Summary
Obesity is an evolutionary, chronic, and relapsing disease that consists of a pathological accumulation of adipose tissue able to increase morbidity for high blood pressure, type 2 diabetes, metabolic syndrome, and obstructive sleep apnea in adults, children, and adolescents. Despite intense research over the last 20 years, obesity remains today a disease with a complex and multifactorial etiology. Recently, long non‐coding RNAs (lncRNAs) are emerging as interesting new regulators as different lncRNAs have been found to play a role in early and late phases of adipogenesis and to be implicated in obesity‐associated complications onset. In this review, we discuss the most recent advances on the role of lncRNAs in adipocyte biology and in obesity‐associated complications. Indeed, more and more researchers are focusing on investigating the underlying roles that these molecular modulators could play. Even if a significant number of evidence is correlation‐based, with lncRNAs being differentially expressed in a specific disease, recent works are now focused on deeply analyzing how lncRNAs can effectively modulate the disease pathogenesis onset and progression. LncRNAs possibly represent new molecular markers useful in the future for both the early diagnosis and a prompt clinical management of patients with obesity.
Keywords: adipogenesis, lncRNAs, metabolic diseases, obesity
Abbreviations
- ADINR
adipogenic differentiation‐induced ncRNA
- AdipoQ AS
adiponectin antisense RNA
- ADNCR
adipocyte differentiation‐associated lncRNA
- AF
atrial fibrillation
- AngII
angiotensin II
- ANRIL
antisense ncRNA in the INK4 Locus
- APF
autophagy promoting factor
- ASMER‐1 and ASMER‐2
adipocyte‐specific metabolic‐related lncRNAs
- BMI
body mass index
- CAIF
cardiac autophagy inhibitory factor
- CARL
cardiac apoptosis‐related lncRNA
- CDKN2B‐AS1
cyclin‐dependent kinase inhibitor 2B antisense RNA 1
- Chaer
cardiac‐hypertrophy‐associated epigenetic regulator
- CHD
coronary heart diseases
- CHRF
cardiac hypertrophy‐related factor
- CIDEC
cell death‐inducing DFF45‐like effector
- CVD
cardiovascular diseases
- DGAT
diacylgycerolacyltransferase
- DN
diabetic nephropathy
- DNMT1
DNA methyl transferase 1
- Giver
Growth factor‐ and pro‐Inflammatory cytokine‐induced Vascular cell‐Expressed lncRNA
- hADSCs
human adipose‐derived stem cells
- HFD
high‐fat diet
- hLMR
human lncRNA metabolic regulators
- HOTAIR
HOX transcript antisense RNA
- HRCR
heart‐related circRNA
- IMFNCR
intramuscular fat‐associated lncRNA
- IR
insulin resistance
- LIPCAR
long intergenic non‐coding RNA predicting cardiac remodeling
- lnc‐ORA
obesity‐related lncRNA
- lncRNA
long non‐coding RNAs
- MALAT1
metastasis‐associated lung adenocarcinoma transcript 1
- MCE
mitotic clonal expansion phase
- MDRL
mitochondrial dynamic‐related lncRNA
- Meg3
maternally expressed gene 3
- MHRT
myosin heavy chain associated RNA transcripts
- MIAT
myocardial infarction‐associated transcript
- MIR221HG
miR‐221 host gene
- MIR31HG
miR‐31 host gene
- MIRT1
myocardial infarction‐associated transcript 1
- NAFLD
nonalcoholic fatty liver disease
- OA
osteoarthritis
- PRC2
Polycomb Repressor Complex 2
- SD
standard deviations
- SRA
steroid receptor RNA activator
- T2D
type 2 diabetes
- TINCR
tissue differentiation‐inducing non‐protein coding RNA
- VSMCs
vascular smooth muscle cells
- WHO
World Health Organization
- Wisper
Wisp2 super‐enhancer‐associated RNA
- βlinc1
β‐cell long intergenic noncoding RNA
1. INTRODUCTION TO OBESITY: CAUSES AND CONSEQUENCES
Obesity is defined by the World Health Organization (WHO) as a condition of abnormal or excessive accumulation of body fat that presents a health risk, increasing both morbidity (for many chronic diseases such as type 2 diabetes [T2D], hypertension, coronary artery disease, dyslipidemia, stroke, osteoarthritis (OA), and even certain forms of cancer) 1 , 2 , 3 , 4 , 5 and mortality. 3 The most recent report of the WHO shows how the worldwide prevalence of obesity nearly tripled between 1975 and 2016, as over 650 million adults clinically were affected by obesity and 41 million children below the age of 5 and over 340 million children and adolescents between 5 and 19 years of age were either overweight or affected by obesity. 3 , 6 Indeed, studies show that 70% of adolescents with obesity will maintain their obese condition as adults, with a significant impact on their physical and physiological health. 3 , 7 , 8 , 9 Specifically, an adult is affected by obesity when his/her body mass index (BMI) is greater than or equal to 30. 3 In the pediatric age, according to the WHO, obesity in children under 5 years of age is defined as weight‐for‐height 3 standard deviations (SD) above the WHO Child Growth Standards reference median. For children aged 5–19 years, obesity is defined as BMI‐for‐age 2 SD above the WHO Growth Standards reference median. 6
Conventional therapies for patients with obesity, such as lifestyle modifications (diet and exercise) and also pharmacotherapy in adults, remain important but are limited by their results in terms of weight loss and weight loss maintenance at long term, and in the next future the development of new combinatory clinical approaches is needed. 10 , 11 , 12 , 13 From a cellular perspective, obesity is caused by the excessive accumulation of adipose cells in different anatomical parts of the body. This is due to an increase in adipocytes' size (hypertrophy), number (hyperplasia) both and even in an imbalance of the adipogenesis process. 14 , 15 At present, it remains thus necessary to continue research on the biological basis of this complex pathology starting from genetic, epigenetic, and molecular pathways as it is not possible to conclude what the relative contribution of genetics and environment are in obesity onset. Indeed, behavior and genes are different levels of the same causal framework, and epigenetics through RNA biology might play a central role in elucidating new targetable pathways. “Classic” epigenetic mechanisms and epigenetic mosaicism, a widespread phenomenon documented in many organisms, that may account for differences in body weight and fat accumulation among people remain to be better investigated, 16 , 17 , 18 taking into account of the role of non‐coding RNAs as possible epigenetic modulator of obesity and secondary co‐morbidities onset. In this review, we aim to discuss the functional roles of long non‐coding RNAs (lncRNAs), focusing on the state of the art and the future clinical implications of lncRNAs in adipogenesis, obesity, and obesity complications onset.
2. LNCRNAS: DEFINITION AND PRINCIPAL FUNCTIONS
In recent years, the role of RNA is changed, and indeed it is now established knowledge that only 1–2% of the human genome codes for protein. 19 , 20 , 21 For this reason, RNAs can be classified for their coding potential in coding RNAs (transcripts that will subsequently be translated into proteins) and non‐coding RNAs that do not code for a polypeptide and whose function is still to be fully understood especially in modulating gene expression. 19 , 20 , 21 Among the non‐coding RNAs, it is possible to distinguish two subclasses: small non‐coding RNAs, molecules smaller than 200 bp, and lncRNAs, defined as non‐coding RNA molecules longer than 200 bp. 22 LncRNAs are poorly conserved, frequently unstable, and/or sometimes present in few copies, and new biological roles have emerged for some lncRNAs. 23 , 24 , 25 In order to facilitate the reader through this mounting evidence in different models, the lncRNAs reported in this work are listed for their homology as summarized in Table S1.
Interestingly, lncRNAs can mediate transcriptional regulation in different ways. Indeed, these molecules can modulate gene expression at multiple levels, ranging from chromatin re‐arrangements to transcriptional regulation or even translational modulation. 26 , 27 , 28 Multiple pieces of evidence suggest that they can operate through distinct modes, including working as signals, scaffolds for protein–protein interactions, molecular decoys, and guides to target elements in the genome or transcriptome. 29 This high degree of complexity in gene expression regulation, and the number of still unknown mechanisms through which lncRNAs could act, indicates a clear need to further investigate these molecules, both in health and disease, as they could provide crucial new insights in cell biology representing promising targets for the development of innovative therapeutic strategies for multiple diseases, with a specific relevance for their epigenetic regulation of metabolic diseases. Indeed, the non‐coding transcriptome is becoming more and more relevant also in the field of adipogenesis, fat mass expansion, and obesity, and in this context lncRNAs represent new potential candidate targets for the development of therapies. 23 , 24 , 25
3. LNCRNAS IN ADIPOGENESIS AND OBESITY
Noncoding RNAs are known to play a regulatory role in many developmental contexts, including adipogenesis. Indeed, lncRNAs have been demonstrated to be involved in adipogenesis with subsequent implications for obesity and obesity‐related complications in adults and children. 30 , 31 , 32 As more and more studies in this field arise every year, there is a need to distinguish between the multiple functions that the lncRNAs could have. Indeed, results are variable, and a full characterization of the role that lncRNAs play in obesity is far from being present. Numerous lncRNAs have been correlated with adipogenesis, and the aim of this section is thus to classify them accordingly to their role in different stages of adipocytes differentiation, subsequently focusing on their role in obesity.
3.1. Role of lncRNAs in the regulation of early adipogenesis master regulators
Adipogenesis is the process of adipocytes formation into fat‐containing cells from stem cells or adipocyte precursors. It involves two phases: determination (considered an early stage) and terminal differentiation (late adipogenesis). 14 , 33 , 34
Early stages of adipogenesis are represented by a mitotic clonal expansion phase (MCE) and by the expression of early regulators such as C/EBPβ and C/EBPδ. 34 , 35 , 36 Among the lncRNAs able to influence this stage of adipogenesis, the lncRNA steroid receptor RNA activator (SRA) was one of the first to be described. 37 Its expression resulted twofolds higher in differentiated murine 3T3‐L1 adipocytes than pre‐adipocytes, but the lncRNA seems to also act in early phases of adipogenesis. 38 Indeed, it can promote S‐phase entry during the MCE of adipogenesis controlling cell cycle genes' expression. 37 Moreover, in the mouse ST2 mesenchymal cell line, SRA is implicated in the regulation of p38/JNK′ phosphorylation inhibition, a crucial step in the early stages of adipogenesis, as well as in stimulating insulin receptor gene expression and downstream signaling. 39 , 40 The obesity‐related lncRNA (lnc‐ORA), whose expression levels increases during adipogenesis in obese mice, also regulates the cell cycle through induction of expression of crucial marker genes such as PCNA, cyclin B, cyclin D1, and cyclin E. 41 Modulation of the cell cycle and thus early stages of adipogenesis can also occur through epigenetic modulation, and indeed the lncRNA slincRAD was found to interact with the DNA methyl transferase 1 (DNMT1) in the S phase of the cell cycle in mouse, facilitating the cell's entry into the MCE phase. 42 Through microarray study a novel lncRNA, the lncRNA‐Adi, has been identified and found to be highly expressed in the MCE phase in rat adipocytes. It exerts its effect through the interaction with miR‐449a, enhancing the expression of the miRNA's target CDK6, a cyclin‐dependent kinase sensitive to high‐fat diet (HFD) and involved in the regulation of cell beige tissue formation. 43 , 44
The genetic location of lncRNAs could be of crucial relevance in identifying their target genes. Three recently discovered lncRNAs, Gm15051, Tmem189, and Cebpd genomically, locate respectively next to Hoxa1, C/EBPβ, and C/EBPδ in mouse, and their expression levels correlate, suggesting that each of them can positively influence the neighboring gene's expression having the role of transcriptional regulators. 45
3.2. Role of lncRNAs in the regulation of late adipogenesis master regulators
As pre‐adipocytes mature into adipocytes, C/EBPβ and δ target the promoters of C/EBPα and PPARγ, master regulators of adipocytes terminal differentiation as they activate genes that are involved in insulin sensitivity, lipogenesis, and lipolysis, with subsequent implications for diseases involving lipid metabolism such as dyslipidemia.
This step is critical for late adipocyte differentiation, and indeed numerous lncRNAs have been found to modulate specifically PPARγ (Figure 1), along with other late‐adipogenesis regulators. SRA also plays a role in this context, as it exerts its function via direct association with the PPARγ protein in murine cells, promoting its transcriptional activity. 37 , 38 Another mode of action through which lncRNAs can modulate PPARγ is through miRNA sponging. This is the case of lncRNA IMFNCR (intramuscular fat‐associated lncRNA), which has been found to promote intramuscular adipocyte differentiation in chicken sponging miR‐128‐3p and miR‐27b‐3p, which directly target PPARγ. 46 There can also be an indirect lncRNA‐miRNA modulation of PPARγ, through other epigenetic modulators. The adipocyte differentiation‐associated lncRNA (ADNCR) can sponge miR‐204, whose target gene, SIRT1, is known to form a complex with modulators such as NCoR and SMART to repress PPARγ activity in bovine adipocytes. 47 An epigenetic modulation can happen at PPARγ's promoter, in sites known as CpG islands that when methylated decrease the expression of the respective downstream genes. Indeed, the lncRNA Plnc1, transcribed 25,000 bp upstream of PPARγ2, can attenuate the methylation status of its promoter increasing subsequent transcription in mouse. 48 PPARγ can also be targeted at the end of specific signal transduction pathways, as demonstrated for STAT3 gene expression regulation. 49 Specifically, adipogenesis is induced by the activation of STAT3, acting as a molecular switch. This effect was counteracted by PPARγ's activation with the agonist troglitazone, suggesting that STAT3 can modulated adipogenic differentiation through a PPARγ upstream regulation. 49 The nuclear lncRNA PVT1 has been found to associate with STAT3 in 3T3‐L1 pre‐adipocytes, and indeed PVT1 has been found to correlate with increased expression of PPARγ, but also C/EBPα, FABP4, and genes related to fatty acid synthesis. 50 Well‐renowned lncRNAs, such as NEAT1, widely implicated in numerous cancers, can also have a function in adipogenesis, and indeed NEAT1 has been found to modulate the splicing of PPARγ, increasing the expression of the isoform 2 through SRp40 association in 3T3‐L1 pre‐adipocyte. 51 PPARγ can itself regulate lncRNA's expression, such as AK079912, which presents three conserved PPARγ binding sites in its promoter region 52 or lnc‐BATE in mouse. 53
FIGURE 1.
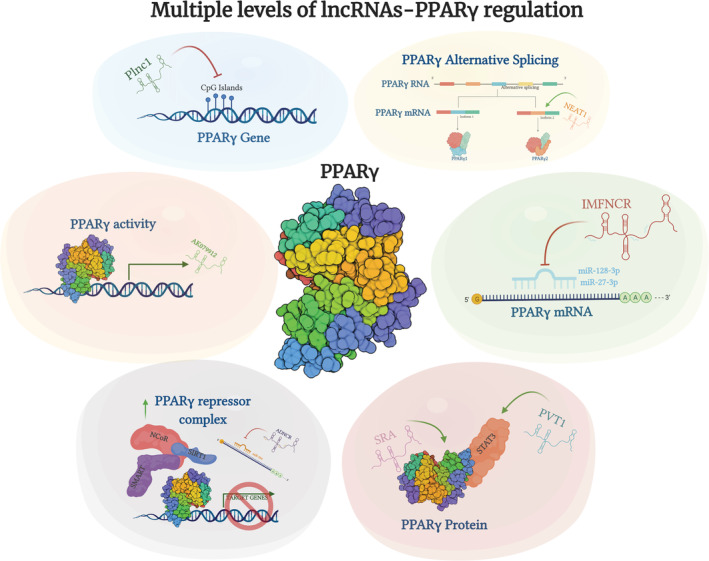
LncRNAs can influence PPARγ's transcription and activity at multiple levels. Specifically, lncRNAs can modulate directly PPARγ by inhibiting DNA methylation. They can also selectively induce a different PPARγ mRNA splicing or sponge‐specific miRNAs which would sequester and lead to degradation of PPARγ's mRNA. They directly bind to the PPARγ protein being able to inhibit its activity through upregulation of the PPARγ repressor complex. Lastly, PPARγ itself can induce the expression of specific lncRNAs. Made in ©BioRender—biorender.com
PPARγ is not the only player in late adipogenesis, and indeed, lncRNAs can modulate other key targets. Specifically, knockdown of the lncRNA HOXA11‐AS1 can result in the inhibition of adipocyte differentiation through a decrease of C/EBPα, diacylgycerolacyltransferase (DGAT) 2, cell death‐inducing DFF45‐like effector (CIDEC), and perilipin. 54 On the other hand, the tissue differentiation‐inducing non‐protein coding RNA (TINCR) can form a feedback loop with miR‐31 and C/EBPα, promoting adipogenesis in human adipose‐derived stem cells (hADSCs). 55 The adipogenic differentiation‐induced ncRNA (ADINR) can activate MLL3/4, epigenetically modulating transcription of C/EBPα in hADSCs. 57 , 58 LncRNAs can also bind epigenetic regulators and upregulate expression of late‐adipogenesis genes, as does miR‐31 host gene (MIR31HG), which is able to promote the binding of H3K4me3 to FABP4's promoter, increasing its expression in hADSCs. 56 The Wnt/β‐catenin signaling is also influenced by a novel nuclear lncRNA, AC092834.1 in hADSCs. This lncRNA directly promoted an increase in the expression of DKK1, which competitively binds to LRP5 to degrade cytosolic β‐catenin, ultimately leading to upregulation of adipogenic transcripts such as PPARγ, FAPB4, and C/EBPα. 57
A specific subclass of lncRNAs, defined as “antisense RNAs,” can modulate the expression of their respective sense gene altering processes in which they are involved. For example, PU.1AS can form a RNA‐duplex with PU1, a molecule that inhibits adipogenesis, hindering its expression and subsequent protein expression with a decreased expression of PPARγ, fatty acid synthase, and adiponectin in mouse. 58 , 59 Similarly, adiponectin antisense RNA (AdipoQ AS) can modulate adiponectin expression and inhibit murine adipogenesis. 60 Although not its antisense, lnc‐leptin is directly correlated with leptin, as it is transcribed from an enhancer region upstream of leptin and their expression directly correlates. 61
The lncRNA's correlation with adipogenesis can also be negative, as some lncRNAs have been found to be decreased in adipogenesis, such as lnc‐U90926 in murine 3T3‐L1 pre‐adipocytes, 62 miR‐221 host gene (MIR221HG) in bovine adipocytes, and lncRNA H19 in human bone marrow mesenchymal stem cells. 63 , 64
Further studies might be needed to clarify specific lncRNA's functions in this process, as controversial evidences are also present. This is the case of maternally expressed gene 3 (Meg3), a novel lncRNA which has been defined as both able to inhibit and promote adipogenesis. 65 , 66 Indeed, a first study reported that silencing of Meg3 promoted adipogenesis through the overexpression of the adipogenesis‐related miR‐140‐5p, PPARγ, and C/EBPα, suggesting that when Meg3 is absent, adipogenesis is induced. 65 On the contrary, another work described Meg3's role in upregulating Dickkpof‐3 through interaction with miR‐217, ultimately leading to an upregulation of adipogenesis via the induction of expression of adipogenesis‐related genes such as FABP4. 66 This might be due to a time‐specific effect of the lncRNA's action, or the different cellular context as the first study was performed in human cells whereas the second in murine 3T3‐L1 pre‐adipocytes.
3.3. Identification of lncRNAs specifically associated with obesity
Specific studies correlate lncRNAs with the obese phenotype and obesogenic models. Among them, SRA has been demonstrated to be strictly associated with obesity, as it has been shown that SRA−/− mice have a phenotype of resistance to HFD‐induced obesity with decreased fat mass, reduced fatty liver, and improved glucose tolerance. 67 High‐throughput techniques such as RNA sequencing allowed the screening of the whole transcriptome in adipose tissue of patients with obesity versus lean individuals, leading to the identification of novel lncRNAs involved in the disease. In one study, two lncRNAs termed adipocyte‐specific metabolic‐related lncRNAs (ASMER‐1 and ASMER‐2) were identified and found to regulate adipogenesis, lipid mobilization, and adiponectin secretion. 68 Screenings were also performed in gluteal subcutaneous adipose tissue on healthy subjects, in which 120 adipose‐derived lncRNAs were identified 69 and in children with obesity, with the identification of 1268 lncRNAs, and a specific relevance for RP11‐20G13.3 has been found. 31 The same has been done in mice, where brown and white adipocytes, pre‐adipocytes, and cultured adipocytes were screened leading to the identification of 175 different lncRNAs that are specifically regulated during adipogenesis in one study 70 and 735 upregulated and 877 downregulated lncRNAs in murine brown versus white adipocytes. 71 Similarly, inguinal white adipose tissue has been screened in obese mice compared to wild type ones, identifying 46 differentially expressed lncRNAs. 41 Moreover, lncRNAs such as PVT1 and Plnc1 were found to be upregulated in obese mice. 48 , 50
From an anatomical point of view, lncRNAs expression can differ in different fat depots, as it is for HOX transcript antisense RNA (HOTAIR) which has been demonstrated to be highly expressed in gluteal‐femoral fat, and mechanical stimulation of this area in human subjects induces exosomal secretion of HOTAIR, which then circulates in the bloodstream resulting in higher serum expression in subjects with obesity and a sedentary lifestyle. 72
4. LNCRNAS IN OBESITY‐ASSOCIATED DISEASES
Given the strong implications of lncRNAs in adipogenesis and adipocytes differentiation, it was a natural evolution to study the role of these molecular modulators in obesity and in the related most common complications. 73 , 74 , 75 The obesity‐associated diseases are numerous, 1 , 2 , 3 and the initiating events start early in childhood. 76 Indeed, very recently numerous lncRNAs have been found to correlate with obesity‐associated inflammatory diseases. 77 The following sections summarize recent advances in identifying lncRNAs implicated in cardiovascular complications (such as myocardial infarction, coronary heart diseases (CHD), cardiac hypertrophy, heart failure, atrial fibrillation (AF), and atherosclerotic thrombosis), endocrine/metabolic complications (such as T2D and nephropathy), and even immune‐related complications (such as OA) which are obesity‐associated and/or regulated.
4.1. Cardiovascular diseases
Cardiovascular diseases (CVD) include myocardial infarction, CHD, cardiac hypertrophy, heart failure, AF, and atherosclerotic thrombosis. 73 , 78 , 79 , 80 Childhood and adolescent obesities play a crucial role in developing CVD risk factors and are linked to higher risk of cardiovascular morbidity and mortality in adulthood. 81 Numerous lncRNAs are implicated in CVD, and among them cardiac autophagy inhibitory factor (CAIF) is downregulated in end‐stage cardiomyopathy and usually could represent a good biomarker of a disease state in humans. 82 CAIF seems to have a protective role through suppression of cardiac autophagy while directly blocking p53. P53 is known to target and upregulate myocardin in myocardial ischemia and reperfusion, and CAIF thus indirectly inhibited myocardin's expression. 83 It has been reported that antisense ncRNA in the INK4 Locus (ANRIL) can sponge miR‐99a and miR‐449 during autophagy processes, subsequently upregulating thrombomodulin and promoting angiogenesis in human umbilical vein endothelial cells. 84 The lncRNA autophagy promoting factor (APF) can also influence autophagic cell death in murine myocardial infarction targeting miR‐188‐3p and autophagy‐related protein 7. 85 A third lncRNA which can modulate murine autophagy through miRNA sponging is AK088388, regulating Beclin‐1 and LC3‐II's expression through miR‐30a. 86
LncRNAs can also target the apoptotic process in cardiomyocytes, which can lead to myocardial infarction. P53 is also implicated in apoptosis modulation, and the lncRNA Meg3 can target p53 and subsequently modulate NF‐κB‐ and ERS‐associated apoptosis in murine ventricular myocytes. 87 Cardiac apoptosis‐related lncRNA (CARL) is able to sponge miR‐539 in mice and thus indirectly upregulate its target PHB2, which modulates apoptosis and mitochondrial fission. 88 Mitochondrial fission and fusion are indeed strictly associated with cardiomyocyte apoptosis. The lncRNA AK009271, named mitochondrial dynamic‐related lncRNA (MDRL), has been proved to be involved in mitochondrial fission and fusion under stress conditions. MDRL can interact with miR‐361 and suppress it, thus reducing mitochondrial fission and apoptosis upon anoxia/reoxygenation treatment in murine cardiomyocytes. 89 , 90 A specific analysis of lncRNAs involved in myocardial infarction has been performed by Chen and colleagues, which reports numerous studies aimed at performing high‐throughput screening of lncRNAs which are differentially expressed in various heart diseases. 91 They also report an implication for the lncRNAs ZFAS1, 92 , 93 HOTAIR, 94 MALAT1, 95 , 96 GAS5, 97 FAF, 98 TTTY15, 99 ECRAR, 100 AK080084, 101 NR_045363, 102 TUG1, 103 and Meg3. 91 , 104
Myocardial infarction can indeed influence a differential lncRNAs expression. Specifically, acute myocardial infarction in mice was associated with the upregulation of two lncRNAs named myocardial infarction‐associated transcript 1 (MIRT1) and 2 (MIRT2), which negatively correlated with infarct size and positively correlated with ejection fraction. MIRT1 and MIRT2 modulate the expression of multiple genes known to be involved in processes affecting left ventricular remodeling, such as extracellular matrix turnover, inflammation, fibrosis, and apoptosis. 105 The lncRNA metastasis‐associated lung adenocarcinoma transcript 1 (MALAT1) has been seen expressed in cardiomyocytes subjected to hypoxia, high glucose, cytokine, and oxidative stress which are all risk factors of CVD in human and murine models, and thus has been suggested to represent a new possible therapeutic target in the disease. 106 The lncRNA myosin heavy chain associated RNA transcripts (MHRT) was upregulated in blood of patients with myocardial infarction and seems to be upregulated in cardiac myocytes in the presence of high levels of reactive oxygen species to exert protective effect on these cells. 107 The lncRNA Wisp2 super‐enhancer‐associated RNA (Wisper) was induced in cardiac fibrosis in both human patients and murine models, where it could be protective through regulation of cardiac fibroblast proliferation, migration, and survival. 108 MIAT has been found to be upregulated in serum of patients with coronary atherosclerotic heart disease. 109 MIAT can also sponge and thus inhibit miR‐133a‐3p, protective in multiple heart diseases for its role in improving cardiac function and decreasing fibrosis in rat models. 110
LncRNAs can also influence cardiac hypertrophy and thus aggravate CVD, as cardiac hypertrophy is a crucial hallmark of heart failure. 111 Indeed, the heart‐enriched lncRNA cardiac‐hypertrophy‐associated epigenetic regulator (Chaer), can epigenetically interact with the Polycomb Repressor Complex 2 (PRC2) and inhibit histone H3 lysine 27 methylation at the promoter regions of genes involved in cardiac hypertrophy, thus inducing the expression of genes involved in cardiac hypertrophy, with studies performed in rat, murine, and human cells. 112 Cardiac hypertrophy can also be induced by the lncRNA cardiac hypertrophy‐related factor (CHRF) in mouse, although in this case the underlying mechanisms involves sponging of miR‐489 and subsequent upregulation of the miRNA's target Myd88, a regulator of cardiomyocyte hypertrophy. 113 The lncR‐UCA1 is upregulated in mice hypertrophic cardiomyocytes, and it can sponge miR‐184, enhancing the expression of HOXA9. 114 A detailed report on lncRNAs in cardiac hypertrophy is reported in the work by Liu and colleagues, 115 which also implicates the lncRNAs MHRT, 116 Meg3, 117 DACH1, 118 H19, 119 Plscr4, 120 SNHG1, 121 TINCR, 122 Uc.323, 123 and Ahit. 124 Other lncRNAs have also been implicated in heart failure, 111 as does the heart‐related circRNA (HRCR), which in mice was found to acts as endogenous sponge to mir‐223, protecting them from hypertrophic stimuli. 125 Moreover, the lncRNA HypERlnc was significantly reduced in human cardiac tissue from patients with heart failure compared with controls. 126 Moreover, the lncRNAs profile was analyzed in plasma of patients with ischemic cardiomyopathy and dilated cardiomyopathy, two major problems which lead to heart failure. 127 This microarray analysis identified 3222 differentially expressed lncRNAs, highlighting also a co‐expression between lncRNAs and mRNAs. 127 Other high‐throughput screening for lncRNAs in heart failure were performed in rat models of ischemic heart failure, 128 in murine models of post‐myocardial infarction, 129 in explanted human heart failure hearts versus control donated ones, 130 and in left ventricle biopsies of patients affected by non‐end‐stage dilated ischemic cardiomyopathy and matched controls 131 highlighting a substantial number of lncRNAs implicated in the pathophysiology of this process. 91 , 132 , 133
Another form of CVD is AF, which is the most common type of arrhythmia. 134 Numerous studies were performed on the role of lncRNAs in this disease, and also in this case high‐throughput screening has allowed the identification of mounting evidences on lncRNAs in this disease. 135 Specifically, a study conducted in right atrium tissue of patients with rheumatic heart diseases and AF or normal sinus rhythm highlighted 182 differentially expressed lncRNAs. 134 Another work identified the transcriptome profile of left and right atrial appendages of patients with AF versus controls and identified NPPA and its antisense as potential regulators of muscle contraction in AF and moreover RP11‐99E15.2 and RP3‐523K23.2 which could modulate extracellular matrix binding and transcription of HSF2 targets, respectively. 136 The atrial tissue was also examined in another study considering three AF patients, highlighting 219 differentially expressed lncRNAs. 137 RNA‐seq performed in lymphocytes of patients with permanent AF versus controls highlighted the differential expression profiles of lncRNAs, ultimately implicating two lncRNAs, ETF1P2 and AP001053.11, in AF pathogenesis. 138 , 139 Also focusing on the relevance of lncRNAs as peripheral biomarkers, another study performed a microarray study on blood from patients with AF and matched controls, highlighting 177 deregulated lncRNAs, with the two most deregulated being NONHSAT040387 and NONHSAT098586. 140 Lastly, a study in atria from AF rabbit highlighted 99,843 putative new lncRNAs, of which TCONS_00075467 was selected to be important for electrical remodeling, possibly through sponging of miR‐328 and subsequent regulation of CACNA1C. 141 Other lncRNAs implicated in AF include TCONS_00202959, 142 AK055347, 143 MIAT, 110 KCNQ1OT1, 144 and others extensively reviewed in previous publications. 135 , 145 , 146 , 147 When focusing on the adipose tissue implication in AF, the number of studies is more restricted, but a very recent work performed a RNA‐sequencing analysis in epicardial adipose tissue samples of patients with persistent non‐valvular AF and sinus rhythm, highlighting 57 differentially expressed lncRNAs. 148
Numerous lncRNAs have also been found deregulated in CHD, with one recent work highlighting a network of 62 lncRNAs, 332 miRNAs, and 366 mRNA differentially expressed in peripheral blood mononuclear cells (PBMCs) of patients with CHD versus controls. 149 The screening led to the identification of two lncRNAs, CTA‐384D8.35 and CTB‐114C7.4, as main players in the disease. 149 Also in this case, an in‐depth classification of both miRNA and lncRNAs involved in CHD was performed by Zhang and colleagues, 150 which specifically report the implicated lncRNAs to be ANRIL, 151 H19, 152 HIF1A‐AS1, 153 linc‐p21, 154 RNCR3, 155 TGFB2‐OT1, 156 lnc‐Ang362, 157 HAS2‐AS1, 158 SMILR, 159 SENCR, 160 Meg3, 161 and lnc‐MKI67IP‐3. 162
Lastly, lncRNAs are also being investigated for their role in atherosclerotic thrombosis, with multiple recent works focusing especially on this topic. 163 , 164 , 165 These include ANRIL, 166 LeXis, 167 RP5‐833A20.1, 168 MeXis, 169 and several more, able to act through numerous processes such as vascular remodeling, endothelial dysfunction, leukocyte recruitment, macrophage apoptosis, and cholesterol metabolism. 165
In conclusion, recent evidence indicates the important roles of lncRNAs in the complex regulatory network of CVD, and many of them could be used as novel therapeutic targets and/or biomarkers for early diagnosis or prognosis for CVD. Indeed, current therapies for CVD such as cardiac hypertrophy currently alleviates symptoms, but new genetic analyses could provide new therapeutic targets. 115 Modulation of lncRNAs such as Meg3, Plscr4, H19, SNHG1, uc.323, or Ahit could attenuate the increasing size of cardiomyocytes. 117 , 119 , 120 , 121 , 123 , 124 Moreover, a specific class of antisense oligonucleotides, GapmeRs, shows great promise in pharmacological silencing of lncRNAs in vivo, 170 and even if no clinical trial has been performed, therapeutic GapmeR injections have been found to modulate lncRNAs such as Chast 171 and Meg3 172 in animal models of pressure overload or Wisper in myocardial infarction. 108 , 173 Moreover, as lncRNAs have been detected in extracellular body fluids, they could be used as biomarkers, and example of this is long intergenic non‐coding RNA predicting cardiac remodeling (LIPCAR), whose plasma levels in humans are associated with left ventricular remodeling after myocardial infarction and with an increased risk of developing heart failure. 174 Other identified predictors are MIAT, 174 SENCR, 174 H19, 174 NFAT, 175 MHRT, 175 ANRIL, 176 lncPPARδ, 177 and CoroMarker. 178 Remarkably, four clinical trials are investigating the role of lncRNAs as biomarkers in patients with CVD, 132 suggesting a strong potentiality for these molecules as disease indicators.
4.2. Hypertension
Multiple lncRNAs have been found to be upregulated in the plasma of patients with hypertension, such as AK125261, AK098656, and TUG1. 74 AK098656, upregulated in hypertensive patients, acts through an increase in proliferation and migration of vascular smooth muscle cells (VSMCs), as it has been shown that it can directly bind to the VSMCs‐specific contractile protein, myosin heavy chain‐11, and an essential component of extracellular matrix, fibronectin‐1, promoting their degradation. 179 Moreover, AK098656‐overexpressing transgenic rats spontaneously progress to hypertension, presenting increased media thickness and reduced arterial lumen. 180 The lncRNA TUG1 can also modulate proliferation and migration of rats VSMCs acting as a sponge for miR‐145‐5p and thus inducing the miRNA's target FGF10 and subsequently activating the Wnt/β‐catenin pathway. 181 Proliferation and migration of VSMCs can also be increased in rats by the lncRNAs XR‐007793 and MRAK048635 P1. 182 , 183 Downregulation of MRAK048635 P1 seems to induce VSMCs phenotypic switching from a contractile to a secretory phenotype, representing a potential therapeutic target in the disease. 182 The lncRNA GAS5 can also modulate PDGF‐induced proliferation and migration of human VSMCs through the sponging of miR‐21, which is indeed able to target platelet‐derived growth factor (PDGF). 184
A second process that can be modulated by lncRNAs in hypertension is indeed muscular remodeling. Vascular remodeling is an active process that involves changes in cellular growth, apoptosis, migration, inflammation, and production of extracellular matrix proteins. The lncRNA GAS5 can also regulate this process as it can remodel arteries such as the caudal, carotid, renal, and thoracic ones. Indeed, GAS5's knockdown regulate the function of endothelial cells and VSMCs through β‐catenin signaling. 185 Another previously mentioned lncRNA involved in this process is MALAT1, highly expressed in myocardial and thoracic aortic vascular tissues of hypertensive rats, where it promotes cardiac remodeling through transcriptional repression of MyoD. 186 The inflammatory process can also be of crucial relevance in the hypertension process. TUG1 also act at this level, as it positively correlates with the expression of inflammatory factors such as PAF, ET‐1, TNF‐α, and hsCRP in the blood serum of hypertensive patients. 187 Moreover, a novel lncRNA has been named Giver (Growth factor‐ and pro‐Inflammatory cytokine‐induced Vascular cell‐Expressed lncRNA), for its action in modulation of inflammation. 188 Giver is induced by angiotensin II (AngII) through the recruitment of Nr4a3 to Giver's promoter, and both Giver and NR4a3 were found increased in AngII‐treated human VSMC and in arteries from hypertensive subjects but attenuated in hypertensive patients treated with angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers. It has been hypothesized that Nox1, a gene involved in oxidative stress, may be one of the key effectors through which Giver may promote cell proliferation and inflammation in VSMCs. 188
Polymorphisms in specific lncRNAs can also induce disease pathology. This is the case of cyclin‐dependent kinase inhibitor 2B antisense RNA 1 (CDKN2B‐AS1), also termed ANRIL and previously mentioned for its implication in CVD, where polymorphisms in its sequence may contribute to higher systolic blood pressure in hypertensive patients. 189 , 190 Specifically, it has been found that the SNPs rs10757274, rs2383207, rs10757278, and rs1333049, particularly those within the CDKN2B‐AS1 gene and related haplotypes, may confer an increased susceptibility to hypertension development. 189
4.3. Type 2 Diabetes
At all ages, the risk of T2D rises with increasing body fat. The prevalence of T2D is 3 to 7 times higher in those who are affected by obesity than in normal‐weight adults. Specifically, T2D is an adult‐onset, non‐ insulin‐dependent type of diabetes and is strictly linked to obesity. 75 In recent years, an increased incidence of T2D among youth is also reported, with obesity and family history of T2D generally present. 191 Also, in this case, lncRNAs could be crucial players in disease onset and its progression and as this review focuses specifically on obesity‐related metabolic diseases, the next paragraph will highlight potential implications of lncRNAs in T2D.
Indeed, lncRNAs can be both upregulated and downregulated during disease progression in different cell types (Figure 2). Expression profiles of lncRNAs in PBMCs from patients with T2D highlighted how several lncRNAs were significantly increased compared to controls, and these included HOTAIR, Meg3, LET, MALAT1, MIAT, CDKN2BAS1/ANRIL, XIST, PANDA, GAS5, Linc‐p21, ENST00000550337.1, PLUTO, and NBR2. 192 The lncRNAs ANRIL and MALAT1 were found increased in the serum of patients with T2D, 193 , 194 and the same was true for NONRATT021972, which also correlated with increased blood glucose and neuropathic pain. 195 Interestingly, LncRNA‐p3134 is highly expressed in serum's exosomes of patients with T2D as studies found that it is secreted by islet β‐cell. 196 Moreover, the lncRNA H19 was found upregulated in plasma of patients with T2D, 197 and the lncRNA KCNQ1OT1 was upregulated in T2D islets. 198 Evidences can also be obtained from murine models of the disease, as is the case of E330013P06, which was found upregulated firstly in macrophages of diet‐induced insulin‐resistant T2D mice and subsequently also found upregulated in monocytes from patients with T2D. 199
FIGURE 2.
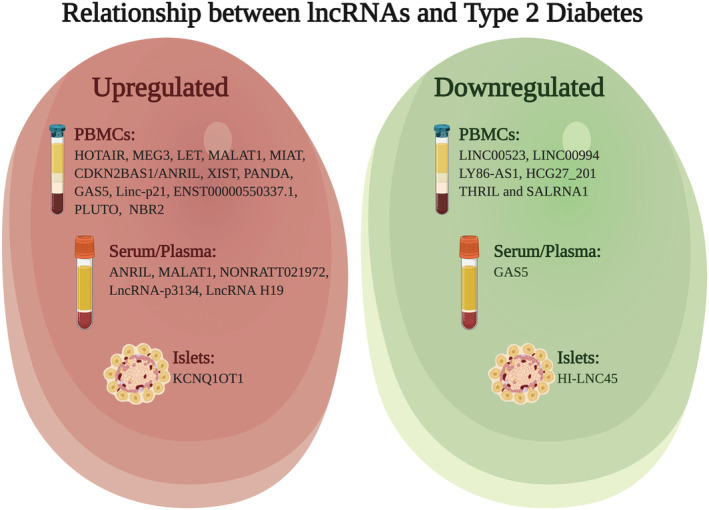
Summary of lncRNAs upregulated or downregulated in specific cell types of patients with T2D. Made in ©BioRender—biorender.com
Interestingly, many lncRNAs have also been reported to be downregulated in patients with T2D. When considering PBMCs screening studies, results showed that multiple lncRNAs were found downregulated. These include LINC00523, LINC00994, 200 LY86‐AS1, HCG27_201, 201 THRIL, and SALRNA1. 192 Moreover, studies showed that levels of GAS5 lncRNA were decreased both in serum and in plasma of patients with T2D. 197 , 202 Lastly, the lncRNA HI‐LNC45 was found downregulated in human T2D islets. 198
Indeed, lncRNAs can modulate the cellular activity of pancreatic β cells. lncRNA‐p3134, found deregulated in human patients and diabetic mice, seems to act as a new signaling molecule that maintains β‐cell mass and enhances insulin synthesis and secretion, and indeed it has been seen that lncRNA‐p3134 can contribute to reverse the insufficient insulin secretion in T2D. 196 Moreover, the lncRNA β‐cell long intergenic noncoding RNA (βlinc1) can coordinate the regulation of neighboring islet‐specific transcription factors, and in fact it is necessary for the specification and function of insulin‐producing β cells. In particular, in adult mice it has been shown that deletion of βlinc1 leads to a defective islet development and disruption of glucose homeostasis. 203 In pediatric age, Liu et al. reported that several lncRNAs involved in regulation of glucose metabolic process and insulin resistance (IR), such as RP11‐559N14.5, RP11‐363E7.4, and RP11‐707P17.1, were significantly upregulated or downregulated in children with obesity compared to controls, even in the absence of diabetes. 31 Considering that hyperglycemia and T2D develop when the pancreas cannot match the increased insulin demands resulting from IR, the lncRNAs could play a crucial role in the onset of the disease.
4.4. Nephropathy
Obesity is a major risk factor for the development of chronic kidney disease, through the direct development of nephropathy. 204 , 205 , 206 Indeed, obesity can cause both a specific renal nephropathy and contribute to renal complications in metabolic syndrome. 206 LncRNAs have also been found to associate with this process. 207 , 208 , 209 Specifically, the role of lncRNAs in diabetic nephropathy (DN), which accounts for approximately 40% of diagnosed end‐stage kidney failure, has been extensively reviewed by Li and collaborators. 207 Specifically, TUG1, 210 , 211 , 212 MIAT, 213 CASC2, 214 ENSMUST00000147869, 215 1700020I14Rik, 216 CYP4B1‐PS1–001, 217 Gm15645, 218 and LINC01619 219 were downregulated in DN, whereas PVT1, MALAT1, 220 Gm4419, 221 Gm15645, 218 NR_033515, 222 Erbb4‐IR, 223 ASncmtRNA‐2, 224 and lnc‐MCG 225 were upregulated in DN. 207
Among the other lncRNAs implicated, Rpph1 was found upregulated in mice with DN, regulating also cell proliferation and inflammatory cytokines production in mesangial cells, through a direct interaction with galectin‐3. 226 LncRNAs can indeed play a role in epigenetic regulation of DN, along with canonical modulators such as histone modifiers and DNA methylation. 227 Indeed, they can act synergistically with miRNAs in the disease pathology, as does RP23, which is induced by TGF‐β1 in mesangial cells along with its containing miRNAs, miR‐216a, and miR‐217. 228 Moreover, in mouse miR‐192 is also co‐regulated by TGF‐β1 in mesangial cells along with its host ncRNA CJ241444, through promoter Smad binding elements and epigenetic regulation via protein C‐ets‐1 and histone acetylation. 227 , 229 Lastly, another study found 21 lncRNAs upregulated in two models of renal fibrosis, subsequently downregulated in Smad3‐knockout mice, suggesting they were induced by this factor. 230
4.5. Osteoarthritis
Obesity can impact tissue types other than the adipose tissue, and indeed it can significantly impact both the musculoskeletal and immune systems, leading to the development of OA. 77 , 231 OA is a debilitative degenerative joint disorder which is characterized by pain, decreased mobility, and an overall negative impact on the quality of life. 231 In recent years, lncRNAs have been found to also be strongly deregulated in this disease, although most studies concern OA development and do not specifically focus on the obesogenic co‐morbidity. 77 These lncRNAs have been extensively reviewed in other works, 77 , 232 specifically classifying them for their role in disease progression, immune response, and even potential therapeutic targets. 77 , 232 It is indeed clear that the main implication of lncRNAs in OA relates to the immune response, and to this end in recent years mounting studies are reporting this correlation, with the implication of, but not limited to, CASC2, 233 SNHG1, 234 DANCR, 235 HOTAIR, 236 H19, 237 SNHG7, 238 MFI2‐AS1, 239 PACER, CILinc01, CILinc02, 240 PVT1, 241 XIST, 242 and FOXD2‐AS1. 243 A high‐throughput screening also reported 3007 upregulated lncRNAs and 1707 downregulated lncRNAs in OA human cartilage compared with normal samples, indicating their significant implication in the diseases. 244 Moreover, another work investigated the role of exosomal lncRNAs from plasma and from synovial fluid in patients at different stages of OA, highlighting a role for PCGEM1 in disease progression. 245
Even so, future works will need to specifically focus on the link between OA, lncRNAs, and obesity. Nanus and co‐authors reported 19 differentially expressed lncRNAs in normal‐weight OA versus non‐OA patient fibroblasts, and these are MALAT1, MIR155HG, SMILR, LINC01426, RP11‐863P13.3, CARMN, RP11‐79H23.3, RP11‐362F19.1, RP11‐290 M5.4, VLDLR‐AS1, RP11‐536 K7.3, HAGLR, LINC01915, RP11‐367F23.2, RP11‐392O17.1, LINC01705, LINC01021, DNAJC27‐AS1, and AF131217.1. 246 Specifically, MALAT1 was rapidly induced upon stimulation of OA synovial fibroblasts with proinflammatory cytokines, and its ablation leads to a reduced expression of IL‐6 and IL‐8. 77 , 246 Moreover, the lncRNA Nespas was found upregulated in human OA chondrocytes, sponging numerous miRNAs which target Acyl‐CoA synthetase 6 (ACSL6), leading to an overall increase in ACSL6. 247 ACSL6 encodes a key enzyme that activates polyunsaturated long‐chain fatty acids, suggesting that this process could modulate lipid metabolism in OA. 247 Overall, these evidences suggest a clear implication for lncRNAs in mediating epigenetic dysregulation in OA, but the specific link with obesity will need further clarification.
4.6. Hepatic metabolic disease
Obesity is also linked with the development of hepatic metabolic disease, as nonalcoholic fatty liver disease (NAFLD) and especially its most severe form (nonalcoholic steatohepatitis) present an increased prevalence in patients with obesity (from 3% to 20–40%). 248 LncRNAs also appear to intervene in this process, with a tight link with obesity development. Indeed, the lncRNA Blnc1, implicated in adipogenesis and obesity, was found upregulated in obese and NAFLD mice, activating SREBP1c and hepatic lipogenesis, thus aggravating disease progression. 249 Gm15622 was also found upregulated in the liver of obese mice fed a HFD, exerting its mechanism of action sponging miR‐742‐3p, subsequently upregulating SREBP1c. 250 Moreover, its inactivation abrogates HFD‐induced hepatic steatosis, suggesting also in this case a therapeutic window. 249 Conversely, lncARSR was found upregulated in high fatty acid‐treated human HepG2 and NAFLD mouse models, binding YAP1 and further increasing lipid accumulation, a mechanism alleviated when lncARSR was silenced. 251 The lncRNA H19 was also upregulated in NAFLD murine models, and again its silencing reduced lipid accumulation in hepatocytes. 252 On the contrary, overexpression of the lncRNA FLRL2 in vivo in murine NAFLD models resolved steatosis, lipogenesis, and inflammation. 253 Similarly, Meg3 was downregulated in HFD mice, and acting as ceRNA for miR‐21 it could help alleviate lipid over‐deposition. 254
Also in this case, RNA sequencing and microarrays allowed the identification of numerous new putative candidates. Indeed, numerous high‐throughput studies were performed in both murine models 255 , 256 , 257 and human tissues, 258 allowing the identification of specific new candidates such as AK012226, 256 NONMMUT010685, 257 and MALAT1. 258 Interestingly, starting from pre‐existing human transcriptome data on NAFLD and liver metabolism, it was also possible to develop a pipeline which identified human lncRNA metabolic regulators (hLMR), with a specific one being strictly involved in cholesterol metabolism. 259 Their potential as biomarkers was investigated analyzing serum samples of patients with mild and severe NAFLD; through microarray analysis several ncRNAs were identified, and specifically the expression of TGFB2/TGFB2‐OT1 allowed advanced fibrosis discrimination. 260 Indeed, the amount of data concerning the role of lncRNAs is becoming increasingly overwhelming, with numerous new evidences each year, and for further reading on the topic we refer the reader to other published review reports. 261 , 262 , 263 , 264 , 265 , 266 , 267
4.7. Dyslipidemia
Obesity is probably the main cause for the development dyslipidemia, which typically consists of increased triglycerides, free fatty acids, apolipoprotein B, and LDL‐C, and decreased HDL‐C. 268 The role of lncRNAs in adipogenesis and thus lipid metabolism has been previously discussed in Section 3, but limited evidence specifically refers to the link between lncRNAs and patients with dyslipidemia. 269 Among all, Blnc1 activation in epididymal fat in HFD‐induced obese mice seems to have a slight impact on dyslipidemia, suggesting a specific link with this pathogenesis. 270 Moreover, a recent work screened the lncRNAs expression in rat livers with hypertriglyceridemia and identified the upregulation of a novel lncRNA: lnc19959.2. The knockdown of lnc19959.2 resulted in triglycerides lowering effects both in vitro and in vivo, and mechanistic studies revealed that lnc19959.2 upregulated ApoA4 expression via ubiquitinated transcription inhibitor factor Purb, while its specific interaction with hnRNPA2B1 was able to downregulate the expression of Cpt1a, Tm7sf2, and Gpam. 271
Indeed, lncRNAs can deeply influence lipid homeostasis, but further studies are required in order to determine whether lncRNAs that regulate lipogenesis, lipolysis, β‐oxidation, adipogenesis, and thermogenesis could also become biomarkers for therapies that target dyslipidemias. 269 , 272
5. CONCLUSIONS
Obesity is a complex disease representing a great burden on the health care system, commonly leading to the development of co‐morbidities also in pediatrics. Epigenetics through RNA biology might play a crucial role in elucidating new targetable pathways, and in this context lncRNAs are emerging as interesting new candidate targets and players. Indeed, obesity‐associated lncRNAs play a crucial role in adipose tissue modulation, but their action is not limited to this, as they have been implicated in modulating obesogenic co‐morbidities influencing the cardiovascular system, the immune system, the liver, and even the musculoskeletal system. 73 , 246 , 265 Moreover, the number of co‐morbidities associated with obesity is extremely significant and includes also diseases which do not strictly correlate with disruption in metabolic pathways. Indeed, multiple numerous tumors are also obesity‐induced, and although no specific correlation between lncRNAs present in patients with obesity and specific cancer has yet been made, one review report summarizes the link between numerous lncRNAs present both in obesity and cancer. 273 Non‐coding RNAs will revolutionize modern medicine making it possible to understand in detail unknown aspects of molecular biology over the coming years, and indeed a deep understanding of lncRNAs' role in adipocytes biology will provide multiple novel therapeutic strategies to better combat obesity and prevent early obesity complications in the near future. There is a need to summarize all the recent advances made in the discovery of the role of lncRNAs in the pathogenesis and progression of this disease, and it appears evident that in future years more and more research efforts will focus on characterization of the specificity of lncRNAs' mechanisms of action in obesity‐related diseases (Table 1). Indeed, further studies will need to analyze in depth the transcriptional deregulation present at a tissue level in patients with obesity and co‐morbidities, in order to identify further deregulated targets. A better understanding of these mechanisms, already from pediatric age, will accompany us in filling the gap from basic research to clinical care of patients with obesity. These molecules, in fact, could act as biomarkers for the early diagnosis of obesity‐linked complications and possibly representing new indicators of risk assessment.
TABLE 1.
Summary of deregulated lncRNAs in obesity and associated diseases
| Disease | LncRNA |
|---|---|
| Obesity | SRA, 67 ASMER‐1 and ASMER‐2, 68 RP11‐20G13.3, 31 PVT1, 50 Plnc1, 48 HOTAIR, 72 lnc19959.2. 271 |
| Cardiovascular diseases | CAIF, 83 CDKN2BAS1/ANRIL, 84 , 151 , 166 , 176 APF, 85 AK088388, 86 Meg3, 87 , 161 CARL, 88 MDRL, 89 , 90 ZFAS1, 92 , 93 HOTAIR, 94 MALAT1, 95 , 96 GAS5, 97 FAF, 98 TTTY15, 99 ECRAR, 100 AK080084, 101 NR_045363, 102 TUG1 103 and Meg3, 91 , 104 , 117 MIRT1 and MIRT2, 105 MALAT1, 106 MHRT, 107 , 116 , 175 Wisper, 108 MIAT, 109 , 110 , 174 Chaer, 112 CHRF, 113 lncR‐UCA1, 114 DACH1, 118 H19, 119 , 152 , 174 Plscr4, 120 SNHG1, 121 TINCR, 122 Uc.323, 123 Ahit, 124 HRCR, 125 HypERlnc 126 RP11‐99E15.2 and RP3‐523 K23.2, 136 ETF1P2 and AP001053.11, 138 , 139 NONHSAT040387 and NONHSAT098586, 140 TCONS_00075467, 141 TCONS_00202959, 142 AK055347, 143 MIAT, 110 KCNQ1OT1, 144 CTA‐384D8.35 and CTB‐114C7.4, 149 HIF1A‐AS1, 153 linc‐p21, 154 RNCR3, 155 TGFB2‐OT1, 156 lnc‐Ang362, 157 HAS2‐AS1, 158 SMILR, 159 SENCR, 160 , 174 lnc‐MKI67IP‐3, 162 LeXis, 167 RP5‐833A20.1, 168 MeXis, 169 lncPPARδ 177 and CoroMarker. 178 |
| Hypertension | AK125261, 74 AK098656, 74 , 179 , 180 TUG1, 74 , 181 , 187 XR‐007793, 183 MRAK048635 P1, 182 GAS5, 184 , 185 MALAT1, 186 Giver, 188 CDKN2BAS1/ANRIL. 189 , 190 |
| Type 2 diabetes | HOTAIR, 192 Meg3, 192 LET, 192 MIAT, 192 XIST, 192 PANDA, 192 GAS5, 192 , 197 , 202 LINC‐p21, 192 ENST00000550337.1, 192 PLUTO, 192 NBR2, 192 MALAT1, 192 , 194 CDKN2BAS1/ANRIL, 192 , 193 NONRATT021972, 195 LncRNA‐p3134, 196 H19, 197 KCNQ1OT1, 198 E330013P06, 199 LINC00523, 200 LINC00994, 200 LY86‐AS1, 201 HCG27_201, 201 THRIL, 192 SALRNA1, 192 HI‐LNC45, 198 lncRNA‐p3134, 196 βlinc1, 203 RP11‐559 N14.5, 31 RP11‐363E7.4, 31 RP11‐707P17. 31 |
| Nephropathy | TUG1, 210 , 211 , 212 MIAT, 213 CASC2, 214 ENSMUST00000147869, 215 1700020I14Rik, 216 CYP4B1‐PS1–001, 217 Gm15645, 218 LINC01619, 219 PVT1, 274 MALAT1, 220 Gm4419, 221 Gm15645, 218 NR_033515, 222 Erbb4‐IR, 223 ASncmtRNA‐2, 224 lnc‐MCG, 225 Rpph1, 226 RP23, 228 CJ241444. 227 , 229 |
| Osteoarthritis | CASC2, 233 SNHG1, 234 DANCR 235 HOTAIR, 236 H19, 237 SNHG7, 238 MFI2‐AS1, 239 PACER, CILinc01, CILinc02, 240 PVT1, 241 XIST, 242 FOXD2‐AS1, 243 PCGEM1, 245 MALAT1, 246 MIR155HG, 246 SMILR, 246 LINC01426, 246 RP11‐863P13.3, 246 CARMN, 246 RP11‐79H23.3, 246 RP11‐362F19.1, 246 RP11‐290 M5.4, 246 VLDLR‐AS1, 246 RP11‐536 K7.3, 246 HAGLR, 246 LINC01915, 246 RP11‐367F23.2, 246 RP11‐392O17.1, 246 LINC01705, 246 LINC01021, 246 DNAJC27‐AS1, 246 AF131217.1, 246 Nespas. 247 |
| Hepatic metabolic disease | Blnc1, 249 Gm15622, 250 lncARSR, 251 H19, 252 FLRL2, 253 Meg3, 254 AK012226, 256 NONMMUT010685, 257 MALAT1, 258 hLMR, 259 TGFB2‐OT1. 260 |
| Dyslipidemia | Blnc1, 270 lnc19959.2 271 |
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Table S1: List of lncRNAs cited in the manuscript listed in alphabetical order with the number of known orthologues and a specific focus on their presence in the most common models (Rattus norvegicus, Mus musculus, and Homo sapiens)
ACKNOWLEDGMENTS
FR would like to acknowledge and thank the Fondazione Fratelli Confalonieri for financial support during her PhD. This work was supported by a grant from the Pediatric Clinical Research Center Fondazione “Romeo and Enrica Invernizzi” to GVZ and SC.
Rey F, Urrata V, Gilardini L, et al. Role of long non‐coding RNAs in adipogenesis: State of the art and implications in obesity and obesity‐associated diseases. Obesity Reviews. 2021;22:e13203. 10.1111/obr.13203
REFERENCES
- 1. Haslam D, Sattar N, Lean M. ABC of obesity. Obesity—time to wake up. BMJ. 2006;333(7569):640‐642. 10.1136/bmj.333.7569.640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawrence VJ, Kopelman PG. Medical consequences of obesity. Clin Dermatol. 2004;22(4):296‐302. 10.1016/j.clindermatol.2004.01.012 [DOI] [PubMed] [Google Scholar]
- 3. WHO . Obesity and overweight. 2020. https://www.who.int/news‐room/fact‐sheets/detail/obesity‐and‐overweight
- 4. O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015/01/01. 2015;16(1):1‐12. 10.1111/obr.12229 [DOI] [PubMed] [Google Scholar]
- 5. Lega IC, Lipscombe LL. Review: diabetes, obesity, and cancer—pathophysiology and clinical implications. Endocr Rev. 2020;41(1):33‐52. 10.1210/endrev/bnz014 [DOI] [PubMed] [Google Scholar]
- 6. WHO . The WHO child growth standards. https://www.who.int/childgrowth/en/
- 7. Maclaren NK, Gujral S, Ten S, Motagheti R. Childhood obesity and insulin resistance. Cell Biochem Biophys. 2007;48(2–3):73‐78. 10.1007/s12013-007-0017-6 [DOI] [PubMed] [Google Scholar]
- 8. Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA Jan. 2010;303(3):242‐249. 10.1001/jama.2009.2012 [DOI] [PubMed] [Google Scholar]
- 9. Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obes Rev. 2004/05/01. 2004;5(s1):4‐85. 10.1111/j.1467-789X.2004.00133.x [DOI] [PubMed] [Google Scholar]
- 10. Montesi L, El Ghoch M, Brodosi L, Calugi S, Marchesini G, Dalle Grave R. Long‐term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes. 2016;9:37‐46. 10.2147/DMSO.S89836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bray GA, Kim KK, Wilding JPH, World Obesity F . Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017/07/01. 2017;18(7):715‐723. 10.1111/obr.12551 [DOI] [PubMed] [Google Scholar]
- 12. Tronieri JS, Wadden TA, Chao AM, Tsai AG. Primary care interventions for obesity: review of the evidence. Curr Obes Rep. Mar. 2019;8:128‐136 10.1007/s13679-019-00341-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dalton B, Campbell IC, Schmidt U. Neuromodulation and neurofeedback treatments in eating disorders and obesity. Curr Opin Psychiatry. 2017;30(6):458‐473. 10.1097/YCO.0000000000000361 [DOI] [PubMed] [Google Scholar]
- 14. Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019/04/01. 2019;20(4):242‐258. 10.1038/s41580-018-0093-z [DOI] [PubMed] [Google Scholar]
- 15. Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov Jun. 2010;9(6):465‐482. 10.1038/nrd3138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stöger R. Epigenetics and obesity. Pharmacogenomics Dec. 2008;9(12):1851‐1860. 10.2217/14622416.9.12.1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loh M, Zhou L, Ng HK, Chambers JC. Epigenetic disturbances in obesity and diabetes: epidemiological and functional insights. Mol Metab. 2019/09/01/. 2019;27:S33‐S41. 10.1016/j.molmet.2019.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allum F, Grundberg E. Capturing functional epigenomes for insight into metabolic diseases. Mol Metab. 2020;38:100936. 10.1016/j.molmet.2019.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mattick JS. The genetic signatures of noncoding RNAs. PLoS Genet Apr. 2009;5(4):e1000459. 10.1371/journal.pgen.1000459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell Feb. 2009;136(4):629‐641. 10.1016/j.cell.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 21. Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature Sep. 2012;489(7414):57‐74. 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. St Laurent G, Wahlestedt C, Kapranov P. The landscape of long noncoding RNA classification. Trends Genet May. 2015;31(5):239‐251. 10.1016/j.tig.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salem ESB, Vonberg AD, Borra VJ, Gill RK, Nakamura TRNA. RNA‐binding proteins in immuno‐metabolic homeostasis and diseases. Front Cardiovasc Med. 2019;6:106–125. 10.3389/fcvm.2019.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Landrier JF, Derghal A, Mounien L. MicroRNAs in obesity and related metabolic disorders. Cell. 08. 2019;8(8). 10.3390/cells8080859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arcinas C, Tan W, Fang W, et al. Adipose circular RNAs exhibit dynamic regulation in obesity and functional role in adipogenesis. Nat Metab. 2019/07/01. 2019;1(7):688‐703. 10.1038/s42255-019-0078-z [DOI] [PubMed] [Google Scholar]
- 26. Yao RW, Wang Y, Chen LL. Cellular functions of long noncoding RNAs. Nat Cell Biol 05. 2019;21(5):542‐551. 10.1038/s41556-019-0311-8 [DOI] [PubMed] [Google Scholar]
- 27. Sun Q, Hao Q, Prasanth KV. Nuclear long noncoding RNAs: key regulators of gene expression. Trends Genet. 02. 2018;34(2):142‐157. 10.1016/j.tig.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen LL. Linking long noncoding RNA localization and function. Trends Biochem Sci 09. 2016;41(9):761‐772. 10.1016/j.tibs.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 29. Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell Sep. 2011;43(6):904‐914. 10.1016/j.molcel.2011.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wei S, Du M, Jiang Z, Hausman GJ, Zhang L, Dodson MV. Long noncoding RNAs in regulating adipogenesis: new RNAs shed lights on obesity. Cell Mol Life Sci May. 2016;73(10):2079‐2087. 10.1007/s00018-016-2169-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Y, Ji Y, Li M, et al. Integrated analysis of long noncoding RNA and mRNA expression profile in children with obesity by microarray analysis. Sci Rep. 2018;8(1):8750. 10.1038/s41598-018-27113-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen C, Cui Q, Zhang X, et al. Long non‐coding RNAs regulation in adipogenesis and lipid metabolism: emerging insights in obesity. Cell Signal. 2018;51:47‐58. 10.1016/j.cellsig.2018.07.012 [DOI] [PubMed] [Google Scholar]
- 33. Lowe CE, O'Rahilly S, Rochford JJ. Adipogenesis at a glance. J Cell Sci Aug. 2011;124(Pt 16):2681‐2686. 10.1242/jcs.079699 [DOI] [PubMed] [Google Scholar]
- 34. Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol Dec. 2006;7(12):885‐896. 10.1038/nrm2066 [DOI] [PubMed] [Google Scholar]
- 35. Rosen E, Eguchi J, Xu Z. Transcriptional targets in adipocyte biology. Expert Opin Ther Targets Aug. 2009;13(8):975‐986. 10.1517/14728220903039706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci U S A Jan. 2003;100(1):44‐49. 10.1073/pnas.0137044100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu B, Gerin I, Miao H, et al. Multiple roles for the non‐coding RNA SRA in regulation of adipogenesis and insulin sensitivity. PLoS One. 2010;5(12):e14199. 10.1371/journal.pone.0014199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sheng L, Ye L, Zhang D, Cawthorn WP, New Insights XB. Into the long non‐coding RNA SRA: physiological functions and mechanisms of action. Front Med (Lausanne). 2018;5:244. 10.3389/fmed.2018.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu S, Xu R, Gerin I, et al. SRA regulates adipogenesis by modulating p38/JNK phosphorylation and stimulating insulin receptor gene expression and downstream signaling. PLoS One. 2014;9(4):e95416. 10.1371/journal.pone.0095416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bost F, Aouadi M, Caron L, Binétruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie Jan. 2005;87(1):51‐56. 10.1016/j.biochi.2004.10.018 [DOI] [PubMed] [Google Scholar]
- 41. Cai R, Tang G, Zhang Q, et al. A novel lnc‐RNA, named lnc‐ORA, is identified by RNA‐Seq analysis, and its knockdown inhibits adipogenesis by regulating the PI3K/AKT/mTOR signaling pathway. Cell. 2019;8(5):477. 10.3390/cells8050477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yi F, Zhang P, Wang Y, et al. Long non‐coding RNA slincRAD functions in methylation regulation during the early stage of mouse adipogenesis. RNA Biol. 2019;16(10):1401‐1413. 10.1080/15476286.2019.1631643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen Y, Li K, Zhang X, Chen J, Li M, Liu L. The novel long noncoding RNA lncRNA‐Adi regulates adipogenesis. Stem Cells Transl Med. 2020;9(9):1053‐1067. 10.1002/sctm.19-0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hou X, Zhang Y, Li W, et al. CDK6 inhibits white to beige fat transition by suppressing RUNX1. Nat Commun. 2018;9(1):1023. 10.1038/s41467-018-03451-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. You LH, Zhu LJ, Yang L, et al. Transcriptome analysis reveals the potential contribution of long noncoding RNAs to brown adipocyte differentiation. Mol Genet Genomics Oct. 2015;290(5):1659‐1671. 10.1007/s00438-015-1026-6 [DOI] [PubMed] [Google Scholar]
- 46. Zhang M, Li F, Sun JW, et al. LncRNA IMFNCR promotes intramuscular adipocyte differentiation by sponging miR‐128‐3p and miR‐27b‐3p. Front Genet. 2019;10:42. 10.3389/fgene.2019.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li M, Sun X, Cai H, et al. Long non‐coding RNA ADNCR suppresses adipogenic differentiation by targeting miR‐204. Biochim Biophys Acta Jul. 2016;1859(7):871‐882. 10.1016/j.bbagrm.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 48. Zhu E, Zhang J, Li Y, Yuan H, Zhou J, Wang B. Long noncoding RNA Plnc1 controls adipocyte differentiation by regulating peroxisome proliferator‐activated receptor γ. FASEB j. 2019;33(2):2396‐2408. 10.1096/fj.201800739RRR [DOI] [PubMed] [Google Scholar]
- 49. Wang D, Zhou Y, Lei W, et al. Signal transducer and activator of transcription 3 (STAT3) regulates adipocyte differentiation via peroxisome‐proliferator‐activated receptor gamma (PPARgamma). Biol Cell Sep. 2009;102(1):1‐12. 10.1042/BC20090070 [DOI] [PubMed] [Google Scholar]
- 50. Zhang L, Zhang D, Qin ZY, Li J, Shen ZY. The role and possible mechanism of long noncoding RNA PVT1 in modulating 3T3‐L1 preadipocyte proliferation and differentiation. IUBMB Life. 2020;72(7):1460‐1467 10.1002/iub.2269 [DOI] [PubMed] [Google Scholar]
- 51. Cooper DR, Carter G, Li P, Patel R, Watson JE, Patel NA. Long non‐coding RNA NEAT1 associates with SRp40 to temporally regulate PPARγ2 splicing during adipogenesis in 3T3‐L1 cells. Genes (Basel) Nov. 2014;5(4):1050‐1063. 10.3390/genes5041050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xiong Y, Yue F, Jia Z, et al. A novel brown adipocyte‐enriched long non‐coding RNA that is required for brown adipocyte differentiation and sufficient to drive thermogenic gene program in white adipocytes. Biochim Biophys Acta Mol Cell Biol Lipids. Apr. 2018;1863(4):409‐419. 10.1016/j.bbalip.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alvarez‐Dominguez JR, Bai Z, Xu D, et al. De novo reconstruction of adipose tissue transcriptomes reveals long non‐coding RNA regulators of brown adipocyte development. Cell Metab May. 2015;21(5):764‐776. 10.1016/j.cmet.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nuermaimaiti N, Liu J, Liang X, et al. Effect of lncRNA HOXA11‐AS1 on adipocyte differentiation in human adipose‐derived stem cells. Biochem Biophys Res Commun. 2018;495(2):1878‐1884. 10.1016/j.bbrc.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 55. Liu Y, Wang Y, He X, et al. LncRNA TINCR/miR‐31‐5p/C/EBP‐α feedback loop modulates the adipogenic differentiation process in human adipose tissue‐derived mesenchymal stem cells. Stem Cell Res. 2018;32:35‐42. 10.1016/j.scr.2018.08.016 [DOI] [PubMed] [Google Scholar]
- 56. Huang Y, Jin C, Zheng Y, et al. Knockdown of lncRNA MIR31HG inhibits adipocyte differentiation of human adipose‐derived stem cells via histone modification of FABP4. Sci Rep. 2017;8:8080. 10.1038/s41598-017-08131-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fan L, Xu H, Li D, Li H, Lu D. A novel long noncoding RNA, AC092834.1, regulates the adipogenic differentiation of human adipose‐derived mesenchymal stem cells via the DKK1/Wnt/β‐catenin signaling pathway. Biochem Biophys Res Commun May. 2020;525(3):747‐754. 10.1016/j.bbrc.2020.02.140 [DOI] [PubMed] [Google Scholar]
- 58. Pang WJ, Lin LG, Xiong Y, et al. Knockdown of PU.1 AS lncRNA inhibits adipogenesis through enhancing PU.1 mRNA translation. J Cell Biochem Nov. 2013;114(11):2500‐2512. 10.1002/jcb.24595 [DOI] [PubMed] [Google Scholar]
- 59. Wei N, Wang Y, Xu RX, et al. PU.1 antisense lncRNA against its mRNA translation promotes adipogenesis in porcine preadipocytes. Anim Genet Apr. 2015;46(2):133‐140. 10.1111/age.12275 [DOI] [PubMed] [Google Scholar]
- 60. Cai R, Sun Y, Qimuge N, et al. Adiponectin AS lncRNA inhibits adipogenesis by transferring from nucleus to cytoplasm and attenuating adiponectin mRNA translation. Biochim Biophys Acta Mol Cell Biol Lipids Apr. 2018;1863(4):420‐432. 10.1016/j.bbalip.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 61. Lo KA, Huang S, Walet ACE, et al. Adipocyte long‐noncoding RNA transcriptome analysis of obese mice identified. Diabetes. 2018;67(6):1045‐1056. 10.2337/db17-0526 [DOI] [PubMed] [Google Scholar]
- 62. Chen J, Liu Y, Lu S, et al. The role and possible mechanism of lncRNA U90926 in modulating 3T3‐L1 preadipocyte differentiation. Int J Obes (Lond). 2017;41(2):299‐308. 10.1038/ijo.2016.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li M, Gao Q, Tian Z, et al. MIR221HG is a novel long noncoding RNA that inhibits bovine adipocyte differentiation. Genes (Basel). 2019;11(1). 10.3390/genes11010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang Y, Zheng Y, Jin C, et al. H19 inhibits adipocyte differentiation of bone marrow mesenchymal stem cells through epigenetic modulation of histone deacetylases. Science Report. 2016;6:28897. 10.1038/srep28897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Li Z, Jin C, Chen S, et al. Long non‐coding RNA MEG3 inhibits adipogenesis and promotes osteogenesis of human adipose‐derived mesenchymal stem cells via miR‐140‐5p. Mol Cell Biochem Sep. 2017;433(1–2):51‐60. 10.1007/s11010-017-3015-z [DOI] [PubMed] [Google Scholar]
- 66. Huang X, Fu C, Liu W, et al. Chemerin‐induced angiogenesis and adipogenesis in 3 T3‐L1 preadipocytes is mediated by lncRNA Meg3 through regulating Dickkopf‐3 by sponging miR‐217. Toxicol Appl Pharmacol. 2019;385:114815. 10.1016/j.taap.2019.114815 [DOI] [PubMed] [Google Scholar]
- 67. Liu S, Sheng L, Miao H, et al. SRA gene knockout protects against diet‐induced obesity and improves glucose tolerance. J Biol Chem. 2014;289(19):13000‐13009. 10.1074/jbc.M114.564658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gao H, Kerr A, Jiao H, et al. Long non‐coding RNAs associated with metabolic traits in human white adipose tissue. EBioMedicine Apr. 2018;30:248‐260. 10.1016/j.ebiom.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang X, Xue C, Lin J, et al. Interrogation of nonconserved human adipose lincRNAs identifies a regulatory role of. Sci Transl Med. 2018;10(446):eaar5987. 10.1126/scitranslmed.aar5987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sun L, Goff LA, Trapnell C, et al. Long noncoding RNAs regulate adipogenesis. PNAS 2013;110(9):3387‐3392. 10.1073/pnas.1222643110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chen J, Cui X, Shi C, et al. Differential lncRNA expression profiles in brown and white adipose tissues. Mol Genet Genomics. 2015;290(2):699‐707. 10.1007/s00438-014-0954-x [DOI] [PubMed] [Google Scholar]
- 72. Lu X, Bai D, Liu X, Zhou C, Yang G. Sedentary lifestyle related exosomal release of Hotair from gluteal‐femoral fat promotes intestinal cell proliferation. Sci Rep. 2017;7:45648. 10.1038/srep45648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yeh CF, Chang YE, Lu CY, Hsuan CF, Chang WT, Yang KC. Expedition to the missing link: long noncoding RNAs in cardiovascular diseases. J Biomed Sci. 2020;27(1):48. 10.1186/s12929-020-00647-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wu G, Jose PA, Zeng C. Noncoding RNAs in the regulatory network of hypertension. Hypertension. 2018;72(5):1047‐1059. 10.1161/HYPERTENSIONAHA.118.11126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Raut SK, Khullar M. The big entity of new RNA world: long non‐coding RNAs in microvascular complications of diabetes. Front Endocrinol (Lausanne). 2018;9:300. 10.3389/fendo.2018.00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Singer K, Lumeng CN. The initiation of metabolic inflammation in childhood obesity. J Clin Invest. 2017;127(1):65‐73. 10.1172/JCI88882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wijesinghe SN, Nicholson T, Tsintzas K, Jones SW. Involvements of long noncoding RNAs in obesity‐associated inflammatory diseases. Obes Rev. 2020;1–14 10.1111/obr.13156 [DOI] [PubMed] [Google Scholar]
- 78. Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part II: variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation. 2001;104(23):2855‐2864. 10.1161/hc4701.099488 [DOI] [PubMed] [Google Scholar]
- 79. Luengo‐Fernández R, Leal J, Gray A, Petersen S, Rayner M. Cost of cardiovascular diseases in the United Kingdom. Heart. 2006;92(10):1384‐1389. 10.1136/hrt.2005.072173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Podolec P, Matusik PT. New clinical classification of rare cardiovascular diseases and disorders: relevance for cardiovascular research. Cardiovasc Res. 2019;115(8):e77‐e79. 10.1093/cvr/cvz142 [DOI] [PubMed] [Google Scholar]
- 81. Joshi SM, Katre PA, Kumaran K, et al. Tracking of cardiovascular risk factors from childhood to young adulthood—the Pune Children's Study. Int J Cardiol. 2014;175(1):176‐178. 10.1016/j.ijcard.2014.04.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wu D, Zhou Y, Fan Y, et al. LncRNA CAIF was downregulated in end‐stage cardiomyopathy and is a promising diagnostic and prognostic marker for this disease. Biomarkers. 2019;24(8):735‐738. 10.1080/1354750X.2019.1677778 [DOI] [PubMed] [Google Scholar]
- 83. Liu CY, Zhang YH, Li RB, et al. LncRNA CAIF inhibits autophagy and attenuates myocardial infarction by blocking p53‐mediated myocardin transcription. Nat Commun. 2018;9(1):29. 10.1038/s41467-017-02280-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zeng R, Song XJ, Liu CW, Ye W. LncRNA ANRIL promotes angiogenesis and thrombosis by modulating microRNA‐99a and microRNA‐449a in the autophagy pathway. Am J Transl Res. 2019;11(12):7441‐7448. [PMC free article] [PubMed] [Google Scholar]
- 85. Wang K, Liu CY, Zhou LY, et al. APF lncRNA regulates autophagy and myocardial infarction by targeting miR‐188‐3p. Nat Commun. 2015;6(1):6779–6790. 10.1038/ncomms7779 [DOI] [PubMed] [Google Scholar]
- 86. Wang JJ, Bie ZD, Sun CF. Long noncoding RNA AK088388 regulates autophagy through miR‐30a to affect cardiomyocyte injury. J Cell Biochem. 2019;120(6):10155‐10163. 10.1002/jcb.28300 [DOI] [PubMed] [Google Scholar]
- 87. Li X, Zhao J, Geng J, et al. Long non‐coding RNA MEG3 knockdown attenuates endoplasmic reticulum stress‐mediated apoptosis by targeting p53 following myocardial infarction. J Cell Mol Med. 2019;23(12):8369‐8380. 10.1111/jcmm.14714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang K, Long B, Zhou LY, et al. CARL lncRNA inhibits anoxia‐induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR‐539‐dependent PHB2 downregulation. Nat Commun Apr. 2014;5(1):3596‐3609. 10.1038/ncomms4596 [DOI] [PubMed] [Google Scholar]
- 89. Huang Y. The novel regulatory role of lncRNA‐miRNA‐mRNA axis in cardiovascular diseases. J Cell Mol Med. 2018;22(12):5768‐5775. 10.1111/jcmm.13866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang K, Sun T, Li N, et al. MDRL lncRNA regulates the processing of miR‐484 primary transcript by targeting miR‐361. PLoS Genet Jul. 2014;10(7):e1004467. 10.1371/journal.pgen.1004467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chen C, Tang Y, Sun H, Lin X, Jiang B. The roles of long noncoding RNAs in myocardial pathophysiology. Biosci Rep. 2019;39(11):1‐17. 10.1042/BSR20190966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wu T, Wu D, Wu Q, et al. Knockdown of long non‐coding RNA‐ZFAS1 protects cardiomyocytes against acute myocardial infarction via anti‐apoptosis by regulating miR‐150/CRP. J Cell Biochem. 2017;118(10):3281‐3289. 10.1002/jcb.25979 [DOI] [PubMed] [Google Scholar]
- 93. Zhang Y, Jiao L, Sun L, et al. LncRNA ZFAS1 as a SERCA2a inhibitor to cause intracellular Ca2+ overload and contractile dysfunction in a mouse model of myocardial infarction. Circ Res. 2018;122(10):1354‐1368. 10.1161/CIRCRESAHA.117.312117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gao L, Liu Y, Guo S, et al. Circulating long noncoding RNA HOTAIR is an essential mediator of acute myocardial infarction. Cell Physiol Biochem. 2017;44(4):1497‐1508. 10.1159/000485588 [DOI] [PubMed] [Google Scholar]
- 95. Guo X, Wu X, Han Y, Tian E, Cheng J. LncRNA MALAT1 protects cardiomyocytes from isoproterenol‐induced apoptosis through sponging miR‐558 to enhance ULK1‐mediated protective autophagy. J Cell Physiol. 2019;234(7):10842‐10854. 10.1002/jcp.27925 [DOI] [PubMed] [Google Scholar]
- 96. Sun R, Zhang L. Long non‐coding RNA MALAT1 regulates cardiomyocytes apoptosis after hypoxia/reperfusion injury via modulating miR‐200a‐3p/PDCD4 axis. Biomed Pharmacother Mar. 2019;111:1036‐1045. 10.1016/j.biopha.2018.12.122 [DOI] [PubMed] [Google Scholar]
- 97. Du J, Yang ST, Liu J, Zhang KX, Leng JY. Silence of LncRNA GAS5 protects cardiomyocytes H9c2 against hypoxic injury via sponging miR‐ 142‐5p. Mol Cells May. 2019;42(5):397‐405. 10.14348/molcells.2018.0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shi HJ, Wang MW, Sun JT, et al. A novel long noncoding RNA FAF inhibits apoptosis via upregulating FGF9 through PI3K/AKT signaling pathway in ischemia‐hypoxia cardiomyocytes. J Cell Physiol. 2019;234(12):21973‐21987. 10.1002/jcp.28760 [DOI] [PubMed] [Google Scholar]
- 99. Huang S, Tao W, Guo Z, Cao J, Huang X. Suppression of long noncoding RNA TTTY15 attenuates hypoxia‐induced cardiomyocytes injury by targeting miR‐455‐5p. Gene Jun. 2019;701:1‐8. 10.1016/j.gene.2019.02.098 [DOI] [PubMed] [Google Scholar]
- 100. Chen Y, Li X, Li B, et al. Long non‐coding RNA ECRAR triggers post‐natal myocardial regeneration by activating ERK1/2 signaling. Mol Ther. 01. 2019;27(1):29‐45. 10.1016/j.ymthe.2018.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ponnusamy M, Liu F, Zhang YH, et al. Long noncoding RNA CPR (cardiomyocyte proliferation regulator) regulates cardiomyocyte proliferation and cardiac repair. Circulation. 2019;139(23):2668‐2684. 10.1161/CIRCULATIONAHA.118.035832 [DOI] [PubMed] [Google Scholar]
- 102. Wang J, Chen X, Shen D, et al. A long noncoding RNA NR_045363 controls cardiomyocyte proliferation and cardiac repair. J Mol Cell Cardiol. 2019;127:105‐114. 10.1016/j.yjmcc.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 103. Wu Z, Zhao S, Li C, Liu C. LncRNA TUG1 serves an important role in hypoxia‐induced myocardial cell injury by regulating the miR‐145‐5p‐Binp3 axis. Mol Med Rep. 2018;17(2):2422‐2430. 10.3892/mmr.2017.8116 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104. Gong L, Xu H, Chang H, Tong Y, Zhang T, Guo G. Knockdown of long non‐coding RNA MEG3 protects H9c2 cells from hypoxia‐induced injury by targeting microRNA‐183. J Cell Biochem. 2018;119(2):1429‐1440. 10.1002/jcb.26304 [DOI] [PubMed] [Google Scholar]
- 105. Zangrando J, Zhang L, Vausort M, et al. Identification of candidate long non‐coding RNAs in response to myocardial infarction. BMC Genomics. 2014;15(1):1‐14. 10.1186/1471-2164-15-460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yan Y, Song D, Song X, Song C. The role of lncRNA MALAT1 in cardiovascular disease. IUBMB Life. 2020;72(3):334‐342. 10.1002/iub.2210 [DOI] [PubMed] [Google Scholar]
- 107. Zhang J, Gao C, Meng M, Tang H. Long noncoding RNA MHRT protects cardiomyocytes against H2O2‐induced apoptosis. Biomol Ther (Seoul). 2016;24(1):19‐24. 10.4062/biomolther.2015.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Micheletti R, Plaisance I, Abraham BJ, et al. The long noncoding RNA Wisper controls cardiac fibrosis and remodeling. Sci Transl Med. 2017;9(395):eaai9118. 10.1126/scitranslmed.aai9118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tan J, Liu S, Jiang Q, Yu T, Huang K. LncRNA‐MIAT increased in patients with coronary atherosclerotic heart disease. Cardiol Res Pract 2019;2019:6280194. 10.1155/2019/6280194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yao L, Zhou B, You L, Hu H, Xie R. LncRNA MIAT/miR‐133a‐3p axis regulates atrial fibrillation and atrial fibrillation‐induced myocardial fibrosis. Mol Biol Rep Apr. 2020;47(4):2605‐2617. 10.1007/s11033-020-05347-0 [DOI] [PubMed] [Google Scholar]
- 111. Gomes CPC, Schroen B, Kuster GM, et al. Regulatory RNAs in heart failure. Circulation. 2020;141(4):313‐328. 10.1161/CIRCULATIONAHA.119.042474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wang Z, Zhang XJ, Ji YX, et al. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat Med. 2016;22(10):1131‐1139. 10.1038/nm.4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wang K, Liu F, Zhou LY, et al. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR‐489. Circ Res Apr. 2014;114(9):1377‐1388. 10.1161/CIRCRESAHA.114.302476 [DOI] [PubMed] [Google Scholar]
- 114. Zhou G, Li C, Feng J, Zhang J, Fang Y. lncRNA UCA1 is a novel regulator in cardiomyocyte hypertrophy through targeting the miR‐184/HOXA9 axis. Cardiorenal Med. 2018;8(2):130‐139. 10.1159/000487204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Liu L, Zhang D, Li Y. LncRNAs in cardiac hypertrophy: from basic science to clinical application. J Cell Mol Med Oct. 2020;24(20):11638‐11645. 10.1111/jcmm.15819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xu Y, Luo Y, Liang C, Zhang T. LncRNA‐Mhrt regulates cardiac hypertrophy by modulating the miR‐145a‐5p/KLF4/myocardin axis. J Mol Cell Cardiol. 2020;139:47‐61. 10.1016/j.yjmcc.2019.12.013 [DOI] [PubMed] [Google Scholar]
- 117. Zhang J, Liang Y, Huang X, et al. STAT3‐induced upregulation of lncRNA MEG3 regulates the growth of cardiac hypertrophy through miR‐361‐5p/HDAC9 axis. Sci Rep. 2019;9(1):1‐11. 10.1038/s41598-018-36369-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Cai B, Zhang Y, Zhao Y, et al. Long noncoding RNA‐DACH1 (dachshund homolog 1) regulates cardiac function by inhibiting SERCA2a (sarcoplasmic reticulum calcium ATPase 2a). Hypertension. 2019;74(4):833‐842. 10.1161/HYPERTENSIONAHA.119.12998 [DOI] [PubMed] [Google Scholar]
- 119. Liu L, An X, Li Z, Song Y., Li L., Zuo S., Liu N., Yang G., Wang H., Cheng X., Zhang Y., Yang X., Wang J. The H19 long noncoding RNA is a novel negative regulator of cardiomyocyte hypertrophy. Cardiovasc Res 2016;111(1):56‐65. 10.1093/cvr/cvw078 [DOI] [PubMed] [Google Scholar]
- 120. Lv L, Li T, Li X, et al. The lncRNA Plscr4 controls cardiac hypertrophy by regulating miR‐214. Mol Ther Nucleic Acids. Mar. 2018;10:387‐397. 10.1016/j.omtn.2017.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yan SM, Li H, Shu Q, Wu WJ, Luo XM, Lu L. LncRNA SNHG1 exerts a protective role in cardiomyocytes hypertrophy via targeting miR‐15a‐5p/HMGA1 axis. Cell Biol Int Apr. 2020;44(4):1009‐1019. 10.1002/cbin.11298 [DOI] [PubMed] [Google Scholar]
- 122. Shao M, Chen G, Lv F, et al. LncRNA TINCR attenuates cardiac hypertrophy by epigenetically silencing CaMKII. Oncotarget Jul. 2017;8(29):47565‐47573. 10.18632/oncotarget.17735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sun Y, Fan W, Xue R, et al. Transcribed ultraconserved regions, Uc.323, ameliorates cardiac hypertrophy by regulating the transcription of CPT1b (carnitine palmitoyl transferase 1b). Hypertension. 2020;75(1):79‐90. 10.1161/HYPERTENSIONAHA.119.13173 [DOI] [PubMed] [Google Scholar]
- 124. Yu J, Yang Y, Xu Z, et al. Long noncoding RNA Ahit protects against cardiac hypertrophy through SUZ12 (suppressor of Zeste 12 protein homolog)‐mediated downregulation of MEF2A (myocyte enhancer factor 2A). Circ Heart Fail. 2020;13(1):e006525. 10.1161/CIRCHEARTFAILURE.119.006525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wang K, Long B, Liu F, et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR‐223. Eur Heart J Sep. 2016;37(33):2602‐2611. 10.1093/eurheartj/ehv713 [DOI] [PubMed] [Google Scholar]
- 126. Bischoff FC, Werner A, John D, et al. Identification and functional characterization of hypoxia‐induced endoplasmic reticulum stress regulating lncRNA (HypERlnc) in pericytes. Circ Res Aug. 2017;121(4):368‐375. 10.1161/CIRCRESAHA.116.310531 [DOI] [PubMed] [Google Scholar]
- 127. Lin F, Gong X, Yu P, et al. Distinct circulating expression profiles of long noncoding RNAs in heart failure patients with ischemic and nonischemic dilated cardiomyopathy. Front Genet. 2019;10:1‐14. 10.3389/fgene.2019.01116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Gao W, Wang ZM, Zhu M, et al. Altered long noncoding RNA expression profiles in the myocardium of rats with ischemic heart failure. J Cardiovasc Med (Hagerstown). 2015;16(7):473‐479. 10.2459/JCM.0b013e32836499cd [DOI] [PubMed] [Google Scholar]
- 129. Ounzain S, Micheletti R, Beckmann T, et al. Genome‐wide profiling of the cardiac transcriptome after myocardial infarction identifies novel heart‐specific long non‐coding RNAs. Eur Heart J. 2015;36(6):53‐68a. 10.1093/eurheartj/ehu180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Di Salvo TG, Guo Y, Su YR, et al. Right ventricular long noncoding RNA expression in human heart failure. Pulm Circ. 2015;5(1):135‐161. 10.1086/679721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Greco S, Zaccagnini G, Perfetti A, et al. Long noncoding RNA dysregulation in ischemic heart failure. J Transl Med. 2016;14(1):1‐14. 10.1186/s12967-016-0926-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hermans‐Beijnsberger S, van Bilsen M, Schroen B. Long non‐coding RNAs in the failing heart and vasculature. Noncoding RNA Res. 2018;3(3):118–130. 10.1016/j.ncrna.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. El Azzouzi H, Doevendans PA, Sluijter JP. Long non‐coding RNAs in heart failure: an obvious lnc. Ann Transl Med. 2016;4(9):182‐187. 10.21037/atm.2016.05.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Mei B, Liu H, Yang S, et al. Long non‐coding RNA expression profile in permanent atrial fibrillation patients with rheumatic heart disease. Eur Rev Med Pharmacol Sci. 2018;22(20):6940‐6947. 10.26355/eurrev_201810_16165 [DOI] [PubMed] [Google Scholar]
- 135. Babapoor‐Farrokhran S, Gill D, Rasekhi RT. The role of long noncoding RNAs in atrial fibrillation. Heart Rhythm. 2020;17(6):1043‐1049. 10.1016/j.hrthm.2020.01.015 [DOI] [PubMed] [Google Scholar]
- 136. Ke ZP, Xu YJ, Wang ZS, Sun J. RNA sequencing profiling reveals key mRNAs and long noncoding RNAs in atrial fibrillation. J Cell Biochem. 2019;121(8‐9):3752‐3763. 10.1002/jcb.29504 [DOI] [PubMed] [Google Scholar]
- 137. Ruan Z, Sun X, Sheng H, Zhu L. Long non‐coding RNA expression profile in atrial fibrillation. Int J Clin Exp Pathol. 2015;8(7):8402‐8410. [PMC free article] [PubMed] [Google Scholar]
- 138. Yu XJ, Zou LH, Jin JH, et al. Long noncoding RNAs and novel inflammatory genes determined by RNA sequencing in human lymphocytes are up‐regulated in permanent atrial fibrillation. Am J Transl Res. 2017;9(5):2314‐2326. [PMC free article] [PubMed] [Google Scholar]
- 139. Wu DM, Zhou ZK, Fan SH, et al. Comprehensive RNA‐Seq data analysis identifies key mRNAs and lncRNAs in atrial fibrillation. Front Genet. 2019;10:908–918. 10.3389/fgene.2019.00908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Xu Y, Huang R, Gu J, Jiang W. Identification of long non‐coding RNAs as novel biomarker and potential therapeutic target for atrial fibrillation in old adults. Oncotarget. 2016;7(10):10803‐10811. 10.18632/oncotarget.7514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Li Z, Wang X, Wang W, et al. Altered long non‐coding RNA expression profile in rabbit atria with atrial fibrillation: TCONS_00075467 modulates atrial electrical remodeling by sponging miR‐328 to regulate CACNA1C. J Mol Cell Cardiol. 2017;108:73‐85. 10.1016/j.yjmcc.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 142. Zhao JB, Zhu N, Lei YH, Zhang CJ, Li YH. Modulative effects of lncRNA TCONS_00202959 on autonomic neural function and myocardial functions in atrial fibrillation rat model. Eur Rev Med Pharmacol Sci. 2018;22(24):8891‐8897. 10.26355/eurrev_201812_16658 [DOI] [PubMed] [Google Scholar]
- 143. Chen G, Guo H, Song Y, et al. Long non‐coding RNA AK055347 is upregulated in patients with atrial fibrillation and regulates mitochondrial energy production in myocardiocytes. Mol Med Rep. 2016;14(6):5311‐5317. 10.3892/mmr.2016.5893 [DOI] [PubMed] [Google Scholar]
- 144. Shen C, Kong B, Liu Y, et al. YY1‐induced upregulation of lncRNA KCNQ1OT1 regulates angiotensin II‐induced atrial fibrillation by modulating miR‐384b/CACNA1C axis. Biochem Biophys Res Commun. 2018;505(1):134‐140. 10.1016/j.bbrc.2018.09.064 [DOI] [PubMed] [Google Scholar]
- 145. Bektik E, Cowan DB, Wang DZ. Long non‐coding RNAs in atrial fibrillation: pluripotent stem cell‐derived cardiomyocytes as a model system. Int J Mol Sci. 2020;21(15):1‐25. 10.3390/ijms21155424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Viereck J, Thum T. Circulating noncoding RNAs as biomarkers of cardiovascular disease and injury. Circ Res. 2017;120(2):381‐399. 10.1161/CIRCRESAHA.116.308434 [DOI] [PubMed] [Google Scholar]
- 147. Franco D, Aranega A, Dominguez JN. Non‐coding RNAs and atrial fibrillation. Adv Exp Med Biol. 2020;1229:311‐325. 10.1007/978-981-15-1671-9_19 [DOI] [PubMed] [Google Scholar]
- 148. Zhao L, Ma Z, Guo Z, Zheng M, Li K, Yang X. Analysis of long non‐coding RNA and mRNA profiles in epicardial adipose tissue of patients with atrial fibrillation. Biomed Pharmacother. 2020;121:109634. 10.1016/j.biopha.2019.109634 [DOI] [PubMed] [Google Scholar]
- 149. Liao J, Wang J, Liu Y, Li J, Duan L. Transcriptome sequencing of lncRNA, miRNA, mRNA and interaction network constructing in coronary heart disease. BMC Med Genomics. 2019;12(1):124‐136. 10.1186/s12920-019-0570-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zhang Y, Zhang L, Wang Y, et al. MicroRNAs or long noncoding RNAs in diagnosis and prognosis of coronary artery disease. Aging Dis. 2019;10(2):353‐366. 10.14336/AD.2018.0617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhou X, Han X, Wittfeldt A, et al. Long non‐coding RNA ANRIL regulates inflammatory responses as a novel component of NF‐κB pathway. RNA Biol. 2016;13(1):98‐108. 10.1080/15476286.2015.1122164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhang Z, Gao W, Long QQ, et al. Increased plasma levels of lncRNA H19 and LIPCAR are associated with increased risk of coronary artery disease in a Chinese population. Sci Rep. 2017;7(1):7491–7500. 10.1038/s41598-017-07611-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zhao Y, Feng G, Wang Y, Yue Y, Zhao W. Regulation of apoptosis by long non‐coding RNA HIF1A‐AS1 in VSMCs: implications for TAA pathogenesis. Int J Clin Exp Pathol. 2014;7(11):7643‐7652. [PMC free article] [PubMed] [Google Scholar]
- 154. Wu G, Cai J, Han Y, et al. LincRNA‐p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 2014;130(17):1452‐1465. 10.1161/CIRCULATIONAHA.114.011675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Shan K, Jiang Q, Wang XQ, et al. Role of long non‐coding RNA‐RNCR3 in atherosclerosis‐related vascular dysfunction. Cell Death Dis. 2016;7(6):e2248‐e2262. 10.1038/cddis.2016.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Huang S, Lu W, Ge D, et al. A new microRNA signal pathway regulated by long noncoding RNA TGFB2‐OT1 in autophagy and inflammation of vascular endothelial cells. Autophagy. 2015;11(12):2172‐2183. 10.1080/15548627.2015.1106663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Leung A, Trac C, Jin W, et al. Novel long noncoding RNAs are regulated by angiotensin II in vascular smooth muscle cells. Circ Res. 2013;113(3):266‐278. 10.1161/CIRCRESAHA.112.300849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Vigetti D, Deleonibus S, Moretto P, et al. Natural antisense transcript for hyaluronan synthase 2 (HAS2‐AS1) induces transcription of HAS2 via protein O‐GlcNAcylation. J Biol Chem. 2014;289(42):28816‐28826. 10.1074/jbc.M114.597401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ballantyne MD, Pinel K, Dakin R, et al. Smooth muscle enriched long noncoding RNA (SMILR) regulates cell proliferation. Circulation. 2016;133(21):2050‐2065. 10.1161/CIRCULATIONAHA.115.021019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Boulberdaa M, Scott E, Ballantyne M, et al. A role for the long noncoding RNA SENCR in commitment and function of endothelial cells. Mol Ther. 2016;24(5):978‐990. 10.1038/mt.2016.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Wu Z, He Y, Li D, et al. Long noncoding RNA MEG3 suppressed endothelial cell proliferation and migration through regulating miR‐21. Am J Transl Res. 2017;9(7):3326‐3335. [PMC free article] [PubMed] [Google Scholar]
- 162. Lin Z, Ge J, Wang Z, et al. Let‐7e modulates the inflammatory response in vascular endothelial cells through ceRNA crosstalk. Sci Rep. 2017;7: 42498. 10.1038/srep42498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Aryal B, Rotllan N, Fernández‐Hernando C. Noncoding RNAs and atherosclerosis. Curr Atheroscler Rep. 2014;16(5):407‐418. 10.1007/s11883-014-0407-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zhang Z, Salisbury D, Sallam T. Long noncoding RNAs in atherosclerosis: JACC review topic of the week. J am Coll Cardiol. 2018;72(19):2380‐2390. 10.1016/j.jacc.2018.08.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Pierce JB, Feinberg MW. Long noncoding RNAs in atherosclerosis and vascular injury: pathobiology, biomarkers, and targets for therapy. Arterioscler Thromb Vasc Biol. 2020;40(9):2002‐2017. 10.1161/ATVBAHA.120.314222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Holdt LM, Hoffmann S, Sass K, et al. Alu elements in ANRIL non‐coding RNA at chromosome 9p21 modulate atherogenic cell functions through trans‐regulation of gene networks. PLoS Genet. 2013;9(7):e1003588‐e1003600. 10.1371/journal.pgen.1003588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Tontonoz P, Wu X, Jones M, Zhang Z, Salisbury D, Sallam T. Long noncoding RNA facilitated gene therapy reduces atherosclerosis in a murine model of familial hypercholesterolemia. Circulation. 2017;136(8):776‐778. 10.1161/CIRCULATIONAHA.117.029002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hu YW, Zhao JY, Li SF, et al. RP5‐833A20.1/miR‐382‐5p/NFIA‐dependent signal transduction pathway contributes to the regulation of cholesterol homeostasis and inflammatory reaction. Arterioscler Thromb Vasc Biol. 2015;35(1):87‐101. 10.1161/ATVBAHA.114.304296 [DOI] [PubMed] [Google Scholar]
- 169. Sallam T, Jones M, Thomas BJ, et al. Transcriptional regulation of macrophage cholesterol efflux and atherogenesis by a long noncoding RNA. Nat Med. 2018;24(3):304‐312. 10.1038/nm.4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Swayze EE, Siwkowski AM, Wancewicz EV, et al. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 2007;35(2):687‐700. 10.1093/nar/gkl1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Viereck J, Kumarswamy R, Foinquinos A, et al. Long noncoding RNA Chast promotes cardiac remodeling. Sci Transl Med. 2016;8(326):326ra22‐326ra35. 10.1126/scitranslmed.aaf1475 [DOI] [PubMed] [Google Scholar]
- 172. Piccoli MT, Gupta SK, Viereck J, et al. Inhibition of the cardiac fibroblast‐enriched lncRNA Meg3 prevents cardiac fibrosis and diastolic dysfunction. Circ Res. 2017;121(5):575‐583. 10.1161/CIRCRESAHA.117.310624 [DOI] [PubMed] [Google Scholar]
- 173. Hobuß L, Bär C, Thum T. Long non‐coding RNAs: at the heart of cardiac dysfunction? Front Physiol. 2019;10:30‐39. 10.3389/fphys.2019.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kumarswamy R, Bauters C, Volkmann I, et al. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ Res May. 2014;114(10):1569‐1575. 10.1161/CIRCRESAHA.114.303915 [DOI] [PubMed] [Google Scholar]
- 175. Xuan L, Sun L, Zhang Y, et al. Circulating long non‐coding RNAs NRON and MHRT as novel predictive biomarkers of heart failure. J Cell Mol Med. 2017;21(9):1803‐1814. 10.1111/jcmm.13101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Wang F, Su X, Liu C, Wu M, Li B. Prognostic value of plasma long noncoding RNA ANRIL for in‐stent restenosis. Med Sci Monit. 2017;23:4733‐4739:4733‐4739. 10.12659/msm.904352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Cai Y, Yang Y, Chen X, et al. Circulating “LncPPARδ” from monocytes as a novel biomarker for coronary artery diseases. Medicine (Baltimore). 2016;95(6):e2360–e2373. 10.1097/MD.0000000000002360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Yang Y, Cai Y, Wu G, et al. Plasma long non‐coding RNA, CoroMarker, a novel biomarker for diagnosis of coronary artery disease. Clin Sci (Lond). 2015;129(8):675‐685. 10.1042/CS20150121 [DOI] [PubMed] [Google Scholar]
- 179. Jin L, Lin X, Yang L, et al. AK098656, a novel vascular smooth muscle cell‐dominant long noncoding RNA, promotes hypertension. Hypertension. 2018;71(2):262‐272. 10.1161/HYPERTENSIONAHA.117.09651 [DOI] [PubMed] [Google Scholar]
- 180. Zhou H, Wang B, Yang YX, Jia QJ, Zhang A, Qi ZW, Zhang JP Long noncoding RNAs in pathological cardiac remodeling: a review of the update literature. Biomed Res Int 2019;2019:7159592. 10.1155/2019/7159592, 1, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Shi L, Tian C, Sun L, Cao F, Meng Z. The lncRNA TUG1/miR‐145‐5p/FGF10 regulates proliferation and migration in VSMCs of hypertension. Biochem Biophys Res Commun. 2018;501(3):688‐695. 10.1016/j.bbrc.2018.05.049 [DOI] [PubMed] [Google Scholar]
- 182. Fang G, Qi J, Huang L, Zhao X. LncRNA MRAK048635_P1 is critical for vascular smooth muscle cell function and phenotypic switching in essential hypertension. Biosci Rep. 2019;39(3):1‐11. 10.1042/BSR20182229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Yao QP, Xie ZW, Wang KX, et al. Profiles of long noncoding RNAs in hypertensive rats: long noncoding RNA XR007793 regulates cyclic strain‐induced proliferation and migration of vascular smooth muscle cells. J Hypertens. 2017;35(6):1195‐1203. 10.1097/HJH.0000000000001304 [DOI] [PubMed] [Google Scholar]
- 184. Liu K, Liu C, Zhang Z. lncRNA GAS5 acts as a ceRNA for miR‐21 in suppressing PDGF‐bb‐induced proliferation and migration in vascular smooth muscle cells. J Cell Biochem. 2019;120(9):15233‐15240. 10.1002/jcb.28789 [DOI] [PubMed] [Google Scholar]
- 185. Wang YN, Shan K, Yao MD, et al. Long noncoding RNA‐GAS5: a novel regulator of hypertension‐induced vascular remodeling. Hypertension. 2016;68(3):736‐748. 10.1161/HYPERTENSIONAHA.116.07259 [DOI] [PubMed] [Google Scholar]
- 186. Li D, Zhang C, Li J, Che J, Yang X, Xian Y, Li X, Cao C Long non‐coding RNA MALAT1 promotes cardiac remodeling in hypertensive rats by inhibiting the transcription of MyoD. Aging (Albany NY). 10 2019;11(20):8792‐8809. 10.18632/aging.102265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Du SS, Zuo XJ, Xin Y, Man JX, Wu ZL. Expression of lncRNA TUG1 in hypertensive patients and its relationship with change state of an illness. Eur Rev Med Pharmacol Sci. 2020;24(2):870‐877. 10.26355/eurrev_202001_20071 [DOI] [PubMed] [Google Scholar]
- 188. Das S, Zhang E, Senapati P, et al. A novel angiotensin II‐induced long noncoding RNA giver regulates oxidative stress, inflammation, and proliferation in vascular smooth muscle cells. Circ Res. 2018;123(12):1298‐1312. 10.1161/CIRCRESAHA.118.313207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Bayoglu B, Yuksel H, Cakmak HA, Dirican A, Cengiz M. Polymorphisms in the long non‐coding RNA CDKN2B‐AS1 may contribute to higher systolic blood pressure levels in hypertensive patients. Clin Biochem. 2016;49(10–11):821‐827. 10.1016/j.clinbiochem.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 190. Huang K, Zhong J, Li Q, et al. Effects of CDKN2B‐AS1 polymorphisms on the susceptibility to coronary heart disease. Mol Genet Genomic Med. 2019;7(11):e955‐e963. 10.1002/mgg3.955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: epidemiology and treatment. Curr Diab Rep. 2014;14(8):508‐508. 10.1007/s11892-014-0508-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Sathishkumar C, Prabu P, Mohan V, Balasubramanyam M. Linking a role of lncRNAs (long non‐coding RNAs) with insulin resistance, accelerated senescence, and inflammation in patients with type 2 diabetes. Hum Genomics. 2018;12(1):41‐50. 10.1186/s40246-018-0173-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Zhang L, Wang YM. Expression and function of lncRNA ANRIL in a mouse model of acute myocardial infarction combined with type 2 diabetes mellitus. J Chin Med Assoc. 2019;82(9):685‐692. 10.1097/JCMA.0000000000000182 [DOI] [PubMed] [Google Scholar]
- 194. Liu SX, Zheng F, Xie KL, Xie MR, Jiang LJ, Cai Y. Exercise reduces insulin resistance in type 2 diabetes mellitus via mediating the lncRNA MALAT1/microRNA‐382‐3p/resistin axis. Mol Ther Nucleic Acids. 2019;18:34‐44. 10.1016/j.omtn.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Yu W, Zhao GQ, Cao RJ, Zhu ZH, Li K. LncRNA NONRATT021972 was associated with neuropathic pain scoring in patients with type 2 diabetes. Behav Neurol 2017;2017:2941297. 10.1155/2017/2941297, 1, 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Ruan Y, Lin N, Ma Q, et al. Circulating LncRNAs analysis in patients with type 2 diabetes reveals novel genes influencing glucose metabolism and islet β‐cell function. Cell Physiol Biochem. 2018;46(1):335‐350. 10.1159/000488434 [DOI] [PubMed] [Google Scholar]
- 197. Fawzy MS, Abdelghany AA, Toraih EA, Mohamed AM. Circulating long noncoding RNAs H19 and GAS5 are associated with type 2 diabetes but not with diabetic retinopathy: a preliminary study. Bosn J Basic Med Sci. Jan. 2020;20(3):365–371. 10.17305/bjbms.2019.4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Morán I, Akerman I, van de Bunt M, et al. Human β cell transcriptome analysis uncovers lncRNAs that are tissue‐specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab. 2012;16(4):435‐448. 10.1016/j.cmet.2012.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Reddy MA, Chen Z, Park JT, et al. Regulation of inflammatory phenotype in macrophages by a diabetes‐induced long noncoding RNA. Diabetes. 2014;63(12):4249‐4261. 10.2337/db14-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Mansoori Z, Ghaedi H, Sadatamini M, et al. Downregulation of long non‐coding RNAs LINC00523 and LINC00994 in type 2 diabetes in an Iranian cohort. Mol Biol Rep. 2018;45(5):1227‐1233. 10.1007/s11033-018-4276-7 [DOI] [PubMed] [Google Scholar]
- 201. Saeidi L, Ghaedi H, Sadatamini M, et al. Long non‐coding RNA LY86‐AS1 and HCG27_201 expression in type 2 diabetes mellitus. Mol Biol Rep. 2018;45(6):2601‐2608. 10.1007/s11033-018-4429-8 [DOI] [PubMed] [Google Scholar]
- 202. Carter G, Miladinovic B, Patel AA, Deland L, Mastorides S, Patel NA. Circulating long noncoding RNA GAS5 levels are correlated to prevalence of type 2 diabetes mellitus. BBA Clin. 2015;4:102‐107. 10.1016/j.bbacli.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Arnes L, Akerman I, Balderes DA, Ferrer J, Sussel L. βlinc1 encodes a long noncoding RNA that regulates islet β‐cell formation and function. Genes Dev. 2016;30(5):502‐507. 10.1101/gad.273821.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Kovesdy CP, Furth SL, Zoccali C. Committee WKDS. Obesity and kidney disease: hidden consequences of the epidemic. Can J Kidney Health Dis. 2017;4:2054358117698669. 10.1177/2054358117698669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Tsuboi N, Okabayashi Y, Shimizu A, Yokoo T. The renal pathology of obesity. Kidney Int Rep. 2017;2(2):251‐260. 10.1016/j.ekir.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Pommer W. Preventive nephrology: the role of obesity in different stages of chronic kidney disease. Kidney Dis (Basel) Nov. 2018;4(4):199‐204. 10.1159/000490247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Li Y, Xu K, Chen S, Cao Y, Zhan H. Roles of identified long noncoding RNA in diabetic nephropathy. J Diabetes Res 2019;2019:5383010. 10.1155/2019/5383010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Leung A, Natarajan R. Long noncoding RNAs in diabetes and diabetic complications. Antioxid Redox Signal. 2018;29(11):1064‐1073. 10.1089/ars.2017.7315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. He X, Ou C, Xiao Y, Han Q, Li H, Zhou S. LncRNAs: key players and novel insights into diabetes mellitus. Oncotarget. 2017;8(41):71325‐71341. 10.18632/oncotarget.19921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Li SY, Susztak K. The long noncoding RNA Tug1 connects metabolic changes with kidney disease in podocytes. J Clin Invest 11. 2016;126(11):4072‐4075. 10.1172/JCI90828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Long J, Badal SS, Ye Z, et al. Long noncoding RNA Tug1 regulates mitochondrial bioenergetics in diabetic nephropathy. J Clin Invest. 2016;126(11):4205‐4218. 10.1172/JCI87927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Duan LJ, Ding M, Hou LJ, Cui YT, Li CJ, Yu DM. Long noncoding RNA TUG1 alleviates extracellular matrix accumulation via mediating microRNA‐377 targeting of PPARγ in diabetic nephropathy. Biochem Biophys Res Commun. 2017;484(3):598‐604. 10.1016/j.bbrc.2017.01.145 [DOI] [PubMed] [Google Scholar]
- 213. Zhou L, Xu DY, Sha WG, Shen L, Lu GY, Yin X. Long non‐coding MIAT mediates high glucose‐induced renal tubular epithelial injury. Biochem Biophys Res Commun. 2015;468(4):726‐732. 10.1016/j.bbrc.2015.11.023 [DOI] [PubMed] [Google Scholar]
- 214. Wang L, Su N, Zhang Y, Wang G. Clinical significance of serum lncRNA cancer susceptibility candidate 2 (CASC2) for chronic renal failure in patients with type 2 diabetes. Med Sci Monit Sep. 2018;24:6079‐6084. 10.12659/MSM.909510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Muller DN, Schmidt C, Barbosa‐Sicard E, et al. Mouse Cyp4a isoforms: enzymatic properties, gender‐ and strain‐specific expression, and role in renal 20‐hydroxyeicosatetraenoic acid formation. Biochem J. 2007;403(1):109‐118. 10.1042/BJ20061328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Li A, Peng R, Sun Y, Liu H, Peng H, Zhang Z. LincRNA 1700020I14Rik alleviates cell proliferation and fibrosis in diabetic nephropathy via miR‐34a‐5p/Sirt1/HIF‐1α signaling. Cell Death Dis. 2018;9(5):461–477. 10.1038/s41419-018-0527-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Wang M, Wang S, Yao D, Yan Q, Lu W. A novel long non‐coding RNA CYP4B1‐PS1‐001 regulates proliferation and fibrosis in diabetic nephropathy. Mol Cell Endocrinol. 2016;426:136‐145. 10.1016/j.mce.2016.02.020 [DOI] [PubMed] [Google Scholar]
- 218. Feng Y, Chen S, Xu J, et al. Dysregulation of lncRNAs GM5524 and GM15645 involved in high‐glucose‐induced podocyte apoptosis and autophagy in diabetic nephropathy. Mol Med Rep. 2018;18(4):3657‐3664. 10.3892/mmr.2018.9412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Bai X, Geng J, Li X, et al. Long noncoding RNA LINC01619 regulates microRNA‐27a/forkhead box protein O1 and endoplasmic reticulum stress‐mediated podocyte injury in diabetic nephropathy. Antioxid Redox Signal. 2018;29(4):355‐376. 10.1089/ars.2017.7278 [DOI] [PubMed] [Google Scholar]
- 220. Li X, Zeng L, Cao C, et al. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR‐23c targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res. 2017;350(2):327‐335. 10.1016/j.yexcr.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 221. Yi H, Peng R, Zhang LY, et al. LincRNA‐Gm4419 knockdown ameliorates NF‐κB/NLRP3 inflammasome‐mediated inflammation in diabetic nephropathy. Cell Death Dis. 2017;8(2):e2583–e2597. 10.1038/cddis.2016.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Gao J, Wang W, Wang F, Guo C. LncRNA‐NR_033515 promotes proliferation, fibrogenesis and epithelial‐to‐mesenchymal transition by targeting miR‐743b‐5p in diabetic nephropathy. Biomed Pharmacother. 2018;106:543‐552. 10.1016/j.biopha.2018.06.104 [DOI] [PubMed] [Google Scholar]
- 223. Sun SF, Tang PMK, Feng M, et al. Novel lncRNA Erbb4‐IR promotes diabetic kidney injury in db/db mice by targeting miR‐29b. Diabetes. 2018;67(4):731‐744. 10.2337/db17-0816 [DOI] [PubMed] [Google Scholar]
- 224. Gao Y, Chen ZY, Wang Y, Liu Y, Ma JX, Li YK. Long non‐coding RNA ASncmtRNA‐2 is upregulated in diabetic kidneys and high glucose‐treated mesangial cells. Exp Ther Med. 2017;13(2):581‐587. 10.3892/etm.2017.4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Kato M, Wang M, Chen Z, et al. An endoplasmic reticulum stress‐regulated lncRNA hosting a microRNA megacluster induces early features of diabetic nephropathy. Nat Commun. 2016;7(1):12864–12880. 10.1038/ncomms12864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Zhang P, Sun Y, Peng R, et al. Long non‐coding RNA Rpph1 promotes inflammation and proliferation of mesangial cells in diabetic nephropathy via an interaction with Gal‐3. Cell Death Dis. 2019;10(7):526–542. 10.1038/s41419-019-1765-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Kato M, Natarajan R. Diabetic nephropathy—emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10(9):517‐530. 10.1038/nrneph.2014.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Kato M, Putta S, Wang M, et al. TGF‐beta activates Akt kinase through a microRNA‐dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11(7):881‐889. 10.1038/ncb1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Kato M, Dang V, Wang M, et al. TGF‐β induces acetylation of chromatin and of Ets‐1 to alleviate repression of miR‐192 in diabetic nephropathy. Sci Signal. 2013;6(278):ra43–ra55. 10.1126/scisignal.2003389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Zhou Q, Chung AC, Huang XR, Dong Y, Yu X, Lan HY. Identification of novel long noncoding RNAs associated with TGF‐β/Smad3‐mediated renal inflammation and fibrosis by RNA sequencing. Am J Pathol. 2014;184(2):409‐417. 10.1016/j.ajpath.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 231. King LK, March L, Anandacoomarasamy A. Obesity & osteoarthritis. Indian J Med Res. 2013;138:185‐193. [PMC free article] [PubMed] [Google Scholar]
- 232. Jiang SD, Lu J, Deng ZH, Li YS, Lei GH. Long noncoding RNAs in osteoarthritis. Joint Bone Spine. 2017;84(5):553‐556. 10.1016/j.jbspin.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 233. Huang T, Wang J, Zhou Y, Zhao Y, Hang D, Cao Y. LncRNA CASC2 is up‐regulated in osteoarthritis and participates in the regulation of IL‐17 expression and chondrocyte proliferation and apoptosis. Biosci Rep. 2019;39(5):1‐7. 10.1042/BSR20182454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Lei J, Fu Y, Zhuang Y, Zhang K, Lu D. LncRNA SNHG1 alleviates IL‐1β‐induced osteoarthritis by inhibiting miR‐16‐5p‐mediated p38 MAPK and NF‐κB signaling pathways. Biosci Rep. 2019;39(9):1‐10. 10.1042/BSR20191523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Zhang L, Zhang P, Sun X, Zhou L, Zhao J. Long non‐coding RNA DANCR regulates proliferation and apoptosis of chondrocytes in osteoarthritis via miR‐216a‐5p‐JAK2‐STAT3 axis. Biosci Rep. 2018;38(6):1‐11. 10.1042/BSR20181228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Mao T, He C, Wu H, Yang B, Li X. Silencing lncRNA HOTAIR declines synovial inflammation and synoviocyte proliferation and promotes synoviocyte apoptosis in osteoarthritis rats by inhibiting Wnt/β‐catenin signaling pathway. Cell Cycle. 2019;18(22):3189‐3205. 10.1080/15384101.2019.1671716 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 237. Hu Y, Li S, Zou Y. Knockdown of LncRNA H19 relieves LPS‐induced damage by modulating miR‐130a in osteoarthritis. Yonsei Med J. 2019;60(4):381‐388. 10.3349/ymj.2019.60.4.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Tian F, Wang J, Zhang Z, Yang J. LncRNA SNHG7/miR‐34a‐5p/SYVN1 axis plays a vital role in proliferation, apoptosis and autophagy in osteoarthritis. Biol Res. 2020;53(1):9–20. 10.1186/s40659-020-00275-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Luo X, Wang J, Wei X, Wang S, Wang A. Knockdown of lncRNA MFI2‐AS1 inhibits lipopolysaccharide‐induced osteoarthritis progression by miR‐130a‐3p/TCF4. Life Sci. 2020;240:117019–117027. 10.1016/j.lfs.2019.117019 [DOI] [PubMed] [Google Scholar]
- 240. Pearson MJ, Philp AM, Heward JA, et al. Long intergenic noncoding RNAs mediate the human chondrocyte inflammatory response and are differentially expressed in osteoarthritis cartilage. Arthritis Rheumatol. 2016;68(4):845‐856. 10.1002/art.39520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Zhao Y, Zhao J, Guo X, She J, Liu Y. Long non‐coding RNA PVT1, a molecular sponge for miR‐149, contributes aberrant metabolic dysfunction and inflammation in IL‐1β‐simulated osteoarthritic chondrocytes. Biosci Rep. 2018;38(5):1‐11. 10.1042/BSR20180576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Xiang S, Li Z, Bian Y, Weng X. Identification of changed expression of mRNAs and lncRNAs in osteoarthritic synovium by RNA‐sequencing. Gene. 2019;685:55‐61. 10.1016/j.gene.2018.10.076 [DOI] [PubMed] [Google Scholar]
- 243. Wang Y, Cao L, Wang Q, Huang J, Xu S. LncRNA FOXD2‐AS1 induces chondrocyte proliferation through sponging miR‐27a‐3p in osteoarthritis. Artif Cells Nanomed Biotechnol Dec. 2019;47(1):1241‐1247. 10.1080/21691401.2019.1596940 [DOI] [PubMed] [Google Scholar]
- 244. Fu M, Huang G, Zhang Z, et al. Expression profile of long noncoding RNAs in cartilage from knee osteoarthritis patients. Osteoarthr Cartil. 2015;23(3):423‐432. 10.1016/j.joca.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 245. Zhao Y, Xu J. Synovial fluid‐derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int Orthop. 2018;42(12):2865‐2872. 10.1007/s00264-018-4093-6 [DOI] [PubMed] [Google Scholar]
- 246. Nanus DE, Wijesinghe SN, Pearson MJ, et al. Regulation of the inflammatory synovial fibroblast phenotype by metastasis‐associated lung adenocarcinoma transcript 1 long noncoding RNA in obese patients with osteoarthritis. Arthritis Rheumatol. 2020;72(4):609‐619. 10.1002/art.41158 [DOI] [PubMed] [Google Scholar]
- 247. Park S, Lee M, Chun CH, Jin EJ. The lncRNA, Nespas, is associated with osteoarthritis progression and serves as a potential new prognostic biomarker. Cartilage. 2019;10(2):148‐156. 10.1177/1947603517725566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Edmison JM, Kalhan SC, McCullough AJ. Obesity, hepatic metabolism and disease. Nestle Nutr Workshop Ser Pediatr Program. 2009;63:163‐172; discussion 172‐6, 259‐68. 10.1159/000209980 [DOI] [PubMed] [Google Scholar]
- 249. Zhao XY, Xiong X, Liu T, et al. Long noncoding RNA licensing of obesity‐linked hepatic lipogenesis and NAFLD pathogenesis. Nat Commun. 2018;9(1):2986–3000. 10.1038/s41467-018-05383-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Ma M, Duan R, Shen L, et al. The lncRNA Gm15622 stimulates SREBP‐1c expression and hepatic lipid accumulation by sponging the miR‐742‐3p in mice. J Lipid Res. 2020;61(7):1052‐1064. 10.1194/jlr.RA120000664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Chi Y, Gong Z, Xin H, Wang Z, Liu Z. Long noncoding RNA lncARSR promotes nonalcoholic fatty liver disease and hepatocellular carcinoma by promoting YAP1 and activating the IRS2/AKT pathway. J Transl Med. 2020;18(1):126–137. 10.1186/s12967-020-02225-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Wang H, Cao Y, Shu L, et al. Long non‐coding RNA (lncRNA) H19 induces hepatic steatosis through activating MLXIPL and mTORC1 networks in hepatocytes. J Cell Mol Med. 2020;24(2):1399‐1412. 10.1111/jcmm.14818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Chen Y, Chen X, Gao J, et al. Long noncoding RNA FLRL2 alleviated nonalcoholic fatty liver disease through Arntl‐Sirt1 pathway. FASEB j. 2019;33(10):11411‐11419. 10.1096/fj.201900643RRR [DOI] [PubMed] [Google Scholar]
- 254. Huang P, Huang FZ, Liu HZ, Zhang TY, Yang MS, Sun CZ. LncRNA MEG3 functions as a ceRNA in regulating hepatic lipogenesis by competitively binding to miR‐21 with LRP6. Metabolism. 2019;94:1‐8. 10.1016/j.metabol.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 255. Chen Y, Huang H, Xu C, Yu C, Li Y. Long non‐coding RNA profiling in a non‐alcoholic fatty liver disease rodent model: new insight into pathogenesis. Int J Mol Sci. 2017;18(1):21–34. 10.3390/ijms18010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Chen X, Xu Y, Zhao D, et al. LncRNA‐AK012226 is involved in fat accumulation in db/db mice fatty liver and non‐alcoholic fatty liver disease cell model. Front Pharmacol. 2018;9:888–900. 10.3389/fphar.2018.00888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Ma TT, Huang C, Ni Y, Yang Y, Li J. ATP citrate lyase and LncRNA NONMMUT010685 play crucial role in nonalcoholic fatty liver disease based on analysis of microarray data. Cell Physiol Biochem. 2018;51(2):871‐885. 10.1159/000495384 [DOI] [PubMed] [Google Scholar]
- 258. Leti F, Legendre C, Still CD, et al. Altered expression of MALAT1 lncRNA in nonalcoholic steatohepatitis fibrosis regulates CXCL5 in hepatic stellate cells. Transl Res. 2017;190:25‐39.e21. 10.1016/j.trsl.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Ruan X, Li P, Ma Y, et al. Identification of human long non‐coding RNAs associated with nonalcoholic fatty liver disease and metabolic homeostasis. J Clin Invest. 2020;131(1):e136336–e136352. 10.1172/JCI136336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Di Mauro S, Scamporrino A, Petta S, et al. Serum coding and non‐coding RNAs as biomarkers of NAFLD and fibrosis severity. Liver Int. 2019;39(9):1742‐1754. 10.1111/liv.14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Huang R, Duan X, Fan J, Li G, Wang B. Role of noncoding RNA in development of nonalcoholic fatty liver disease. Biomed Res Int 2019;2019:8690592. 10.1155/2019/8690592, 1, 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Ji E, Kim C, Kim W, Lee EK. Role of long non‐coding RNAs in metabolic control. Biochim Biophys Acta Gene Regul Mech. 2020;1863(4):194348–194361. 10.1016/j.bbagrm.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 263. Zhao Y, Wu J, Liangpunsakul S, Wang L. Long non‐coding RNA in liver metabolism and disease: current status. Liver Res. 2017;1(3):163‐167. 10.1016/j.livres.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Giroud M, Scheideler M. Long non‐coding RNAs in metabolic organs and energy homeostasis. Int J Mol Sci. 2017;18(12):2578‐2595. 10.3390/ijms18122578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Sulaiman SA, Muhsin NIA, Jamal R. Regulatory non‐coding RNAs network in non‐alcoholic fatty liver disease. Front Physiol. 2019;10:279‐290. 10.3389/fphys.2019.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Hanson A, Wilhelmsen D, DiStefano JK. The role of long non‐coding RNAs (lncRNAs) in the development and progression of fibrosis associated with nonalcoholic fatty liver disease (NAFLD). Noncoding RNA. 2018;4(3):18‐33. 10.3390/ncrna4030018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Rohilla S, Awasthi A, Kaur S, Puria R. Evolutionary conservation of long non‐coding RNAs in non‐alcoholic fatty liver disease. Life Sci. 2020;264:118560‐118571. 10.1016/j.lfs.2020.118560 [DOI] [PubMed] [Google Scholar]
- 268. Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218‐1240. 10.3390/nu5041218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Chen Z. Progress and prospects of long noncoding RNAs in lipid homeostasis. Mol Metab. 2016;5(3):164‐170. 10.1016/j.molmet.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Tang S, Zhu W, Zheng F, et al. The long noncoding RNA Blnc1 protects against diet‐induced obesity by promoting mitochondrial function in white fat. Diabetes Metab Syndr Obes. 2020;13:1189‐1201:1189‐1201. 10.2147/DMSO.S248692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Wang J, Xiang D, Mei S, et al. The novel long noncoding RNA Lnc19959.2 modulates triglyceride metabolism‐associated genes through the interaction with Purb and hnRNPA2B1. Mol Metab. 2020;37:100996‐101009. 10.1016/j.molmet.2020.100996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Muret K, Désert C, Lagoutte L, et al. Long noncoding RNAs in lipid metabolism: literature review and conservation analysis across species. BMC Genomics. 2019;20(1):882‐900. 10.1186/s12864-019-6093-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Yau MY, Xu L, Huang CL, Wong CM. Long non‐coding RNAs in obesity‐induced cancer. Noncoding RNA. 2018;4(3):19‐36. 10.3390/ncrna4030019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Hanson RL, Craig DW, Millis MP, Yeatts KA, Kobes S, Pearson JV, Lee AM, Knowler WC, Nelson RG, Wolford JK Identification of PVT1 as a candidate gene for end‐stage renal disease in type 2 diabetes using a pooling‐based genome‐wide single nucleotide polymorphism association study. Diabetes. 2007;56(4):975‐983. 10.2337/db06-1072 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: List of lncRNAs cited in the manuscript listed in alphabetical order with the number of known orthologues and a specific focus on their presence in the most common models (Rattus norvegicus, Mus musculus, and Homo sapiens)


