Abstract
Coral bleaching is the single largest global threat to coral reefs worldwide. Integrating the diverse body of work on coral bleaching is critical to understanding and combating this global problem. Yet investigating the drivers, patterns, and processes of coral bleaching poses a major challenge. A recent review of published experiments revealed a wide range of experimental variables used across studies. Such a wide range of approaches enhances discovery, but without full transparency in the experimental and analytical methods used, can also make comparisons among studies challenging. To increase comparability but not stifle innovation, we propose a common framework for coral bleaching experiments that includes consideration of coral provenance, experimental conditions, and husbandry. For example, reporting the number of genets used, collection site conditions, the experimental temperature offset(s) from the maximum monthly mean (MMM) of the collection site, experimental light conditions, flow, and the feeding regime will greatly facilitate comparability across studies. Similarly, quantifying common response variables of endosymbiont (Symbiodiniaceae) and holobiont phenotypes (i.e., color, chlorophyll, endosymbiont cell density, mortality, and skeletal growth) could further facilitate cross‐study comparisons. While no single bleaching experiment can provide the data necessary to determine global coral responses of all corals to current and future ocean warming, linking studies through a common framework as outlined here, would help increase comparability among experiments, facilitate synthetic insights into the causes and underlying mechanisms of coral bleaching, and reveal unique bleaching responses among genets, species, and regions. Such a collaborative framework that fosters transparency in methods used would strengthen comparisons among studies that can help inform coral reef management and facilitate conservation strategies to mitigate coral bleaching worldwide.
Keywords: common framework, coral bleaching, coral heat stress, cross‐study comparisons, experimental design methods, feeding, flow, light, phenotype, standardization, temperature
Introduction
Temperature stress from ocean warming due to climate change is now the single largest threat to coral reefs globally (Veron et al. 2009, Cantin et al. 2010, Frieler et al. 2012, Hughes et al. 2018). Reef ecosystems are experiencing unprecedented declines in coral colony abundance, coral diversity, and reef growth as a result of temperature‐induced coral bleaching, a phenomenon that is becoming more frequent and severe (e.g., Hoegh‐Guldberg et al. 2007, Eakin et al. 2009, Veron et al. 2009, Hoegh‐Guldberg 2011). By the end of this century, tropical seawater temperatures are expected to rise by 1°C–3°C (IPCC 2013), and severe bleaching is expected to occur annually in some regions by 2030 and globally by 2055 (van Hooidonk et al. 2014). Coral bleaching is the visual manifestation of the breakdown in the symbiosis between the coral host and its endosymbiotic dinoflagellates (family Symbiodiniaceae; LaJeunesse et al. 2018) whereby the coral loses its endosymbiotic algae or pigments resulting in a pale or “bleached” appearance. Bleaching results in decreased coral health, growth, and reproductive output, as well as increased coral susceptibility to disease and mortality (e.g., Brown 1997, Hoegh‐Guldberg 1999, Omori et al. 1999, Buddemeier et al. 2004, Jokiel 2004, Maynard et al. 2015).
Despite the wide impact of bleaching events, the magnitude and extent of bleaching can vary substantially across scales, ranging from the individual colony to the ocean basin (e.g., Rowan et al. 1997, Fitt et al. 2000, Loya et al. 2001, Grottoli et al. 2006, 2014, Palumbi et al. 2014, Muller et al. 2018, Morikawa and Palumbi 2019). Although it is well documented that temperature and irradiance are key drivers of coral bleaching, the processes causing broad variation in bleaching susceptibility and recovery across reefs, corals, and colonies are not fully resolved. Manipulative experiments remain a critical tool for elucidating the underlying mechanisms and responses of corals to thermal stress (McLachlan et al. 2020). However, few studies conduct detailed comparisons of results across data sets because it is not always straightforward to ascertain whether the variation in bleaching and recovery responses are due to (1) differences in experimental design (e.g., differences in light, baseline temperature, rate of temperature increase, experimental duration, etc.), (2) differences in bleaching and recovery measurements, (3) differences in coral biology, or (4) some combination of these differences.
A detailed review of coral bleaching experiments by McLachlan et al. (2020) revealed that many important details about how experiments are designed and executed are sometimes missing from published papers, making comparisons between studies challenging. For example, knowing experimental heating temperature, heating duration, and lighting conditions are essential for cross‐study comparisons because all three variables can influence coral bleaching responses. In addition, some bleaching studies use a heat‐hold or heat‐pulse strategy of heating that mimics daily heat stress over a mid‐day low tide (Oliver and Palumbi 2011), whereas others mimic the onset and duration of a natural reef‐wide bleaching event with gradual increases in temperature and prolonged temperature exposure (Rodrigues and Grottoli 2007). Whether corals are exposed to pulse or gradual exposure may influence responses (Mayfield et al. 2013b ). Therefore, clear reporting of experimental details and results is necessary for meaningful comparisons among studies (Gerstner et al. 2017) and for reliably identifying patterns in coral bleaching and recovery across species, habitats, reefs, and regions.
One way to increase comparability and transparency among ongoing and future coral bleaching studies is to develop a common framework for reporting the conditions and results of coral bleaching experiments, while neither being overly prescriptive nor diminishing scientific innovation. A common framework for coral bleaching should include consideration of coral provenance, experimental conditions, and husbandry. Similar approaches have been successful in advancing other fields (e.g., ocean acidification research; Riebesell et al. 2010, Cornwall and Hurd 2015), while also allowing for the rapid development of creative approaches to understanding underlying mechanisms. Doing so for experimental coral bleaching research will markedly improve our ability to detect important trends, identify species vulnerabilities and tolerances, and help coral researchers and managers devise solutions for coral persistence over the coming decades (Warner et al. 2016).
The state of coral bleaching experimental design and methods
Prior to the 1970s, the phenomenon of coral bleaching was relatively unknown. In 1971, coral bleaching was reported on a Hawaiian nearshore reef adjacent to a power plant that discharged warm water (Jokiel and Coles 1974). The first experimental research connecting coral bleaching with high‐temperature stress followed (Jokiel and Coles 1977). One of the first records of large‐scale heat‐induced coral bleaching was in Panama, which was attributed to a thermal anomaly associated with the 1982–1983 El Niño event at that time (Glynn 1983). Since then, experimental research on coral bleaching has accelerated, with at least 243 peer‐reviewed journal articles published since 1990, two‐thirds of which were published in the last 10 years alone (McLachlan et al. 2020). Manipulative experiments have been, and remain, critical for elucidating the triggers and responses of the coral holobiont to thermal stress and assessing their subsequent recovery. Research to date reveals that bleaching susceptibility and recovery vary among coral species, populations, seasons, reef habitats, and genetically distinct individuals (i.e., genets, Box 1) as well as among corals harboring similar or different algal endosymbionts or bacteria (e.g., Rowan et al. 1997, Fitt et al. 2000, Loya et al. 2001, Grottoli et al. 2006, 2014, Palumbi et al. 2014, Ziegler et al. 2017, Muller et al. 2018, Morikawa and Palumbi 2019, Voolstra et al. 2020). Yet, it is unclear how much of the variation in bleaching responses is a consequence of biological differences in bleaching among coral holobionts, differences in experimental conditions (e.g., differences in light, baseline temperature, rate of temperature increase, experimental duration, flow, etc.), or methodologically inherent biases in how coral bleaching is measured (McLachlan et al. 2020). We know that the scientific understanding of coral bleaching relies heavily on experimental outcomes from three coral species (Pocillopora damicornis, Stylophora pistillata, and Acropora millepora), that experimental conditions are sometimes not reported (e.g., missing information on water flow, experimental location, heating rate), and that measurements of bleaching phenotypes are weighted heavily by responses of the endosymbiotic algae (McLachlan et al. 2020). Thus, direct comparisons among studies can be challenging. While experimental methods ultimately depend on the research question, this paper outlines a strategy for providing a common framework for coral bleaching experiments to enhance cross‐comparisons and strengthen coral bleaching meta‐analyses. The details were developed by 27 coral research scientists from 21 institutions, spanning research expertise in biological, geological, physical, and computational disciplines, who participated in the first Coral Bleaching Research Coordination Network (CBRCN) workshop at The Ohio State University in May of 2019.
Box 1. Glossary of Terms.
Ambient temperature: temperature at time of collection.
Baseline temperature: temperature from which heat‐stress offset is calculated (typically MMM).
MMM: maximum monthly mean (i.e., average daily temperature of the hottest month of the year for the previous several years).
Genets†: formed by sexual reproduction. All colonies and tissue that can trace their ancestry back to the same fertilization event belong to the same genet (Appendix S1: Fig. S1).
Genotype†: the genetic makeup of a sample for a given (set of) genetic marker(s). When enough markers are assayed, a sample can be assigned to a genet based on its genotype.
Ramets†: physically independent modules arising from colony fragmentation or other asexual means of dispersion. A genet can have one or many ramets. Ramets can be experimentally generated nubbins, naturally occurring fragments, or attached colonies (Appendix S1: Fig. S1).
Phenotype: the set of observable characteristics of an individual resulting from the interaction of its genotype with the environment.
Water flow rate: volumetric water flow rate per unit time (L/s−1). In a tank, this would be the fluid output from the exhaust of the pump or tank outflow in flow‐through systems.
Water turnover time: time required to replace the entire volume of water in a tank(s), assuming the tank is continuously well mixed. Calculated by dividing the tank volume by the flow rate.
Water flow velocity: motion of water relative to sessile coral (cm/s−1).
†Baums et al. (2019).
Experiments were separated into three temporally defined categories: (1) short‐term and acute (0–7 d of thermal stress), (2) moderate duration (8–30 d of thermal stress), and (3) long‐term and chronic (>31 d of thermal stress) experiments. The methods used and the experiments conducted within each category are clearly different from each other (McLachlan et al. 2020) and thus were considered separately. A summary of the common framework for coral bleaching experiments in each category is given in Table 1 (see details in the Proposed Common Framework section). Our summary is not intended to be prescriptive, but instead should be considered as a heuristic guide to help facilitate and strengthen comparisons among studies. One common finding that emerged from discussions of all three experimental categories was to provide guidance on the number of replicates in experiments. This topic will be discussed first as it applies to all experimental categories. In addition, we find that including measurements for common coral response variables in coral bleaching experiments would further enhance cross‐study comparisons by providing common physiological reference points across studies. A list of potential response variables is provided at the end of Table 1. A brief review of common methods for measuring each listed variable is provided in Appendix S1. A full discussion of the proposed common framework is detailed below.
Table 1.
Framework for coral bleaching experimental methods and coral response variables.
| Variable | Appendix section | Suggested target or range | ||
|---|---|---|---|---|
| Acute and short‐term experiments(<7 d at BST) | Moderate duration experiments (7–30 d at BST) | Long‐term experiments (>30 d at BST) | ||
| No. genets | S1.1 | 5 minimum; >5 if possible | 5 minimum; >5 if possible | ≥5 |
| No. replicate tanks per treatment | Minimum two tanks per treatment | ANOVA design, minimum of 3 tanks per treatment factor; regression design, gradient study with >3 treatment levels; avoid pseudo‐replication | Avoid pseudo‐replication | |
| Acclimatization to experimental tanks | Typically none | 7–12 d following fragmentation and mounting | 7–12 d following fragmentation and mounting | |
| Control temperature | S1.2 | Ambient temperature at collection site at time of collection | Ambient temperature at collection site at time of collection | Ambient temperature at collection site during the experimental period |
| Baseline temperature | S1.2 | MMM and/or rapid temperature profiles corresponding to in situ temperature patterns if appropriate | MMM | MMM |
| Bleaching stress temperature above local MMM | Typically +3 to +9°C; increase temperature from MMM until death is observed, then set target temperature lower; if the goal is to observe phenotypic variability, expose corals to several temperatures to find the temperature at which half of the corals bleach; stress exposure should happen at the same time of day; temperature stress duration should be standardized within experiments | +1 to +4°C depending on local ecological relevance and species, may need to be higher in extreme environments | +1°C or more depending on local ecological relevance and species | |
| Temperature ramp‐up rate | None recommended as it will depend on temperature stress duration; heating rates should be adjusted to take the same time across treatment temperatures | 0.1–1°C/d | Mimics increase in temperature rate observed during previous bleaching events at that site | |
| Temperature modulation | Temperature ramp‐up to static elevated temperature, followed by recovery at baseline temperatures; profiles can be run once or multiple times | May be static or diurnally modulated; choice of modulation should be the same in treatments and controls | Static or diurnal for indoor experiments; diurnal and seasonal for outdoor experiments | |
| Control conditions | At ambient temperature; exact same conditions as treatment, except for temperature | At ambient temperature; exact same conditions as treatment, except for temperature | At ambient temperature; exact same conditions as treatment, except for temperature; mimics natural conditions | |
| Light | S1.3 | Ideally, static light conditions for short‐term thermal exposures (with no light at night) or possibly diurnal variability if over several days; light levels match natural light conditions; minimum 250–500 µmol photons·m−2·s−1 | Ideally, diurnal variability with 80% of maximum PAR light at collection site; minimum 250–500 µmol photons·m−2·s−1 | Indoor tanks, diurnal variability (with moonlight cycles); outdoor tanks, apply shade to mimic PAR at collection depth; minimum 250 µmol photons·m−2·s−1 |
| Flow | ||||
| Flow system | S1.4 | |||
| Flow‐through | Report pump rate in liters pumped per hour | 2–20 cm/s | 2–20 cm/s, mimic natural conditions | |
| Closed | Report pump rate in liters pumped per hour, tank volume | Record flow rates, pump size, tank volume, and try to base the flow rate on in situ data | Record flow rates, pump size, tank volume | |
| Tank volume turnover | S1.4 | |||
| Flow‐through | 100% within 3–6 h | 1–4 times per day | 1–4 times per day | |
| Closed | 100% within 3–6 h | Case‐dependent and depends on system biomass | Case‐dependent and depends on system biomass | |
| Feeding | S1.5 | None typically | Minimum once per week to satiation; report feeding amount, rate, and food type | Minimum once per week to satiation; ideally feed up to three times per week; report feeding amount, rate, and food type; mimic food availability in nature |
| Seawater | S1.6 | Filtered or unfiltered; natural or artificial | Filtered or unfiltered; natural or artificial | Filtered or unfiltered; natural or artificial |
| Post heat‐stress monitoring | Hours to a few days (longer than the stress duration); this doubles the number of fragments needed | If possible, immediate (0.2–1 month) and long‐term monitoring (>1 month) depending on the question | 0.2–3 months depending on the question | |
| Other environmental conditions | ||||
| Salinity | S1.7 | |||
| Nutrients | S1.8 | |||
| pH | S1.9 | |||
| Dissolved oxygen | S1.10 | |||
| Coral bleaching responses | ||||
| Bleaching phenotype | ||||
| Image analysis of color | S2.1a | |||
| Chlorophyll concentration | S2.1b | |||
| Symbiodiniaceae cell density | S2.1c | |||
| Holobiont phenotype | ||||
| Mortality | S2.2a | |||
| Skeletal growth | S2.2b | |||
| Other | ||||
| Active chlorophyll fluorescence† | S2.3a | |||
| Symbiodiniaceae identity | S2.3b | |||
BST, bleaching stress temperature; MMM, maximum monthly mean (i.e., men temperature of the warmest month; ANOVA, analysis of variance; PAR, photosynthetically active radiation. A review of commonly used methods is summarized in Appendix S1. Glossary of terms is given in Box 1.
E.g., PAM fluorometry.
Proposed Common Framework
Number of genets and ramets
For all types of coral bleaching experiments, it is essential to control for potential sources of variation in the response of experimental corals across scales of biological organization. For example, there may be measurable differences in performance among genets when comparing the performance of their ramets (i.e., fragments, asexually produced, originating from the same genet) in different experimental conditions (Appendix S1: Fig. S1; Box 1) (Parkinson et al. 2018, Muller et al. 2018, Jury and Toonen 2019, Morikawa and Palumbi 2019, Wright et al. 2019, Voolstra et al. 2020). Investigating multiple ramets of the same genet across treatments allows for a more direct inference of treatment effects. Such “identical twin”‐type designs have proven useful in short‐, moderate‐, and long‐term bleaching studies (Grottoli et al. 2014, Ziegler et al. 2017). Furthermore, there is increasing evidence that heritable genetic effects that are attributable to distinct coral genets can significantly affect holobiont physiology and thermal tolerance (Meyer et al. 2009, Dixon et al. 2015, Kenkel et al. 2015, Kuffner et al. 2017, Jury et al. 2019). To control for this source of variation, genets and their ramets should be identified and tracked, and sufficient numbers of genets should be included in a given study.
Recent work by Baums et al. (2019) indicated that for Caribbean corals, four genets capture the most common genetic diversity within a population (though this minimum could vary for corals in other ocean basins). Thus, a minimum of five genets from each species, population, region, or habitat would add sufficient representation across each experimental treatment and allow for a minimum of four genets if one genet is lost due to unforeseen circumstances. A larger sample size would more effectively characterize a population, especially if the experimental goals include measuring the variance as well as the mean responses. We recognize that this minimum recommendation may not be sufficient in some cases and power analyses prior to the start of the experiment would facilitate determining the appropriate number of genets needed.
Tracking the identity of each genet and ramet throughout the duration of an experiment is useful for survival analysis, which can factor into variance among genets (see methods for tracking genets and ramets in Appendix S1: Section S1.1). Ideally, unique genets are confirmed with genetic markers, but we recognize that this may not be a reasonable expectation in many studies. Alternatively, distinct colonies sampled at least 5 m apart on the reef decreases the chances that collections will include clonal ramets (Baums et al. 2019). For species known to engage more heavily in asexual proliferation, particularly Acroporids (Baums et al. 2006, Gorospe et al. 2015, Manzello et al. 2019), even greater spacing of field‐sampled corals may be needed, or secondary genetic analysis performed, to verify the uniqueness of the sourced corals (Gorospe et al. 2015, Riginos 2015, Manzello et al. 2019).
Acute and short‐term (0–7 d) thermal‐stress experiments
Acute and short‐term thermal‐stress experiments are here defined as those designed to be completed in 0–7 d. The advantages of such experiments are three‐fold. First, many corals can be rapidly tested for their responses to a variety of temperatures and their responses can be compared among species, populations, genets, and experimental treatments. Quick testing of hypotheses further allows for the rapid validation of interesting and unexpected results. Second, the phenotype of the coral of interest is captured soon after collection, thereby avoiding potential behavioral and physiological changes arising from acclimation in captivity. Third, these experiments can be used to mimic strong, rapid swings in temperature that some corals are exposed to in shallow‐water settings, especially in localities with large tidal cycles (Green et al. 2019). Corals exposed to the latter are among some of the most heat resistant (Oliver and Palumbi 2011) and serve as important subjects to understand thermal tolerance and stress resilience. Overall, short‐term experiments provide a mechanism to test a large number of colonies and reef sites for their immediate and extreme physiological responses to acute‐heat exposure that are not possible in longer experiments.
However, the short‐duration and fast‐temperature‐ramping rates inherent to these types of experiments do not mimic most natural bleaching events, and care must be taken when using results from acute and short‐term bleaching experiments to infer outcomes or make predictions about natural bleaching. These experiments are also limited by the types of responses that can be quantified over short periods of time. For instance, pigmentation and –omics level responses are easily quantified, but processes such as calcification that typically require more time to measure are not as amenable to short heat‐stress studies. Thus, acute and short‐term thermal‐stress experiments may be most ecologically relevant for understanding corals from reef flats and shallow lagoons that experience natural short‐term heating associated with low tide (Brown et al. 2002, Palumbi et al. 2014, Herdman et al. 2015, van Oppen et al. 2018). The extent to which acute‐stress experimental outcomes relate to results obtained from long‐term heat‐stress experiments, and how both inform our knowledge of thermal resilience in situ is under active investigation. Results from one study suggest that the thermal tolerance of corals in acute heat‐stress studies are indicative of thermal resilience of corals to natural heat‐stress events (Voolstra et al. 2020).
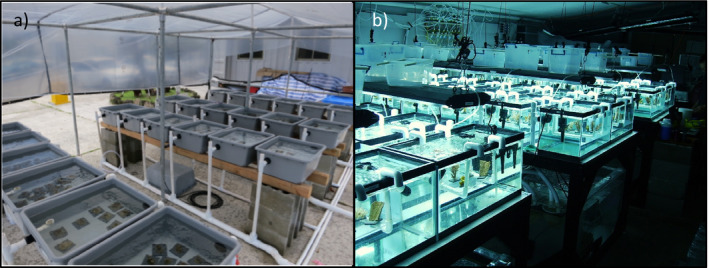
Mechanistically, acute and short‐term thermal‐stress experiments use small‐scale, highly portable instrumentation that house small tanks where physical variables such as temperature, light, and flow can be highly controlled, facilitating downstream comparisons among studies (Fig. 1). While these experiments can be done with as few as two tanks per treatment, four to six tanks provide additional statistical power (Table 1) and serve as a fail‐safe in case a tank malfunctions. The relatively simple design is flexible and more repeatable than moderate and long‐term experiments, amenable to deployment in remote locations, and accessible to those working with limited resources. These features may make acute and short‐term thermal‐stress experiments readily adoptable by researchers, teachers, and students. In addition, acute and short‐term studies typically use small coral ramets allowing for conservative use of coral material and the opportunity to obtain repeatable phenotype diagnostics with a large number of samples at a relatively low effort per sample. Reporting the average and range of as many physicochemical conditions as possible in an experiment enhances comparisons among studies since differences in any one of the non‐temperature variables can influence how corals respond to temperature stress. A common framework for acute and short‐duration coral bleaching experiments is summarized in Table 1.
Fig. 1.

Two examples of acute and short‐term coral heat‐stress experimental setups. Photo in panel (a) by S. Palumbi and panel (b) by C. R.Voolstra.
Acute and short‐term thermal‐stress experimental conditions
1. 1. Temperature.
In all heat‐stress experiments, treatment temperatures are typically based on in situ temperature measurements or previous bleaching records from the coral collection site. Given the dramatic heat‐stress conditions in short‐term and acute studies, pilot studies to empirically assess coral responses to a range of temperature levels are helpful in determining the exposure temperature at which the corals in question bleach and die. These pilot experiments are relevant for setting a target temperature and should be set below the temperature that caused mortality (Table 1). Treatment temperature may fall within a range of +3°C to +9°C above the monthly mean maximum (MMM; Voolstra et al. 2020). This initial testing is particularly important when in situ temperature data are lacking. Reef temperature at the time of collection should provide the most realistic control temperature. Precision and accuracy of temperature in control and treatment tanks is achieved by using continuous temperature logging devices (Appendix S1: Section S1.2), which enhance the ability to compare results across studies.
Temperature profiles of acute and short‐term heat‐stress experiments are either of a heat‐pulse or a heat‐hold design (Fig. 2; Mayfield et al. 2011, Parkinson et al. 2018, Morikawa and Palumbi 2019, Voolstra et al. 2020). Heat‐pulse experiments are often designed to mimic natural temperature fluctuations over diel cycles, across tidal cycles, and during internal wave or upwelling events, but may also be used to rapidly test the response of corals to a range of elevated temperatures that are not typically recorded in a natural setting (Fig. 2a). The profile encompasses cycles of ramp‐up heating, exposure to a target high temperature, and ramp‐down cooling, often followed by a recovery phase (i.e., with the latter often lasting longer than the heat cycle(s) themselves). In the simplest case, heat‐pulse experiments run through one such cycle, but any number of cycles may be explored (e.g., to assess the effect of repeated heat exposures on recovery and resilience). They can also explore the maximum thermal tolerance of corals with multiple tanks at temperatures ranging from MMM to +9°C (Fig. 2b). Heat‐pulse designs explicitly allow the exploration of the holobiont response to thermal extremes, as well as examination of the potential for acclimation, given that the heat‐stress exposures are brief. Starting and stopping times typically mimic natural diel variability, are only run during the day, and ideally finish at the same time of the day each day. Consistency in ramp duration (minutes–hours) and heating duration at the target temperatures helps to facilitate comparisons among heat‐pulse coral‐bleaching studies. We recognize that this protocol may result in variable temperature ramp rates (°C/h) to reach the desired heat‐stress target temperatures (Table 1; Fig. 2b).
Fig. 2.
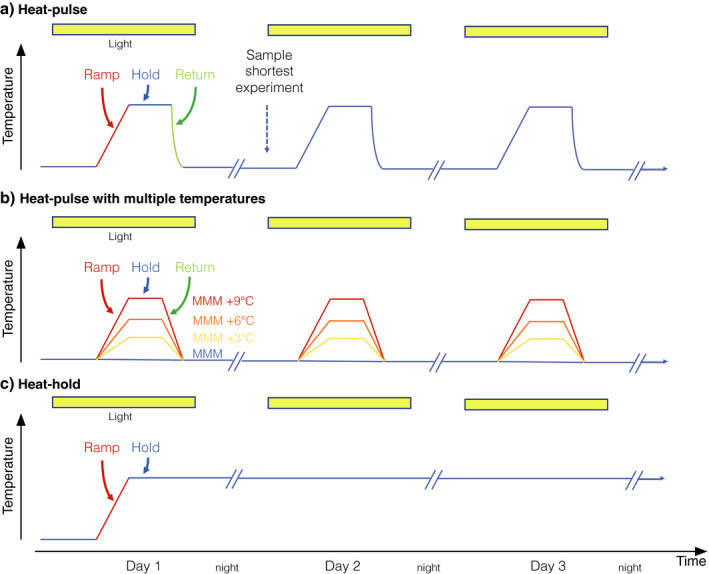
Temperature profiles of coral (a) heat‐pulse, (b) heat‐pulse with multiple temperatures, and (c) heat‐hold acute and short‐term thermal stress experiments. Number of days will depend on the specific study. Yellow bars indicate light cycles. Line breaks indicate night. MMM, maximum monthly mean temperature.
In heat‐hold experiments, the temperature ramp‐up rate is high compared with long‐term experiments, and the duration of heating at the target temperature is extended compared to heat‐pulse experiments (Fig. 2c). For this type of experiment, thermal stress is continuously accumulated, and could be considered a short‐term model for bleaching events in which the entire water column is rapidly heated.
2. 2. Light
Coral bleaching is a response to both temperature and light (Jokiel and Coles 1990, Brown et al. 1994, Warner et al. 1999, Brown et al. 2002). Natural bleaching often correlates strongly with maximal light conditions (Mumby et al. 2001), and there is often a relationship between temperature‐related photodamage to Symbiodiniaceae and light intensity (Warner and Suggett 2016). Artificial light that is modulated over day/night cycles (see yellow bars in Fig. 2) mimics the diel cycle providing realistic light cues for these photosynthetically active animals with strong circadian rhythms (Hoadley et al. 2016). If light is not a dependent variable, in situ light data from the coral collection site can be used to determine the maximum irradiance on a clear cloudless day and thus the maximum experimental light levels. If replicating natural light conditions is not possible, minimum light levels from 250 to 500 μmol photons·m−2·s−1 are typically sufficient to stimulate maximal photosynthesis (P max; Warner et al. 1999, Falkowski and Raven 2007, Suggett et al. 2013; Table 1; Appendix S1: Section S1.3). Given the short nature of acute heat‐stress experiments, use of static light intensities during the day is more practical over fluctuating light levels that incorporate dawn and dusk. Light levels that are standardized within experiments enhance comparability of results among runs.
3. Seawater flow and turnover
Adequate flow within the tanks is important as static water creates temperature, pH, and oxygen gradients, chemical changes, and pockets of high microbial growth (Mass et al. 2010, Osinga et al. 2017), whereas higher current flow reduces bleaching (Nakamura and van Woesik 2001, Nakamura et al. 2003, Lenihan et al. 2008, Schmidt et al. 2016, Fujimura and Riegl 2017). Thus, adequate flow as well as consistent flow rates among tanks are needed for valid comparisons within and among studies. Thus, flow and tank volume turnover need to be sufficient in acute and short‐term studies (Table 1) where flow effects may manifest quickly. Water flow velocity can be measured with a velocimeter (i.e., distance travelled per unit time) and seawater turnover rate within tanks can be estimated by measuring the volume exchanged over a defined time period. Submersible water pumps can provide additional circulation in cases where tank turnover and/or flow is limited for logistical reasons. In flow‐through systems, we suggest a 100% water turnover rate every 3–6 h (Table 1).
3. 4. Feeding
Unlike long‐term experiments, direct feeding is not critical in acute and short‐duration studies (assuming sufficient light is provided to the corals; Table 1). However, the type of seawater used (i.e., filtered, unfiltered, natural, artificial) is important as the chemical composition and particulate organic matter content can vary substantially among different seawater types.
4. 5. Applications for early life stages
Acute and short‐term thermal‐stress experiments allow for the assessment of temperature stress on early‐life stages of coral larvae and juveniles. In the estimated 85% of coral species where eggs are not provisioned with algal symbionts by the parent colony, larvae provide access to naturally aposymbiotic tissue, which can be used to understand the coral host response to temperature stress (Voolstra et al. 2009, Baums et al. 2013, Dixon et al. 2015), albeit against the background of ontogenetic change. Endosymbiont‐host associations are often manipulated more easily during larval and juvenile stages when the coral may be able to associate with a wider array of symbionts than during the adult stage (Abrego et al. 2009, van Oppen 2015, Quigley et al. 2017, Poland and Coffroth 2019). Furthermore, the small size of coral larvae allows for comparison across many individuals in the same experiment.
Moderate‐duration (8–30 d) thermal‐stress experiments
Moderate‐duration thermal‐stress experiments are defined as those in which thermal stress lasts between 8 and 30 d above the baseline temperature (Glynn and D'Croz 1990; Table 1). These experiments typically seek to simulate natural conditions by assessing the coral phenotypic responses while maximizing biological realism and ecological relevance. For experiments conducted at remote field sites, moderate duration experiments are often more practical and cost‐effective than long‐term experiments. Key advantages of moderate‐duration experiments is that they can be used to measure compensatory mechanisms, holobiont responses, mortality, and recovery that are typically included in long‐term experiments, but with a more ecologically relevant heat‐stress duration than acute and short‐term experiments. In addition, moderate‐duration experiments do not limit the range and type of coral responses that can be quantified and are sufficiently long to detect genet‐level responses.
Mechanistically, moderate‐duration thermal‐stress experiments are typically conducted using standard indoor or outdoor aquaria where physical variables such as temperature and flow can be reasonably constrained, facilitating subsequent comparisons between studies (Fig. 3). Light conditions may be natural or artificial (see Light section below) and tank replication of at least three tanks per treatment reduces the problem of tank effects. Coral ramets in these studies are typically medium to large in size (e.g., 3–8 cm tall), making them easy to manipulate experimentally and providing sufficient material for a large number of downstream analyses. Coral ramets are typically allowed to recover for 7–12 d after fragmentation providing time for initial wound healing (Traylor‐Knowles 2016, Edmunds and Yarid 2017, Counsell et al. 2019). It is generally assumed that 7–12 d is sufficient time for acclimation to the experimental conditions prior to the start of the experiment. Mimicking natural conditions in terms of baseline temperature, light, flow, salinity, pH, nutrient levels, and dissolved oxygen, as closely as is reasonably possible, helps to provide ecologically relevant findings. Reporting the average and range of as many physicochemical conditions as possible in an experiment enhances comparisons among studies since differences in any one of the non‐temperature variables can influence how corals respond to temperature stress (Finelli et al. 2006, Anthony et al. 2008, Wiedenmann et al. 2013, Vega Thurber et al. 2014). A common framework for moderate‐duration coral bleaching experiments is outlined in Table 1.
Fig. 3.
Example of an (a) outdoor and (b) indoor moderate‐duration coral heat‐stress experiment setup. Long‐term experimental setups are similar. Photo in panel (a) by D. Kemp and panel (b) by A. Grottoli.
Moderate‐duration thermal‐stress experimental conditions
1. 1. Temperature
The duration and severity of thermal stress is determined by the experimental question. Thermal stress of +1–4°C above the local thermal baseline (i.e., MMM) typically produces a bleaching response within 30 d (Jokiel and Coles 1990, Fitt et al. 2001, Grottoli et al. 2006, Mayfield et al. 2013b ; Table 1; Appendix S1: Section S1.2). The upper temperature threshold depends on what is realistic for the species studied, and what is ecologically relevant for that location. Gradual temperature ramp‐up rates over several days minimizes the chances of heat‐shock and mimics the rate of warming in natural bleaching events (Table 1). In general, a temperature ramp‐up rate of no more than 1°C/d can prevent an acute stress response, although this is still rapid in relation to many natural bleaching events (Jokiel 2004, Ainsworth et al. 2016, Bahr et al. 2017). Ideally, the warming rate should simulate natural profiles when possible so as not to induce an acute stress response (Table 1; Appendix S1: Section S1.2). How long corals are experimentally maintained at bleaching stress temperatures will depend on the desired phenotypic response (i.e., such as disruption of photosynthesis, loss of pigmentation/endosymbionts, or onset of mortality), but without unintended mortality over the course of the experiment.
2. Light
Similar to the recommendations above for acute experiments (see Acute and short‐term thermal‐stress experimental conditions: Light ), light requires special consideration in moderate‐duration experiments as well (Table 1; Appendix S1: Section S1.3). When light is not an experimental treatment, light conditions that mimic natural irradiance conditions as closely as possible at the depth from which the colonies were collected will be most ecologically relevant. For outdoor experiments, neutral‐density shade cloth is useful for attenuating full sunlight and to ensure that light intensity mimics photosynthetic available radiation (PAR) experienced at the depth from which the corals were collected (Grottoli et al. 2014, Jury and Toonen 2019). Recommended peak PAR levels should follow the same guidelines provided in Acute and short‐term thermal‐stress experimental conditions: Light . For indoor systems, diurnal light cycling is most realistic though it is often difficult to generate daytime light levels that are as high as those experienced in shallow reef environments. When replicating natural light conditions is not possible, minimum light levels close to saturating photosynthesis are typically sufficient (Acute and short‐term thermal‐stress experimental conditions: Light), but this is dependent on the collection location and ideally empirically tested prior to starting experiments. For corals from deeper locations, maximum light levels are more easily matched to those at the collection site. Since high light can modulate bleaching responses in corals (Anthony et al. 2007, Ferrier‐Pagès et al. 2007, Hawkins et al. 2015), an adequate acclimation period is especially important in experimental systems where light conditions differ from those at the collection sites.
2. 3. Seawater flow and turnover
Adequate water flow minimizes unwarranted temperature gradients and localized pH or chemical changes in experimental tanks. For comparative purposes clear reporting of the various flow parameters is useful (i.e., circulating pump size, brand, and model, the tank volume, water flow, and tank volume turnover time; Table 1; Appendix S1: Section S1.4). For many reef environments, near‐bottom water velocities are on the order of 2–20 cm/s (Nakamura and van Woesik 2001, Hench et al. 2008, Lowe et al. 2009, Hench and Rosman 2013) depending on the location (e.g., lagoon vs. barrier reef crest). Velocity variability due to wave exposure can be quantified using the root mean squared (rms) velocity (Reidenbach et al. 2006, Falter et al. 2007, Lowe et al. 2008). Flow rates within experimental tanks should attempt to replicate flow conditions at the corals collection site to minimize any unintended flow effects. Complete water exchange (i.e., tank volume turnover) is also important for ensuring adequate mixing and temporally stable physicochemical conditions in tanks during an experiment. Tank volume turnover times of once per day may be all that is feasible for some types of experiments, although higher daily turnover is better for providing physicochemical conditions in the system that are more consistent with natural reef environments (Table 1, Appendix S1: Section S1.4).
3. 4. Feeding and post heat‐stress recovery
Corals are mixotrophic, relying on both autotrophy and heterotrophy for proper nourishment. Heterotrophic feeding on zooplankton, particulate, and dissolved organic particles is a natural part of their diet and an essential source of nutrition, especially when stressed (Anthony 2000, Grottoli et al. 2006, Houlbreque and Ferrier‐Pages 2009, Edmunds 2011, Hughes and Grottoli 2013, Baumann et al. 2014). In moderate‐duration heat‐stress experiments, supplemental feeding at least once a week to satiation provides corals with some of that essential nutrition (though coral have access to zooplankton nightly on the reef, so up to three times a week is more realistic; Tables 1; Appendix S1: Section S1.5). Even if using natural seawater flow‐through systems, corals will likely not be getting zooplankton or adequate nutritional resources, necessitating supplemental feeding. Little to no zooplankton are available in many natural seawater flow‐through systems (A. G. Grottoli, personal observation), although there can be fine particulate and dissolved organic carbon available. Finally, moderate‐duration experiments present an opportunity to monitor responses to post heat‐stress treatment (i.e., recovery; Table 1). How corals physiologically recover from heat‐stress is an understudied area of research (McLachlan et al. 2020), yet vital to understanding how corals might recover or continue to decline following bleaching events (Hughes and Grottoli 2013, Grottoli et al. 2014).
Long‐term and chronic (>31 d) thermal‐stress experiments
Long‐term bleaching experiments are here defined as those in which thermal stress above the baseline temperature (i.e., MMM temperature) lasts for 31 d or more. These experiments may include a single prolonged heat‐stress, multiple heat‐stress events with similar or different heating profiles (i.e., repeat or annual bleaching), and/or preconditioning and recovery periods (Mayfield et al. 2013a , Grottoli et al. 2014; Fig. 3). These experiments are best‐suited for reproducing naturally occurring heat‐stress conditions and bleaching events followed by observations on recovery. As such, long‐term and chronic experiments have maximum ecological relevance and provide real‐world responses of coral phenotypes to thermal stress. Experiments on these timescales can capture seasonal variability and evaluate acclimatization responses that integrate over long timespans, which include photo‐acclimation, changes in gene expression, symbiont shuffling, calcification, changes in energy reserves, and feeding behaviors. In addition, the long‐term nature of these studies also enables time‐series analysis and can facilitate more collaborative and comprehensive measurements.
Despite the advantages of long‐term heat‐stress experiments, they require much more investment in resources and effort than short‐term and moderate‐duration experiments. Long‐term studies also have a greater risk of tank effects that compound over time (although these problems can be minimized by rotating treatments among experimental tanks, or rotating corals among tanks of the same treatment), or other unforeseen issues that may cause the experimental conditions to deviate from those that are realistic in nature (e.g., an outbreak of algae, micro‐predator, or disease). Therefore, backup equipment, maintenance of power, adequate plumbing, robust scientific equipment, and careful monitoring are critical for these types of experiments.
Mechanistically, long‐term experiments are typically conducted in outdoor tank systems where ambient light and flow‐through seawater best replicate conditions on the reef. Alternatively, they are conducted in an indoor laboratory setting where conditions are carefully controlled to mimic natural environments. However, since this can be expensive and difficult, outdoor settings are typically more practical. In most studies, pseudoreplication is avoided by including two or more tanks per treatment (Table 1). As with moderate‐duration experiments, sufficient time for wound healing post‐collection under control conditions ensures corals can acclimate to the system prior to experimentation (Table 1). Coral ramets in these studies typically start off as small to medium in size but can grow to be very large in studies lasting more than a year. This allows for many downstream analyses, but the projected growth of the corals should be taken into account in the planning stages of long‐term experiments. Since these types of experiments are designed to mimic naturally occurring heat‐stress events, the physical conditions other than those being experimentally manipulated are ecologically relevant when they mimic local conditions as closely as possible. When local environmental data are not available for the area where the experimental corals were sourced, data from nearby or comparable sites are often used to establish the physical conditions in the experiment. Measuring and reporting as many physicochemical conditions (i.e., temperature, light, flow, salinity, pH, etc.) at the highest resolution possible is especially important in longer studies as their changes can have cumulative effects over the course of the study and influence the measured coral response variables. A common framework for long‐term duration coral bleaching experiments is outlined in Table 1.
Long‐term and chronic thermal‐stress experimental conditions
1. 1. Temperature
Control temperatures are most realistic when they mimic the ambient diel temperature and the seasonal variability where the corals were collected (Table 1; Appendix S1: Section S1.2). While this is reasonable for outdoor flow‐through systems, it can be challenging in an indoor environment. The heat‐stress temperature will depend on the local ecological relevance and species of interest. An MMM +1°C or more (i.e., enough to elicit a bleaching response without being so severe as to cause unintended mortality over the experimental duration) often realistically mimics natural bleaching events (Table 1). Likewise, the rate of thermal ramping will depend on the observed natural warming rate observed in one or more previous local bleaching events (Table 1).
2. 2. Light
Optimal experimental light conditions mimic natural irradiance at the coral collection depth and site, including the daily light integral for the region on both diel and seasonal timescales. The lighting requirements in long‐term experiments are the same as those for moderate heat‐stress experiments and discussed in Moderate‐duration thermal‐stress experimental conditions: Light above. Due to the longer duration of these studies, indoor systems that also simulate moonlight provide an important regulator of coral physiology, particularly reproduction, over longer timescales (Table 1).
3. 3. Seawater flow and turnover
The common framework structure for flow and turnover in long‐term heat‐stress experiments is the same as those for moderate heat‐stress experiments and discussed in Moderate‐duration thermal‐stress experimental conditions: Seawater flow and turnover above.
4. 4. Feeding and post heat‐stress recovery
The common framework structure for feeding and monitoring of recovery are the same in long‐term heat‐stress studies as for moderate‐duration heat‐stress studies and are discussed in Moderate‐duration thermal‐stress experimental conditions: Feeding and post heat‐stress recovery .
Common Currencies for Quantifying Coral Bleaching Responses
Bleaching is often based on characteristics of the algal endosymbionts (i.e., color, appearance) or the coral holobiont (i.e., growth, mortality). Yet, in some experiments, no quantified measure of bleaching is reported (McLachlan et al. 2020). This creates difficulty in comparing coral bleaching studies because there is no common experimental “currency” among them. For example, one study might measure the microbiome and endosymbiont algal density, whereas another study might measure calcification and gene expression. Even if the two studies are on the same coral species from the same location, without a common response variable between them it is more difficult to compare and draw inferences. This is especially true when there are different bleaching thresholds among different genets of the same species, or different species that are morphologically indistinguishable (Boulay et al. 2014, Johnston et al. 2018). Reporting one or more common currency measures of coral bleaching could provide a quantitative reference to enhance physiological comparisons among studies and provide greater potential for meta‐analyses. Examples of measurements that could serve as common currencies include color image analysis, chlorophyll concentration, Symbiodiniaceae cell density, mortality rate, and skeletal growth rate. While there are many other methods for quantifying coral bleaching, the response variables listed in Table 1 were prioritized for their effectiveness in quantifying bleaching and holobiont phenotypes as well as for their ease of measurement, minimal training necessary to execute the measurements, and low per sample cost, making them accessible to as many researchers as possible. Measuring and reporting at least one endosymbiont response variable (i.e., color, chlorophyll, cell density) and one holobiont response variable (i.e., mortality, growth) would be a valuable means of establishing common physiological reference points between studies (Table 1; Appendix S1: Sections S2.1, S2.2). Reporting these response variables in International System of Units (SI units), as opposed to percentage change, would further facilitate cross‐study comparisons, future data reuse, and statistical analyses. If resources permit, measurements of active chlorophyll fluorescence (e.g., pulse‐amplitude modulating [PAM] fluorometry) can be an effective and non‐destructive way of quantifying endosymbiont photosystem performance. Further, Symbiodiniaceae diversity (i.e., genus, species, or strain) can provide incredibly insightful information as it is an important correlate of bleaching severity and recovery (Table 1; Appendix S1: Section S2.3). We recognize that the latter two analyses require substantial instrumentation, cost, and training, and therefore may not be feasible in many instances.
Implications of Accurate Reporting for Meta‐Analysis
McLachlan et al. (2020) noted that many basic environmental and experimental conditions are underreported in coral bleaching experiments. For example, at least 95% of the studies examined do not report any measure of flow (i.e., flow within tanks or tank turnover rates), 25% do not report light intensity, and 21% do not provide any quantitative measurement of the bleaching phenotype or the precise geographic location of the study. Yet, flow and light can have dramatic interactive effects on thermal‐stress responses (Nakamura and van Woesik 2001, Nakamura et al. 2003, McLanahan et al. 2005, Nakamura et al. 2005). A quantitative measure of bleaching severity can have a profound effect on how the results might be interpreted, and the geographic location is critical for placing results into a broader ecological context (e.g., bleaching threshold temperature above MMM of corals in the Red Sea are a lot higher than elsewhere, Bellworthy and Fine 2017, Osman et al. 2018). Being able to effectively compare findings among studies requires accurate reporting of experimental conditions. Thus, we have compiled a summary of some metadata that are valuable to accurately report in Table 2 to increase transparency in experimental methods, enhance comparability among studies, and facilitate a more global understanding of coral bleaching patterns across space and time. We recognize that not all metadata types will apply to all experiments.
Table 2.
Summary of metadata that can be reported in coral bleaching experiment research to increase cross‐study comparisons.
| Metadata type | Conditions or methods | Units or other identifier(s) |
|---|---|---|
| Coral collection | Latitude and longitude at collection site | Decimal degrees |
| Collection depth | Meters | |
| Collection date(s) | Year‐month‐day | |
| Coral species | ||
| Coral morphology (i.e., plating, encrusting, mounding, branching, foliose) | ||
| Symbiodiniaceae for all coral colonies† | ||
| Acclimation post collection prior to experiment | Days | |
| Experimental design | Name of location | Institution, city, state/province, country |
| Bleaching stress temperature period | Start and end dates in year‐month‐day | |
| System type (flow‐through or recirculating, outdoor or indoor) | ||
| No. tanks per treatment | ||
| No. coral genets (colonies) per treatment | ||
| No. coral genets (colonies) per tank within treatments | ||
| No. recovery days post heat‐stress | ||
| Experimental temperature conditions‡ | Heat stress temperature above MMM per treatment | °C |
| Control temperature | °C | |
| Baseline temperature (MMM) | °C | |
| Temperature ramp‐up rate | °C/h or °C/d | |
| Duration at heat stress temperature | Hours or days | |
| Temperature modulation | Static, diurnal, seasonal | |
| Other experimental conditions | Light conditions§ | µmol photons·m−2·s−1 |
| Light cycle | Static, diurnal, seasonal | |
| Water flow velocity¶ or tank volume with pump circulating capacity | Flow rate, cm/s | |
| Tank turnover¶ | No./d or L/min | |
| Seawater filtration | Filtered or unfiltered | |
| Seawater source# | Natural or artificial | |
| Salinity|| | ||
| Nutrient concentrations†† (i.e., ammonia, nitrite, nitrate, phosphate) | ||
| Feeding‡‡ (i.e., fed/not fed, frequency, concentration, and food type) | ||
| pH§§ | ||
| Dissolved oxygen¶¶ |
MMM, maximum monthly mean (i.e., mean temperature of the warmest month). A review of commonly used methods for many of the measurements and analyses is included in Appendix S1. Not all conditions or methods will apply to all studies.
Appendix S1: Section S2.3b.
Appendix S1: Section S1.2.
Appendix S1: Section S1.3.
Appendix S1: Section S1.4.
Appendix S1: Section S1.6.
Appendix S1: Section S1.7.
Appendix S1: Section S1.8.
Appendix S1: Section S1.5.
Appendix S1: Section S1.9.
Appendix S1: Section S1.10.
Beyond Coral Bleaching Experiments
While the development of a common framework for coral bleaching experiments is a step in the right direction, there is more to consider. Every year, researchers conduct coral bleaching experiments, measure some response variable(s) of interest, and publish their results. Too often, remaining coral material is disposed of, or not archived in a way that could be utilized or made available to other researchers for additional studies. The next step for the coral research community is to evaluate how coral samples are collected, preserved, and archived to determine how researchers might effectively share existing coral material to conduct additional complementary research without duplicative experimentation. This approach has the advantage of limiting the amount of wild coral material harvested for research, increasing the return on investment for a given experiment, fostering new collaborations and exchanges of ideas, and reducing the time to discovery. Sample preservation and archiving are strategies that have been effectively used in other communities (e.g., International Ocean Drilling Program) and are models for coral researchers to consider developing.
Conclusions
The common framework for coral bleaching experiments outlined in this paper provides some insights and suggestions that could help increase comparability among coral bleaching experiments. We recognize that studies are driven by specific research questions that may differ in scope or have requirements that are outside the framework parameters outlined here. Nevertheless, it is our hope that the common framework discussed here will encourage researchers to consider measuring and reporting more of the physicochemical conditions and variables (Table 1), better appreciate the value of reporting all of the relevant metadata (Table 2), and perhaps incorporate new analytical techniques or approaches in their research (see Appendix S1). The broad adoption of a common framework for coral bleaching experiments would increase the comparability of studies and enhance collaboration, which would have the net effect of increasing the efficacy and creativity of coral bleaching research. As coral reefs continue to change globally, every effort we can make to accelerate the pace of discovery will bring us that much closer to innovative solutions for protecting and restoring coral reefs.
Supporting information
Appendix S1
Acknowledgments
All authors participated in the Coral Bleaching Research Coordination Network workshop in May 2019 where the content of this manuscript was developed. All participants contributed to the writing and revising of the manuscript. A. G. Grottoli was the director of the workshop, wrote 40% of the text, coordinated all writing efforts, compiled all of the components of the manuscript, and incorporated all revisions and edits. Many thanks to Kathleen Weathers for advice on how to run a workshop. Funding was provided by the National Science Foundation Division of Biological Oceanography (1838667). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Grottoli, A. G. , R. et al. 2021. Increasing comparability among coral bleaching experiments. Ecological Applications 31(4):e02262. 10.1002/eap.2262
Corresponding Editor: David S. Schimel.
Literature Cited
- Abrego, D. , van Oppen M. J. H., and Willis B. L.. 2009. Highly infectious symbiont dominates initial uptake in coral juveniles. Molecular Ecology 18:3518–3531. [DOI] [PubMed] [Google Scholar]
- Ainsworth, T. , Heron S., Ortiz J., Mumby P., Grech A., Ogawa D., Eakin M., and Leggat W.. 2016. Climate change disables coral bleaching protection on the Great Barrier Reef. Science 352:338–342. [DOI] [PubMed] [Google Scholar]
- Anthony, K. R. N. 2000. Enhanced particle‐feeding capacity of corals. Coral Reefs 19:59–67. [Google Scholar]
- Anthony, K. R. N. , Connolly S. R., and Hoegh‐Guldberg O.. 2007. Bleaching, energetics, and coral mortality risk: Effects of temperature, light, and sediment regime. Limnology and Oceanography 52:716–726. [Google Scholar]
- Anthony, K. R. N. , Kline D. I., Diaz‐Pulido G., Dove S., and Hoegh‐Guldberg O.. 2008. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proceedings of the National Academy of Sciences USA 105:17442–17446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr, K. , Rodgers K., and Jokiel P.. 2017. Impact of three bleaching events on the reef resiliency of Kāne‘ohe Bay, Hawai‘i. Frontiers in Marine Science 4:398. [Google Scholar]
- Baumann, J. , Grottoli A. G., Hughes A. D., and Matsui Y.. 2014. Photoautotrophic and heterotrophic carbon in bleached and non‐bleached coral lipid acquisition and storage. Journal of Experimental Biology and Ecology 461:469–478. [Google Scholar]
- Baums, I. B. , Devlin‐Durante M., Polato N., Xu D., Giri S., Altman N., Ruiz D., Parkinson J., and Boulay J.. 2013. Genotypic variation influences reproductive success and thermal stress tolerance in the reef building coral, Acropora palmata . Coral Reefs 32:703–717. [Google Scholar]
- Baums, I. , Miller M., and Hellberg M. E.. 2006. Geographic variation in clonal structure in a reef‐building Caribbean coral, Acropora palmata . Ecological Monographs 76:503–519. [Google Scholar]
- Baums, I. B. , et al. 2019. Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecological Applications 29:e01978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellworthy, J. , and Fine M.. 2017. Beyond peak summer temperatures, branching corals in the Gulf of Aqaba are resilient to thermal stress but sensitive to high light. Coral Reefs 36:1071–1082. [Google Scholar]
- Boulay, J. , Hellberg M., Cortes J., and Baums I.. 2014. Unrecognized coral species diversity masks differences in functional ecology. Proceedings of the Royal Society B 281:20131580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, B. E. 1997. Coral bleaching: causes and consequences. Coral Reefs 16(Suppl):s129–s138. [Google Scholar]
- Brown, B. E. , Dunne R. P., Goodson M. S., and Douglas A. E.. 2002. Experience shapes the susceptibility of a reef coral to bleaching. Coral Reefs 21:119. [Google Scholar]
- Brown, B. E. , Dunne R. P., Scoffin T. P., and Le Tissier M. D. A.. 1994. Solar damage in intertidal corals. Marine Ecology Progress Series 105:219–230. [Google Scholar]
- Buddemeier, R. W. , Kleypas J. A., and Aronson R. B.. 2004. Coral reefs & global climate change: Potential contributions of climate change to stresses on coral reef ecosystems. Pew Center on Global Climate Change, Arlington, Virginia, USA. www.pewclimate.org [Google Scholar]
- Cantin, N. , Cohen A. L., Karnauskas K., Tarrant A., and McCorkle D.. 2010. Ocean warming slows coral growth in the Central Red Sea. Science 329:322–325. [DOI] [PubMed] [Google Scholar]
- Cornwall, C. , and Hurd C.. 2015. Experimental design in ocean acidification research: problems and solutions. ICES Journal of Marine Science 73:572–581. [Google Scholar]
- Counsell, C. , Johnston E., and Sale T.. 2019. Colony size and depth affect wound repair in a branching coral. Marine Biology 166:148. [Google Scholar]
- Dixon, G. , Davies S., Aglyamova G., Meyer E., Bay L., and Matz M.. 2015. Genomic determinants of coral heat tolerance across latitudes. Science 348:1460–1462. [DOI] [PubMed] [Google Scholar]
- Eakin, C. M. , Lough J. M., and Heron S. F.. 2009. Climate variability and change: monitoring data and evidence for increased coral bleaching stress. Pages 41–67 in van Oppen M. J. H. and Lough J. M., editors. Coral bleaching: patterns, processes, causes and consequences. Springer‐Verlag, Berlin, Germany. [Google Scholar]
- Edmunds, P. J. 2011. Zooplanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnology and Oceanography 56:2402–2410. [Google Scholar]
- Edmunds, P. J. , and Yarid A.. 2017. The effects of ocean acidification on wound repair in the coral Porites spp. Journal of Experimental Marine Biology and Ecology 486:98–104. [Google Scholar]
- Falkowski, P. G. , and Raven J. A.. 2007. Aquatic photosynthesis. Princeton University Press, Princeton, New Jersey, USA. [Google Scholar]
- Falter, J. , Atkinson M., Lowe R., Monismith S., and Koseff J.. 2007. Effects of nonlocal turbulence on mass transfer of dissolved species to coral reefs. Limnology and Oceanography 52:274–285. [Google Scholar]
- Ferrier‐Pagès, C. , Richard C., Forcioli D., Allemand D., Pichon M., and Shick J. M.. 2007. Effects of temperature and UV radiation increases on the photosynthetic efficiency in four scleractinian coral species. Biological Bulletin 213:76–87. [DOI] [PubMed] [Google Scholar]
- Finelli, C. M. , Helmuth B. S. T., Pentcheff N. D., and Wethey D. S.. 2006. Water flow influences oxygen transport and photosynthetic efficiency in corals. Coral Reefs 25:47–57. [Google Scholar]
- Fitt, W. K. , Brown B. E., Warner M. E., and Dunne R. P.. 2001. Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs 20:51–56. [Google Scholar]
- Fitt, W. K. , McFarland F. K., Warner M. E., and Chilcoat G. C.. 2000. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnology and Oceanography 45:677–685. [Google Scholar]
- Frieler, K. , Meinshausen M., Golly A., Mengel M., Lebek K., Donner S. D., and Hoegh‐Guldberg O.. 2012. Limiting global warming to 2°C is unlikely to save most coral reefs. Nature Climate Change 3:165–170. [Google Scholar]
- Fujimura, A. , and Riegl M.. 2017. Effects of water flow on intra‐ and intercolonial variable in bleaching of the zoanthis, Palythoa caribaeorum . Journal of Experimental Marine Biology 490:29–33. [Google Scholar]
- Gerstner, K. , Moreno‐Mateos D., Gurevitch J., Beckmann M., Kambach S., Jones H., and Seppelt R.. 2017. Will your paper be used in a meta‐analysis? Make the reach of your research broader and longer lasting. Methods in Ecology and Evolution 8:777–784. [Google Scholar]
- Glynn, P. W. 1983. Extensive ‘bleaching’ and death of reef corals on the Pacific coast of Panama. Environmental Conservation 10:149–154. [Google Scholar]
- Glynn, P. W. , and D'Croz L.. 1990. Experimental evidence for high temperature stress as the cause of El Nino‐coincident coral mortality. Coral Reefs 8:181–191. [Google Scholar]
- Gorospe, K. , Donahue M., and Karl S.. 2015. The importance of sampling design: spatial patterns and clonality in estimating the genetic diversity of coral reefs. Marine Biology 162:917–928. [Google Scholar]
- Green, R. H. , Lowe R. J., Buckley M. L., Foster T., and Gilmour J. P.. 2019. Physical mechanisms influencing localized patterns of temperature variability and coral bleaching within a system of reef atolls. Coral Reefs 38:759–771. [Google Scholar]
- Grottoli, A. G. , Rodrigues L. J., and Palardy J. E.. 2006. Heterotrophic plasticity and resilience in bleached corals. Nature 440:1186–1189. [DOI] [PubMed] [Google Scholar]
- Grottoli, A. G. , Warner M. E., Levas S. J., Aschaffenburg M. D., Schoepf V., McGinley M., Baumann J., and Matsui Y.. 2014. The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Global Change Biology 20:3823–3833. [DOI] [PubMed] [Google Scholar]
- Hawkins, T. D. , Krueger T., Wilkinson S. P., Fisher P. L., and Davy S. K.. 2015. Antioxidant responses to heat and light stress differ with habitat in a common reef coral. Coral Reefs 34:1229–1241. [Google Scholar]
- Hench, J. , Leichter J. J., and Monismith S.. 2008. Episodic circulation and exchange in a wave‐driven coral reef and lagoon system. Limnology & Oceanography 53:2681–2694. [Google Scholar]
- Hench, J. , and Rosman J.. 2013. Observations of spatial flow patterns at the coral colony scale on a shallow reef flat. Journal of Geophysical Research: Oceans 118:1142–1156. [Google Scholar]
- Herdman, L. M. M. , Hench J. L., and Monismith S. G.. 2015. Heat balances and thermally driven lagoon‐ocean exchanges on a tropical coral reef system (Moorea, French Polynesia). Journal of Geophysical Research: Oceans 120:1233–1252. [Google Scholar]
- Hoadley, K. , Vize P., and Pyott S.. 2016. Current understanding of the circadian clock within Cnidaria. Pages 511–520 in Goffredo S. and Dubinsky Z., editors. The Cnidaria: Past, present and future. Springer, Cham, Switzerland. [Google Scholar]
- Hoegh‐Guldberg, O. 1999. Climate change, coral bleaching and the future of the world's coral reefs. Marine Freshwater Research 50:839–866. [Google Scholar]
- Hoegh‐Guldberg, O. , et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742. [DOI] [PubMed] [Google Scholar]
- Hoegh‐Guldberg, O. 2011. The impact of climate change on coral reef ecosystems. Pages 391–403 in Dubinsky Z. and Stambler N., editors. Corals reefs: an ecosystem in transition. Springer Science+Business Media, Berlin, Germany. [Google Scholar]
- Houlbreque, F. , and Ferrier‐Pages C.. 2009. Heterotrophy in tropical scleractinian corals. Biological Reviews 84:1–17. [DOI] [PubMed] [Google Scholar]
- Hughes, A. , and Grottoli A. G.. 2013. Heterotrophic compensation: a possible mechanism for resilience of coral reefs to global warming or a sign of prolonged stress? PLoS ONE 8:e81172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, T. , et al. 2018. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83. [DOI] [PubMed] [Google Scholar]
- IPCC 2013. Summary for policymakers. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Johnston, E. C. , Forsman Z. H., and Toonen R. J.. 2018. A simple molecular technique for distinguishing species reveals frequent misidentification of Hawaiian corals in the genus Pocillopora . PeerJ 6:e4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokiel, P. L. 2004. Temperature stress and coral bleaching. Pages 401–425 in Rosenberg E. and Loya Y., editors. Coral health and disease. Springer, Berlin, Germany. [Google Scholar]
- Jokiel, P. L. , and Coles S. L.. 1974. Effects of heated effluent on hermatypic corals at Kahe Point, Oahu. Pacific Science 28:1–18. [Google Scholar]
- Jokiel, P. L. , and Coles S. L.. 1977. Effects of temperature on the mortality and growth of Hawaiian reef corals. Marine Biology 43:201–208. [Google Scholar]
- Jokiel, P. L. , and Coles S. L.. 1990. Response of Hawaiian and other Indo‐Pacific reef corals to elevated temperature. Coral Reefs 8:155–162. [Google Scholar]
- Jury, C. , Delano M., and Toonen R.. 2019. High heritability of coral calcification rates and evolutionary potential under ocean acidification. Scientific Reports 9:20419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jury, C. , and Toonen R. 2019. Adaptive responses and local stressor mitigation drive coral resilience in warmer, more acidic oceans. Proceedings of the Royal Society B 286:20190614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel, C. , Setta S., and Matz M. V.. 2015. Heritable differences in fitness‐related traits among populations of the mustard hill coral, Porites astreoides . Heredity 115:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffner, I. B. , Bartels E., Stathakopoulos A., Enochs I., Kolodzeij G., Toth L., and Manzello D.. 2017. Plasticity in skeletal characteristics of nursery‐raised staghorn coral, Acropora cervicornis . Coral Reefs 36:679–684. [Google Scholar]
- LaJeunesse, T. C. , Parkinson J., Barielson P., Jeong H., Reimer J., Voolstra C. R., and Santos S.. 2018. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Current Biology 28:2570–2580. [DOI] [PubMed] [Google Scholar]
- Lenihan, H. , Adjeroud M., Kotchen M., Hench J., and Nakamura T.. 2008. How reef structure regulates small‐scale spatial variation in coral bleaching. Marine Ecology Progress Series 370:127–141. [Google Scholar]
- Lowe, R. , Falter J., Monismith S., and Atkinson M.. 2009. Wave‐driven circulation of a coastal reef–lagoon system. Journal of Physical Oceanography 39:873–893. [Google Scholar]
- Lowe, R. , Shavit U., Falter J., Koseff J., and Monismith S.. 2008. Modeling flow in coral communities with and without waves: a synthesis of porous media and canopy flow approaches. Limnology and Oceanography 53:2668–2680. [Google Scholar]
- Loya, Y. , Sakai K., Yamazato K., Nakano Y., Sambali H., and van Woesik R.. 2001. Coral bleaching: the winners and the losers. Ecology Letters 4:122–131. [Google Scholar]
- Manzello, D. , Matz M. V., Enochs I., Valentino L., Carlton R. G., Kolodzeij G., Serrano X., Towle E., and Jankulak M.. 2019. Role of host genetics and heat‐tolerant algal symbionts in sustaining populations of the endangered coral Orbicella faveolata in the Florida Keys with ocean warming. Global Change Biology 25:1016–1031. [DOI] [PubMed] [Google Scholar]
- Mass, T. , Genin A., Shavit U., Grinstein M., and Tchernov D.. 2010. Flow enhances photosynthesis in marine benthic autotrophs by increasing the efflux of oxygen from the organism to the water. Proceedings of the National Academy of Sciences USA 107:2527–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield, A. , Fan T., and Chen C.. 2013a. Physiological acclimation to elevated temperature in a reef‐building coral from an upwelling environment. Coral Reefs 32:909–921. [Google Scholar]
- Mayfield, A. B. , Chen M., Meng P., Lin H., Chen C., and Liu P.. 2013b. The physiological response of the reef coral Pocillopora damicornis to elevated temperature: results from coral reef mesocosm experiments in Southern Taiwan. Marine Environmental Research 86:1–11. [DOI] [PubMed] [Google Scholar]
- Mayfield, A. , Wang L., Tang P., Fan T., Hsiao Y., Tsai C., and Chen C.. 2011. Assessing the impacts of experimentally elevated temperature on the biological composition and molecular chaperone gene expression of a reef coral. PLoS ONE 6:e26529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard, J. , et al. 2015. Projections of climate conditions that increase coral disease susceptibility and pathogen abundance and virulence. Nature Climate Change 5:688–695. [Google Scholar]
- McLachlan, R. H. , Price J., Solomon S., and Grottoli A. G.. 2020. Thirty years of coral heat‐stress experiments: a review of methods. Coral Reefs 39:885–902. [Google Scholar]
- McLanahan, T. , Maina J., Moothien‐Pillay R., and Baker A. C.. 2005. Effects of geography, taxa, water flow, and temperature variation on coral bleaching intensity in Mauritius. Marine Ecology Progress Series 298:131–142. [Google Scholar]
- Meyer, E. , Davies S., Wang S., Willis B., Abrego D., Juenger T., and Matz M. V.. 2009. Genetic variation in responses to a settlement cue and elevated temperature in the reef‐building coral Acropora millepora . Marine Ecology Progress Series 392:81–92. [Google Scholar]
- Morikawa, M. , and Palumbi S.. 2019. Using naturally occurring climate resilient corals to construct bleaching‐resistant nurseries. Proceedings of the National Academy of Sciences USA 116:10586–10591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, E. , Bartels E., and Baums I. B.. 2018. Bleaching causes loss of disease resistance within the threatened coral species Acropora cervicornis . eLife 7:e35066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby, P. J. , Chisholm J. R. M., Edwards A. J., Andrefouet S., and Jaubert J.. 2001. Cloudy weather may have saved Society Island reef corals during the 1998 ENSO event. Marine Ecology Progress Series 222:209–216. [Google Scholar]
- Nakamura, T. , and van Woesik R.. 2001. Water‐flow rates and passive diffusion partially explain differential survival of corals during the 1998 bleaching event. Marine Ecology Progress Series 212:301–304. [Google Scholar]
- Nakamura, T. , van Woesik R., and Yamasaki H.. 2005. Photoinhibition of photosynthesis is reduced by water flow in the reef‐building coral Acropora digitifera . Marine Ecology Progress Series 301:109–118. [Google Scholar]
- Nakamura, T. , Yamasaki H., and van Woesik R.. 2003. Water flow facilitates recovery from bleaching in the coral Stylophora pistillata . Marine Ecology Progress Series 256:287–291. [Google Scholar]
- Oliver, T. , and Palumbi S.. 2011. Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30:429–440. [Google Scholar]
- Omori, M. , Fukami H., Kobinata H., and Hatta M.. 1999. Significant drop of fertilization of Acropora corals in 1999: An after‐effect of heavy coral bleaching? Limnology and Oceanography 46:704–706. [Google Scholar]
- Osinga, R. , Derksen‐Hooijberg M., Wijgerde T., and Verreth J.. 2017. Interactive effects of oxygen, carbon dioxide and flow on photosynthesis and respiration in the scleractinian coral Galaxea fascicularis . Journal of Experimental Biology 220:2236–2242. [DOI] [PubMed] [Google Scholar]
- Osman, E. , Smith D. J., Ziegler M., Kürten B., Conrad C., El Haddad K. M., Voolstra C. R., and Suggett D. J.. 2018. Thermal refugia against coral bleaching throughout the northern Red Sea. Global Change Biology 234:e474–e484. [DOI] [PubMed] [Google Scholar]
- Palumbi, S. , Barshis D., Traylor‐Knowles N., and Bay R.. 2014. Mechanisms of reef coral resistance to future climate change. Science 344:895–898. [DOI] [PubMed] [Google Scholar]
- Parkinson, J. , Bartels E., Devlin‐Durante M., Lustic C., Nedimyer K., Schopmeyer S., Lirman D., LaJeunesse T., and Baums I.. 2018. Extensive transcriptional variation poses a challenge to thermal stress biomarker development for endangered corals. Molecular Ecology 27:1103–1119. [DOI] [PubMed] [Google Scholar]
- Poland, D. , and Coffroth M. A.. 2019. Host growth and survivorship varies with endosymbiotic algal partner in developing cnidarians. Marine Ecology Progress Series 612:87–100. [Google Scholar]
- Quigley, K. , Willis B., and Bay L.. 2017. Heritability of the Symbiodinium community in vertically‐ and horizontally‐transmitting broadcast spawning corals. Scientific Reports 7:8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidenbach, M. , Koseff J., Monismith S., Steinbuck J., and Genin A.. 2006. The effects of waves and morphology on mass transfer within branched reef corals. Limnology and Oceanography 51:1134–1141. [Google Scholar]
- Riebesell, U. , Fabry V., Hansson L., and Gattuso J.. 2010. Guide to best practices for ocean acidification research and data reporting. Office of the European Union, Luxembourg, Luxembourg. [Google Scholar]
- Riginos, C. 2015. Clones in space—How sampling can bias genetic diversity estimates in corals: editorial comment on the feature article by Gorospe et al. Marine Biology 162:913–915. [Google Scholar]
- Rodrigues, L. J. , and Grottoli A. G.. 2007. Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnology and Oceanography 52:1874–1882. [Google Scholar]
- Rowan, R. , Knowlton N., Baker A., and Jara J.. 1997. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388:265–269. [DOI] [PubMed] [Google Scholar]
- Schmidt, G. , Wall M., Taylor M., Jantzen C., and Richter C.. 2016. Large‐amplitude internal waves sustain coral health during thermal stress. Coral Reefs 35:869–881. [Google Scholar]
- Suggett, D. J. , Dong L. F., Lawson T., Lawrenz E., Torres L., and Smith D. J.. 2013. Light availability determines susceptibility of reef building corals to ocean acidification. Coral Reefs 32:327–337. [Google Scholar]
- Traylor‐Knowles, N. 2016. Distinctive wound‐healing characteristics in the corals Pocillopora damicornis and Acropora hyacinthus found in two different temperature regimes. Marine Biology 163:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooidonk, R. , Maynard J., Manzello D., and Planes S.. 2014. Opposite latitudinal gradients in projected ocean acidification and bleaching impacts on coral reefs. Global Change Biology 20:103–112. [DOI] [PubMed] [Google Scholar]
- van Oppen, M. 2015. In vitro establishment of symbiosis in Acropora millpora planulae. Coral Reefs 20:200. [Google Scholar]
- van Oppen, M. J. H. , Bongaerts P., Frade P., Peplow L., Boyd S., Nim H., and Bay L.. 2018. Adaptation to reef habitats through selection on the coral animal and its associated microbiome. Molecular Ecology 27:2956–2971. [DOI] [PubMed] [Google Scholar]
- Vega Thurber, R. , Burkepile D., Fuchs C., Shantz A., McMinds R., and Zaneveld J.. 2014. Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Global Change Biology 20:544–554. [DOI] [PubMed] [Google Scholar]
- Veron, J. E. N. , Hoeg‐Guldberg O., Lenton T. M., Lough J. M., Obura D. O., Pearce‐Kelly P., Sheppard C. R. C., Spalding M., Stafford‐Smith M. G., and Rogers A. D.. 2009. The coral reef crisis: the critical importance of <350 ppm CO2 . Marine Pollution Bulletin 58:1428–1436. [DOI] [PubMed] [Google Scholar]
- Voolstra, C. R. , Buitrago‐Lopez C., Perna G., Carenas A., Hume B. C. C., Raedecker N., and Barshis D. J.. 2020. Standardized short‐term acute heat stress assays resolve historical differences in coral thermotolerance across microhabitat reef sites. Global Change Biology 26:4328–4343. [DOI] [PubMed] [Google Scholar]
- Voolstra, C. , Schnetzer J., Peshkin L., Randall C., Szmant A., and Medina M.. 2009. Effects of temperature on gene expression in embryos of the coral Montastraea faveolata . BCM Genomics 10:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner, M. , Barshis D., Davies S., Grottoli A. G., LaJeunesse T. C., and Van Woesik R.. 2016. Investigating coral bleaching in a changing climate: Our state of understanding and opportunities to push the field forward. June 17–18, 2016. Pages 26. Hawaii Prince Hotel, Honolulu, Hawaii, USA. [Google Scholar]
- Warner, M. E. , Fitt W. K., and Schmidt G. W.. 1999. Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proceedings of the National Academy of Sciences USA 96:8007–8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner, M. E. , and Suggett D. J.. 2016. The photobiology of Symbiodinium spp.: linking physiological diversity to the implications of stress and resilience. Pages 489–509 in Goffredo S., and Dubinsky Z., editors. The Cnidaria, past, present and future. Springer International Publishing, Cham, Switzerland. [Google Scholar]
- Wiedenmann, J. , D'Angelo C., Smith E. G., Hunt A. N., Legiret F.‐E., Postle A. D., and Achterberg E. P.. 2013. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nature Climate Change 3:160–164. [Google Scholar]
- Wright, R. , Mera H., Kenkel C., Nayfa M., Bay L., and Matz M.. 2019. Positive genetic associations among fitness traits support evolvability of a reef‐building coral under multiple stressors. Global Change Biology 25:3294–3304. [DOI] [PubMed] [Google Scholar]
- Ziegler, M. , Seneca F., Yum L., Palumbi S., and Voolstra C. R.. 2017. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nature Communications 8:14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


