Abstract
Immunoglobulin G (IgG) is important in clearance and recognition of previously presented antigens and after activation, IgGs can interact with the Fc gamma receptors (FcγRs) on haematopoietic cells, including bone‐resorbing osteoclasts. The pathogenicity of IgG, that is the ability to elicit stimulatory effects via FcγRs, can be modulated by attachment of sugar moieties, including sialic acids. Human IgGs and autoantibodies are associated with bone loss in autoimmune disease. However, the impact of polyclonal murine IgG via FcγRs on bone loss is poorly understood. Here, we investigate if heat‐aggregated activated murine polyclonal IgG complexes have any direct effects on murine osteoclasts and if they modulate arthritis‐mediated bone loss. Using cell cultures of murine osteoclasts, we show that IgG complexes without sialic acids (de‐IgG complexes) enhance receptor activator of nuclear factor kappa‐Β ligand (RANKL)‐stimulated osteoclastogenesis, an effect associated with increased FcγRIII expression. Using an in vivo model of arthritis‐mediated bone loss, where IgG complexes were injected into arthritic knees, no effect on the severity of arthritis or the degree of arthritis‐mediated bone loss was detected. Interestingly, injection of de‐IgG complexes into non‐arthritic knees increased osteoclast formation and enhanced bone erosions. Our findings show that activated de‐IgG complexes have no additive effect on arthritis‐mediated bone loss. However, de‐IgG complexes potentiate murine osteoclastogenesis and enhance local bone erosion in non‐arthritic bones, further confirming the link between the adaptive immune system and bone.
Keywords: antibodies/immunoglobulins, experimental animals, fc receptors, in vitro, osteoclast
1. INTRODUCTION
Polyclonal immunoglobulin G (IgG) is a secretory product of the adaptive immune system and constitutes the major class of antibodies found in serum. IgGs consist of variable antigen‐binding fragment (Fab) parts that interact with specific antigens and a constant Fc part that after antigen activation or complex formation, binds to cell surface Fc gamma receptors (FcγRs). FcγRs are expressed on all haematopoietic cells, including osteoclasts, which originate from bone marrow derived myeloid lineage cells and are the cells responsible for bone resorption. 1 , 2 , 3 There are four different types of murine FcγRs, where I, III and IV activate and IIb inhibits immune action. 4 Signalling via both FcγRIII 3 and FcγRIV 2 in osteoclasts has been demonstrated to regulate murine bone remodelling in transgenic mice. Rheumtoid arthrthis (RA) specific autoantibodies towards citrullinated proteins (ACPAs) are strongly associated with induction of osteoclastogenesis and bone destruction 5 , 6 , 7 , 8 and we recently demonstrated a direct role of mutated citrullinated vimentin (MCV) or antibodies against this protein, ACPAs, for local arthritis‐mediated bone loss in the same model as used in the present study. 7 Several studies support the hypothesis that IgGs affect osteoclasts via its Fc part after interaction with FcγR 1 , 2 , 3 , 6 , 9 but there are studies showing that the Fab part also can be of importance. 6 , 8 Citrullinated vimentin is highly expressed on the surface on myeloid cells and the expression further increases during osteoclast differentiation 10 and the Fab part can mediate direct effects on osteoclasts via recognition of citrullinated vimentin on the osteoclast surface. 6 , 8
Recent studies have highlighted that IgG undergoes glycosylation, a post‐translational modification that strongly influences its stability, conformation and sizeof the protein. Certain glycans attached to the conserved N‐linked glycosylation site (Asn‐297) in the Fc part of IgGs affect the binding capability to FcγRs and thereby the pathogenicity of IgGs. 11 , 12 , 13 The glycan is a biantennary structure with one heptameric stem consisting of mannose and N‐acetylglucosamine residues, where variable terminal sugar residues, such as galactose, fucose and sialic acid, can attach. 14 A high degree of sialic acid on the IgG Fc part counteracts the binding potential to FcγRs, which leads to less inflammation, and is thereby an anti‐inflammatory property. Conversely, desialylated IgGs, where terminal sialic acid is lacking, have enhanced affinity to FcγR, and are thereby pro‐inflammatory. 15 , 16 Changes in glycosylation of antibodies have been linked to various autoimmune diseases, and the degree of sialylation has been correlated to the incidence RA, 17 , 18 Sjogren's disease, 19 lupus erythematosus, 20 multiple sclerosis 21 and inflammatory bowel disease. 22 Interestingly, it has been found that RA‐specific autoantibodies, ACPAs, have a lower level of sialic acids attached to their Fc parts compared to other IgGs. 23 It has been suggested that changes in glycosylation grade of IgG occur shortly before the onset of RA, and that this change could be involved in the transitional phase of the induction of the disease. 23 , 24 , 25 , 26
IgGs can mediate effects via FcγR, either after activation via antigen binding to the Fab parts of the protein, or via complex formation. Complexes can be formed between antibodies and soluble antigens, but activated complexes can also be formed by heat‐aggregation of purified IgGs without the presence of antigens. 9 Modulation of sugar moieties on heat‐aggregated human IgG complexes has been shown to affect receptor activator of nuclear factor kappa‐B ligand (RANKL)‐induced differentiation of human osteoclasts and induce bone loss in vivo. 9 Bone homoeostasis is maintained by a balanced action of bone‐resorbing osteoclasts and bone forming osteoblasts. Osteoclast formation is regulated by specific cytokines such as macrophage colony‐stimulating factor (M‐CSF), which stimulates progenitor cell proliferation and enhances their survival, and RANKL, which induces differentiation along the osteoclastic lineage through activation of its cognate receptor RANK. RANK stimulation as well as activation of other co‐stimulatory signals, like FcγR and DAP12, act in concert to activate several transcription factors including the osteoclast master transcription factor nuclear factor of activated T‐cells, cytoplasmic 1 (NFATc1).
Antigen‐induced arthritis (AIA), is a monoarthritis model used to determine effects on local bone loss, including periarticular bone loss and bone erosions. The model is characterized by this local bone loss, leucocyte infiltration, cytokine production and synovitis. 27 , 28 We injected polyclonal murine IgG immune complexes, with or without sialic acids into both arthritic and non‐arthritic knees, to determine the role of IgG complexes in both arthritic and non‐arthritic conditions.
The present study aimed to evaluate the role of heat‐aggregated activated murine polyclonal IgG complexes in osteoclast differentiation and bone loss, and to determine if this effect was affected by alteration of the sugar moieties on the Fc part of the IgGs.
2. METHODS
2.1. Isolation of immunoglobulin G complexes
Polyclonal immunoglobulin G (IgG) antibodies were isolated from pooled serum of naïve C57BL/6J mice (Taconic) three times, generating three different batches using a protein G spin column (GE Healthcare, GE28‐9031‐34) according to the manufacturer's instructions. 1 mg of murine IgG was incubated with 5U of neuraminidase (Sigma‐Aldrich, N3001‐10UN) and dissolved in 0.1 M acetate buffer for 24h at 37°C for desialylation (de). Untreated (sialylated) IgG was incubated with only acetate buffer for 24h at 37°C. The digested de‐IgG and untreated IgG was purified using a protein G spin column (GE Healthcare, GE28‐9031‐34) according to the manufacturer's instructions. Protein concentration was determined using Mouse IgG ELISA Quantitation set (Bethyl Laboratories, Inc, E90‐131) and detergent compatible (DC) protein assay (Bio‐Rad, 5 000 112) according to the manufacturer's instructions. For assessing IgG sialylation, a plate was coated with goat‐anti‐mouse IgG‐F(ab’)2 fragment (Sigma‐Aldrich, M0659‐0.5ML) and blocked with 3% gelatine buffer (Carl Roth GmbH, 4308.4 ) followed by wash with TBS and lectin buffer (TBS 1mM MgCl2, 1mM CaCl2, 1mM MnCl2) before incubation with the purified IgG. Sialic acids were captured by biotinylated Sambucus Nigra lectin (Vector Laboratories, B‐1305‐2) and displayed by streptavidin‐HRP (R&D Systems, DY998). The de‐IgG had lower levels of sialic acids compared to the untreated IgG in all three batches (Supp Figure S1). Activated IgG complexes, that have the possibility to interact with FcγR without binding to antigens, were obtained by heat‐aggregation of the untreated and desialylated IgG at 63°C for 30 minutes. 9
2.2. Generation and stimulation of osteoclasts
Murine osteoclast differentiation was studied in vitro by RANKL‐induced differentiation of bone marrow macrophages (BMM). Bone marrow cells were isolated from naïve C57BL/6J mice (Taconic) by flushing bone marrow from femoral and tibial bones with α‐MEM medium (Invitrogen) using a syringe and 30G needle. BMMs were expanded in complete α‐MEM medium containing 10% foetal bovine serum (Sigma‐Aldrich, 12103C‐500ML), 50 µg·ml‐1 gentamicin (Gibco, 15 750 060), 1% penicillin/streptomycin (Gibco, 15 140 122), 2mM GlutaMAX (Gibco, 35 050 061) with 30 ng·ml‐1 M‐CSF (R&D, 416‐ML‐050) in suspension culture dishes (Corning Costar Ins, 430 591), at 37°C. 29 After 2 days, non‐adherent cells were washed away with PBS and 0.02% EDTA in PBS was used to detach the adherent BMMs from the culture dish. BMMs were spot seeded in the centre of the wells at a density of 5000 cells per well in 96‐well plates and incubated with 30 ng·ml‐1 M‐CSF alone or in combination with 2 ng·ml‐1 RANKL (R&D, 462‐TEC) (MRL) to induce osteoclast differentiation. After 3 days the media was changed, a time point at which the cells were mono‐ or bi‐nucleated preosteoclasts, and after an additional 2‐3 days of incubation, osteoclast formation was evaluated by tartrate resistant acid phosphatase (TRAP) staining (Sigma‐Aldrich, 386A‐1KT) according to the manufacturer's instructions. The RANKL was used at a sub‐maximally effective concentration in order to increase the ability of the IgG complexes to enhance RANKL‐induced osteoclast formation. Untreated IgG complexes or desialylated IgG complexes (de‐IgG complexes), were added in a concentration of 0.1 mg·ml‐1 during different parts of the cultures, I; continuously throughout the entire culture period (Supp Figure S2A), II; only during the 3 first days of cultures (Supp Figure S2B) or III; only during the last 2‐3 days of culture (Figure 1). As an additional control culture, IV; 0.1 mg·mL−1 monomeric IgGs as well as desialylated monomeric IgGs were added the 2 last days (Supp Figure S3A). The monomeric IgGs were not activated by heat‐aggregation and therefore have to be activated via antigen interaction to be able to recognize FcγRs. Numbers of osteoclasts per well were counted using a microscope (Nikon) and an image analysis system (Osteomeasure; OsteoMetrics). TRAP positive cells containing three or more nuclei were counted as osteoclasts. Stimulation I, II, III and IV were performed in cells from three different naïve C57BL/6 mice stimulated with two different batches of IgG complexes and all experiments displayed similar results. Stimulation III was performed four times, each time using cells from one or two C57BL/6 mice and two different batches of IgG complex.
Figure 1.
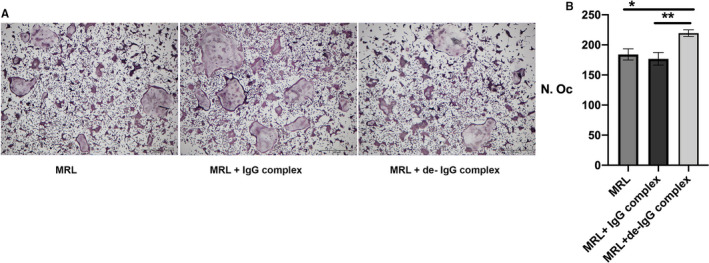
A, Representative images of osteoclasts cultured in the presence or absence of heat‐aggregated IgG complexes, untreated and desialylated (de) during the last 24 hours of culture. Scale bar, 500 µm. B, TRAP positive cells with ≥ 3 nuclei were counted as osteoclasts. Results are shown as mean ± SEM, n = 8. Statistical analysis was performed using one‐way ANOVA followed by Tukey's multiple comparison test. MRL = macrophage colony‐stimulating factor + receptor activator of nuclear kappa‐B ligand (RANKL) stimulation
2.3. RNA isolation and quantitative RT‐PCR analysis
Total RNA was isolated from cultured osteoclasts stimulated with untreated or de‐IgG complexes. The cultures were performed as described above with 2‐days for isolation of adhesive BMMs, 3 days with only M‐CSF or M‐CSF + RANKL (MRL) for the cells to become mono‐ or bi‐nucleated preosteoclasts and then 2 days with only M‐CSF or MRL. Additional stimuli with untreated or de‐IgG complexes was added during the two final days. Total RNA was isolated using RNeasy Micro Kit (Qiagen, 74 004) according to the manufacturer's instructions and cDNA was synthesized using a High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, 4 368 814). Quantitative real‐time PCR (qPCR) analyses were performed using predesigned Taqman Assays and Taqman Fast Advance Master Mix (Thermo Fisher Scientific, 4 444 556). The following predesigned real‐time PCR assays were used for gene expression analysis: Nuclear factor of activated T‐cells (Nfatc1, Mm00479445_m), Cathepsin K (Ctsk, Mm00484036_m), Receptor activator of nuclear factor κ B (Rank, Tnfrs11a, Mm00437135_m1), Fcγ receptor I (FcγRI) (Fcgr1, Mm00438874_m1), Fcγ receptor IIb (FcγRiib) (Fcgr2b, Mm00438875_m1), Fcγ receptor III (FcγRIII) (Fcgr3, Mm00438882_m1), Fcγ receptor IV (FcγRIV) (Fcgr4, Mm00519988_m1). The house‐keeping gene 18S (Thermo Fisher Scientific, cat no 4310893E) was used as endogenous control in all analyses. These experiments were performed with cells from four naïve C57BL/6 mice, each mouse stimulated with two separate batches of untreated and de‐IgG complexes. Results are displayed as per cent change from M‐CSF stimulated cells from two mice, in Table 1. Data repeated with similar results from two additional mice.
Table 1.
Expression Pattern of Bone‐Associated Genes and FcyR‐Associated Genes
| M‐CSF |
M‐CSF RANKL |
M‐CSF RANKL untreated IgG complex |
M‐CSF RANKL de‐IgG complex |
|
|---|---|---|---|---|
| NFATc1 | 100 ± 12 | 401 ± 95*** | 424 ± 97*** | 395 ± 92*** |
| CTSK | 100 ± 57 | 238 ± 105* | 324 ± 79*** | 427 ± 105 *** # |
| RANK | 100 ± 12 | 141 ± 11** | 135 ± 15** | 131 ± 13 ** |
| Fcgr1 | 100 ± 22 | 799 ± 350 *** | 1020 ± 285 *** | 1048 ± 278 *** |
| Fcgr3 | 100 ± 23 | 230 ± 73** | 279 ± 125 *** | 355 ± 80 **, # |
Stimulation is presented as per cent of expression in M‐CSF stimulated cells, set to 100% ±SEM, n = 4, experiment repeated once with similar results.
M‐CSF; macrophage colony‐stimulating factor, RANKL; receptor activator of nuclear kappa‐B ligand, IgG; immunoglobulin G, de‐IgG; desialylated IgG
P < .05,
P < .01,
P < .001 vs M‐CSF,
P < .05 vs M‐CSF + RANKL analysed using one‐way ANOVA followed by Tukey's comparison with all groups.
2.4. Animals and induction of antigen‐induced arthritis
Three‐month‐old female C57BL/6J mice (Taconic) were kept 5 animals per cage under standard environmental conditions and fed a standard chow with tap water ad libitum. The mice were subjected to antigen‐induced arthritis (AIA) where a local mono‐arthritis was induced in one knee joint by injecting methylated murine bovine serum albumin (mBSA; Sigma‐Aldrich, A1009‐1G) (arthritic knee), while the other knee was injected with PBS (the non‐arthritic knee) as previously described 27 (Figure 2A). Using this experimental design, we could investigate the impact of untreated and de‐IgG complexes in both arthritic and non‐arthritic conditions. Shortly, 0.1 mg·ml‐1 mBSA was emulsified in an equal volume of complete Freund's adjuvant (CFA) (Sigma‐Aldrich, 344289‐1SET) containing 1 mg·mL−1 heat‐inactivated Mycobacterium tuberculosis. On day 0, the mice were immunized by injecting 0.1 ml of the emulsion intra‐dermally at the base of the tail (0.05 ml on each side). After 7 days, the mice were randomly allocated into three groups (n;7‐8), group I received an injection of 0.15 mg mBSA in one knee (arthritic control) and the other knee was injected with PBS (non‐arthritic control), group II received an injection of 0.15 mg mBSA + 0.15 mg untreated IgG complexes in one knee (arthritic + untreated IgG complexes) and the other knee received PBS + 0.15 mg of untreated IgG complexes (non‐arthritic + untreated IgG complexes), group III received an injection of 0.15 mg mBSA + 0.15 mg de‐IgG complexes in one knee (arthritic + de‐IgG complexes) and the other knee received PBS + 0.15 mg de‐IgG complexes (non‐arthritic + de‐IgG complexes) (Figure 2A). Four naïve mice, injected with PBS both at the primary and the secondary (intra‐articular) immunization, served as additional controls (naïve control). The joint swelling was determined using a caliper where the knee diameter was measured for 7 days until study termination. After termination, the bones were dissected for radiological and histological analyses. Animal studies were performed at the Sahlgrenska Academy, Laboratory of Experimental Biomedicine, Gothenburg, Sweden, and approved by the animal ethics committee in Gothenburg (Sweden).
Figure 2.
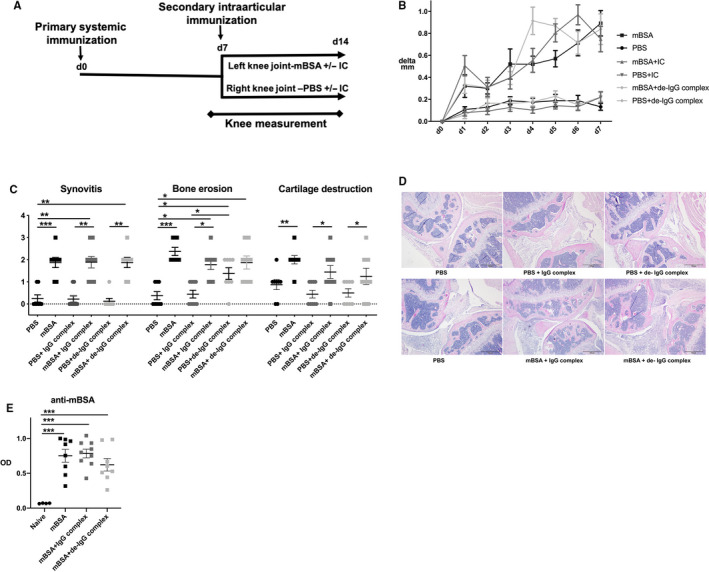
A, Flow‐chart of antigen‐induced arthritis. Mice were systemically immunized with mBSA. After 7 days, intra‐articular antigen challenge was induced with mBSA alone or together with heat‐aggregated IgG complexes, untreated and desialylated (de), or with PBS alone or together with untreated IgG complexes or de‐IgG complexes. Knee joint swelling was measured daily over 7 days. B) Differences in knee joint swelling from baseline in micrometres (µm) are displayed as Kaplan‐Meier curves. Results are shown as mean ± SEM, n = 7. For differences analysed on respective day, the statistical comparison was done using ordinary one‐way ANOVA followed by Tukey's multiple comparison post hoc test, C) Histological scoring (0‐3) of synovitis, bone erosions and cartilage destruction after 7 days. Results are shown as mean ± SEM n = 7. Statistical analysis was performed with Kruskal‐Wallis test followed by Dunn's post hoc test. D) Representative images of haematoxylin‐eosin stained knee joints. E) Serum levels of anti‐mBSA antibodies compared to naïve mice. The results are shown as mean ± SEM; naïve; n = 4, treatment groups; n = 7. Statistical comparison using ordinary one‐way ANOVA followed by Tukey's multiple comparison post hoc test
2.5. Dual‐energy‐x‐ray absorptiometry
At termination, mice were anaesthetized with a Ketador/Dexdomitor (Salfarm Scandinavia AB/Orion Pharma AB Animal Health) cocktail and scanned using Faxitron UltraFocus dual‐energy x‐ray absorptiometry of 40 kV and 0.28 mA for 2.53 s with the spatial resolution of 24 µm and 2X geometric magnification (Faxitron Bioptics). Bone mineral density (BMD) in total body, lumbar spine, and the epiphyseal part of the proximal tibia close to the injected joint were analysed.
2.6. Peripheral quantitative computed tomography
To determine periarticular bone loss, skin was removed from the hind limb with the knee intact, and the leg was fixed in 4% formalin. Computed tomography analysis was performed with the Stratec peripheral quantitative computed tomography (pQCT) XCT Research M (software version 5.4; Norland, Fort Atkinson, WI) at a resolution of 70 μm. Total and trabecular BMD was determined by scans of the metaphysis at a distance of 0.4 mm from the proximal growth plate in the distal direction in the tibia. The trabecular bone region was defined by setting an inner area to 45% of the total cross‐sectional area. Cortical thickness was determined by scans analysed in the mid‐diaphyseal region of the tibia.
2.7. Histological examination
The formalin fixed knee joints were decalcified in EDTA (Sigma‐Aldrich, ED‐1KG), embedded in paraffin and sectioned (0.02 mm) (sectioning was performed at Histocenter, Mölndal Sweden). Sections were stained with haematoxylin and eosin. Synovitis, bone erosion and cartilage destruction were graded in a blinded manner by two examiners (ES and CE) displaying similar results. A 3‐graded histological scoring system was used where 1 = mild, 2 = moderate, and 3 = severe. Bone erosions were only evaluated on the bone surfaces in the knee joint as described by Liphardt et al 30 For quantification of osteoclast number and osteoclast surface attached to the bone, the sections were stained and counted for TRAP positive osteoclasts using a Leukocyte Acid Phosphatase Kit (Sigma‐Aldrich, 387A‐1KT). Osteoclasts were quantified in the epiphyseal part of tibia, the area above the growth plate near the affected knee joint. All analyses were performed using a microscope (Nikon) and the image analysis system Osteomeasure (OsteoMetrics).
2.8. ELISA
Serum was prepared from blood taken at study termination and stored at ‐ 20°C. For assessment of mBSA specific IgG, ELISA plates (Thermo scientific, 152 503) were coated with 0.1 mg·ml‐1 mBSA, washed and blocked with 2% milk powder (Merck, 70166‐500G) in PBS, as previously described. 27 Serum was analysed in triplicates and horseradish peroxidase (HRP)‐conjugated rabbit‐anti mouse IgG (Dako/Agilent Technologies, P0260) detected bound anti‐mBSA antibodies.
2.9. Statistical analysis
All statistical analyses were performed with GraphPad Prism software (Graph Pad Software inc., La Jolla, CA, USA). For the in vitro data, one‐way ANOVA was used for comparison of; M‐CSF, M‐CSF + RANKL, M‐CSF + RANKL+IgG complexes and M‐CSF + RANKL+de‐IgG complexes, followed by Tukey's post hoc analysis. For the in vivo data, when comparing the antibody levels and the BMD at lumbar spine, each mouse was counted separately and one‐way ANOVA was performed followed by Tukey's post hoc analysis. When comparing the knees, each knee was counted separately. We first determined that there was no difference between knees in the naïve mice and knees from the PBS side of mBSA immunized mice, using a two‐sided t test, except for parameters based on ordinal scale data where the Mann‐Whitney test was used. Following this, the knees from all treatments were separately compared; PBS, mBSA, PBS + untreated IgG complexes, mBSA + untreated IgG complexes, PBS + de‐IgG complexes and mBSA + de‐IgG complexes, using one‐way ANOVA followed of Tukey's post hoc analysis. The non‐parametric Kruskal‐Wallis test was used for ordinal scale comparisons, followed by Dunn's post hoc test where differences in rank sum are displayed and χ2 tables. Data on knee swelling is presented as Kaplan‐Meier curves as differences from baseline. All statistical results are presented as means ± standard error of the mean (SEM), P ≤ .05 was considered significant.
3. RESULTS
3.1. Desialylated IgG complexes stimulate osteoclastogenesis in vitro
Given the important role of sialic acid on activated IgG, in regulating general pathogenicity and in the activation of osteoclastogenesis, via binding to FcγRs in humans, we aimed to investigate the importance of sialic acids on polyclonal murine IgG for osteoclastogenesis. Adherent mouse BMMs were differentiated into osteoclasts in the presence of M‐CSF and RANKL and either de‐IgG complexes or untreated IgG complexes was added to the cultures at different stages. The addition of both untreated IgG and de‐IgG complexes during the entire 4‐5 days of culture inhibited the preosteoclast differentiation into multinucleated cells (Supp Figure S2A). Inhibition of osteoclastogenesis was also seen when untreated IgG and de‐IgG complexes were added to the cultures during the three first days together with M‐CSF and RANKL (Supp Figure S2B). In contrast, an increased number of osteoclasts was seen when de‐IgG complexes were added to mono‐ or bi‐nucleated osteoclast precursors after three days of culture (Fig 1A‐B). This stimulatory effect was not observed after adding untreated IgG complexes (Fig 1A‐B) or desialylated or untreated monomeric IgGs (Supp Figure S3A‐B).
To investigate how this potentiation of RANKL‐mediated osteoclastogenesis is mediated by the de‐IgG complexes, we isolated RNA from osteoclast cultures at the end of the cultivation when mature osteoclasts are present. NFATc1 and RANK expression levels were elevated following stimulation with RANKL, when compared to only M‐CSF stimulation, but this was not further affected by either untreated IgG or de‐IgG complexes (Table 1). Cathepsin K expression was also elevated following RANKL stimulation compared to M‐CSF alone, and addition of de‐IgG complexes, but not untreated IgG complexes, resulted in a significantly higher expression compared to only M‐CSF + RANKL stimulation (Table 1). Expression of all four FcγR were investigated, but only FcγRI and FcγRIII were expressed in the mature osteoclasts, and expression was increased by addition of RANKL compared to stimulation with only M‐CSF. Expression of FcγRIII was significantly stimulated after addition of de‐IgG complexes, compared to M‐CSF + RANKL stimulation. In addition, there was a tendency for increased expression of FcγRI after addition of both untreated IgG (P = .13) and de‐IgG complexes (P = .11) to the M‐CSF + RANKL stimulation compared to stimulation with only M‐CSF + RANKL.
3.2. IgG complexes induce local bone erosion but do not affect antigen‐induced arthritis
Next, we aimed to investigate whether murine IgG complexes can influence local bone loss and arthritis using a standard in vivo mouse model of local monoarthritic antigen‐induced arthritis (AIA) 27 (outline displayed in Figure 2A). Induction of arthritis after mBSA injection compared to the non‐arthritic, PBS‐injected knee, was macroscopically confirmed by swelling over the arthritic knee between day 1 and termination of the mice at day 7 (Figure 2B). The swelling mediated by mBSA was elevated after co‐injection with de‐IgG complexes at day 4 compared to mBSA injection alone, but after 7 days all arthritic knees were at the same level. There was no swelling in the non‐arthritic, PBS‐injected knees, and addition of untreated or de‐IgG complexes did not affect the swelling (Figure 2B). As expected, neither non‐arthritic knees with an intra‐articular PBS injection in mice primary immunized with mBSA, nor naïve controls, showed any signs of synovitis, bone erosions or cartilage destruction (data not shown). In contrast, arthritic knees injected with mBSA, showed signs of synovitis, bone erosions and cartilage destruction compared to the non‐arthritic control side (Figure 2C‐D). This is in line with previous studies using this model of AIA. 7 , 27 , 30 The presence of untreated or de‐IgG complexes together with mBSA did not affect arthritis induction (Figure 2C‐D). However, bone erosions were detected in non‐arthritic knees after injection of de‐IgG complexes compared to only PBS injected non‐arthritic control knees and non‐arthritic knees injected with untreated IgG complexes, while synovitis and cartilage destruction was absent. Interestingly, a similar degree of bone erosions was seen in the non‐arthritic and arthritic knees treated with de‐IgG complexes (Figure 2C). Immune activation against mBSA, investigated by the presence of anti‐mBSA antibodies, was seen in all mBSA injected mice compared to the naïve controls (Figure 2E).
3.3. IgG complexes do not change arthritis‐mediated bone loss in AIA but increase the number of osteoclasts in close proximity to the injection in the non‐arthritic condition
Considering that de‐IgG complexes promoted osteoclastogenesis in vitro (Figure 1) and bone erosions on the bone surface in the joint in the non‐arthritic condition (Figure 2), we wanted to investigate whether de‐IgG complexes also affect periarticular bone loss in the model of AIA. As expected, arthritis induction with or without untreated or de‐IgG complexes, did not affect total body BMD (data not shown) or lumbar spine BMD compared to the naïve controls (Figure 3A). To investigate periarticular bone loss, we analysed the areal BMD in the epiphyseal part of the tibia which is close to the affected joint using dual‐energy‐x‐ray absorptiometry (DXA). We found that induction of arthritis reduced the tibial epiphyseal BMD, demonstrated by a decrease in BMD in arthritic knees compared to non‐arthritic knees (Figure 3A‐B). This effect was not altered by the presence of untreated IgG or de‐IgG complexes (Figure 3A‐B). To further verify these results, the proximal metaphyseal part of the tibial bone was investigated by peripheral quantitative computed tomography (pQCT), which measures volumetric bone density. Arthritis‐mediated periarticular bone loss was found in the metaphyseal part of the tibia in all arthritic knees, compared to the non‐arthritic knees, while no effect was seen on tibial cortical thickness (Figure 3C‐D). However, similar as seen on areal BMD in the tibia epiphysis, no effect on volumetric BMD in the tibia metaphysis was seen after injection of de‐IgG complexes in non‐arthritic knees compared to non‐arthritic controls (Figure 3A‐D). In line with the reduction of trabecular BMD following mBSA injection, an induction of osteoclast parameters was histologically visible in the epiphyseal part of the tibia. Arthritis induction with mBSA increased both the osteoclast surface and osteoclast number compared to the PBS‐injected control side (Figure 3E‐F). In line with the finding that de‐IgG complexes result in bone erosions on the bone surfaces of the tibia and femur in non‐arthritic joints, injection of de‐IgG complexes in the non‐arthritic joint resulted in increased number of osteoclasts and a strong tendency in increased osteoclast surface compared to the non‐arthritic PBS control (Figure 3E‐F). Comparing the non‐arthritic knees injected with de‐IgG with non‐arthritic knees injected with untreated IgG complexes, there was also a significant difference for osteoclast number and a tendency for osteoclast surface (Figure 3E‐F).
Figure 3.
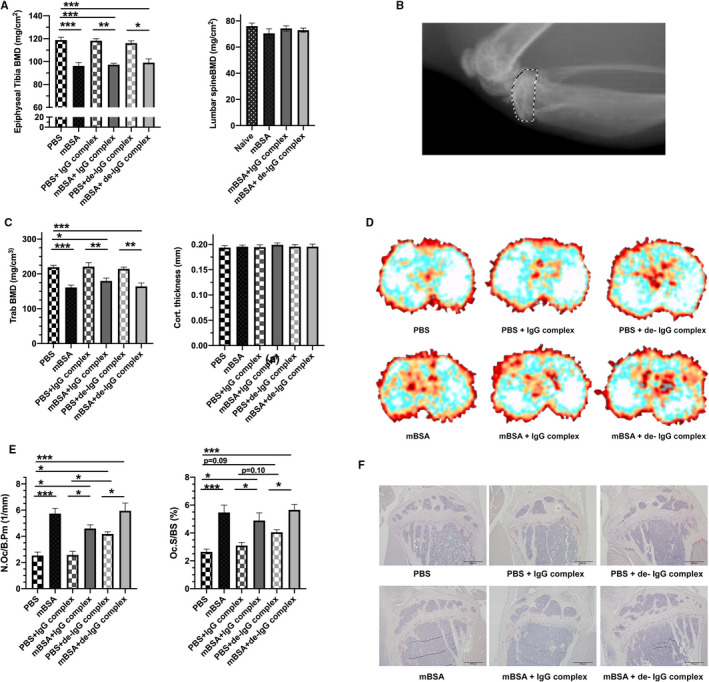
Bone was investigated in primary immunized mice followed by an intra‐articular antigen (mBSA) challenge alone or together with untreated and de‐IgG complexes, or with PBS alone or together with untreated or de‐IgG complexes. A, Areal bone mineral density (BMD) was determined by DXA measurements. Proximal epiphyseal part of tibia and lumbar spine is shown. B, Representative image of the proximal epiphyseal part of tibia. C, Periarticular tibial bone composition was determined by pQCT. Trabecular bone mineral density and cortical thickness are displayed. D, Representative images of the trabecular bone in the tibia. E, Number of osteoclasts/bone perimeter (N.Oc/B.pm), osteoclast surface/bone surface (Oc.S/BS) in the epiphyseal part of tibia. F, Representative micro images of TRAP‐stained osteoclasts in tibial sections. Results are shown as mean ± SEM; n = 7. Statistical comparison using one‐way ANOVA followed of Tukey's multiple comparison post hoc test
4. DISCUSSION
Bone loss and osteoclast formation are regulated by activated polyclonal IgG complexes 1 , 2 , 3 , 9 and RA‐specific autoantibodies. 5 , 6 , 7 , 8 Changes in glycosylation of the Fc part of IgG alter the binding capability to FcγRs and desialylated IgG complexes have been shown to display a higher binding affinity to FcγRs. Desialylated human polyclonal heat‐aggregated IgG complexes, which completely lose their antigen‐binding capacity, affect in vitro human osteoclastogenesis as well as induce bone loss in vivo in mice. 5 Furthermore, a strong correlation has been established in RA patients between reduced trabecular bone volume and the glycosylation grade of general IgGs, the binding of the Fc part to FcγRs is highly involved in mediating the effects of IgGs. In this study, we show for the first time that desialylated murine polyclonal IgG complexes, which can activate FcγRs, affect osteoclastogenesis of adherent murine bone marrow macrophages, while they have no effect on arthritis or arthritis‐mediated bone loss in a mouse mono‐arthritis model.
In the present study, we hypothesize that activated murine IgG complexes may play an important role in murine in vivo potentiation of RANKL‐mediated osteoclastogenesis. We further hypothesize that this effect could be dependent on the glycosylation level, especially on sialic acid, which alters the binding capability to FcγRs. We show that treatment with murine IgG complexes, lacking terminal sialic acid, results in an accelerated osteoclastogenesis with an increase in osteoclast numbers in vitro, while no effect is displayed after treatment with untreated, normally sialylated, IgG complexes. In line with human in vitro studies performed by Harre et al, monomeric IgGs, with or without sialic acids, did not affect the murine osteoclastogenesis. Monomeric IgGs can only interact with FcγRs after antigen activation, and the lack of effect in the cultivation experiments indicates the importance of FcγR activation. However, in line with human osteoclastogenesis stimulation, as demonstrated by Harre et al, we only observed an increasing effect when desialylated IgG complexes were added to the cultures at the stage of committed mono‐ and bi‐nucleated preosteoclasts, and we found an inhibitory effect when added to un‐committed progenitors. This bi‐functional effect on osteoclastogenesis have also been reported after activation of Toll‐like Receptor (TLR) 2 and 4, where an inhibitory role is seen in cultures of un‐committed progenitors but an accelerating effect is seen on committed osteoclast progenitors as well as on osteoclasts in vivo. 31 This may be dependent on regulation of cytokine production by the osteoclasts. TLR stimulation of BMMs induces not only pro‐osteoclastogenic cytokines, like TNF and IL‐6, but also IL‐12 and type 1 interferon, which inhibit osteoclast formation. 32 , 33 Whether the inhibitory effect overwhelms the later stimulatory effect in un‐committed osteoclast progenitors needs to be further verified both regarding TLR as well as IgG complex modulation of RANKL‐mediated osteoclastogenesis.
Murine FcγRI, FcγRIII as well as FcγRIV have previously been demonstrated to influence osteoclast development in mice. 2 , 3 We detected a significant induction of FcγRIII mRNA expression following stimulation with desialylated IgG complexes and a strong tendency to increased expression of FcγRI, after stimulation with both untreated as well as desialylated IgG complexes. This may, at least partly, explain the increased number of osteoclasts in the cultures. We did not see any effects after stimulation with desialylated IgG complexes on the expression of the master transcription factor NFATc1 or RANK, but the mRNA levels have only been investigated at a late stage of osteoclastogenesis and these factors might be altered at earlier time points. However, desialylated IgG complexes increased the expression of Cathepsin K, which is primarily stimulated by NFATc1 and the NF‐kB pathways. This induction could be dependent on the induced number of osteoclasts after stimulation with desialylated IgG complexes.
To investigate if the observed stimulatory effect on osteoclastogenesis in vitro, could be observed in vivo and if IgG complexes may affect arthritis or arthritis‐mediated bone loss, we used the AIA model. Addition of desialylated IgG complexes to the arthritic knee only resulted in a limited transient increase in swelling over the knee, and no difference could be observed between the groups at termination. There were no differences in synovitis or joint destruction in our in vivo model of arthritis in the presence of neither untreated nor desialylated IgG complexes, indicating that IgG complexes do not have any additive effect on arthritis induction. Addition of IgG complexes did not influence the arthritis‐mediated bone loss and a similar bone loss was seen in all arthritic knees compared to their non‐arthritic control, irrespective of additional treatment. The reason that IgG complexes did not lead to increased arthritis or arthritis‐mediated bone loss might be that the inflammation itself in this model gives such a massive induction of osteoclasts and that this cannot be further enhanced by the addition of IgG complexes. Intriguingly, we have previously shown in Engdahl et al 2017, that addition of murine ACPAs in the AIA model results in more severe bone loss as well as a limited, but still significant, increase in arthritis induction and synovitis. 9 This distinction compared to the current study could be caused by different actions of ACPAs and IgG complexes. ACPAs can cause effects, both via interaction between their Fab parts towards citrullinated vimentin placed on the surface on preosteoclast, 6 , 8 and via interaction of their Fc part with FcγRs. How the stimulatory effect of the ACPAs was mediated in Engdahl et al 2017 was not investigated, but it has been shown that the stimulatory effect of ACPAs might not only be mediated via stimulation through FcγR and the binding ability of the Fab parts of the ACPAs to citrullinated proteins could be of importance. 6 , 8 In the current study, we found a stimulatory effect on bone erosions of desialylated IgG complexes in the non‐arthritic joints as well as an increased number of osteoclasts in the tibial epiphysis. 7 However, no periarticular trabecular bone loss, as seen after the addition of ACPAs to the non‐arthritic joint in Engdahl et al 2017, was detected after injection with desialylated IgG complexes. This difference may alternatively also depend on the concentration of antibodies in the knee. It is difficult to estimate how much of the antibodies that remain in the knee joint. Three times more antibodies were injected in the present study compared to Engdahl et al 2017, however, the ACPAs could find their antigen of interest in the knee area, and thereby stay within the area and continuously activate FcγRs. In addition, the IgG complexes might evaporate easier and thereby mainly mediate the effect during a short period of time and repeated injections of IgG complexes might result in a more robust effect and cause metaphyseal trabecular bone loss.
Different specific IgGs, targeting either inflammatory cytokines or cancer cells, are a new breakthrough in modern medicine. The possibility that these activated antibodies may play a role in the regulation of bone is neglected and needs further investigation. Furthermore, by altering the glycosylation of the antibodies, the bone effects may be affected and therefore, it is important to further investigate the glycosylation of the therapeutic antibodies.
In summary, desialylated IgG complexes potentiate murine osteoclastogenesis in vitro but do not affect arthritis‐mediated bone loss in vivo. The lack of additive or synergistic effects in the presence of arthritis is possibly due to the strong local inflammation seen in this model. The observed stimulatory effect on local bone erosions and induction of osteoclasts by the desialylated IgG complexes in the non‐arthritic condition in our murine setting further confirms the link between the adaptive immune system and bone.
CONFLICT OF INTEREST
The author displays no conflict of interest.
AUTHOR CONTRIBUTIONS
ES PH and CE designed and performed experiment and analysed data. ES AW PH and CE performed in vivo stimulation. ES MKL and CE performed the animal experiment. MKL performed the pQCT. UHL and HC provided valuable material and intellectual input. E.S and C.E wrote the manuscript with input from all co‐authors.
Supporting information
Supplement Figure S1‐S3
ACKNOWLEDGMENT
The authors acknowledge the excellent technical assistance from Dr Jianyao Wu, Elin Elvesten, and Carolina Johansson. This study was supported by the Swedish Research Council (2013‐07370 and 2019‐01852), the Swedish Association for Medical Research, Swedish state under the agreement between the Swedish government and the country councils, the ALF‐agreement (770351), Konung Gustav V stiftelse, Tore Nilsson Foundation, the Swedish Association Against Rheumatism and Åke Wiberg stiftelse.
Sehic E, Westerlund A, Lagerquist MK, et al. Immunoglobulin G complexes without sialic acids enhance osteoclastogenesis but do not affect arthritis‐mediated bone loss. Scand J Immunol.2021;93:e13009. 10.1111/sji.13009
REFERENCES
- 1. Grevers LC, de Vries TJ, Everts V, et al. Immune complex‐induced inhibition of osteoclastogenesis is mediated via activating but not inhibitory Fcγ receptors on myeloid precursor cells. Ann Rheum Dis. 2013;72(2):278‐285. [DOI] [PubMed] [Google Scholar]
- 2. Seeling M, Hillenhoff U, David JP, et al. Inflammatory monocytes and Fcγ receptor IV on osteoclasts are critical for bone destruction during inflammatory arthritis in mice. Proc Natl Acad Sci U S A. 2013;110(26):10729‐10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Negishi‐Koga T, Gober HJ, Sumiya E, et al. Immune complexes regulate bone metabolism through FcRγ signalling. Nat Commun. 2015;6:6637. [DOI] [PubMed] [Google Scholar]
- 4. Bruhns P, Jönsson F. Mouse and human FcR effector functions. Immunol Rev. 2015;268(1):25‐51. [DOI] [PubMed] [Google Scholar]
- 5. Kleyer A, Finzel S, Rech J, et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis. 2014;73(5):854‐860. [DOI] [PubMed] [Google Scholar]
- 6. Harre U, Georgess D, Bang H, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest. 2012;122(5):1791‐1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Engdahl C, Bang H, Dietel K, et al. Periarticular bone loss in arthritis is induced by autoantibodies against citrullinated vimentin. J Bone Miner Res. 2017;32(8):1681‐1691. [DOI] [PubMed] [Google Scholar]
- 8. Krishnamurthy A, Joshua V, Haj Hensvold A, et al. Identification of a novel chemokine‐dependent molecular mechanism underlying rheumatoid arthritis‐associated autoantibody‐mediated bone loss. Ann Rheum Dis. 2016;75(4):721‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harre U, Lang SC, Pfeifle R, et al. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat Commun. 2015;6:6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arita K, Hashimoto H, Shimizu T, et al. Structural basis for Ca2+‐induced activation of human PAD4. Nat Struct Mol Biol. 2004;11(8):777‐783. [DOI] [PubMed] [Google Scholar]
- 11. Li H, Sethuraman N, Stadheim TA, et al. Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nat Biotechnol. 2006;24(2):210‐215. [DOI] [PubMed] [Google Scholar]
- 12. Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N‐linked oligosaccharide improves binding to human Fcgamma RIII and antibody‐dependent cellular toxicity. J Biol Chem. 2002;277(30):26733‐26740. [DOI] [PubMed] [Google Scholar]
- 13. Kaneko Y, Nimmerjahn F, Ravetch JV. Anti‐inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313(5787):670‐673. [DOI] [PubMed] [Google Scholar]
- 14. Böhm S, Schwab I, Lux A, Nimmerjahn F. The role of sialic acid as a modulator of the anti‐inflammatory activity of IgG. Semin Immunopathol. 2012;34(3):443‐453. [DOI] [PubMed] [Google Scholar]
- 15. Biermann MHC, Griffante G, Podolska MJ, et al. Sweet but dangerous ‐ the role of immunoglobulin G glycosylation in autoimmunity and inflammation. Lupus. 2016;25(8):934‐942. [DOI] [PubMed] [Google Scholar]
- 16. Scallon BJ, Tam SH, McCarthy SG, et al. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol Immunol. 2007;44(7):1524‐1534. [DOI] [PubMed] [Google Scholar]
- 17. Gudelj I, Salobc PP, Trbojević‐Akmačića I, et al. Low galactosylation of IgG associates with higher risk for future diagnosis of rheumatoid arthritis during 10 years of follow‐up. Biochim Biophy Acta. 2018;1864(6, Part A):2034‐2039. [DOI] [PubMed] [Google Scholar]
- 18. Parekh RB, Dwek RA, Sutton BJ, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316(6027):452‐457. [DOI] [PubMed] [Google Scholar]
- 19. Youinou P, Pennec Y‐L, Casburn‐Budd R, et al. Galactose terminating oligosaccharides of IgG in patients with primary Sjögren's syndrome. J Autoimmun. 1992;5(3):393‐400. [DOI] [PubMed] [Google Scholar]
- 20. Vučković F, Krištić J, Gudelj I, et al. Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol. 2015;67(11):2978‐2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wuhrer M, Selman MHJ, McDonnell LA, et al. Pro‐inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J Neuroinflamm. 2015;12:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miyoshi E, Shinzaki S, Fujii H, et al. Role of aberrant IgG glycosylation in the pathogenesis of inflammatory bowel disease. Proteomics Clin Appl. 2016;10(4):384‐390. [DOI] [PubMed] [Google Scholar]
- 23. Scherer HU, van der Woude D, Ioan‐Facsinay A, et al. Glycan profiling of anti‐citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 2010;62(6):1620‐1629. [DOI] [PubMed] [Google Scholar]
- 24. Rombouts Y, Ewing E, van de Stadt LA, et al. Anti‐citrullinated protein antibodies acquire a pro‐inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis. 2015;74(1):234‐241. [DOI] [PubMed] [Google Scholar]
- 25. Scherer HU, Wang J, Toes RE, et al. Immunoglobulin 1 (IgG1) Fc‐glycosylation profiling of anti‐citrullinated peptide antibodies from human serum. Proteomics Clin Appl. 2009;3(1):106‐115. [DOI] [PubMed] [Google Scholar]
- 26. Pfeifle R, Rothe T, Ipseiz N, et al. Regulation of autoantibody activity by the IL‐23‐TH17 axis determines the onset of autoimmune disease. Nat Immunol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Engdahl C, Lindholm C, Stubelius A, et al. Periarticular bone loss in antigen‐induced arthritis. Arthritis Rheum. 2013;65(11):2857‐2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petrow PK, Thoss K, Henzgen S, Katenkamp D, Bräuerf R. Limiting dilution analysis of the frequency of autoreactive lymph node cells isolated from mice with antigen‐induced arthritis. J Autoimmun. 1996;9(5):629‐635. [DOI] [PubMed] [Google Scholar]
- 29. Takeshita S, Kaji K, Kudo A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res. 2000;15(8):1477‐1488. [DOI] [PubMed] [Google Scholar]
- 30. Liphardt AM, et al. Changes in mechanical loading affect arthritis‐induced bone loss in mice. Bone. 2020;131:115149. [DOI] [PubMed] [Google Scholar]
- 31. Souza PPC, Lerner UH. Finding a Toll on the Route: The Fate of Osteoclast Progenitors After Toll‐Like Receptor Activation. Front Immunol. 2019;10:1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Amcheslavsky A, Bar‐Shavit Z. Interleukin (IL)‐12 mediates the anti‐osteoclastogenic activity of CpG‐oligodeoxynucleotides. J Cell Physiol. 2006;207(1):244‐250. [DOI] [PubMed] [Google Scholar]
- 33. Takami M, et al. Stimulation by toll‐like receptors inhibits osteoclast differentiation. J Immunol. 2002;169(3):1516‐1523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure S1‐S3


