Abstract
Context
Understanding factors involved in the rate of C-peptide decline is needed to tailor therapies for type 1 diabetes (T1D).
Objective
Evaluate factors associated with rate of C-peptide decline after a T1D diagnosis in young children.
Design
Observational study.
Setting
Academic centers.
Participants
A total of 57 participants from the Environmental Determinants of Diabetes in the Young (TEDDY) study who were enrolled at 3 months of age and followed until T1D, and 56 age-matched children diagnosed with T1D in the community.
Intervention
A mixed meal tolerance test was used to measure the area under the curve (AUC) C-peptide at 1, 3, 6, 12, and 24 months postdiagnosis.
Outcome
Factors associated with rate of C-peptide decline during the first 2 years postdiagnosis were evaluated using mixed effects models, adjusting for age at diagnosis and baseline C-peptide.
Results
Adjusted slopes of AUC C-peptide decline did not differ between TEDDY subjects and community controls (P = 0.21), although the former had higher C-peptide baseline levels. In univariate analyses combining both groups (n = 113), younger age, higher weight and body mass index z-scores, female sex, an increased number increased number of islet autoantibodies, and IA-2A or ZnT8A positivity at baseline were associated with a higher rate of C-peptide loss. Younger age, female sex, and higher weight z-score remained significant in multivariate analysis (all P < 0.02). At 3 months after diagnosis, higher HbA1c became an additional independent factor associated with a higher rate of C-peptide decline (P < 0.01).
Conclusion
Younger age at diagnosis, female sex, higher weight z-score, and HbA1c were associated with a higher rate of C-peptide decline after T1D diagnosis in young children.
Keywords: C-peptide, pediatric type 1 diabetes, new onset, beta cell decline, risk factors
Type 1 diabetes (T1D) is an autoimmune disease resulting from the progressive immune-mediated destruction of beta cells. Clinical trials employing immunotherapies in T1D have sought to prevent beta cell loss at each of Stage 1, 2, and 3 of the disease (ie, both before and after clinical diagnosis) (1). Preservation of beta cell function has been associated with lower risk of hypoglycemia and lower risk of long-term complications such as microalbuminuria and retinopathy (2, 3). Notably, clinical trials using rituximab (anti-CD20), abatacept (CTLA4-Ig), alefacept (anti-CD2), teplizumab (anti-CD3), and low-dose antithymocyte globulin (ATG) (4–8) have demonstrated transient preservation of C-peptide in new onset T1D. In addition, teplizumab recently demonstrated the ability to delay, by more than 2 years, progression from Stage 2 to Stage 3 T1D in high-risk subjects (9). In double islet autoantibody positive subjects with first phase insulin below the threshold, oral insulin immunomodulation delayed progression to Stage 3 by 2 years (10). Improved understanding of the factors involved in the rate of C-peptide decline could help tailor immunomodulatory therapies to specific groups or subjects and increase the success rates of these clinical trials (11).
An older age and a higher body mass index (BMI) have been previously associated with higher C-peptide levels at diagnosis of T1D (12, 13). The decline in stimulated C-peptide during the first year after the diagnosis has been highly variable, from 0% to 58% (14, 15), and factors involved in this variability are still only partially understood. While older age, lower HbA1c, and higher BMI at diagnosis have been reported to predict a slower loss of C-peptide (16–18), the SEARCH study showed a progressive decline in beta cell function independent of age, sex, HbA1c, and BMI (19).
Children participating in prospective studies, such as the Environmental Determinants of Diabetes in the Young (TEDDY) study, typically have a less severe clinical presentation at diabetes onset, with less diabetic ketoacidosis and diabetes symptoms (20, 21). We have previously shown that children diagnosed through the TEDDY study have higher C-peptide levels at onset compared with community-diagnosed children, and their C-peptide levels stayed higher throughout the first 12 months following the onset of diabetes (22). The goal of the current study was to evaluate factors associated with the rate of C-peptide decline in the first 2 years after the diagnosis of clinical T1D in a cohort of young children.
Materials and Methods
Study population
From September 2004 to February 2010, the TEDDY study identified 8676 infants at increased risk for T1D through genetic screening for diabetes-susceptible HLA DR-DQ genotypes at sites in Sweden, Finland, Germany, Colorado, Washington State, and Florida/Georgia. Those enrolled are followed prospectively from birth to 15 years of age, with study visits beginning at 3 months of age, then every 3 months until 4 years of age, then every 6 months thereafter. Children positive for islet autoantibodies are followed every 3 months. The details of screening and follow-up have been previously published (23, 24). The JDRF follow-up study recruited TEDDY study children diagnosed with T1D from January 2012 to December 2016; a total of 161 TEDDY study children were diagnosed with T1D during that time. For this study, 70 TEDDY study children and 60 age-matched children diagnosed with T1D from the community were enrolled by December 2016. Among the 130 enrolled subjects, 113 subjects had complete C-peptide baseline data available and were therefore included in the analysis. Control subjects from the community were matched to TEDDY subjects by age of diabetes diagnosis within 1 year and were required to have at least 1 positive islet autoantibody at diagnosis. Type 1 diabetes was defined according to American Diabetes Association criteria for diagnosis (25).
After diagnosis of T1D, all participants had visits with HbA1c and a mixed meal tolerance test (MMTT) within 1 month of onset, then at 3, 6, and 12 months after diagnosis, and biannually thereafter. The primary outcome measure was the area under the curve (AUC) for serum C-peptide in response to a 2-hour MMTT. The goal was to follow all subjects for at least 2 years after diagnosis or until the loss of detectable endogenous C-peptide. Parents (or legal guardian) of the subjects provided written informed consent, and the children assent when applicable. The study was approved by the ethical review boards of all participating institutions.
Study visits
Subjects came in fasting for MMTT, which consisted of a standardized liquid meal, Boost High Protein (Nestle Health Care Nutrition, Inc., Bridgewater, New Jersey) given at 6 ml/kg to a maximum of 360 ml. HbA1c was measured by a Tosoh G8 HPLC Analyzer (Tosoh Bioscience Inc., San Francisco, California) at the Diabetes Diagnostic Laboratory at the University of Missouri, Columbia. C-peptide (ng/ml) was measured using Tosoh reagents on a TOSOH 2000 autoanalyzer (Tosoh Bioscience Inc., San Francisco, California) at the Northwest Lipid Research Laboratories at the University of Washington.
Islet autoantibodies
Autoantibodies to GAD65, IA-2, and ZnT8 were measured in 2 reference laboratories by standard radiobinding assays (26). For sites in the United States, all serum samples were assayed at the Barbara Davis Center for Diabetes at the University of Colorado. In Europe, all sera were assayed at the University of Bristol, United Kingdom. Both laboratories have previously shown high assay sensitivity and specificity, as well as concordance (27). Positive samples were reanalyzed in the other laboratory for confirmation (US samples in Europe and vice versa).
Statistical analysis
Data were analyzed using the Statistical Analysis System software (version 9.4; SAS Institute, Cary, North Carolina). C-peptide was measured at time points 0, 15, 30, 60, 90, and 120 minutes. These timed values were combined using the trapezoidal rule to approximate the AUC; the reported value is the AUC divided by 120 minutes, which is an estimate of the mean of the C-Peptide level over the 2-hour period. Insulin-dose adjusted HbA1c (IDAA1C), an alternate measure of residual beta cell function (28), was calculated as HbA1c (%) + (4 x insulin dose (units/kg/day)).
Mixed-effects models were used to analyze the longitudinal data of C-peptide AUC, where AUC C-peptide values were normalized using a log-transformation on the value in the unit of ng/mL plus 1(log (x + 1)). The matching of case-control was modeled as a random effect and within-subject correlation was modeled using a first-order autoregressive structure. The time from diagnosis (months) was a covariate in the models and its corresponding coefficient was interpreted as the slope of C-peptide AUC change from diagnosis (ie, the negative value of the rate of C-peptide AUC loss). The effect of a potential factor on the rate of C-peptide AUC loss was modeled by examining an interaction term between the factor and the time from diagnosis. All the analyses were adjusted for age at diagnosis (years), C-peptide AUC at baseline, and the factor being examined. C-peptide AUC at baseline and autoantibody status (number of positive autoantibodies) were the measure from the baseline visit or from the 3-month visit if the baseline data was missing.
The effect of each of the potential factors on the rate of C-peptide AUC loss was examined individually. Factors with P < 0.05 for their effects on the slope were further analyzed in multivariate analysis using backward elimination procedure. In the backward elimination procedure, factors having effects on the rate of C-peptide AUC loss with P > 0.10 were eliminated. All participants with missing data specified as part of a particular analysis were omitted from that analysis. Two-tailed P-values < 0.05 were considered to be statistically significant.
Results
Characteristics at diagnosis of diabetes of the 57 TEDDY study and 56 community control children are described in Table 1. Mean age of diagnosis was 6.5 ± 1.8 years in these children and did not differ between TEDDY cases and community controls. As expected, the TEDDY study children had a higher frequency of the high-risk HLA DR3/4 genotype (56% vs 18%, P < 0.001). None of the TEDDY study children presented with diabetic ketoacidosis (DKA) at diagnosis, while 16% of the community children did (P = 0.001), with an overall low frequency of DKA for this cohort of young children. The low rate of DKA in the community group could potentially be explained by the willingness of more medically aware or committed community control families to enroll in this intensive follow-up study with multiple MMTTs during the 1st year postdiagnosis. The mean weight and BMI z-scores were higher at diagnosis in TEDDY study children versus community controls (0.6 vs 0.1, P = 0.02 and 0.2 vs -0.4, P = 0.01, respectively). TEDDY study children had a lower HbA1c at diagnosis and higher C-peptide AUC at baseline than community controls (6.9% [52 mmol/mol] vs 10.2% [88 mmol/mol], P < 0.001 and 1.7 ng/mL vs 1.3 ng/mL, P = 0.007, respectively).
Table 1.
Characteristics of study participants
| TEDDY Case (N = 57) | Control (N = 56) | P-valuea | |
|---|---|---|---|
| Female, N (%) | 25 (43.9) | 30 (53.6) | 0.349 |
| FDR with T1D, N (%) | 9 (15.8) | 5 (8.9) | 0.393 |
| HLA DR3/4, DQB1*0302, N (%) | 32 (56.1) | 10 (17.9) | <0.001 |
| Age at Diagnosis (years) | 6.4 ± 1.8 | 6.7 ± 1.9 | 0.378 |
| Diabetic ketoacidosis, N (%) | 0 (0.0) | 9 (16.1) | 0.001 |
| Weight z-score at diagnosis | 0.6 ± 0.9 | 0.1 ± 0.9 | 0.021 |
| Height z-score at diagnosisb | 0.8 ± 1.0 | 0.6 ± 1.0 | 0.208 |
| BMI z-score at diagnosisb | 0.2 ± 1.1 | -0.4 ± 1.2 | 0.010 |
| HbA1c at diagnosisc (%) | 6.9 ± 1.5 | 10.2 ± 2.3 | <0.001 |
| HbA1c at diagnosis (mmol/mol) | 52 ± 16 | 88 ± 25 | – |
| Positive autoantibodies,d N (%) | – | – | 1 |
| 0–1 Ab | 14 (24.6) | 13 (23.2) | – |
| ≥2 Ab | 43 (75.4) | 43 (76.8) | – |
| AUC C-Peptide at baselined (ng/mL) | 1.7 ± 0.8 | 1.3 ± 0.6 | 0.007 |
Means ± standard deviations are shown unless specified otherwise. Weight, height, and BMI were converted to SD units (Z scores) using the CDC growth chart.
Abbreviations: Ab, autoantibodies; AUC, area under the curve; BMI, body mass index; FDR, first-degree relative; HbA1c, hemoglobin A1c; HLA, human leukocyte antigen; TEDDY, The Environmental Determinants of Diabetes in the Young; T1D, type 1 diabetes.
a Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for proportions.
bEight subjects with missing information were omitted from the analysis.
cOne subject with missing HbA1c was omitted from the analysis.
dData from baseline visit and if missing from 3-month visit.
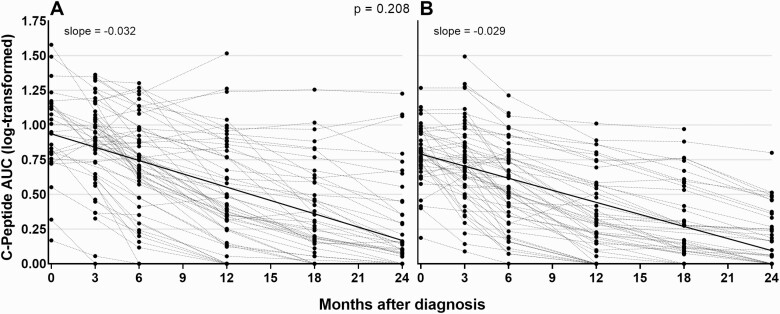
Individual trajectories of AUC C-peptide over time in TEDDY study children and community controls are shown in Fig. 1. The slope of the C-peptide AUC change was -0.030 per month overall (95% CI: -0.033, -0.028) in log scale, adjusted for age at diagnosis and AUC C-peptide at baseline. Adjusted slopes of C-peptide AUC change did not differ between TEDDY study subjects and community controls (slope = -0.032 vs. -0.029 per month, respectively, P = 0.208).
Figure 1.
Individual trajectories of AUC C-peptide (log-transformed) over time in TEDDY children (A) and community controls (B). Abbreviation: AUC, area under the curve.
The following factors were examined for association with the rate of C-peptide loss: sex, first-degree relative with T1D, HLA DR3-DQ2/DR4-DQ8 genotype, age at diagnosis, presence of DKA, and/or diabetes symptoms at diagnosis as well as HbA1c, weight, height, and BMI z-scores at diagnosis. Additional factors from the baseline and 3-month visits included autoantibody status (IA-2A, GADA, and ZnT8A), C-peptide, HbA1c, and insulin dose-adjusted HbA1c. In univariate analyses at baseline, C-peptide, female gender, younger age, higher weight, and BMI z-scores as well as an increased number of autoantibodies, presence of IA-2A or ZnT8A autoantibodies were all associated with a higher rate of C-peptide loss; only factors that were significantly associated with a higher rate of C-peptide loss are shown in Table 2. Three of these 8 factors remained significant in multivariate analysis adjusted for C-peptide at baseline: younger age at diagnosis, female gender, and higher weight z-score (all P < 0.02, Table 3).
Table 2.
Factors associated with slope of AUC C-peptide change (univariate baseline analyses)
| Factor | Estimated Effect (95% CI) on the Slope of AUC C-peptide (ng/mL/month) | P-value |
|---|---|---|
| C-peptide AUC (per 1 unit log) | -0.011 (-0.021, -0.001) | 0.037 |
| Female sex | -0.006 (-0.011, -0.002) | 0.010 |
| Age at diagnosis (per year) | 0.002 (0.000, 0.003) | 0.008 |
| Weight z-score at Dx (per SD) | -0.005 (-0.008, -0.002) | 0.001 |
| BMI z-score at Dx (per SD)a | -0.003 (-0.005, -0.001) | 0.013 |
| Number of positive autoantibodies | -0.006 (-0.009, -0.003) | <0.001 |
| IA-2A positive | -0.009 (-0.015, -0.003) | 0.004 |
| ZnT8A positive | -0.007 (-0.012, -0.002) | 0.009 |
A negative coefficient in these analyses corresponds to a faster rate of C-peptide loss. Analyses were adjusted for age at diagnosis (years) and C-peptide AUC at baseline.
Abbreviations: AUC, area under the curve; BMI, body mass index; CI, confidence interval; SD, standard deviation.
a Eight subjects with a missing BMI z-score were omitted from the analysis.
Table 3.
Factors associated with slope of AUC C-peptide change (multivariate baseline analyses)
| Factor | Estimated Effect (95% CI) on the Slope of AUC C-peptide (ng/mL/month) | P-value |
|---|---|---|
| Female | -0.006 (-0.011, -0.001) | 0.014 |
| Age at diagnosis (per year) | 0.002 (0.001, 0.003) | 0.005 |
| Weight z-score at Dx (per SD) | -0.003 (-0.006, -0.001) | 0.014 |
| IA-2A positive | -0.006 (-0.012, 0.000) | 0.061 |
| ZnT8A positive | -0.005 (-0.010, 0.000) | 0.065 |
A negative coefficient in these analyses corresponds to a faster rate of C-peptide loss. Analyses were adjusted for age at diagnosis (years) and C-peptide AUC at baseline.
Abbreviations: AUC, area under the curve; CI, confidence interval; SD, standard deviation.
The same univariate and multivariate analyses at 3 months after diagnosis are shown in Tables 4 and 5. At that time point, C-peptide, female gender, younger age, higher weight and BMI, an increased number of autoantibodies, IA-2A positivity and ZnT8A positivity as well as higher HbA1c were all associated with a higher rate of C-peptide loss (Table 4). In multivariate-adjusted analyses, higher HbA1c, in addition to younger age, female gender, and higher weight, was also associated with a higher rate of C-peptide decline (all P < 0.02, Table 5).
Table 4.
Factors associated with slope of AUC C-peptide change (univariate 3 month visit analyses)
| Factor | Estimated Effect (95% CI) on the Slope of AUC C-peptide (ng/mL/month) | P-value |
|---|---|---|
| C-peptide AUC (per 1 unit log) | -0.011 (-0.021, -0.001) | 0.037 |
| Female | -0.006 (-0.011, -0.002) | 0.010 |
| Age at diagnosis (per year) | 0.002 (0.000, 0.003) | 0.008 |
| Weight z-score (per SD)a | -0.006 (-0.009, -0.002) | 0.001 |
| BMI z-score (per SD)a | -0.005 (-0.008, -0.002) | 0.002 |
| Number of positive autoantibodiesb | -0.006 (-0.009, -0.003) | <0.001 |
| IA-2A positivec | -0.010 (-0.016, -0.003) | 0.002 |
| ZnT8A positivec | -0.006 (-0.012, -0.001) | 0.018 |
| HbA1c (per %)d | -0.003 (-0.006, -0.001) | 0.018 |
A negative coefficient in these analyses corresponds to a faster rate of C-peptide loss. Analyses were adjusted for age at diagnosis (years) and C-peptide AUC at baseline.
Abbreviations: AUC, area under the curve; BMI, body mass index; CI, confidence interval; HbA1c, hemoglobin A1c; SD, standard deviation.
a Three subjects with missing information were omitted from the analysis.
b Six subjects with missing information were omitted from the analysis.
c Four subjects with missing information were omitted from the analysis.
d Three subjects with missing HbA1c were omitted from the analysis.
Table 5.
Factors associated with slope of AUC C-peptide change (multivariate 3 month visit analyses)
| Factor | Estimated Effect (95% CI) on the Slope of AUC C-peptide (ng/mL/month) | P-value |
|---|---|---|
| Female | -0.006 (-0.010, -0.001) | 0.017 |
| Age at diagnosis (per year) | 0.002 (0.001, 0.003) | 0.001 |
| Weight z-score (per SD) | -0.005 (-0.008, -0.001) | 0.009 |
| HbA1c (per %) | -0.004 (-0.007, -0.001) | 0.005 |
| IA-2A positive | -0.006 (-0.012, 0.000) | 0.051 |
| ZnT8A positive | -0.005 (-0.010, 0.000) | 0.052 |
A negative coefficient in these analyses corresponds to a faster rate of C-peptide loss. Analyses were adjusted for age at diagnosis (years) and C-peptide AUC at baseline. Five subjects with missing information on HbA1c, IA-2A or ZnT8A status were omitted from the analysis.
Abbreviations: AUC, area under the curve; CI, confidence interval; HbA1c, hemoglobin A1c; SD, standard deviation.
Discussion
In this international cohort of children diagnosed with T1D at a young age (mean age 6.5 years), we have analyzed factors involved in the rate of C-peptide decline during the first 2 years after the onset of disease. We have previously shown that children diagnosed through the TEDDY study have higher C-peptide levels at onset compared with community-diagnosed children and that C-peptide levels stay higher throughout the first 12 months following the onset of T1D (22). Here we found that rates of C-peptide AUC loss (slope), adjusted for age at diagnosis and C-peptide AUC at baseline, did not differ between TEDDY study subjects and community controls, and that younger age at diagnosis, female sex, higher weight z-score, and higher HbA1c were associated with a faster decline of C-peptide over the first 2 years after diagnosis.
While age and BMI have been associated with C-peptide levels at diagnosis (13, 17, 18, 29), the rate of C-peptide decline after diagnosis has been highly variable in previous studies, and factors involved in the rate of C-peptide decline have not been consistent across studies. Data from 481 individuals with recent onset T1D enrolled in TrialNet studies showed that age at diagnosis and baseline C-peptide were significant predictors of the rate of C-peptide loss (11). A study looking at the rate of stimulated C-peptide decline in 446 children with new onset T1D from Scandinavia, Europe, and North America between 1982 and 2009 found that both the initial C-peptide and rate of C-peptide decline seemed to have increased over this 27-year time period (30). In that study, younger age, positivity for GADA, IAA, or both—but not BMI z-score, gender, or initial C-peptide—were associated with a faster rate of C-peptide decline during the 15 months after diagnosis. Of note, mean age varied from 9 years to 12.8 years for the 5 cohorts included, while this cohort of children was younger (mean age of 6.5 years at diagnosis). After adjusting for age at diagnosis and C-peptide AUC at baseline, IA-2A positivity and ZnT8A positivity in this study were associated with a higher rate of C-peptide loss in univariate analyses (<0.01), with P = 0.06 in multivariate analyses. Islet autoantibody positivity and levels have been shown to be associated with the rate of progression to T1D in children followed in prospective birth cohort studies such as TEDDY and the Diabetes Autoimmunity Study in the Young (31, 32). Therefore, it seems likely that ongoing autoimmunity as noted by autoantibody positivity would also play a role in rate of beta cell loss after diagnosis.
Preservation of C-peptide is known to be associated with a lower risk of hypoglycemia and a lower risk of long-term microvascular complications (2, 3). More recently, a study showed that DKA at diagnosis of T1D in children predicts persistently elevated HbA1c levels and poor long-term glycemic control independent of demographic and socioeconomic factors (33). In our current study, both higher HbA1c and a higher weight z-score were associated with a higher rate of C-peptide decline over the first 2 years after diagnosis, suggesting that metabolic factors could also play a role in the preservation of beta cell function. Diagnosis at a young age and poor metabolic control (higher HbA1c) could imply a more severe autoimmune process leading to an early presentation of the disease in those at risk. While some studies have reported preserved beta-cell function with a higher BMI close to diabetes onset (16), other studies have shown greater C-peptide decline over 1 year postdiagnosis with a higher BMI (18, 34) or no effect of BMI on the rate of C-peptide decline postdiagnosis (17, 19). At diagnosis of diabetes in subjects <18 years old, HbA1c levels have been shown to inversely correlate with AUC C-peptide as well as measures of the timing of C-peptide responses in OGTT, including peak C-peptide and early and late C-peptide responses (35). These various metabolic measures are obviously intertwined, but may help develop endpoints for beta cell preservation trials and tailor therapies to specific individuals or groups of individuals.
Limitations of this study include a relatively small number of subjects diagnosed with T1D with follow-up limited to 2 years after diagnosis. This could influence the results of some of the factors that were borderline, such as IA-2A and ZnT8A positivity. In addition, as baseline visits happened about a month after diagnosis, IAA levels were not available for community control subjects and therefore could not be analyzed and were not included in the analysis of either group. Although the rate of C-peptide decline was not statistically different between the 2 groups, there are important differences between the groups, such as higher T1D genetic load and earlier diagnosis in TEDDY subjects. Therefore, it is possible that known or unknown factors that regulate beta cell function decline were different between these 2 groups.
In summary, this study of young children diagnosed with T1D shows that adjusted rates of C-peptide loss were similar between TEDDY study subjects and community controls. Younger age at diagnosis, female gender, a higher weight z-score, and a higher HbA1c were associated with a higher rate of C-peptide decline over the first 2 years after diagnosis, while positivity for IA-2A and ZnT8A autoantibodies might also contribute to C-peptide decline in young children.
Acknowledgments
Financial Support: The TEDDY Study is funded by U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63863, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63863, UC4 DK95300, UC4 DK100238, UC4 DK106955, UC4 DK112243, UC4 DK117483, and Contract No. HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Centers for Disease Control and Prevention (CDC), and JDRF. This work supported in part by the NIH/NCATS Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR002535). The JDRF Follow-up study is funded by grant number 17-2011-274 from the Juvenile Diabetes Research Foundation (JDRF).
Author Contributions: A.K.S designed the study, wrote the manuscript, contributed to the discussion; X.L. researched data, reviewed/edited the manuscript; J.K. designed the study, contributed to the discussion, reviewed/edited the manuscript; M.J.H. reviewed/edited the manuscript; R.V. contributed to the discussion, reviewed/edited the manuscript; M.L. contributed to the discussion, reviewed/edited the manuscript; S.A. contributed to the discussion, reviewed/edited the manuscript; B.A. contributed to the discussion, reviewed/edited the manuscript; J.T. contributed to the discussion, reviewed/edited the manuscript; W.A.H reviewed/edited the manuscript; M.J.R designed the study, contributed to the discussion, reviewed/edited the manuscript; H.E.L. contributed to the discussion, reviewed/edited the manuscript.
The TEDDY Study Group
Colorado Clinical Center: Marian Rewers, MD, PhD, PI,1,4,5,6,9,10 Aaron Barbour, Kimberly Bautista,11 Judith Baxter,8,911 Daniel Felipe-Morales, Kimberly Driscoll, PhD,8 Brigitte I. Frohnert, MD,2,13 Marisa Stahl, MD,12 Patricia Gesualdo,2,6,11,13 Michelle Hoffman,11,12,13 Rachel Karban,11 Edwin Liu, MD,12,Jill Norris, PhD,2,3,11 Stesha Peacock, Hanan Shorrosh, Andrea Steck, MD,3,13 Megan Stern, Erica Villegas,2 Kathleen Waugh6,7,11. University of Colorado, Anschutz Medical Campus, Barbara Davis Center for Childhood Diabetes, Aurora, Colorado 80045.
Finland Clinical Center: Jorma Toppari, MD, PhD, PI,¥,^,1,4,10,13 Olli G Simell, MD, PhD, Annika Adamsson, PhD,^,11 Suvi Ahonen, *,±,§ Mari Åkerlund, *,±,§ Leena Hakola, * Anne Hekkala, MD,µ,¤ Henna Holappa, µ,¤ Heikki Hyöty, MD, PhD,*,±,6 Anni Ikonen, µ,¤ Jorma Ilonen, MD, PhD,¥,¶,3 Sinikka Jäminki, *,±, Sanna Jokipuu, ^ Leena Karlsson, ^ Jukka Kero, MD, PhD,¥,^ Miia Kähönen, µ,¤,11,13 Mikael Knip, MD, PhD,*,±,5 Minna-Liisa Koivikko, µ,¤ Merja Koskinen, *,± Mirva Koreasalo, *,±,§,2 Kalle Kurppa, MD, PhD,*,±,12 Jarita Kytölä, *,± Tiina Latva-aho, µ,¤ Katri Lindfors, PhD,*,12 Maria Lönnrot, MD, PhD,*,±,6 Elina Mäntymäki, ^ Markus Mattila, * Maija Miettinen, §,2 Katja Multasuo, µ,¤ Teija Mykkänen, µ,¤ Tiina Niininen, ±,*,11 Sari Niinistö, ±,§,2 Mia Nyblom, *,± Sami Oikarinen, PhD,*,± Paula Ollikainen, µ,¤ Zhian Othmani, ^ Sirpa Pohjola, µ,¤ Petra Rajala, ^ Jenna Rautanen, ±,§ Anne Riikonen, *,±,§,2 Eija Riski, ^ Miia Pekkola, *,± Minna Romo, ^ Satu Ruohonen, ^ Satu Simell, MD, PhD,¥,12 Maija Sjöberg, ^ Aino Stenius, µ,¤,11 Päivi Tossavainen, MD,µ,¤ Mari Vähä-Mäkilä, ¥ Sini Vainionpää, ^ Eeva Varjonen, ^,11 Riitta Veijola, MD, PhD,µ,¤,13 Irene Viinikangas, µ,¤ Suvi M Virtanen, MD, PhD.*,±,§,2 ¥University of Turku, 20500 Turku, Finland; *Tampere University, 33100 Tampere, Finland; µUniversity of Oulu, 90570 Oulu, Finland; ^Turku University Hospital, Hospital District of Southwest Finland, 20521 Turku, Finland; ±Tampere University Hospital, 33520 Tampere, Finland; ¤Oulu University Hospital, 90220 Oulu, Finland, §National Institute for Health and Welfare, FI-00271 Helsinki, Finland; ¶University of Kuopio, 70210 Kuopio, Finland.
Georgia/Florida Clinical Center: Jin-Xiong She, PhD, PI,1,3,4,10 Desmond Schatz, MD,*,4,5,7,8 Diane Hopkins, 11 Leigh Steed, 11,12,13 Jennifer Bryant, 11 Katherine Silvis, 2 Michael Haller, MD,*,13 Melissa Gardiner, 11 Richard McIndoe, PhD, Ashok Sharma, Stephen W Anderson, MD,^ Laura Jacobsen, MD,*,13 John Marks, DHSc,*,11,13 and *PD Towe. Center for Biotechnology and Genomic Medicine, Augusta University, Augusta, Georgia 30912. *University of Florida, Gainesville, Florida 32610, ^Pediatric Endocrine Associates, Atlanta, Georgia 30342.
Germany Clinical Center: Anette G Ziegler, MD, PI,1,3,4,10 Ezio Bonifacio, PhD,*,5 Cigdem Gezginci, Anja Heublein, Eva Hohoff, ¥,2 Sandra Hummel, PhD,2 Annette Knopff, 7 Charlotte Koch, Sibylle Koletzko, MD,¶,12 Claudia Ramminger, 11 Roswith Roth, PhD,8 Jennifer Schmidt, Marlon Scholz, Joanna Stock, 8,11,13 Katharina Warncke, MD,13 Lorena Wendel, Christiane Winkler, PhD,2,11 Forschergruppe Diabetes e.V. and Institute of Diabetes Research, Helmholtz Zentrum München, Forschergruppe Diabetes, and Klinikum rechts der Isar, Technische Universität München, 85764 Neuherberg, Germany; *Center for Regenerative Therapies, TU Dresden, 01307 Dresden, Germany, ¶Dr. von Hauner Children’s Hospital, Department of Gastroenterology, Ludwig Maximillians University Munich, 80337 München, Germany, ¥University of Bonn, Department of Nutritional Epidemiology, 53012 Bonn, Germany.
Sweden Clinical Center: Åke Lernmark, PhD, PI,1,3,4,5,6,8,9,10 Daniel Agardh, MD, PhD,6,12 Carin Andrén Aronsson, PhD,2,11,12 Maria Ask, Rasmus Bennet, Corrado Cilio, PhD, MD,5,6 Susanne Dahlberg, Helene Engqvist, Emelie Ericson-Hallström, Annika Björne Fors, Lina Fransson, Thomas Gard, Monika Hansen, Hanna Jisser, Fredrik Johansen, Berglind Jonsdottir, MD, PhD,11 Helena Elding Larsson, MD, PhD6,13, Marielle Lindström, Markus Lundgren, MD, PhD,13 Marlena Maziarz, PhD, Maria Månsson-Martinez, Jessica Melin, 11 Zeliha Mestan, Caroline Nilsson, Karin Ottosson, Kobra Rahmati, Anita Ramelius, Falastin Salami, Anette Sjöberg, Birgitta Sjöberg, Carina Törn, PhD,3 Åsa Wimar.13 Lund University, SE-221 00 Lund, Sweden.
Washington Clinical Center: William A Hagopian, MD, PhD, PI,1,3,4,5,6,7,10,12,13 Michael Killian, 6,7,11,12 Claire Cowen Crouch, 11,13 Jennifer Skidmore 2, Masumeh Chavoshi, Arlene Meyer, Jocelyn Meyer, Denise Mulenga, 11 Nole Powell, Jared Radtke, Matei Romancik, Shreya Roy, Davey Schmitt, Sarah Zink . Pacific Northwest Research Institute, Seattle, Washington, DC 98122.
Pennsylvania Satellite Center: Dorothy Becker, MD, Margaret Franciscus, MaryEllen Dalmagro-Elias Smith, 2 Ashi Daftary, MD, Mary Beth Klein , Chrystal Yates . Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, Pennsylvania 15224.
Data Coordinating Center: Jeffrey P Krischer, PhD, PI,1,4,5,9,10 Sarah Austin-Gonzalez, Maryouri Avendano, Sandra Baethke, Brant Burkhardt, PhD,5,6 Martha Butterworth 2, Joanna Clasen, David Cuthbertson, Christopher Eberhard, Steven Fiske, 8 Jennifer Garmeson, Veena Gowda, Kathleen Heyman, Belinda Hsiao, Christina Karges, Francisco Perez Laras, Qian Li, PhD,2,3 Shu Liu, Xiang Liu, PhD,2,3,8,13 Kristian Lynch, PhD5,6,8, Colleen Maguire, Jamie Malloy, Cristina McCarthy, 11 Hemang Parikh, PhD3, Cassandra Remedios, Chris Shaffer, Laura Smith, PhD,8,11 Susan Smith 11, Noah Sulman, PhD, Roy Tamura, PhD,1,2,11,12,13 Dena Tewey, Michael Toth, Ulla Uusitalo, PhD,2 Kendra Vehik, PhD,4,5,6,8,13 Ponni Vijayakandipan, Jimin Yang, PhD, RD.2Past staff: Michael Abbondondolo, Lori Ballard, Rasheedah Brown, Stephen Dankyi, David Hadley, PhD, Hye-Seung Lee, PhD, Wendy McLeod, Aubrie Merrell, Steven Meulemans, Ryan Quigley . University of South Florida, Tampa, Florida 33620.
Autoantibody Reference Laboratories: Liping Yu, MD,^,5 Dongmei Miao, MD,^ Polly Bingley, MD, FRCP,*,5 Alistair Williams, * Kyla Chandler, * Ilana Kelland, * Yassin Ben Khoud, * Huma Zahid, * Matthew Randell, *. ^Barbara Davis Center for Childhood Diabetes, University of Colorado, Anschutz Medical Campus, Aurora, Colorado 80045. *Bristol Medical School, University of Bristol, Bristol BS8 1TL, UK.
HbA1c Laboratory: Randie R Little, PhD, Curt Rohlfing . Diabetes Diagnostic Laboratory, Dept. of Pathology, University of Missouri School of Medicine, Columbia, Missouri 65212.
HLA Reference Laboratory: William Hagopian, MD, PhD,3 Masumeh Chavoshi, Jared Radtke, Sarah Zink . Pacific Northwest Research Institute, Seattle, Washington, DC 98122. (Previously Henry Erlich, PhD,3 Steven J Mack, PhD, Anna Lisa Fear . Center for Genetics, Children’s Hospital Oakland Research Institute, Oakland, California 94609.)
OGTT/MMTT Laboratory: Santica M Marcovina, PhD, ScD, Andrew N Hoofnagle, MD, PhD, Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington, Seattle, Washington, DC 98195.
Repository: Sandra Ke, Niveen Mulholland, PhD, NIDDK Biosample Repository at Fisher BioServices, Rockville, Maryland 20850.
Project Scientist: Beena Akolkar, PhD.1,3,4,5,6,7,9,10 National Institutes of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland 20892.
Other contributors: Kasia Bourcier, PhD; *,5 Thomas Briese, PhD; ¥,6 Suzanne Bennett Johnson, PhD; μ,8,11 and Eric Triplett, PhD.¶,6 *National Institutes of Allergy and Infectious Diseases, Bethesda, Maryland 20892; ¥Columbia University, New York, NY 10032., μFlorida State University, Tallahassee, Florida 32306; ¶University of Florida, Gainesville, Florida 32603.
Committees: 1Ancillary Studies, 2Diet, 3Genetics, 4Human Subjects/Publicity/Publications, 5Immune Markers, 6Infectious Agents, 7Laboratory Implementation, 8Psychosocial, 9Quality Assurance, 10Steering, 11Study Coordinators, 12Celiac Disease, 13Clinical Implementation.
Glossary
Abbreviations
- AUC
area under the curve
- CI
confidence interval
- BMI
body mass index
- FDR
first-degree relative
- HbA1c
hemoglobin A1c
- HLA
human leukocyte antigen
- JDRF
Juvenile Diabetes Research Foundation
- SD
standard deviation
- SEARCH
SEARCH for Diabetes in Youth study
- TEDDY
The Environmental Determinants of Diabetes in the Young
- T1D
type 1 diabetes
Additional Information
Disclosure Summary : The authors have nothing to disclose.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26(3):832–836. [DOI] [PubMed] [Google Scholar]
- 3. Lachin JM, McGee P, Palmer JP; DCCT/EDIC Research Group . Impact of C-peptide preservation on metabolic and clinical outcomes in the diabetes control and complications trial. Diabetes. 2014;63(2):739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. ; Type 1 Diabetes TrialNet Anti-CD20 Study Group . Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Orban T, Bundy B, Becker DJ, et al. ; Type 1 Diabetes TrialNet Abatacept Study Group . Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rigby MR, Harris KM, Pinckney A, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2015;125(8):3285–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hagopian W, Ferry RJ Jr, Sherry N, et al. ; Protégé Trial Investigators . Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62(11):3901–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haller MJ, Long SA, Blanchfield JL, et al. ; Type 1 Diabetes TrialNet ATG-GCSF Study Group . Low-dose anti-thymocyte globulin preserves C-peptide, reduces HbA1c, and increases regulatory to conventional T-cell ratios in new-onset type 1 diabetes: two-year clinical trial data. Diabetes. 2019;68(6): 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herold KC, Bundy BN, Long SA, et al. ; Type 1 Diabetes TrialNet Study Group . An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. 2019;381(7):603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study G, Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA 2017; 318(19):1891–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bundy BN, Krischer JP; Type 1 Diabetes TrialNet Study Group . A model-based approach to sample size estimation in recent onset type 1 diabetes. Diabetes Metab Res Rev. 2016;32(8):827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu P, Qian X, Schatz DA, Cuthbertson D, Krischer JP; DPT-1 Study Group . Distribution of C-peptide and its determinants in North American children at risk for type 1 diabetes. Diabetes Care. 2014;37(7):1959–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sosenko JM, Geyer S, Skyler JS, et al. The influence of body mass index and age on C-peptide at the diagnosis of type 1 diabetes in children who participated in the diabetes prevention trial-type 1. Pediatr Diabetes. 2018;19(3):403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palmer JP. C-peptide in the natural history of type 1 diabetes. Diabetes Metab Res Rev 2009;25(4):325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown RJ, Sinaii N, Rother KI. Too much glucagon, too little insulin: time course of pancreatic islet dysfunction in new-onset type 1 diabetes. Diabetes Care. 2008;31(7):1403–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenbaum CJ, Anderson AM, Dolan LM, et al. ; SEARCH Study Group . Preservation of beta-cell function in autoantibody-positive youth with diabetes. Diabetes Care. 2009;32(10):1839–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hao W, Gitelman S, DiMeglio LA, Boulware D, Greenbaum CJ; Type 1 Diabetes TrialNet Study Group . Fall in C-peptide during first 4 years from diagnosis of type 1 diabetes: variable relation to age, HbA1c, and insulin dose. Diabetes Care. 2016;39(10):1664–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ludvigsson J, Carlsson A, Deli A, et al. Decline of C-peptide during the first year after diagnosis of Type 1 diabetes in children and adolescents. Diabetes Res Clin Pract. 2013;100(2):203–209. [DOI] [PubMed] [Google Scholar]
- 19. Dabelea D, Mayer-Davis EJ, Andrews JS, et al. Clinical evolution of beta cell function in youth with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia. 2012;55(12):3359–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elding Larsson H, Vehik K, Bell R, et al. ; TEDDY Study Group; SEARCH Study Group; Swediabkids Study Group; DPV Study Group; Finnish Diabetes Registry Study Group . Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34(11):2347–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elding Larsson H, Vehik K, Gesualdo P, et al. ; TEDDY Study Group . Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr Diabetes. 2014;15(2):118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steck AK, Larsson HE, Liu X, et al. ; and the TEDDY Study Group . Residual beta-cell function in diabetes children followed and diagnosed in the TEDDY study compared to community controls. Pediatr Diabetes. 2017;18(8):794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kiviniemi M, Hermann R, Nurmi J, et al. ; TEDDY Study Group . A high-throughput population screening system for the estimation of genetic risk for type 1 diabetes: an application for the TEDDY (the Environmental Determinants of Diabetes in the Young) study. Diabetes Technol Ther. 2007;9(5):460–472. [DOI] [PubMed] [Google Scholar]
- 24. Hagopian WA, Erlich H, Lernmark A, et al. ; TEDDY Study Group . The environmental determinants of diabetes in the young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes. 2011;12(8):733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 26. Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab. 2010;95(7):3360–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Törn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ; Participating Laboratories . Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia. 2008;51(5):846–852. [DOI] [PubMed] [Google Scholar]
- 28. Mortensen HB, Hougaard P, Swift P, et al. ; Hvidoere Study Group on Childhood Diabetes . New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care. 2009;32(8):1384–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wallensteen M, Dahlquist G, Persson B, et al. Factors influencing the magnitude, duration, and rate of fall of B-cell function in type 1 (insulin-dependent) diabetic children followed for two years from their clinical diagnosis. Diabetologia. 1988;31(9):664–669. [DOI] [PubMed] [Google Scholar]
- 30. Max Andersen ML, Nielsen LB, Svensson J, et al. Disease progression among 446 children with newly diagnosed type 1 diabetes located in Scandinavia, Europe, and North America during the last 27 yr. Pediatr Diabetes. 2014;15:345–354. [DOI] [PubMed] [Google Scholar]
- 31. Steck AK, Vehik K, Bonifacio E, et al. ; TEDDY Study Group . Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: the environmental determinants of diabetes in the Young (TEDDY). Diabetes Care. 2015;38(5):808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steck AK, Johnson K, Barriga KJ, et al. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care. 2011;34(6):1397–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long-term glycemic control. Diabetes Care. 2017;40(9):1249–1255. [DOI] [PubMed] [Google Scholar]
- 34. Lauria A, Barker A, Schloot N, et al. BMI is an important driver of beta-cell loss in type 1 diabetes upon diagnosis in 10 to 18-year-old children. Eur J Endocrinol. 2015;172(2):107–113. [DOI] [PubMed] [Google Scholar]
- 35. Ismail HM, Evans-Molina C, DiMeglio LA, et al. ; Type 1 Diabetes Trial Net and Diabetes Prevention Trial-Type-1 (DPT-1) Study Groups . Associations of HbA1c with the timing of C-peptide responses during the oral glucose tolerance test at the diagnosis of type 1 diabetes. Pediatr Diabetes. 2019;20(4):408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.