Summary
The role of serotonin in food intake has been studied for decades. Food intake is mainly regulated by two brain circuitries: (i) the homeostatic circuitry, which matches energy intake to energy expenditure, and (ii) the hedonic circuitry, which is involved in rewarding and motivational aspects of energy consumption. In the homeostatic circuitry, serotonergic signaling contributes to the integration of metabolic signals that convey the body's energy status and facilitates the ability to suppress food intake when homeostatic needs have been met. In the hedonic circuitry, serotonergic signaling may reduce reward‐related, motivational food consumption. In contrast, peripherally acting serotonin promotes energy absorption and storage. Disturbed serotonergic signaling is associated with obesity, emphasizing the importance to understand the role of serotonergic signaling in food intake. However, unraveling the serotonin‐mediated regulation of food intake is complex, as the effects of serotonergic signaling in different brain regions depend on the regional expression of serotonin receptor subtypes and downstream effects via connections to other brain regions. We therefore provide an overview of the effects of serotonergic signaling in brain regions of the homeostatic and hedonic regulatory systems on food intake. Furthermore, we discuss the disturbances in serotonergic signaling in obesity and its potential therapeutic implications.
Keywords: food intake, obesity, serotonin
Abbreviations
- 5‐HT
5‐hydroxytryptamine
- AgRP
agouti‐related protein
- ARC
arcuate nucleus
- BBB
blood–brain barrier
- BDNF
brain‐derived neurotrophic factor
- BMI
body mass index
- CCK
cholecystokinin
- CeA
central nucleus of the amygdala
- CGRP
calcitonin gene‐related peptide
- CSF
cerebrospinal fluid
- DMN
dorsomedial nucleus
- DRN
dorsal raphe nucleus
- FFA
free fatty acids
- GABA
gamma‐aminobutyric acid
- GLP1
glucagon‐like peptide 1
- LHA
lateral hypothalamic area
- MC3R
melanocortin 3 receptor
- MC4R
melanocortin 4 receptor
- MCH
melanin‐concentrating hormone
- MRN
median raphe nucleus
- MSH
α‐melanocyte‐stimulating hormone
- NAc
nucleus accumbens
- NPY
neuropeptide Y
- NTS
nucleus tractus solitarius
- PBN
parabrachial nucleus
- PET
positron emission tomography
- PFC
prefrontal cortex
- POMC
proopiomelanocortin
- PVN
paraventricular nucleus
- PVT
paraventricular thalamic nucleus
- SERT
serotonin transporter
- SF‐1
steroidogenic factor 1
- SPECT
single‐photon emission computed tomography
- SSRIs
selective serotonin reuptake inhibitors
- VMN
ventral medial nucleus
- VTA
ventral tegmental area
1. INTRODUCTION
Energy status of the body is a crucial determinant of survival and reproduction. An energy deficit is counteracted by an increase in food intake, a behavioral response that is orchestrated by the brain. Studies in the early 20th century showed that hypothalamic lesions in rats exerted region‐specific effects on food intake, either resulting in obesity or severe anorexia that ultimately led to death. 1 , 2 , 3 , 4 These findings pointed to an important role for the hypothalamus in the regulation of food intake. Since then, decades of basic and clinical research have resulted in the identification of complex neuronal networks that are involved in the regulation of food intake and body weight.
Briefly, two major pathways regulate feeding behavior: the hedonic or reward pathway and the homeostatic pathway. Hedonic pathways are mainly located in the corticolimbic areas, and homeostatic pathways include the hypothalamus and brainstem. The main roles for the hedonic system are to promote food‐seeking behavior and signal reward after a meal; the homeostatic pathways integrate metabolic feedback about energy stores into an appropriate feeding response and adjustment in energy expenditure. Multiple neurotransmitters, including gamma‐aminobutyric acid (GABA), glutamate, acetylcholine, dopamine, and serotonin (5‐hydroxytryptamine [5‐HT]), are involved in these processes. With the discovery of leptin, an adipose tissue‐secreted neuroendocrine hormone that increases in parallel with adipose mass and adipocyte size, it became clear that metabolic signals from the body directly modulate neuronal cell activity and influence food intake. 5 In addition to leptin, energy deficit and surplus are signaled to the brain by hormones, substrates, and afferent input from organs involved in nutrient processing. The constellation of metabolic signals that reflect the body's energy state is complex. In terms of substrates, during fasting, glucose levels drop, and free fatty acids (FFAs) and ketone bodies increase. 6 This is accompanied by low insulin and leptin levels and increased glucagon, growth hormone, and catecholamines. 7 , 8 Also, gastrointestinal hormone levels are low in the fasting state, except for ghrelin, which signals hunger and is secreted by the stomach. 9 The metabolic signature of fasting thus provides the brain with feedback to restore energy levels by food‐seeking behavior and increasing food intake. An opposite response occurs in the satiated and energy‐sufficient state.
Given the current obesity epidemic, it has become apparent that central body weight regulation can be—and often is—overruled, and resistance to metabolic feedback likely underlies this phenomenon. In that regard, much attention has been given to leptin resistance, because humans with obesity are characterized by high leptin levels, but do not decrease food intake. 10 , 11 Resistance to other metabolic signals that reflect the energy state has also been studied intensively. Overall, it has been shown that reward and homeostatic pathways are altered in individuals with obesity, with multiple effectors and neuronal pathways involved. 12 , 13 , 14 This review will focus on the role of the neurotransmitter serotonin in the regulation of food intake and development of obesity.
2. SEROTONIN SIGNALING AFFECTS EATING BEHAVIOR
Several lines of evidence support a role for serotonin signaling in the regulation of eating behavior and long‐term body weight. Firstly, the experimental modulation of multiple serotonin receptor subtypes has been shown to affect food intake and/or body weight regulation in animal models. The subtype‐specific effects of receptor knockout, agonism, or antagonism are summarized in Table 1. Secondly, molecular neuroimaging studies, which allow for the spatial visualization and quantification of central serotonin receptor or transporter (SERT) availability, support that human obesity is often associated with decreased serotonergic signaling, 15 although further research is needed to determine causality. Thirdly, systemic serotonin administration decreases food intake. 16 Fourthly, treatment with antidepressants, including the selective serotonin reuptake inhibitors (SSRIs), affects food intake in both animals and humans, and a recent meta‐analysis showed that these therapies are associated with increased risk of weight gain over 10‐year follow‐up. 17 Finally, several novel weight loss drugs selectively influence the serotonin system. 18 , 19
TABLE 1.
Modulation of serotonin receptor subtypes affects food intake and/or body weight
| Receptor subtype | Effect of | ||
|---|---|---|---|
| Receptor knockout | Agonist | Antagonist | |
| 5‐HT1A | Inconsistent/= FI and BW 24 , 25 , 26 |
↓ FI (NAc) 30 |
↓ palatable FI 27 |
| 5‐HT1B | Slightly ↑ FI and BW 25 , 31 | ||
| 5‐HT2A | = FI and BW 38 |
↓ FI 39 ↑ FI (CeA; via direct 5‐HT2A + neuron activation) 40 |
|
| 5‐HT2C | ↑ FI and BW 41 , 42 |
↓ FI (POMC neurons/VMN) 36 , 44 , 45 ↓ motivational FI (NAc/VTA; via direct 5‐HT2C + neuron activation) 30 , 46 |
↑ FI 47 |
| 5‐HT3A | = FI and BW 48 |
= FI 48 ↓ FI [NTS (AgRP‐ablated)] ↓ FI (VTA) 49 ↑ FI (NAc) 49 |
= FI 48 ↓ CCK‐mediated inhibition of gastric emptying 50 ↑ FI (rostral NTS) |
| 5‐HT4 |
= basal FI 51 ↓ stress‐induced hypophagia 51 |
↓ FI (NAc) 52 | ↑ FI in the fed state (NAc) 53 |
| 5‐HT6 | ↓ FI and BW on high‐fat diet 54 |
↓ FI and BW 55 ↑ (NAc) 30 |
↓ FI and BW 56 |
| 5‐HT7 | = BW 57 |
↓ (NAc at higher dose) 30 ↑ (NAc at lower dose) 30 |
|
Note: Presented are the effects of systemic receptor agonism/antagonism, unless a region is specified.
Abbreviations: 5‐HT, 5‐hydroxytryptamine; AgRP, agouti‐related peptide; BW, body weight; CCK, cholecystokinin; CeA, central nucleus of the amygdala; FI, food intake; NAc, nucleus accumbens; NTS, nucleus tractus solitaries; NPY, neuropeptide Y; PBN, parabrachial nucleus; POMC, proopiomelanocortin; VMN, ventral medial nucleus; VTA, ventral tegmental area.
Notably, the serotonin system spans across the central and peripheral nervous system, and the central and peripheral components have opposing effects on energy homeostasis. 20 , 21 , 22 Overall, central serotonergic signaling is anorexigenic, and it increases energy expenditure via the stimulation of thermogenesis in brown adipose tissue. 21 , 23 In contrast, peripheral serotonergic signaling promotes energy absorption and storage; peripheral effects may account for the SSRI‐associated increase in body weight. 22 For in‐depth discussions of the role of peripheral serotonergic signaling in energy homeostasis, we refer to the available literature. 22 In the following sections, we will discuss the (neuro)anatomy and physiology of central serotonin‐mediated regulation of food intake as well as its role in obesity development.
3. FUNCTIONAL ORGANIZATION OF SEROTONERGIC SIGNALING IN EATING BEHAVIOR
Eating behavior is regulated by a complex, partially elucidated interplay between numerous brain regions, neurotransmitters, neuropeptides, and peripheral input and effectors. To understand the physiological mechanisms by which serotonin signaling influences energy intake, we first need to provide an overview of the involved brain circuits.
Food intake can be driven by homeostatic and/or hedonic stimuli. The homeostatic regulatory circuit aims to match energy intake to energy expenditure in order to maintain a stable energy balance and body weight. In contrast, the hedonic motivation for food intake is driven by the reward circuit. In times of body energy depletion, these regulatory systems work synergistically to increase the likelihood of food consumption. Evolutionarily, a high motivational drive for food is beneficial, because this increases the chance to survive and reproduce during times of scarcity. Nowadays, obesity is thought to develop when the hedonic drive overrides the homeostatic regulation and when the homeostatic system is resistant to metabolic feedback, thereby promoting the consumption of food beyond nutritional needs. Therefore, in the modern obesogenic environment, where high‐energy nutrients are readily available, a strong hedonic drive for food has become a disadvantage.
Serotonin signaling is involved in multiple brain regions that play a role in the homeostatic and hedonic circuits of food intake regulation. An overview of the brain regions and their proposed functions in serotonin‐mediated regulation of feeding behavior is provided in Table 2. A schematic overview of the interconnections between these brain regions is presented in Figure 1.
TABLE 2.
Overview of brain regions involved in the serotonergic modulation of feeding behavior
| Brain region | Function in the regulation of food intake | Effect of local serotonergic signaling (mechanism) |
|---|---|---|
| Hypothalamus | ||
| ARC | Integration of circulating nutritional and hormonal signals; origin of POMC and AgRP/NPY neurons | Anorexigenic (stimulation of POMC neurons; inhibition of AgRP/NPY neurons) |
| PVN | Major downstream target of POMC and AgRP neurons | Anorexigenic (projections to brainstem regions; synthesis of neuropeptides that modulate food intake) |
| VMN | Downstream target of POMC and AgRP neurons | Anorexigenic (activation of BDNF‐expressing neurons) |
| DMN | Downstream target of POMC and AgRP neurons | Anorexigenic (unknown) |
| LHA | Drives food consumption during hunger; integration with reward centers | Anorexigenic (inhibition of orexin and MCH neurons) |
| Brainstem | ||
| Raphe nuclei | Origin of serotonergic projections toward a variety of brain regions involved in the regulation of feeding behavior | Orexigenic (by inhibition of serotonergic projections via auto‐inhibition) |
| NTS | Integration of peripheral visceral afferents and hormonal signals related to energy status (normally anorexigenic) | Anorexigenic (excitation of POMC neurons and stimulation of glutamatergic projections toward the PBN) |
| PBN | Responds to satiation and noxious signals and controls meal termination; origin of CGRP neurons | Anorexigenic (partly unknown; indirect excitation of CGRP neurons) |
| Thalamus | ||
| PVT | Relay or integration of homeostatic and hedonic control | Unknown |
| Mesolimbic circuitry | ||
| VTA | Origin of dopaminergic projections to mesolimbic circuitry | Anorexigenic (suppression of the motivation for food intake) |
| NAc | Receives most VTA dopaminergic projections; response to food‐predictive cues; incentive motivation for food | Anorexigenic (suppression of the motivation for food intake) Orexigenic through 5‐HT3 and 5‐HT6 receptors |
| CeA | Reward value prediction by integration of homeostatic, cognitive, and visceral inputs | Unknown (serotonin may increase feeding behavior via 5‐HT2A‐expressing CeA neurons) |
Abbreviations: 5‐HT, 5‐hydroxytryptamine; AgRP, agouti‐related protein; ARC, arcuate nucleus; BDNF, brain‐derived neurotrophic factor; CCK, cholecystokinin; CeA, central nucleus of the amygdala; CGRP, calcitonin gene‐related peptide; DMN, dorsomedial nucleus; GLP1, glucagon‐like peptide 1; LHA, lateral hypothalamic area; MCH, melanin‐concentrating hormone; NAc, nucleus accumbens; NPY, neuropeptide Y; NTS, nucleus tractus solitarius; PBN, parabrachial nucleus; PFC, prefrontal cortex; POMC, proopiomelanocortin; PVN, paraventricular nucleus; PVT, paraventricular thalamic nucleus; VMN, ventral medial nucleus; VTA, ventral tegmental area.
FIGURE 1.
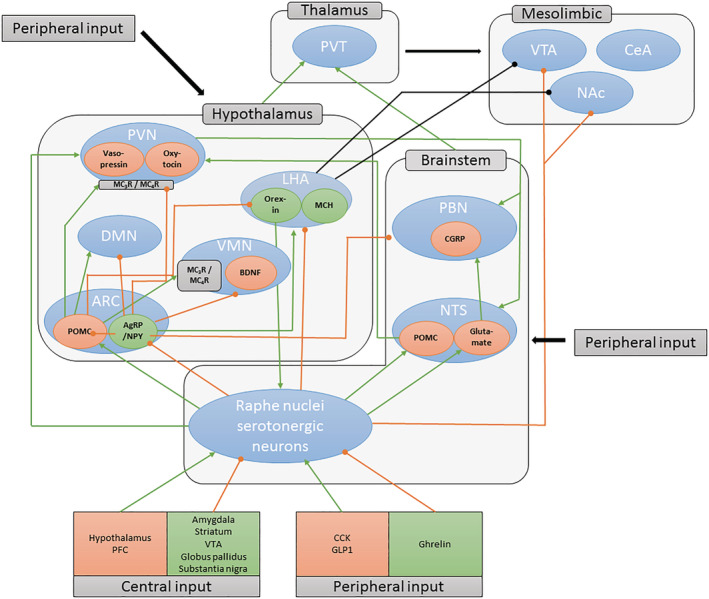
Serotonergic pathways involved in regulation of food intake. Blue ovals indicate brain regions or nuclei. Orange ovals indicate anorexigenic neuronal populations. Green ovals indicate orexigenic neuronal populations. Green lines indicate excitatory projections. Orange lines indicate inhibitory projections. Most ascending serotonergic projects arise from the dorsal and medial raphe nuclei. Note that connections to the mesolimbic (hedonic) and other brain regions have been simplified for clarity. AgRP, agouti‐related protein; ARC, arcuate nucleus; BDNF, brain‐derived neurotrophic factor; CCK, cholecystokinin; CeA, central nucleus of the amygdala; CGRP, calcitonin gene‐related peptide; DMN, dorsomedial nucleus; GLP1, glucagon‐like peptide 1; LHA, lateral hypothalamic area; MCH, melanin‐concentrating hormone; NAc, nucleus accumbens; NPY, neuropeptide Y; NTS, nucleus tractus solitarius; PBN, parabrachial nucleus; PFC, prefrontal cortex; POMC, proopiomelanocortin; PVN, paraventricular nucleus; PVT, paraventricular thalamic nucleus; VMN, ventral medial nucleus; VTA, ventral tegmental area
3.1. Serotonin signaling in the homeostatic circuitry
Failure to appropriately suppress food intake when body energy stores are sufficient results in the (over)consumption of food beyond nutritional needs and, subsequently, weight gain. Decreased serotonin signaling in the homeostatic circuit has been suggested to contribute to this pathophysiological state. 58 In fact, due to its assumed role in obesity development, serotonin and the homeostatic regulation of food intake have been extensively studied over the past decades. We note, however, that this functional circuit involves multiple neurotransmitters across multiple brain regions.
The hypothalamus and brainstem are considered the primary brain regions of the homeostatic regulation of food intake. 21 In these key regions, central and peripheral inputs on hunger, satiety, and whole‐body nutrient availability are integrated in order to adequately adapt subsequent feeding behavior to the present nutritional state. Both regions can be subdivided into several nuclei, of which the most important for food intake regulation are the raphe nuclei, nucleus tractus solitarius (NTS), and parabrachial nucleus (PBN) of the brainstem and the arcuate nucleus (ARC), paraventricular nucleus (PVN), ventral medial nucleus (VMN), dorsomedial nucleus (DMN), and lateral hypothalamic area (LHA) of the hypothalamus. These, in turn, are strongly (inter)connected and receive central and peripheral input (Figure 1).
3.1.1. Raphe nuclei
Almost all ascending serotonergic projections involved in nutritional homeostasis originate from the dorsal raphe nucleus (DRN) and median raphe nucleus (MRN). 59 The activity of serotonergic neurons in the DRN increases directly after food intake, whereas the activity of serotonergic neurons in the MRN does not. This suggests that the DRN is specifically involved in postprandial satiation signaling. 60 In contrast, MRN serotonergic neurons may be involved in the motivational control of eating behavior, because these project to several brain regions in the reward system. 61 The DRN and MRN, sometimes referred to as the rostral caudate nuclei, contain the majority of central serotonergic neurons; much smaller, the caudal raphe nuclei, including the raphe pallidus, the raphe magnus, and raphe obscurus nuclei, are also activated under satiated conditions. 62 Serotonergic projections from the latter nuclei project to the NTS and contribute to the suppression of food intake. 63
Raphe nuclei serotonergic neurons are under regulatory control of central and peripheral input. Prominent projections to the DRN and MRN include pathways from the hypothalamus, prefrontal cortex (PFC), amygdala, ventral tegmental area (VTA), and the basal ganglia, which include the striatum, globus pallidus, and substantia nigra. 64 The caudal raphe nuclei receive innervation from the amygdala and hypothalamic nuclei. 65 These connections likely enable higher brain functions to influence the sensitivity of homeostatic control. In addition, peripheral input on the body's nutritional state is integrated with the serotonin system within the raphe nuclei. 66 The gastrointestinal hormones cholecystokinin (CCK) and glucagon‐like peptide 1 (GLP1) are secreted from the gastrointestinal tract in response to nutrient ingestion. These hormones stimulate serotonergic neurons in the DRN and mediate anorexigenic effects. 67 , 68 In contrast, ghrelin is secreted from the stomach during fasting and stimulates food intake; it may also interact with serotonin receptors in the DRN. 69
Finally, there is also evidence that serotonin signaling toward the DRN may be involved in food intake regulation: experimental administration of serotonin or SSRI to the DRN stimulates food intake in rats, presumably via the activation of inhibitory autoreceptors on DRN serotonergic neurons in the homeostatic circuit. 70
3.1.2. Arcuate nucleus and nucleus tractus solitarius
The ARC in the hypothalamus and NTS in the brainstem are among those brain regions that receive serotonergic projections from the DRN. The ARC is in close proximity to the third ventricle and has a leaky blood–brain barrier (BBB). It is optimally equipped to read out circulating nutritional and hormonal signals. The ARC then uses this input to modify feeding behavior via downstream projections to neighboring hypothalamic nuclei and reward‐related mesolimbic areas. 71 The NTS also contributes to the integration of peripheral visceral afferents, including the vagal afferents, and hormonal signals related to energy status. 72
These two regions have been extensively studied in relation to food intake, because they contain neuronal populations pivotal in nutritional homeostasis: proopiomelanocortin (POMC)‐expressing neurons and neurons that express agouti‐related peptide (AgRP) and neuropeptide Y (NPY). 73 Both POMC neurons in the ARC (POMCARC) and NTS (POMCNTS) mediate their anorexigenic effects via the melanocortin pathway, 35 which will be discussed below. The importance of POMC neurons in the regulation of food intake has been well established. In mice, POMC knockout results in hyperphagia and obesity, 74 whereas activation of POMC neurons suppresses food intake. 75 Humans with POMC deficiency display severe and early‐onset hyperphagia and obesity. 76
There has been some controversy regarding the effects of ARC serotonin release. In one early rodent study, 77 serotonin signaling to the ARC was found to favor food intake, and inhibition of serotonin signaling in the ARC was suggested to mediate the anorexigenic effects of leptin. However, these results have never been replicated, and the overall majority of subsequent studies now show that serotonin release in the ARC and NTS activates POMC neurons, resulting in acute (within minutes) or long‐term suppression of food intake. 75 Only about 25% of adult mouse POMC neurons express the 5‐HT2C receptor, 78 but activation of POMC neurons via this receptor has been shown to account for the anorexigenic effects of several serotonergic drugs. 44 , 45 In addition, selectively reintroducing the 5‐HT2C receptor to POMC neurons in whole‐body 5‐HT2C receptor knockout mice is sufficient to completely negate the knockout phenotype. 44 Interestingly, it has been suggested that POMCARC regulate the long‐term anorexigenic effects of serotonin signaling by integrating long‐term adiposity signals from the hypothalamus, whereas POMCNTS activation is responsible for the acute effects of serotonin signaling by integrating short‐term satiety signals from the brainstem. 45 , 75 Both POMC populations may thus act synergistically, but via different mechanisms. In support of this, POMCNTS and POMCARC receive additional neuronal input from distinct brain regions: whereas projections to POMCNTS predominantly originate from other brainstem regions, those to POMCARC predominantly come from other hypothalamic regions. 79 Leptin also stimulates POMCARC. 80
The AgRP/NPY neurons in the ARC mediate an opposite, orexigenic effect via a dual mechanism. Firstly, activation of AgRP/NPY neurons promotes the release of AgRP, which is a direct antagonist of the melanocortin pathway. 35 Secondly, AgRP/NPY neurons directly inhibit POMCARC via GABAergic projections. Serotonin signaling to AgRP/NPY neurons via the 5‐HT1B receptor causes hyperpolarization, which results in a reduced likelihood of AgRP release and decreased GABAergic inhibition of POMC neurons. 35 Moreover, a glutamatergic, oxytocin receptor‐expressing ARC population has recently been described to mediate postprandial satiety via projections to anorexigenic neurons in the PVN, that is, the same downstream target of POMCARC and AgRP/NPY neurons. 81 Whether serotonergic signaling affects this glutamatergic population remains to be established. Finally, the NTS contains a glutamatergic population that expresses the 5‐HT3 receptor and inhibits feeding via activation of anorexigenic neurons in the PBN. 63
3.1.3. Paraventricular nucleus
Lesions to the hypothalamic PVN result in hyperphagia and obesity, suggesting an important role for this nucleus in the regulation of food intake and energy homeostasis. 82 The PVN receives dense projections from POMCARC/NTS and AgRP/NPY neurons. These activate melanocortin 3 and 4 receptors (MC3R and MC4R, respectively) on PVN neurons by releasing α‐melanocyte‐stimulating hormone (MSH) and inhibit PVN neurons by releasing AgRP and NPY, respectively. 83 Melanocortin receptors are highly expressed in the PVN, and the importance of the melanocortin pathway downstream of POMCARC/NTS and AgRP/NPY neurons is illustrated by several lines of evidence. In mice, MC4R knockout causes obesity, whereas selective reintroduction of MC4R to PVN neurons attenuates the obese phenotype. 82 Surgical disruption of the ARC ➔ PVN tract causes obesity. 84 In humans, MC4R deficiency or heterozygous mutations cause hyperphagia, similarly to POMC deficiency. 76 , 85 Upon its activation, the PVN mediates its anorexigenic effects via descending projections (back) to the NTS and PBN of the brainstem as well as via the synthesis of anorexigenic neuropeptides including arginine vasopressin and oxytocin. 86 , 87
In addition to POMCARC/NTS and AgRP/NPY signaling, serotonin may directly act on PVN neurons: several serotonin receptor subtypes are expressed, and the PVN receives direct serotonergic projections from the DRN. 88 Mice that lack the 5‐HT1B receptor have impaired activation of PVN neurons by fenfluramine, a stimulator of serotonin release and serotonin reuptake inhibitor, and impaired suppression of food intake by 5‐HT1A/B agonism. 32 Cannabinoids may mediate some of their orexigenic effects by inhibiting serotonin release and reducing serotonergic signaling via the 5‐HT1A/B receptors in the PVN. 89 Selective 5‐HT6 receptor antagonism promotes the activation of PVN neurons, which may contribute to the anorexigenic effect of 5‐HT6 receptor antagonists. 90
3.1.4. Ventral medial nucleus
The VMN was once regarded as the “satiety center” of the hypothalamus: an early study found that destruction of this hypothalamic nucleus resulted in voracious eating behavior. 91 This was later refuted: lesions restricted to the VMN were neither sufficient nor necessary for hyperphagia, and the previously observed effect was attributed to damage outside of the VMN. 92 More recent studies have attributed specific functions related to nutritional homeostasis, thereby “resurrecting” interest in this region. 93
One neuronal population of interest expresses steroidogenic factor 1 (SF‐1). 94 These neurons are exclusively found in the VMN, express the 5‐HT2C receptor, and are directly targeted by serotonergic projections from the brainstem. 24 , 94 However, SF‐1 neurons seem to primarily contribute to energy homeostasis by modulating energy expenditure and glucose homeostasis (rather than energy intake). 94 The VMN also receives POMCARC and AgRP/NPY neuronal projections from the ARC. 95 Here, POMCARC are believed to activate VMN brain‐derived neurotrophic factor (BDNF)‐expressing neurons via the MC4R. 95 Administration of BDNF to the VMN decreases food intake and body weight, and this effect was most pronounced for NPY‐induced feeding. 96 Finally, direct VMN administration of serotonin selectively reduces carbohydrate preference, 97 whereas direct administration of a 5‐HT1B/2C receptor agonist reduces overall food intake. 36 It is likely that other and/or more specific functions of the VMN have yet to be discovered.
3.1.5. Dorsomedial nucleus
Although lesion studies strongly support a role for the DMN in feeding, drinking, and body weight regulation, 98 it is not clear whether serotonin signaling is involved. Like the VMN, this hypothalamic nucleus also receives POMCARC and AgRP/NPY neuronal projections from the ARC 99 and expresses MC4R. 100 It may thus be possible for serotonin signaling to exert some anorexigenic effects through the DMN. Direct serotonin infusion into the DMN decreases subsequent energy intake, but this effect is quite small in comparison with other brain regions. 97
3.1.6. Lateral hypothalamus
Electrical stimulation of the LHA induces compulsive and hedonic eating behavior, whereas LHA inhibition causes hypophagia. 2 , 4 The LHA is targeted by POMCARC/NTS and AgRP/NPY neurons, and serotonergic signaling thus indirectly acts on the LHA. 101 , 102 Additionally, raphe nuclei serotonergic neurons also project to the LHA, 103 and extracellular serotonin levels in the LHA increase upon food intake. 104 Notably, serotonin inhibits orexin neurons, 105 , 106 an LHA‐exclusive neuronal population that promotes reward‐related behavior and arousal. 107 Orexin neurons are involved in hedonically motivated feeding. 108 , 109 , 110 , 111 The effect on energy homeostasis seems to be context specific, that is, under hypoglycemic conditions, activation of orexin neurons increases short‐term food intake, 112 , 113 whereas under (long‐term) high‐fat diet conditions, the activation of orexin neurons may actually protect against diet‐induced weight gain by enhancing spontaneous physical activity. 114 Orexin neurons express several serotonin receptor subtypes, 115 but blocking the 5‐HT1A receptor is sufficient to completely negate serotonin‐mediated inhibition of orexin neurons. 106 Orexin neurons, in turn, activate serotonergic neurons in the DRN and may thus partake in a complex negative feedback loop within the homeostatic circuit. 116 Another neuronal population in the LHA expresses melanin‐concentrating hormone (MCH). Intracerebroventricular MCH injections increase food intake, 117 whereas MCH receptor antagonists reduce food intake and may even cause weight loss. 118 Serotonin hyperpolarizes MCH neurons, thereby desensitizing this mechanism. 119
3.1.7. Parabrachial nucleus
This brainstem nucleus consists of several subpopulations of neurons that relay sensory information to other forebrain structures. 120 Interest in the role of the PBN in nutritional homeostasis was first stirred following the observations that dexfenfluramine, an indirect serotonin agonist, increased c‐Fos immunoreactivity in the lateral PBN 121 and bilateral PBN lesions attenuated dexfenfluramine‐induced anorexia. 122 The 5‐HT2C receptor is required for the anorexic effects of dexfenfluramine. 123 In addition, infusion of a 5‐HT1B receptor agonist into the PBN dose‐dependently reduces food intake. 37 Thus, anorexic effects of serotonergic signaling in the PBN may be partially mediated by the 5‐HT1B and 5‐HT2C receptors, but the downstream mechanisms remain to be determined.
More recently, another anorexigenic neuronal population, one that expresses calcitonin gene‐related peptide (CGRP), has been identified in the PBN. 124 These are inhibited by ARC AgRP neurons and project to the central nucleus of the amygdala (CeA). 125
3.1.8. Thalamus
The paraventricular thalamic nucleus (PVT) receives serotonergic projections from the raphe nuclei 103 as well as extensive feeding‐related input from the hypothalamic nuclei. 126 It is unknown how thalamic serotonin signaling is functionally involved in the regulation of feeding behavior. Human neuroimaging studies that compare lean subjects against subjects with obesity do not show consistent trends to support increased or decreased serotonergic activity in this region, 15 and it is likely more complicated. Thalamic SERT availability is decreased in insulin‐resistant humans, as compared with insulin‐sensitive humans with obesity, 127 suggesting that the relationship between obesity and thalamic SERT availability may, at least in part, be driven by changes in metabolic health. Weight loss and weight gain also affect thalamic SERT availability, but these effects are dependent on meal timing during weight loss and meal composition during weight gain. The consumption of a large breakfast and small dinner during weight loss is associated with increased thalamic SERT availability, 128 suggesting that serotonin signaling may contribute to reported favorable effects of meal timing on weight maintenance. In contrast, a 6‐week high‐fat/high‐sugar snacking diet decreases SERT availability. 129 Because this occurs early during weight gain, it potentially contributes to the progression of obesity. Interestingly, the PVT has dense connections with the limbic system, 130 suggesting that it may play a role in the relaying or integration of homeostatic and reward circuitries.
3.2. Serotonin signaling in the reward circuitry
Serotonin and dopamine have been considered the most important neurotransmitters in the homeostatic and hedonic systems, respectively, but emerging evidence supports a role for serotonin in reward‐related, motivational food consumption as well. 131 In the reward circuitry, serotonergic signaling interplays with dopaminergic signaling and signaling through other neurotransmitters.
The mesolimbic system, including the VTA, the nucleus accumbens (NAc) of the ventral striatum, and the CeA, is sometimes referred to as the reward pathway. These regions have also been proposed to partake in the interaction between homeostatic and hedonic regulation of food intake. In addition, serotonin receptors are expressed in the dorsal striatum, 132 a key region in the development of habitual (eating) behavior. 133 When the availability of food is time restricted, the dorsal striatum is proposed to function as a food‐entrained oscillator, resulting in food anticipatory activity with a circadian rhythm that is synced to the food availability time schedule. 134 The role of serotonin signaling in food anticipatory activity is debated: where previous studies have suggested that whole‐brain serotonin signaling suppresses the development of food anticipatory activity, 135 , 136 a more recent study reported food anticipatory activity to be independent of serotonin signaling. 137 Serotonergic signaling in the dorsal striatum is more strongly related to the regulation of motor behavior, and perturbed serotonergic signaling within this region is associated with several pathological motor conditions. 138 , 139
3.2.1. Ventral tegmental area
Most dopaminergic projections to the mesolimbic brain areas originate from the VTA. Just like the hypothalamic nuclei of the homeostatic system, the VTA itself is innervated by serotonergic neurons from the DRN. The VTA also receives direct projections from the LHA, a neural circuit that controls compulsive sugar consumption. 140
Subsets of both dopaminergic and GABAergic neurons in the VTA express the 5‐HT2C receptor, 46 , 141 , 142 and systemic administration of lorcaserin, a 5‐HT2C receptor agonist, increases the activity of GABAergic, but not of dopaminergic, neurons in the VTA. 46 In fact, activation of the 5‐HT2C receptor in the VTA results in decreased dopaminergic signaling 143 and reduced motivational food intake. 46 When specifically activating 5‐HT2C receptor‐expressing dopaminergic VTA neurons, they increase their firing rate and suppress binge eating behavior in mice. 141 Such observations indicate that, overall, serotonergic signaling in the VTA may decrease the hedonic drive for eating behavior, primarily via GABAergic inhibition of VTA dopaminergic neurons.
3.2.2. Nucleus accumbens
The NAc, located in the ventral part of the striatum, is a major target for downstream VTA dopaminergic projections. Dopamine release in the NAc increases the incentive motivation for palatable food. 144 Increases in NAc dopamine levels are induced by food‐predictive cues, and this dopaminergic response is amplified in underweight and/or fasted animals. 145 , 146
A proposed hypothalamic–thalamic–striatal axis links the NAc to the homeostatic system. The NAc receives direct homeostatic input from the raphe nuclei and LHA as well as indirect input via the PVT; the NAc reciprocally projects to the LHA. 130 , 147 This axis would allow hypothalamic serotonergic signaling to influence NAc dopaminergic signaling, but its precise role remains unclear. Injection of serotonin into the NAc reduces the motivation for food. 148 In addition, injection of either CCK or serotonin into the PVN limits dopamine release and synergistically promotes acetylcholine release in the NAc. 149 However, the modulation of specific serotonin receptors on NAc neurons has differential effects: agonists for the 5‐HT1/7, 5‐HT2C, and 5‐HT4 receptors decrease food intake, whereas agonists for the 5‐HT3 and 5‐HT6 receptors increase food intake. 30 , 49 , 53 This demonstrates the complexity of serotonergic modulation of the hedonic circuitry. Interestingly, experimental stimulation of serotonergic signaling using D‐fenfluramine or lorcaserin suppresses the binge‐like eating induced by stimulation of μ‐opioid receptors in the NAc, 150 pointing to another pathway where serotonin signaling may influence the regulation of food intake via the reward circuitry.
3.2.3. Central nucleus of the amygdala
The CeA contains many distinct cellular and neurochemical populations, 151 complicating investigations into its role in feeding behavior. At least two known neuronal populations, a GABAergic population that expresses the 5‐HT2A receptor 40 and another GABAergic population that expresses protein kinase Cδ, 152 play a role in the regulation of feeding behavior. Experimental activation of 5‐HT2A receptor‐expressing CeA neurons increases food intake, but not the motivational drive for food. 40 It is unknown to what extent endogenous serotonergic signaling to these cells occurs. However, decreased SERT availability in the CeA has been associated with resistance to chronic high‐fat diet‐induced obesity in mice, providing further evidence that CeA serotonergic signaling may affect the regulation of food intake. 153
4. DISTURBED SEROTONERGIC SIGNALING IN OBESITY AND THERAPEUTIC IMPLICATIONS
Obesity results from an energy intake that exceeds energy expenditure. In this regard, attenuated homeostatic inhibition and/or increased hedonic drive for energy consumption has been postulated to contribute to the consumption of food beyond homeostatic needs. Because serotonergic signaling fulfills an important role in the regulation of food intake, disruption in serotonergic signaling may contribute to the pathogenesis of disturbed feeding behavior in individuals with chronic overweight or obesity. Indeed, data from multiple studies indicate that serotonergic signaling is disturbed in animals and humans with obesity.
Hypothalamic baseline serotonin release is reduced in animal models of obesity. 154 , 155 Feeding rats an obesogenic diet for 7 weeks results in changes in binding to 5‐HT1A, 5‐HT1B, and 5‐HT2A receptors (using quantitative autoradiography), 156 in accordance with reduced serotonin release and decreased activity of the serotonergic neurons. Attenuation of meal‐induced hypothalamic serotonin release occurs as early as after 1 week of high‐fat feeding, and it progresses over time to a complete absence of meal‐stimulated hypothalamic serotonin release. 157 Apparently, diet‐induced changes in serotonergic signaling precede the onset of obesity. Given the role for serotonin in food intake, it is not surprising that specific serotonin receptors serve as therapeutic targets to reduce food intake in individuals with obesity.
In humans, it is impossible to study the central serotonin system in vivo directly. Postmortem immunohistochemistry of brain tissue, analysis of serotonin and its metabolites in cerebrospinal fluid (CSF), and molecular neuroimaging techniques (positron emission tomography [PET] and single‐photon emission computed tomography [SPECT]) have been applied to assess changes in serotonergic signaling associated with human obesity. 15 Decreased levels of SERT protein were observed in the infundibular nucleus (equivalent to the ARC in rodents) in postmortem hypothalamic tissue of humans with overweight/obesity. 158 In addition, women with obesity have lower levels of serotonin and its metabolites in CSF compared with lean women. 159 Interestingly, studies measuring serotonin receptor or SERT availability using either PET or SPECT consistently support decreased serotonin levels/signaling in a variety of brain regions in individuals with obesity. 58 , 160 , 161 , 162 , 163 , 164
Whether these findings are the consequence or cause of obesity remains a point of discussion. Because most studies observe no (curvi)linear correlation between body mass index (BMI) and these indirect measures of central serotonin signaling, it is likely that obesity‐associated factors, such as diet composition and meal timing, at least partially account for the obesity‐associated changes in serotonin measures. 15 A role for meal timing is supported by the observations that thalamic SERT increased following a 4‐week hypocaloric diet when most daily calories were consumed during breakfast and decreased when most daily calories were consumed during dinner. 128 In addition, following a 6‐week hypercaloric high‐fat/high‐sugar snacking diet, a reduction in diencephalic SERT was observed in lean men. 129 These studies indicate that changes in serotonergic signaling may develop early during the overconsumption of food in humans and may therefore contribute to the development and/or persistence of obesity.
On the basis of the evident role of serotonergic signaling in feeding behavior and translational observations supporting reduced serotonergic signaling in human obesity, the stimulation of central serotonergic signaling emerged as a therapeutic target for obesity well over a decade ago. Initially, fenfluramine and, later, dexfenfluramine and sibutramine were successfully marketed as obesity treatments. Unfortunately, the success of serotonergic drugs in the treatment of obesity has so far been limited by peripheral side effects due to the stimulation of serotonin receptors in peripheral tissues. More recently, the 5‐HT2C receptor agonist lorcaserin was shown to be effective in reducing food intake, body weight, and cardiometabolic complications in individuals with obesity. 165 It was used in clinical practice across the United States from 2012 to 2020, at which time the FDA requested it be withdrawn from the market due to concerns about increased cancer incidence. 166
Finally, given the peripheral side effects of centrally acting serotonin stimulators and given the overall effects of peripheral serotonin stimulation on energy balance per se, the targeted inhibition of peripheral serotonin has also been proposed as a potential therapeutic target for prevention or treatment of obesity, 167 although we are not aware of any clinical trials assessing this mechanism at this moment. In summary, several serotonergic drugs have shown promising results in humans with obesity, but they have also been associated with severe side effects; further research is required to determine if this system can be safely targeted in humans with obesity.
5. CONCLUDING REMARKS
The brain's serotonergic system plays a pivotal role in the control of food intake and whole‐body energy homeostasis. Multiple complex neuronal networks and serotonin receptor subtypes participate in this regulatory system, together eliciting an appropriate feeding response depending on the actual metabolic state. Human obesity is associated with reduced serotonergic signaling. Early changes in serotonergic signaling occur during overconsumption, and these may contribute to the onset and/or persistence of overweight and obesity. Pharmacotherapy aimed at specific serotonin receptor subtypes affects food intake and body weight, but peripheral side effects have thus far limited their use.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest associated with the manuscript.
van Galen KA, ter Horst KW, Serlie MJ. Serotonin, food intake, and obesity. Obesity Reviews. 2021;22:e13210. 10.1111/obr.13210
REFERENCES
- 1. Hetherington AW. Nutrition classics. The anatomical record, volume 78, 1940: hypothalamic lesions and adiposity in the rat. Nutr Rev. 1983;41(4):124‐127. [DOI] [PubMed] [Google Scholar]
- 2. Anand BK, Brobeck JR. Localization of a “feeding center” in the hypothalamus of the rat. Proc Soc Exp Biol Med Soc Exp Biol Med (New York, NY). 1951;77(2):323‐324. [DOI] [PubMed] [Google Scholar]
- 3. Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24(2):123‐140. [PMC free article] [PubMed] [Google Scholar]
- 4. Delgado JM, Anand BK. Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am J Physiol. 1953;172(1):162‐168. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425‐432. [DOI] [PubMed] [Google Scholar]
- 6. Kerndt PR, Naughton JL, Driscoll CE, Loxterkamp DA. Fasting: the history, pathophysiology and complications. West J Med. 1982;137(5):379‐399. [PMC free article] [PubMed] [Google Scholar]
- 7. Hartman ML, Veldhuis JD, Johnson ML, et al. Augmented growth hormone (GH) secretory burst frequency and amplitude mediate enhanced GH secretion during a two‐day fast in normal men. J Clin Endocrinol Metab. 1992;74(4):757‐765. [DOI] [PubMed] [Google Scholar]
- 8. Webber J, Macdonald IA. The cardiovascular, metabolic and hormonal changes accompanying acute starvation in men and women. Br J Nutr. 1994;71(3):437‐447. [DOI] [PubMed] [Google Scholar]
- 9. Muller TD, Nogueiras R, Andermann ML, et al. Ghrelin. Mol Metab. 2015;4(6):437‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet‐induced resistance to leptin action. Nat Med. 1995;1(12):1311‐1314. [DOI] [PubMed] [Google Scholar]
- 11. Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight‐reduced subjects. Nat Med. 1995;1(11):1155‐1161. [DOI] [PubMed] [Google Scholar]
- 12. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661‐671. [DOI] [PubMed] [Google Scholar]
- 13. Berthoud HR, Munzberg H, Morrison CD. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology. 2017;152(7):1728‐1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tulloch AJ, Murray S, Vaicekonyte R, Avena NM. Neural responses to macronutrients: hedonic and homeostatic mechanisms. Gastroenterology. 2015;148(6):1205‐1218. [DOI] [PubMed] [Google Scholar]
- 15. van Galen KA, Ter Horst KW, Booij J, la Fleur SE, Serlie MJ. The role of central dopamine and serotonin in human obesity: lessons learned from molecular neuroimaging studies. Metabolism. 2018;85:325‐339. [DOI] [PubMed] [Google Scholar]
- 16. Fletcher PJ, Burton MJ. Microstructural analysis of the anorectic action of peripherally administered 5‐HT. Pharmacol Biochem Behav. 1986;24(4):1133‐1136. [DOI] [PubMed] [Google Scholar]
- 17. Gafoor R, Booth HP, Gulliford MC. Antidepressant utilisation and incidence of weight gain during 10 years' follow‐up: population based cohort study. BMJ. 2018;361(k1951):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bello NT, Liang NC. The use of serotonergic drugs to treat obesity‐‐is there any hope? Drug Des Devel Ther. 2011;5:95‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oh CM, Park S, Kim H. Serotonin as a new therapeutic target for diabetes mellitus and obesity. Diabetes Metab J. 2016;40(2):89‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakatani Y, Sato‐Suzuki I, Tsujino N, et al. Augmented brain 5‐HT crosses the blood‐brain barrier through the 5‐HT transporter in rat. Eur J Neurosci. 2008;27(9):2466‐2472. [DOI] [PubMed] [Google Scholar]
- 21. Donovan MH, Tecott LH. Serotonin and the regulation of mammalian energy balance. Front Neurosci. 2013;7(36):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yabut JM, Crane JD, Green AE, Keating DJ, Khan WI, Steinberg GR. Emerging roles for serotonin in regulating metabolism: new implications for an ancient molecule. Endocr Rev. 2019;40(4):1092‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGlashon JM, Gorecki MC, Kozlowski AE, et al. Central serotonergic neurons activate and recruit thermogenic brown and beige fat and regulate glucose and lipid homeostasis. Cell Metab. 2015;21(5):692‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yadav VK, Oury F, Suda N, et al. A serotonin‐dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138(5):976‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bechtholt AJ, Smith K, Gaughan S, Lucki I. Sucrose intake and fasting glucose levels in 5‐HT(1A) and 5‐HT(1B) receptor mutant mice. Physiol Behav. 2008;93(4–5):659‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci U S A. 1998;95(18):10734‐10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moreau JL, Griebel G, Jenck F, Martin JR, Widmer U, Haefely WE. Behavioral profile of the 5HT1A receptor antagonist (S)‐UH‐301 in rodents and monkeys. Brain Res Bull. 1992;29(6):901‐904. [DOI] [PubMed] [Google Scholar]
- 28. Hutson PH, Dourish CT, Curzon G. Evidence that the hyperphagic response to 8‐OH‐DPAT is mediated by 5‐HT1A receptors. Eur J Pharmacol. 1988;150(3):361‐366. [DOI] [PubMed] [Google Scholar]
- 29. Dourish CT, Hutson PH, Kennett GA, Curzon G. 8‐OH‐DPAT‐induced hyperphagia: its neural basis and possible therapeutic relevance. Appetite. 1986;7(Suppl):127‐140. [DOI] [PubMed] [Google Scholar]
- 30. Pratt WE, Schall MA, Choi E. Selective serotonin receptor stimulation of the medial nucleus accumbens differentially affects appetitive motivation for food on a progressive ratio schedule of reinforcement. Neurosci Lett. 2012;511(2):84‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bouwknecht JA, van der Gugten J, Hijzen TH, Maes RA, Hen R, Olivier B. Male and female 5‐HT(1B) receptor knockout mice have higher body weights than wildtypes. Physiol Behav. 74(4‐5):507‐516. [DOI] [PubMed] [Google Scholar]
- 32. Lucas JJ, Yamamoto A, Scearce‐Levie K, Saudou F, Hen R. Absence of fenfluramine‐induced anorexia and reduced c‐Fos induction in the hypothalamus and central amygdaloid complex of serotonin 1B receptor knock‐out mice. J Neurosci. 1998;18(14):5537‐5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Halford JC, Blundell JE. The 5‐HT1B receptor agonist CP‐94,253 reduces food intake and preserves the behavioural satiety sequence. Physiol Behav. 1996;60(3):933‐939. [DOI] [PubMed] [Google Scholar]
- 34. Lee MD, Simansky KJ. CP‐94, 253: a selective serotonin1B (5‐HT1B) agonist that promotes satiety. Psychopharmacology (Berl). 131(3):264‐270. [DOI] [PubMed] [Google Scholar]
- 35. Heisler LK, Jobst EE, Sutton GM, et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51(2):239‐249. [DOI] [PubMed] [Google Scholar]
- 36. Hikiji K, Inoue K, Iwasaki S, Ichihara K, Kiriike N. Local perfusion of mCPP into ventromedial hypothalamic nucleus, but not into lateral hypothalamic area and frontal cortex, inhibits food intake in rats. Psychopharmacology (Berl). 2004;174(2):190‐196. [DOI] [PubMed] [Google Scholar]
- 37. Lee MD, Aloyo VJ, Fluharty SJ, Simansky KJ. Infusion of the serotonin1B (5‐HT1B) agonist CP‐93,129 into the parabrachial nucleus potently and selectively reduces food intake in rats. Psychopharmacology (Berl). 1998;136(3):304‐307. [DOI] [PubMed] [Google Scholar]
- 38. Jaggar M, Weisstaub N, Gingrich JA, Vaidya VA. 5‐HT2A receptor deficiency alters the metabolic and transcriptional, but not the behavioral, consequences of chronic unpredictable stress. Neurobiol Stress. 2017;7:89‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fox MA, French HT, LaPorte JL, Blackler AR, Murphy DL. The serotonin 5‐HT(2A) receptor agonist TCB‐2: a behavioral and neurophysiological analysis. Psychopharmacology (Berl). 2010;212(1):13‐23. [DOI] [PubMed] [Google Scholar]
- 40. Douglass AM, Kucukdereli H, Ponserre M, et al. Central amygdala circuits modulate food consumption through a positive‐valence mechanism. Nat Neurosci. 2017;20(10):1384‐1394. [DOI] [PubMed] [Google Scholar]
- 41. Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin‐independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5‐HT2C receptor gene. Nat Med. 1998;4(10):1152‐1156. [DOI] [PubMed] [Google Scholar]
- 42. Tecott LH, Sun LM, Akana SF, et al. Eating disorder and epilepsy in mice lacking 5‐HT2c serotonin receptors. Nature. 1995;374(6522):542‐546. [DOI] [PubMed] [Google Scholar]
- 43. Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo‐controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363(3):245‐256. [DOI] [PubMed] [Google Scholar]
- 44. Xu Y, Jones JE, Kohno D, et al. 5‐HT2CRs expressed by pro‐opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60(4):582‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. D'Agostino G, Lyons D, Cristiano C, et al. Nucleus of the solitary tract serotonin 5‐HT2C receptors modulate food intake. Cell Metab. 2018;28(4):619‐630.e615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Valencia‐Torres L, Olarte‐Sanchez CM, Lyons DJ, et al. Activation of ventral tegmental area 5‐HT2C receptors reduces incentive motivation. Neuropsychopharmacology. 2017;42(7):1511‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schuhler S, Clark A, Joseph W, et al. Involvement of 5‐HT receptors in the regulation of food intake in Siberian hamsters. J Neuroendocrinol. 2005;17(5):276‐285. [DOI] [PubMed] [Google Scholar]
- 48. Smit‐Rigter LA, Wadman WJ, van Hooft JA. Impaired social behavior in 5‐HT(3A) receptor knockout mice. Front Behav Neurosci. 2010;4(169):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pratt WE, Lin P, Pierce‐Messick Z, Ilesanmi AO, Clissold KA. Contrasting effects of 5‐HT(3) receptor stimulation of the nucleus accumbens or ventral tegmentum on food intake in the rat. Behav Brain Res. 2017;323:15‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hayes MR, Savastano DM, Covasa M. Cholecystokinin‐induced satiety is mediated through interdependent cooperation of CCK‐A and 5‐HT3 receptors. Physiol Behav. 2004;82(4):663‐669. [DOI] [PubMed] [Google Scholar]
- 51. Compan V, Zhou M, Grailhe R, et al. Attenuated response to stress and novelty and hypersensitivity to seizures in 5‐HT4 receptor knock‐out mice. J Neurosci. 2004;24(2):412‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jean A, Conductier G, Manrique C, et al. Anorexia induced by activation of serotonin 5‐HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci. 104(41):16335‐16340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jean A, Conductier G, Manrique C, et al. Anorexia induced by activation of serotonin 5‐HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104(41):16335‐16340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Frassetto A, Zhang J, Lao JZ, et al. Reduced sensitivity to diet‐induced obesity in mice carrying a mutant 5‐HT6 receptor. Brain Res. 2008;1236:140‐144. [DOI] [PubMed] [Google Scholar]
- 55. Fisas A, Codony X, Romero G, et al. Chronic 5‐HT6 receptor modulation by E‐6837 induces hypophagia and sustained weight loss in diet‐induced obese rats. Br J Pharmacol. 148(7):973‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dudek M, Marcinkowska M, Bucki A, Olczyk A, Kolaczkowski M. Idalopirdine ‐ a small molecule antagonist of 5‐HT6 with therapeutic potential against obesity. Metab Brain Dis. 2015;30(6):1487‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guscott M, Bristow LJ, Hadingham K, et al. Genetic knockout and pharmacological blockade studies of the 5‐HT7 receptor suggest therapeutic potential in depression. Neuropharmacology. 2005;48(4):492‐502. [DOI] [PubMed] [Google Scholar]
- 58. Hesse S, van de Giessen E, Zientek F, et al. Association of central serotonin transporter availability and body mass index in healthy Europeans. Eur Neuropsychopharmacol. 2014;24(8):1240‐1247. [DOI] [PubMed] [Google Scholar]
- 59. Dahlstroem A, Fuxe K. Evidence for the existence of monoamine‐containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl. 1964;232:231‐255. [PubMed] [Google Scholar]
- 60. Flores RA, da Silva ES, Ribas AS, et al. Evaluation of food intake and Fos expression in serotonergic neurons of raphe nuclei after intracerebroventricular injection of adrenaline in free‐feeding rats. Brain Res. 1678;2018:153‐163. [DOI] [PubMed] [Google Scholar]
- 61. Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J Comp Neurol. 1999;407(4):555‐582. [PubMed] [Google Scholar]
- 62. Takase LF, Nogueira MI. Patterns of fos activation in rat raphe nuclei during feeding behavior. Brain Res. 2008;1200:10‐18. [DOI] [PubMed] [Google Scholar]
- 63. Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483(7391):594‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pollak Dorocic I, Furth D, Xuan Y, et al. A whole‐brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron. 2014;83(3):663‐678. [DOI] [PubMed] [Google Scholar]
- 65. Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003;26(4):331‐343. [DOI] [PubMed] [Google Scholar]
- 66. Voigt JP, Fink H. Serotonin controlling feeding and satiety. Behav Brain Res. 2015;277:14‐31. [DOI] [PubMed] [Google Scholar]
- 67. Boden PR, Woodruff GN, Pinnock RD. Pharmacology of a cholecystokinin receptor on 5‐hydroxytryptamine neurones in the dorsal raphe of the rat brain. Br J Pharmacol. 1991;102(3):635‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Anderberg RH, Richard JE, Eerola K, et al. Glucagon‐like peptide 1 and its analogs act in the dorsal raphe and modulate central serotonin to reduce appetite and body weight. Diabetes. 2017;66(4):1062‐1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hansson C, Alvarez‐Crespo M, Taube M, et al. Influence of ghrelin on the central serotonergic signaling system in mice. Neuropharmacology. 2014;79:498‐505. [DOI] [PubMed] [Google Scholar]
- 70. Fletcher PJ, Davies M. Dorsal raphe microinjection of 5‐HT and indirect 5‐HT agonists induces feeding in rats. Eur J Pharmacol. 1990;184(2–3):265‐271. [DOI] [PubMed] [Google Scholar]
- 71. Ciofi P. The arcuate nucleus as a circumventricular organ in the mouse. Neurosci Lett. 2011;487(2):187‐190. [DOI] [PubMed] [Google Scholar]
- 72. Grill HJ, Hayes MR. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes (2005). 2009;33(Suppl 1):S11‐S15. [DOI] [PubMed] [Google Scholar]
- 73. Millington GW. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr Metab (Lond). 2007;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro‐opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5(9):1066‐1070. [DOI] [PubMed] [Google Scholar]
- 75. Zhan C, Zhou J, Feng Q, et al. Acute and long‐term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci. 2013;33(8):3624‐3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Krude H, Biebermann H, Gruters A. Mutations in the human proopiomelanocortin gene. Ann N Y Acad Sci. 2003;994(1):233‐239. [DOI] [PubMed] [Google Scholar]
- 77. Yadav VK, Oury F, Tanaka KF, et al. Leptin‐dependent serotonin control of appetite: temporal specificity, transcriptional regulation, and therapeutic implications. J Exp Med. 2011;208(1):41‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro‐opiomelanocortin neurons via TRPC channels. Neuron. 2011;71(3):488‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang D, He X, Zhao Z, et al. Whole‐brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front Neuroanat. 2015;9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cone RD. The central melanocortin system and energy homeostasis. Trends Endocrinol Metab. 1999;10(6):211‐216. [DOI] [PubMed] [Google Scholar]
- 81. Fenselau H, Campbell JN, Verstegen AM, et al. A rapidly acting glutamatergic ARC → PVH satiety circuit postsynaptically regulated by alpha‐MSH. Nat Neurosci. 2017;20(1):42‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Balthasar N, Dalgaard LT, Lee CE, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493‐505. [DOI] [PubMed] [Google Scholar]
- 83. Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24(1):155‐163. [DOI] [PubMed] [Google Scholar]
- 84. Bell ME, Bhatnagar S, Akana SF, Choi S, Dallman MF. Disruption of arcuate/paraventricular nucleus connections changes body energy balance and response to acute stress. J Neurosci. 2000;20(17):6707‐6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085‐1095. [DOI] [PubMed] [Google Scholar]
- 86. Blevins JE, Ho JM. Role of oxytocin signaling in the regulation of body weight. Rev Endocr Metab Disord. 2013;14(4):311‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pei H, Sutton AK, Burnett KH, Fuller PM, Olson DP. AVP neurons in the paraventricular nucleus of the hypothalamus regulate feeding. Mol Metab. 2014;3(2):209‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sawchenko PE, Swanson LW, Steinbusch HW, Verhofstad AA. The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res. 1983;277(2):355‐360. [DOI] [PubMed] [Google Scholar]
- 89. Cruz‐Martinez AM, Tejas‐Juarez JG, Mancilla‐Diaz JM, Floran‐Garduno B, Lopez‐Alonso VE, Escartin‐Perez RE. CB1 receptors in the paraventricular nucleus of the hypothalamus modulate the release of 5‐HT and GABA to stimulate food intake in rats. Eur Neuropsychopharmacol. 2018;28(11):1247‐1259. [DOI] [PubMed] [Google Scholar]
- 90. Garfield AS, Burke LK, Shaw J, Evans ML, Heisler LK. Distribution of cells responsive to 5‐HT(6) receptor antagonist‐induced hypophagia. Behav Brain Res. 2014;266:201‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brobeck JR, Tepperman J, Long CN. Experimental hypothalamic hyperphagia in the albino rat. Yale J Biol Med. 1943;15(6):831‐853. [PMC free article] [PubMed] [Google Scholar]
- 92. Gold RM. Hypothalamic obesity: the myth of the ventromedial nucleus. Science (New York, NY). 1973;182(4111):488‐490. [DOI] [PubMed] [Google Scholar]
- 93. King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87(2):221‐244. [DOI] [PubMed] [Google Scholar]
- 94. Choi YH, Fujikawa T, Lee J, Reuter A, Kim KW. Revisiting the ventral medial nucleus of the hypothalamus: the roles of SF‐1 neurons in energy homeostasis. Front Neurosci. 2013;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Xu B, Goulding EH, Zang K, et al. Brain‐derived neurotrophic factor regulates energy balance downstream of melanocortin‐4 receptor. Nat Neurosci. 2003;6(7):736‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang C, Bomberg E, Levine A, Billington C, Kotz CM. Brain‐derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1037‐R1045. [DOI] [PubMed] [Google Scholar]
- 97. Leibowitz SF, Weiss GF, Suh JS. Medial hypothalamic nuclei mediate serotonin's inhibitory effect on feeding behavior. Pharmacol Biochem Behav. 1990;37(4):735‐742. [DOI] [PubMed] [Google Scholar]
- 98. Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav. 2002;76(3):431‐442. [DOI] [PubMed] [Google Scholar]
- 99. Haskell‐Luevano C, Chen P, Li C, et al. Characterization of the neuroanatomical distribution of agouti‐related protein immunoreactivity in the rhesus monkey and the rat. Endocrinology. 1999;140(3):1408‐1415. [DOI] [PubMed] [Google Scholar]
- 100. Harrold JA, Widdowson PS, Williams G. Altered energy balance causes selective changes in melanocortin‐4(MC4‐R), but not melanocortin‐3 (MC3‐R), receptors in specific hypothalamic regions: further evidence that activation of MC4‐R is a physiological inhibitor of feeding. Diabetes. 1999;48(2):267‐271. [DOI] [PubMed] [Google Scholar]
- 101. Elias CF, Saper CB, Maratos‐Flier E, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402(4):442‐459. [PubMed] [Google Scholar]
- 102. Betley JN, Cao ZF, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155(6):1337‐1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Moore RY, Halaris AE, Jones BE. Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol. 1978;180(3):417‐438. [DOI] [PubMed] [Google Scholar]
- 104. Schwartz DH, McClane S, Hernandez L, Hoebel BG. Feeding increases extracellular serotonin in the lateral hypothalamus of the rat as measured by microdialysis. Brain Res. 1989;479(2):349‐354. [DOI] [PubMed] [Google Scholar]
- 105. Yamanaka A, Muraki Y, Tsujino N, Goto K, Sakurai T. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun. 2003;303(1):120‐129. [DOI] [PubMed] [Google Scholar]
- 106. Muraki Y, Yamanaka A, Tsujino N, Kilduff TS, Goto K, Sakurai T. Serotonergic regulation of the orexin/hypocretin neurons through the 5‐HT1A receptor. J Neurosci. 2004;24(32):7159‐7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci. 2013;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cole S, Mayer HS, Petrovich GD. Orexin/hypocretin‐1 receptor antagonism selectively reduces cue‐induced feeding in sated rats and recruits medial prefrontal cortex and thalamus. Sci Rep. 2015;5(1):16143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cason AM, Aston‐Jones G. Role of orexin/hypocretin in conditioned sucrose‐seeking in female rats. Neuropharmacology. 2014;86:97‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Borgland SL, Chang SJ, Bowers MS, et al. Orexin A/hypocretin‐1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29(36):11215‐11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. 2010;67(8):753‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cai XJ, Evans ML, Lister CA, et al. Hypoglycemia activates orexin neurons and selectively increases hypothalamic orexin‐B levels: responses inhibited by feeding and possibly mediated by the nucleus of the solitary tract. Diabetes. 2001;50(1):105‐112. [DOI] [PubMed] [Google Scholar]
- 113. Sweet DC, Levine AS, Billington CJ, Kotz CM. Feeding response to central orexins. Brain Res. 1999;821(2):535‐538. [DOI] [PubMed] [Google Scholar]
- 114. Zink AN, Bunney PE, Holm AA, Billington CJ, Kotz CM. Neuromodulation of orexin neurons reduces diet‐induced adiposity. Int J Obes (2005). 2018;42(4):737‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Jalewa J, Joshi A, McGinnity TM, Prasad G, Wong‐Lin K, Holscher C. Neural circuit interactions between the dorsal raphe nucleus and the lateral hypothalamus: an experimental and computational study. PLoS ONE. 2014;9(2):e88003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology. 2001;40(3):457‐459. [DOI] [PubMed] [Google Scholar]
- 117. Qu D, Ludwig DS, Gammeltoft S, et al. A role for melanin‐concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380(6571):243‐247. [DOI] [PubMed] [Google Scholar]
- 118. Borowsky B, Durkin MM, Ogozalek K, et al. Antidepressant, anxiolytic and anorectic effects of a melanin‐concentrating hormone‐1 receptor antagonist. Nat Med. 2002;8(8):825‐830. [DOI] [PubMed] [Google Scholar]
- 119. van den Pol AN, Acuna‐Goycolea C, Clark KR, Ghosh PK. Physiological properties of hypothalamic MCH neurons identified with selective expression of reporter gene after recombinant virus infection. Neuron. 2004;42(4):635‐652. [DOI] [PubMed] [Google Scholar]
- 120. Palmiter RD. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 2018;41(5):280‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Li BH, Rowland NE. Dexfenfluramine induces Fos‐like immunoreactivity in discrete brain regions in rats. Brain Res Bull. 1993;31(1–2):43‐48. [DOI] [PubMed] [Google Scholar]
- 122. Li BH, Spector AC, Rowland NE. Reversal of dexfenfluramine‐induced anorexia and c‐Fos/c‐Jun expression by lesion in the lateral parabrachial nucleus. Brain Res. 1994;640(1–2):255‐267. [DOI] [PubMed] [Google Scholar]
- 123. Trifunovic R, Reilly S. Medial parabrachial nucleus neurons modulate d‐fenfluramine‐induced anorexia through 5HT2C receptors. Brain Res. 2006;1067(1):170‐176. [DOI] [PubMed] [Google Scholar]
- 124. Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503(7474):111‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Essner RA, Smith AG, Jamnik AA, Ryba AR, Trutner ZD, Carter ME. AgRP neurons can increase food intake during conditions of appetite suppression and inhibit anorexigenic parabrachial neurons. J Neurosci. 2017;37(36):8678‐8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Lee JS, Lee EY, Lee HS. Hypothalamic, feeding/arousal‐related peptidergic projections to the paraventricular thalamic nucleus in the rat. Brain Res. 2015;1598:97‐113. [DOI] [PubMed] [Google Scholar]
- 127. Versteeg RI, Koopman KE, Booij J, et al. Serotonin transporter binding in the diencephalon is reduced in insulin resistant obese humans. Neuroendocrinology. 2016;105(2):141‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Versteeg RI, Schrantee A, Adriaanse SM, et al. Timing of caloric intake during weight loss differentially affects striatal dopamine transporter and thalamic serotonin transporter binding. FASEB J. 2017;31(10):4545‐4554. [DOI] [PubMed] [Google Scholar]
- 129. Koopman KE, Booij J, Fliers E, Serlie MJ, la Fleur SE. Diet‐induced changes in the lean brain: hypercaloric high‐fat‐high‐sugar snacking decreases serotonin transporters in the human hypothalamic region. Molecular metabolism. 2013;2(4):417‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic‐thalamic‐striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493(1):72‐85. [DOI] [PubMed] [Google Scholar]
- 131. Meguid MM, Fetissov SO, Varma M, et al. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition (Burbank, Los Angeles County, Calif). 2000;16(10):843‐857. [DOI] [PubMed] [Google Scholar]
- 132. Beliveau V, Ganz M, Feng L, et al. A high‐resolution in vivo atlas of the human brain's serotonin system. J Neurosci. 2017;37(1):120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science (New York, NY). 2004;304(5669):452‐454. [DOI] [PubMed] [Google Scholar]
- 134. de Lartigue G, McDougle M. Dorsal striatum dopamine oscillations: setting the pace of food anticipatory activity. Acta Physiol (Oxf). 2019;225(1):e13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Rozenblit‐Susan S, Chapnik N, Genzer Y, Froy O. Serotonin suppresses food anticipatory activity and synchronizes the food‐entrainable oscillator during time‐restricted feeding. Behav Brain Res. 2016;297:150‐154. [DOI] [PubMed] [Google Scholar]
- 136. Shibata S, Ono M, Minamoto Y, Watanabe S. Attenuating effect of serotonin receptor antagonists on impairment of mealtime‐associated activity rhythm in old rats. Pharmacol Biochem Behav. 1995;51(2–3):541‐544. [DOI] [PubMed] [Google Scholar]
- 137. Gallardo CM, Martin CS, Steele AD. Food anticipatory activity on circadian time scales is not dependent on central serotonin: evidence from tryptophan hydroxylase‐2 and serotonin transporter knockout mice. Front Mol Neurosci. 2020;13:534238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Virk MS, Sagi Y, Medrihan L, Leung J, Kaplitt MG, Greengard P. Opposing roles for serotonin in cholinergic neurons of the ventral and dorsal striatum. Proc Natl Acad Sci U S A. 2016;113(3):734‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Miguelez C, Morera‐Herreras T, Torrecilla M, Ruiz‐Ortega JA, Ugedo L. Interaction between the 5‐HT system and the basal ganglia: functional implication and therapeutic perspective in Parkinson's disease. Front Neural Circuits. 2014;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Nieh EH, Matthews GA, Allsop SA, et al. Decoding neural circuits that control compulsive sucrose seeking. Cell. 2015;160(3):528‐541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Xu P, He Y, Cao X, et al. Activation of serotonin 2C receptors in dopamine neurons inhibits binge‐like eating in mice. Biol Psychiatry. 2017;81(9):737‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bubar MJ, Cunningham KA. Distribution of serotonin 5‐HT2C receptors in the ventral tegmental area. Neuroscience. 2007;146(1):286‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Navailles S, Moison D, Cunningham KA, Spampinato U. Differential regulation of the mesoaccumbens dopamine circuit by serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: an in vivo microdialysis study with cocaine. Neuropsychopharmacology. 2008;33(2):237‐246. [DOI] [PubMed] [Google Scholar]
- 144. Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117(2):202‐211. [DOI] [PubMed] [Google Scholar]
- 145. Avena NM, Rada P, Hoebel BG. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience. 2008;156(4):865‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward‐predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci. 2011;34(12):1997‐2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624(1–2):188‐198. [DOI] [PubMed] [Google Scholar]
- 148. Fletcher PJ, Azampanah A, Korth KM. Activation of 5‐HT(1B) receptors in the nucleus accumbens reduces self‐administration of amphetamine on a progressive ratio schedule. Pharmacol Biochem Behav. 2002;71(4):717‐725. [DOI] [PubMed] [Google Scholar]
- 149. Helm KA, Rada P, Hoebel BG. Cholecystokinin combined with serotonin in the hypothalamus limits accumbens dopamine release while increasing acetylcholine: a possible satiation mechanism. Brain Res. 2003;963(1–2):290‐297. [DOI] [PubMed] [Google Scholar]
- 150. Blumenthal SA, Pratt WE. d‐Fenfluramine and lorcaserin inhibit the binge‐like feeding induced by mu‐opioid receptor stimulation of the nucleus accumbens in the rat. Neurosci Lett. 2018;687:43‐48. [DOI] [PubMed] [Google Scholar]
- 151. Day HE, Curran EJ, Watson SJ Jr, Akil H. Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin‐1beta. J Comp Neurol. 1999;413(1):113‐128. [PubMed] [Google Scholar]
- 152. Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC‐delta(+) neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci. 2014;17(9):1240‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Huang XF, Huang X, Han M, Chen F, Storlien L, Lawrence AJ. 5‐HT2A/2C receptor and 5‐HT transporter densities in mice prone or resistant to chronic high‐fat diet‐induced obesity: a quantitative autoradiography study. Brain Res. 2004;1018(2):227‐235. [DOI] [PubMed] [Google Scholar]
- 154. Meguid MM, Fetissov SO, Blaha V, Yang ZJ. Dopamine and serotonin VMN release is related to feeding status in obese and lean Zucker rats. Neuroreport. 2000;11(10):2069‐2072. [DOI] [PubMed] [Google Scholar]
- 155. Routh VH, Stern JS, Horwitz BA. Serotonergic activity is depressed in the ventromedial hypothalamic nucleus of 12‐day‐old obese Zucker rats. Am J Physiol. 1994;267(3 Pt 2):R712‐R719. [DOI] [PubMed] [Google Scholar]
- 156. Park S, Harrold JA, Widdowson PS, Williams G. Increased binding at 5‐HT(1A), 5‐HT(1B), and 5‐HT(2A) receptors and 5‐HT transporters in diet‐induced obese rats. Brain Res. 1999;847(1):90‐97. [DOI] [PubMed] [Google Scholar]
- 157. Banas SM, Rouch C, Kassis N, Markaki EM, Gerozissis K. A dietary fat excess alters metabolic and neuroendocrine responses before the onset of metabolic diseases. Cell Mol Neurobiol. 2009;29(2):157‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Borgers AJ, Koopman KE, Bisschop PH, et al. Decreased serotonin transporter immunoreactivity in the human hypothalamic infundibular nucleus of overweight subjects. Front Neurosci. 2014;8:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Strombom U, Krotkiewski M, Blennow K, Mansson JE, Ekman R, Bjorntorp P. The concentrations of monoamine metabolites and neuropeptides in the cerebrospinal fluid of obese women with different body fat distribution. Int J Obes (Lond). 1996;20(4):361‐368. [PubMed] [Google Scholar]
- 160. Koskela AK, Kaurijoki S, Pietilainen KH, et al. Serotonin transporter binding and acquired obesity ‐‐ an imaging study of monozygotic twin pairs. Physiol Behav. 2008;93(4–5):724‐732. [DOI] [PubMed] [Google Scholar]
- 161. Haahr ME, Fisher PM, Jensen CG, et al. Central 5‐HT4 receptor binding as biomarker of serotonergic tonus in humans: a [11C]SB207145 PET study. Mol Psychiatry. 2014;19(4):427‐432. [DOI] [PubMed] [Google Scholar]
- 162. Hesse S, Rullmann M, Luthardt J, et al. Central serotonin transporter availability in highly obese individuals compared with non‐obese controls: a [(11)C] DASB positron emission tomography study. Eur J Nucl Med Mol Imaging. 2016;43(6):1096‐1104. [DOI] [PubMed] [Google Scholar]
- 163. Versteeg RI, Serlie MJ, Kalsbeek A, la Fleur SE. Serotonin, a possible intermediate between disturbed circadian rhythms and metabolic disease. Neuroscience. 2015;301:155‐167. [DOI] [PubMed] [Google Scholar]
- 164. Wu CH, Chang CS, Yang YK, Shen LH, Yao WJ. Comparison of brain serotonin transporter using [I‐123]‐ADAM between obese and non‐obese young adults without an eating disorder. PLoS ONE. 2017;12(2):e0170886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bohula EA, Wiviott SD, McGuire DK, et al. Cardiovascular safety of lorcaserin in overweight or obese patients. N Engl J Med. 2018;379(12):1107‐1117. [DOI] [PubMed] [Google Scholar]
- 166. Sharretts J, Galescu O, Gomatam S, Andraca‐Carrera E, Hampp C, Yanoff L. Cancer risk associated with lorcaserin ‐ the FDA's review of the CAMELLIA‐TIMI 61 trial. N Engl J Med. 2020;383(11):1000‐1002. [DOI] [PubMed] [Google Scholar]
- 167. Oh CM, Namkung J, Go Y, et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat Commun. 2015;6:6794. [DOI] [PMC free article] [PubMed] [Google Scholar]


