Abstract
Variation among functionally similar species in their response to environmental stress buffers ecosystems from changing states. Functionally similar species may often be cryptic species representing evolutionarily distinct genetic lineages that are morphologically indistinguishable. However, the extent to which cryptic species differ in their response to stress, and could therefore provide a source of response diversity, remains unclear because they are often not identified or are assumed to be ecologically equivalent. Here, we uncover differences in the bleaching response between sympatric cryptic species of the common Indo‐Pacific coral, Pocillopora. In April 2019, prolonged ocean heating occurred at Moorea, French Polynesia. 72% of pocilloporid colonies bleached after 22 d of severe heating (>8oC‐days) at 10 m depth on the north shore fore reef. Colony mortality ranged from 11% to 42% around the island four months after heating subsided. The majority (86%) of pocilloporids that died from bleaching belonged to a single haplotype, despite twelve haplotypes, representing at least five species, being sampled. Mitochondrial (open reading frame) sequence variation was greater between the haplotypes that experienced mortality versus haplotypes that all survived than it was between nominal species that all survived. Colonies > 30 cm in diameter were identified as the haplotype experiencing the most mortality, and in 1125 colonies that were not genetically identified, bleaching and mortality increased with colony size. Mortality did not increase with colony size within the haplotype suffering the highest mortality, suggesting that size‐dependent bleaching and mortality at the genus level was caused instead by differences among cryptic species. The relative abundance of haplotypes shifted between February and August, driven by declines in the same common haplotype for which mortality was estimated directly, at sites where heat accumulation was greatest, and where larger colony sizes occurred. The identification of morphologically indistinguishable species that differ in their response to thermal stress, but share a similar ecological function in terms of maintaining a coral‐dominated state, has important consequences for uncovering response diversity that drives resilience, especially in systems with low or declining functional diversity.
Keywords: bleaching 2019, colony size, cryptic species, degree heating days, Moorea, Pocillopora
Introduction
Rapid human‐induced climate change has led to an urgent need to identify the genetic, species, and trait diversity associated with the ability of ecosystems to avoid regime shifts (Webster et al. 2017). An important element of diversity that maintains ecosystem states is response diversity (sensu Elmqvist et al. 2003), where species sharing similar ecological functions differ in their response to perturbations (Chapin et al. 1997, Yachi and Loreau 1999). If functionally similar species respond negatively and in the same way to common stressors, the capacity for biological communities to withstand these effects and avoid shifts to different states is reduced (Baskett et al. 2014). However, overlooking cryptic species within functional groups may miss an important source of variation contributing to response diversity, and limit the success of management and intervention approaches aimed at protecting biological systems (Bickford et al. 2007, Stat et al. 2012). Therefore, the identification of response diversity within groups of closely related and morphologically indistinguishable taxa is essential to complement existing trait‐based approaches based on functional diversity determined by morphological variation (Madin et al. 2016).
There is widespread evidence that cryptic genetic species (evolutionarily distinct genetic lineages that are “hidden” by morphological similarity and plasticity) exist within many recognized “species” (Bickford et al. 2007, Stat et al. 2012, Arrigoni et al. 2019, Cowman et al. 2020). Yet studies on how biological diversity contributes to the ability of an ecosystem to maintain a desired state following disturbance (resilience; Holling 1973) often do not identify cryptic species, or assume they are ecologically equivalent (Elmqvist et al. 2003). Response diversity among cryptic species could increase resilience by increasing the capacity for one species to compensate for the temporary loss of another in terms of the function it provides (Chapin et al. 1997, Elmqvist et al. 2003, Baskett et al. 2014). Resilience via response diversity, and, the continued coexistence of cryptic species, depends on niche partitioning or a lack of a competitive hierarchy among cryptic species, as well as disturbances varying over spatial scales that are smaller than the expected spatial scale of dispersal (Baskett et al. 2014).
Corals in the genus Pocillopora, which dominate reefs throughout much of the Indo‐Pacific, are well known for exhibiting morphological plasticity and overlapping morphological phenotypes that frequently make species identification based on gross morphology unreliable (Pinzón et al. 2013, Marti‐Puig et al. 2014, Paz‐García et al. 2015, Edmunds et al. 2016). As a result, it is often unknown if analyses of purportedly distinct populations in fact contain multiple species (Bickford et al. 2007, Stat et al. 2012). While the existence of morphologically similar yet genetically divergent lineages complicates the study of population and community biology in the field, it also provides a previously unrecognized source of diversity potentially important for uncovering differences in responses to environmental perturbations.
Furthermore, thermal regimes causing coral bleaching can vary over horizontal and vertical scales of meters to kilometers, and timescales of hours to days (Safaie et al. 2018, Wyatt et al. 2020). Importantly, such fine‐scale variation is not accurately characterized by sea surface temperature (SST) data collected remotely and averaged over several kilometers (Leichter et al. 2006). Spatial variability in thermal stress and mortality over scales of a few kilometers increases the potential for larval dispersal over scales greater than a few kilometers to supply recruits from less impacted sites to more impacted sites (Baskett et al. 2014). Few studies have adequately linked the magnitude and timescales of thermal variability in shallow tropical waters that occurs over scales of meters to kilometers to bleaching impacts on coral reefs (though see Penin et al. 2007, Safaie et al. 2018, Wyatt et al. 2020) and response diversity. Furthermore, bleaching can also affect larger colonies more than smaller colonies (Nakamura and van Woesik 2001, Shenkar et al. 2005, McClanahan et al. 2008, Brandt 2009, van Woesik et al. 2011). Response diversity could therefore manifest as differences among functionally similar species in their size‐dependent response to thermal stress, in addition to the recognized differences among morphologically identified species (Glynn 1993, McClanahan et al. 2005, van Woesik et al. 2011, Pratchett et al. 2013, Guest et al. 2016).
In 2019, a severe bleaching event occurred on the reefs at Moorea, French Polynesia. Prior to bleaching, the cover of hard coral on the fore reef was dominated by broadcast spawning colonies in the genus Pocillopora (Adjeroud et al. 2018, Holbrook et al. 2018). Since at least the 1970s, Pocillopora colonies have been very abundant on the fore reefs of Moorea, but have come to dominate reefs in the last two decades (Berumen and Pratchett 2006, McWilliam et al. 2020). The relatively recent dominance of Pocillopora highlights the importance of studying this genus to better understand why it has been relatively more successful than other genera (e.g., Acropora) that have declined in abundance at this location (Berumen and Pratchett 2006, McWilliam et al. 2020). Hidden diversity (Souter 2010, Schmidt‐Roach et al. 2013) in this genus, field observations of size‐dependent bleaching (see also K. E. Speare et al., unpublished manuscript), and spatial variation in bleaching prevalence provided the impetus to study response diversity among cryptic genetic species. Our goals were to (1) quantify the source of the stress by quantifying the regime of temperature variability and how it differed between sites several kilometers apart, (2) quantify the impact of the stress on the Pocillopora community in terms of bleaching prevalence, severity, and mortality, and (3) quantify response diversity within the Pocillopora community as the extent to which bleaching mortality differed among cryptic genetic species. We uncovered differences in the bleaching response of cryptic genetic species occurring in sympatry (response diversity). We also found differences in bleaching mortality among sites that related to local differences in the thermal regime, providing a way for response diversity to influence resilience.
Methods
Temperature data
Continuous in situ temperature time‐series were collected as part of the Moorea Coral Reef Long‐Term Ecological Research (MCR‐LTER) program. Water temperatures were logged at 2‐minute sampling intervals using a temperature recorder mounted onto a plate directly affixed to the reef surface at 10 m depth at each of three sites around the island (Fig. 1). Temperature loggers were adjacent (within 15 m) to the location of the biological sampling. Temperature data from these loggers were used to characterize temperature over a ~ 50‐m distance along the 10‐m depth contour at each site for which there were temperature loggers. Seabird Electronics (Bellevue, Washington, USA) SBE39 or SBE56 temperature recorders were used with an accuracy several orders of magnitude higher than relevant to this study (0.002°C accuracy, 0.0001°C resolution, <10‐s response time). In addition to raw in situ temperatures (Fig. 2a), we show cumulative temperature variations using variance within a temporal window scaled to capture the local inertial period (~40 h) summed over the preceding 12 d (Fig. 2b; see Wyatt et al. 2020 for a full description of this calculation). Following Wyatt et al. (2020), we quantified heat accumulation using degree heating days (DHD) for the period of the present study (Fig. 2c). DHD has a higher, and perhaps more ecologically relevant, temporal resolution than DHW (for more information on the calculation of DHD, see Appendix S1). For comparison to in situ temperatures, daily satellite‐derived sea‐surface temperatures (SST) were derived within a 2° × 2° area around Moorea using the CoralTemp product produced by the National Oceanic and Atmospheric Administration (NOAA) Coral Reef Watch (CRW).
Fig. 1.
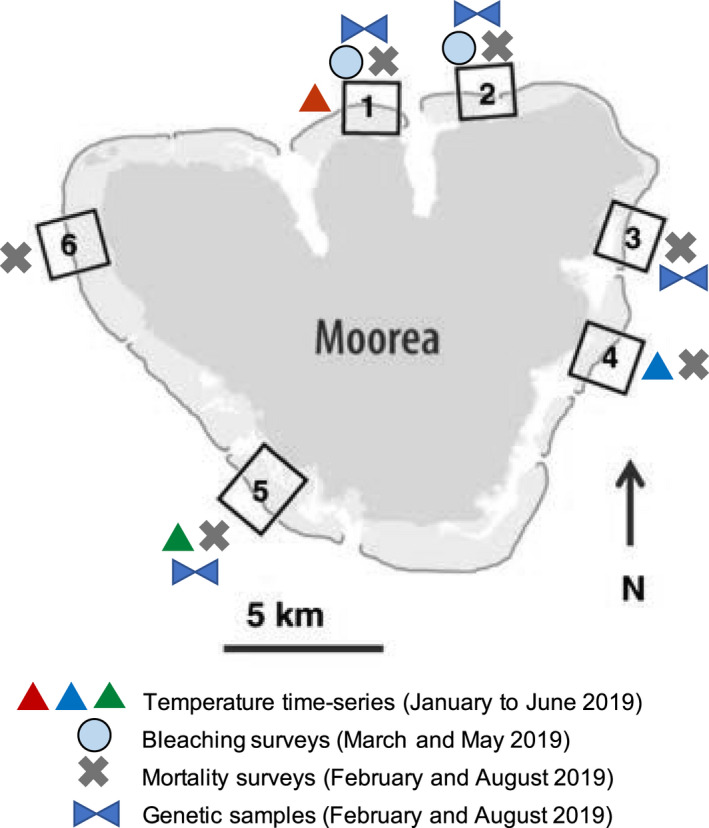
Map of the island of Moorea (French Polynesia, 17°32′ S, 149°50′ W) showing the sites where time‐series data on water temperature, bleaching surveys from photoquadrats, mortality surveys of fixed photoquadrats, and tissue for genetic analysis were collected in 2019. The color of the triangles represents different sites and is the same color as that used in Fig. 2.
Fig. 2.
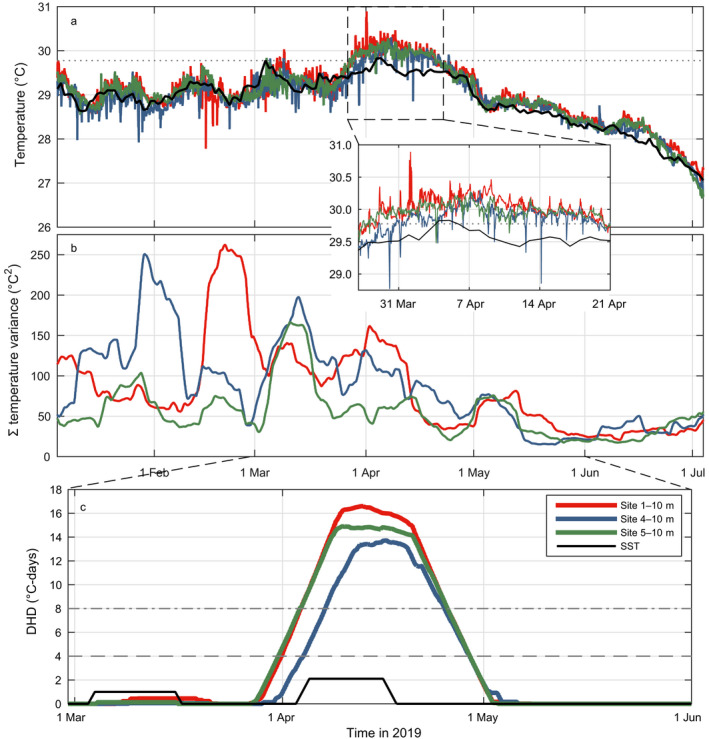
In situ (a) temperatures (°C), (b) cumulative temperature variance (°C2), and (c) degree heating days (DHD; °C‐days) at 10 m depth on the fore reef around Moorea (red, north shore [Site 1]; blue, east shore [Site 4]; green, west shore [Site 5]) during the 2019 heating event centered on the sea surface temperature (SST) peak (5 April 2019). Solid black lines in panels a and c show values for satellite‐derived SST. Dashed line in panel a shows the local bleaching threshold (MMM + 1°C) based on Moorea’s maximum monthly mean (MMM) SST of 28.8°C. Variance in panel b was calculated within a moving window the size of the local inertial frequency (39.6 h) and summed over the preceding 12 d. Dashed and dotted‐dashed lines in panel c show moderate (4°C‐days, dash) and severe (8 °C‐days, dash‐dot) bleaching thresholds, respectively.
Bleaching prevalence
Bleaching prevalence in Pocillopora spp. corals was quantified from photoquadrats (0.5 × 0.5 m) taken on the fore reef in March and May 2019 (Appendix S1: Figs. S1 and S2). At this time, the cover of Pocillopora spp. was low in the back reef (<2% on the north shore), in part because of the consequences of a bleaching event in 2016 (Donovan et al. 2020) that affected the back reef, but had minor effects on the fore reef (Edmunds 2017). As a result, there was little opportunity to compare back reef and fore reef habitats, which differ markedly in their temperature, light, and nutrient regimes. Photoquadrats to quantify bleaching were recorded at 10 m depth at two sites on the fore reef of the north shore of Moorea (Fig. 1; 38 from Site 1 and 40 from Site 2). Photoquadrats were recorded at locations that initially were randomly selected along a ~50‐m transect in 2005, but were resampled thereafter. The transect and photoquadrat placement was the same as that for the permanent sites used for annual sampling of the coral community by the MCR‐LTER program and were marked with rebar (Holbrook et al. 2018). A total of 1,043 Pocillopora spp. colonies in these quadrats in March, and 1,023 corals in May, were assigned to one of four bleaching severity categories (Appendix S1: Fig. S1): 0, no bleaching; 1, partial bleaching (many pale branch tips); 2, moderate bleaching (white branch tips, bleaching extends down the branch); 3, severe bleaching (whole colonies white). We also assigned corals as not bleached (category 0) vs. bleached (category 1–3 combined).
Bleaching mortality
Photoquadrats used to estimate bleaching mortality were different to the photoquadrats used to estimate bleaching prevalence. Photoquadrats (0.7 × 0.7 m) were recorded at 10 m depth on the fore reef at six sites (24 quadrats from each of six sites covering the north, east, and west shore, Fig. 1). Photoquadrats used to estimate bleaching mortality were collected along a transect that was immediately adjacent (~2 m) to the MCR‐LTER transects, which the photoquadrats used to estimate bleaching prevalence came from at site 1 and 2. Bleaching mortality was measured by following the fate of individual Pocillopora spp. colonies in photoquadrats (marked with 30‐cm stainless steel threaded rods) taken in February 2019, before the bleaching event, and again in August 2019, approximately four months after the peak of the bleaching event. Because we sampled the same photoquadrat in February and August, the status and fate of 1,186 individual colonies could be tracked over time.
Colony size
Colony size was quantified as the diameter (in centimeters) along the longest axis of the coral in planar view from the photoquadrat images (van Woesik et al. 2011). The size of a colony was not recorded if the colony was only partially in the frame and the longest axis could not be identified. Excluding these colonies will only reduce the precision of size‐dependent bleaching and mortality. As a result, size was measured for 641 out of 1,023 (62%) colonies assigned to a bleaching category in May 2019, and 1,125 out of 1,186 (95%) of colonies observed in February 2019 and categorized as alive or dead in August 2019. Size was estimated using ImageJ software (Schneider et al. 2012). Each photograph included a reference scale with which each image was individually size calibrated. The smallest colony size detectable in the images was ~2 cm. For assessment of the repeatability of colony size estimates from images, see Appendix S1.
Genetic analysis and identification of genetic lineages
Tissue was collected from a subset of colonies present in the photoquadrats used to estimate mortality. Tissue was collected in February from a total of 68 colonies from Sites 1 (n = 7), 2 (n = 30), 3 (n = 19), and 5 (n = 12). Of these 68 colonies, 51 colonies were tracked between February 2019 (before the bleaching) and August 2019 (after the bleaching). Tissue was collected in August from a total of 394 colonies from Sites 1 (n = 65), 2 (n = 69), 3 (n = 42), 4 (n = 83), 5 (n = 68), and 6 (n = 67; Table 1).
Table 1.
Mitochondrial Open Reading Frame (mtORF) haplotypes (type 1–8 after Pinzón and LaJeunesse [2011], Pinzón et al. [2013] and type 10 and 11 after Forsman et al. [2013]), nominal species (after Gélin et al. [2017] and Johnston et al. [2017]), and sample sizes from February and August 2019 used in this study.
| Nominal species | mtORF haplotype | No. sampled | |||
|---|---|---|---|---|---|
| In Feb | In Feb, relocated in Aug | In Feb, alive in Aug | In Aug | ||
| P. eydouxi | 1a | 3 | 2 | 2 | 32 |
| P. meandrina | 1a | 20 | 13 | 13 | 179 |
| P. meandrina | 1c† | 1 | 0 | 2 | |
| P. meandrina | 1d† | 1 | 1 | 1 | 1 |
| P. meandrina | 1e† | 2 | |||
| P. cf. effusus (PSH 1) | 2 | 4 | 3 | 1 | 4 |
| P. verrucosa (PSH 13) | 3a | 1 | 1 | 1 | 6 |
| P. verrucosa (PSH 13) | 3b | 11 | |||
| P. verrucosa (PSH 16) | 3f | 1 | |||
| P. verrucosa (PSH 13) | 3h | 2 | |||
| PSH6 | 8a | 4 | 3 | 3 | 42 |
| 10 | 15 | 11 | 11 | 83 | |
| 11 | 19 | 17 | 5 | 29 | |
Following extensive previous genetic studies of Pocillopora throughout the Indo‐Pacific (Flot and Tillier 2007, Souter 2010, Pinzón and LaJeunesse 2011, Pinzón et al. 2013, Forsman et al. 2013, Marti‐Puig et al. 2014, Schmidt‐Roach et al. 2014, Gélin et al. 2017, Johnston et al. 2017, 2018), we used the mitochondrial Open Reading Frame (mtORF) marker, using the FATP6.1 and RORF primers found in Flot et al. (2008), to haplotype individuals (see Appendix S1 for details on the genetic methods). This marker is the most widely used and informative marker currently available for Pocillopora (Johnston et al. 2017). We differentiated P. meandrina and P. eydouxi using a restriction fragment length polymorphism (RFLP) gel‐based assay following Johnston et al. (2018). Samples were identified to haplotype based on previously published sequences of mtORF haplotypes, using the naming conventions in Pinzón and LaJeunesse (2011), Pinzón et al. (2013), Forsman et al. (2013), Edmunds et al. (2016), and Johnston et al. (2017).
Statistical analyses
The effects of colony size on the probability of bleaching and the probability of whole colony mortality were estimated using binomial generalized linear models. For colonies that were not genetically identified, the effects of colony size on the probability of bleaching and the probability of mortality were compared across sites. For colonies that were genetically identified, sites were pooled for analyses because there were not enough genetically identified samples at each site to directly estimate the interactive effects of site and haplotype identity on the probability of colony mortality (see Appendix S1: Fig. S4 for analyses at Site 2). Each colony was assigned a category of 0 for alive, or 1 for dead. We modelled the probability of bleaching using two approaches. The first approach follows that used in multinomial models. For each bleaching severity category (i = 1, 2, and 3), we modelled the probability of bleaching as the log of the odds of bleaching in category i vs. not bleaching (i = 0) as: . In the second approach, we simply distinguished between those corals that did (i = 1) or did not (i = 0) bleach. Since colony size was only estimated on a subset of colonies for which bleaching and mortality were recorded, the effects of site on bleaching and mortality included a larger sample of colonies than the effects of colony size on bleaching and mortality. The effects of colony size were tested using χ2 log‐likelihood ratio tests of models with and without the slope coefficient. Spatial and temporal variation in the relative abundance of haplotypes was assessed using Principal Coordinates Analysis.
Results
Temperature
The timing of the peak 2019 subsurface heating around Moorea varied among shores (Fig. 2). In situ logger‐derived water temperatures at 10 m depth exceeded the maximum monthly mean (MMM) satellite‐derived sea surface temperature (SST) for Moorea of 28.8ºC for a maximum of 61, 36, and 48 consecutive days at Sites 1, 4, and 5, respectively. Degree heating days (DHD) peaked at 17, 14, and 15ºC‐days at Sites 1, 4, and 5, respectively (Fig. 2c). Heating above the severe bleaching threshold (8ºC‐days) occurred for a slightly shorter period on the east (Site 4; 18 d) than the west (Site 5; 22 d) and north (Site 1; 22 d) shores. The period of elevated temperature coincided with reduced cumulative variance in water temperatures summed over the preceding ~40 d (Fig. 2b). Higher variance at Site 1 compared to Site 4 and 5 during the heating event reflected daily upward spikes in temperature and a reduction in cooling from internal waves (downward spikes in temperature) that was evident at all sites earlier in the Austral summer (Fig. 2b). Importantly, satellite‐derived SST (solid gray lines in Fig. 2) suggested a substantially milder heating event than measured in situ at 10 m depth.
Bleaching
By May 2019, 71% (67–75, 95% CI) and 72% (68–76, 95% CI) of pocilloporid corals exhibited some degree of bleaching on the fore reef at 10 m depth at sites 1 and 2, respectively (Fig. 3, Appendix S1: Fig. S1). For corals that bleached, most were severely bleached (category 3) rather than partially (category 1) or moderately (category 2) bleached (Fig. 3). 55% (49 – 60, 95% CI) and 60% (55 – 65, 95% CI) of pocilloporid corals at site 1 (n = 546 colonies sampled) and 2 (n = 477 colonies sampled), respectively, severely bleached.
Fig. 3.
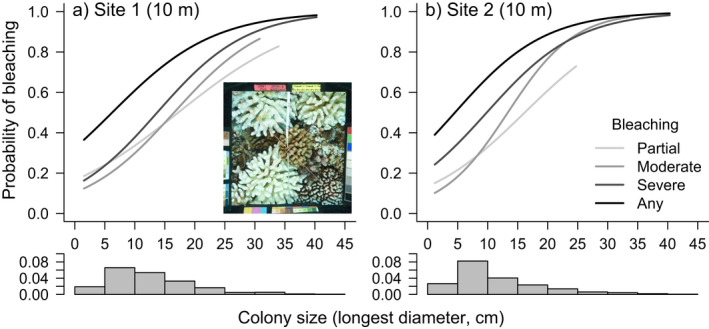
The relationship between Pocillopora spp. colony size (longest diameter in cm) and the probability of bleaching in each bleaching severity category (partial, moderate, severe bleaching, examples shown in Appendix S1: Fig. S1, see also Appendix S1: Fig. S2) in May 2019 at (a) Site 1 (n = 328) and (b) Site 2 (n = 313) at 10 m depth on the north shore of Moorea. The “any bleaching” category is all three bleaching categories combined. Lines indicate fits from binomial generalized linear models. The histogram below each x‐axis indicates the size‐frequency distribution of the observed Pocillopora spp. colonies. Inset shows a representative photoquadrat showing an example of colonies that bleached.
The probability of bleaching in Pocillopora spp. increased with colony size (Fig. 3). The relationship between colony size and bleaching probability was consistent between sites 1 and 2 in May 2019 on the north shore fore reef of Moorea (size site: = −0.2, P = 0.65). Most pocilloporid corals greater than ~ 20cm diameter (and up to the largest size observed of ~40 cm diameter) severely bleached. At both sites, the odds of bleaching to any degree increased by 13% (10–17, 95% CI) for every cm increase in colony diameter (Fig. 3).
Mortality
By August 2019, bleaching mortality was highest on the north shore (Site 1, 33% [28 – 38, 95% CI]; Site 2, 42% [37 – 47, 95% CI]), lowest on the east shore (Site 3, 14% [8 – 25, 95% CI]; Site 4, 11% [7 – 17, 95% CI]), and intermediate on the west shore (Site 5, 22% [16 – 30, 95% CI]; Site 6, 25% [18 – 33, 95% CI]) (Fig. 4). Whole colonies were either clearly dead from the recent bleaching (covered in turf algae) or had color and otherwise appeared “normal” (Appendix S1: Fig. S3). Only 74 out of 1,186 (6%) colonies exhibited partial mortality. There was no other known or obvious cause of mortality (e.g., no crown‐of‐thorns seastars, and no obvious disease).
Fig. 4.
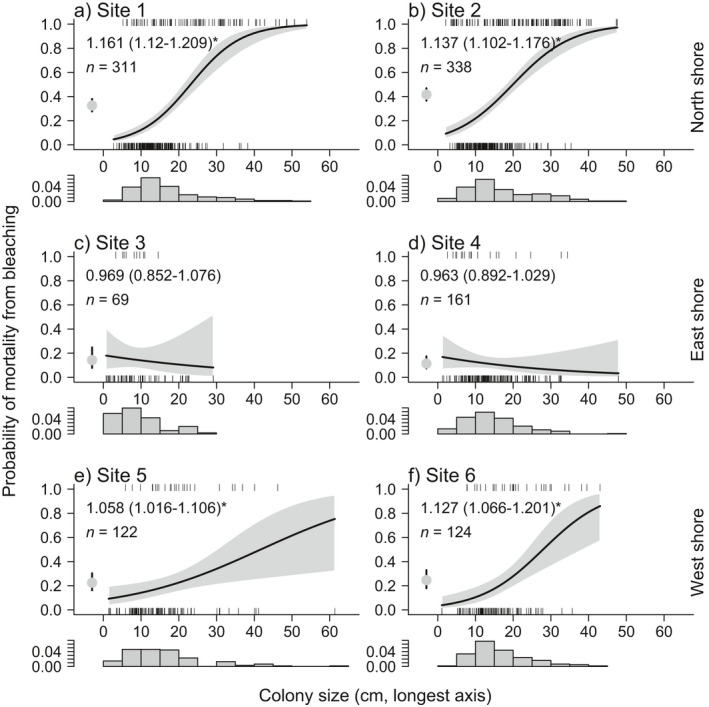
The relationship between Pocillopora spp. colony size (longest diameter in cm) and the probability of whole colony bleaching mortality between February and August 2019 at Site 1–6 (a–f, respectively) at 10 m depth around the island of Moorea. The dot, with 95% confidence interval, indicates the average probability of whole colony mortality at each site. The gray bands indicate 95% confidence intervals estimated from binomial generalized linear models. Numbers in the top left of each plot indicate the factor change in the odds ratio of mortality, with 95% confidence interval in parentheses, for an increase in diameter of 1cm. Asterisks indicate where the factor change in the odds ratio is significantly different from 1 (i.e., a significant relationship between colony size and mortality [P < 0.05]). n denotes the number of colonies sampled at each site. The histogram below each x‐axis indicates the size‐frequency distribution of Pocillopora spp. colony sizes at each site in February 2019 (prior to bleaching).
The relationship between coral size (longest diameter) and mortality varied among sites (size site: = −39.5, P < 0.0001; Fig. 4). On the north and west shore, where mortality was > 21%, the probability of colony mortality increased with colony size (Fig. 4).
Response diversity
The apparent relationship between colony size and colony mortality at the genus level was attributable to higher mortality within a single haplotype (haplotype 11), which contained the largest colony sizes. The 462 corals identified genetically contained 12 mtORF haplotypes (all but three have been documented in previous mtORF analyses of Pocillopora) and at least five nominal species (Table 1; Gélin et al. 2017, Johnston et al. 2017). Of the 51 colonies tracked between February 2019 (before the bleaching) and August 2019 (after the bleaching), 14 died. Of the 14 that died, 12 were haplotype 11, and 2 were haplotype 2 (P. cf. effusus, which is most closely related to haplotype 11). Therefore, the mortality rate for haplotype 11 was 71% (47–88, 95% CI), whereas all colonies from the remaining haplotypes survived (aside from two out of three colonies from haplotype 2 that died). In particular, colonies of the next most abundant haplotypes, P. meandrina (haplotype 1; n = 14) and haplotype 10 (n = 11), experienced no mortality by August. It is not yet known if haplotype 11 is a distinct species, but the mtORF sequence of haplotype 11 and 2 is more divergent from other haplotypes than mtORF sequences are between nominal species that all survived (see haplotype networks in Forsman et al. [2013] and E. C. Johnston et al., unpublished manuscript). That we even detected differences in mortality among haplotypes with relatively low sample size for any given haplotype underscores the large effect size.
In addition to the direct evidence for bleaching mortality being higher in haplotype 11 compared to other haplotypes (Fig. 5), there are several lines of indirect evidence. First, for the 51 colonies sampled in February 2019 that were genetically identified, 91% (10 out of 11) of colonies that were >26 cm diameter were haplotype 11 (Fig. 5a), and for the 1125 of colonies that were not genetically identified, both bleaching and mortality increased with colony size at sites that had many colonies greater than ~30 cm diameter (Fig. 4). Within haplotype 11, there was no evidence of whole colony mortality increasing with colony size ( = −0.027, P = 0.87; Fig. 5b), indicating that all size classes were equally affected and that size‐dependent bleaching and mortality at the genus level was driven by higher mortality in haplotype 11. Colonies of P. meandrina (haplotype 1a) and haplotype 10 were all 26 cm diameter when sampled in February.
Fig. 5.
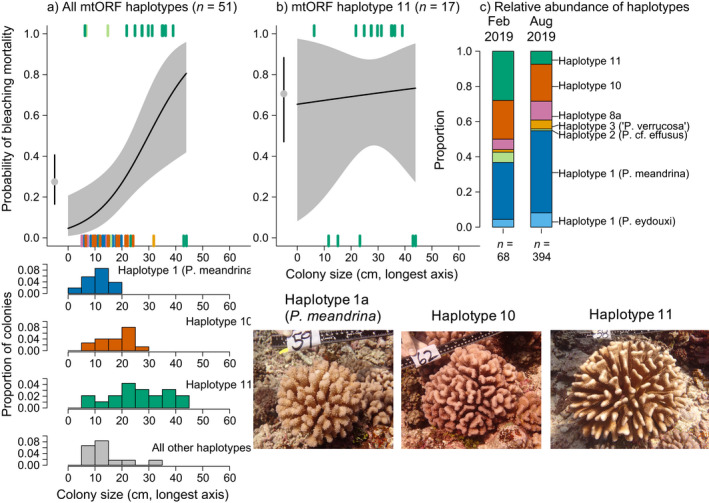
The relationship between Pocillopora spp. colony size (longest diameter in cm) and the probability of colony bleaching mortality between February and August 2019 for (a) all mtORF haplotypes pooled and (b) mtORF haplotype 11 only at 10 m depth around Moorea. Gray dots indicate the average probability of whole colony mortality. All intervals indicate 95% confidence intervals estimated from binomial generalized models. The rugs denote individual colonies colored by their mtORF haplotype. (c) Indicates the relative abundance of mtORF haplotypes from all 68 samples collected in February 2019 and all 394 samples collected in August 2019. The histograms in panel a indicate the size‐frequency distribution of colony sizes for the three most common haplotypes (examples of which are shown in the images).
Second, haplotype 11 was the dominant haplotype in February 2019 (Fig. 5c), and the relative abundance of haplotype 11 in February (before bleaching) was much higher than it was in August (after bleaching), when it was no longer the dominant haplotype (Fig. 5c). Furthermore, the relative abundance of haplotypes at sites 1 and 5, where heat accumulation was greatest, shifted between February and August, driven by declines in haplotype 11 (Fig. 6). In contrast, the relative abundance of haplotypes did not change between February and August at site 3, where there was a dominance of haplotype 10 (Fig. 6), no colonies sampled greater than 30 cm diameter (Fig. 5a), and which is on the east side of the island a few kilometers from site 4 where heat accumulation was the least (Fig. 2).
Fig. 6.
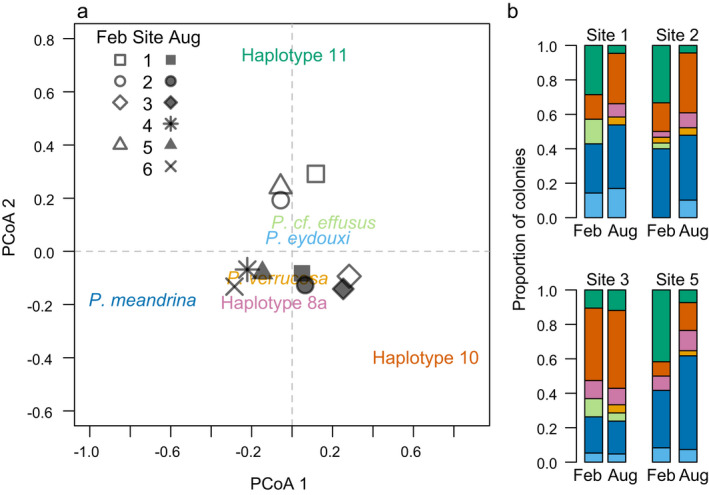
(a) Biplot of Principal Coordinate Axes (PCoA) and (b) barplots visualizing differences in the relative abundance of haplotypes before (February 2019) and after bleaching (August 2019) at 10 m depth at multiple sites on the fore reef of Moorea.
Discussion
Understanding variability in the response of functionally similar species to environmental stress is critical to understand how diversity increases community or ecosystem‐level resilience to disturbances, such as those linked to global change. Previous studies on a range of taxa, such as plants (Laliberte et al. 2010), microbes (Wohl et al. 2004), and fishes (Nash et al. 2016), have shown how response diversity stabilizes ecosystems. In most cases however, species are easily identified. Overlooking cryptic species in population and community studies may therefore miss an important source of variation that could lead to such stability and resilience. For example, instead of changes in population size‐structure that would be expected from not recognizing cryptic genetic species, our results show that bleaching has shifted the relative abundance of species within the Pocillopora assemblage, driven by a decline in haplotype 11. By genetically identifying colonies in the field and censusing them before and after a major bleaching event, we have uncovered previously unrecognized response diversity among cryptic coral species that dominate coral cover on the fore reefs of Moorea (Elmqvist et al. 2003, Baskett et al. 2014). Response diversity within Pocillopora corals may have contributed to this system being able to maintain coral dominance, despite losing diversity in functional traits associated with declines in acroporid corals (Adjeroud et al. 2018, McWilliam et al. 2020). Response diversity was further enhanced by differential heat accumulation, measured as degree heating days using in situ seawater temperature records (and not SST, which is the most common approach), among sites separated by several kilometers. Mortality at the genus level was lower at sites with less heat accumulation, providing the opportunity for less impacted sites to supply recruits to impacted sites in the coming years. Importantly, regardless of whether symbiont identity or composition could offer an explanation for the causes of mortality differences among species, our results have important consequences for understanding how response diversity can maintain coral dominance.
Response diversity enhances ecological resilience when species that can withstand thermal stress occupy different habitats, or are not competitively inferior, compared to species that are susceptible to thermal stress but can recover quickly (Baskett et al. 2014). The three taxa of Pocillopora that dominated the fore reef at Moorea in February 2019 (P. meandrina, haplotype 10, and haplotype 11; Fig. 5c) differed in their bleaching mortality. These three taxa also appear to exhibit differences in their relative abundance across different depths (E. C. Johnston et al., unpublished manuscript), as well as similarities in the net outcome of survival, growth, and fecundity. An outbreak of crown‐of‐thorns sea stars (ending around 2009) followed by Cyclone Oli in 2010 resulted in the near complete loss of live coral (to <5% cover at all fore reef sites around Moorea; Adam et al. 2011). The near complete absence of live coral after these disturbances suggests that the relative abundance of Pocillopora lineages recorded in February 2019 (Fig. 5c) reflects possible lineage differences in the net outcome of recruitment, growth, and survival between 2010 and 2019. If so, P. meandrina, haplotype 10, and haplotype 11 may have exhibited the highest recruitment, growth, or survival compared to other lineages, thereby rapidly increasing in abundance between 2010 and 2019 to become some of the dominant lineages on the north shore by 2019 (Fig. 5c). We speculate that the high recruitment of pocilloporid corals that occurred around 2011 on the fore reef of Moorea (Edmunds 2017) was dominated by P. meandrina, haplotype 10, and haplotype 11. Furthermore, the larger sizes of haplotype 11 colonies compared to colonies from all other lineages in 2019 (Fig. 5a) also suggests that haplotype 11 colonies grew faster than colonies belonging to the other haplotypes, or dominated recruitment for the first few years of recovery. These hypotheses, of course, require testing using methods that estimate population vital rates (Edmunds and Riegl 2020).
A conclusion that bleaching mortality varied among species is not a new discovery (Glynn 1993, McClanahan et al. 2005, van Woesik et al. 2011, Pratchett et al. 2013, Guest et al. 2016). What is novel, however, is that bleaching differed among common species that are closely related, living in sympatry, are members of a relatively young (<3 Mya) monophyletic radiation (Johnston et al. 2017), and cannot be reliably identified in the field based on gross morphology (Pinzón et al. 2013, Marti‐Puig et al. 2014, Schmidt‐Roach et al. 2014, Paz‐García et al. 2015). The latter, in particular, has forced researchers in the past to pool corals within a genus, preventing a full understanding of the impacts of bleaching in prior events, both in Moorea (Pratchett et al. 2013) and in other locations (van Woesik et al. 2011, Guest et al. 2016). More generally, response diversity has received much attention because it underlies ecosystem stability and resilience (Elmqvist et al. 2003, Baskett et al. 2014), but empirical examples in natural populations are exceedingly rare.
Available evidence leads to the hypothesis that haplotype 11 suffered the greatest mortality in the recent bleaching in Moorea because it is a “cooler water”‐adapted species. Haplotype 11 has so far only been documented at Moorea and surrounding islands (~17°–18° S; Forsman et al. 2013, Edmunds et al. 2016). Haplotype 11 is closely related to haplotype 6a (P. ligulata), differing by only two base pair substitution (mtORF sequences 99.8% identical over 935 bp), but is morphologically most similar to P. eydouxi. Haplotype 6a (P. ligulata) has so far only been documented in the Hawaiian Islands (~20°–30° N), and is most common at subtropical islands in the northernmost section of this island chain (Johnston et al. 2018). Very little is known about haplotype 6a (P. ligulata), or whether it is a distinct species to haplotype 11. In our photographs from March and May 2019, we do not know the identity of bleached and unbleached Pocillopora colonies, or what proportion of colonies that bleached subsequently died or recovered (the colonies surveyed in March and May were different to the colonies surveyed between February and August). We do not, therefore, know if colonies from all lineages bleached and only haplotype 11 subsequently died, or if only haplotype 11 bleached. We speculate that that size‐dependent bleaching affecting Pocillopora in both 2007 (Pratchett et al. 2013) and 2019 (Fig. 3) was driven by higher susceptibility to bleaching in haplotype 11.
Heat accumulation and mortality from bleaching varied among sites separated by several kilometers, and larval dispersal greater than this scale increases the potential for dispersal to connect locations that experience different levels of thermal stress (Baskett et al. 2014). The potential for spatial variation in coral bleaching over meters to kilometers is well recognized (Hoegh‐Guldberg and Salvat 1995, Penin et al. 2007, Lenihan et al. 2008). In particular, the shallow back reef habitat at Moorea has a different regime of thermal variability than adjacent the fore reef, notably between night and day (Putnam and Edmunds 2011), and the threats from bleaching are quite different in the back reef than on the fore reef. Our results suggest that spatial variation in bleaching mortality in Pocillopora corals on the fore reef, specifically, could be driven by spatial differences in heat accumulation, spatial differences in the relative abundance of host haplotypes, or both. However, our sampling of genetic material in February 2019 was too limited to directly estimate the interactive effects of site and haplotype identify on the probability of colony mortality. Heat accumulation was highest on the north shore (Site 1) and lowest on the east shore (Site 4) in 2019. The cause of differences in heat accumulation among sites is possibly related to local differences in the internal wave climate and the extent to which internal waves, which normally bring cooler water to the reef, were reduced at each site during 2019 (Leichter et al. 2012, Wyatt et al. 2020). Similarly, mortality at the genus level was highest on the north shore and lowest on the east shore by August. Mortality did not increase with size on the east shore (Site 3 and 4), but all colonies sampled at Site 3 were <30 cm diameter and only 4% of the colonies at Site 4 were >30 cm diameter. Furthermore, site 3 was dominated by haplotype 10 and the relative abundance of haplotypes did not change before and after bleaching.
The advent of genomic methods to advance the understanding of species boundaries and evolutionarily distinct units has important implications beyond better estimates of biodiversity (Knowlton 1993, Bickford et al. 2007, Stat et al. 2012). There is a long history of identifying corals in the field based on morphology, but it is becoming increasingly clear that morphology may not be a sufficient or reliable means of delineating many closely related coral species for the purpose of studying population and community dynamics (Stat et al. 2012, Arrigoni et al. 2019, Cowman et al. 2020). As shown here, delineating cryptic genetic species in field studies using verified genetic markers is essential to uncover ecological and evolutionary processes that are hidden by analyses at the genus, morphological, or trait level. For example, all the Pocillopora lineages we focus on are thought to reproduce via broadcast spawning (Schmidt‐Roach et al. 2012). Reproductive mode is a common way to categorize corals for the purpose of predicting changes in community structure (Hughes et al. 2019). However, not all of the common lineages sampled here responded to bleaching in 2019 in the same way. Without delineating cryptic genetic species in the field, studies will be limited to descriptions of decline in percent cover at coarse taxonomic levels. The results presented here have important implications for identifying ecological portfolios (Webster et al. 2017) at Moorea and surrounding islands, where diversity in environmental conditions, habitat types, phenotypes, and genotypes would buffer the capacity for Pocillopora to absorb impacts of climate change (van Woesik 2017, Webster et al. 2017). Since at least the 1970s, coral cover at Moorea has seen large declines and recovery on multiple occasions, where coral cover has become increasingly dominated by corals in the genus Pocillopora after each recovery period (Berumen and Pratchett 2006, Adjeroud et al. 2018, Holbrook et al. 2018, McWilliam et al. 2020). The presence of multiple co‐occurring cryptic species of Pocillopora that differ in bleaching mortality, and differences in heat accumulation over kilometer scales driven by differences in local oceanographic phenomena, may underlie the seemingly unusual patterns of resilience known for Pocillopora at Moorea and perhaps explain at least some of the success of Pocillopora relative to Acropora (Edmunds 2017, Adjeroud et al. 2018, Holbrook et al. 2018).
Open Research
Data and R code (Burgess et al. 2021) to reproduce the figures are available on the Dryad Digital Repository: https://doi.org/10.5061/dryad.fqz612js0
Supporting information
Appendix S1
Acknowledgments
We thank four anonymous reviewers for comments that improved the manuscript. This work was funded by a National Science Foundation (NSF) grant to S. C Burgess (OCE 18‐29867) and P. J. Edmunds (OCE: 18‐29898 and 16‐37396). We thank J. Powell and C. Peters for invaluable assistance in the field, C. Peters and the Florida State University Dive Program for facilitating field work on SCUBA, N. Johnson and C. Kotscher for help analyzing the photoquadrat images, the staff of the UC Berkeley Richard B. Gump South Pacific Research Station for facilitating our research, and M. Hay for logistical support with samples. The authors declare no conflicts of interest. Research was completed under permits issued by the French Polynesian Government (Délégation à la Recherche), the Haut‐Commissariat de la République en Polynésie Française (DTRT) (Protocole d’Accueil 2019), and the U.S. Fish and Wildlife Service.
Burgess, S. C. , Johnston E. C., Wyatt A. S. J., Leichter J. J., and Edmunds P. J.. 2021. Response diversity in corals: hidden differences in bleaching mortality among cryptic Pocillopora species. Ecology 102(6):e03324. 10.1002/ecy.3324
Corresponding Editor: Mark Hixon.
Literature Cited
- Adam, T. C. , Schmitt R. J., Holbrook S. J., Brooks A. J., Edmunds P. J., Carpenter R. C., and Bernardi G.. 2011. Herbivory, connectivity, and ecosystem resilience: response of a coral reef to a large‐scale perturbation. PLoS ONE 6:e23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjeroud, M. , Kayal M., Iborra‐Cantonnet C., Vercelloni J., Bosserelle P., Liao V., Chancerelle Y., Claudet J., and Penin L.. 2018. Recovery of coral assemblages despite acute and recurrent disturbances on a South Central Pacific reef. Scientific Reports 8:9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni, R. , Berumen M. L., Stolarski J., Terraneo T. I., and Benzoni F.. 2019. Uncovering hidden coral diversity: a new cryptic lobophylliid scleractinian from the Indian Ocean. Cladistics 35:301–328. [DOI] [PubMed] [Google Scholar]
- Baskett, M. L. , Fabina N. S., and Gross K.. 2014. Response diversity can increase ecological resilience to disturbance in coral reefs. American Naturalist 184:E16–E31. [DOI] [PubMed] [Google Scholar]
- Berumen, M. L. , and Pratchett M. S.. 2006. Recovery without resilience: persistent disturbance and long‐term shifts in the structure of fish and coral communities at Tiahura Reef, Moorea. Coral Reefs 25:647–653. [Google Scholar]
- Bickford, D. , Lohman D. J., Sodhi N. S., Ng P. K. L., Meier R., Winker K., Ingram K. K., and Das I.. 2007. Cryptic species as a window on diversity and conservation. Trends in Ecology & Evolution 22:148–155. [DOI] [PubMed] [Google Scholar]
- Brandt, M. E. 2009. The effect of species and colony size on the bleaching response of reef‐building corals in the Florida Keys during the 2005 mass bleaching event. Coral Reefs 28:911–924. [Google Scholar]
- Burgess, S. C. , Johnston E. C., Leichter J. J., Wyatt A. S. J., and Edmunds P. J.. 2021. Response diversity in corals: hidden differences in bleaching mortality among cryptic Pocillopora species. Dryad, data set. 10.5061/dryad.fqz612js0 [DOI] [PMC free article] [PubMed]
- Chapin, F. S. , Walker B. H., Hobbs R. J., Hooper D. U., Lawton J. H., Sala O. E., and Tilman D.. 1997. Biotic control over the functioning of ecosystems. Science 277:500–504. [Google Scholar]
- Cowman, P. F. , Quattrini A. M., Bridge T. C. L., Watkins‐Colwell G. J., Fadli N., Grinblat M., Roberts T. E., McFadden C. S., Miller D. J., and Baird A. H.. 2020. An enhanced target‐enrichment bait set for Hexacorallia provides phylogenomic resolution of the staghorn corals (Acroporidae) and close relatives. Molecular Phylogenetics and Evolution 153:106944. [DOI] [PubMed] [Google Scholar]
- Donovan, M. K. , Adam T. C., Shantz A. A., Speare K. E., Munsterman K. S., Rice M. M., Schmitt R. J., Holbrook S. J., and Burkepile D. E.. 2020. Nitrogen pollution interacts with heat stress to increase coral bleaching across the seascape. Proceedings of the National Academy of Sciences USA 117:5351–5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds, P. J. 2017. Unusually high coral recruitment during the 2016 El Niño in Mo’orea. French Polynesia. PLoS ONE 12:e0185167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds, P. J. , Leichter J. J., Johnston E. C., Tong E. J., and Toonen R. J.. 2016. Ecological and genetic variation in reef‐building corals on four Society Islands. Limnology and Oceanography 61:543–557. [Google Scholar]
- Edmunds, P. , and Riegl B.. 2020. Urgent need for coral demography in a world where corals are disappearing. Marine Ecology Progress Series 635:233–242. [Google Scholar]
- Elmqvist, T. , Folke C., Nyström M., Peterson G., Bengtsson J., Walker B., and Norberg J.. 2003. Response diversity, ecosystem change, and resilience. Frontiers in Ecology and the Environment 1:488–494. [Google Scholar]
- Flot, J.‐F. , Magalon H., Cruaud C., Couloux A., and Tillier S.. 2008. Patterns of genetic structure among Hawaiian corals of the genus Pocillopora yield clusters of individuals that are compatible with morphology. Comptes Rendus Biologies 331:239–247. [DOI] [PubMed] [Google Scholar]
- Flot, J.‐F. , and Tillier S.. 2007. The mitochondrial genome of Pocillopora (Cnidaria: Scleractinia) contains two variable regions: The putative D‐loop and a novel ORF of unknown function. Gene 401:80–87. [DOI] [PubMed] [Google Scholar]
- Forsman, Z. H. , Johnston E. C., Brooks A. J., Adam T. C., and Toonen R. J.. 2013. Genetic evidence for regional isolation of Pocillopora corals from Moorea. Oceanography 26:153–155. [Google Scholar]
- Gélin, P. , Postaire B., Fauvelot C., and Magalon H.. 2017. Reevaluating species number, distribution and endemism of the coral genus Pocillopora Lamarck, 1816 using species delimitation methods and microsatellites. Molecular Phylogenetics and Evolution 109:430–446. [DOI] [PubMed] [Google Scholar]
- Glynn, P. W. 1993. Coral reef bleaching: ecological perspectives. Coral Reefs 12:1–17. [Google Scholar]
- Guest, J. R. et al. 2016. Coral community response to bleaching on a highly disturbed reef. Scientific Reports 6:20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegh‐Guldberg, O. , and Salvat B.. 1995. Periodic mass‐bleaching and elevated sea temperatures: bleaching of outer reef slope communities in Moorea, French Polynesia. Marine Ecology Progress Series 121:181–190. [Google Scholar]
- Holbrook, S. J. , Adam T. C., Edmunds P. J., Schmitt R. J., Carpenter R. C., Brooks A. J., Lenihan H. S., and Briggs C. J.. 2018. Recruitment drives spatial variation in recovery rates of resilient coral reefs. Scientific Reports 8:7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holling, C. S. 1973. Resilience and stability of ecological systems. Annual Review of Ecology and Systematics 4:1–23. [Google Scholar]
- Hughes, T. P. et al. 2019. Global warming impairs stock–recruitment dynamics of corals. Nature 568:387–390. [DOI] [PubMed] [Google Scholar]
- Johnston, E. C. , Forsman Z. H., Flot J.‐F., Schmidt‐Roach S., Pinzón J. H., Knapp I. S. S., and Toonen R. J.. 2017. A genomic glance through the fog of plasticity and diversification in Pocillopora . Scientific Reports 7:5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, E. C. , Forsman Z. H., and Toonen R. J.. 2018. A simple molecular technique for distinguishing species reveals frequent misidentification of Hawaiian corals in the genus Pocillopora . PeerJ 6:e4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton, N. 1993. Sibling species in the sea. Annual Review of Ecology and Systematics 24:189–216. [Google Scholar]
- Laliberte, E. et al. 2010. Land‐use intensification reduces functional redundancy and response diversity in plant communities. Ecology Letters 13:76–86. [DOI] [PubMed] [Google Scholar]
- Leichter, J. J. , Helmuth B., and Fischer A. M.. 2006. Variation beneath the surface: Quantifying complex thermal environments on coral reefs in the Caribbean, Bahamas and Florida. Journal of Marine Research 64:563–588. [Google Scholar]
- Leichter, J. J. , Stokes M. D., Hench J. L., Witting J., and Washburn L.. 2012. The island‐scale internal wave climate of Moorea, French Polynesia. Journal of Geophysical Research: Oceans 117:608. [Google Scholar]
- Lenihan, H. , Adjeroud M., Kotchen M., Hench J., and Nakamura T.. 2008. Reef structure regulates small‐scale spatial variation in coral bleaching. Marine Ecology Progress Series 370:127–141. [Google Scholar]
- Madin, J. S. et al. 2016. A trait‐based approach to advance coral reef science. Trends in Ecology & Evolution 31:419–428. [DOI] [PubMed] [Google Scholar]
- Marti‐Puig, P. , Forsman Z. H., Haverkort‐Yeh R. D., Knapp I. S. S., Maragos J. E., and Toonen R. J.. 2014. Extreme phenotypic polymorphism in the coral genus Pocillopora; micro‐morphology corresponds to mitochondrial groups, while colony morphology does not. Bulletin of Marine Science 90:211–231. [Google Scholar]
- McClanahan, T. R. , Ateweberhan M., and Omukoto J.. 2008. Long‐term changes in coral colony size distributions on Kenyan reefs under different management regimes and across the 1998 bleaching event. Marine Biology 153:755–768. [Google Scholar]
- McClanahan, T. , Maina J., Moothien‐Pillay R., and Baker A.. 2005. Effects of geography, taxa, water flow, and temperature variation on coral bleaching intensity in Mauritius. Marine Ecology Progress Series 298:131–142. [Google Scholar]
- McWilliam, M. , Pratchett M. S., Hoogenboom M. O., and Hughes T. P.. 2020. Deficits in functional trait diversity following recovery on coral reefs. Proceedings of the Royal Society B 287:20192628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, T. , and van Woesik R.. 2001. Water‐flow rates and passive diffusion partially explain differential survival of corals during the 1998 bleaching event. Marine Ecology Progress Series 212:301–304. [Google Scholar]
- Nash, K. L. , Graham N. A. J., Jennings S., Wilson S. K., and Bellwood D. R.. 2016. Herbivore cross‐scale redundancy supports response diversity and promotes coral reef resilience. Journal of Applied Ecology 53:646–655. [Google Scholar]
- Paz‐García, D. A. , Hellberg M. E., García‐de‐León F. J., and Balart E. F.. 2015. Switch between morphospecies of Pocillopora corals. American Naturalist 186:434–440. [DOI] [PubMed] [Google Scholar]
- Penin, L. , Adjeroud M., Schrimm M., and Lenihan H. S.. 2007. High spatial variability in coral bleaching around Moorea (French Polynesia): patterns across locations and water depths. Comptes Rendus Biologies 330:171–181. [DOI] [PubMed] [Google Scholar]
- Pinzón, J. H. , and LaJeunesse T. C.. 2011. Species delimitation of common reef corals in the genus Pocillopora using nucleotide sequence phylogenies, population genetics and symbiosis ecology. Molecular Ecology 20:311–325. [DOI] [PubMed] [Google Scholar]
- Pinzón, J. H. , Sampayo E., Cox E., Chauka L. J., Chen C. A., Voolstra C. R., and LaJeunesse T. C.. 2013. Blind to morphology: genetics identifies several widespread ecologically common species and few endemics among Indo‐Pacific cauliflower corals (Pocillopora, Scleractinia). Journal of Biogeography 40:1595–1608. [Google Scholar]
- Pratchett, M. S. , McCowan D., Maynard J. A., and Heron S. F.. 2013. Changes in bleaching susceptibility among corals subject to ocean warming and recurrent bleaching in Moorea, French Polynesia. PLoS ONE 8:e70443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam, H. M. , and Edmunds P. J.. 2011. The physiological response of reef corals to diel fluctuations in seawater temperature. Journal of Experimental Marine Biology and Ecology 396:216–223. [Google Scholar]
- Safaie, A. , Silbiger N. J., McClanahan T. R., Pawlak G., Barshis D. J., Hench J. L., Rogers J. S., Williams G. J., and Davis K. A.. 2018. High frequency temperature variability reduces the risk of coral bleaching. Nature Communications 9:1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt‐Roach, S. , Lundgren P., Miller K. J., Gerlach G., Noreen A. M. E., and Andreakis N.. 2013. Assessing hidden species diversity in the coral Pocillopora damicornis from Eastern Australia. Coral Reefs 32:161–172. [Google Scholar]
- Schmidt‐Roach, S. , Miller K. J., Lundgren P., and Andreakis N.. 2014. With eyes wide open: a revision of species within and closely related to the Pocillopora damicornis species complex (Scleractinia; Pocilloporidae) using morphology and genetics. Zoological Journal of the Linnean Society 170:1–33. [Google Scholar]
- Schmidt‐Roach, S. , Miller K. J., Woolsey E., Gerlach G., and Baird A. H.. 2012. Broadcast spawning by Pocillopora species on the Great Barrier Reef. PLoS ONE 7:e50847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, C. A. , Rasband W. S., and Eliceiri K. W.. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkar, N. , Fine M., and Loya Y.. 2005. Size matters: bleaching dynamics of the coral Oculina patagonica . Marine Ecology Progress Series 294:181–188. [Google Scholar]
- Souter, P. 2010. Hidden genetic diversity in a key model species of coral. Marine Biology 157:875–885. [Google Scholar]
- Stat, M. et al. 2012. Molecular delineation of species in the coral holobiont. Advances in Marine Biology 63:1–65. [DOI] [PubMed] [Google Scholar]
- van Woesik, R. 2017. Contemporary coral bleaching: why diversity matters. Biodiversity 18:16–18. [Google Scholar]
- van Woesik, R. , Sakai K., Ganase A., and Loya Y.. 2011. Revisiting the winners and the losers a decade after coral bleaching. Marine Ecology Progress Series 434:67–76. [Google Scholar]
- Webster, M. S. , Colton M. A., Darling E. S., Armstrong J., Pinsky M. L., Knowlton N., and Schindler D. E.. 2017. Who should pick the winners of climate change? Trends in Ecology & Evolution 32:167–173. [DOI] [PubMed] [Google Scholar]
- Wohl, D. L. , Arora S., and Gladstone J. R.. 2004. Functional redundancy supports biodiversity and ecosystem function in a closed and constant environment. Ecology 85:1534–1540. [Google Scholar]
- Wyatt, A. S. J. , Leichter J. J., Toth L. T., Miyajima T., Aronson R. B., and Nagata T.. 2020. Heat accumulation on coral reefs mitigated by internal waves. Nature Geoscience 13:28–34. [Google Scholar]
- Yachi, S. , and Loreau M.. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proceedings of the National Academy of Sciences USA 96:1463–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


