris‐aminophenylmethane (Ark Pharm Inc., Arlington Heights, IL, USA) and 0.07% p‐cresol. 20
For immunocytochemical detection of oxytocin, sections that had been processed for immunocytochemical detection of c‐Fos protein were incubated with guinea pig anti‐oxytocin antibody (diluted 1:1 000 000; T‐5021; Peninsula Laboratories LLC, San Carlos, CA, USA; RRID:AB_518526) at 4°C for 2 days, followed by incubation with biotinylated anti‐guinea pig IgG (diluted 1:750; BA‐7000; Vector Laboratories) at room temperature for 2 hours, and avidin biotinylated horseradish peroxidase complex (diluted 1:50; Elite ABC kit; Vector Laboratories) at room temperature for 30 minutes. Oxytocin immunoreactivity was visualised as a brown cytoplasmic precipitate with DAB.
Pre‐absorption of the c‐Fos antibody with the synthetic peptide human c‐Fos (amino acid residues 4‐17 of human c‐Fos) blocked immunostaining. 19 No immunostaining was also found with the GFP antibody in brain sections of wild‐type mice. 18 There was also no immunostaining in brain sections in which the oxytocin antibody was omitted or in sections in which the antibody was pre‐adsorbed with 100 μmol L‐1 oxytocin at 4°C for 24 hours prior to section incubation. Pre‐adsorption with 100 μmol L‐1 Arg8‐vasopressin did not impair immunostaining. 19
The numbers of sections for detection of c‐Fos protein and Venus were 3 for the anterior olfactory nucleus, 5 for the prelimbic cortex, 5 for the infralimbic cortex, 18 for the cingulate cortex, 26 for the insular cortex, 6 for the dorsal part of the lateral septum, 6 for the ventral part of the lateral septum, 11 for the medial amygdala, 7 for the nucleus accumbens, 6 for the posterior intralaminar thalamic nucleus, 4 for the medial preoptic area, 7 for the ventrolateral part of the ventromedial hypothalamus, 8 for the ventrolateral periaqueductal grey and 4 for the nucleus tractus solitarii in each animal. The numbers of sections for detection of c‐Fos protein and oxytocin were 3 for the bed nucleus of the stria terminalis (BNST), 9 for hypothalamic supraoptic nucleus (SON) and 12 for hypothalamic paraventricular nucleus (PVN) in each animal. The intervals between sections were 90 μm for each of the areas. Each brain region was identified according to the coordinates of the Paxinos mouse brain atlas. 21
2.5. Statistical analysis
Statistical analyses were performed using Prism, version 9 (GraphPad Software Inc., San Diego, CA, USA). Effect sizes were calculated using HAD. 22 The behavioural and histochemical data showed a non‐normal distribution and non‐parametric statistics. The Mann‐Whitney U test and Spearman’s correlation were used for data analysis. For analysis of allogrooming, side‐by‐side contact and sniffing behaviour, P values were adjusted by Holm‐Sidak correction for multiple comparisons. The percentages of c‐Fos‐positive oxytocin receptor‐expressing cells in fourteen brain regions were analysed to exploratorily detect activated brain regions during allogrooming behaviour and P values were not adjusted for multiple comparisons. P < 0.05 was considered statistically significant. Data are presented as medians and interquartile ranges.
3. RESULTS
3.1. Behaviours towards socially defeated cage mates
During the 3‐minute exposure to aggressors, the intruder female mice received attacks (median = 24.8, 25th percentile = 3.4, 75th percentile = 37 seconds) with short latency (median = 2.2, 25th percentile = 1.6, 75th percentile = 3.8 seconds) but no mounting and showed social defeat posture (median = 13.8, 25th percentile = 0, 75th percentile = 32 seconds), suggesting that exposure to the aggressors caused social defeat stress in females in the present experimental conditions.
To determine whether female mice show allogrooming and side‐by‐side contact toward distressed cage mates, we first examined the behaviours of female mice toward socially defeated cage mates. Oxytocin receptor‐Venus knock‐in heterozygous female mice were exposed to socially defeated cage mates. Female mice exposed to defeated cage mates spent a longer time in allogrooming behaviour toward their defeated cage mates and a longer time in side‐by‐side contact behaviour with their socially defeated cage mates than the time spent in those behaviours toward non‐distressed cage mates (Figure 1) (allogrooming: U = 6, P = 0.0011, effect size r = 0.75; side‐by‐side: U = 15, P = 0.015, effect size r = 0.59). There was no significant difference between the time spent sniffing defeated cage mates and the time spent sniffing non‐defeated cage mates (Figure 1) (U = 28, P = 0.11, effect size r = 0.37).
FIGURE 1.
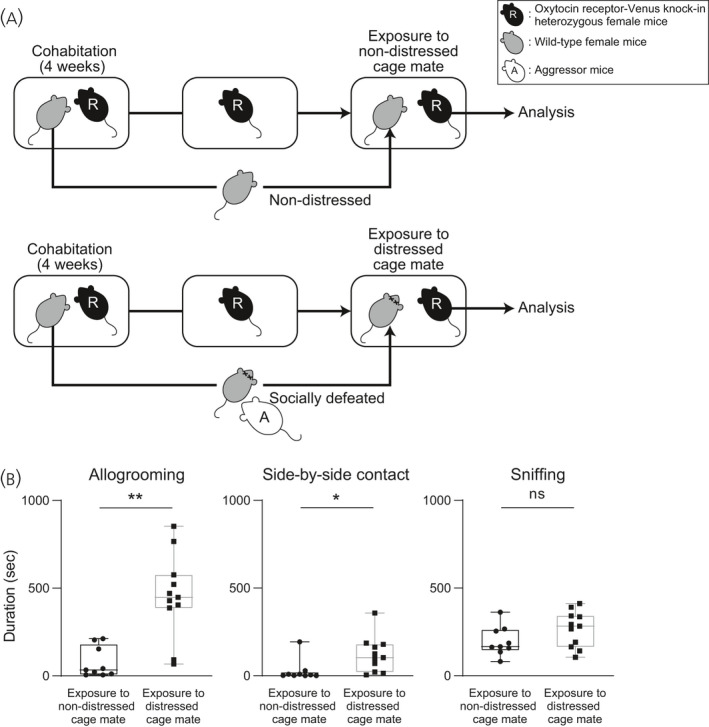
Times spent for allogrooming, side‐by‐side contact and sniffing behaviours of oxytocin receptor‐Venus knock‐in heterozygous female mice toward distressed cage mates. A, Scheme of the behaviour test. Pairs of resident test mice (R, oxytocin receptor‐Venus knock‐in heterozygous female mice) and cage mates were housed in cages for 4 weeks. In the control group, cage mates were removed from their home cages after 4 weeks of cohabitation, kept isolated overnight and then returned to their previous home cages. Thus, control resident test mice were exposed to non‐distressed cage mates (upper). In a group of distressed cage mates, cage mates were removed from their home cages after 4 weeks of cohabitation, kept isolated overnight, placed in cages of aggressive CD1 mice, and then returned to their previous home cages. Thus, resident test mice in the group of distressed cage mates were exposed to cage mates that had been defeated by aggressor CD1 mice (A) (lower). B, Times spent for allogrooming, side‐by‐side contact and sniffing behaviours of female mice toward non‐distressed or distressed cage mates. Female mice spent a longer time for allogrooming behaviour and a longer time for side‐by‐side contact behaviour toward distressed cage mates than the times spent for those behaviours toward non‐distressed cage mates. Time spent for sniffing behaviour toward distressed conspecifics was not significantly different from the time spent for that behaviour toward non‐stressed conspecifics. The numbers of animals in the groups for non‐distressed control cage mates and for distressed cage mates were 9 and 11, respectively. The box and whiskers plot shows the maximum, upper quartile, median, lower quartile and minimum values. *P < 0.05, **P < 0.01 vs non‐distressed control cage mates (Mann‐Whitney U test). NS, not significant
We also examined behaviours towards distressed unfamiliar mice. Female mice also spent a longer time in allogrooming behaviour and a longer time in sniffing behaviour toward distressed unfamiliar female mice than the times spent in those behaviours toward non‐distressed unfamiliar mice (Figure 2) (allogrooming: U = 3, P = 0.030, effect size r = 0.70; side‐by‐side: U = 14, P = 0.59, effect size r = 0.17; sniffing: U = 2, P = 0.026, effect size r = 0.75).
FIGURE 2.
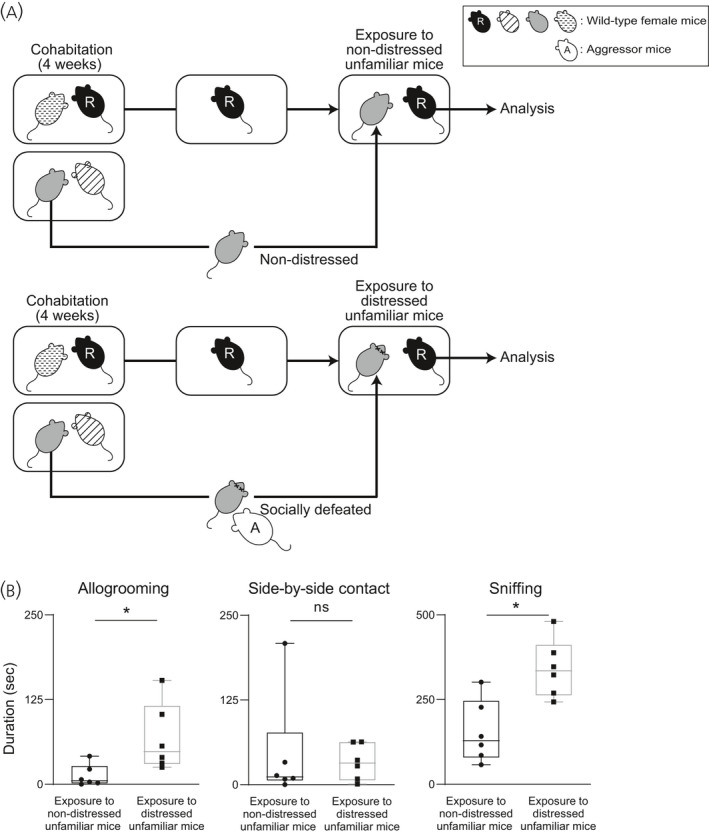
Times spent for allogrooming, side‐by‐side contact and sniffing behaviours toward unfamiliar distressed female mice. A, Scheme of the behaviour test. Pairs of resident test mice (R) and cage mates were housed in cages for 4 weeks. Resident test mice were exposed to non‐distressed unfamiliar mice or to unfamiliar mice that had been defeated by aggressor CD1 mice (A). B, Times spent for allogrooming, side‐by‐side contact and sniffing behaviours toward non‐distressed or distressed unfamiliar female mice. Times spent for allogrooming and sniffing behaviours toward distressed unfamiliar female mice were significantly longer than those toward non‐distressed unfamiliar mice. The numbers of animals in the groups for non‐distressed control mice and for distressed mice were 6 and 6, respectively. The maximum, upper quartile, median, lower quartile and minimum values are shown. *P < 0.05 vs non‐distressed control. NS, not significant (Mann‐Whitney U test)
3.2. Activation of oxytocin receptor‐expressing neurones in mice exposed to socially defeated cage mates
The oxytocin receptor is expressed in various brain regions, including social brain areas. To determine whether oxytocin receptor‐expressing neurones were activated during allogrooming or side‐by‐side behaviour toward distressed cage mates, we examined the expression of c‐Fos protein in oxytocin receptor‐expressing neurones of oxytocin receptor‐Venus knock‐in heterozygous female mice after exposure to socially defeated cage mates. Expression of c‐Fos protein in oxytocin‐immunoreactive neurones in the hypothalamus and the BNST was also examined.
We first confirmed the specificity of the c‐Fos antibody used in c‐Fos‐deficient mice. No immunoreactive staining was found in brain sections of c‐Fos‐deficient mice (Figure 3), indicating that the c‐Fos antibody had specificity to c‐Fos protein.
FIGURE 3.
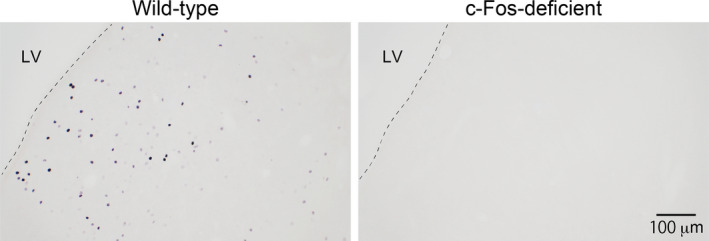
No detection of c‐Fos protein immunoreactivity in brain sections of c‐Fos‐deficient mice. Photographs of sections containing the septum region are shown. Brain sections were processed for detection of c‐Fos protein as described in the Materials and methods. No c‐Fos protein‐immunoreactive profiles (black nuclear profiles) were observed in sections of c‐Fos‐deficient mice. The numbers of wild‐type and c‐Fos‐deficient mice were 3 and 5, respectively. LV, lateral ventricle. Scale bar = 20 μm
We then examined the expression of c‐Fos protein after exposure to defeated cage mates. After exposure to defeated cage mates, the percentages of oxytocin receptor‐expressing neurones showing immunoreactivity of c‐Fos protein were increased in the anterior olfactory nucleus, cingulate cortex, insular cortex, dorsal part of the lateral septum, ventral part of the lateral septum and medial amygdala compared to those after exposure to non‐distressed cage mates (Figures 4 and 5) (anterior olfactory nucleus: U = 9, P = 0.0059, effect size r = 0.64; cingulate cortex: U = 23, P = 0.047, effect size r = 0.45; insular cortex: U = 10, P = 0.0056, effect size r = 0.64; dorsal part of the lateral septum: U = 11, P = 0.0023, effect size r = 0.66; ventral part of the lateral septum: U = 5, P = 0.0002, effect size r = 0.77; medial amygdala: U = 0, P < 0.0001, effect size r > 0.99).
FIGURE 4.
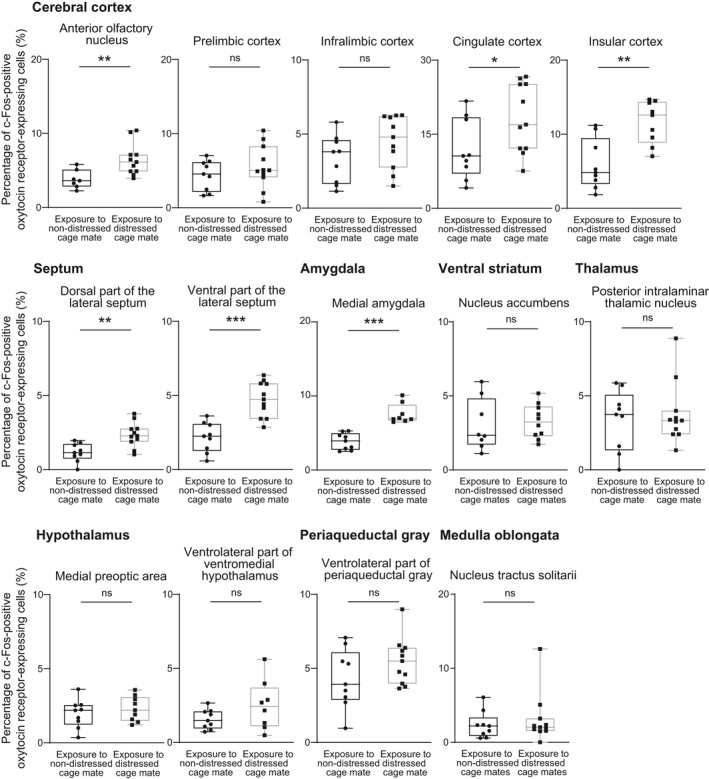
Percentages of oxytocin receptor‐expressing neurones expressing c‐Fos immunoreactivity after exposure to distressed cage mates in oxytocin receptor‐Venus knock‐in heterozygous female mice. Oxytocin receptor‐expressing cells were detected by Venus immunoreactivity in oxytocin receptor‐Venus knock‐in mice. Exposure to distressed cage mates activated oxytocin receptor‐expressing neurones in the anterior olfactory nucleus, cingulate cortex, insular cortex, lateral septum and medial amygdala. The numbers of animals in the groups for non‐distressed control cage mates and for distressed cage mates were 7‐9 and 8‐11, respectively. The maximum, upper quartile, median, lower quartile and minimum values are shown. *P < 0.05, **P < 0.01, ***P < 0.001 vs non‐distressed control cage mates (Mann‐Whitney U test). NS, not significant
FIGURE 5.
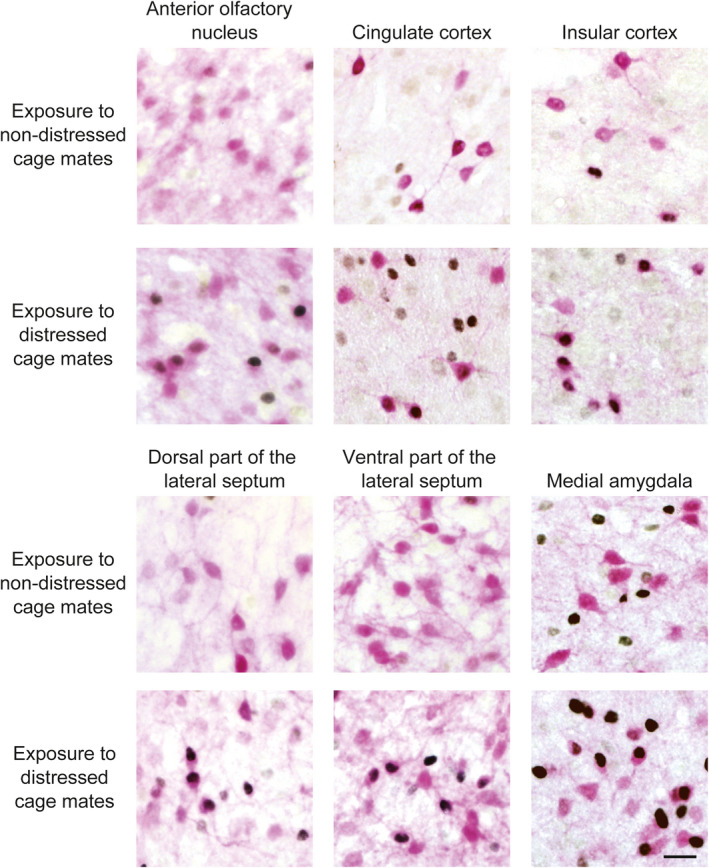
Expression of c‐Fos protein in oxytocin receptor‐expressing neurones in oxytocin receptor‐Venus knock‐in heterozygous female mice after exposure to distressed cage mates. Photographs showing c‐Fos immunoreactivity (black nuclear profiles) in oxytocin receptor‐expressing neurones (pink cell body profiles) of the anterior olfactory nucleus, cingulate cortex, insular cortex, lateral septum and medial amygdala. Exposure to socially defeated cage mates activated oxytocin receptor‐expressing neurones in these areas. The numbers of animals in the groups for non‐distressed control cage mates and for distressed cage mates were 7 or 9 and 8‐11, respectively. Scale bar = 20 μm
The percentages of oxytocin receptor‐expressing neurones showing immunoreactivity of c‐Fos protein in the prelimbic cortex, infralimbic cortex, nucleus accumbens, posterior intralaminar thalamic nucleus, medial preoptic area, ventrolateral part of the ventromedial hypothalamus, ventrolateral periaqueductal grey and nucleus tractus solitarii were not significantly different between animals exposed to distressed cage mates and those exposed to non‐distressed cage mates (Figure 4) (prelimbic cortex: U = 39, P = 0.46, effect size r = 0.17; infralimbic cortex: U = 28, P = 0.11, effect size r = 0.37; nucleus accumbens: U = 32.5, P = 0.53, effect size r = 0.15; posterior intralaminar thalamic nucleus: U = 46, P = 0.82, effect size r = 0.070; medial preoptic area: U = 33, P = 0.55, effect size r = 0.15; ventrolateral part of the ventromedial hypothalamus: U = 22, P = 0.20, effect size r = 0.32; ventrolateral periaqueductal grey: U = 32, P = 0.20, effect size r = 0.30; nucleus tractus solitarii: U = 46, P = 0.82, effect size r = 0.052). The numbers of oxytocin receptor‐expressing cells (Venus‐immunoreactive cells in oxytocin receptor‐Venus knock‐in heterozygous mice) were not significantly different between mice exposed to distressed cage mates and those exposed to non‐distressed cage mates in these brain regions (Figure 6 and Table 1). These results indicate that oxytocin receptor‐expressing neurones in the anterior olfactory nucleus, cingulate cortex, insular cortex, dorsal part of the lateral septum, ventral part of the lateral septum and medial amygdala were activated by exposure to distressed cage mates.
FIGURE 6.
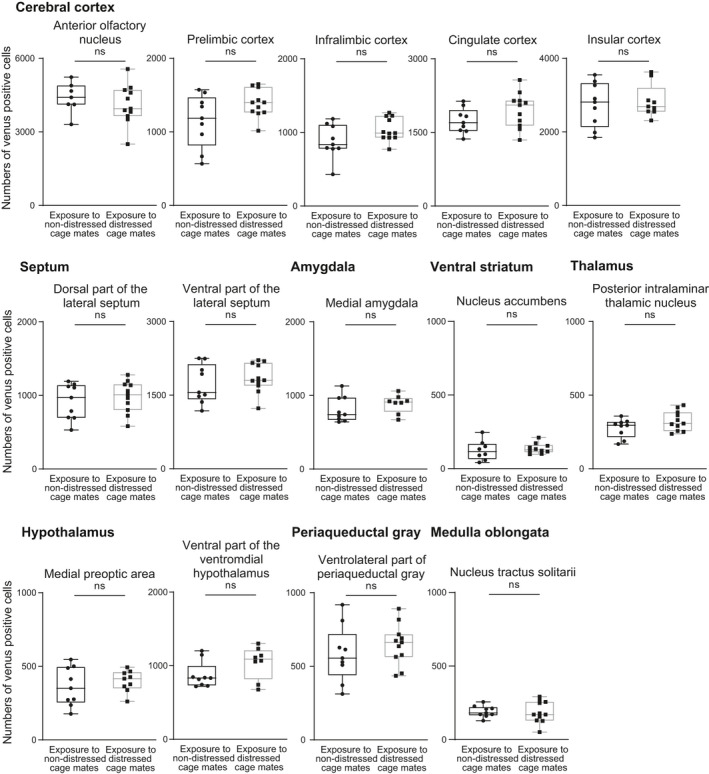
Numbers of oxytocin receptor‐expressing cells in various brain regions in oxytocin receptor‐Venus knock‐in heterozygous female mice. The numbers of oxytocin receptor‐expressing cells (Venus‐immunoreactive cells in oxytocin receptor‐Venus knock‐in heterozygous mice) were not significantly different between mice exposed to distressed cage mates and those exposed to non‐distressed cage mates in the anterior olfactory nucleus, prelimbic cortex, infralimbic cortex, cingulate cortex, insular cortex, dorsal part of the lateral septum, ventral part of the lateral septum, medial amygdala, nucleus accumbens, posterior intralaminar thalamic nucleus, medial preoptic area, ventromedial hypothalamus, periaqueductal grey and nucleus tractus solitarii. The numbers of animals in the groups for non‐distressed control cage mates and for distressed cage mates were 7‐9 and 8‐11, respectively. The maximum, upper quartile, median, lower quartile and minimum values are shown. NS, not significant (Mann‐Whitney U test)
TABLE 1.
U values, P values and effect size r of the Mann‐Whitney U test between the numbers of oxytocin receptor‐expressing cells in mice exposed to distressed cage mates and those in mice exposed to non‐distressed cage mates in various brain regions
| Brain regions | U | P value | Effect size r |
|---|---|---|---|
| Anterior olfactory nucleus | 29 | .43 | 0.22 |
| Prelimbic cortex | 28 | .11 | 0.37 |
| Infralimbic cortex | 27 | .095 | 0.38 |
| Cingulate cortex | 28 | .11 | 0.37 |
| Insular cortex | 38 | .86 | 0.043 |
| Dorsal part of the lateral septum | 41 | .55 | 0.14 |
| Ventral part of the lateral septum | 40 | .50 | 0.16 |
| Medial amygdala | 25 | .32 | 0.25 |
| Nucleus accumbens | 32 | .50 | 0.16 |
| Posterior intralaminar thalamic nucleus | 32.5 | .21 | 0.29 |
| Medial preoptic area | 34 | .60 | 0.13 |
| Ventrolateral part of ventromedial hypothalamus | 24 | .28 | 0.28 |
| Ventrolateral part of periaqueductal grey | 34 | .26 | 0.26 |
| Nucleus tractus solitarii | 44.5 | .72 | 0.096 |
On the other hand, the percentages of c‐Fos protein‐expressing oxytocin‐immunoreactive neurones in the BNST, SON and PVN were not significantly changed after exposure to distressed cage mates (Figure 7) (BNST: U = 35, P = 0.43, effect size r = 0.18; SON: U = 26, P = 0.56, effect size r = 0.16; PVN: U = 38, P = 0.60, effect size r = 0.13).
FIGURE 7.
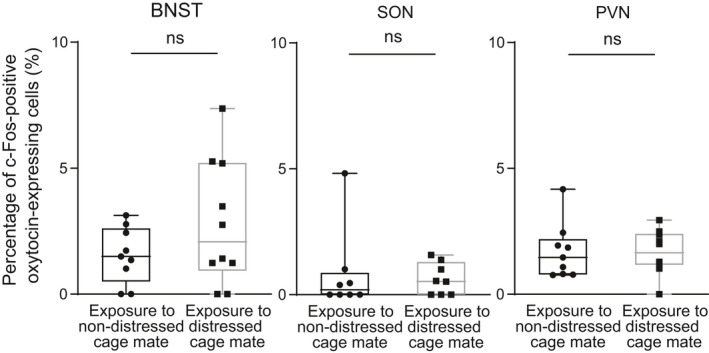
Percentages of oxytocin‐immunoreactive neurones expressing c‐Fos immunoreactivity after exposure to distressed cage mates in oxytocin receptor‐Venus knock‐in heterozygous female mice. Exposure to distressed cage mates did not significantly change the percentages of oxytocin‐immunoreactive neurones expressing c‐Fos immunoreactivity. The numbers of animals in the groups for non‐distressed control cage mates and for distressed cage mates were 8 or 9 and 8 or 10, respectively. The maximum, upper quartile, median, lower quartile and minimum values are shown. NS, not significant as compared with non‐distressed control cage mates (Mann‐Whitney U test), BNST, bed nucleus of the stria terminalis; SON, supraoptic nucleus; PVN, hypothalamic paraventricular nucleus
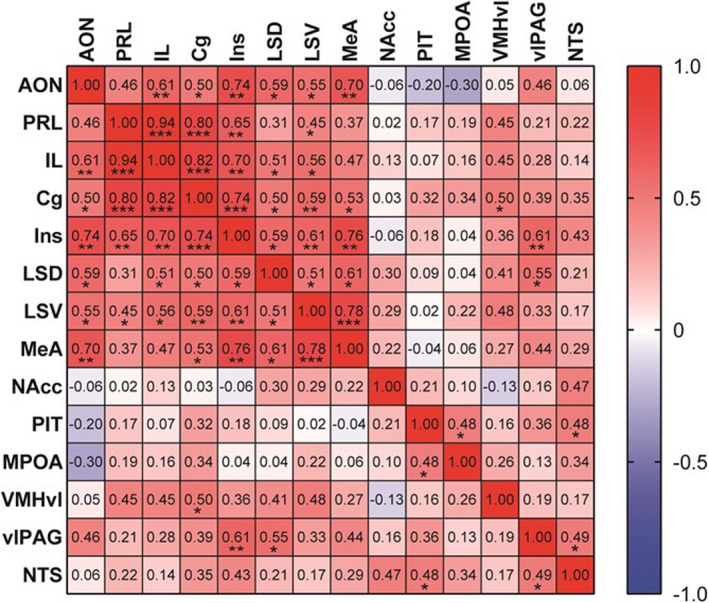
We also investigated the relationship between allogrooming behaviour, side‐by‐side contact or sniffing behaviour and percentages of c‐Fos‐positive oxytocin receptor‐expressing neurones in the anterior olfactory nucleus, cingulate cortex, insular cortex dorsal and ventral parts of the lateral septum and medial amygdala. The duration of allogrooming was significantly correlated with percentages of c‐Fos‐positive oxytocin receptor‐expressing neurones in the anterior olfactory nucleus, insular cortex, dorsal and ventral parts of the lateral septum, and medial amygdala but not in the cingulate cortex (Figure 8 and Table 2). Significant correlations were found among percentages of c‐Fos‐positive oxytocin receptor‐expressing neurones in these brain areas (Table 3). There were high correlation coefficients between the percentage of c‐Fos protein‐positive oxytocin receptor‐expressing neurones in the insular cortex and percentages in other forebrain areas.
FIGURE 8.
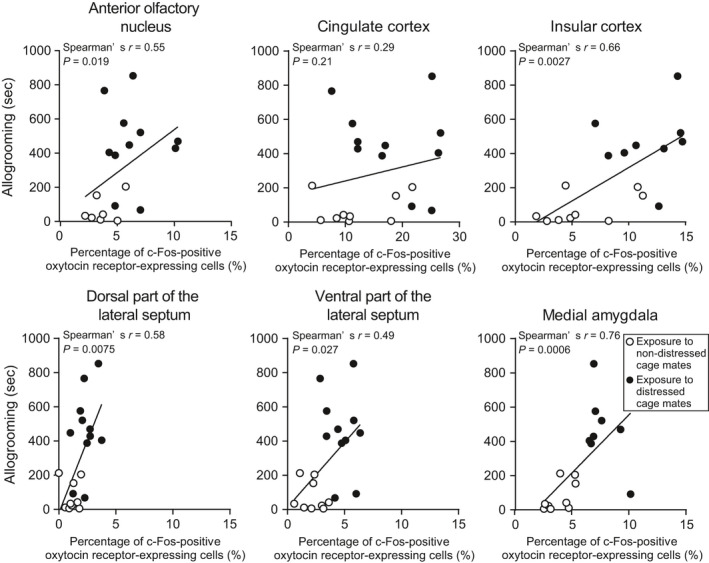
Correlation coefficients between duration of allogrooming and percentages of c‐Fos‐positive oxytocin receptor‐expressing neurones in oxytocin receptor‐Venus knock‐in heterozygous female mice. There were significant correlations between duration of allogrooming and percentages of c‐Fos‐positive oxytocin receptor‐expressing neurones in the anterior olfactory nucleus, insular cortex, lateral septum and medial amygdala but not in the cingulate cortex. The numbers of animals in the groups for non‐distressed control cage mates and for distressed cage mates were 7 or 9 and 8‐11, respectively. Data were analysed by Spearman’s correlation
TABLE 2.
Spearman’s correlations between time duration spent for behaviours toward distressed cage mates and percentages of oxytocin receptor‐expressing neurones showing c‐Fos protein in various brain regions in oxytocin receptor‐Venus knock‐in heterozygous female mice
| Brain regions | Allogrooming | Side‐by‐side contact | Sniffing | |||
|---|---|---|---|---|---|---|
| Spearman’s r | P value | Spearman’s r | P value | Spearman’s r | P value | |
| Anterior olfactory nucleus | 0.55 | .019 | 0.42 | .080 | −0.0062 | .98 |
| Cingulate cortex | 0.29 | .21 | 0.18 | .44 | 0.36 | .12 |
| Insular cortex | 0.66 | .0027 | 0.33 | .18 | 0.022 | .93 |
| Dorsal part of the lateral septum | 0.58 | .0075 | 0.39 | .094 | 0.13 | .58 |
| Ventral part of the lateral septum | 0.49 | .027 | 0.25 | .29 | 0.58 | .0079 |
| Medial amygdala | 0.76 | .0006 | 0.45 | .073 | 0.16 | .55 |
TABLE 3.
Spearman’s correlations among percentages of oxytocin receptor‐expressing neurones showing c‐Fos protein in various brain regions of oxytocin receptor‐Venus knock‐in heterozygous female mice. Spearman’s r values are shown. *P < 0.05, **P < 0.01, ***P < 0.001
No significant correlation coefficients were found between side‐by‐side contact or sniffing and percentages of c‐Fos‐positive oxytocin receptor‐expressing neurones in the anterior olfactory nucleus, cingulate cortex, insular cortex dorsal and ventral parts of the lateral septum, and medial amygdala, except between sniffing and percentage of c‐Fos protein‐positive oxytocin receptor‐expressing neurones in the ventral part of the lateral septum (Table 2). These results are consistent with the view that activation of oxytocin receptor‐expressing neurones in the anterior olfactory nucleus, insular cortex, dorsal and ventral parts of the lateral septum, and medial amygdala is involved in allogrooming behaviour toward socially distressed cage mates.
3.3. Effects of oxytocin receptor deficiency on allogrooming behaviour and side‐by‐side contact of female mice toward distressed cage mates
To determine the roles of the oxytocin receptor in allogrooming behaviour and side‐by‐side contact toward distressed cage mates, we examined behaviours of oxytocin receptor‐deficient female mice toward their socially defeated cage mates. Oxytocin receptor‐deficient female mice spent a much shorter time in allogrooming behaviour toward their distressed cage mates than did wild‐type mice (Figure 9) (U = 3, P = 0.0037, effect size r = −0.77). On the other hand, there was no significant difference in the time spent for side‐by‐side contact or the time spent sniffing (Figure 9) (side‐by‐side contact: U = 26, P = 0.61, effect size r = 0.16; sniffing: U = 21, P = 0.51, effect size r = 0.27). All of these results suggest that the oxytocin receptor was indispensable for allogrooming behaviour of female mice toward distressed cage mates.
FIGURE 9.
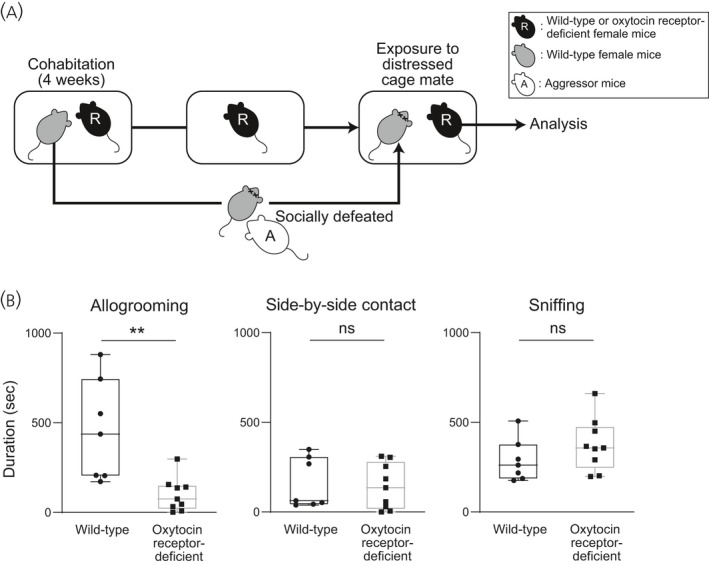
Effects of oxytocin receptor deficiency on durations of allogrooming, side‐by‐side contact and sniffing behaviours toward distressed cage mates in wild‐type and oxytocin receptor‐deficient female mice. A, Scheme of the behaviour test. Pairs of resident test mice (R, wild‐type or oxytocin receptor‐deficient mice) and cage mates were housed in cages for 4 weeks. After 4 weeks, cage mates were removed from their home cages, kept isolated overnight, and then returned to their previous home cages. Resident test mice were exposed to cage mates that had been defeated by aggressor CD1 mice (A). B, Times spent for allogrooming, side‐by‐side contact and sniffing behaviours toward distressed cage mates of wild‐type and oxytocin receptor‐deficient female mice. The duration of allogrooming behaviour toward defeated cage mates was significantly shorter in oxytocin receptor‐deficient females than in wild‐type females, suggesting that deficiency in the oxytocin receptor blocked allogrooming behaviour toward distressed cage mates. The numbers of wild‐type and oxytocin receptor‒deficient females were 7 and 9, respectively. The maximum, upper quartile, median, lower quartile and minimum values are shown. **P < 0.01 vs wild‐type females (Mann‐Whitney U test). NS, not significant
In oxytocin receptor‐deficient male mice, the time spent for allogrooming behaviour, the time spent for side‐by‐side contact behaviour and the time spent for sniffing behaviour were not significantly different from the times spent for those behaviours in wild‐type mice (Figure 10) (allogrooming: U = 26, P = 0.89, effect size r = 0.19; side‐by‐side contact: U = 31, P = 0.99, effect size r = 0.063; sniffing: U = 33, P > 0.99, effect size r = 0.00), suggesting that the oxytocin receptor is not essential for allogrooming, side‐by‐side contact and sniffing behaviours of male mice toward their distressed cage mates.
FIGURE 10.
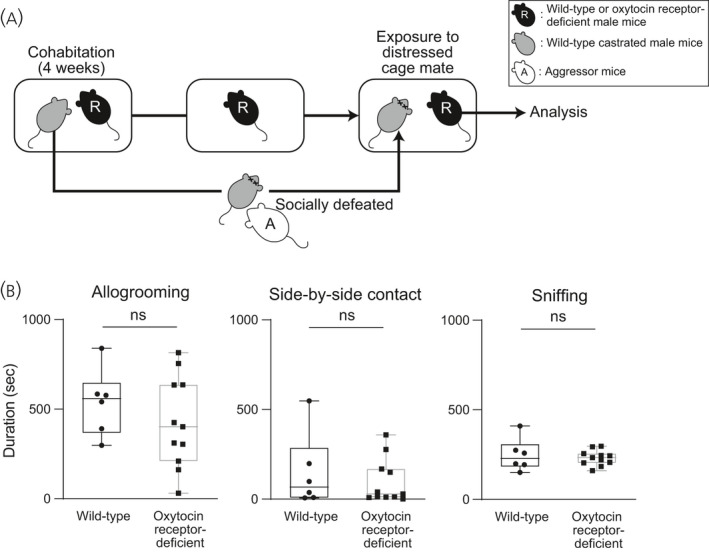
Times spent for allogrooming, side‐by‐side contact and sniffing behaviours of oxytocin receptor‐deficient male mice toward distressed cage mates. A, Scheme of the behaviour test. Pairs of resident test mice (R) and cage mates were housed in cages for 4 weeks. After 4 weeks, cage mates were removed from their home cages, kept isolated overnight, and then returned to their previous home cages. Resident test mice (R, wild‐type or oxytocin receptor‐deficient mice) were exposed to cage mates that had been defeated by aggressor CD1 mice (A). B, Times spent for allogrooming, side‐by‐side contact and sniffing behaviours toward distressed cage mates of wild‐type and oxytocin receptor‐deficient male mice. Times spent for allogrooming, side‐by‐side contact and sniffing behaviours toward distressed cage mates were not significantly different between wild‐type and oxytocin receptor‐deficient males. The numbers of wild‐type and oxytocin receptor‐deficient males were 6 and 11, respectively. The maximum, upper quartile, median, lower quartile and minimum values are shown. NS, not significant (Mann‐Whitney U test)
4. DISCUSSION
Monogamous voles, but not promiscuous voles, have been reported to show affiliative allogrooming behaviour toward cage mates that are distressed with conditioned fear in an oxytocin receptor‐dependent manner. 5 In the present study, we found that commonly used laboratory mice showed allogrooming behaviour and side‐by‐side contact behaviour toward socially distressed cage mates. We also obtained data that are consistent with the view of the existence of a sexual difference in the underlying mechanisms of allogrooming behaviour toward distressed conspecifics in mice. Genetic deficiency of the oxytocin receptor impaired allogrooming behaviour toward distressed cage mates in female mice but not in male mice, suggesting that the oxytocin receptor is indispensable for allogrooming behaviour only in females. These findings suggest that female mice show allogrooming behaviour toward distressed cage mates in an oxytocin receptor‐dependent way, whereas males show allogrooming via different mechanisms.
In the present study, allogrooming toward distressed cage mates was reduced in oxytocin receptor‐deficient female mice but not in oxytocin receptor‐deficient male mice. The apparent sexual differences observed in the present study suggests a possible sexual difference in the mechanisms of allogrooming toward distressed conspecifics. However, the protocols of social defeat stress were different for male and female mice. The maximum exposure times to aggressor mice were 3 minutes in female mice and 10 minutes in male mice. Female mice received aggression from mice of the opposite sex (male) and male mice received aggression from mice of the same sex (male). The difference in the protocols might have affected behaviours of demonstrator mice and then those of observer mice, although female and male observer mice showed comparable amounts of allogrooming behaviour toward distressed conspecifics. Further study is necessary to fully clarify the effects of the difference in the protocols on the apparent sexual difference in oxytocin receptor‐dependency of allogrooming toward distressed conspecifics.
Allogrooming has been categorised as an affiliative behaviour for promoting positive social interaction in mice. 23 On the other hand, allogrooming, which eventually results in alopecia of the recipients, has been reported to be exhibited in mice by dominant animals toward subordinate cage mates. 24 , 25 The precise roles of allogrooming remain to be clarified in mice. However, allogrooming has been proposed to be a coping behaviour to reduce stress. 26 Gentle touch stimuli activate a low‐threshold mechanoreceptor to induce pleasant sensation in mice 27 and tactile stimuli reduce stress responses in rats. 4 In prairie voles, allogrooming induces anxiolytic actions. 5 It is thus interesting to speculate that allogrooming behaviour in the female mice observed in the present study had stress‐reducing actions and is categorised as consolation‐related behaviour.
In the present study, oxytocin receptor‐deficient female mice showed a reduction in allogrooming behaviour toward distressed cage mates. The oxytocin receptor has been shown to be involved in social interaction, 28 reduction in anxiety 10 and social recognition. 29 Oxytocin receptor‐deficient male mice have been shown to have deficits in social interactions, including a reduction in huddling and allogrooming 28 and to show social amnesia. 19 , 29 It is thus possible that a general reduction in social interaction, increased anxiety and social amnesia as a result of deficiency in the oxytocin receptor might have contributed to the reduced allogrooming of female mice observed in the present study. However, side‐by‐side contact and sniffing behaviour did not significantly change in the present study, suggesting that that possible a general reduction in social interaction and social amnesia were not the main causes for a selective reduction in allogrooming.
In the present study, oxytocin receptor heterozygous knockout female mice (oxytocin receptor‐Venus heterozygous mice) showed allogrooming toward distressed cage mates similarly to wild‐type mice, whereas oxytocin receptor‐deficient female mice showed a low level of allogrooming. Oxytocin receptor heterozygous knockout mice have been shown to exhibit impaired social behaviour but exhibit normal aggressive behaviour and no cognitive inflexibility, whereas oxytocin receptor‐deficient mice show impaired social behaviour, increased aggressive behaviour and cognitive inflexibility. 30 In the present study, experiments for directly comparing amounts of allogrooming between wild‐type female and oxytocin receptor‐heretozygous knockout female mice were not performed and thus it remains to be determined whether oxytocin receptor heterozygous mice show any impairment in alllogrooming and consolation‐related behaviours as compared to wild‐type mice.
Promiscuous meadow voles do not show allogrooming behaviour toward familiar conspecifics that are distressed with conditioned fear. 5 In the present study, non‐monogamous mice showed allogrooming behaviour toward socially distressed conspecifics. Monogamous voles showed allogrooming behaviour toward familiar but not unfamiliar animals. 3 In the present study, female mice showed allogrooming behaviour toward distressed unfamiliar mice, suggesting that familiarity is not essential for inducing allogrooming behaviour in female mice. Consistent with the idea that familiarity is not essential for empathy‐related behaviours, prior pain experience enhances consolation‐related allogrooming behaviour in rats toward unfamiliar conspecifics that have received noxious stimuli. 31 Further studies are necessary to clarify the precise roles of familiarity in consolation‐related behaviours.
Female rats show approach behaviour to distressed familiar conspecifics. 6 , 7 In the present study, female mice showed allogrooming behaviour toward distressed conspecifics. Signals that are emitted by distressed animals and induce allogrooming behaviour in resident animals remain to be determined. Rodents can transmit their affective states via olfactory signals such as chemosignals, 32 , 33 auditory signals such as vocalisations, 34 , 35 and visual signals such as freezing behaviours and facial expression. 36
Detection of alarm signals emitted by other individuals and avoidance of distressed conspecifics may be adaptive because they help to avoid a possible threat. Male rats have been shown to avoid distressed same‐sex familiar or unfamiliar adult conspecifics, whereas male rats approach distressed juveniles. 37 However, social animals sometimes approach and perform affiliative behaviour toward distressed conspecifics in certain conditions. 38 Staying close to distressed conspecifics has been shown to attenuate stress responses in rats 39 , 40 and mice 41 , 42 of both sexes, a phenomenon called social buffering. Some gregarious animals show not only passive behaviours such as staying close to distressed conspecifics but also active behaviours such as making friendly contact with distressed conspecifics, behaviours that reduce stress responses in distressed conspecifics 43 and are considered to be consolation‐related behaviours. 44 Consolation‐related behaviours such as grooming, gentle touching and embracing have been documented in both primates and non‐primates, including gorillas, 45 chimpanzees, 12 , 44 bonobos, 46 monkeys, 47 elephants, 48 dogs, 49 wolves, 50 monogamous prairie voles 5 and corvids. 48 These empathy‐related behaviours are assumed to be associated with cooperation among group members and to contribute to the formation and maintenance of groups. 38 The results of the present study showing allogrooming behaviour toward distressed conspecifics are consistent with the view that mice can show consolation‐related behaviour toward distressed conspecifics. 8
Empathy‐related behaviours are assumed to be evolutionally originated from maternal care, and the involvement of oxytocin has been suggested. 14 , 51 , 52 , 53 Postpartum females show caregiving behaviours toward their young, including pup retrieval, crouching, lactating and licking, 54 and it has been demonstrated that deficiency in the oxytocin receptor results in deficits in maternal behaviours, 29 suggesting the involvement of oxytocin in parental behaviours. Social buffering, which attenuates stress responses of cage mates, has also been shown to depend on oxytocin. 55 The results of the present study showing that deficiency of the oxytocin receptor in female mice impaired allogrooming behaviour toward distressed conspecifics suggest the involvement of the oxytocin receptor in allogrooming behaviour toward distressed conspecifics in female mice. However, the precise site of the oxytocin receptor responsible for allogrooming behaviour toward distressed conspecifics remains unknown. The oxytocin receptor in the medial preoptic nucleus has been shown to play an important role in maternal behaviours. 54 During maternal behaviours, which were induced by repeated exposures to pups, oxytocin receptor‐expressing neurones have been shown to be activated in the medial preoptic area. 56 However, in the present study, oxytocin receptor‐expressing neurones in the medial preoptic area were not activated during allogrooming behaviour toward distressed cage mates, suggesting that the oxytocin receptor in the medial preoptic area is not essential for allogrooming behaviour toward distressed conspecifics and being consistent with the view that neuronal mechanisms for consolation‐related behaviours toward distressed conspecifics are different from those for maternal affiliative behaviours. On the other hand, oxytocin receptor‐expressing neurones in the anterior olfactory nucleus, insular cortex, lateral septum and medial amygdala were activated after exposure to distressed cage mates, and the activation in those areas was correlated with the duration of allogrooming in the present study, although it remains to be determined whether activation of oxytocin receptor‐expressing neurones was caused by stimulation of the oxytocin receptor. Furthermore, allogrooming behaviour toward distressed mice was impaired by oxytocin receptor deficiency in female mice. All of these findings suggest that activation of oxytocin receptor‐expressing neurones in these areas of female mice is involved in allogrooming behaviour toward socially distressed cage mates. Furthermore, neurones in these forebrain areas have anatomical and physiological connections with each other. 57 , 58 High correlation coefficients were found in c‐Fos protein expression of oxytocin receptor‐expressing neurones among these forebrain areas, especially between the insular cortex and other forebrain areas, suggesting the possible importance of the insular cortex. Consistent with the present results, the insular cortex has been shown to be involved in empathy in humans. 59 , 60 The oxytocin receptor in the insular cortex of mice has been reported to be necessary for approaching behaviour to distressed conspecifics. 37 On the other hand, the oxytocin receptor in the medial amygdala has been shown to play an important role in processing of social olfactory signals. 19 , 61 , 62 Although the precise function of the oxytocin receptor remains unclear, it is possible that the oxytocin receptor is involved in detection and/or processing of signals emitted by distressed conspecifics and/or in elicitation of allogrooming behaviour toward distressed conspecifics. Further studies are necessary for clarification of the responsible sites and functions of the oxytocin receptor for allogrooming behaviours toward socially distressed conspecifics.
In the present study, no significant increase in the expression of c‐Fos protein was found in oxytocin neurones of the hypothalamus and the BNST. However, the absence of c‐Fos protein expression in oxytocin neurones does not necessarily indicate the absence of increased activity of oxytocin neurones or increased oxytocin release. 63 , 64 , 65 A recent electrophysiological study has shown that social interactions and social physical contact activate hypothalamic oxytocin neurones. 64 Hypothalamic oxytocin neurones send considerable amounts of axonal projections to the forebrain areas, 18 where activated oxytocin receptor‐expressing neurones were found in the present study. It is thus tempting to speculate the possible involvement of hypothalamic oxytocin‐forebrain oxytocin receptor systems in allogrooming behaviour toward distressed conspecifics.
The oxytocin receptor in the anterior cingulate cortex has been shown to be involved in allogrooming behaviour toward partners distressed with conditioned fear in monogamous prairie voles. 5 Freezing behaviour in response to exposure to conspecifics distressed with conditioned fear, which is called observational fear, has also been reported to be facilitated by activation of oxytocin neurones 66 and is attenuated by administration of an oxytocin receptor antagonist in the anterior cingulate cortex. 67 In the present study, oxytocin receptor‐expressing neurones were activated by exposure to distressed cage mates, although activation of the neurones in the cingulate cortex was not significantly correlated with the duration of allogrooming behaviour toward socially distressed cage mates. Further studies are necessary to clarify the roles of the oxytocin receptor in the cingulate cortex in allogrooming behaviour toward distressed conspecifics in mice.
The results of the present study suggest the involvement of the oxytocin receptor possibly in the anterior olfactory nucleus, insular cortex, lateral septum and medial amygdala for induction of allogrooming behaviour toward distressed conspecifics in female mice. Consolation‐related behaviour reduces anxiety not only in the receiver, but also in the giver of consolation. 5 The present mouse model of allogrooming behaviours is expected to contribute to clarification of neural mechanisms of empathy‐related behaviours.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Makiya Matsumoto: Investigation; Methodology; Writing – review & editing. Masahide Yoshida: Conceptualisation; Funding acquisition; Investigation; Methodology; Supervision; Writing – review & editing. Buddhini Wimarsha Jayathilake: Investigation; Methodology. Ayumu Inutsuka: Funding acquisition; Investigation; Methodology; Writing – review & editing. Katsuhiko Nishimori: Resources. Yuki Takayanagi: Funding acquisition; Investigation; Methodology; Writing – review & editing. Tatsushi Onaka: Conceptualisation; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing.
ACKNOWLEDGEMENTS
This work was supported in part by JSPS KAKENHI (Grants‐in‐Aid for Scientific Research) (17H04026, 17K19636 and 20H03419 to TO; 17K08574 and 20K07264 to MY; 17K08573 and 20K07278 to YT; and 16K08527, 19H05026 and 19K06910 to AI), by Takeda Science Foundation to TO and YT, by JMU Graduate Student Research Award to MM, and by JMU Graduate Student Start‐Up Grant for Young Investigators to MM. KN was supported by Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and Development (AMED; 18dm0107076h0003, 2016–2020).
Matsumoto M, Yoshida M, Jayathilake BW, et al. Indispensable role of the oxytocin receptor for allogrooming toward socially distressed cage mates in female mice. J Neuroendocrinol. 2021;33:e12980. 10.1111/jne.12980
Contributor Information
Masahide Yoshida, Email: y-masa@jichi.ac.jp, Email: tonaka@jichi.ac.jp.
Tatsushi Onaka, Email: tonaka@jichi.ac.jp.
DATA AVAILABILITY STATEMENT
The data are available from the corresponding authors upon reasonable request.
REFERENCES
- 1. Dunbar RI. The social role of touch in humans and primates: behavioural function and neurobiological mechanisms. Neurosci Biobehav Rev. 2010;34:260‐268. [DOI] [PubMed] [Google Scholar]
- 2. Schino G. Grooming, competition and social rank among female primates: a meta‐analysis. Anim Behav. 2001;62:265‐271. [Google Scholar]
- 3. Young LJ, Winslow JT, Wang Z, et al. Gene targeting approaches to neuroendocrinology: oxytocin, maternal behavior, and affiliation. Horm Behav. 1997;31:221‐231. [DOI] [PubMed] [Google Scholar]
- 4. Kiyokawa Y, Hennessy MB. Comparative studies of social buffering: a consideration of approaches, terminology, and pitfalls. Neurosci Biobehav Rev. 2018;86:131‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ. Oxytocin‐dependent consolation behavior in rodents. Science. 2016;351:375‐378. 10.1126/science.aac4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Langford DJ, Tuttle AH, Brown K, et al. Social approach to pain in laboratory mice. Soc Neurosci. 2010;5:163‐170. 10.1080/17470910903216609 [DOI] [PubMed] [Google Scholar]
- 7. Rogers‐Carter MM, Djerdjaj A, Culp AR, Elbaz JA, Christianson JP. Familiarity modulates social approach toward stressed conspecifics in female rats. PLoS ONE. 2018;13:e0200971. 10.1371/journal.pone.0200971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Du R, Luo W‐J, Geng K‐W, et al. Empathic contagious pain and consolation in laboratory rodents: species and sex comparisons. Neurosci Bull. 2020;36:649‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214:443‐449. 10.1016/j.bbr.2010.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Onaka T, Takayanagi Y. Role of oxytocin in the control of stress and food intake. J Neuroendocrinol. 2019;31:e12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amico JA, Vollmer RR, Karam JR, et al. Centrally administered oxytocin elicits exaggerated grooming in oxytocin null mice. Pharmacol Biochem Behav. 2004;78:333‐339. [DOI] [PubMed] [Google Scholar]
- 12. Romero T, Castellanos MA, de Waal FB. Consolation as possible expression of sympathetic concern among chimpanzees. Proc Natl Acad Sci USA. 2010;107:12110‐12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silva BA, Mattucci C, Krzywkowski P, et al. Independent hypothalamic circuits for social and predator fear. Nat Neurosci. 2013;16:1731‐1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christov‐Moore L, Simpson EA, Coude G, Grigaityte K, Iacoboni M, Ferrari PF. Empathy: gender effects in brain and behavior. Neurosci Biobehav Rev. 2014;46:604‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoshida M, Takayanagi Y, Inoue K, et al. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29:2259‐2271. 10.1523/jneurosci.5593-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guenthner Casey J, Miyamichi K, Yang Helen H, Heller HC, Luo L. Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron. 2013;78:773‐784. 10.1016/j.neuron.2013.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Golden SA, Covington HE 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nasanbuyan N, Yoshida M, Takayanagi Y, et al. Oxytocin‐oxytocin receptor systems facilitate social defeat posture in male mice. Endocrinology. 2018;159:763‐775. 10.1210/en.2017-00606 [DOI] [PubMed] [Google Scholar]
- 19. Takayanagi Y, Yoshida M, Takashima A, et al. Activation of supraoptic oxytocin neurons by secretin facilitates social recognition. Biol Psychiatry. 2017;81:243‐251. 10.1016/j.biopsych.2015.11.021 [DOI] [PubMed] [Google Scholar]
- 20. Kaneko T, Caria MA, Asanuma H. Information processing within the motor cortex. II. Intracortical connections between neurons receiving somatosensory cortical input and motor output neurons of the cortex. J Comp Neurol. 1994;345:172‐184. [DOI] [PubMed] [Google Scholar]
- 21. Paxinos G, Keith BJ. Paxinos and Franklin's The Mouse Brain in Stereotaxic Coordinates. London: Academic Press; 2012. [Google Scholar]
- 22. Shimizu H. An introduction to the statistical free software HAD: suggestions to improve teaching, learning and practice data analysis. J Media Inform Commun. 2016;1:59‐73. [Google Scholar]
- 23. McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism‐like behavioral phenotypes in BTBR T+tf/J mice. Genes, Brain Behav. 2008;7:152‐163. 10.1111/j.1601-183x.2007.00330.x [DOI] [PubMed] [Google Scholar]
- 24. Long SY. Hair‐nibbling and whisker‐trimming as indicators of social hierarchy in mice. Anim Behav. 1972;20:10‐12. [DOI] [PubMed] [Google Scholar]
- 25. Lee W, Fu J, Bouwman N, Farago P, Curley JP. Temporal microstructure of dyadic social behavior during relationship formation in mice. PLoS ONE. 2019;14:e0220596. 10.1371/journal.pone.0220596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dufour BD, Garner JP. An ethological analysis of barbering behavior. In: Kalueff AV, Bergner CL, La Porte JL, eds. Neurobiology of Grooming Behavior. Cambridge: Cambridge University Press; 2010:184‐225. [Google Scholar]
- 27. McGlone F, Wessberg J, Olausson H. Discriminative and affective touch: sensing and feeling. Neuron. 2014;82:737‐755. [DOI] [PubMed] [Google Scholar]
- 28. Pobbe RLH, Pearson BL, Defensor EB, et al. Oxytocin receptor knockout mice display deficits in the expression of autism‐related behaviors. Horm Behav. 2012;61:436‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takayanagi Y, Yoshida M, Bielsky IF, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor‐deficient mice. Proc Natl Acad Sci USA. 2005;102:16096‐16101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sala M, Braida D, Donzelli A, et al. Mice heterozygous for the oxytocin receptor gene (Oxtr(+/‐)) show impaired social behaviour but not increased aggression or cognitive inflexibility: evidence of a selective haploinsufficiency gene effect. J Neuroendocrinol. 2013;25:107‐118. [DOI] [PubMed] [Google Scholar]
- 31. Luo WJ, Li CL, Geng KW, et al. The similar past pain experience evokes both observational contagious pain and consolation in stranger rat observers. Neurosci Lett. 2020;722:134840. [DOI] [PubMed] [Google Scholar]
- 32. Valenta JG, Rigby MK. Discrimination of the odor of stressed rats. Science. 1968;161:599‐601. 10.1126/science.161.3841.599 [DOI] [PubMed] [Google Scholar]
- 33. Kiyokawa Y. Social odors: alarm pheromones and social buffering. Curr Top Behav Neurosci. 2017;30:47‐65. [DOI] [PubMed] [Google Scholar]
- 34. Brudzynski SM. Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr Opin Neurobiol. 2013;23:310‐317. [DOI] [PubMed] [Google Scholar]
- 35. Warren MR, Clein RS, Spurrier MS, Roth ED, Neunuebel JP. Ultrashort‐range, high‐frequency communication by female mice shapes social interactions. Sci Rep. 2020;10: 2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Langford DJ, Bailey AL, Chanda ML, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447‐449. 10.1038/nmeth.1455 [DOI] [PubMed] [Google Scholar]
- 37. Rogers‐Carter MM, Varela JA, Gribbons KB, et al. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci. 2018;21:404‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Waal FBM, Preston SD. Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci. 2017;18:498‐509. [DOI] [PubMed] [Google Scholar]
- 39. Kiyokawa Y, Takeuchi Y, Mori Y. Two types of social buffering differentially mitigate conditioned fear responses. Eur J Neuorsci. 2007;26:3606‐3613. 10.1111/j.1460-9568.2007.05969.x [DOI] [PubMed] [Google Scholar]
- 40. Ishii A, Kiyokawa Y, Takeuchi Y, Mori Y. Social buffering ameliorates conditioned fear responses in female rats. Horm Behav. 2016;81:53‐58. [DOI] [PubMed] [Google Scholar]
- 41. Liu H, Yuan TF. Physical interaction is required in social buffering induced by a familiar conspecific. Sci Rep. 2016;6:39788. 10.1038/srep39788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gutzeit VA, Ahuna K, Santos TL, et al. Optogenetic reactivation of prefrontal social neural ensembles mimics social buffering of fear. Neuropsychopharmacology. 2020;45:1068‐1077. 10.1038/s41386-020-0631-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Han Y, Bruls R, Soyman E, et al. Bidirectional cingulate‐dependent danger information transfer across rats. PLoS Biol. 2019;17:e3000524. 10.1371/journal.pbio.3000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fraser ON, Stahl D, Aureli F. Stress reduction through consolation in chimpanzees. Proc Natl Acad Sci. 2008;105:8557‐8562. 10.1073/pnas.0804141105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mallavarapu S, Stoinski TS, Bloomsmith MA, Maple TL. Postconflict behavior in captive western lowland gorillas (Gorilla gorilla gorilla). Am J Primatol. 2006;68:789‐801. [DOI] [PubMed] [Google Scholar]
- 46. Clay Z, de Waal FB. Bonobos respond to distress in others: consolation across the age spectrum. PLoS ONE. 2013;8:e55206. 10.1371/journal.pone.0055206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Palagi E, Dall’Olio S, Demuru E, Stanyon R. Exploring the evolutionary foundations of empathy: consolation in monkeys. Evol Hum Behav. 2014;35:341‐349. [Google Scholar]
- 48. Plotnik JM, de Waal FB. Asian elephants (Elephas maximus) reassure others in distress. PeerJ. 2014;2:e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cools AK, Vam Hout AJM, Nelissen MH. Canine reconciliation and third‐party‐initiated postconflict affiliation: do peacemaking social mechanisms in dogs rival those of higher primates? Ethology. 2008;114:53‐63. [Google Scholar]
- 50. Cordoni G, Palagi E. Being a victim or an aggressor: different functions of triadic post‐conflict interactions in wolves (Canis lupus lupus). Aggress Behav. 2015;41:526‐536. [DOI] [PubMed] [Google Scholar]
- 51. MacLean PD. Brain evolution relating to family, play, and the separation call. Arch Gen Psychiatry. 1985;42:405‐417. [DOI] [PubMed] [Google Scholar]
- 52. Baron‐Cohen S, Knickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310:819‐823. [DOI] [PubMed] [Google Scholar]
- 53. Robinson KJ, Bosch OJ, Levkowitz G, Busch KE, Jarman AP, Ludwig M. Social creatures: model animal systems for studying the neuroendocrine mechanisms of social behaviour. J Neuroendocrinol. 2019;31:e12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yoshihara C, Numan M, Kuroda KO. Oxytocin and parental behaviors. Curr Top Behav Neurosci. 2018;35:119‐153. [DOI] [PubMed] [Google Scholar]
- 55. Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiat. 2014;76:281‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Okabe S, Tsuneoka Y, Takahashi A, et al. Pup exposure facilitates retrieving behavior via the oxytocin neural system in female mice. Psychoneuroendocrinology. 2017;79:20‐30. [DOI] [PubMed] [Google Scholar]
- 57. Nieuwenhuys R. The insular cortex: a review. Prog Brain Res. 2012;195:123‐163. [DOI] [PubMed] [Google Scholar]
- 58. Rogers‐Carter MM, Christianson JP. An insular view of the social decision‐making network. Neurosci Biobehav Rev. 2019;103:119‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gu X, Hof PR, Friston KJ, Fan J. Anterior insular cortex and emotional awareness. J Comp Neurol. 2013;521:3371‐3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bernhardt BC, Singer T. The neural basis of empathy. Annu Rev Neurosci. 2012;35:1‐23. [DOI] [PubMed] [Google Scholar]
- 61. Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278‐8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yao S, Bergan J, Lanjuin A, Dulac C. Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. eLife. 2017;6:e31373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hoffman GE, Lyo D. Anatomical markers of activity in neuroendocrine systems: are we all ‘fos‐ed out’? J Neuroendocrinol. 2002;14:259‐268. [DOI] [PubMed] [Google Scholar]
- 64. Tang Y, Benusiglio D, Lefevre A, et al. Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Nat Neurosci. 2020;23:1125‐1137. [DOI] [PubMed] [Google Scholar]
- 65. Fenelon VS, Poulain DA, Theodosis DT. Oxytocin neuron activation and Fos expression: a quantitative immunocytochemical analysis of the effect of lactation, parturition, osmotic and cardiovascular stimulation. Neuroscience. 1993;53:77‐89. [DOI] [PubMed] [Google Scholar]
- 66. Pisansky MT, Hanson LR, Gottesman II, Gewirtz JC. Oxytocin enhances observational fear in mice. Nat Commun. 2017;8:2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sakaguchi T, Iwasaki S, Okada M, Okamoto K, Ikegaya Y. Ethanol facilitates socially evoked memory recall in mice by recruiting pain‐sensitive anterior cingulate cortical neurons. Nat Commun. 2018;9:3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the corresponding authors upon reasonable request.


