Abstract
Psoriasis mainly affects the skin and joints and has serious impacts on the physical, emotional, and financial life of patients. Recent studies have demonstrated that other comorbidities are frequently detected in psoriatic patients. A strong association with the development of cardiovascular diseases, such as hypertension, myocardial infarction, and stroke is responsible for the shortened (by 4.5‐5 years) life expectancy of severe psoriatic patients. Systemic inflammation plays an important role in the interrelationship between psoriasis and atherosclerotic plaque formation, which is a common immunopathogenic pathway that explains the multiorgan involvement in psoriasis. As far life‐threatening cardiovascular diseases are very often symptom‐free, the treating dermatologist's responsibility is to initiate interdisciplinary holistic patient care, which may lead to directly saved patients' lives. Holistic care of severe psoriatic patients should include regular cardiac monitoring using cardiovascular imaging modalities and functional testing to detect even subclinical coronary artery disease. Effective anti‐inflammatory treatment with biologic therapies may have beneficial effects on the cardiovascular state and may reduce the incidence of cardiac events. The authors review the latest findings on the shared immunopathogenic background of psoriasis and cardiovascular diseases and discuss the available data about the cardiovascular responses to the currently used biologic treatments.
Keywords: atherosclerosis, cardiovascular diseases, imaging modalities, inflammation, psoriasis
Abbreviations
- ACS
acute coronary syndrome
- Aix
augmentation index
- ASCVD
atherosclerotic cardiovascular disease
- CACS
calcium score
- CAD
coronary artery disease
- CANTOS
the canakinumab anti‐inflammatory thrombosis outcome study
- CARIMA
evaluation of cardiovascular risk markers in psoriasis patients treated with secukinumab
- CCTA
coronary computed tomography angiography
- CFR
coronary flow reserve
- cIMT
carotid intima media thickness
- CTA
computed tomography angiography
- CVD
cardiovascular disease
- CVE
cardiovascular event
- EAT
epicardial adipose tissue
- FAI
perivascular fat attenuation index
- FDA
US Food and Drug Administration
- 18FDG‐PET/CT
18F‐fluoro‐deoxyglucose‐positron emission tomography/computed tomography
- fIMT
femoral intima media thickness
- FMD
flow‐mediated dilatation
- GLS
global longitudinal strain
- GLSR
global longitudinal strain rate
- HDL
high‐density lipoprotein
- hs‐CRP
high‐sensitivity C‐reactive protein
- HU
Hounsfield unit
- ICAM‐1
intracellular adhesion molecule‐1
- IFN‐γ
interferon‐γ
- IL
interleukin
- LDL
low‐density lipoprotein
- LV
left ventricular
- LVF
left ventricular function
- mAb
monoclonal antibody
- MACE
major adverse cardiovascular event
- MCP‐1
monocyte chemoattractant protein‐1
- MDC
macrophage‐derived chemokine
- MMP‐9
matrix metallopeptidase 9)
- MPO
myeloperoxidase
- MRI
magnetic resonance imaging
- MTX
methotrexate
- NT‐proBNP
N‐terminal‐pro hormone‐B‐type natriuretic peptide
- OS
oxidative stress
- oxHDL
oxidized high‐density lipoprotein
- oxLDL
oxidized low‐density lipoprotein
- PWV
pulse wave velocity
- RHI
hyperaemia‐peripheral arterial tonometry index
- SA
subclinical atherosclerosis
- TGF‐β
transforming growth factor‐β
- Th cell
T helper cell
- TNF‐α
tumor necrosis factor‐α
- tPAI‐1
tissue plasminogen activator inhibitor‐1
- TTE
transthoracic echocardiography
- VCAM‐1
vascular cell adhesion molecule‐1
1. INTRODUCTION
Psoriasis is a chronic, immune‐mediated autoinflammatory disorder that affects approximately 2% to 3% of the population worldwide. 1 Psoriasis represents a serious burden for patients, has skin and potentially joint manifestations (arthritis psoriatica) and is associated with several cardiovascular comorbidities. 2
Psoriasis and cardiovascular diseases (CVDs) share a common self‐reinforcing immunopathogenic pathway, providing possible explanations for their cooccurrence. Environmental factors associated with genetic predisposition (PSORS 1‐9 genes) lead to cytokine and T cell recruitment. 3 Th1 cell‐produced tumor necrosis factor‐α (TNF‐α), interferon‐γ (IFN‐γ), and interleukin (IL)‐12 have been thought to have pivotal roles in psoriatic development, inducing keratinocytes to produce proinflammatory IL‐6, ‐8, and ‐12, as well as TNF‐α. IL‐23/Th17 axis‐produced cytokines are now considered central proteins (IL‐17A‐IL‐17F). IL‐17A and IL‐17F recruit neutrophils, induce keratinocyte proliferation through IL‐22 expression, and with IFN‐γ enhance keratinocytes to produce proinflammatory cytokines, thus leading to increases in T cells in the skin. 4 In the “psoriatic march” systemic inflammation has an effect on the vasculature, increasing the risk of CVDs. 5 Cardiac involvement may shorten life expectancy by 4.5 to 5 years. 6 , 7 Anti‐psoriatic treatments, which target common cytokines, might improve not only skin and joint manifestations, but also systemic atherosclerosis.
The aim of this review is to describe the available data about the immunopathogenic link between psoriasis and CVDs and the effect of the currently used biologic therapies in the cardiovascular system.
2. IMMUNOPATHOGENIC LINK BETWEEN PSORIASIS AND ATHEROSCLEROSIS
CVD is the leading cause of mortality worldwide (16.7 million deaths/year). 8
The five key steps in the atherosclerotic pathomechanism are: endothelial dysfunction, lipid layer/fatty streak formation within the intima and media, leukocyte and smooth cell migration into the vessel wall, foam cell formation, and extracellular matrix degradation. In coronary atherosclerosis, plaque formation progressively narrows the artery lumen and causes stable coronary artery disease (CAD) or results in acute coronary syndrome (ACS) due to plaque rupture or erosion and sudden intracoronary thrombus formation.
The psoriatic and atherosclerotic processes are interconnected through several biochemical pathways, including elevated oxidative stress (OS), endothelial dysfunction, monocyte and neutrophil modulation, T cell activation (Th1 and Th17 cells), increased expression of endothelins, adhesion molecules, increased angiogenesis, and hypercoagulability. 9 , 10 Th1 cell‐produced TNF‐α and IFN‐γ are responsible for plaque growth, while Th17 cell‐produced IL‐17A is responsible for plaque vulnerability with intraplaque angiogenesis and hemorrhage. Th1 and Th17 cells impair the function of Treg cells; therefore, low levels of cardioprotective IL‐10 and transforming growth factor‐β (TGF‐β) can be measured. 10 The IL‐23R rs6682925T/C polymorphism and inheritance of the HLA, FUT2, UBE2L3, and SH2B3 gene variants increase the risk of major adverse cardiovascular events (MACE) in psoriasis. 11 , 12 IL‐1β, IL‐6, and high‐sensitivity C‐reactive protein (hs‐CRP) are involved in the pathogenesis of both psoriasis and atherosclerosis. 13 The canakinumab anti‐inflammatory thrombosis outcome study (CANTOS) showed that canakinumab (an anti‐IL‐1β antibody) therapy has decreased inflammation (IL‐6 and hs‐CRP levels) and the risk of MACEs. 14 Monocyte chemoattractant protein‐1 (MCP‐1) and macrophage‐derived chemokine (MDC) are known biomarkers of atherosclerotic CVD (ASCVD) and are significantly elevated in psoriasis. 15 Inflammation affects the oxidation states of high‐density lipoprotein (HDL) and low‐density lipoprotein (LDL) and transforms cardioprotective HDL to oxHDL (oxidized‐HDL). This transformation results in an atherogenic profile with decreased cholesterol efflux ability. OxLDL (oxidized‐LDL) plays an important role in the atherosclerotic plaque formation. 16
Severe psoriasis increases the risk of MACE within 10 years by 6.2% compared to that of the average population. 17 The risk of MI is 3‐fold higher in psoriatic patients than in healthy individuals. 18
3. MONITORING ATHEROSCLEROSIS IN PSORIASIS
Commonly available imaging tests are suitable for detecting subclinical atherosclerosis (SA) in psoriatic patients.
Carotid intima media thickness (cIMT) is a potential indicator of SA and can be measured by ultrasound. Psoriatic patients have significantly increased cIMTs. 19 Femoral IMT (fIMT) is more informative and has a stronger association with CAD than cIMT based on autopsy studies. A recent investigation showed a significantly higher prevalence of femoral plaques in psoriatic patients than in controls, and the prevalence was 2‐fold higher than carotid plaque prevalence. 20
Flow‐mediated dilatation (FMD) is a potential marker of endothelial dysfunction and SA and is measured by ultrasound. Psoriasis causes significant impairments in the FMD. 21
Vascular ultrasound examinations are reproducible, inexpensive, noninvasive, and based on nonionizing radiation. Therefore, these examinations are perfectly suitable for cardiovascular screening to evaluate the state of the vasculature and monitor changes induced by the applied therapy. If an adverse CVD state is detected (intermediate risk category), we may consider performing CT angiography (CTA).
18F‐fluoro‐deoxyglucose (FDG)‐positron emission tomography (PET)/computed tomography (CT) is a novel, highly sensitive procedure to evaluate vascular inflammation. SA can be identified through aortic uptake of 18FDG by PET/CT and aortic wall thickness measurement by magnetic resonance imaging (MRI). In psoriasis increased aortic vascular uptake of 18F‐FDG can be measured. 22
Epicardial adipose tissue (EAT) can be measured by native CT, MRI, and transthoracic echocardiography (TTE). 23 Previous studies have documented a strong association between EAT, obesity, and ASCVD, including CAD. 24 , 25 EAT thickness was compared between psoriatic patients and age‐sex‐body mass index‐matched healthy individuals with no history of CVD. EAT and hs‐CRP levels were significantly higher in psoriatic patients and correlated with disease severity. 26
The perivascular fat attenuation index (FAI) is a noninvasive marker of the early stage of coronary atherosclerosis. Abnormal perivascular FAI indicates a 6‐to‐9‐fold increased risk of MACE. 27 Novel anti‐inflammatory therapies could also reduce coronary inflammation in more advanced stages of psoriasis and thus could possibly inhibit plaque progression.
Coronary CTA is a reliable, noninvasive method to detect obstructive or nonobsturctive CAD. CTA can describe the presence, severity, and extent of CAD. For asymptomatic patients, noncontrast CT imaging is acceptable to quantify calcified atherosclerotic plaque burden. The higher the CACS, the greater the risk of MACE. 28 Psoriasis is associated with an increased CACS and elevated noncalcified and total plaque burdens on CTA. Additionally, growing body of evidence suggests that systemic anti‐psoriatic treatment can simultaneously improve disease severity and coronary plaque burden.
4. IMMUNOPATHOGENIC LINK BETWEEN PSORIASIS AND HYPERTENSION
Hypertension is more common among psoriatic patients than nonpsoriatic patients. The incidence of newly diagnosed hypertension in psoriasis is 94.3/1000 patient‐years (control: 80.6/1000 patient‐years). 29 Hypertension has well‐known consequences, including MI, transient ischemic attack, stroke, and hypertensive crisis. 30
The need for the parallel use of 3 to 4 types of antihypertensive drugs is 16.5 to 19.9 times higher than that of nonpsoriatic hypertonic controls. 31
In the shared pathogenesis of psoriasis and hypertension, different dysregulated pathways can be discussed. It is known that metabolic syndrome is highly prevalent in psoriatic patients. Adipose tissue is increased around not only the organs but also the vessel walls and is the source of angiotensinogen. Through enzymatic steps, angiotensinogen is converted to angiotensin II, which is responsible for salt retention and stimulates the differentiation of Th17 cells, which produce IL‐17, the main cytokine associated with the pathogenesis of hypertension through vascular dysfunction. 10 Perivascular fat is also a robust niche for macrophages and activated T cells.
Figure 1 shows the comprehensive immunopathogenic link between psoriasis and cardiovascular diseases.
FIGURE 1.
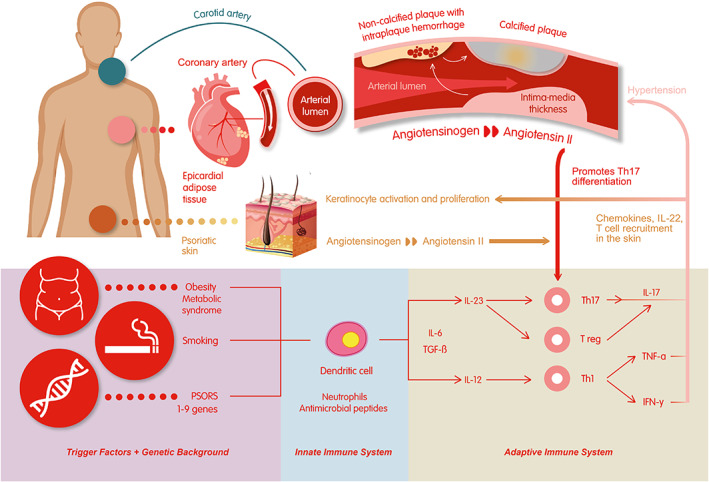
Comprehensive immunopathogenic link between psoriasis and cardiovascular diseases
5. THE EFFECTS OF BIOLOGIC ANTI‐PSORIATIC TREATMENTS ON CARDIOVASCULAR MORBIDITY
5.1. Anti‐TNF‐α
The FDA‐approved TNF‐α inhibitors (etanercept, infliximab, adalimumab, and certolizumab‐pegol) were the most widespread systemic anti‐psoriatic drugs in the recent decade for the treatment of moderate‐to‐severe psoriasis. Several studies showed no increased risk of CVD during anti‐TNF‐α therapy. 32 , 33 Anti‐TNF‐α therapies reduce serum levels of CRP, VCAM‐1, ICAM‐1, E‐selectin, IL‐8, MMP‐9, MPO, MCP‐1, and tPAI‐1, thus reducing the risk of atherosclerosis. 34 , 35 Several studies revealed significant differences in vascular inflammation during therapy. Our previous study measured carotid and brachial IMT before the initiation of anti‐TNF‐α therapy and 6 months later. The majority of patients had increased IMT levels compared to those of age‐adjusted normal healthy controls. Significantly reduced IMT levels were observed in the younger cohort of patients. 19 , 36 Anti‐TNF‐α also induced significant differences in FMD after treatment compared to the baseline state. 37 The hyperaemia‐peripheral arterial tonometry index (RHI) was measured before the first and third infliximab infusions. Only therapeutic nonresponders showed decreased RHI values before the third infusion compared to that of the initiation state. This value can be a predictor of the long‐term, not preferred effect of infliximab. 38 Arterial stiffness and wave reflections predict CVEs and can be determined by arterial (carotid [c]) pulse wave velocity (PWV). Adalimumab therapy significantly improved cPWV values after 6 months of therapy. 39 Vascular inflammation was assessed by 18FDG‐PET/CT and showed improvements after 1 year of therapy. 40
In contrast, some studies did not find significant differences in vascular inflammation or the cIMT during therapy. 41 , 42
5.2. Anti‐IL‐12/23
The FDA‐approved anti‐IL‐12/23 treatment is ustekinumab. The literature is conflicting about its effects on CVDs. Ustekinumab ameliorated myocardial function and coronary effects. After 4 months of therapy, the GLS, LV twist, coronary flow reserve (CFR), malondyaldehide, and NT‐proBNP levels (OS biomarkers) improved by 25%, 27%, 14%, 27%, and 26%, respectively. The PWV and AIx were also improved. 43 Ustekinumab showed beneficial effects on the CACS compared to that of the controls. 44 18FDG‐PET/CT imaging showed improved glucose up‐take and decreased inflammatory biomarker levels. 45 The classic conception about the psoriatic pathological mechanism was associated with the IL‐12/Th1 pathway. Now, this idea has been revised and places IFN‐γ in the early steps of psoriatic development. The current model is based on the IL‐23/Th17 axis. 46
5.3. Anti‐IL‐17
Ixekizumab is a humanized IgG4 monoclonal antibody (mAb), secukinumab is a fully human mAb, and brodalumab is a human anti‐IL17RA mAb that blocks IL17RA, 17A/F, and 17E. In the 52‐week‐long CARIMA (evaluation of cardiovascular risk markers in psoriasis patients treated with secukinumab) study, brachial artery FMD was measured among moderate‐to‐severe psoriatic patients. Significant FMD improvements were observed compared to those at baseline. A 1% FMD increase indicated a 13% CVE risk decrease. 47 A prospective cohort study compared the effect of biologic therapy (anti‐TNF‐α, anti‐IL‐12/23, and anti‐IL‐17) to topical/phototherapy among psoriatic patients with a low Framingham risk score (median: 3%). CCTA was performed before the therapy initiation and 1 year later. Anti‐IL‐17 therapy induced a significant decrease in the FAI from −71.22HU to‐76.09HU, which was a 6.8% improvement (P < .001). The improvement was increased in the anti‐IL‐17 group. 27 In another study, coronary plaques in psoriatic patients with low Framingham risk scores (median: 3%) were assessed by CTA before and 1 year after biologic therapy (anti‐TNF‐α, anti‐IL‐12/23, and anti‐IL‐17) or topical/phototherapy. The decrease in all plaques was 5% in patients receiving biologic therapy, and the improvement was greater (6%) in noncalcified plaques. Anti‐IL‐17 therapy resulted in the greatest (12%) improvements. 48 Secukinumab improved left ventricular function (LVF) and decreased OS. LVF in response to three different anti‐psoriatic drugs (MTX, cyclosporine, and secukinumab) was assesses by Doppler echocardiography before treatment and 4 and 12 months later. After 1 year, secukinumab showed the greatest improvement in the GLS, GLSR, GLSR at early diastole, LV twist, CFR, and PWV (14%, 41%, 28%, 19%, and 11%) compared to those of the two other treatments. Malondialdehyde and protein carbonyl levels were only decreased in response to anti‐IL‐17 treatment. 49 In a Japanese case study on a 64‐year‐old man who had had psoriasis for 40 years, CTA‐controlled coronary constriction and interruption showed significant improvements during 2 years of secukinumab treatment with unchanged antihypertensive and statin treatment. 50
5.4. Anti‐IL‐23
IL‐23 inhibitors are the latest anti‐psoriatic drugs. Guselkumab is a human IgG1λ mAb, while tildrakizumab is a humanized IgG1 κ mAb against the IL‐23 p19 subunit. The latest anti‐IL‐23 drugs are mirikizumab (human mAb) and risankizumab (humanized mAb). The only available data regarding anti‐IL‐23 and cardiovascular comorbidities are adverse event reporting from clinical studies.
Table 1 shows the summary of available clinical data about cardiovascular effects of biologic therapies based on cardiovascular imaging modalities.
TABLE 1.
Summary of available clinical data about cardiovascular effects of biologic therapies based on cardiovascular imaging modalities
| Biologic drug | Molecule | CV imaging technique | Detected feature | References |
|---|---|---|---|---|
|
TNF‐α inhibitor |
Etanercept, infliximab, adalimumab | Ultrasound | Brachial and/or carotid IMT | 19 , 36 |
| Etanercept, infliximab | Ultrasound | Brachial FMD | 37 | |
| adalimumab | Ultrasound | cPWV | 39 | |
| Infliximab, etanercept, adalimumab, golimumab, or certolizumab | 18FDG‐PET/CT | Vascular inflammation | 40 | |
|
IL‐12/23 inhibitor |
Ustekinumab |
Echocardiography |
GLS, LV twist, coronary flow reserve (CFR), PWV, and AIx | 43 |
| CTA | CACS | 44 | ||
| 18FDG‐PET/CT | Vascular inflammation | 45 | ||
|
IL‐17 inhibitor |
Secukinumab | Ultrasound | Brachial FMD | 47 |
| Secukinumab, ixekizumab | CCTA | FAI | 27 | |
| Secukinumab, ixekizumab | CCTA | Plaque burden | 48 | |
| Secukinumab |
Echocardiography |
GLS, GLSR, GLSR at early diastole, LV twist, CFR, PWV | 49 | |
| Secukinumab (case study) | CTA | Resolved coronary constriction and interruption | 50 |
Abbreviations: Aix, augmentation index; CACS, calcium score; CCTA, coronary computed tomography angiography; CFR, coronary flow reserve; cPWV, carotid pulse wave velocity; CTA, computed tomography angiography; FAI, perivascular fat attenuation index; 18FDG‐PET/CT,18F‐fluoro‐deoxyglucose‐positron emission tomography/computed tomography; FMD, flow‐mediated dilatation; GLS, global longitudinal strain; GLSR, global longitudinal strain rate; IL, interleukin; IMT, intima media thickness; LV twist, left ventricular twist; PWV, pulse wave velocity; TNF‐α, Tumor Necrosis Factor‐alfa.
6. CONCLUSION
Systemic inflammation promotes cardiovascular comorbidities and significantly limits life expectancy. The latest data support that effective systemic anti‐inflammatory treatment improves not only cutaneous manifestations but also the incidence and severity of comorbidities, including CAD. Novel biologic therapies may show beneficial effects on CVEs by blocking pathogenically important, commonly targeted cytokines. Prospective studies on the prognostic value of biological therapies for CVEs could further improve multidisciplinary care for psoriatic patients. Real‐world data with large populations are needed to estimate the long‐term cardiovascular effects of these treatments. There is also a need to specifically describe the patient group of moderate‐to‐severe psoriatic patients who are in need of further cardiology care and screening. Dermatologists in the everyday practice are rarely in the situation to save lives. Focusing on the possible cardiovascular complications in severe psoriatic patients ‐which is the responsibility also of the treating dermatologist‐ may change this, and can lead to directly saved patient‐years, worldwide.
Dermatologists should expand their knowledge about the cardiovascular burden and the possible noninvasive investigative modalities during a dermatologist initialized interdisciplinary holistic patient care. Ultrasound and CT imaging can identify SA and facilitate the use of preventative therapies for those at increased risk.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Designing the study: Éva Anna Piros, Péter Holló; Generating the data for the study: Éva Anna Piros, Péter Holló; Gathering the data for the study: Éva Anna Piros, Péter Holló; Analysis of the data: Éva Anna Piros, Péter Holló; Had access to all of the raw data of the study: Éva Anna Piros, Péter Holló; Wrote the majority of the original draft of the article: Éva Anna Piros; Participated in writing article: Éva Anna Piros, Bálint Szilveszter, Borbála Vattay, Pál Maurovich‐Horvat, Edit Dósa, Péter Holló; Approved the final version of the article: Éva Anna Piros, Klára Szalai, Bálint Szilveszter, Borbála Vattay, Béla Merkely, Pál Maurovich‐Horvat, Edit Dósa, Péter Holló; Reviewed the pertinent raw data on which the results and conclusions of this study are based: Éva Anna Piros, Péter Holló; Guarantee that all individuals who meet the Journal's authorship criteria are included as authors of this article: Éva Anna Piros, Péter Holló.
Piros ÉA, Szilveszter B, Vattay B, et al. Novel anti‐inflammatory therapies to reduce cardiovascular burden of psoriasis. Dermatologic Therapy. 2021;34:e14721. 10.1111/dth.14721
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- 1. Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145(6):700‐703. [DOI] [PubMed] [Google Scholar]
- 2. Visalli E, Crispino N, Foti R. Multidisciplinary management of psoriatic arthritis: the benefits of a comprehensive approach. Adv Ther. 2019;36(4):806‐816. [DOI] [PubMed] [Google Scholar]
- 3. Ayala‐Fontánez N, Soler DC, McCormick TS. Current knowledge on psoriasis and autoimmune diseases. Psoriasis (Auckland, NZ). 2016;6:7‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogawa E, Sato Y, Minagawa A, Okuyama R. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45(3):264‐272. [DOI] [PubMed] [Google Scholar]
- 5. Boehncke WH, Boehncke S, Tobin AM, Kirby B. The 'psoriatic march': a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol. 2011;20(4):303‐307. [DOI] [PubMed] [Google Scholar]
- 6. Navarini AA, French LE. Survival of second‐line biologics in psoriasis: the British BADBIR registry data informs daily practice. J Invest Dermatol. 2018;138(4):726‐728. [DOI] [PubMed] [Google Scholar]
- 7. Rencz F, Hollo P, Karpati S, et al. Moderate to severe psoriasis patients' subjective future expectations regarding health‐related quality of life and longevity. J Eur Acad Dermatol Venereol. 2015;29(7):1398‐1405. [DOI] [PubMed] [Google Scholar]
- 8. Schaftenaar F, Frodermann V, Kuiper J, Lutgens E. Atherosclerosis: the interplay between lipids and immune cells. Curr Opin Lipidol. 2016;27(3):209‐215. [DOI] [PubMed] [Google Scholar]
- 9. Caiazzo G, Fabbrocini G, di Caprio R, et al. Psoriasis, cardiovascular events, and biologics: lights and shadows. Front Immunol. 2018;9:1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Egeberg A, Gisondi P, Carrascosa JM, Warren RB, Mrowietz U. The role of the interleukin‐23/Th17 pathway in cardiometabolic comorbidity associated with psoriasis. J Eur Acad Dermatol Venereol. 2020;34:1695‐1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang M, Cai ZR, Zhang B, et al. Functional polymorphisms in interleukin‐23 receptor and susceptibility to coronary artery disease. DNA Cell Biol. 2014;33(12):891‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choudhary S, Patel R, Pradhan D, et al. Psoriasis and cardiovascular disorders: association or epiphenomenon? Meta‐analysis of observational studies. 3 Biotech. 2020;10(3):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kothiwala SK, Khanna N, Tandon N, et al. Prevalence of metabolic syndrome and cardiovascular changes in patients with chronic plaque psoriasis and their correlation with disease severity: a hospital‐based cross‐sectional study. Indian J Dermatol Venereol Leprol. 2016;82(5):510‐518. [DOI] [PubMed] [Google Scholar]
- 14. Aksentijevich M, Lateef SS, Anzenberg P, Dey AK, Mehta NN. Chronic inflammation, cardiometabolic diseases and effects of treatment: psoriasis as a human model. Trends Cardiovasc Med. 2020;30(8):472‐478. 10.1016/j.tcm.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehta NN, Li K, Szapary P, Krueger J, Brodmerkel C. Modulation of cardiometabolic pathways in skin and serum from patients with psoriasis. J Transl Med. 2013;11:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siddiqi HK, Ridker PM. Psoriasis and atherosclerosis. Circ Res. 2018;123(11):1183‐1184. [DOI] [PubMed] [Google Scholar]
- 17. Mehta NN, Yu Y, Pinnelas R, et al. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124(8):775.e1‐775.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furue M, Tsuji G, Chiba T, Kadono T. Cardiovascular and metabolic diseases comorbid with psoriasis: beyond the skin. Intern Med. 2017;56(13):1613‐1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jokai H, Szakonyi J, Kontar O, et al. Impact of effective tumor necrosis factor‐alfa inhibitor treatment on arterial intima‐media thickness in psoriasis: results of a pilot study. J Am Acad Dermatol. 2013;69(4):523‐529. [DOI] [PubMed] [Google Scholar]
- 20. Gonzalez‐Cantero A, Gonzalez‐Cantero J, Sanchez‐Moya AI, et al. Femoral artery ultrasound for improving the detection of atherosclerosis in psoriasis. J Am Acad Dermatol. 2019;80(3):784‐786. [DOI] [PubMed] [Google Scholar]
- 21. Fang N, Jiang M, Fan Y. Association between psoriasis and subclinical atherosclerosis: a meta‐analysis. Medicine (Baltimore). 2016;95(20):e3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Groenendyk JW, Shukla P, Dey AK, et al. Association of aortic vascular uptake of (18)FDG by PET/CT and aortic wall thickness by MRI in psoriasis: a prospective observational study. Eur J Nucl Med Mol Imaging. 2019;46(12):2488‐2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Villasante Fricke AC, Iacobellis G. Epicardial adipose tissue: clinical biomarker of cardio‐metabolic risk. Int J Mol Sci. 2019;20(23):5989. 10.3390/ijms20235989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wong CX, Sun MT, Odutayo A, et al. Associations of epicardial, abdominal, and overall adiposity with atrial fibrillation. Circ Arrhythm Electrophysiol. 2016;9(12):e004378. 10.1161/CIRCEP.116.004378. [DOI] [PubMed] [Google Scholar]
- 25. Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22(11):450‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bacaksiz A, Tasal A, Sevgili E, et al. Epicardial fat thickness in patients with psoriasis vulgaris. Turk Kardiyol Dern Ars. 2014;42(1):47‐54. [DOI] [PubMed] [Google Scholar]
- 27. Elnabawi YA, Oikonomou EK, Dey AK, et al. Association of biologic therapy with coronary inflammation in patients with psoriasis as assessed by perivascular fat attenuation index. JAMA Cardiol. 2019;4:885‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kivelevitch D, Schussler Jeffrey M, Menter A. Coronary plaque characterization in psoriasis. Circulation. 2017;136(3):277‐280. [DOI] [PubMed] [Google Scholar]
- 29. Feldman SR, Hur P, Zhao Y, et al. Incidence rates of comorbidities among patients with psoriasis in the United States. Dermatol Online J. 2018;24(10):13030/qt2m18n6vj. [PubMed] [Google Scholar]
- 30. Parisi R, Rutter MK, Lunt M, et al. Psoriasis and the risk of major cardiovascular events: cohort study using the clinical practice research datalink. J Invest Dermatol. 2015;135(9):2189‐2197. [DOI] [PubMed] [Google Scholar]
- 31. Armstrong AW, Lin SW, Chambers CJ, Sockolov ME, Chin DL. Psoriasis and hypertension severity: results from a case‐control study. PloS One. 2011;6(3):e18227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rungapiromnan W, Yiu ZZN, Warren RB, Griffiths CEM, Ashcroft DM. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta‐analysis of randomized controlled trials. Br J Dermatol. 2017;176(4):890‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti‐inflammatory drugs in patients with severe psoriasis: 5‐year follow‐up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol. 2015;29(6):1128‐1134. [DOI] [PubMed] [Google Scholar]
- 34. Sigurdardottir G, Ekman AK, Stahle M, Bivik C, Enerback C. Systemic treatment and narrowband ultraviolet B differentially affect cardiovascular risk markers in psoriasis. J Am Acad Dermatol. 2014;70(6):1067‐1075. [DOI] [PubMed] [Google Scholar]
- 35. Gkalpakiotis S, Arenbergerova M, Gkalpakioti P, Potockova J, Arenberger P, Kraml P. Impact of adalimumab treatment on cardiovascular risk biomarkers in psoriasis: results of a pilot study. J Dermatol. 2017;44(4):363‐369. [DOI] [PubMed] [Google Scholar]
- 36. Ramonda R, Puato M, Punzi L, et al. Atherosclerosis progression in psoriatic arthritis patients despite the treatment with tumor necrosis factor‐alpha blockers: a two‐year prospective observational study. Joint Bone Spine. 2014;81(5):421‐425. [DOI] [PubMed] [Google Scholar]
- 37. Mazzoccoli G, Notarsanto I, de Pinto GD, et al. Anti‐tumor necrosis factor‐alpha therapy and changes of flow‐mediated vasodilatation in psoriatic and rheumatoid arthritis patients. Intern Emerg Med. 2010;5(6):495‐500. [DOI] [PubMed] [Google Scholar]
- 38. Nakao M, Nakamura K, Fukasawa T, et al. Assessment of endothelial function during the loading phase of infliximab in psoriasis: a potential predictor of its drug survival. Int J Dermatol. 2019;58(1):54‐59. [DOI] [PubMed] [Google Scholar]
- 39. Pina T, Corrales A, Lopez‐Mejias R, et al. Anti‐tumor necrosis factor‐alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6‐month prospective study. J Dermatol. 2016;43(11):1267‐1272. [DOI] [PubMed] [Google Scholar]
- 40. Eder L, Joshi AA, Dey AK, et al. Association of tumor necrosis factor inhibitor treatment with reduced indices of subclinical atherosclerosis in patients with psoriatic disease. Arthritis Rheumatol. 2018;70(3):408‐416. [DOI] [PubMed] [Google Scholar]
- 41. Bissonnette R, Harel F, Krueger JG, et al. TNF‐alpha antagonist and vascular inflammation in patients with psoriasis vulgaris: a randomized placebo‐controlled study. J Invest Dermatol. 2017;137(8):1638‐1645. [DOI] [PubMed] [Google Scholar]
- 42. Bergstrom U, Jovinge S, Persson J, Jacobsson LTH, Turesson C. Effects of treatment with adalimumab on blood lipid levels and atherosclerosis in patients with rheumatoid arthritis. Curr Ther Res Clin Exp. 2018;89:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ikonomidis I, Papadavid E, Makavos G, et al. Lowering interleukin‐12 activity improves myocardial and vascular function compared with tumor necrosis factor‐a antagonism or cyclosporine in psoriasis. Circ Cardiovasc Imaging. 2017;10(9):e006283. 10.1161/CIRCIMAGING.117.006283. [DOI] [PubMed] [Google Scholar]
- 44. Hjuler KF, Bottcher M, Vestergaard C, Botker HE, Iversen L, Kragballe K. Association between changes in coronary artery disease progression and treatment with biologic agents for severe psoriasis. JAMA Dermatol. 2016;152(10):1114‐1121. [DOI] [PubMed] [Google Scholar]
- 45. Kim BS, Lee WK, Pak K, et al. Ustekinumab treatment is associated with decreased systemic and vascular inflammation in patients with moderate‐to‐severe psoriasis: feasibility study using (18)F‐fluorodeoxyglucose PET/CT. J Am Acad Dermatol. 2019;80(5):1322‐1331. [DOI] [PubMed] [Google Scholar]
- 46. Chiricozzi A, Romanelli P, Volpe E, Borsellino G, Romanelli M. Scanning the Immunopathogenesis of psoriasis. Int J Mol Sci. 2018;19(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. von Stebut E, Reich K, Thaci D, et al. Impact of Secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Invest Dermatol. 2019;139(5):1054‐1062. [DOI] [PubMed] [Google Scholar]
- 48. Elnabawi YA, Dey AK, Goyal A, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. 2019;115(4):721‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Makavos G, Ikonomidis I, Andreadou I, et al. Effects of interleukin 17A inhibition on myocardial deformation and vascular function in psoriasis. Can J Cardiol. 2020;36(1):100‐111. [DOI] [PubMed] [Google Scholar]
- 50. Yamazaki F, Takehana K, Tamashima M, Okamoto H. Improvement in abnormal coronary arteries estimated by coronary computed tomography angiography after secukinumab treatment in a Japanese psoriatic patient. J Dermatol. 2019;46(2):e51‐e52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


