Abstract
To evaluate the efficacy and safety of onion extract (OE) gel on scar management, a systematic review was performed by searching Embase, PubMed, Medline, and the Cochrane Library databases, and a meta‐analysis was conducted according to Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement guidelines. Finally, 13 randomised controlled trails were enrolled for meta‐analysis. OE gel increased the total improvement scores assessed by investigators (P < .00001) and patients (P < .00001) than no treatment, but no differences were detected between OE gel and other commonly used topical treatments assessed by investigators (P = .56) and patients (P = .39). Moreover, OE in silicone gel increased the total improvement scores assessed by investigators (P < .00001) and patients (P = .0007) than other treatments. OE gel increased the incidence of total adverse effects compared with no treatment (P < .0001) and other treatments (P = .008) by a fixed‐effects model, and increased the incidence of dropping out caused by intolerance of treatments (P = .0002). OE gel not only has no superiority to commonly used topical treatments, but also has the potential to increase the incidence of adverse effects on scar management; OE in silicone gel might be the optimal topical choice for scar treatment; however, more evidences are needed to strength these conclusions.
Keywords: gel, meta‐analysis, onion extract, scar
1. INTRODUCTION
Cutaneous scars are caused by an excessive deposition of collagen during wound healing that result from abnormal response to thermal, postsurgical, and traumatic injuries. 1 Although full maturation of a scar may take up to 2 years, typically, the new scars mature and become lighter and narrower in a few weeks. 2 In some cases, the scars become hypertrophic or result in keloids. The scars can cause pain, itching, discomfort, contracture, and a lessening of self‐esteem. 2 , 3 Thus, promoting wound healing without noticeable scarring and improving scars are important aspects of cosmesis.
Owing to the reported effects of anti‐inflammatory, antimicrobial, antiproliferative, and regenerative activities of onion extract (OE), 4 its gel modality has been commercially available for treating, preventing, and reducing dermatologic scars and keloids in clinic many years. 5 Several clinical trials found this gel was well tolerated and helpful for preventing pathological scarring and improving preexisting scars. 5 , 6 , 7 , 8 , 9 Thus, it was recommended for clinical scar management by the International Advisory Panel on Scar Management in 2014. 10 However, large‐sized randomised controlled trials (RCTs) and the evidence‐based data are still lacking. The purpose of this meta‐analysis was to evaluate the efficacy and safety of OE gel on scar management and to provide reliable evidence for clinical application.
2. METHODS
This meta‐analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement guidelines. 11 All analyses are based on previous articles; therefore, no ethical approvals are required. This protocol was registered with the International Platform of Registered Systematic Review and Meta‐Analysis Protocols (INPLASY202080103).
2.1. Search strategy
Two independent authors searched Embase, PubMed, Medline, and the Cochrane Library from their inception to August 2020 by the following keywords in combination with Boolean operators AND: “Allium cepa OR onion OR onions OR onion extract”, “scar OR scars OR cicatrix OR cicatrice OR cicatrices OR keloid OR keloids OR cicatricle”. No limitations were imposed on language. Search strategy can be found in Appendix A. The bibliographies of retrieved trials and other relevant publications were cross‐referenced to identify additional articles. The search results are shown in Figure 1.
FIGURE 1.
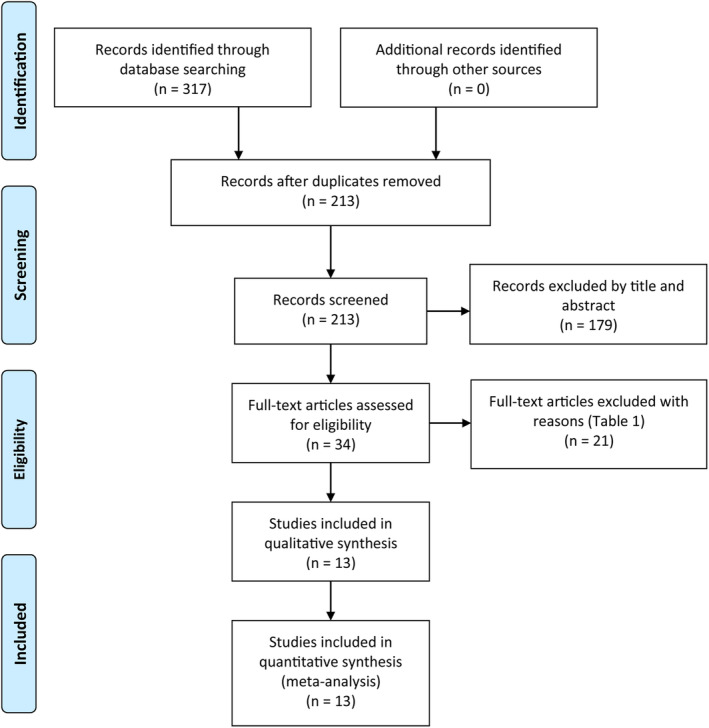
The search results and selection procedure
2.2. Inclusion criteria
Two authors independently evaluated the trials according to the PICOS (patients, intervention, comparator, outcome, study design) criteria. (a) Patients: patients with wounds or scars. (b) Intervention: topical gel with OE. (c) Comparator: no topical treatment, placebo gel, or other commonly used topical therapy for scar management. (d) Reported at least one of the following outcomes: quantitative evaluation of the results by one scale, such as the visual analog scale, vancouver scar scale, and Manchester scar scale, the width and/or volume of the scars, patient satisfaction, and adverse events. (e) Study design: RCT. In cases of disagreement, a consensus was reached through discussion with the third author.
2.3. Data extraction
Two reviewers independently extracted the data by a standard extraction form. These data included author, publication date, country, study design, sample size, age, gender, type and location of scars or wounds, details of intervention and comparator, methods and results of outcome evaluation, adverse events, and the conclusions. The primary outcomes were the total improvement scores by a recognised scale evaluated by investigators and patients. Additional outcomes were the width or volume of the scars, patient satisfaction, and adverse events. We also contacted the corresponding authors of the included studies to obtain the primary data, but no answer was received. In this case, the improvement score was calculated by the baseline score or the highest score of each item minus the endpoint score when it was not reported in the article directly.
2.4. Assessment of methodological quality
Two authors independently assessed the quality of included RCTs based on the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions. A “risk of bias” table that included the following contents was created: details on methods of random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other bias. The overall quality of each study was evaluated as “low risk of bias”, “high risk of bias”, or “unclear risk of bias”. Moreover, a modified Jadad 7‐point scale was used to quantitative assess the quality of RCTs, and the study with the Jadad score ≥ 4 is considered to be of high quality. 12
2.5. Statistical analysis
Meta‐analysis was conducted by Review Manager software 5.3. Continuous outcomes were assessed by mean difference (MD) with 95% confidence intervals (CIs), while dichotomous outcomes were evaluated by odds ratio (OR) with 95% CIs. Separate statistics was combined by the inverse variance or Mantel‐Haenszel method. P and I 2 values were used to assess heterogeneity among included studies. If I 2 < 50% and P > .1, a fixed‐effects model was applied; otherwise, a random‐effects model was applied. Sensitivity analysis was conducted by omitting one study in turn. Subgroup analysis of patients with wounds or scars and the control group with or without other topical treatments were performed. P < .05 were considered statistically significant.
Reliability and conclusiveness of the available evidence were examined by trial sequential analysis (TSA) software 0.9.5.10 according to the previous meta‐analysis, 13 , 14 which can reduce type I errors caused by multiple testing and sparse data. When the cumulative Z curve crossed the TSA boundary, a sufficient level of evidence for the anticipated intervention effect is reached and no further studies are needed; otherwise, when the Z curve failed to cross the TSA boundary and the required information size (RIS) was not reached, the evidence to reach a conclusion is insufficient. Two‐sided tests with a type I error of 5%, a power of 80%, and a low bias or a user‐defined MD were used to calculate the RIS.
3. RESULTS
3.1. Search results
A total of 213 studies were identified and 179 studies were excluded by reading the title and abstract. After reading the full text of remaining 34 articles, 21 studies were excluded with reasons in Table 1. Finally, 13 RCTs 1 , 2 , 3 , 7 , 8 , 9 , 15 , 16 , 17 , 18 , 19 , 20 , 21 were taken into our meta‐analysis. The basic characteristics and interventions are summarised in Table 2. Based on the different control groups, we divided the enrolled RCTs into three groups (Table 3): (a) five studies 2 , 3 , 7 , 18 , 19 compared OE gel versus no treatment concluded that OE gel is superior to no treatment; (b) seven studies 1 , 3 , 8 , 9 , 15 , 17 , 20 compared OE gel vs other commonly used topical treatments (placebo lotion, silicone lotion, silicone gel, and silicone gel sheet) in scar management did not reach consistent conclusions; (c) three studies 9 , 16 , 21 compared OE in silicone gel vs other treatments concluded that OE in silicone gel is superior to placebo gel and silicone gel.
TABLE 1.
Reasons of full‐text article excluded
| Number | Reason |
|---|---|
| 8 | Full‐text was unavailable |
| 5 | Conference abstract |
| 4 | Non‐randomised controlled trail |
| 1 | Patients with alopecia areata, neither scars nor wounds |
| 1 | Intralesional injection of onion extract, not topical treatment |
| 1 | Used topical silicone gel plus herbal extract gel (Allium cepa, Centella asiatica, Aloe vera and paper mulberry) |
| 1 | Published in 2008 by Draelos, which was similar with another study published in 2012 by himself |
TABLE 2.
The characteristics of included studies
| Study (year) | Country | Study design | Wounds or scars | Location | Arm 1/Arm 2/Arm 3 | Study arms and the number of wounds or scars received treatment | Follow‐up | Jadad score | |
|---|---|---|---|---|---|---|---|---|---|
| Cases (female%) | Age (years) | ||||||||
| Chanprapaph 2012 8 | Thailand | RCT, internally controlled, split‐scar study | Caesarean wounds at 1 week after surgery | Abdomen | 16 (100) | 19–43 |
Arm 1: one‐third of the 16 wounds received OE gel (Erase gel, ABCA Pharma Lab) Arm 2: one‐third of the 16 wounds received vehicle‐based gel |
12 wk | 6 |
| Chung 2006 20 | America | RCT, internally controlled, split‐scar study | New surgical wounds after suture removal | Head, trunk, extremities | 24 (29) | 65 ± 11.25 |
Arm 1: half of the 14 wounds received OE gel; Arm 2: half of the 14 wounds received petrolatum ointment |
12 wk | 6 |
| Draelos 2012 7 | America | RCT, internally controlled | Two bilateral chest wounds after re‐epithelialisation completed | Chest | 44 (27) | 57 ± 6.75 |
Arm 1: 44 wounds received OE gel (Merderma, Merz Pharmaceuticals) Arm 2: 44 wounds received no treatment |
8 wk | 4 |
| Ho 2006 19 | Hong Kong | RCT | Wounds after tattoo removal | Unavailable | 60 (38)/60 (44) | 37.7 ± 6.8/37.6 ± 6.2 |
Arm 1: 61 wounds received OE gel (Contractubex, Merz Pharma) Arm 2: 68 wounds received no treatment |
13 to 20 mo | 3 |
| Hosnuter 2007 9 | Turkey | RCT | Hypertrophic and keloid scars with duration from 1 mo to 6 mo | Unavailable | 24/24/24 (60) | 40.3 ± 9.6 |
Arm 1: 21 scars received OE gel (Contractubex, Merz Pharma) Arm 2: 19 scars received silicone gel sheet Arm 3: 20 scars received combination treatment |
6 mo | 3 |
| Jenwitheesuk 2012 21 | Thailand | RCT | Median sternotomy wounds at 1 wk after surgery | Chest | 28 (39)/26 (50) | 51 ± 12.50/48 ± 14.47 |
Arm 1: 28 wounds received OE in silicone derivative gel (Cybele Scagel, Bangkok Botanica) Arm 2: 26 wounds received placebo gel |
12 wk | 5 |
| Jorge 2014 18 | Mexico | RCT | Caesarean wounds after suture removal | Abdomen | 31/30 (100) | 24.3 ± 6.0/ 23.7 ± 6.3 |
Arm 1: 31 wounds received OE gel (Contractubex, Merz Pharma) Arm 2: 30 wounds received no treatment |
12 wk | 4 |
| Karagoz 2009 1 | Turkey | RCT | Postburn scars less than 6 mo | Head, trunk, extremities | 32 (63) | 24 ± 13 |
Arm 1: 15 scars received OE gel (Contractubex, Merz Pharma) Arm 2: 15 scars received silicone gel (Scarfade1, Hanson Medical Inc) Arm 3: 15 scars received silicone gel sheet (Epi‐DermTM, Biodermis) |
6 mo | 5 |
| Owji 2018 15 | Iran | RCT, internally controlled | Upper blepharoplasty wounds after suture removal | Upper blephar | 36 (100) | 51.5 ± 6.5 |
Arm 1: 26 wounds received OE gel (Contractubex, Merz Pharmaceuticals) Arm 2: 26 wounds received petroleum jelly |
6 mo | 5 |
| Perez 2010 17 | America | RCT | Keloids or hypertrophic scars with a diameter of 0.5 to 2.5 cm | Unavailable | 10 (40)/10 (25)/10 (40) | 42/45/40 |
Arm 1: 5 scars received OE gel (Mederma, Merz Pharma) Arm 2: 5 scars received 0.5% hydrocortisone, silicone and vitamin E lotion (Scarguard, Scarguard Labs) Arm 3: 5 scars received placebo (Cethaphil moisturising lotion, Galderma Laboratories) |
16 wk | 5 |
| Prager 2018 2 | Germany | RCT, internally controlled | Two wounds within 3 weeks postsurgery on similar regions | Unavailable | 124 (45) | 48.6 ± 15.7 |
Arm 1: 113 wounds received OE gel (Contractubex, Merz Pharmaceuticals) Arm 2: 113 wounds received no treatment |
12–24 wk | 4 |
| Song 2018 3 | Republic of Korea | RCT | New surgical wounds after suture removal | Abdomen | 30/30/30 (90) | 39 ± 6.0/38 ± 6.5/39 ± 6.7 |
Arm 1: 30 wounds received OE gel (Contractubex, Merz Pharma) Arm 2: 30 wounds received silicone gel (Kelo‐cort, Advanced Bio‐Technologies) Arm 3: 30 wounds received no treatment |
12 wk | 7 |
| Wananukul 2013 16 | Thailand | RCT, internally controlled, split‐scar study | Median sternotomy wounds at 1 week after surgery | Chest |
30 (43) |
4.3 ± 3.63 |
Arm 1: 30 wounds received OE in silicone derivative gel (Cybele Scagel, Bangkok Botanica) Arm 2: 30 wounds received placebo gel |
6 mo | 6 |
Abbreviations: OE, onion extract; RCT: randomised controlled trial.
TABLE 3.
Outcomes and conclusions of the included studies
| Study | Outcomes | Conclusion |
|---|---|---|
| Group 1: studies compared OE gel versus no treatment | ||
| Draelos 2012 7 | The improvement in overall appearance, texture, redness, softness by a 4‐point scale (investigator and patients); complications | OE gel is superior to no treatment |
| Ho 2006 19 | Clinical clearance (investigator) and complications | OE gel is superior to no treatment |
| Jorge 2014 18 | POSAS (investigator and patients); complications | OE gel is superior to no treatment |
| Prager 2018 2 | POSAS (investigator and patients); global aesthetic improvement scale (investigator); global comfort assessment scale and satisfaction with scar appearance (patients); complications | OE gel is superior to no treatment |
| Song 2018 3 | VSS and image panel scale (investigator); body image scale and cosmetic scale (patients); complications | OE gel is superior to no treatment |
| Group 2: studies compared OE gel versus other treatments | ||
| Chanprapaph 2012 8 | Skin redness index; pliability by a 6‐point scale and height by a 4‐point scale (investigator); overall cosmetic improvement, pain, itching, discomfort, tightness, hardness by a 4‐point scale (patients); complications | OE gel is superior to placebo gel |
| Chung 2006 20 | Cosmetic appearance, redness, thickness by a 10‐point VAS scale (investigator); redness, itchiness, burning, pain by a 10‐point VAS scale (patients); overall cosmetic appearance by phone (patients) | OE gel is similar with petrolatum ointment |
| Owji 2018 15 | Manchester Scar Scale and the overall scar appearance by a 10‐point VAS scale (investigator); redness and appearance by phone (patients); complications | OE gel is similar with petroleum jelly |
| Song 2018 3 | VSS and image panel scale (investigator); body image scale and cosmetic scale (patients); complications | OE gel is similar with silicone gel |
| Hosnuter 2007 9 | Colour, height, hardness, itching by a 4‐point or 3‐point scale (patients); complications | OE gel is similar with silicone gel sheet |
| Karagoz 2009 1 | VSS (investigator); complications | Silicone gel and silicone gel sheet are superior to OE gel |
| Perez 2010 17 | Scar induction, erythema, pigmentation alteration, pain, itching, tenderness, cosmetic appearance by a 100‐point VAS scale (investigator and patients); scar volume; patient satisfaction; complications | OE gel is similar with silicone lotion, both two are superior to placebo lotion |
| Group 3: studies compared OE in silicone gel versus other treatments | ||
| Jenwitheesuk 2012 21 | VSS (investigator); pruritus and pain scores by a 10‐point scale (patients); complications | OE in silicone gel is superior to placebo gel |
| Wananukul 2013 16 | VSS (investigator); complications | OE in silicone gel is superior to placebo gel |
| Hosnuter 2007 9 | Colour, height, hardness, itching by a 4‐point or 3‐point scale (patients); complications | OE in silicone gel is superior to silicone gel sheet |
Abbreviations: OE, onion extract; POSAS, patient and observer scar assessment scale; VAS, visual analog scale; VSS, vancouver scar scale.
3.2. Quality assessment
Study quality was assessed by the risk of bias (Figures 2 and 3) and Jadad score (Table 2). Seven RCTs 2 , 3 , 7 , 8 , 16 , 17 , 18 recorded using computer‐generated or other appropriate methods for randomisation, but only two 3 , 17 described allocation concealment. Six studies 1 , 3 , 8 , 15 , 16 , 20 were carried out in a double‐blind method; 10 studies 2 , 3 , 7 , 8 , 15 , 16 , 17 , 19 , 20 , 21 described the implementation of blinding of outcomes; 8 studies 1 , 3 , 7 , 8 , 9 , 16 , 18 , 21 presented complete data; and only 1 study 17 showed selective reporting. Two studies 9 , 19 with a Jadad score < 4 were considered as of low quality. The publication bias was assessed by a funnel plot diagram (Supplemental Figures [Link], [Link]). The asymmetrical funnel plot diagrams indicated that there were potential risks of publication bias of the total improvement scores between OE gel and no treatment (Supplemental Figure 1), between OE gel and other treatments (Supplemental Figure 2), and the incidence of adverse effects (Supplemental Figure 3).
FIGURE 2.
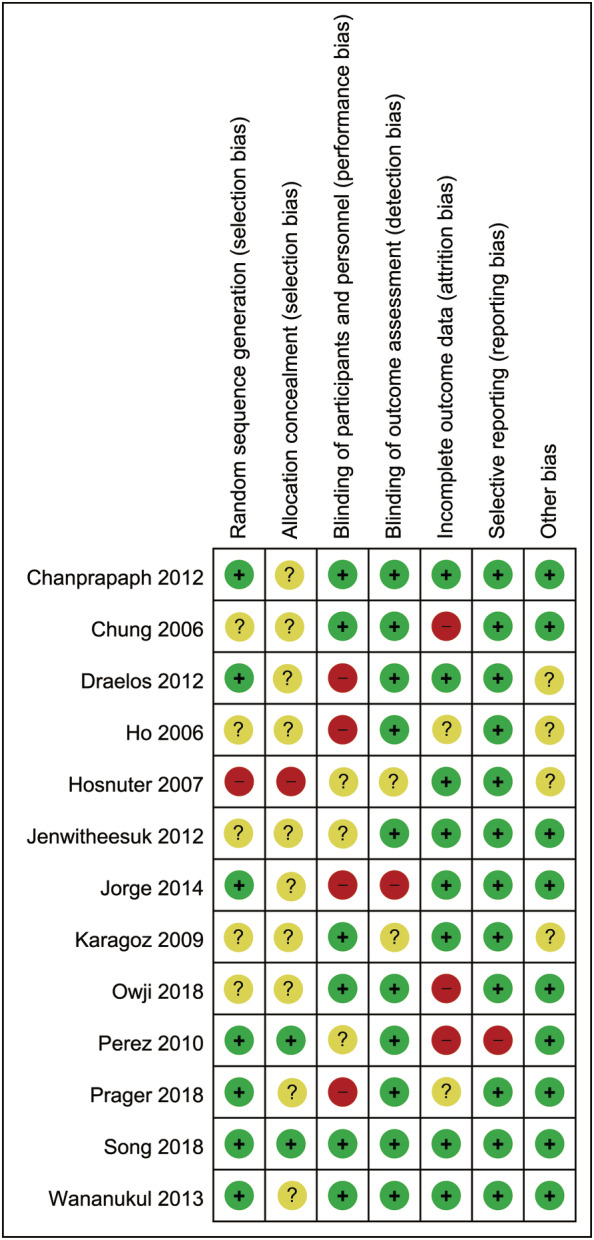
Risk‐of‐bias summary of the included studies
FIGURE 3.
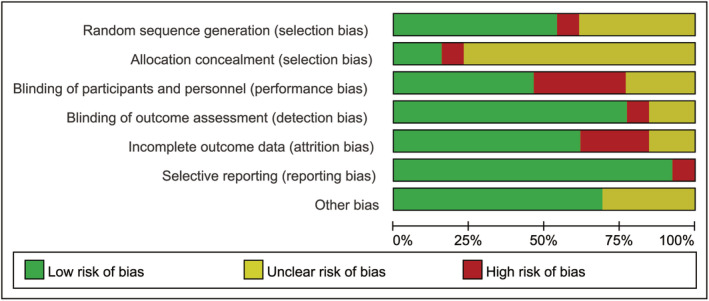
Risk‐of‐bias graph of the included studies
3.3. Meta‐analysis of the primary outcomes
Owing to different scales being used to evaluate the scars in the included studies and none of the scale was able to integrate all of the results (Table 3); thus, we calculated and meta‐analysed the total improvement scores evaluated by investigators and patients, respectively. Compared with no treatment, OE gel significantly increased the total improvement scores assessed by investigators [MD = 1.63, 95% CI: (1.04, 2.22), P < .00001] and patients [MD = 1.93, 95% CI: (1.20, 2.66), P < .00001] (Figure 4), but significant heterogeneities were observed (I 2 = 89% and I 2 = 81%, Figure 4). Furthermore, significant differences remained when omitting one study in turn for the sensitivity analysis (data not shown); heterogeneities were greatly decreased (I 2 = 49% and I 2 = 0%, data not shown) when omitting the study of Prager 2018. Moreover, The TSA of OE gel vs no treatment assessed by investigators (Supplemental Figure 4A) and patients (Supplemental Figure 4B) indicated that the Z curve crossed the conventional boundary, TSA boundary, and RIS.
FIGURE 4.
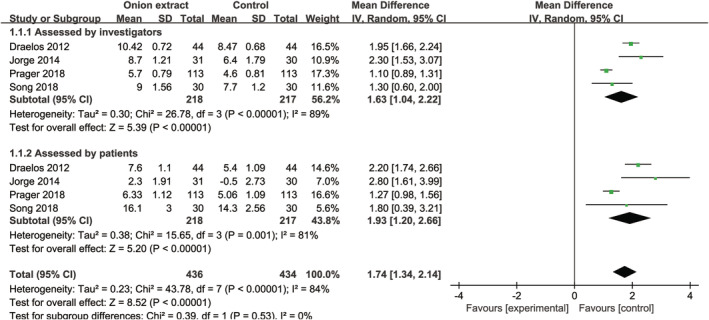
Forest plot diagram showing the total improvement scores between onion extract gel and no treatment
No significant differences were detected between OE gel and other treatments (placebo gel or lotion, petroleum ointment or jelly, silicone lotion, and silicone gel or gel sheet), at the total improvement scores assessed by investigators [MD = −0.16, 95% CI: (−0.70, 0.38), P = .56] and patients [MD = 0.37, 95% CI: (−0.47, 1.20), P = .39] (Figure 5). When omitting one study in turn for sensitivity analysis, there were still no intergroup differences (data not shown). Furthermore, subgroup analysis of surgical wounds assessed by investigators [MD = 0.09, 95% CI: (−0.43, 0.61), P = .74] and patients [MD = 0.74, 95% CI: (−0.78, 2.27), P = .34] and scars assessed by patients [MD = −1.05, 95% CI: (−4.33, 2.22), P = .53] (Figure 6) found no significant differences between OE gel and other treatments, but scars assessed by investigators found OE gel had a lower total improvement score than other treatments [MD = −1.99, 95% CI: (−3.26, −0.73), P = .002] (Figure 6). Moreover, the TSA of OE gel vs other treatments assessed by investigators (Supplemental Figure 5A) and patients (Supplemental Figure 5B) indicated that the Z curve failed to cross the conventional boundary, TSA boundary, and RIS.
FIGURE 5.
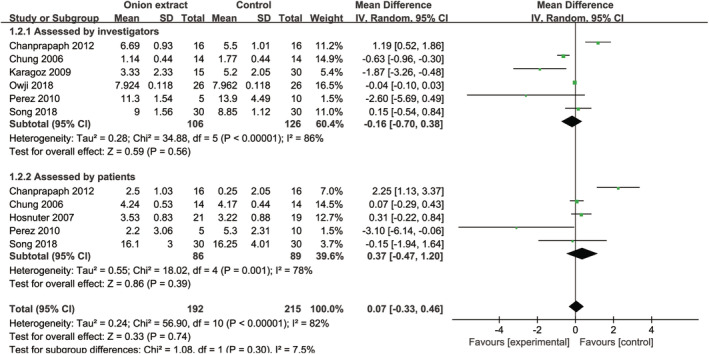
Forest plot diagram showing the total improvement scores between onion extract gel and other treatments
FIGURE 6.
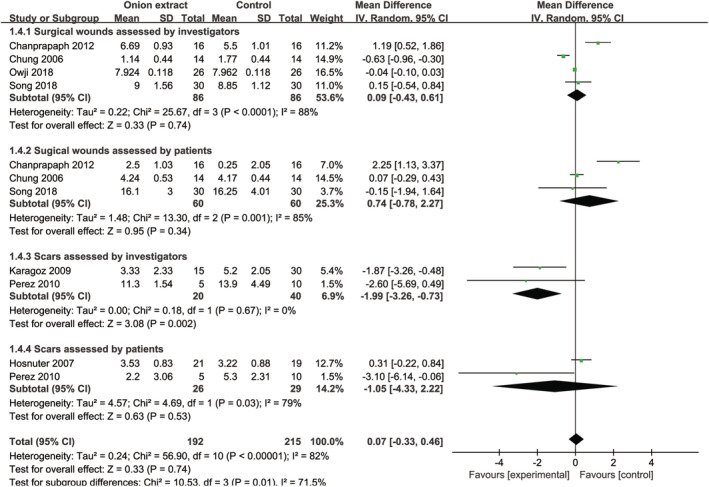
Forest plot diagram showing the subgroup analysis of total improvement scores between onion extract gel and other treatments
Compared with other treatments (placebo gel or silicone gel sheet), OE in silicone gel significantly increased the total improvement scores assessed by investigators [MD = 0.43, 95% CI: (0.29, 0.57), P < .00001] and patients [MD = 1.22, 95% CI: (0.51, 1.92), P = .0007] (Figure 7). For only two studies were included, sensitivity and subgroup analyses were not applicable.
FIGURE 7.
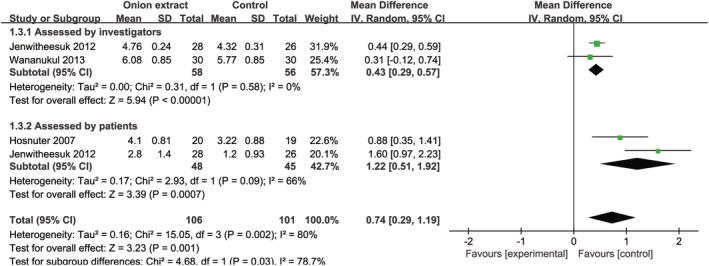
Forest plot diagram showing the total improvement scores between onion extract in silicone gel and other treatments
3.4. Meta‐analysis of the secondary outcomes
Except the study of Chung 2006, 20 all of the remaining studies recorded the adverse effects and all of them were topical side effects, including itching, burning, stinging, and contact dermatitis. Compared with no treatment [OR = 6.86, 95% CI: (2.86, 16.48), P < .00001] or other treatments [OR = 3.72, 95% CI: (1.41, 9.84), P = .008] (Figure 8), OE gel significantly increased the incidence of total adverse effects when analysed by a fixed‐effects model. However, no significant differences were detected between OE gel with no treatment [OR = 6.16, 95% CI: (0.95, 39.73), P = .06] or other treatments [OR = 2.92, 95% CI: (0.64, 13.38), P = .17] (Supplemental Figure 6) by a random‐effects model, and between OE in silicone gel and other treatments by a fixed‐effects model [OR = 5.42, 95% CI: (0.61, 47.71), P = .13] (Figure 8) or a random‐effects model [OR = 5.14, 95% CI: (0.56, 46.83), P = .15] (Supplemental Figure 6). Moreover, we found the incidence of dropping out caused by the intolerance of treatments in OE gel was significantly higher than control group [OR = 12.03, 95% CI: (3.19, 45.38), P = .0002] (Figure 9). Other outcomes, such as the volume of the scar and patient satisfaction, due to the limited number of studies, could not be integrated.
FIGURE 8.
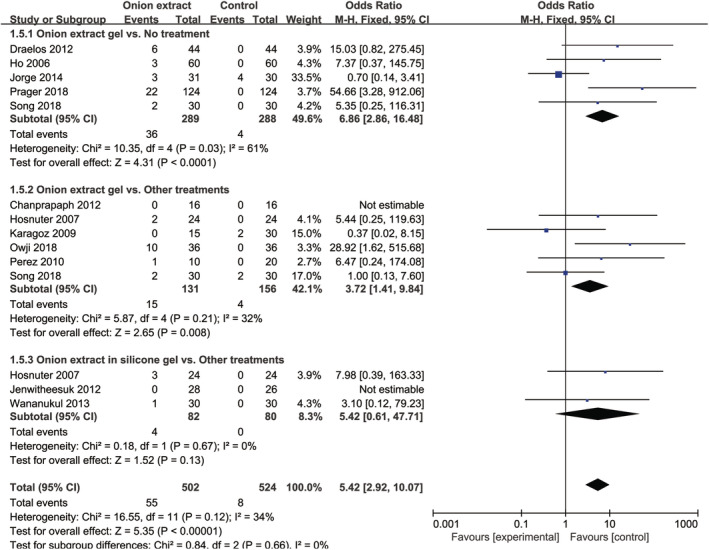
Forest plot diagram showing the incidence of total adverse effects by a fixed‐effects model
FIGURE 9.
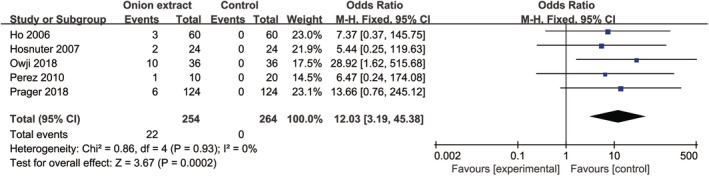
Forest plot diagram showing the incidence of dropping out caused by the intolerance of treatments
4. DISCUSSION
This meta‐analysis aimed to evaluate the efficacy and safety of OE gel in scar management. The data from 13 RCTs found OE gel were better than no treatment, but did not show superiority to other treatments (petroleum ointment or jelly, and silicone lotion, silicone gel, or gel sheet) in the management of scarring. Moreover, this meta‐analysis found OE in silicone gel was superior to other treatments (placebo gel or silicone gel sheet) in scar treatment. Finally, OE gel had a potential to increase the incidence of treatment‐induced adverse effects, and increased the incidence of dropping out caused by the intolerance of treatments, when compared with no treatment and other commonly used topical treatments.
The total improvement score was the primary outcome in this meta‐analysis. Compared with no treatment, OE gel significantly increased the total improvement scores assessed by investigators and patients. These data were further confirmed by sensitivity analysis and TSA. In addition, our data are also consistent with the conclusions in the included studies as well as those studies 5 , 22 , 23 excluded for no full‐text available or non‐RCT, which all concluded that OE gel was superior to no treatment. Taken together, these evidences strongly demonstrate that OE gel is beneficial for scar management when compared with no treatment.
However, in this study, OE gel did not show superiority to other treatments, including petroleum ointment or jelly, silicone lotion, and silicone gel or gel sheet. This result is consistent with most of the included studies that concluded OE gel had similar effect with petroleum ointment or jelly, silicone lotion, and silicone gel or gel sheet. Furthermore, subgroup analysis of scars assessed by investigators found OE gel had a lower total improvement score than other treatments; Karagoz et al 1 concluded that OE gel was inferior to silicone gel and silicone gel sheet on scar treatment; one non‐RCT 24 found petrolatum ointment was better than OE gel on scar treatment. In summary, OE gel is not superior to those treatment (petroleum ointment or jelly, silicone lotion, and silicone gel or gel sheet) on scar management, but the TSA of OE gel versus other treatments indicates more trails are needed to confirm this conclusion.
Furthermore, this meta‐analysis found OE in silicone gel significantly increased the total improvement scores than placebo gel and silicone gel sheet, which is consistent with all of three included RCTs that OE in silicone gel was superior to placebo gel and silicone gel sheet. One similar RCT 25 reported that topical silicone gel plus herbal extract gel (Allium cepa, Centella asiatica, Aloe vera, and paper mulberry) had better vascularity and pigmentation than silicone gel. Although topical silicone gel beneficial for scar prevention has been concluded by two meta‐analyses with weak evidence, 26 , 27 the performance of topical silicone gel and other non‐silicone topical treatment is also similar. 27 Thus, OE in silicone gel seems an optimal choice for scar treatment, but still needs more studies to strength this evidence.
Concerning the safety, although OE gel had a potential to increase the incidence of total adverse effects when compared with no treatment and other treatments, most of the adverse effects were itching, burning, stinging, or contact dermatitis at the site of application, mild to moderate in severity, and resolved spontaneously. However, the increased incidence of dropping out caused by the intolerance of OE gel, indicating that OE gel is not suitable to all patients and the optimal topical choice for scar treatment should base on the topical reactions.
The limitations in our meta‐analysis: (a) only three studies assessed the effect of OE in silicone gel; thus, the sample size was small to reach firm conclusion about OE in silicone gel in scar management. Moreover, TSA indicates more trails are needed to strength the conclusion about OE gel vs other topical treatments. (b) Heterogeneity among the included studies was visible, especially the different scales to assess the scars, different controls (no treatment, placebo gel, petroleum ointment or jelly, and silicone lotion, gel or gel sheet), and different study design (internally controlled or split‐scar study). (c) Scales including different items, such as redness, itchiness, burning, and pain, were used to evaluate the scars in the enrolled studies, and these items could not be meta‐analysed separately to get more detailed information. (d) The asymmetrical funnel plot diagrams indicated that there were potential risks of publication bias in the included studies.
5. CONCLUSIONS
Based on this meta‐analysis of RCTs, OE gel not only has no superiority to commonly used topical treatments, but also has the potential to increase the incidence of adverse effects on scar management; OE in silicone gel might be the optimal topical choice for scar treatment; however, more evidences are needed to strength these conclusions.
CONFLICT OF INTEREST
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Supporting information
Supplemental Figure 1 A funnel plot of the total improvement scores between onion extract gel and no treatment.
Supplemental Figure 2 A funnel plot of the total improvement scores between onion extract gel and other treatments.
Supplemental Figure 3 A funnel plot of the total adverse effects by a fixed‐effects model.
Supplemental Figure 4 Trial sequential analysis of the total improvement scores between onion extract gel and no treatment. (A) Assessed by investigators, (B) assessed by patients.
Supplemental Figure 5 Trial sequential analysis of the total improvement scores between onion extract gel and other treatments. (A) Assessed by investigators, (B) assessed by patients.
Supplemental Figure 6 Forest plot diagram showing the total adverse effects by a random‐effects model.
ACKNOWLEDGEMENTS
This study was supported by Youth Talent Supported Program of Southwest Hospital (No. SWH2018QNLC‐11).
Appendix A. Appendices (Search Strategy)
PubMed
#1 Allium cepa[TIAB] OR onion[TIAB] OR onions[TIAB] OR onion extract[TIAB]
#2 scar[TIAB] OR scars[TIAB] OR cicatrix[TIAB] OR cicatrice[TIAB] OR cicatrices[TIAB] OR keloid[TIAB] OR keloids[TIAB] OR cicatricle[TIAB]
#3 #1 AND #2
Medline
#1 TS=(Allium cepa) OR TS=(onion) OR TS=(onions) OR TS=(onion extract)
#2 TS=(scar) OR TS=(scars) OR TS=(cicatrix) OR TS=(cicatrice) OR TS=(cicatrices) OR TS=(keloid) OR TS=(keloids) OR TS=(cicatricle)
#3 #1 AND #2
Embase and Cochrane Library
#1 Allium cepa:ti,ab,kw OR onion:ti,ab,kw OR onions:ti,ab,kw OR onion extract:ti,ab,kw
#2 scar:ti,ab,kw OR scars:ti,ab,kw OR cicatrix:ti,ab,kw OR cicatrice:ti,ab,kw OR cicatrices:ti,ab,kw OR keloid:ti,ab,kw OR keloids:ti,ab,kw OR cicatricle:ti,ab,kw
#3 #1 AND #2
Yuan X, Shen J, Chen L, Wang L, Yan Q, Zhang J. Onion extract gel is not better than other topical treatments in scar management: A meta‐analysis from randomised controlled trails. Int Wound J. 2021;18:396–409. 10.1111/iwj.13542
Funding information Youth Talent Supported Program of Southwest Hospital, Grant/Award Number: SWH2018QNLC‐11
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are included within the article.
REFERENCES
- 1. Karagoz H, Yuksel F, Ulkur E, Evinc R. Comparison of efficacy of silicone gel, silicone gel sheeting, and topical onion extract including heparin and allantoin for the treatment of postburn hypertrophic scars. Burns. 2009;35(8):1097‐1103. [DOI] [PubMed] [Google Scholar]
- 2. Prager W, Gauglitz GG. Effectiveness and safety of an overnight patch containing Allium cepa extract and allantoin for post‐dermatologic surgery scars. Aesthetic Plast Surg. 2018;42(4):1144‐1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song T, Kim KH, Lee KW. Randomised comparison of silicone gel and onion extract gel for post‐surgical scars. J Obstet Gynaecol. 2018;38(5):702‐707. [DOI] [PubMed] [Google Scholar]
- 4. Sidgwick GP, McGeorge D, Bayat A. A comprehensive evidence‐based review on the role of topicals and dressings in the management of skin scarring. Arch Dermatol Res. 2015;307(6):461‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Willital GH, Heine H. Efficacy of Contractubex gel in the treatment of fresh scars after thoracic surgery in children and adolescents. Int J Clin Pharmacol Res. 1994;14(5–6):193‐202. [PubMed] [Google Scholar]
- 6. Willital GH, Simon J. Efficacy of early initiation of a gel containing extractum cepae, heparin, and allantoin for scar treatment: an observational noninterventional study of daily practice. J Drugs Dermatol. 2013;12(1):38‐42. [PubMed] [Google Scholar]
- 7. Draelos ZD, Baumann L, Fleischer AB Jr, Plaum S, Avakian EV, Hardas B. A new proprietary onion extract gel improves the appearance of new scars: a randomized, controlled, blinded‐investigator study. J Clin Aesthet Dermatol. 2012;5(6):18‐24. [PMC free article] [PubMed] [Google Scholar]
- 8. Chanprapaph K, Tanrattanakorn S, Wattanakrai P, Wongkitisophon P, Vachiramon V. Effectiveness of onion extract gel on surgical scars in asians. Dermatol Res Pract. 2012;2012:212945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hosnuter M, Payasli C, Isikdemir A, Tekerekoglu B. The effects of onion extract on hypertrophic and keloid scars. J Wound Care. 2007;16(6):251‐254. [DOI] [PubMed] [Google Scholar]
- 10. Gold MH, McGuire M, Mustoe TA, et al. Updated international clinical recommendations on scar management: part 2—algorithms for scar prevention and treatment. Dermatol Surg. 2014;40(8):825‐831. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg. 2010;8(5):336‐341. [DOI] [PubMed] [Google Scholar]
- 12. Gong S, Xu W, Wang R, et al. Patient‐specific instrumentation improved axial alignment of the femoral component, operative time and perioperative blood loss after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2019;27(4):1083‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li S, Kwong JS, Zeng XT, et al. Plasmakinetic resection technology for the treatment of benign prostatic hyperplasia: evidence from a systematic review and meta‐analysis. Sci Rep. 2015;5:12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miao S, Shi M, Zou L, Wang G. Effect of intrathecal dexmedetomidine on preventing shivering in cesarean section after spinal anesthesia: a meta‐analysis and trial sequential analysis. Drug des Devel Ther. 2018;12:3775‐3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Owji N, Khademi B, Khalili M. Effectiveness of topical onion extract gel in the cosmetic appearance of blepharoplasty scar. J Clin Aesthet Dermatol. 2018;11(10):31‐35. [PMC free article] [PubMed] [Google Scholar]
- 16. Wananukul S, Chatpreodprai S, Peongsujarit D. Lertsapcharoen P. a prospective placebo‐controlled study on the efficacy of onion extract in silicone derivative gel for the prevention of hypertrophic scar and keloid in median sternotomy wound in pediatric patients. J Med Assoc Thai. 2013;96(11):1428‐1433. [PubMed] [Google Scholar]
- 17. Perez OA, Viera MH, Patel JK, et al. A comparative study evaluating the tolerability and efficacy of two topical therapies for the treatment of keloids and hypertrophic scars. J Drugs Dermatol. 2010;9(5):514‐518. [PubMed] [Google Scholar]
- 18. Ocampo‐Candiani J, Vazquez‐Martinez OT, Iglesias Benavides JL, Buske K, Lehn A, Acker C. The prophylactic use of a topical scar gel containing extract of Allium cepae, Allantoin., and heparin improves symptoms and appearance of cesarean‐section scars compared with untreated scars. J Drugs Dermatol. 2014;13(2):176‐182. [PubMed] [Google Scholar]
- 19. Ho WS, Ying SY, Chan PC, Chan HH. Use of onion extract, heparin, allantoin gel in prevention of scarring in chinese patients having laser removal of tattoos: a prospective randomized controlled trial. Dermatol Surg. 2006;32(7):891‐896. [DOI] [PubMed] [Google Scholar]
- 20. Chung VQ, Kelley L, Marra D, Jiang SB. Onion extract gel versus petrolatum emollient on new surgical scars: a prospective double‐blinded study. Dermatol Surg. 2006;32(2):193‐197. [DOI] [PubMed] [Google Scholar]
- 21. Jenwitheesuk K, Surakunprapha P, Jenwitheesuk K, Kuptarnond C, Prathanee S, Intanoo W. Role of silicone derivative plus onion extract gel in presternal hypertrophic scar protection: a prospective randomized, double blinded, controlled trial. Int Wound J. 2012;9(4):397‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maragakis M, Willital GH, Michel G, Gortelmeyer R. Possibilities of scar treatment after thoracic surgery. Drugs Exp Clin Res. 1995;21(5):199‐206. [PubMed] [Google Scholar]
- 23. Campanati A, Savelli A, Sandroni L, et al. Effect of Allium cepa‐allantoin‐pentaglycan gel on skin hypertrophic scars: clinical and video‐capillaroscopic results of an open‐label, controlled, nonrandomized clinical trial. Dermatol Surg. 2010;36(9):1439‐1444. [DOI] [PubMed] [Google Scholar]
- 24. Jackson BA, Shelton AJ. Pilot study evaluating topical onion extract as treatment for postsurgical scars. Dermatol Surg. 1999;25(4):267‐269. [DOI] [PubMed] [Google Scholar]
- 25. Surakunprapha P, Winaikosol K, Chowchuen B, Jenwithaesuk K, Jenwitheesuk K. Adding herbal extracts to silicone gel on post‐sternotomy scar: a prospective randomised double‐blind study. J Wound Care. 2020;29:S36‐S42. [DOI] [PubMed] [Google Scholar]
- 26. O'Brien L, Jones DJ. Silicone gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2013;12(9):CD003826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang F, Li X, Wang X, Jiang X. Efficacy of topical silicone gel in scar management: a systematic review and meta‐analysis of randomised controlled trials. Int Wound J. 2020;17(3):765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 A funnel plot of the total improvement scores between onion extract gel and no treatment.
Supplemental Figure 2 A funnel plot of the total improvement scores between onion extract gel and other treatments.
Supplemental Figure 3 A funnel plot of the total adverse effects by a fixed‐effects model.
Supplemental Figure 4 Trial sequential analysis of the total improvement scores between onion extract gel and no treatment. (A) Assessed by investigators, (B) assessed by patients.
Supplemental Figure 5 Trial sequential analysis of the total improvement scores between onion extract gel and other treatments. (A) Assessed by investigators, (B) assessed by patients.
Supplemental Figure 6 Forest plot diagram showing the total adverse effects by a random‐effects model.
Data Availability Statement
The data used to support the findings of this study are included within the article.


