Abstract
Diabetic foot ulcers (DFUs) present a substantial clinical and economic burden to healthcare systems around the world, with significant reductions in quality of life for those affected. We aimed to analyse the clinical and economic burden of DFU via a 5‐year longitudinal multi‐ethnic cohort study. A longitudinal analysis of inpatient and outpatient DFUs data over 5 years from a university tertiary hospital in Singapore was performed. Data included baseline characteristics, clinical outcomes, hospitalisation, and outpatient details. Descriptive statistics, Kaplan–Meier survival analyses, and Cox proportional hazard models were performed. Patients treated for DFUs (n = 1729, mean patient age of 63·4 years) were assessed. The cohort consists of Chinese (61.4%), Malay (13.5%), and Indian (18.4%) patients. Common comorbidities included peripheral arterial disease (74.8%), peripheral neuropathy (14.5%), and a median haemoglobin A1c of 9.9%. Patients underwent toe(s) amputation (36.4%), transmetatarsal amputations (16.9%), or major amputations (6·5%). The mean length of inpatient stay for ulcer‐only, minor amputation, and major amputation was 13.3, 20.5, and 59.6 days, respectively. Mean cost per patient‐year was US $3368 (ulcer‐only), US $10468 (minor amputation), and US $30131 (major amputation). Minor amputation‐free survival was 80.9% at 1 year and 56.9% at 5 years, while major amputation‐free survival was 97.4% at 1 year and 91.0% at 5 years. In conclusion, within our multi‐ethnic cohort of patients from the tropics, there was significant clinical and economic burden of DFUs, with a high wound per patient ratio and escalating healthcare costs corresponding to more proximal amputation levels.
Keywords: diabetic foot, healthcare costs, inpatient, outpatient, tropics
1. INTRODUCTION
In 2014, there were 422 million people across the globe living with diabetes, with a prevalence of 8.5%. 1 Among adults with diabetes, the lifetime risk of developing a diabetic foot ulcer (DFU) is 15% to 25%. 2 DFUs present a substantial clinical and economic burden to health systems around the world, with significant reductions in quality of life for those affected 3 . In a 2017 systematic review and meta‐analysis, DFU prevalence around the world was estimated at 6.3%, with prevalence in Asia at 5.5%. 4 In 2016, an estimated 131 million people (1.8% of the global overall population) had diabetes related lower extremity complications (DRLEC), with overall age‐standardised rates increase of 15.9% between 1990 and 2016. 5 The largest increases from 1990 to 2016 were in Southern Sub‐Saharan Africa, South Asia, and Southeast Asia.
In 2017, within our healthcare cluster in Singapore (comprising two hospitals and six polyclinics, which service almost one third of the Singapore population), 6 the gross healthcare costs for all inpatient wound episodes stand at US $216 million within hospital care and US $596000 within primary care. 7 Majority of patients who suffer from neuro‐ischaemic ulcers (NIU) had diabetes (97.2%) and there was a 54% increase of NIU‐related admissions between 2013 (8/1000 inpatient episodes) and 2017 (12.3/1000 inpatient episodes), with 30.5% of patients requiring two or more NIU‐related admission episodes for the index wound in 2017. The direct healthcare cost per patient for hospital care (inpatient and specialist outpatient) and primary care in 2017 was US $16 920. 6 This evaluation provided a broad overview on the healthcare burden of DFU, with no granular analysis. Hence, building upon our previous study, we aimed to analyse the clinical and economic burden of DFUs via a 5‐year longitudinal multi‐ethnic cohort study.
2. MATERIALS AND METHODS
2.1. Study design, population, and setting
An observational analysis of inpatient and outpatient data from January 2013 to December 2017 was performed at a university tertiary hospital in Singapore with over 1700 acute inpatient beds and 9000 healthcare staff, 2700 outpatient visits, and 450 emergency department attendances daily. 6 Data of Clinical, administrative, and healthcare costs were retrieved from the Population Health Registry by the Health Services Outcomes Research Unit using International Classification of Diseases (ICD9 and ICD10) diagnosis codes, surgical procedure codes, and service codes (Appendix 1). These data were then matched with an institutional wound‐specific electronic medical record system (eWounds), which contained more than 500 000 wound‐related entries between 2013 and 2017. The direct healthcare costs were calculated from a patient's perspective (prior to government subsidies), which include physician fees, inpatient hospital stay, procedures, supportive dressings, and adjuvant therapy. Singapore's healthcare system adopts a mixed financing system, whereby healthcare subsidies are derived from nationalised life insurance schemes and deductions from the compulsory savings plan via the Central Provident Fund. 8
The study population consisted of patients with a diagnosis of DFU, who were treated at the hospital between January 2013 and December 2017. In general, a patient with DFU presented to the hospital either at Specialist Outpatient Clinic (SOC) or Emergency Department (ED) and received subsequent treatments in inpatient or outpatient care settings. Patients were included in the study if they had ≥1 SOC visit or ≥1 inpatient episode. Only SOC visits and inpatient episodes relating to DFU treatments were considered. These included DFU‐related admissions such as surgical debridement, minor or major amputations, antibiotic therapy, or revascularisation. DFU‐related SOC visits to podiatry, wound nurses, vascular surgery, orthopaedics surgery, or endocrinology were also included in the analysis. Patient's age, sex, race, wound anatomy, comorbidities, and clinical biochemical markers present at the date of index DFU diagnosis are reported.
2.2. DFU management protocol
The study site adopts a multi‐disciplinary team (MDT) approach in managing patients with DFU, with podiatry, vascular surgery, and endocrinology as core care team members. Support members included wound nursing, physiotherapy, occupational therapy, prosthesis and orthosis department, and plastics and reconstructive surgeons. At SOC, patients were reviewed by the MDT lower extremity amputation prevention program clinic. 9 During inpatient episodes, patients were managed under the local diabetic mellitus foot inpatient pathway. As per international working group for diabetic foot guidelines, patients with neuropathic ulcers received medical optimisation, wound care, and appropriate off‐loading; while patients with ischaemic ulcers received medical optimisation, revascularisation, wound care, and appropriate off‐loading. 10 When indicated, patients underwent surgical debridement, toe amputations, or trasmetatarsal amputations (TMA). In accordance with definitions within the literature, a lower extremity “minor amputation” was defined as amputations distal to the ankle (eg, toe amputations or TMA), while a lower extremity “major amputation” was defined as amputation proximal to the ankle (eg, below knee trans‐tibial amputation [BKA] or above knee trans‐femur amputation [AKA]).
2.3. Outcomes measurements
The incidence of hospitalisations (including length of stay), ED visits, SOC visits, and surgeries attributable to DFU, were filtered from the Population Health Registry based on clinical expert guidance and patient pathway. For instance, admissions for amputation or surgical debridement in the foot were considered relevant to DFU, whereas admissions for arteriovenous fistula creation or coronary artery bypass were considered not related. For SOC visits, only those visits to vascular surgery, orthopaedic surgery, and podiatry were included. The gross amount charged per visit and/or admission and its related procedures were retrieved from the Population Health Registry for cost calculations. Economic outcomes evaluated include total healthcare resource utilisation and costs during the 5‐year follow‐ up period for DFU patients, with resource utilisation and costs categorised by inpatient care, SOC visit, ED visits, and DFU‐related procedures. Clinical factors and outcomes evaluated include baseline patient characteristics and comorbidities, wound status, amputation rates (wound status, major, and minor), and survival rates (amputation‐free and all‐cause). Hospitalisation and outpatient details evaluated include procedures performed, length of stay, re‐admissions, and clinic visits.
2.4. Statistical analysis
All continuous data were expressed as mean ± standard deviation (SD) for normally distributed data and median + interquartile range (IQR) for data not normally distributed. Categorical data were expressed as percentages (%). No data imputations were conducted for reported clinical biochemical markers. The number and costs of resource utilisations were reported as the total for the study period and per patient‐year. All DFU‐related services were calculated as the count of discrete inpatient stay, ED visits, SOC visits, and procedures performed. Patient‐year was calculated as the number of days a patient added to the denominator (ie, days from index date until cessation of therapy, death, or end of study period) divided by 365. The unadjusted survival probabilities for all‐cause mortality and major amputation were estimated using the Kaplan–Meier method. In addition, the stepwise Cox‐proportional hazard model was performed to identify the risk factors for major amputation and all‐cause mortality. Risk factors investigated include age, sex, peripheral arterial disease (PAD), history of stroke, ischaemic heart disease (IHD), end‐stage renal failure (ESRF), and major amputation. A dummy coding of 0 and 1 was used to enter the nominal independent variables except for age. The proportional hazards (PH) assumption was met and was tested using statistical tests and graphical diagnostics based on the scaled Schoenfeld residuals. Variance inflation factor (VIF) analysis was also performed to check for multicollinearity, but none was observed. The significance level was predetermined at P < .05 for all tests. All statistical analyses were performed using Microsoft Excel 2016 (Microsoft, Redmond, Washington) and R software version 3.6.1 (R Foundation, Vienna, Austria).
2.5. Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the study report. Both first authors and the corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. This study had been approved by the institution ethics review board (National Healthcare Group Domain Specific Review Board 2019/00813).
3. RESULTS
3.1. Patient demographics
Between January 2013 and December 2017, there were a total of 1729 patients who underwent treatment for DFU (see Table 1). Mean age was 63·4 years (SD 12.59), with more than half aged between 55 and 74 years (56.5%). The average follow‐up time of patients during the 5‐year observation period was 2.9 years. Most of the patients were male (64.4%) and there was a greater proportion of patients of Indian ethnicity (18.4%), as compared to the Singapore general population (9.0% in 2018). 11 Almost three quarters of patients had underlying PAD (74.8%) and one sixth of patients (14.5%) had peripheral neuropathy. The study population generally had good lipid control with median cholesterol level at 4.8 mmol/L (IQR 2.1) but poor glycaemic control with median haemoglobin A1c (HbA1c) at 9.9% (IQR 14.8). Each patient had a mean of 6.1 documented wounds (total wound documentation n = 10 490), with majority of documented wounds occurring at the toes (39.2%) or foot plantar surface (34.4%). In this cohort, on average, one patient had 2.12 DFUs per year.
TABLE 1.
Patient characteristics (at date of index DFU diagnosis)
| All (n = 1729) | Ulcer only (n = 1108) | Minor amputation (n = 513) | Major amputation (n = 108) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | n | % | n | % | n | % | n | % | P value |
| Age, mean (SD), years | 63.4 (12.59) | 63.9 (12.97) | 62.0 (12.09) | 65.2 (10.09) | .004 a | ||||
| 18 to 34 | 22 | 1.3 | 14 | 1.3 | 8 | 1.6 | 0 | 0 | |
| 35 to 44 | 97 | 5.6 | 64 | 5.8 | 31 | 6.0 | 2 | 1.9 | |
| 45 to 54 | 318 | 18.4 | 192 | 17.3 | 108 | 21.1 | 18 | 16.7 | |
| 55 to 64 | 529 | 30.6 | 335 | 30.2 | 164 | 32.0 | 30 | 27.8 | |
| 65 to 74 | 448 | 25.9 | 275 | 24.8 | 134 | 26.1 | 39 | 36.1 | |
| 75+ | 315 | 18.2 | 228 | 20.6 | 68 | 13.3 | 19 | 17.6 | |
| Sex | <.001 b | ||||||||
| Male | 1113 | 64.4 | 675 | 60.6 | 366 | 63.6 | 72 | 70.1 | |
| Female | 616 | 35.6 | 433 | 39.4 | 147 | 36.4 | 36 | 29.9 | |
| Ethnicity | .626 b | ||||||||
| Chinese | 1061 | 61.4 | 670 | 60.9 | 317 | 61.6 | 74 | 69.0 | |
| Indian | 318 | 18.4 | 211 | 18.9 | 91 | 18.2 | 16 | 16.6 | |
| Malay | 234 | 13.5 | 147 | 13.2 | 75 | 14.5 | 12 | 11.0 | |
| Others | 116 | 6.7 | 80 | 7.0 | 30 | 5.7 | 6 | 3.5 | |
| Comorbidities | |||||||||
| Diabetic retinopathy | 764 | 44.0 | 469 | 42.5 | 248 | 48.3 | 47 | 39.0 | .076 b |
| Hypertension | 1534 | 88.7 | 963 | 86.7 | 468 | 91.9 | 103 | 95.2 | .003 b |
| Dyslipidaemia | 1618 | 93.6 | 1034 | 93.5 | 481 | 94.0 | 103 | 95.1 | .695 b |
| Ischaemic heart disease | 855 | 49.9 | 519 | 47.1 | 262 | 51.6 | 74 | 68.9 | <.001 b |
| History of stroke | 425 | 25.6 | 271 | 26.3 | 121 | 23.0 | 33 | 30.1 | .307 b |
| Chronic kidney disease | 1031 | 61.6 | 661 | 61.3 | 318 | 64.4 | 52 | 50.6 | .029 b |
| End‐stage renal failure | 408 | 23.2 | 233 | 20.9 | 127 | 24.3 | 48 | 40.5 | <.001 b |
| Peripheral arterial disease | 1292 | 74.8 | 703 | 62.8 | 482 | 95.1 | 107 | 99.2 | <.001 b |
| Peripheral neuropathy | 261 | 14.5 | 177 | 15.5 | 66 | 12.2 | 18 | 15.9 | .238 b |
| Biochemical markers | |||||||||
| Cholesterol (median, IQR), n = 1341 | 4.8 (2.1) | 4.9 (2.0) | 4.6 (2.0) | 4.8 (2.2) | .062 a | ||||
| Hba1c (median, IQR) n = 1542 | 9.9 (14.8) | 9.8 (4.3) | 10.1 (4.2) | 10.0 (3.7) | .127 a | ||||
| CRP (median, IQR), n = 1596 | 43.4 (378.1) | 31.5 (98.4) | 58.6 (136.0) | 70.7 (134.0) | <.001 a | ||||
| Documented wound anatomy (n = 10 490) | |||||||||
| Toes | 4109 | 39.2 | — | — | — | — | — | — | — |
| Foot plantar | 3613 | 34.4 | — | — | — | — | — | — | — |
| Foot dorsum | 1187 | 11.3 | — | — | — | — | — | — | — |
| Heel | 1008 | 9.6 | — | — | — | — | — | — | — |
| Ankle | 573 | 5.5 | — | — | — | — | — | — | — |
Kruskal‐Wallis test.
Chi‐square test.
Subgroup analysis of patient characteristics for patients with either ulcer only, minor amputations, or major amputations showed that patients who underwent major amputations were more likely to be older (mean age 65.2 years, P = .004), male (70.1%, P < .001), with comorbidities of hypertension (95.2%, P = .003), IHD (68.9%, P < .001), chronic kidney disease (50.6%, P = .029), ESRF (40.5%, P < .001), PAD (99.2%, P < .001), and with raised C‐reactive protein (CRP) levels (median 70.7 mg/L, P < .001).
3.2. Mean healthcare costs for DFU care
Within the study population, the mean healthcare cost for hospital (inpatient and outpatient) DFU care was US $6615437 (SG $9381748) per year, with respective mean cost per patient‐year for ulcer only, minor amputation, and major amputation at US $3368 (SG $4776), US $10468 (SG $14845), and US $30131 (SG $42730) (see Table 2). Mean ED visit was 1.0 per patient‐year, mean SOC visit at 8.1 per patient‐year, and mean inpatient admission at 0.58 per patient‐year, with 30‐day re‐admission rates at 12.7%. The mean inpatient length of stay (LOS) for ulcer only, minor amputation, and major amputation was 13.3, 20.5, and 59.6 days, respectively. More than one in three patients underwent toe(s) amputation (36.4%), more than one in six patients underwent TMA (16.9%) while more than one in 15 patients underwent major amputation (6.5%).
TABLE 2.
Costs and healthcare utilisation
| Components | Costs | |
| Total healthcare costs, US $ (SGD$) | ||
| Cumulative 2013 to 2017 | 33 077 183 (46908742) | |
| Mean cost per year | 6 615 437 (9381748) | |
| Costs, per patient year, US $ (SGD$) | ||
| Mean | 7152 (10142) | |
| Per ulcer | 3368 (4776) | |
| Per minor amputation | 10 468 (14845) | |
| Per major amputation | 30 131 (42730) | |
| Components | Episodes | Mean utilisation |
| Services | ||
| Emergency department | 4605 | 1.0 |
| Specialist outpatient clinic | 37 447 | 8.1 |
| Inpatient admission | 2679 | 0.58 |
| 30‐day re‐admission rate | 12.7% | — |
| Mean inpatient length of stay (days) | 16.6 | — |
| Per ulcer only | 13.3 | — |
| Per minor amputation | 20.5 | — |
| Per major amputation | 59.6 | — |
| Components | Episodes | |
| Interventions | ||
| Debridement | 1803 | |
| Revascularisation | 667 | |
| Minor amputation | ||
| Toe(s) amputations | 630 (36.4%) | |
| Transmetatarsal | 293 (16.9%) | |
| Major amputation | 113 (6.5%) |
3.3. Patient 1‐year and 5‐year survival outcomes
In terms of time to event (survival outcomes), major amputation‐free survival was 97.4% at 1 year and 91.0% at 5 years, while minor amputation‐free survival was 80.9% at 1 year and 56.9% at 5 years (see Table 3, P < .001). Overall survival was 93.8% at 1 year and 62.1% at 5 years. Subgroup survival analysis showed higher mortality signals among patients who underwent major amputations (5‐year survival at 32.9%) (see Figure 1, P < .001) were older (see Figure 2, P < .001) with PAD (5‐year survival at 58.9%), IHD (5‐year survival at 50.1%), previous stroke (5‐year survival at 52.3%), and ESRF (5‐year survival at 50.8%) (see Figure 3, P < .001).
TABLE 3.
Survival probabilities for major amputation, major amputation, and overall
| Probability of surviving, % (event‐major amputation) | Probability of surviving, % (event‐minor amputation) | Probability of surviving, % (event‐death) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | 1‐year | 3‐year | 5‐year | 1‐year | 3‐year | 5‐year | 1‐year | 3‐year | 5‐year |
| Overall | 97.4 | 94.7 | 91.0 | 80.9 | 67.9 | 56.9 | 93.8 | 79.5 | 62.1 |
| Gender | |||||||||
| Male | 97.5 | 94.9 | 90.8 | 79.3 | 65.0 | 52.7 | 94.2 | 80.0 | 62.6 |
| Female | 97.1 | 94.2 | 91.5 | 83.7 | 73.0 | 64.2 | 92.9 | 78.6 | 61.2 |
| Age groups | |||||||||
| 18 < 35 | 100 | 100 | 100 | 77.3 | 72.7 | 62.3 | 100 | 100 | 94.7 |
| 35 < 45 | 99.0 | 99.0 | 95.7 | 84.5 | 66.2 | 61.9 | 97.9 | 92.2 | 87.0 |
| 45 < 55 | 97.4 | 96.2 | 91.6 | 78.9 | 66.4 | 53.6 | 96.1 | 88.3 | 72.4 |
| 55 < 65 | 97.5 | 95.1 | 92.3 | 81.2 | 69.5 | 58.2 | 95.8 | 85.2 | 70.0 |
| 65 < 75 | 97.2 | 92.7 | 87.7 | 81.2 | 65.8 | 53.1 | 94.7 | 78.1 | 58.0 |
| ≥75 | 96.6 | 93.3 | 91.7 | 81.1 | 69.4 | 62.9 | 84.8 | 56.5 | 33.8 |
| Amputation | |||||||||
| Major | — | — | — | — | — | — | 88.8 | 61.7 | 32.9 |
| Minor | — | — | — | — | — | — | 94.7 | 83.0 | 62.8 |
| None | — | — | — | — | — | — | 93.8 | 79.5 | 64.7 |
| IHD | |||||||||
| Yes | 96.1 | 92.8 | 87.6 | 81.0 | 65.4 | 51.8 | 92.3 | 72.9 | 50.1 |
| No | 98.6 | 96.6 | 94.5 | 80.8 | 70.3 | 62.0 | 95.2 | 86.4 | 76.6 |
| PAD | |||||||||
| Yes | 96.5 | 92.9 | 88.5 | 75.6 | 59.2 | 47.0 | 93.2 | 76.9 | 58.9 |
| No | 99.5 | 99.5 | 99.5 | 96.8 | 94.3 | 89.5 | 95.5 | 87.7 | 74.1 |
| Stroke | |||||||||
| Yes | 96.6 | 92.8 | 90.2 | 81.5 | 67.5 | 55.0 | 91.9 | 71.8 | 52.3 |
| No | 97.6 | 95.3 | 91.3 | 80.7 | 68.0 | 57.4 | 94.4 | 82.1 | 65.8 |
| ESRF | |||||||||
| Yes | 94.4 | 91.0 | 83.5 | 83.0 | 67.1 | 49.3 | 92.0 | 74.6 | 50.8 |
| No | 98.3 | 95.9 | 93.6 | 80.3 | 68.2 | 59.7 | 94.3 | 81.1 | 66.6 |
FIGURE 1.
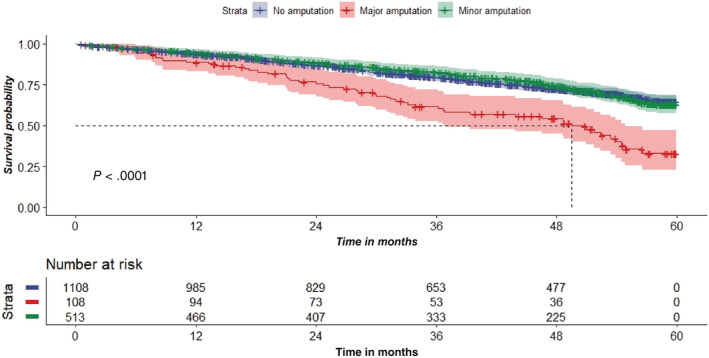
Survival analysis comparing patients with ulcers only, minor amputations, and major amputation
FIGURE 2.

Survival analysis by age groups
FIGURE 3.
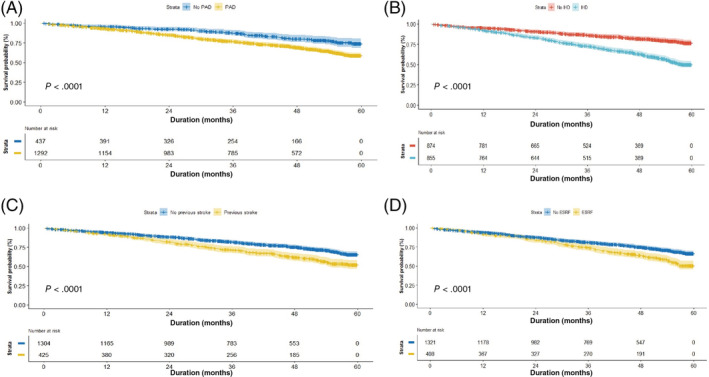
Survival analysis in patients with and without PAD (A), IHD (B), previous stroke (C), and ESRF (D)
3.4. Independent predictors for mortality and amputation
Independent predictors for all‐cause mortality were age (hazard ratio [HR] 1.1, P < .001), major amputation (HR 1.8, P < .001), PAD (HR 1.4, P = .015), IHD (HR 10.8, P < .001), previous stroke (HR 1.2, P = .041), and ESRF (HR 1.4, P = .002) (see Table 4).
TABLE 4.
Multivariate Cox proportional‐hazards model for all‐cause mortality, minor, and major amputation
| 95% CI | |||||
|---|---|---|---|---|---|
| Covariates | exp (coef) | Lower | Upper | z | P value |
| All‐cause mortality a | |||||
| Age (years) | 1.07 | 1.05 | 1.08 | 9.53 | <.001 |
| Major amputation | 1.80 | 1.35 | 2.40 | 4.03 | <.001 |
| PAD | 1.37 | 1.06 | 1.77 | 2.43 | .015 |
| IHD | 10.77 | 3.28 | 35.34 | 3.92 | <.001 |
| Previous stroke | 1.22 | 1.01 | 1.48 | 2.05 | .041 |
| ESRF | 1.37 | 1.12 | 1.68 | 3.04 | .002 |
| Age*IHD | 0.97 | 0.96 | 0.99 | −2.99 | .003 |
| Major amputation b | |||||
| Age (years) | 1.04 | 1.01 | 1.07 | 3.04 | .002 |
| IHD | 12.27 | 1.21 | 124.19 | 2.12 | .033 |
| PAD | 27.95 | 3.98 | 200.64 | 3.31 | <.001 |
| ESRF | 1.92 | 1.27 | 2.90 | 3.11 | .002 |
| Age*IHD | 0.97 | 0.94 | 1.00 | −1.85 | .064 |
| Minor amputation c | |||||
| PAD | 7.39 | 5.15 | 10.61 | 10.84 | <.001 |
| Male | 1.35 | 1.13 | 1.60 | 3.34 | <.001 |
Abbreviations: ESRF, end‐stage renal failure; IHD, ischaemic heart disease; PAD, peripheral arterial disease.
Likelihood ratio test = 279.3, P = <.001; Wald test = 228.6, P = <.001.
Likelihood ratio test = 80.6, P < .001; Wald test = 36.7, P < .001.
Likelihood ratio test = 232.9, P = <.001; Wald test = 130.1, P = <.001.
With regard to major amputations, overall rate was 6.5% with independent predictors being age (HR 1.0, P = .002), PAD (HR 27.9, P < .001), IHD (HR 12.3, P = .033), and ESRF (HR 1.9, P = .002) (see Table 4). With regard to minor amputations (including toes or TMA), overall rate was 53.3% with independent predictors being male (HR 1.3, P < .001) and PAD (HR 7.4, P < .001) (see Table 4).
4. DISCUSSION
DFUs present a substantial burden to global health systems and patients. In 2017, within our healthcare cluster, the direct healthcare cost per patient for hospital care (inpatient and specialist outpatient) and primary care in 2017 was US $16 920 with 30.5% of patients requiring two or more NIU‐related admission episodes. 6 This initial evaluation provided a broad overview on the burden of DFUs; however, a DFU‐focused analysis of patient data was needed. We present clinical and economic analysis of the largest longitudinal cohort of patients in the tropics with DFUs to date with Faglia et al's Italian series of 993 patients as the next largest single‐centred series. 12
Similar to the global prevalence of DFU, we report a larger proportion of male patients with DFU. 4 This cohort subset included a significantly higher percentage of patients who underwent major amputations. Across the world, a higher proportion of male patients with DFU had been consistently reported from both developed countries 13 and developing countries, 14 , 15 with a possible hypothesis in the gender difference due to increased physical work in males. 16 The patient population analysed showed a high percentage of patients with Indian ethnicity. This is consistent with a 2008 study examining the epidemiology of diabetic foot problems in another Singapore hospital. 17 Nather et al reported a significantly increased incidence of diabetic foot problems in patients of Indian decent. This is likely secondary to the higher prevalence of diabetes among Indians within the local Singapore population. 18
Patients within our study population had poor glycaemic control with median HbA1c at 9.9% (IQR 14.8) with a significant proportion suffering from macro (IHD, stroke, ESRF, PAD) and micro (diabetic retinopathy, peripheral neuropathy) vascular complications. In a meta‐analysis of six studies with 109 933 patients, Zhou et al found that the odds ratio for lower extremity amputations incidence was 1.229 (95% CI: 1.169–1.292) for every 1% HbA1c increase. 19 Specifically, it has been reported that for each 1·0% point increase in HbA1c, the daily wound‐area healing rate decreased by 0.028 cm2/day (95% CI: 0.003, 0.0054, P = .027). 20 As result of poor glycaemic control, it was not surprising that each patient within our study population had a mean of 6.1 documented wounds within the 5‐year study period. A majority of DFUs also occurred on the toes or plantar region. In a review of 19 compatible studies on incidence rates for DFU recurrence, Armstrong et al estimated that 40% of patients have DFU recurrence within 1 year after ulcer healing, almost 60% within 3 years and 65% within 5 years. 21 Within the multi‐centre prospective Eurodiale study, significant independent predictors for recurrence were plantar ulcer location; presence of osteomyelitis; HbA1c > 7.5%, and CRP > 5 mg/L. 13 Hence, a holistic approach of home monitoring of foot temperature, pressure‐relieving therapeutic footwear, and certain surgical interventions may be effective in preventing up to 60% to 75% of DFU recurrence. 21
In terms major amputations, overall rates within our study cohort was 6·5%, with independent risk factors being age, PAD, IHD, and ESRF. These risk factors are similar to those reported within the literature. Within United States, up to 20% of moderately or severely infected DFU eventually lead to some level of amputation. 22 It is known that the presence of PAD independently increases the risk of non‐healing ulcers, infection, and amputation. Similarly within Asia, data from the Japanese OLIVE registry revealed that age, body mass index, ESRF, and Rutherford grade 6 classification were identified as predictors of major amputation or death. 23 Within patients of Chinese ethnicity, overall amputation rate among DFU was 21·5%, with stepwise logistic regression analysis revealing PAD (as one of four significant risk factors. 24 Traditionally, it is well known that ESRF patients have a higher risk of limb loss after revascularisation and a poorer survival. 25 Significantly, mortality after DFU‐related amputation exceeds 70% at 5 years, with mortality rates even higher at 74% at 2 years for patients with ESRF. 26 However, it is still unclear if such high mortality rates are due to a combination of premorbid conditions (including amputation perioperative risks), lack of activity, and/or deconditioning.
With regard to mortality, data within the literature states that 5‐year mortality rates were estimated at 45%, 18%, and 55% for neuropathic, neuroischaemic, and ischaemic ulcers, respectively. 26 Similarly, for patients post minor and major amputations, 5‐year mortality was estimated at 46% and 57%, respectively, which is comparable to 5‐year pooled mortality rates for all reported cancer at 31%. 22 Within our study population, overall 5‐year mortality was 37.9%, with subgroup analysis showing higher mortality signals among patients who underwent major amputations, were older, with PAD, IHD, previous stroke, and ESRF. It is known that patients with DFU were older, had a longer diabetic duration, and had more hypertension, diabetic retinopathy, and smoking history than patients without DFU. 4 Earlier data from our institution also revealed that patients with neuroischaemic ulcers also had multiple comorbidities and were the frailest group of patients. 7 Globally, between 1990 and 2016, age‐standardised years lived with disability rates of all DRLECs increased by 14·6% to 31·0% from 1990 estimates. DRLECs are a large and growing contributor to the disability burden worldwide and disproportionately affect males and middle‐ to older‐aged populations. 5 Thankfully, contemporary data on 5‐year follow‐up on patients with DFU from 2009 to 2010 in France showed a lower than expected mortality rates, suggesting that with increasing awareness, international guidelines, and multi‐disciplinary team approach, progress has been and can be made in the management of patients with DFU. 27
In Singapore, the cost of diabetes mellitus was estimated at US $700 million (SG $1 billion) in 2010, with the figure projected to rise to US $1.75 billion (SG $2.5 billion) by 2050. 28 The cost per working‐age person with diabetes in Singapore is also projected to increase from US $5400 (SG $7678) in 2010 to US $7423 (SG $10596) in 2050. Within our current study, we found that the mean healthcare cost for hospital (inpatient and outpatient) DFU care was US $6615437 (SG $9381748) per year, with respective mean cost per patient‐year for ulcer only, minor amputation, and major amputation at US $3368 (SG $4776), US $10468 (SG $14845) and US $30131 (SG $42730). These data are similar to that from the United States. In 1998, the total direct cost for healing of infected DFUs not requiring amputation was estimated at US $17 500 per patient while the cost for lower‐extremity amputations, depending on the level of amputation, was between US $30 000 and $33 500 per patient. 29 In a patient with DFU, the potential economic benefits of lower extremity amputation prevention strategies are estimated between US $2900 and $4442 per patient over 3 years. Similarly, within our study population, the difference in the mean cost per patient‐year between ulcer only and minor amputation was US $7100 while the difference between minor and major amputations was US $19663. When comparing against other diseases, direct cost of care for patients with DRLEC are comparable with cancer. In 2017, direct cost of care for diabetes in general was estimated at US $237 billion, with up to one‐third attributed to the lower extremity, while direct costs of care for cancer in 2015 was estimated at US $80 billion. 22 Hence, active measures to prevent DFU will help to decrease the economic burden of diabetic foot disease. Evidence and guideline‐based management of DFU improves survival, reduces DRLEC, and is cost‐effective when compared with standard care. 30
Limitations of our study include its registry‐based retrospective design from a single healthcare cluster, with associated selection and information biases. Local healthcare structure meant that DFU‐related admissions at hospitals were well coded, but DFU‐related presentations at primary care was lacking. Hence, contrary to studies from other global data, 3 , 4 , 5 our study population was identified from tertiary care, instead of primary care. In addition, there are multiple and often overlapping diagnosis codes for diabetic foot ulcers, related aetiology (such as peripheral arterial disease, critical limb ischaemia, and neuropathy), and complications (such as gangrene, foot abscess, and osteomyelitis). Coupled with the fact that patients often have comorbidities with active issues (such as ESRF, diabetes, and IHD), this may result in underreporting on the true clinical burden of DFU or result in misclassification bias in terms of diagnosis codes, surgical procedure codes, or service codes.
5. CONCLUSION
Similar to global data, there is a high clinical and economic burden of DFU within Southeast Asia and the tropics. Within our study cohort, patients have poor DM control, resulting in high wounds per patient ratio with escalating healthcare costs corresponding to more proximal amputation levels. Patients have a high re‐admission rate and required multiple SOC visits. Primary prevention via DM control should be a focus for population health interventions. Patients with PAD are at a significantly higher risk for mortality, major, and minor amputations and should be the subset of patients for early and aggressive limb salvage interventions.
CONFLICT OF INTEREST
All authors contributed to and approved the final manuscript. The authors declare no conflict of interest and all authors contributed sufficiently to be credited at co‐authors.
AUTHOR CONTRIBUTIONS
All authors contributed to and approved the final manuscript. Zhiwen Joseph Lo and Naren Kumar Surendra: data analysis and writing the manuscript. Zhiwen Joseph Lo: guarantor. Akshar Saxena and Josip Car: data analysis, reviewing, and editing the manuscript.
ACKNOWLEDGEMENTS
The authors thank Edin Nuhiji, Christine Bongards and the 3M+KCI team for aiding with manuscript formatting.
Skin Research Institute of Singapore, Agency for Science, Technology and Research (A*STAR) under its Industry Alignment Fund—Pre‐Positioning Programme (IAF‐PP) grant number H18/01/a0/MM9 and H18/01/a0/ZZ9 as part of Wound Care Innovation for the Tropics (WCIT) Programme and a collaborative research agreement with KCI Asia Medical Pte Ltd.
Appendix A.
TABLE A1.
International Classification of Diseases (ICD9 and ICD10) diagnosis codes, surgical procedure codes, and service codes utilised for data retrieval
| Categories | ICD9 | ICD10 | Surgical codes | Service codes |
|---|---|---|---|---|
| Inpatient discharge primary diagnosis and secondary diagnosis |
2507′ 25 070′ 25 071′ 25 072′ 25 073′ 4402′ 44 020′ 44 021′ 44 023′ 44 024′ 44 029′ 4403′ 44 030′ 44 031′ 44 032′ 443′ 4438′ 44 389′ 4439′ 4442′ 7854′ |
E09.0 E09.01 E09.02 E09.5 E09.51 E09.52 E10.51 E10.52 E10.73 E11.51 E11.52 E11.73 E13.51 E13.52 E13.73 E14.51 E14.52 E14.73 I70.2 I70.20 I70.21 I70.23 I70.24 I73 I73.8 I73.9 I74.3 I74.4 I79.2 |
||
| Tertiary outpatient wound nurse care |
WC001 WC006 |
|||
| Primary care wound visits |
NBF017 NHF018 NBF026 NBF030 |
|||
| Angioplasty |
SD720A SD721A SD722A XRM019 |
|||
| Surgical bypass |
SD713A SD714A SD810V SD814V SD807A |
|||
| Minor amputations |
SB708T SB830T SB707T SB829T |
|||
| Major amputations | SB809L | |||
| ABPI arterial duplex |
VS0020 VS0005 VS0006 |
Abbreviation: ABPI, arterial‐brachial pressure index.
Lo ZJ, Surendra NK, Saxena A, Car J. Clinical and economic burden of diabetic foot ulcers: A 5‐year longitudinal multi‐ethnic cohort study from the tropics. Int Wound J. 2021;18:375–386. 10.1111/iwj.13540
Zhiwen Joseph Lo and Naren Kumar Surendra are both declared as first authors.
Funding information KCI Asia Medical Pte Ltd; Skin Research Institute of Singapore, Agency for Science, Technology and Research, Grant/Award Numbers: H18/01/a0/MM9, H18/01/a0/ZZ9
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analysed during this study are available from the corresponding author on reasonable request.
REFERENCES
- 1. World Health Organization World Health Organization Fact Sheet on Diabetes. 2019. https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed June 1, 2020.
- 2. Amin N, Doupis J. Diabetic foot disease: from the evaluation of the “foot at risk” to the novel diabetic ulcer treatment modalities. World J Diabetes. 2016;7:153‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boulton AJ, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719‐1724. [DOI] [PubMed] [Google Scholar]
- 4. Zhang P, Lu J, Jing Y, Tang S, Zhu BY. Global epidemiology of diabetic foot ulceration: a systematic review and meta‐analysis. Ann Med. 2017;49(2):106‐116. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y, Lazzarini PA, McPhail SM, van Netten JJ, Armstrong DG, Pacella RE. Global disability burdens of diabetes‐related lower‐extremity complications in 1990 and 2016. Diabetes Care. 2020;43:964‐974. [DOI] [PubMed] [Google Scholar]
- 6. National Healthcare Group Corporate Yearbook FY2018 . 2019. https://corp.nhg.com.sg/Lists/Corporate%20Year%20Book/Attachments/67/NHG%20Yearbook%202019%20Lowres%20Single1.pdf.
- 7. Lo ZJ, Lim X, Eng D, et al. Clinical and economic burden of wound care in the tropics: a 5‐year institutional population health review. Int Wound J. 2020;17:790‐803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ministry of Health Singapore . Singapore's Healthcare System. 2020. https://moh.gov.sg/home/our-healthcare-system.
- 9. Loke Y, Ng I, ZJ Lo QH, Tan GWL, Chandrasekar S. Implementation of a multidisciplinary team approach in lower extremity amputation prevention program for diabetic foot ulcer referral from primary healthcare to a tertiary center vascular surgery clinic: initial experience in an Asian population. J Vasc Surg. 2018;68(5S): LEA11. e117. [Google Scholar]
- 10. International Working Group on Diabetic Foot (IWGDF) 2019 guidelines. 2020. https://www.iwgdfguidelines.org
- 11. Singapore Government . Singapore residents by age group, Ethnic Group and Gender https://data.gov.sg/dataset/resident-population-by-ethnicity-gender-and-age-group.
- 12. Faglia E, Dalla Paola L, Clerici G, et al. Peripheral angioplasty as the first‐choice revascularization procedure in diabetic patients with critical limb ischemia: prospective study of 993 consecutive patients hospitalized and followed between 1999 and 2003. Eur J Vasc Endovasc Surg. 2005;29:620e7. [DOI] [PubMed] [Google Scholar]
- 13. Dubsky M, Jirkovska A, Bem R, et al. Risk factors for recurrence of diabetic foot ulcers: prospective follow‐up analysis in the Eurodiale subgroup. Int Wound J. 2012;10(5):555‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parisi MCR, Moura Neto A, Menezes FH, et al. Baseline characteristics and risk factors for ulcer, amputation and severe neuropathy in diabetic foot at risk: the BRAZUPA study. Diabetol Metab Syndr. 2016;8(25):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Banik PC, Barua L, Moniruzzaman M, Mondal R, Zaman F, Ali L. Risk of diabetic foot ulcer and its associated factors amoong Bangladeshi subjects: a multicentric cross‐sectional study. BMJ Open. 2020;10:e034058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siddiqui MA, Khan MF, Carline TE. Gender differences in living with diabetes mellitus. Mater Sociomed. 2013;25(2):140‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nather A, Chionk SB, Chan YH, et al. Epidemiology of diabetic foot problems in predictive factors for limb loss. J Diabetes Complications. 2008;22:77‐82. [DOI] [PubMed] [Google Scholar]
- 18. Ministry of Health Singapore . Prevalence of Diabetes in Singapore. https://data.gov.sg/dataset/prevalence-of-hypertension-diabetes-high-total-cholesterol-obesity-and-daily-smoking.
- 19. Zhou ZY, Liu YK, Chen HL, Yang HL, Fan L. HbA1c and lower extermity amputation risk in patients with with diabetes: a meta‐analysis. Int J Lower Extremity Wounds. 2015;14(2):168‐177. [DOI] [PubMed] [Google Scholar]
- 20. Christman AL, Selvin E, Margolis DJ, Lazarus GS, Garza LA. Hemoglobin A1c is a predictor of healing rate in diabetic wounds. J Invest Dermatol. 2011;131(10):2121‐2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367‐2375. [DOI] [PubMed] [Google Scholar]
- 22. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13(16):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iida O, Nakamura M, Yamauchi Y, et al. 3‐year outcomes of the OLIVE registry, a prospective multicenter study of patients with critical limb ischemia. JACC Cadiovasc Interv. 2015;8:1493e502. [DOI] [PubMed] [Google Scholar]
- 24. Li X, Xiao T, Wang Y, et al. Incidence, risk factors for amputation among patients with diabetic foot ulcer in a Chinese tertiary hospital. Diabetes Res Clin Pract. 2011;93:26‐30. [DOI] [PubMed] [Google Scholar]
- 25. Lavery LA, Hunt NA, Ndip A, Lavery DC, Van Houtum W, Boulton AJ. Impact of chronic kidney disease on survival after amputation in individuals with diabetes. Diabetes Care. 2010;33:2365‐2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new‐onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003;26(2):491‐494. [DOI] [PubMed] [Google Scholar]
- 27. Amadou C, Carlier A, Amouyal C, et al. Five‐year mortality in patients with diabetic foot ulcer during 2009–2010 was lower than expected. Diabetes Metab. 2019;46(3:230‐235. [DOI] [PubMed] [Google Scholar]
- 28. Png ME, Yoong J, Pham TP, Wee HL. Current and future economic burden of diabetes among working‐age adults in Asia:conservative estimates for Singapore from 2010‐2050. BMC Public Health. 2016;16:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ragnarson Tennvall G, Apelqvist J. Health‐economic consequences of diabetic foot lesions. Clin Infect Dis. 2004;39(2):S132‐S139. [DOI] [PubMed] [Google Scholar]
- 30. Ortegon MM, Redelop WK, Niessen LW. Cost‐effectiveness of prevention and treatment of the diabetic foot. Diabetes Care. 2004;27:901‐907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during this study are available from the corresponding author on reasonable request.


