Abstract
Background
Gaps of knowledge still exist about the potential association between severe thrombocytopenia and increased risk of procedure‐associated bleeding in patients with liver disease.
Methods
In this narrative review, we aimed at examining the association between procedure‐related bleeding risk and platelet count in patients with cirrhosis and severe thrombocytopenia in various settings. We updated to 2020 a previously conducted literature search using MEDLINE/PubMed and EMBASE. The search string included clinical studies, adult patients with chronic liver disease and thrombocytopenia undergoing invasive procedures, any interventions and comparators, and haemorrhagic events of any severity as outcome.
Results
The literature search identified 1276 unique publications, and 15 studies met the inclusion criteria and were analysed together with those identified by the previous search. Most of the new studies included in our analysis did not assess the association between post‐procedural bleeding risk and platelet count alone in patients with chronic liver disease. Furthermore, some results could have been biased by prophylactic platelet transfusions. A few studies found that severe thrombocytopenia may be predictive of bleeding following percutaneous liver biopsy, dental extractions, percutaneous ablation of liver tumours and endoscopic polypectomy.
Conclusions
Currently available literature cannot support definitive conclusions about the appropriate target platelet counts to improve the risk of bleeding in cirrhotic patients who underwent invasive procedures; moreover, it showed enormous variability in the use of prophylactic platelet transfusions.
Keywords: biopsy, liver cirrhosis, platelet count, platelet transfusions, thrombocytopenia
1. INTRODUCTION
Thrombocytopenia is a very common complication of chronic liver disease. 1 However, severity of the underlying liver disease and differences in definition criteria of low platelet count cut‐off makes the prevalence of thrombocytopenia widely variable. 2 , 3 Available data show a prevalence of thrombocytopenia (i.e. platelet count < 150 × 109/L) ranging from 6% to 78%, with lower percentages in noncirrhotic patients, which become progressively greater in patients with compensated and decompensated cirrhosis. 1 , 4 , 5 Also, moderate (i.e. platelet count between 50 and 75 × 109/L) and severe (<50 × 109/L) thrombocytopenia has been reported in 13% and 1% of patients with cirrhosis, respectively. 6
Despite the possible coexistence of coagulopathy, mild‐to‐moderate thrombocytopenia (i.e. platelet count between 50 and 150 × 109/L) rarely represents a critical condition in patients without complication of liver disease (e.g. infections and renal failure). On the contrary, a platelet count < 50 × 109/L could have a negative impact on the clinical management of patients with advanced liver disease, since it may lead to postponement or cancellation of invasive procedures and may be associated with an increased risk of procedure‐associated bleeding. 6 , 7 , 8
De Pietri et al 9 categorized procedures based on the associated bleeding risk: procedures were defined at high or low risk if associated with a bleeding risk > 3% (variceal band ligation, hepatic resection, abdominal surgery, endoscopic polypectomy, radio‐frequency ablation, liver biopsy, biopsy of sites other than liver, abdominal drainage and endoscopic retrograde cholangiopancreatography with sphincterotomy or thoracotomy) or <3% (paracentesis, thoracentesis, central vein cannulation and transjugular intrahepatic portosystemic shunts), respectively.
Literature analysis conducted by the working group of the Position Paper of the Italian Association for the Study of Liver Diseases (AISF) and the Italian Society of Internal Medicine (SIMI) 8 found that the bleeding risk in cirrhotic patients was low (3%) following paracentesis, thoracentesis, and percutaneous or transjugular liver biopsy, and moderate (<10%) following endoscopic variceal ligation, endoscopic polypectomy and minor abdominal surgery (i.e. cholecystectomy and hernioplasty). The Position Paper concluded that, despite the limitations of the studies analysed, a platelet count < 50‐60 × 109/L may be predictive of procedure‐associated bleeding. 8
The aim of this narrative review was to update the literature search conducted by the AISF/SIMI working group and examine the association between procedure‐related bleeding risk and the platelet count in patients with cirrhosis and severe thrombocytopenia in different settings.
2. LITERATURE SEARCH
Starting from the literature search conducted by the working group of the Position Paper AISF/SIMI, which covered relevant evidence on ‘Risk of bleeding following invasive procedures or surgery’ until 2014, 8 we conducted a new search using MEDLINE/PubMed and EMBASE with the aim to update data from 2014 to 2020 (last accessed on 4 March 2020). The search string has been designed on the basis of the PICOS scheme and included clinical (RCT and observational) studies conducted on adult patients with chronic liver disease and thrombocytopenia undergoing invasive procedures, with any interventions and comparators, that had haemorrhagic events of any severity as outcome.
Two independent investigators conducted the literature search; the revision and the selection of the studies were performed by the working group based on title/abstract and subsequently on full text. Study screening flow diagram is reported in Figure 1.
FIGURE 1.
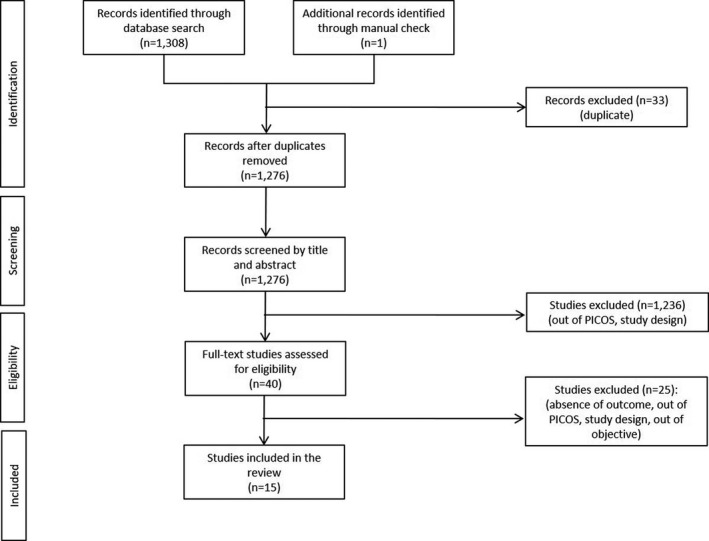
Study screening flow diagram
Since in the Position Paper AISF/SIMI selection criteria were not specified, we assumed they were the same ones we applied in our literature search. Therefore, the 15 studies, which met the inclusion criteria, 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 were analysed together with those identified by the working group AISF/SIMI. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68
3. PROCEDURE‐RELATED BLEEDING RISK IN DIFFERENT SETTINGS
Table 1 summarizes the studies included in the analysis.
TABLE 1.
Summary of the studies included in the analysis
| Author, year | Study design | Procedures/patients (n.) | PLT count or PLT cut‐off | Findings |
|---|---|---|---|---|
| Paracentesis | ||||
| Webster et al, 1996 24 | Retrospective | 179 outpatients | Not specified | 4 haemorrhagic complications in patients with PLT > 80 × 109/L |
| Grabau et al, 2004 25 | Retrospective | 1100 in 628 pts | PLT < 50 × 109/L in 55.64% of procedures | No bleedings in procedures performed with PLT < 50 × 109/L |
| Pache et al, 2005 26 | Retrospective | 4729 paracenteses | Not specified | Severe haemorrhagic complications (6 haemoperitoneum, 3 abdominal wall haematoma) in 0.2% of procedures without association with PLT count |
| Lin et al, 2005 27 | Prospective observational | 410 in 163 pts | PLT < 50 × 109/L in 13% of procedures | Minor bleeding rate (1 local ecchymosis, 1 cutaneous bleeding) in 0.5% of procedures in patients with PLT = 50‐100 × 109/L |
| De Gottardi et al, 2009 28 | Prospective observational | 515 in 171 pts | PLT < 50 × 109/L in 10% and PLT < 100 × 109/L in 40% of pts | Association between PLT < 50 × 109/L and increased risk of overall complications (P =.07). Association with bleeding risk not reported |
| Rowley et al, 2019 10 | Retrospective | 3116 in 123 pts | PLT < 50 × 109/L in 12% of pts | Overall bleeding rate: 0.2%. No bleeding with PLT < 50 × 109/L |
| Liver biopsy | ||||
| Piccinino et al, 1986 29 | Retrospective | 68 276 percutaneous biopsies | PLT > 50 × 109/L in all biopsies | Overall rate of major haemorrhagic events: 0.06%. Association between bleeding and PLT not evaluated |
| Caturelli et al, 1996 30 | Retrospective | NR (only abstract available) | Not specified | Overall rate of haemorrhagic complications: 0.13%. Association between bleeding and PLT not evaluated |
| Actis et al, 2007 31 | Retrospective | 835 pts | Not specified | Overall rate of major haemorrhagic events: 0.12%. Association between bleeding and PLT not evaluated |
| West et al, 2010 32 | Retrospective | 61 187 pts | Not specified | Overall rate of major haemorrhagic events: 0.65%. Association between bleeding and PLT not evaluated |
| Seeff et al, 2010 33 | Retrospective | 2740 percutaneous biopsies | Pts with PLT < 50 × 109/L were excluded | Overall haemorrhagic rate: 0.6%. Pts with PLT = 50‐60 × 109/L was significantly higher than pts with PLT > 60 × 109/L (5.3% vs 0.4%). |
| Kalambokis et al, 2007 34 | Review | 7649 transjugular biopsies in 7189 pts | Cut‐off 60 × 109/L | Haemorrhagic rate < 2% (minor bleeding). No association with PLT count |
| Alessandria et al, 2008 35 | Retrospective | 306 transjugular biopsies | Not specified | No major complications. No association between bleeding rate and PLT count |
| Mammen et al, 2008 36 | Retrospective | 601 transjugular biopsies | PLT < 60 × 109/L in 20.3% of pts | Haemorrhagic rate = 0.9%. No association with PLT count |
| Procopet et al, 2012 37 | Prospective | 75 transjugular biopsies | Not specified | Haemorrhagic rate = 1.3%. No association with PLT count |
| Takyar et al, 2017 11 | Retrospective | 3357 percutaneous biopsies | Cut‐off 100 × 109/L | Haemorrhagic rate: 0.69% (fatal in 0.09%). PLT < 100 × 109/L was an independent risk factors for post‐biopsy bleeding, but % pts with PLT < 60 × 109/L were not different between groups |
| Potretzke et al, 2018 12 | Retrospective | 1876 percutaneous biopsies in 1732 pts | Cut‐off 70 × 109/L | Haemorrhagic rate = 0.69%. No association with PLT count |
| Dentistry | ||||
| Ward et al, 2006 38 | Retrospective | 35 procedures in 30 pts | Cut‐off 35‐50 × 109/L (depending on the risk group) | No association between PLT count and prolonged postoperative bleeding |
| Perdigao et al, 2012 39 | Prospective observational | 35 procedures in 23 pts | PLT < 50 × 109/L in 34% of pts | 1 postoperative bleeding (2.9%) in pts with PLT = 50 × 109/L. No haemorrhagic complication during procedures (n = 12) with PLT = 30‐49 × 109/L |
| Cocero et al, 2017 13 | Retrospective | 1,183 extractions in 381 pts | Cut‐off PLT ≤ 40 × 109/L | Haemorrhagic rate: 0.4% in pts with PLT > 40x109/L and INR < 2.5; 5.88% in pts with PLT ≤ 40 × 109/L |
| Medina et al, 2018 14 | Retrospective | 190 extractions | PLT < 150 × 109/L in 96.3% of pts | Overall haemorrhagic rate: 6.3%. Intra‐operative bleeding was associated with low count of platelets. However, this counting could explain only 16% of the cases of bleeding. |
| Endoscopic variceal ligation | ||||
| Vieira da Rocha et al, 2009 40 | Prospective observational | 150 pts | PLT < 50 × 109/L in 12% of pts | Severe post‐procedural ulcer bleeding in 7.33% of pts. Risk of bleeding was not associated with PLT count. |
| Vanbiervliet et al, 2010 41 | Retrospective | 837 ligations in 605 pts | Not specified | Post‐procedural bleeding rate: 2.75%. No association between PLT count and bleeding but high platelet ratio index was an independent predictive factor of bleeding |
| Endoscopic polypectomy | ||||
| Jeon et al, 2012 42 | Retrospective | 66 in 30 pts | Not specified | Post‐procedural bleeding in 3% of procedures. No association between bleeding and PLT count |
| Lee et al, 2014 43 | Retrospective | 89 pts w/ liver cirrhosis + 348 w/o liver disease | Not specified | Post‐procedural bleeding in 14.61% of pts. Association between bleeding and PLT not evaluated |
| Soh et al, 2020 15 | Retrospective | 1267 patients | Cut‐off 50 × 109/L | Haemorrhagic rate (immediate + delayed): 7.5%. PLT < 50 × 109/L significantly associated with immediate post‐procedural bleeding (rate: 27.5%; OR = 6.6) |
| Percutaneous ablation | ||||
| Cammà et al, 2005 44 | Retrospective | 202 pts | ≥40 × 109/L in all pts | Haemorrhagic rate: 0.50%. Association between bleeding and PLT not evaluated |
| Livraghi et al, 2008 45 | Retrospective | 218 pts | ≥40 × 109/L in all pts | Haemorrhagic rate: 0.92%. Association between bleeding and PLT not evaluated |
| Goto et al, 2010 46 | Retrospective | 4133 in 2154 pts | Mean PLT count = 125 ± 33 × 109/L (50‐669) | Haemorrhagic complications rate: 1.5%. Low PLT count was a significant risk factor for haemoperitoneum (PLT ≥ 50 × 109/L was an inclusion criteria) |
| Park et al, 2017 16 | Retrospective | 1843 in 1211 patients | Mean PLT count = 140 ± 85 × 109/L |
Post‐procedural bleeding rate was 0.6%, and the risk was significantly greater in patients with PLT < 50 × 109/L (OR = 8.79) |
| Liver transplantation | ||||
| McCluskey et al, 2006 47 | Retrospective | 460 pts | Not specified | Incidence of massive blood transfusion: 42%. PLT < 70 × 109/L was an independent predictor of massive blood transfusion (+32% vs PLT > 70 × 109/L) |
| Massicotte et al, 2008 48 | Prospective + retrospective | 200 pts | <50 × 109/L in 18% of pts; <30 × 109/L in 4% of pts | No association between PLT count and transfusion rate |
| Massicotte et al, 2012 49 | Retrospective | 503 pts | Not specified | No significant association between PLT count and blood loss |
| Esmat Gamil et al, 2012 50 | Prospective observational | 286 pts | Not specified | No significant association between PLT count and blood loss |
| Li et al, 2014 17 | Retrospective | 241 pts | Not specified | Postoperative bleeding in 4.98% of pts. No significant association between PLT count and bleeding risk |
| Akamatsu et al, 2015 18 | Retrospective | 403 pts | Mean PLT count 86 ± 70 × 109/L | Haemorrhagic episodes in 8.68% of pts. No significant association between PLT count and blood loss |
| Eghbal et al, 2019 19 | Retrospective | 754 pts | Not specified | PLT count was inversely correlated with total bleeding |
| Liver surgery | ||||
| Wei et al, 2003 51 | Retrospective | 155 pts | Median PLT count 205 × 109/L (82‐473) | Postoperative intra‐abdominal haemorrhage in 5% of patients. Association between bleeding and PLT not evaluated |
| Kubo et al, 2007 52 | Retrospective | 100 pts | Not specified | Postoperative bleeding in 4% of patients. Association between bleeding and PLT not evaluated |
| Palavecino et al, 2009 53 | Retrospective | 1557 resections in 1477 pts | Median PLT count 232 × 109/L (64.0‐775.0) | PLT < 100 × 109/L (1% of pts) was an independent risk factors for blood transfusion (OR = 8.8) |
| Hsu et al, 2009 54 | Retrospective | 1027 resections | Not specified | PLT < 100 × 109/L was correlated with perioperative mortality in the univariate analysis but not in the multivariate one |
| Cockbain et al, 2010 55 | Retrospective | 589 pts | Cut‐off 150 × 109/L | No association between PLT > 150 × 109/L and lower transfusion rate |
| Yang et al, 2011 56 | Retrospective | 305 | Not specified | Haemorrhagic complications in 2.62% of pts. PLT count < 100 × 109/L was independently correlated with postoperative morbidity and hospital mortality |
| Vascular catheter insertion | ||||
| Fisher et al, 1999 57 | Retrospective | 658 cannulations in 283 pts | PLT < 50 × 109/L in ~ 25% pts | 1 haemothorax in pts with PLT = 68 × 109/L. PLT ≤ 10 × 109/L significantly associated with superficial haematoma vs PLT > 50 × 109/L (4,8% vs. 1,6%, respectively) |
| Estcourt et al, 2015 20 | Systematic review | ‐ | Not specified | No evidence from RCTs to determine whether PLT transfusions are required prior to central line insertion in patients with thrombocytopenia, and, if a PLT transfusion is required, what is the correct threshold. |
| HVPG measurement | ||||
|
Bosch et al 2009 58 |
Review + single‐centre experience | 12 000 measurement | Not reported | Major complications have been limited to local injury at the puncture site and include leakage, haematoma and rarely fistulae or Horner syndrome |
|
Woolfson et al, 2013 59 |
Retrospective | 52 HVPG measurements in 49 children | PLT < 100 × 109/L in 28 pts | Variceal bleeding and variceal bleeding + ascites occurred each in 1/7 patients with cirrhosis. Association between bleeding and PLT not evaluated |
| Cholecystectomy | ||||
| Sleeman et al, 1998 60 | Retrospective | 25 pts | PLT < 100 × 109/L in 36% | Association between bleeding and PLT not evaluated |
|
Da Silveira et al 2006 61 |
Retrospective | 99 pts | Not reported | Association between bleeding and PLT not evaluated |
|
Delis et al, 2010 62 |
Retrospective |
220 procedures |
Transfusion when PLT < 50 × 109/L | Intra‐operative bleeding rate: 8%. Association between bleeding and PLT not evaluated |
| Herniotomy | ||||
| Carbonell et al, 2005 63 | Retrospective | 32 033 procedures | Not reported | Association between bleeding and PLT not evaluated. |
| Ammar et al, 2010 64 | Prospective | 80 pts | Not reported | Association between bleeding and PLT not evaluated |
| Thoracentesis | ||||
|
Castellote et al, 2001 65 |
Retrospective | 245 thoracentesis in 69 cirrhotic pts | Not reported | Haemorrhagic rate: 2%. Association between bleeding and PLT not evaluated |
| Xiol et al, 2001 66 | Retrospective |
215 thoracenteses in 60 cirrhotic pts |
Not reported | Association between bleeding and PLT not evaluated |
| Urological surgery | ||||
| Nielsen et al, 2001 67 | Retrospective | 180 pts | Not reported | Association between bleeding and PLT not evaluated |
| Lund et al, 2003 68 | Retrospective | 611 | Not reported | Association between bleeding and PLT not evaluated |
| Miscellaneous | ||||
| Shah et al, 2015 21 | Prospective observational | 380 pts | Cut‐off 50 × 109/L | Clinically significant bleeding following high‐risk procedures occurred in 3 patients with significant coagulopathy and 0 patients without significant coagulopathy (P =.061) |
| De Pietri et al, 2016 9 | RCT open‐label ITT | 60 pts | Cut‐off 50 × 109/L | Bleeding occurred in 1.7% of patients (1/60) following paracentesis (PLT = 111 × 109/L) |
| Napolitano et al, 2017 22 | Prospective observational | 852 procedures in 363 pts | Cut‐off 50 × 109/L |
Overall bleeding complication rate: 2.75%. No bleeding in 90 procedures with PLT count < 50 × 109 L and no association were identified between PLT count and bleeding risk |
| Vuyyuru et al, 2020 23 | RCT open‐label | 60 pts | Cut‐off 50 × 109/L |
No bleeding in 58 procedures with PLT count < 50 × 109 L |
Abbreviations: HVPG, hepatic venous pressure gradient; INR, international normalized ratio; OR, odds ratio; PLT, platelet; pts, patients.
3.1. Paracentesis
In clinical practice, paracentesis is usually performed in cirrhotic patients with significant portal hypertension and thrombocytopenia. However, according to the literature, the incidence of post‐paracentesis haemorrhagic events was extremely low, and since the presence of portal hypertension is associated with bleeding regardless of platelet count, it was probably related to the patient clinical condition rather than the platelet count. 10 , 24 , 25 , 26 , 27 , 28 Even in the most numerous samples with a clear evaluation of platelet count, no bleeding was recorded in paracentesis performed with platelet count < 50 × 109/L. 10 , 25
3.2. Liver biopsy
Bleeding risk associated with percutaneous liver biopsy was about 0.6% in different studies including numerous sample sizes, but also very heterogeneous populations in terms of stage of liver disease. 11 , 12 , 32 , 33 Due to the fact that the presence of severe thrombocytopenia proxies advanced liver disease, thus obviating the need for histological confirmation of the presence of cirrhosis, and to the perceived potential bleeding risk, in clinical practice percutaneous liver biopsy is usually performed in patients without portal hypertension and platelet count > 50 × 109/L. The HALT‐C trial 33 represented the largest sample of patients with advanced liver disease who underwent percutaneous liver biopsy. Even if the bleeding complications were rare (overall haemorrhagic rate = 0.6%), the study highlighted an increased risk of post‐procedural bleeding in patients with platelet count ≤ 60 × 109/L (4/76; 5.3%) compared to patients with a platelet count > 60 × 109/L (11/2578; 0.4%), even though in this study a platelet count < 50 × 109/L was an exclusion criterion.
Transjugular liver biopsy is a procedure related to the operator expertise and in clinical practice is usually performed in patients with advanced liver disease, portal hypertension and thrombocytopenia. In spite of this, bleeding rate from studies was <2% and was mainly represented by the occurrence of haematoma at the site of insertion. 34 , 35 , 36 , 37 None of the studies evaluated the association between platelet count and post‐procedural bleeding rate.
3.3. Dentistry
Most of the evidence on this topic is provided by retrospective studies in which bleeding risk seemed to be inherently related to the procedure, or the number of teeth extraction, rather than to platelet count. 14 , 38 , 39 Furthermore, the study of Ward et al 38 was severely biased by massive transfusions before the procedure, thus making unfeasible any interpretation of the results in regard to the potential association between bleeding and severe thrombocytopenia. An association between platelet count and post‐procedural bleeding was found in the study of Cocero et al, 13 in which the haemorrhagic rate was 0.4% for patients with platelet count > 40 × 109/L and international normalized ratio (INR) <2.5, and 5.88% in patients with platelet count ≤ 40 × 109/L. Finally, in the only prospective study 39 postoperative bleeding occurred in only one procedure (2.9%) performed in a patient with liver cirrhosis, INR = 2.50 and platelet count = 50 × 109/L, whereas no bleeding occurred in procedures performed in patients with platelets = 30‐49 × 109/L.
3.4. Endoscopic variceal ligation
In the two studies analysed, the post‐procedural bleeding rate ranged from 2.75% in the case‐control study of Vanbiervliet et al, 41 to 7.33% in the prospective study of Viera da Rocha et al 40 In both cases, there was no association between bleeding risk and platelet count. In general, post‐ligation bleeding was related to technical problem occurred during the procedure, late bleeding or portal hypertension.
3.5. Endoscopic polypectomy
All the studies identified were retrospective and potentially biased by the heterogeneity of the investigated population including both cirrhotic and noncirrhotic patients. 15 , 42 , 43 Only the study by Soh et al 15 identified a correlation between post‐procedural bleeding and platelet count: while the overall haemorrhagic rate was 7.5%, in patients with platelets <50 × 109/L, the immediate post‐procedural bleeding rate was 27.5% with a relative risk of about 6.
3.6. Percutaneous radio‐frequency ablation of hepatocellular carcinoma
In clinical practice, percutaneous radio‐frequency ablation of hepatocellular carcinoma (HCC) is rarely performed in patients with platelets <50 × 109/L and is usually preceded by platelet transfusions and a close monitoring of platelet count. Therefore, the rate of bleeding following the radio‐frequency ablation of HCC was lower than 1. 16 , 44 , 45 Only the study of Park et al 16 found a correlation between a platelet count < 50 × 109/L and an increased risk of post‐procedural bleeding rate (OR = 8.79). However, the study was biased by prophylactic platelet transfusion in patients with platelets <50 × 109/L. Finally, the study of Goto et al 46 showed a haemorrhagic complication rate of 1.5% in 4133 radio‐frequency ablations (not only HCC), and thrombocytopenia was identified as significant risk factor for hemoperitoneum, even though patients with severe thrombocytopenia (platelets <50 × 109/L) were not included in the study.
3.7. Liver transplantation
The risk and extent of bleeding during liver transplantation were difficult to quantify and were generally reported only as indirect evidence (i.e. number of transfused blood products or amount of blood loss). None of the studies showed an association between platelet count and intra‐ or post‐transplantation bleeding. 17 , 18 , 19 , 47 , 48 , 49 , 50 Indeed, in this setting, the bleeding risk cannot be evaluated on the basis of blood coagulation parameters, since it may be influenced by other recipient's conditions, technical difficulties and portal hypertension control. Besides improvements in surgical experience and techniques in liver transplantation, strategies to reduce the use of blood products termed ‘patient blood management’ are increasingly adopted. For monitoring of haemostasis disturbances, thromboelastography (TEG) or thromboelastometry (TEM) is indicated as the best blood tests that can guide the application of plasma components, platelets and antifibrinolytics. 69
3.8. Liver surgery
In liver surgery, portal hypertension is the main determinant of outcome; in a large series published in 2011, even mild thrombocytopenia (platelet count of less than 150 × 109/L) predicted major postoperative complications and mortality after resection of HCC independently of functional scores such as Child‐Pugh or MELD score. 70
However, in this setting, bleeding rate and risk factors were very difficult to identify due to heterogeneous populations (i.e. inclusion of cirrhotic and noncirrhotic patients). All the studies were retrospective, and none evaluated the association between platelet count and bleeding risk in liver surgery. 51 , 52 , 53 , 54 , 55 , 56 This was probably due to the fact that in clinical practice moderate‐to‐severe thrombocytopenia is often considered a contraindication to liver surgery and patients are treated with pre‐ or intra‐operative platelet transfusions.
3.9. Abdominal surgery and other invasive procedures
The vascular catheter insertion and the hepatic venous pressure gradient measurement are procedures related to the operator expertise and are usually performed in patients at high risk of bleeding due to advanced liver disease, portal hypertension and thrombocytopenia. However, the available studies were not sufficient to determine a relationship between platelet count and bleeding risk following these two procedures. 20 , 57 , 58 , 59
Regarding cholecystectomy and herniotomy, the wide heterogeneity in the management of blood coagulation parameters in the pre‐procedural phases made the relationship between thrombocytopenia and haemorrhagic risk not evaluable. Furthermore, the available studies did not evaluate this association. 60 , 61 , 62 , 63 , 64 Finally, also the available evidence related to thoracentesis 65 , 66 and urological surgery 67 , 68 was not sufficient to assess the association between platelet count and post‐procedural bleeding risk.
3.10. Miscellaneous
Some studies evaluated the overall risk of bleeding in cirrhotic patients submitted to different procedures and the association with platelet count and/or coagulopathy. 9 , 13 , 15 , 23 In the open‐label, intention‐to‐treat trial of De Pietri et al 9 cirrhosis and significant coagulopathy (defined as INR > 1.8 and/or platelet count < 50 × 109/L) did not expose to an increased procedure‐related bleeding risk, regardless of the procedure (i.e. high‐ or low‐risk procedures), although the cohort included was small (i.e. 30 patients per arm) and all patients with severe thrombocytopenia in the standard of care arm received prophylactic platelet transfusions.
Similarly, the prospective case series of Napolitano et al 15 did not identify any association between platelet count and post‐procedural bleeding risk, not even in patients with a platelet count < 50 × 109/L. In this case, the only parameter associated with the risk of bleeding was the number of invasive procedures sequentially performed in each single patient: 3 events following 598 single procedures (bleeding rate: 0.5%) and 7 events following multiple procedures (1.5%). 15
Also, in the randomized controlled trial of Vuyyuru et al 23 no bleeding complications occurred following 58 procedures in cirrhotic patients with platelet count < 50 × 109/L, whereas in the prospective multicentre study of Shah et al, 13 in which none of the patients received peri‐procedural correction of abnormal coagulation parameters, the occurrence of clinically significant bleeding following high‐risk procedures (i.e. cholecystectomy, splenectomy, chemoembolization, central vein cannulation, percutaneous liver biopsy and endoscopic polypectomy) tended to be greater in patients with significant coagulopathy (defined as INR ≥ 1.5 and/or platelet count ≤ 50 × 109/L) as compared to patients without significant coagulopathy (3 vs 0, P = .061), although it was not possible to single out the role played by thrombocytopenia alone on the bleeding risk in this study.
4. INTERPRETATION OF THE RESULTS AND POTENTIAL CLINICAL IMPLICATIONS
Despite the lack of solid evidence identified in the previous consensus conference and the call for prospective studies to address the issue of procedure‐related bleeding risk in patients with liver disease, even most of the new studies included in our analysis had the limitation of not adequately assessing the association between post‐procedural bleeding risk and platelet count in patients with chronic liver disease. We also found other limitations of the available literature. The first is that the majority of studies that investigated the role of platelet count were retrospective and heterogeneous in terms of population (i.e. inclusion of cirrhotic and noncirrhotic patients). Also, it is important to note that in clinical practice moderate‐to‐severe thrombocytopenia is often considered a contraindication to some procedures (e.g. percutaneous radio‐frequency ablation of HCC, liver biopsy and liver surgery) and that patients are frequently treated with plasma and pre‐ or intra‐operative platelet transfusion in order to mitigate the risk of bleeding. 8 , 71 , 72 , 73 , 74 Therefore, it could be possible that some results were biased by these prophylactic interventions.
Only a few studies, among those who assessed the risk of bleeding in relation to platelet count, found that thrombocytopenia may be predictive of bleeding following percutaneous liver biopsy, 11 , 33 dental extractions, 13 , 14 percutaneous ablation of liver tumours 16 , 46 and endoscopic polypectomy. 15 Noteworthy, none of the prospective studies included in this review highlighted a significant correlation between post‐procedural haemorrhagic rate and platelet count. 9 , 21 , 22 , 23
Despite the above limitations, that would require the conduction of prospective studies properly designed to evaluate the bleeding risk in patients with chronic liver disease undergoing invasive procedures, according to platelet count, the available literature highlighted that severe thrombocytopenia is one of the most frequent issues to exclude cirrhotic patients to invasive procedures, which could be, in some cases, life‐saving, such as percutaneous radio‐frequency ablation in malignant lesions. However, available evidence had also the strength to confirm that there is no platelet count threshold at which bleeding is predictable, as other factors contribute to bleeding risk.
Indeed, it has been shown that in patients with chronic liver disease, even at an advanced stage, platelet count alone cannot be considered the only predictor of increased risk of bleeding, 75 , 76 while platelet count should be properly considered in the presence of other risk factors such as sepsis and acute kidney injury, in order to provide a more accurate estimate of the bleeding risk of the patients. 21 , 77
In addition, in cirrhotic patients the aetiology and severity of the disease can influence the haemostatic balance and co‐morbidities could alter the feeble haemostatic equilibrium in patients with advanced liver disease. 8 , 77
Despite the limited evidence available, several Position Papers and Guidelines of Scientific Societies recommend the correction of thrombocytopenia in patients with chronic liver disease and platelet count < 50 × 109/L who are scheduled to undergo invasive procedures. 8 , 71 , 72 , 73 , 74 In these patients, although there is no solid evidence of its efficacy on raising and maintaining an adequate platelet count level, 22 , 78 the standard of care is platelet transfusion. On the contrary, in patients with advanced liver disease there is evidence that prophylactic blood products transfusions, and the resultant volume expansion in a short timeframe, may aggravate portal hypertension and therefore paradoxically determine an increase in bleeding risk. 79 In this context, in cirrhotic patients with high risk of bleeding, thrombopoietin receptor agonists (TPO‐RAs) may represent an advantageous therapeutic alternative. 80 , 81 , 82 TPO‐RAs may improve patient clinical management as they are able to increase patient's platelet counts in a predictable fashion, thus allowing to plan invasive procedures and avoiding the risk of postponement or cancellation of procedures due to an inadequate increase in platelet count, as is often observed with platelet transfusions. 80 , 81 , 82 However, the use of TPO‐RAs has been associated with venous thromboembolism in patients with chronic liver disease, probably because of too high a rise in the platelet count and platelet hyperactivity in liver cirrhosis patients. 75 More in detail, as reported by Loffredo et al, 83 a statistically significant association between thrombotic risk and TPO‐RAs use was observed only in patients treated with eltrombopag.
Therefore, after the early termination of the clinical trial of eltrombopag, due to the increased thrombotic risk, 84 lusutrombopag was the first oral drug approved by EMA for the treatment of severe thrombocytopenia in patients with chronic liver disease undergoing invasive procedures. 85 Lusutrombopag showed efficacy in reducing the need for platelet transfusion, raising the platelet count > 50 × 109/L at the time of procedures and maintaining an adequate platelet level following the procedures, thus granting a safe lingering effect that may theoretically protect from delayed bleeding or allow to perform repeated invasive procedures. 80 , 81 In the pivotal studies L‐PLUS 1 and L‐PLUS 2, in fact, lusutrombopag allowed to reach the platelet threshold of 50 × 109/L in about 9 days, to maintain it for a median of 20.9 days, and safely performed invasive procedures (Figures 2 and 3). 80 , 81
FIGURE 2.
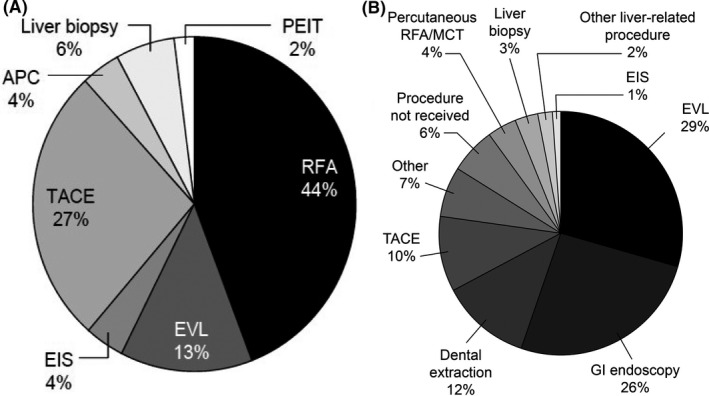
Invasive procedures performed in the lusutrombopag group during the (A) L‐PLUS 1 and (B) L‐PLUS 2 studies. APC = argon plasma coagulation; EIS = endoscopic injection sclerosis; EVL = endoscopic variceal ligation; GI = gastrointestinal; MCT = microwave coagulation therapy; PEIT = percutaneous ethanol injection therapy; RFA = radio‐frequency ablation; TACE = transcatheter arterial chemoembolization. (A) From: Hidaka H et al. Lusutrombopag Reduces Need for Platelet Transfusion in Patients With Thrombocytopenia Undergoing Invasive Procedures. Clin Gastroenterol Hepatol. 2019;17(6):1192‐1200 (B) Elaborated from: Peck‐Radosavljevic et al Lusutrombopag for the Treatment of Thrombocytopenia in Patients With Chronic Liver Disease Undergoing Invasive Procedures (L‐PLUS 2). Hepatology. 2019;70(4):1336‐1348
FIGURE 3.
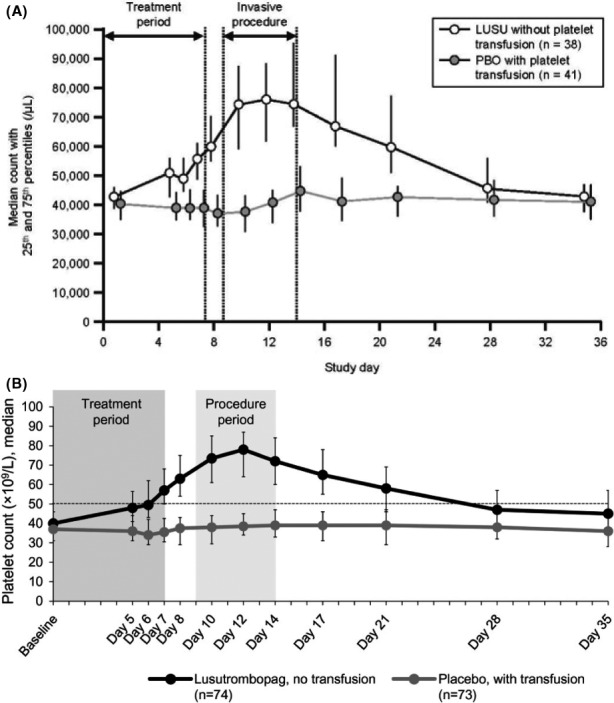
Median PCs over time for patients treated with lusutrombopag (without platelet transfusion) or placebo (with platelet transfusion) in the (A) PLUS‐1 and (B) PLUS‐2 studies. From: (A) Hidaka H et al’s Lusutrombopag Reduces Need for Platelet Transfusion in Patients With Thrombocytopenia Undergoing Invasive Procedures. Clin Gastroenterol Hepatol. 2019;17(6):1192‐1200 (B) Peck‐Radosavljevic et al Lusutrombopag for the Treatment of Thrombocytopenia in Patients With Chronic Liver Disease Undergoing Invasive Procedures (L‐PLUS 2). Hepatology. 2019;70(4):1336‐1348
Furthermore, the aggregated data showed that the use of lusutrombopag was associated with a numerical lower rate of post‐procedural bleeding (6.7% vs 10.6%) without increased risk of thrombosis. 80 , 81 , 85 Platelet increase with lusutrombopag was in fact more moderate than with other TPO‐RAs (median highest platelet count eltrombopag vs lusutrombopag: 140 × 109/L vs 80 × 109/L). 80 , 84
In the ADAPT‐1 and ADAPT‐2, pivotal studies on avatrombopag patients with low (<40 × 109/L) and high (40‐50 × 109/L) baseline platelet count received avatrombopag 40 and 60 mg, respectively. 82 In both cohorts, the proportion of patients who did not require a platelet transfusion after randomization and up to 7 days after the procedure was higher in those who received avatrombopag, compared with placebo. Moreover, in both avatrombopag treatment groups, platelet count increase was observed from day 4, reaching a maximum at days 10‐13. The mean platelet count remained at or above 50 × 109/L at day 17, and only 3 patients reach platelet count > 200 × 109/L. 82
5. CONCLUSIONS AND FUTURE DIRECTIONS
Despite several studies were conducted in the past, there is still a lack of adequate and solid data depicting the risk of bleeding following invasive procedures in patients with advanced liver disease, and its potential association with decreased platelet count. This notwithstanding, the best evidence currently available points to an association between severe thrombocytopenia and an increased risk of bleeding in patients with advanced cirrhosis undergoing procedures, in particular in subjects who undergo ‘closed procedures’ such as biopsies of parenchymal organs or liver tumour ablations. In this regard, international guidelines suggest that severe thrombocytopenia should be corrected before procedures in these patients, nevertheless, due to the lack of literature to support definitive conclusions about the appropriate target platelet counts to improve the risk of bleeding, there is an enormous variability in the use of prophylactic platelet transfusions. 86 It is, however, well established that the prophylactic use of platelet transfusions for these patients is of unpredictable efficacy and biased by potential adverse events including transfusion reactions, sepsis, refractoriness to further platelet transfusions, prolonged hospitalization and increased costs. Specifically, refractoriness to further platelet transfusions may add complexity to the management of these patients and reduce treatment options for bleeding associated with invasive procedures and/or surgery including liver transplant.
For all the above, research for therapeutic options alternative to platelet transfusion is welcome and TPO‐RAs seem to represent a valid option given the safety and efficacy, simplifying the clinical management of these patients. It is important to underline that, unlike platelet transfusion, the use of TPO‐RAs represents the only strategy capable of obtaining a real significant increase in the platelet count. Furthermore, the use of TPO‐RAs may be associated with the improvement in global healthcare resource utilisation, as blood product transfusions—and in particular platelets—are quite often used in clinical practice to increase platelets in patients undergoing procedures, and therefore, in this setting a treatment alternative may increase platelet availability for other clinical purposes. 86 , 87
In this context, still to be detailed is for which invasive procedure TPO‐RA prescription can be allocated and if some liver‐related disease complication may question the therapeutic efficacy. Despite a clear indication of the use of platelet growth factor in cirrhotic patients undergoing procedures at particular risk of bleeding, a valid therapeutic address to be considered is in liver transplant candidates, although in advanced stage of Child‐Pugh score the safety of TPO‐RAs has not yet been assessed. Routine procedures such as dental extraction, endoscopic polypectomy, ligation of oesophageal varices, transjugular intrahepatic portosystemic shunt (TIPS) and HCC transarterial chemoembolization (TACE) could benefit from a single period of drug therapy with the maximum result in terms of lower flow overload (compared with platelet transfusion), fewer days of hospitalization (due to ineffective increase platelet with transfusions) and lower risk of bleeding due to the achievement of a more adequate level of platelets in the plasma by using platelet growth factors.
In the next future, well‐designed studies may disclose whether the use of TPO‐RAs may actually be associated with a decreased risk of bleeding following procedures in patients with liver disease as compared to platelet transfusions, although planning such studies may represent a difficult task due to the variability of clinical situations, the vast array of potential procedures and their different risk of bleeding, and the overall low risk of bleeding that should call for the enrolment of very large cohorts of patients. Furthermore, real‐life data could add important information on the effectiveness and safety of TPO‐RAs in the management of invasive procedures in cirrhotic patients at high risk of bleeding, thus providing the basis of a potential new standard of care.
CONFLICT OF INTEREST
DA declares research grant and consultant fee from Intercept Pharma, research grant from Vesta (USA) and consultant fee from Aboca. NC declares consultant fee from Shionogi. EGG declares consultant fee from Shionogi, BMS, Gilead Sciences, AbbVie, Bayer and GSK. AI and PT have nothing to disclose. MM declares consultant fee from Shionogi, Gilead Sciences, AbbVie and Biotest. FV declares consultant fee from Shionogi.
AUTHOR CONTRIBUTIONS
The review topic was proposed by all authors. Literature selection and analysis of data were undertaken by all authors. Critical revisions of the manuscript were conducted by all authors. Final approval for the version to be published was obtained from all authors. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We would like to thank Ombretta Bandi, from SEEd Medical Publishers, who provided writing assistance and journal styling services, and Lorenzo Pradelli, from AdRes—Health Economics & Outcomes Research, who performed literature search. Editorial services and literature search were funded by Shionogi srl. Shionogi srl had no role in study design, nor in collection, analysis and interpretation of data.
Alvaro D, Caporaso N, Giannini EG, et al; The PReBRiC (Procedure‐Related Bleeding Risk in Cirrhosis) group . Procedure‐related bleeding risk in patients with cirrhosis and severe thrombocytopenia. Eur J Clin Invest. 2021;51:e13508. 10.1111/eci.13508
REFERENCES
- 1. Qamar AA, Grace ND, Groszmann RJ, et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2009;7(6):689‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louie KS, Micallef JM, Pimenta JM, Forssen UM. Prevalence of thrombocytopenia among patients with chronic hepatitis C: a systematic review. J Viral Hepat. 2011;18(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 3. Peck‐Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int. 2017;37(6):778‐793. [DOI] [PubMed] [Google Scholar]
- 4. Giannini EG. Review article: thrombocytopenia in chronic liver disease and pharmacologic treatment options. Aliment Pharmacol Ther. 2006;23(8):1055‐1065. [DOI] [PubMed] [Google Scholar]
- 5. Bashour FN, Teran JC, Mullen KD. Prevalence of peripheral blood cytopenias (hypersplenism) in patients with nonalcoholic chronic liver disease. Am J Gastroenterol. 2000;95(10):2936‐2939. [DOI] [PubMed] [Google Scholar]
- 6. Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48(6):1000‐1007. [DOI] [PubMed] [Google Scholar]
- 7. Giannini EG, Greco A, Marenco S, Andorno E, Valente U, Savarino V. Incidence of bleeding following invasive procedures in patients with thrombocytopenia and advanced liver disease. Clin Gastroenterol Hepatol. 2010;8(10):899‐e109. [DOI] [PubMed] [Google Scholar]
- 8. Andriulli A, Tripodi A, Angeli P, et al. Under the auspices of the Italian Association for the Study of Liver Diseases (AISF) and the Italian Society of Internal Medicine (SIMI). Hemostatic balance in patients with liver cirrhosis: report of a consensus conference. Dig Liver Dis. 2016;48(5):455‐467. [DOI] [PubMed] [Google Scholar]
- 9. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography‐guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63(2):566‐573. [DOI] [PubMed] [Google Scholar]
- 10. Rowley MW, Agarwal S, Seetharam AB, Hirsch KS. Real‐time ultrasound‐guided paracentesis by radiologists: near zero risk of hemorrhage without correction of coagulopathy. J Vasc Interv Radiol. 2019;30(2):259‐264. [DOI] [PubMed] [Google Scholar]
- 11. Takyar V, Etzion O, Heller T, et al. Complications of percutaneous liver biopsy with Klatskin needles: a 36‐year single‐centre experience. Aliment Pharmacol Ther. 2017;45(5):744‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Potretzke TA, Saling LJ, Middleton WD, Robinson KA. Bleeding complications after percutaneous liver biopsy: do subcapsular lesions pose a higher risk? Am J Roentgenol. 2018;211(1):204‐210. [DOI] [PubMed] [Google Scholar]
- 13. Cocero N, Bezzi M, Martini S, Carossa S. Oral surgical treatment of patients with chronic liver disease: assessments of bleeding and its relationship with thrombocytopenia and blood coagulation parameters. J Oral Maxillofac Surg. 2017;75(1):28‐34. [DOI] [PubMed] [Google Scholar]
- 14. Medina JB, Andrade NS, de Paula EF, et al. Bleeding during and after dental extractions in patients with liver cirrhosis. Int J Oral Maxillofac Surg. 2018;47(12):1543‐1549. [DOI] [PubMed] [Google Scholar]
- 15. Soh H, Chun J, Hong SW, et al. Child‐Pugh B or C cirrhosis increases the risk for bleeding following colonoscopic polypectomy [published online ahead of print, 2019 Dec 11]. Gut Liv. 2020;14(6):755‐764. 10.5009/gnl19131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park JG, Park SY, Tak WY, et al. Early complications after percutaneous radiofrequency ablation for hepatocellular carcinoma: an analysis of 1,843 ablations in 1,211 patients in a single centre: experience over 10 years. Clin Radiol. 2017;72(8):692.e9‐692.e15. [DOI] [PubMed] [Google Scholar]
- 17. Li C, Wen TF, Yan LN, Li B. Risk factors for abdominal bleeding after living‐donor liver transplant. Exp Clin Transplant. 2014;12(5):424‐428. [DOI] [PubMed] [Google Scholar]
- 18. Akamatsu N, Sugawara Y, Nakazawa A, et al. Hemostatic status in liver transplantation: association between preoperative procoagulants/anticoagulants and postoperative hemorrhaging/thrombosis. Liver Transpl. 2015;21(2):258‐265. [DOI] [PubMed] [Google Scholar]
- 19. Eghbal MH, Samadi K, Khosravi MB, et al. The impact of preoperative variables on intraoperative blood loss and transfusion requirements during orthotopic liver transplant. Exp Clin Transplant. 2019;17(4):507‐512. [DOI] [PubMed] [Google Scholar]
- 20. Estcourt LJ, Desborough M, Hopewell S, Trivella M, Doree C, Stanworth S. Comparison of different platelet transfusion thresholds prior to insertion of central lines in patients with thrombocytopenia. Cochrane Database Syst Rev. 2015;2015(6):CD011771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shah A, Amarapurkar D, Dharod M, et al. Coagulopathy in cirrhosis: a prospective study to correlate conventional tests of coagulation and bleeding following invasive procedures in cirrhotics. Indian J Gastroenterol. 2015;34(5):359‐364. [DOI] [PubMed] [Google Scholar]
- 22. Napolitano G, Iacobellis A, Merla A, et al. Bleeding after invasive procedures is rare and unpredicted by platelet counts in cirrhotic patients with thrombocytopenia. Eur J Intern Med. 2017;38:79‐82. [DOI] [PubMed] [Google Scholar]
- 23. Vuyyuru SK, Singh AD, Gamanagatti SR, Rout G, Gunjan D, Shalimar. A randomized control trial of thromboelastography‐guided transfusion in cirrhosis for high‐risk invasive liver‐related procedures. Dig Dis Sci. 2020;65(7):2104‐2111. [DOI] [PubMed] [Google Scholar]
- 24. Webster ST, Brown KL, Lucey MR, Nostrant TT. Hemorrhagic complications of large volume abdominal paracentesis. Am J Gastroenterol. 1996;91(2):366‐368. [PubMed] [Google Scholar]
- 25. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484‐488. [DOI] [PubMed] [Google Scholar]
- 26. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525‐529. [DOI] [PubMed] [Google Scholar]
- 27. Lin CH, Shih FY, Ma MH, Chiang WC, Yang CW, Ko PC. Should bleeding tendency deter abdominal paracentesis? Dig Liver Dis. 2005;37(12):946‐951. [DOI] [PubMed] [Google Scholar]
- 28. De Gottardi A, Thévenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906‐909. [DOI] [PubMed] [Google Scholar]
- 29. Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2(2):165‐173. [DOI] [PubMed] [Google Scholar]
- 30. Caturelli E, Giacobbe A, Facciorusso D, et al. Percutaneous biopsy in diffuse liver disease: increasing diagnostic yield and decreasing complication rate by routine ultrasound assessment of puncture site. Am J Gastroenterol. 1996;91(7):1318‐1321. [PubMed] [Google Scholar]
- 31. Actis GC, Olivero A, Lagget M, Pellicano R, Smedile A, Rizzetto M. The practice of percutaneous liver biopsy in a gastrohepatology day hospital: a retrospective study on 835 biopsies. Dig Dis Sci. 2007;52(10):2576‐2579. [DOI] [PubMed] [Google Scholar]
- 32. West J, Card TR. Reduced mortality rates following elective percutaneous liver biopsies. Gastroenterology. 2010;139(4):1230‐1237. [DOI] [PubMed] [Google Scholar]
- 33. Seeff LB, Everson GT, Morgan TR, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT‐C trial. Clin Gastroenterol Hepatol. 2010;8(10):877‐883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalambokis G, Manousou P, Vibhakorn S, et al. Transjugular liver biopsy–indications, adequacy, quality of specimens, and complications–a systematic review. J Hepatol. 2007;47(2):284‐294. [DOI] [PubMed] [Google Scholar]
- 35. Alessandria C, Debernardi‐Venon W, Rizzetto M, Marzano A. Transjugular liver biopsy: a relatively simple procedure with an indefinite past and an expected brilliant future. J Hepatol. 2008;48(1):171‐173. [DOI] [PubMed] [Google Scholar]
- 36. Mammen T, Keshava SN, Eapen CE, et al. Transjugular liver biopsy: a retrospective analysis of 601 cases. J Vasc Interv Radiol. 2008;19(3):351‐358. [DOI] [PubMed] [Google Scholar]
- 37. Procopet B, Bureau C, Métivier S, et al. Tolerance of liver biopsy in a tertiary care center: comparison of the percutaneous and the transvenous route in 143 prospectively followed patients. Eur J Gastroenterol Hepatol. 2012;24(10):1209‐1213. [DOI] [PubMed] [Google Scholar]
- 38. Ward BB, Weideman EM. Long‐term postoperative bleeding after dentoalveolar surgery in the pretransplant liver failure patient. J Oral Maxillofac Surg. 2006;64(10):1469‐1474. [DOI] [PubMed] [Google Scholar]
- 39. Perdigão JP, de Almeida PC, Rocha TD, et al. Postoperative bleeding after dental extraction in liver pretransplant patients. J Oral Maxillofac Surg. 2012;70(3):e177‐e184. [DOI] [PubMed] [Google Scholar]
- 40. Vieira da Rocha EC, D'Amico EA, Caldwell SH, et al. A prospective study of conventional and expanded coagulation indices in predicting ulcer bleeding after variceal band ligation. Clin Gastroenterol Hepatol. 2009;7(9):988‐993. [DOI] [PubMed] [Google Scholar]
- 41. Vanbiervliet G, Giudicelli‐Bornard S, Piche T, et al. Predictive factors of bleeding related to post‐banding ulcer following endoscopic variceal ligation in cirrhotic patients: a case‐control study. Aliment Pharmacol Ther. 2010;32(2):225‐232. [DOI] [PubMed] [Google Scholar]
- 42. Jeon JW, Shin HP, Lee JI, et al. The risk of postpolypectomy bleeding during colonoscopy in patients with early liver cirrhosis. Surg Endosc. 2012;26(11):3258‐3263. [DOI] [PubMed] [Google Scholar]
- 43. Lee S, Park SJ, Cheon JH, et al. Child‐Pugh score is an independent risk factor for immediate bleeding after colonoscopic polypectomy in liver cirrhosis. Yonsei Med J. 2014;55(5):1281‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cammà C, Di Marco V, Orlando A, et al. Treatment of hepatocellular carcinoma in compensated cirrhosis with radio‐frequency thermal ablation (RFTA): a prospective study. J Hepatol. 2005;42(4):535‐540. [DOI] [PubMed] [Google Scholar]
- 45. Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47(1):82‐89. [DOI] [PubMed] [Google Scholar]
- 46. Goto E, Tateishi R, Shiina S, et al. Hemorrhagic complications of percutaneous radiofrequency ablation for liver tumors. J Clin Gastroenterol. 2010;44(5):374‐380. [DOI] [PubMed] [Google Scholar]
- 47. McCluskey SA, Karkouti K, Wijeysundera DN, et al. Derivation of a risk index for the prediction of massive blood transfusion in liver transplantation. Liver Transpl. 2006;12(11):1584‐1593. [DOI] [PubMed] [Google Scholar]
- 48. Massicotte L, Beaulieu D, Thibeault L, et al. Coagulation defects do not predict blood product requirements during liver transplantation. Transplantation. 2008;85(7):956‐962. [DOI] [PubMed] [Google Scholar]
- 49. Massicotte L, Denault AY, Beaulieu D, et al. Transfusion rate for 500 consecutive liver transplantations: experience of one liver transplantation center. Transplantation. 2012;93(12):1276‐1281. [DOI] [PubMed] [Google Scholar]
- 50. Esmat Gamil M, Pirenne J, Van Malenstein H, et al. Risk factors for bleeding and clinical implications in patients undergoing liver transplantation. Transplant Proc. 2012;44(9):2857‐2860. [DOI] [PubMed] [Google Scholar]
- 51. Wei AC, Tung‐Ping Poon R, Fan ST, Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90(1):33‐41. 10.1002/bjs.4018 [DOI] [PubMed] [Google Scholar]
- 52. Kubo S, Takemura S, Yamamoto S, et al. Risk factors for massive blood loss during liver resection for hepatocellular carcinoma in patients with cirrhosis. Hepatogastroenterology. 2007;54(75):830‐833. [PubMed] [Google Scholar]
- 53. Palavecino M, Kishi Y, Chun YS, et al. Two‐surgeon technique of parenchymal transection contributes to reduced transfusion rate in patients undergoing major hepatectomy: analysis of 1,557 consecutive liver resections. Surgery. 2010;147(1):40‐48. [DOI] [PubMed] [Google Scholar]
- 54. Hsu KY, Chau GY, Lui WY, Tsay SH, King KL, Wu CW. Predicting morbidity and mortality after hepatic resection in patients with hepatocellular carcinoma: the role of Model for End‐Stage Liver Disease score. World J Surg. 2009;33(11):2412‐2419. [DOI] [PubMed] [Google Scholar]
- 55. Cockbain AJ, Masudi T, Lodge JP, Toogood GJ, Prasad KR. Predictors of blood transfusion requirement in elective liver resection. HPB (Oxford). 2010;12(1):50‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang T, Zhang J, Lu JH, Yang GS, Wu MC, Yu WF. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg. 2011;35(9):2073‐2082. [DOI] [PubMed] [Google Scholar]
- 57. Fisher NC, Mutimer DJ. Central venous cannulation in patients with liver disease and coagulopathy–a prospective audit. Intensive Care Med. 1999;25(5):481‐485. [DOI] [PubMed] [Google Scholar]
- 58. Bosch J, Abraldes JG, Berzigotti A, García‐Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6(10):573‐582. [DOI] [PubMed] [Google Scholar]
- 59. Woolfson J, John P, Kamath B, Ng VL, Ling SC. Measurement of hepatic venous pressure gradient is feasible and safe in children. J Pediatr Gastroenterol Nutr. 2013;57(5):634‐637. [DOI] [PubMed] [Google Scholar]
- 60. Sleeman D, Namias N, Levi D, et al. Laparoscopic cholecystectomy in cirrhotic patients. J Am Coll Surg. 1998;187(4):400‐403. [DOI] [PubMed] [Google Scholar]
- 61. da Silveira EB. Outcome of cirrhotic patients undergoing cholecystectomy: applying Bayesian analysis in gastroenterology. J Gastroenterol Hepatol. 2006;21(6):958‐962. [DOI] [PubMed] [Google Scholar]
- 62. Delis S, Bakoyiannis A, Madariaga J, Bramis J, Tassopoulos N, Dervenis C. Laparoscopic cholecystectomy in cirrhotic patients: the value of MELD score and Child‐Pugh classification in predicting outcome. Surg Endosc. 2010;24(2):407‐412. [DOI] [PubMed] [Google Scholar]
- 63. Carbonell AM, Wolfe LG, DeMaria EJ. Poor outcomes in cirrhosis‐associated hernia repair: a nationwide cohort study of 32,033 patients. Hernia. 2005;9(4):353‐357. [DOI] [PubMed] [Google Scholar]
- 64. Ammar SA. Management of complicated umbilical hernias in cirrhotic patients using permanent mesh: randomized clinical trial. Hernia. 2010;14(1):35‐38. [DOI] [PubMed] [Google Scholar]
- 65. Castellote J, Xiol X, Cortés‐Beut R, Tremosa G, Rodríguez E, Vázquez S. Complications of thoracentesis in cirrhotic patients with pleural effusion. Rev Esp Enferm Dig. 2001;93(9):566‐575. [PubMed] [Google Scholar]
- 66. Xiol X, Castellote J, Cortes‐Beut R, Delgado M, Guardiola J, Sesé E. Usefulness and complications of thoracentesis in cirrhotic patients. Am J Med. 2001;111(1):67‐69. [DOI] [PubMed] [Google Scholar]
- 67. Nielsen SS, Thulstrup AM, Lund L, Vilstrup H, Sørensen HT. Postoperative mortality in patients with liver cirrhosis undergoing transurethral resection of the prostate: a Danish nationwide cohort study. BJU Int. 2001;87(3):183‐186. [DOI] [PubMed] [Google Scholar]
- 68. Lund L, Jepsen P, Vilstrup H, Sørensen HT. Thirty‐day case fatality after nephrectomy in patients with liver cirrhosis–a Danish population‐based cohort study. Scand J Urol Nephrol. 2003;37(5):433‐436. 10.1080/00365590310006219 [DOI] [PubMed] [Google Scholar]
- 69. Tobin JM, Tanaka KA, Smith CE. Factor concentrates in trauma. Curr Opin Anaesthesiol. 2015;28(2):217‐226. [DOI] [PubMed] [Google Scholar]
- 70. Maithel SK, Kneuertz PJ, Kooby DA, et al. Importance of low preoperative platelet count in selecting patients for resection of hepatocellular carcinoma: a multi‐institutional analysis. J Am Coll Surg. 2011;212(4):638‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cinnella G, Pavesi M, De Gasperi A, et al. Standards clinici per il Patient Blood Management e per il management della coagulazione e dell’emostasi nel perioperatorio Position paper della Società Italiana di Anestesia, Analgesia, Rianimazione e Terapia Intensiva (SIAARTI). 2018. http://www.siaarti.it/SiteAssets/Ricerca/Standards‐clinici‐per‐il‐Patient‐Blood‐Management/SIAARTI%20standard%20PBM.pdf. Accessed October 5, 2020
- 72. Intagliata NM, Argo CK, Stine JG, Lisman T, Caldwell SH, Violi F. Concepts and Controversies in haemostasis and thrombosis associated with liver disease: proceedings of the 7th international coagulation in liver disease conference. Thromb Haemost. 2018;118(08):1491‐1506. 10.1055/s-0038-1666861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liumbruno G, Bennardello F, Lattanzio A, Piccoli P, Rossetti G. Raccomandazioni SIMTI sul corretto utilizzo degli emocomponenti e dei plasmaderivati. Edizioni SIMTI; 2018. [Google Scholar]
- 74. British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122(1):10‐23.12823341 [Google Scholar]
- 75. Violi F, Basili S, Raparelli V, Chowdary P, Gatt A, Burroughs AK. Patients with liver cirrhosis suffer from primary haemostatic defects? Fact or fiction? J Hepatol. 2011;55(6):1415‐1427. [DOI] [PubMed] [Google Scholar]
- 76. Basili S, Raparelli V, Napoleone L, et al. Platelet count does not predict bleeding in cirrhotic patients: results from the PRO‐LIVER study. Am J Gastroenterol. 2018;113(3):368‐375. [DOI] [PubMed] [Google Scholar]
- 77. Hung A, Garcia‐Tsao G. Acute kidney injury, but not sepsis, is associated with higher procedure‐related bleeding in patients with decompensated cirrhosis. Liver Int. 2018;38(8):1437‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tripodi A, Primignani M, Chantarangkul V, et al. Global hemostasis tests in patients with cirrhosis before and after prophylactic platelet transfusion. Liver Int. 2013;33(3):362‐367. [DOI] [PubMed] [Google Scholar]
- 79. Giannini EG, Stravitz RT, Caldwell SH. Correction of hemostatic abnormalities and portal pressure variations in patients with cirrhosis. Hepatology. 2014;60(4):1442. [DOI] [PubMed] [Google Scholar]
- 80. Hidaka H, Kurosaki M, Tanaka H, et al. Lusutrombopag reduces need for platelet transfusion in patients with thrombocytopenia undergoing invasive procedures. Clin Gastroenterol Hepatol. 2019;17(6):1192‐1200. [DOI] [PubMed] [Google Scholar]
- 81. Peck‐Radosavljevic M, Simon K, Iacobellis A, et al. Lusutrombopag for the treatment of thrombocytopenia in patients with chronic liver disease undergoing invasive procedures (L‐PLUS 2). Hepatology. 2019;70(4):1336‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Terrault N, Chen YC, Izumi N, et al. Avatrombopag before procedures reduces need for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology. 2018;155(3):705‐718. [DOI] [PubMed] [Google Scholar]
- 83. Loffredo L, Violi F. Thrombopoietin receptor agonists and risk of portal vein thrombosis in patients with liver disease and thrombocytopenia: a meta‐analysis. Dig Liver Dis. 2019;51(1):24‐27. [DOI] [PubMed] [Google Scholar]
- 84. Afdhal NH, Giannini EG, Tayyab G, et al. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med. 2012;367(8):716‐724. [DOI] [PubMed] [Google Scholar]
- 85. Committee for Medicinal Products for Human Use (CHMP) . Assessment report – Lusutrombopag Shionogi. EMA/CHMP/817852/2018. https://www.ema.europa.eu/en/documents/assessment‐report/lusutrombopag‐shionogi‐epar‐public‐assessment‐report_en.pdf. Accessed October 5, 2020
- 86. Fortea JI, Puente Á, Ezcurra I, et al. Management of haemostatic alterations and associated disorders in cirrhosis in Spain: a national survey. Dig Liver Dis. 2019;51(1):95‐103. [DOI] [PubMed] [Google Scholar]
- 87. Desborough MJ, Hockley B, Sekhar M, et al. Patterns of blood component use in cirrhosis: a nationwide study. Liver Int. 2016;36(4):522‐529. [DOI] [PubMed] [Google Scholar]


