Abstract
Activation of the venous muscle pumps by neuromuscular stimulation of the common peroneal nerve has been previously shown to increase venous and arterial flow in the legs of healthy subjects. The aim of this study is to determine whether a similar effect is observed in patients with chronic venous leg ulcers. 1 Hz intermittent electrostimulation of the common peroneal nerve was applied to 14 patients with ulcers between 1 and 10 cm in diameter, eliciting a small, painless, regular, muscular twitch of the leg. Flow was measured using Duplex ultrasound in the popliteal vein and the popliteal artery. Peak arterial velocity increased from 57 to 78 cm/s (P = .001) in sitting position, and from 79 to 98 cm/s in recumbent position (P = .001). Peak venous velocity increased from 10 to 33 cm/s (P = .001) sitting, and from 14 to 47 cm/s (P = .001) recumbent. Significant increases were observed in both venous and arterial blood flow in the lower limb. This suggestsed that activation of the venous muscle pump and improvement of arterial flow assisted oxygen delivery at the wound site. Moreover this may be a worthwhile intervention to assist in the healing of venous leg ulcers, and may provide a mechanistic explanation for the increased healing rates previously reported with neuromuscular stimulation of the common peroneal nerve.
Keywords: common peroneal nerve, duplex ultrasound, neuromuscular stimulation, venous ulcers
1. INTRODUCTION
Venous leg ulcers are principally distinguished by their aetiology. 1 Chronic venous insufficiency, as a result of occlusion or failure of the venous pump system leads to elevated ambulatory and resting venous pressures in the leg, with accompanying microvascular and macrovascular disturbances and oedema. 2 It is also well recognised that calf and foot muscle pump dysfunction contributes significantly to the development of chronic venous insufficiency and venous ulcers. 3 Treatment for venous ulcers is primarily based on interventions at the macrovascular level, most commonly compression, which aims to reduce oedema and aid venous return. 4 Local wound care, however, remains of secondary importance.
Activation of the venous muscle pumps of the leg by means of 1 Hz intermittent neuromuscular electrical stimulation (NMES) of the common peroneal nerve has been shown to increase not only venous flow, but also to augment arterial flow and skin microvascular flow in healthy individuals. 5 , 6 It has also been shown to augment venous flow in the femoral vein of patients with venous insufficiency. 7 One of the author (SD) was privileged to be the assessor for early stage assessment for PhD by Miss Katie William, who is well known for her original work on NMES device.
In addition to being one of the most common symptoms of venous disease, oedema 8 also plays a causative role in the pathophysiology 9 of chronic venous insufficiency, and many different therapies including NMES are prescribed to reduce oedema. 10 NMES has also been shown to reduce oedema in patients with lymphedema, 11 and in patients following total hip replacement surgery. 12 NMES has been used successfully to treat hard‐to‐heal leg ulcers 13 , 14 and has been shown to promote a significant increase in leg ulcer healing rate. 15
This is a single centre open label study measuring the effect of neuromuscular stimulation on lower limb arterial and venous blood flow in patients with venous leg ulcers. Innate baseline blood flow was compared with blood flow during 1 Hz neuromuscular electrostimulation of the common peroneal nerve.
2. AIMS
The aim of this study is to determine whether 1 Hz intermittent NMES of the common peroneal nerve increases venous and arterial blood flow in the lower limbs of patients with venous ulcers.
3. METHODS
The study was approved by the National and local ethic committee with the study reference number: 16/LO/1091. IRAS project number: 185818.
3.1. Study population
Fourteen outpatients with venous leg ulcers attending the wound clinic at Ealing Hospital participated in the study. Patients signed an informed consent form, and underwent an examination for vital signs, past medical history, and demographics, for screening according to the following criteria:
3.2. Inclusion criteria
Age ≥ 18 years
Intact healthy skin at the site of device application
Able to understand the Patient Information Sheet
Willing and able to give informed consent
Willing and able to follow the requirements of the protocol
Subjects who had a chronic venous leg ulcer (i.e. >6 weeks, CEAP classification of C6) greater than 1 cm2, greater than 2 cm minimum diameter, and less than 10 cm in maximum diameter
ABPI of ≥0.8
Patients treated with Class II below the knee compression
3.3. Exclusion criteria
Wound infection either acute or chronic
History of significant haematological disorders or DVT within the preceding 6 months
pregnant
Pacemakers or implantable defibrillators
Use of any other neuro‐modulation device
Current use of TENS in the pelvic region, back or legs
Use of investigational drug or device within the past 4 weeks that may interfere with this study
Recent surgery that may affect the study (such as abdominopelvic, or lower limb) in the opinion of the investigator.
Recent trauma to the lower limbs
Size of leg incompatible with the NMES device.
Obesity (BMI > 34)
Any medication deemed to be significant by the Investigator
Diabetes
Clinical evidence of peripheral arterial disease (i.e. signs or symptoms, in the opinion of the researcher)
The somewhat lengthy list of exclusion criteria served to reduce confounders and provide homogeneity to the patient group insofar as possible.
3.4. Study procedure
All the measurements were performed by an accredited vascular sonographer and the procedure was completed in all the 14 patients with meaningful data for analysis. Median patient age was 68 years, with an interquartile range of 61 to 79. The Median BMI was 27.9, with an interquartile range of 25.9 to 29.5. All patients were determined by Duplex ultrasound to have venous insufficiency.
The geko (T‐2 and R‐2) devices (Firstkind Ltd., Daresbury, UK) are small disposable, internally powered, NMES devices that are applied externally to the leg. The T‐2 device has a fixed 27 mA current and the R‐2 current is fixed at 54 mA. The R‐2 delivers a higher current to the patient and is intended for patients who do not achieve visible twitch stimulation with the T‐2 device. The devices are self‐adhesive and are applied to the outer/posterior aspect of the knee, as shown in Figure 1, such that the dotted reference line on the device is aligned with the fibular head. This positioning enables integral electrodes to apply a stimulus to the lateral popliteal nerve (often additionally termed the common peroneal) immediately proximal to its anterior/posterior branch. This nerve controls the contraction of several muscles in the lower leg: tibialis anterior, extensor hallucis longus, extensor digitorum longus, peroneous longus, brevis and tertius, extensor digitorum brevis, and extensor hallucis brevis. 16 Intermittent (1 Hz) stimulation of this nerve causes a reciprocating contraction of the muscles in the leg, activating the leg venous muscle pump. Device setting was adjusted to the minimum stimulus to achieve a visible twitch of the foot. The device is a commercially available and CE marked (GB12/87339; SGS, United Kingdom Ltd. notified body CE1639) device.
FIGURE 1.
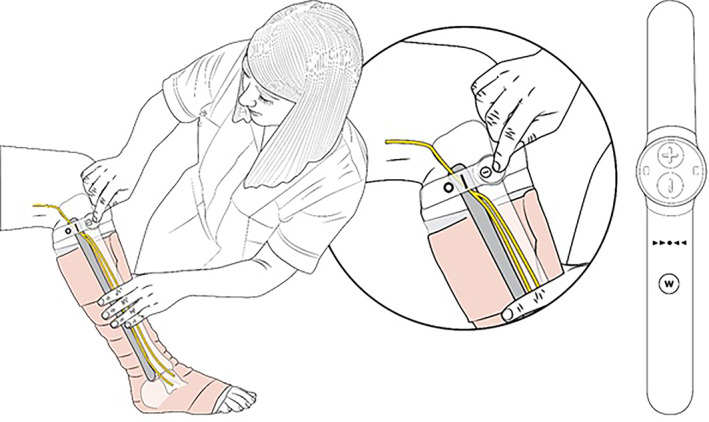
Fitting the NMES device to the leg in the presence of compression bandaging
3.5. Venous and arterial parameters
Venous and arterial flow parameters were measured using Imaging Doppler Ultrasound (GE Logic e Premium BT11 Ultrasound, GE Healthcare) with a linear probe (GE Probe ‐ 9 –RS, GE Healthcare). Measurements were made of Peak Velocity (PV), Volume Flow (VF), and Vessel Diameter (D). The transducer was placed on the popliteal vein distal to the bifurcation. Venous flow was verified to be phasic with respiration. The probe was placed with the sample gate at 45° within the vein, and sample volume matched the vein diameter. Three samples were taken, each with a 6 second duration. This process was then repeated for the popliteal artery. Measurements were taken in both the seated (with knee bent and leg dependant) and the recumbent supine position (with leg straight and horizontal). Measurements were not made in the standing position, because it was anticipated that patients would be unable to stand for the 30 minutes duration of the measurements without fatigue or movement artefact. Measurements were made according to the timeline in Figure 2.
FIGURE 2.
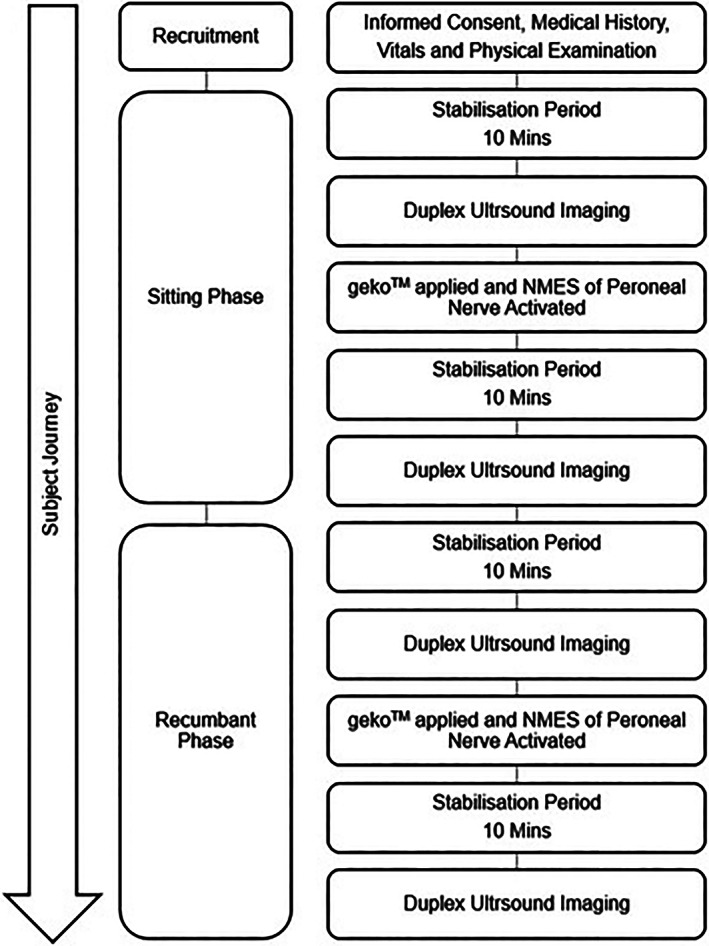
Timeline of measurements
Differences between intervention and baseline were tested using paired sample Student's t‐test.
4. RESULTS
4.1. Arterial blood flow
Figure 3 shows the peak arterial velocity (mean for all subjects +/− Standard error of the mean SEM). In both the seated position and the recumbent position, a very significant (P < .001) increase can be seen when the NMES device is switched on.
FIGURE 3.
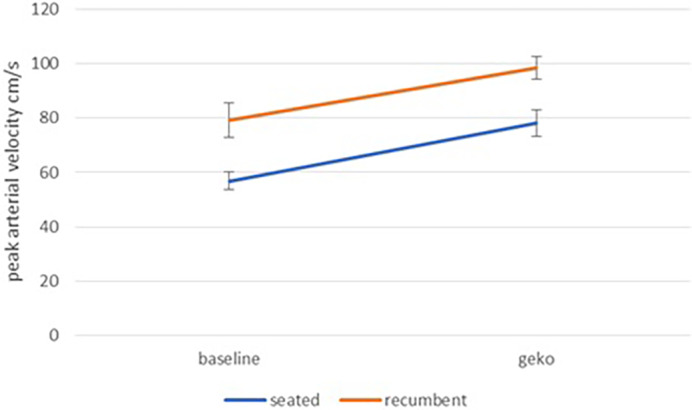
Peak Arterial Velocity at baseline and during NMES stimulation for seated and recumbent subjects
The arterial volume flow in the seated position exhibits the same pattern, with NMES showing a very significant (P < .001) increase over baseline (Figure 4). The arterial volume flow in the recumbent supine position exhibits substantial noise in both baseline and NMES data. Nevertheless, NMES showed a significant (P < .04) increase.
FIGURE 4.
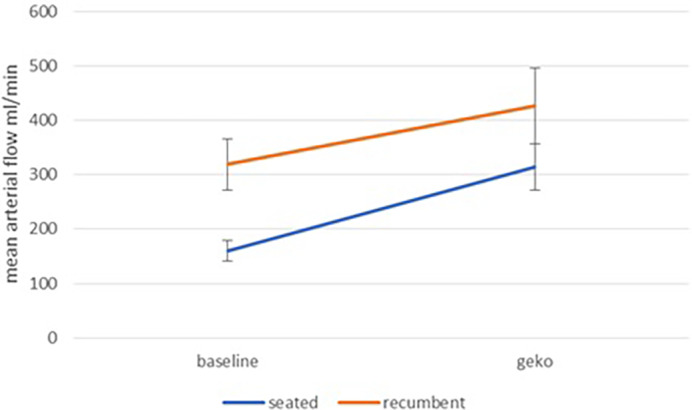
Mean Arterial volume flow at baseline and during NMES stimulation for seated and recumbent subjects
4.2. Venous blood flow
Peak venous velocity with NMES showed a highly significant (P < .001) increase over baseline, both in the seated position and in the recumbent position (Figure 5). Referring to Figure 6, in both the seated position and the recumbent position the mean value for venous flow is higher with NMES, but the high level of noise renders this difference statistically insignificant.
FIGURE 5.
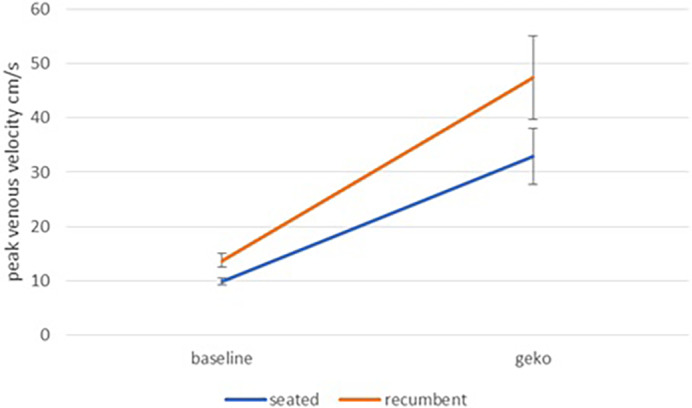
Peak venous velocity at baseline and during NMES stimulation for seated and recumbent subjects
FIGURE 6.
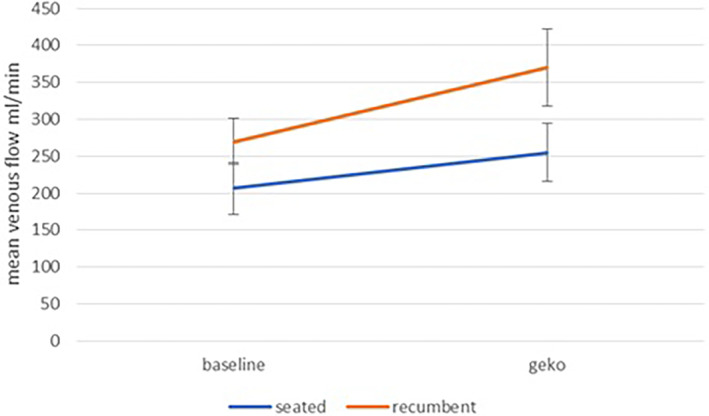
Mean venous volume flow at baseline and during NMES stimulation for seated and recumbent subjects
5. DISCUSSION
Several systematic reviews have concluded that electrical stimulation has a beneficial effect in healing chronic wounds 17 , 18 , 19 However, these reviews have included many different modalities of electrical stimulation. Points of variance included the positioning of electrodes (overlaid on the wound, across the wound, adjacent to the wound, or remote to the wound), current waveform (constant current, pulsed current, high frequency, low frequency), and stated mechanism of action. A systematic review comparing outcomes for different modalities found that pulsed electrical stimulation was significantly more effective than continuous current. 20
The mechanism of action for the geko device is distinct from the mechanism of the electrical stimulation devices reported in these reviews. Accessing the common peroneal nerve at a point just proximal to the deep/superficial branch, it stimulates both branches, eliciting a momentary twitch in a complex of muscles in the leg compartment. By delivering this stimulation intermittently (once per second) as a single pulse, a reciprocating action is achieved, which activates the venous muscle pump of the leg.
In this study, muscle pump activation in patients with venous leg ulcers produced substantial and highly significant augmentation of both arterial and venous velocity in the large vessels of the leg. This augmentation was unaffected by postural changes, being observed in both seated and recumbent positions. Additionally, arterial mean volume flow showed a significant increase. While the increase in mean venous volume flow was not statistically significant, this is likely attributable to the large amount of noise present in the signal for this measurement. Typically, patients with poorly functioning venous valves exhibit substantial retrograde flow in the intervals between activations of the muscle pump, and although substantially elevated forward flows are observed this introduces a wide scatter to the distribution of mean flow throughout the cycle.
Although this increase in blood flow as a result of intermittent NMES has been previously demonstrated in healthy subjects, patients with venous leg ulcers are known to have compromised function of the venous muscle pump, 21 thus playing an important role in the pathophysiology of venous disease. 22 Therefore, it is of considerable interest that this augmentative effect of NMES has been confirmed in this patient group.
Physical exercises aimed at activating the muscle pump 23 such as toe raises have been used with some success for healing venous leg ulcers, 24 and it has been shown that even minor movements of the foot are effective. 25 Furthermore, it has been found that poor muscle pump function can be improved by regular activation. 26 However, typically poor adherence to exercise regimens has been reported 27 , 28 with consequent reduction in efficacy. For exercise regimes to be effective, costly supervision is required. 28 , 29 , 30 The NMES device tested, which mimics the effect of exercise in activating the leg muscle pump, was informally observed to be well tolerated by patients with no complaints of discomfort, and quick and easy to apply. It has the advantage of being self‐contained and self‐adhesive, with no wires to obstruct ambulation. Good patient adherence has been reported 10 , 31 previously.
The substantial increases to macrovascular flow achieved by activating the muscle pumps in patients with incompetent valves and venous disease suggest that intermittent NMES may be a worthwhile intervention to assist in the healing of venous leg ulcers; this may explain the increased healing rates with NMES previously reported. 8 , 9 , 10
It is of interest that NMES demonstrated improvement in venous and arterial circulation in this group of patients with chronic venous disease and incompetent valves. Whether improvement in arterial circulation has any beneficial effect in the healing of venous ulcers remains to be established. This study is not a randomised controlled trial and does not follow patients up in the long term to establish the effect of NMES on healing or recurrence rates of venous leg ulcers. A randomised controlled trial to examine this would be of great value.
6. CONCLUSIONS
Activation of lower limb muscle pumps by 1 Hz intermittent neuromuscular stimulation of the common peroneal nerve provides substantial augmentation of venous and arterial flow in the lower limb of patients with venous leg ulcers. This has important implications in the management of patients with venous leg ulcers.
ACKNOWLEDGEMENTS
The authors acknowledge the assistance of Firstkind ltd, Daresbury for sponsoring the study.
Das SK, Dhoonmoon L, Chhabra S. Neuromuscular stimulation of the common peroneal nerve increases arterial and venous velocity in patients with venous leg ulcers. Int Wound J. 2021;18:187–193. 10.1111/iwj.13510
Funding information Firstkind ltd, Daresbury
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Collins L, Seraj S. Diagnosis and treatment of venous ulcers. Am Fam Physician. 2010;81(8):989‐996. [PubMed] [Google Scholar]
- 2. Grey JE, Harding KG, Enoch S. Venous and arterial leg ulcers. BMJ. 2006;332(7537):347‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Araki CT, Back TL, Padberg FT, et al. The significance of calf muscle pump function in venous ulceration. J Vasc Surg. 1994;20(6):872‐879. 10.1016/0741-5214(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 4. Ratliff CR, Yates S, McNichol L, Gray M. Compression for primary prevention, treatment, and prevention of recurrence of venous leg ulcers: an evidence‐and consensus‐based algorithm for care across the continuum. J Wound Ostomy Continence Nurs. 2016;43(4):347‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tucker A, Maass A, Bain D, et al. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. Int J Angiol. 2010;19(1):e31‐e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Warwick DJ, Shaikh A, Worsley P, et al. Microcirculation in the foot is augmented by neuromuscular stimulation via the common peroneal nerve in different lower limb postures: a potential treatment for leg ulcers. Int Angiol. 2015;34(2):158‐165. [PubMed] [Google Scholar]
- 7. Williams KJ. Chapter 7.1. NMES in the management of chronic venous disease (VeINS). Neuromuscular Stimulation of the Leg. Thesis submitted to Imperial College London for the degree of Doctor of Philosophy April 2017. 258–269.
- 8. Patel SK, Surowiec SM. Venous Insufficiency. [Updated 2020 Feb 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Jan‐.
- 9. Mansilha A, Sousa J. Pathophysiological mechanisms of chronic venous disease and implications for venoactive drug therapy. Int J Mol Sci. 2018;19(6):1669. 10.3390/ijms19061669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allaert FA. Meta‐analysis of the impact of the principal venoactive drugs agents on malleolar venous edema. Int Angiol. 2012;31:310‐315. [PubMed] [Google Scholar]
- 11. Choi YD, Lee JH. Edema and pain reduction using transcutaneous electrical nerve stimulation treatment. J Phys Ther Sci. 2016;28(11):3084‐3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wainwright TW, Burgess LC. Middleton RGA feasibility randomised controlled trial to evaluate the effectiveness of a novel neuromuscular electro‐stimulation device in preventing the formation of oedema following total hip replacement surgery. Heliyon. 2018;4(7):e00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones NJ, Ivins N, Ebdon V, Hagelstein S, Harding KG. Neuromuscular electrostimulation on lower limb wounds. Br J Nurs. 2018;27(20):S16‐S21. [DOI] [PubMed] [Google Scholar]
- 14. Harris C, Duong R, Vanderheyden G, et al. Evaluation of a muscle pump‐activating device for non‐healing venous leg ulcers. Int Wound J. 2017;14(6):1189‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris C, Ramage D, Boloorchi A, Vaughan L, Kuilder G, Rakas S. Using a muscle pump activator device to stimulate healing for non‐healing lower leg wounds in long‐term care residents. Int Wound J. 2019;16(1):266‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krishna G. Front, lateral, and medial sides of leg and dorsum of foot. In: Chaurasia BD, ed. Human Anatomy (Regional and Applied Dissection and Clinical) Volume 2 Chapter 8: Lower Limb, Abdomen, and Pelvis. 5th ed. England: CBS Publishers and Distributors Pvt Ltd; 2010:104‐106. [Google Scholar]
- 17. Barnes R, Shahin Y, Gohil R, Chetter I. Electrical stimulation vs. standard care for chronic ulcer healing: a systematic review and meta‐analysis of randomised controlled trials. Eur J Clin Invest. 2014;44(4):429‐440. 10.1111/eci.12244. [DOI] [PubMed] [Google Scholar]
- 18. Lala D, Spaulding SJ, Burke SM, Houghton PE. Electrical stimulation therapy for the treatment of pressure ulcers in individuals with spinal cord injury: a systematic review and meta‐analysis. Int Wound J. 2016;13(6):1214‐1226. 10.1111/iwj.12446 Epub 2015 Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arora M, Harvey LA, Glinsky JV, et al. Electrical stimulation for treating pressure ulcers. Cochrane Database of Systematic Reviews. 2020;1:CD012196. 10.1002/14651858.CD012196.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu L, Moody J, Quantitative GAA. Pooled analysis and systematic review of controlled trials on the impact of electrical stimulation settings and placement on pressure ulcer healing rates in persons with spinal cord injuries. Ostomy Wound Manage. 2016;62(7):16‐34. [PubMed] [Google Scholar]
- 21. Valencia IC, Falabella A, Kirsner RS. Eaglstein WH chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol. 2001;44(3):401‐421. [DOI] [PubMed] [Google Scholar]
- 22. Simka M. Calf muscle pump impairment and delayed healing of venous leg ulcers: air plethysmographic findings. J Dermatol. 2007;34(8):537‐544. [DOI] [PubMed] [Google Scholar]
- 23. Yang D, Vandongen YK, Stacey MC. Effect of exercise on calf muscle pump function in patients with chronic venous disease. Br J Surg. 1999;86(3):338‐341. [DOI] [PubMed] [Google Scholar]
- 24. Jull A, Slark J, Parsons J. Prescribed exercise with compression vs compression alone in treating patients with venous leg ulcers: a systematic review and meta‐analysis. JAMA Dermatol. 2018;154(11):1304‐1311. 10.1001/jamadermatol.2018.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergan JJ, Schmid‐Schönbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355(5):488‐498. [DOI] [PubMed] [Google Scholar]
- 26. Padberg FT Jr, Johnston MV, Sisto SA. Structured exercise improves calf muscle pump function in chronic venous insufficiency: a randomized trial. J Vasc Surg. 2004;39(1):79‐87. [DOI] [PubMed] [Google Scholar]
- 27. Roaldsen KS, Biguet G, Elfving B. Physical activity in patients with venous leg ulcer—between engagement and avoidance. A patient perspective. Clin Rehabil. 2011;25(3):275‐286. 10.1177/0269215510371424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams KJ, Ravikumar R, Gaweesh AS, et al. A review of the evidence to support neuromuscular electrical stimulation in the prevention and management of venous disease. Adv Exp Med Biol. 2016;906:377‐386. [DOI] [PubMed] [Google Scholar]
- 29. O'Brien J, Finlayson K, Kerr G, Edwards H. Evaluating the effectiveness of a self‐management exercise intervention on wound healing, functional ability and health‐related quality of life outcomes in adults with venous leg ulcers: a randomised controlled trial. Int Wound J. 2017;14(1):130‐137. 10.1111/iwj.12571 Epub 2016 Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klonizakis M, Tew GA, Gumber A, et al. Supervised exercise training as an adjunct therapy for venous leg ulcers: a randomized controlled feasibility trial. Br J Dermatol. 2018;178(5):1072‐1082. 10.1111/bjd.16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Williams J, Natarajan I, Moss C, et al. Effect of the introduction of a new pathway for prevention of venous thromboembolism (VTE) including neuromuscular electrical stimulation (NMES) on symptomatic VTE in immobile stroke patients. Stroke. 2019;50(Suppl. 1):WP367. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


