Abstract
Context
Up to 40% of patients with polycystic ovary syndrome (PCOS) have prediabetes; an optimal pharmacotherapy regimen for diabetes prevention in PCOS is yet to be established.
Objective
To evaluate clinical efficacy of exenatide (EX), metformin (MET), or combination (COM) for prediabetes in PCOS.
Design
Randomized, open-label, parallel-group controlled trial.
Setting
Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine.
Patients
PCOS with prediabetes (fasting plasma glucose 5.6-6.9 mmol/L and/or 2 hour post glucose 7.8-11.0 mmol/L on oral glucose tolerance test [OGTT]). A total of 150 out of 183 eligible enrollees completed the study.
Intervention
EX (10-20μg daily), MET (1500-2000 mg daily), or COM (EX plus MET) for 12 weeks.
Main Outcome Measures
Sustained remission rate of prediabetes (primary endpoint, a normal OGTT after 12 weeks of treatment followed by 12 weeks of washout on no drug treatment) along with anthropometric, hormonal, metabolic, and pancreatic β-cell function parameters (secondary endpoints) and potential mechanisms were assessed.
Results
Impaired glucose tolerance was found the dominant prediabetes phenotype. Overall sustained prediabetes remission rate was 50.7%. Remission rate of COM group (64%, 32/50) or EX group (56%, 28/50) was significantly higher than that of the MET group (32%, 16/50) (P = .003 and .027, respectively). EX was associated with superior suppression of 2-hour glucose increment in OGTT. A 2-step hyperglycemic clamp study revealed that EX had led to higher postprandial insulin secretion than MET, potentially explaining the higher remission rate.
Conclusions
Compared with MET monotherapy, EX or COM achieved higher rate of remission of prediabetes among PCOS patients by improving postprandial insulin secretion.
Keywords: exenatide, metformin, prediabetes, polycystic ovary syndrome
Polycystic ovary syndrome (PCOS), characterized by hyperandrogenism and reproductive dysfunction, is a common endocrine disorder in premenopausal women [1]. Importantly, PCOS is also associated with a wide range of metabolic abnormalities including prediabetes [2, 3], which is defined by the presence of impaired fasting glucose (IFG, or fasting plasma glucose [FBG] levels between 5.6 and 6.9 mmol/L) and/or impaired glucose tolerance (IGT, or 2-hour postprandial glucose [PPG] levels during the 75-g oral glucose tolerance test (OGTT) between 7.8 and 11.0 mmol/L) and/or hemoglobin A1C (A1C) 5.7-6.4% (39-47 mmol/mol) [4]. It is estimated that approximately 30% to 40% of patients with PCOS have prediabetes [5], and up to 70% of PCOS patients with prediabetes will progress to type 2 diabetes (T2DM) in later life [6]. Prediabetes also puts these patients at an increased risk of adverse cardiovascular events [7]. In addition, women with PCOS and IGT early in pregnancy are at greater risk for gestational diabetes as well [8].
While OGTT and A1C are equally appropriate test options for prediabetes in the general population, the Endocrine Society has recommended OGTT over A1C to detect prediabetes in PCOS patients, since IGT (which can be missed by A1C) specifically is associated with increased cardiovascular disease in women [9, 10]. Of importance, data from Legro et al. [11] have suggested that IGT is the predominant subtype of prediabetes in PCOS subjects. Insulin resistance is believed to be 1 of the main underlying pathophysiological mechanisms of prediabetes in the general population. This is also widely assumed to be the case among PCOS patients with prediabetes. Pharmacological intervention with metformin (MET), in addition to lifestyle modification, delays the progress of T2DM in prediabetic subjects in the general population [12-14]. Similarly, MET is also the recommended drug of choice for PCOS patients with impaired glucose metabolism [15-19].
There are, however, an increasing number of studies focused on the contribution of insulin secretion defects in women with PCOS [20, 21]. By utilizing the hyperglycemic clamp technique, Kim et al. reported deficient insulin secretion in PCOS patients with obesity and glucose metabolism abnormality [22]. Consistent with these findings, we have recently reported that Chinese PCOS patients have defects in early-phase insulin secretion [23].
Incretin-based therapies are widely used to treat T2DM [24]. Glucagon-likepeptide (GLP)-1 analogues such as exenatide (EX) reduce plasma glucose levels by enhancing β-cell insulin secretion in an oral glucose–dependent manner. They also lead to weight loss and improved insulin sensitivity [25, 26]. Among type 2 diabetes patients, EX [27], or DPP4 inhibitor sitagliptin [28], when used in combination with MET, improves glycemic control as well as β-cell function compared with MET monotherapy. Long-term EX and MET combination therapy might also be able to relieve chronic inflammation associated with T2DM [29], and improve adipocytokines profile as well [30].
Based on our preliminary findings of IGT being the predominant type of prediabetes in PCOS patients and an associated postprandial insulin secretion defect, we have carried out a randomized, open-labeled, parallel-group controlled study to evaluate EX, MET, and combination (COM) of the 2 for their clinical efficacy for prediabetes in the PCOS population. The primary end point was remission rate of prediabetes defined as normal OGTT after 12 weeks of treatment followed by 12 weeks of washout, and secondary end points included changes in anthropometric, hormonal, and metabolic measures. Pancreatic β-cell function and insulin secretion were studied with OGTT, and confirmed by 2-step hyperglycemic clamp studies in a randomly selected subset of study subjects.
Materials and Methods
Study design
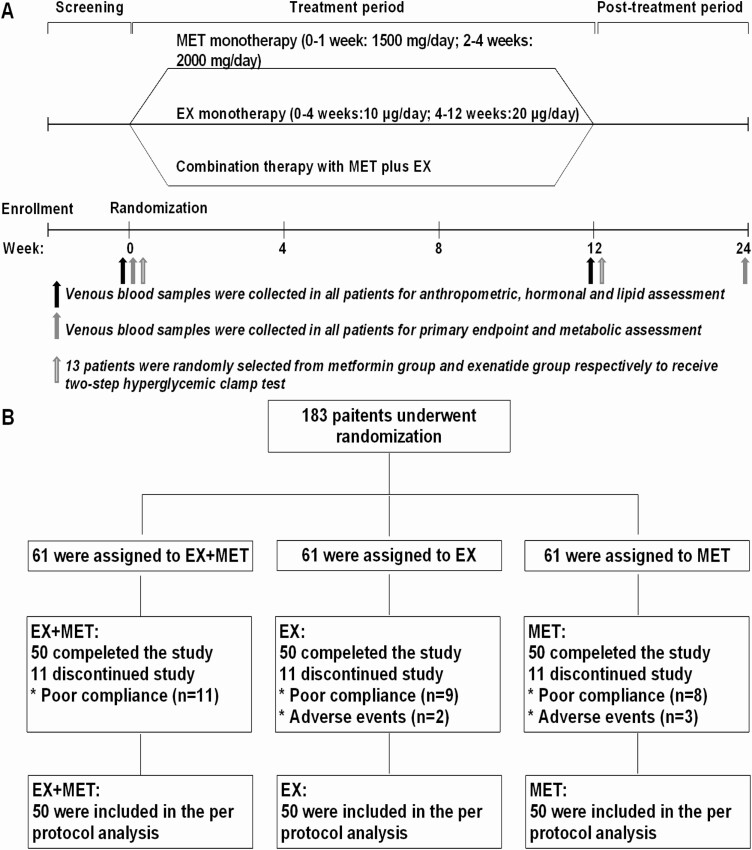
This prospective, randomized, open-label, parallel-group controlled trial enrolled 183 overweight/obese PCOS patients with prediabetes from November 2017 to July 2018 at Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine. This trial included 3 treatment groups, in which patients received exenatide (Byetta®, EX), metformin (Glucophage®, MET) or combination therapy (Fig. 1). The Institutional Review Board of Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine approved the study (ethics number: 2017-077). Written, informed consent was obtained from all subjects prior to any study-related procedures. This trial (ClinicalTrials.gov, NCT03352869) was conducted in accordance with Declaration of Helsinki and the Good Clinical Practice Guidelines.
Figure 1.
Study schema (A) and patient flowchart (B). EX, exenatide; MET, metformin.
Patients
The patient inclusion criteria were as follows: (1) Patients diagnosed with PCOS according to the Rotterdam 2003 criteria [31]; (2) and also met prediabetes criteria. Prediabetes is defined by the presence of IFG and/or IGT and/or A1C 5.7% to 6.4% (39-47 mmol/mol) [4]. IFG is defined as FBG levels between 5.6 and 6.9 mmol/L (100 and 125 mg/dL) and IGT as PPG levels for the 75-g OGTT between 7.8 and 11.0 mmol/L (140 and 199 mg/dL) [4]; (3) premenopausal patients aged between 18 and 45 years; (4) with a body mass index (BMI) ≥25 kg/m2 as defined by the World Health Organization-Western Pacific Region [32]; (5) patients with their first onset of PCOS that have not received any hypoglycemic drugs in 3 months prior to this trial; or have been treated with dietary and behavioral intervention for 3 months but still met OGTT criteria for prediabetes.
Exclusion criteria were as follows: subjects with self-reported allergy to either glucagon-like peptide 1 receptor agonists (GLP-1RA) or MET; subjects with severe liver function test abnormality (defined as alanine aminotransferase [ALT] 2.5 times or higher than the upper limit of normal range), or renal dysfunction (serum creatinine >132 µmol/L, and/or estimated glomerular filtration rate <60 mL/min/1.73 m2), hypertension (>160/100 mmHg), active infection, secondary diabetes, and subjects with active alcohol misuse, pregnancy, or breast feeding.
Treatment protocol
All eligible patients were randomly assigned to 1 of 3 intervention groups in a 1:1:1 ratio using the random number created by a computer-generated coding system. Patients received either EX (10-20 μg daily injection), MET (1500-2000 mg orally daily), or combination therapy (EX 10-20 μg daily injection plus MET 1500-2000 mg orally daily) for 12 weeks (Fig. 1). For all patients receiving EX, the initial injected dose was 10 μg/day and increased to 20 μg/day after 1 month. For all patients receiving oral MET, the initial dose was 500 mg 3 times daily before meals for 1 week and increased to 1000 mg twice daily before meals (breakfast and dinner) after 1 week (Fig. 1A). All patients received guidance concerning diet and exercise as appropriate.
Study measurements and analyses
At baseline and the end of the study, all patients underwent standard anthropometric measurements: height, weight, and waist circumference (WC). The height and weight of each subject wearing light clothing were measured to the nearest 0.1 cm and 0.1 kg, respectively. BMI was calculated as the body weight (kg) divided by the height (m) squared. The WC was measured to the nearest 0.1 cm by placing a measuring tape around the body in a horizontal position at a level midway between the lower rib margin and the iliac crest. The visceral adiposity index (VAI) was calculated using a standard sex-specific formula [WC/(36.58 + (1.89 × BMI))] × (triglyceride, TG/0.81) × (1.52/ high-density lipoprotein, HDL), where the TG and HDL concentrations are expressed in mmol/L [33]. Anthropometric data measurements were performed by a trained clinician.
All laboratory evaluations were performed on subjects in a fasting state between 7:00 and 8:00 am. A standard 75-g OGTT was conducted in all participants at screening, baseline, and 12 weeks after treatment completion. OGTT was performed in the morning (starting at 8:00 am) after a 12-hour overnight fast for analysis of FBG. After baseline blood sample collection, a 75-g oral glucose load was administered. Additional blood samples were drawn 30, 60, 90, and 120 minutes later for analysis of PPG and insulin levels. All measurements, including FBG, PPG, TG, total cholesterol (TC), HDL, and low-density lipoprotein (LDL) were performed using an automatic analyzer (Roche/Hitachi modular® analytics D2400 and E170 module, Roche, USA). Insulin, luteinizing hormone (LH), follicle stimulating hormone (FSH), sex hormone binding globulin (SHBG), dehydroepiandrosterone (DHEAS), and androstenedione (A2) were detected by chemiluminescence (Elecsys Auto analyzer, Roche, USA). Total testosterone (T) was measured by liquid chromatography mass spectrometry according to protocols previously reported [34]. The intra-assay and interassay coefficients of variation were <6% and 10%, respectively, for all analyses. The free androgen index (FAI) was calculated as 100 × T/SHBG [35]. The outcome measures of insulin sensitivity included fasting insulin, postprandial insulin, homeostasis model assessment of insulin resistance (HOMA-IR, for estimation of fasting insulin sensitivity), and Matsuda index (SIOGTT, for estimation of OGTT-derived insulin sensitivity). HOMA-IR was derived from fasting baseline blood glucose and insulin concentrations using the following formula: HOMA-IR = FBG (mmol/L) × fasting insulin (μIU/mL)/22.5 [36], and SIOGTT was derived from both fasting and stimulated values of glucose and insulin from the 2-h OGTT [37]. The glucose and insulin response to glucose were assessed by calculating the area under curve (AUC) during OGTT performance for glucose (AUCglu) and insulin (AUCins) using a trapezoidal rule [38].
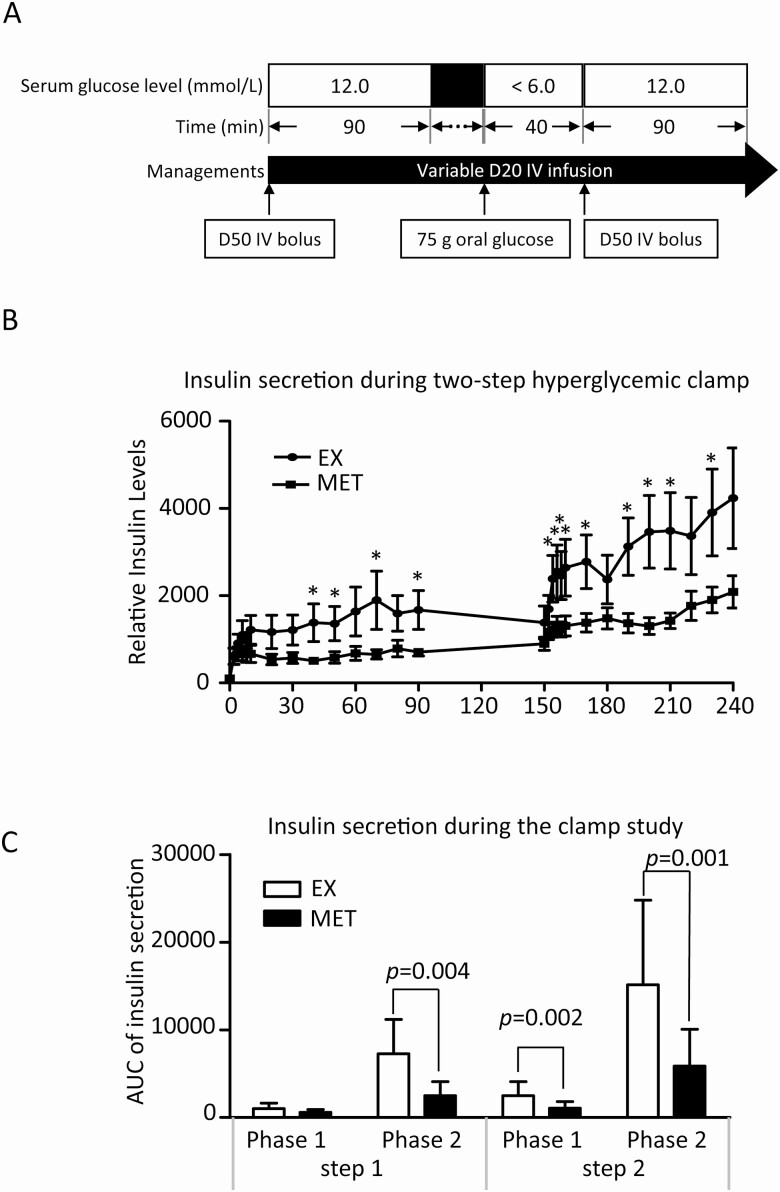
The 2-step hyperglycemic clamp test was performed at baseline and after drug treatment completion to quantitatively assess pancreatic β-cell function as previously described [39]. The 2-step hyperglycemic clamp consisted of 3 clamp periods (step 1, bridge step, and step 2). In step 1, a 50% glucose bolus was administered to quickly achieve a blood glucose value of 12 mmol/L and subject was then continued on a 20% glucose infusion to maintain blood glucose values at 12 mmol/L throughout the 90-minute hyperglycemic period. In the bridge step (euglycemic period), the infusion rate of 20% glucose was adjusted to quickly decrease glucose levels to <6 mmol/L. Subjects were administered 75 g of glucose orally to induce incretin. Forty minutes after oral glucose administration, step 2 of the clamp study was started by 50% glucose bolus followed by 20% glucose infusion to raise and maintain the blood glucose level at 12 mmol/L for another 90 minutes. During step 1 and step 2, blood samples were collected at 5-minute intervals for blood glucose determination. For insulin level determination, blood samples were collected at 2-minute intervals during the first 10 minutes, then at 10-minute intervals during the remaining 80 minutes of the clamp. β-Cell responses for both steps were computed using the AUC for phase 1 (0-10 minutes) and phase 2 (10-90 minutes) insulin secretion. Insulin secretion enhanced by incretin was then distinguished from that mediated purely by circulating blood glucose. Fig. 3A depicts the study protocol. Given the interaction effect on the secretion of incretin and insulin between EX and MET, patients who received combination therapy were excluded from this study. Thirteen patients were randomly selected from each group of MET and EX to receive the 2-step hyperglycemic clamp technique. Out of the 13 patients from each group, 7 subjects from EX group and 9 subjects from MET group successfully completed the 2-step hyperglycemic clamp study. Reasons for dropout included 6 subjects (EX, n = 3; MET, n = 3) refused to continue onto the second clamp after completing the first 1; 1 subject in EX group had phlebotomy difficulty; and finally 3 subjects (MET, n = 1; EX, n = 2) had adverse events (metallic taste and flushing) which led to early termination of the clamp study.
Figure 3.
(A) Study protocol of the 2-step hyperglycemic clamp study: During step 1 of the study, plasma glucose level was acutely raised and maintained at 12 mmol/L by an initial 50% dextrose bolus followed by 20% glucose infusion. After 90 minutes, 20% glucose was withdrawn, allowing plasma glucose to return to baseline. A 75-g of oral glucose was then administered to induce incretin. Forty minutes later, step 2 of the clamp study was started by 50% dextrose bolus followed by 20% glucose infusion to raise and maintain plasma glucose level at 12mmol/L for another 90 minutes. Plasma insulin concentrations were measured at 2-minute intervals during the first 10 minutes, then at 10 minute intervals during the remaining 80 minutes of each step of the clamp. (B) The average relative insulin concentrations (baseline insulin concentrations at time of zero of clamp study were set to 100) at each time points during the 2-step clamp for both groups. Asterisks indicate time points when insulin concentrations were statistically different between the 2 groups. (C)AUC of phase 1 (0-10 minutes) and phase 2 (10-90 minutes) insulin secretion during the two-step hyperglycemic clamp study. P values of the differences between the 2 groups are indicated.
Efficacy endpoints
The primary efficacy endpoint was the remission rate of prediabetes after treatment. Prediabetes remission was defined as a return to normal glucose tolerance (NGT) at 12 weeks after all antidiabetic medications were completed (or 24 weeks after initiating the medication regimen—12 weeks of treatment followed by 12 weeks of washout on no drug treatment) [40]. The prediabetes remission rate was defined as the proportion of patients with NGT among all patients who completed treatment. Secondary endpoints included anthropometric measurements (weight and BMI), hormonal changes (LH, FSH, T, FAI, SHBG, DHEAS, and A2), lipid profiles, and pancreatic β-cell function. Anthropometric, hormonal, and lipid parameters were evaluated at the completion of drug treatment. Insulin sensitivity, glucose, and pancreatic β-cell function were evaluated after 12 weeks of drug withdrawal.
Statistical analysis
To estimate sample size, we made assumptions based on our unpublished pilot data and a previous study that combined EX with MET in prediabetic individuals [41]. The sample size calculation was based on the assumption that differences in prediabetes remission rates between groups would be detected. We assumed that prediabetes remission rates were 50% in the EX group, 33% in the MET group, and 65% in the COM group, respectively. Based on these assumptions, a sample size of 46 per group would provide 80% power (β = 0.20) to detect this difference using a significance level of 0.05. We estimated a loss to follow-up of 20% and therefore increased the sample size by 20% (to 61 subjects per group) to allow for dropouts. The per-protocol population was defined as 150 randomized subjects who completed the study and received at least 90% of study drug injections and/or oral medications.
Statistical analyses were performed using SPSS software 23.0 (IBM, USA). Normality of variables was analyzed using the Kolmogorov–Smirnov test. Continuous variables were presented using either mean ± standard deviation (or standard error if otherwise specified) if the variable was in normal distribution among all comparing groups, or median (interquartile range) if a given variable was with non-normal distribution in 1 or more study groups that were compared. Comparisons of continuous variables with normal distribution and equal variance in all comparing groups were carried out using 1-way analysis of variance with the Bonferroni post hoc test. If these criteria were not satisfied, the Kruskal–Wallis test with the Bonferroni correction for post hoc tests was instead used. Student’s t tests were used to compare the means of 2 sets of data. Categorical variables were described by number and frequency, comparisons of which were performed using the chi-squared test. Significance was set at P < .05.
Results
Patient characteristics
Of the 183 eligible patients enrolled in this study, 150 (50 in each of the EX, MET, or COM treatment group) completed the study (Fig. 1B). Reasons for not completing the study were poor compliance (completion of less than 80% of medication dosages), including self-reducing the dosage without consent (EX, n = 8; COM, n = 6; MET, n = 8), medication administration timing not as prescribed (EX, n = 1; COM, n = 5), and adverse events (nausea and vomiting [MET, n = 2; EX, n = 2] and headache [MET, n = 1]) (Fig. 1B). The demographic characteristics, baseline metabolic measures, and hormonal levels are summarized (all supplementary material and figures are located in a digital research materials repository [42]). None of the baseline features were statistically different among the 3 groups, confirming appropriate randomization. All 150 subjects of the current study cohort had IGT, 27 cases were IFG + IGT, and none of them had isolated IFG.
Sustained remission rates of prediabetes
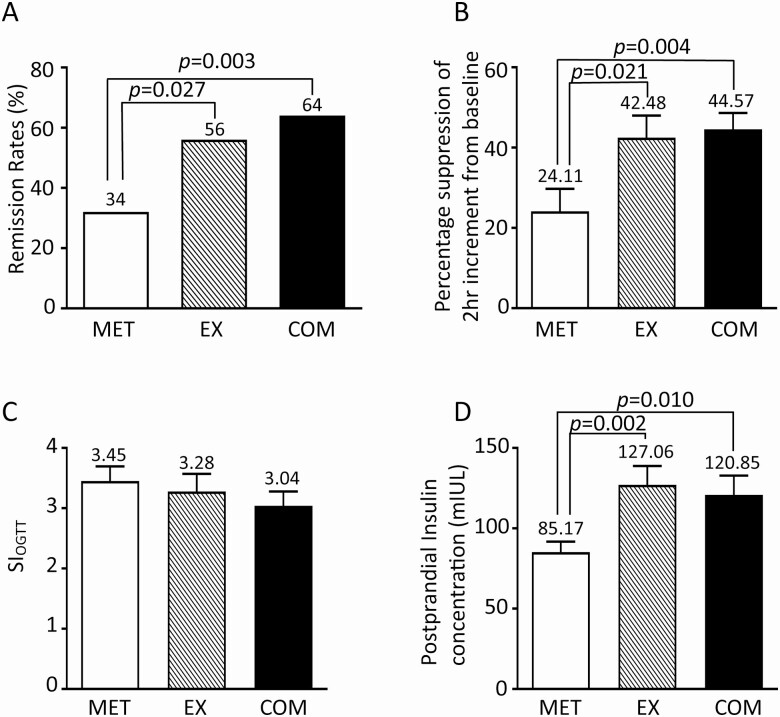
Sustained remission of prediabetes was seen in 76 out of the 150 (50.7%) subjects. The remission rate was 64% (32/50) in the COM group and 56% (28/50) in the EX group, both of which were significantly higher than that in the MET group (32% or 16/50), with P values of .003 and .027, respectively (Fig. 2A). The remission rates were not different between COM and EX groups (P = .414) by chi-squared tests [42].
Figure 2.
(A) Remission rate of patients treated with MET, EX, or COM. (B) Percentage suppression of 2-hour glucose increment by each treatment. Two-hour glucose increments at baseline (before treatment) in each group were set to 100%. (C) The SIOGTT among patients treated with MET, EX, or COM. (D) Serum insulin levels at 2-hour post 75 g of oral glucose in patients treated with MET, EX, or COM.
Potential clinical determinants of the remission
Univariate and multivariate logistic regression analyses were performed to identify potential determinants of prediabetes remission.
As summarized in Table 1, baseline AUCglu was found to be significantly related with remission in both univariate and multivariate analyses, with P values of .003 and .001, respectively. Univariate logistics regression analysis also showed that baseline AUCins was also likely significantly related with the remission rates with a P value of .051. Various forms of treatment were also found associated with remission. Compared with MET, EX and COM were significantly related with remission in both univariate and multivariate logistic analyses (all P < .05).
Table 1.
Univariate and multivariate logistic regression analyses of risk factors for the prediabetes remission
| Independent factors | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| EX Group (MET Group as reference) | 3.45 (1.52-7.85) | .030 | 4.52 (1.89-10.79) | .001 |
| COM Group (MET Group as reference) | 2.47 (1.10-5.55) | .028 | 3.12 (1.32-7.37) | .010 |
| AUCglu | 0.81 (0.70-0.93) | .003 | 0.77 (0.66-0.89) | .001 |
| AUCins | 1.00 (1.00-1.00) | .051 | ||
| BMI | 0.99 (0.92-1.06) | .682 | ||
| FAT | 1.00 (0.95-1.05) | .923 | ||
| FAI | 1.00 (0.97-1.02) | .854 | ||
| SIOGTT | 0.99 (0.90-1.09) | .790 | ||
| HOMA-IR | 0.94 (0.86-1.02) | .137 | ||
All variables were derived from baseline data.
Abbreviations: AUCglu, area under curve for OGTT glucose. AUCins, area under curve for OGTT insulin; BMI, body mass index; FAT, body fat (%); FAI, free androgen index; SIOGTT, oral glucose tolerance test–derived insulin sensitivity (Matsuda index); HOMA-IR, homeostasis model assessment of insulin resistance; CI, confidence interval.
Two-hour glucose increment of OGTT tests
The notion of IGT being the predominant phenotype of prediabetes among PCOS had led us to evaluate the 2-hour glucose increment in OGTT, which is defined as the difference of glucose levels between 0 and 2-hour time points, among the 3 treatment groups.
The 2-hour increments were similar among the 3 groups at baseline. At 12 weeks after completion of treatment, however, they were significantly lower in COM (2.25 ± 0.17 mmol/L [mean ± SE]) and EX (2.38 ± 0.21 mmol/L) groups than in MET group (3.02 ± 0.21 mmol/L), with P values of .006 and .035, respectively. Figure 2B shows significantly higher percentage suppression of 2-hour increments from baseline in COM: 44.57 ± 4.05 (from 4.03 ± 0.15 mmol/L to 2.25 ± 0.17 mmol/L) and EX: 42.48 ± 5.47 (from 4.24 ± 0.13 mmol/L to 2.38 ± 0.21 mmol/L) as opposed to MET: 24.11 ± 5.61 (from 4.12 ± 0.14 mmol/L to 3.02 ± 0.21 mmol/L).
FBG and insulin sensitivity
Despite inferior suppression on a 2-hour increment, MET effectively improved the FBG level. Treatment-specific effects on FBG were the following: MET, reduced FBG from 5.42 ± 0.04 mmol/L to 4.74 ± 0.07 mmol/L; COM, from 5.43 ± 0.13 mmol/L to 5.10 ± 0.10 mmol/L; and EX, from 5.20 ± 0.13 mmol/L to 4.85 ± 0.08 mmol/L. The post-treatment FBG levels were not different between MET and EX (P = .29), or between COM and EX (P = .70), but were rather slightly but statistically significantly lower in the MET group than the COM group (4.74 ± 0.07 mmol/L vs 5.10 ± 0.10 mmol/L, P = .003). The post-treatment fasting insulin levels were not different among the MET (15.66 ± 0.22 mIU/L), EX (16.47 ± 0.96 mIU/L), or COM (18.69 ± 1.66 mIU/L) groups.
Steady fasting state insulin sensitivities, estimated by HOMA-IR, were not different between MET (3.36 ± 0.24) and EX (4.13 ± 0.55, P = .26) or between MET and COM (4.26 ± 0.41, P = .06) groups. Consistent with HOMA-IR, the SIOGTT, which assesses insulin sensitivity using OGTT and correlates well with clamp-derived insulin sensitivity [37], were also similar among the 3 groups: COM, 3.04 ± 0.24; EX, 3.32 ± 0.29; and MET, 3.45 ± 0.24; further arguing against difference in insulin sensitivity among the three groups (Fig. 2C).The differences in 2-hour glucose increments were thus not explained by insulin resistance.
Oral glucose-induced insulin secretion
Serum insulin levels were measured at fasting and 2-hour post oral glucose challenge during OGTT at 12 weeks after treatment completion. While the fasting insulin levels were not different among the 3 groups, postprandial insulin levels were 120.85 ± 12.02 mIU/L in COM and 127.06 ± 11.81 mIU/L in EX groups, both of which were significantly higher than that in MET group (85.17 ± 6.47 mIU/L) with P values of .01 and .002, respectively (Fig. 2D).
Pancreatic β-cell function
The above data suggest that EX might be able to enhance oral glucose induced insulin secretion and contribute to higher prediabetes remission rate in PCOS wherein IGT is the dominant glucose abnormality. To further test this hypothesis, we performed 2-step hyperglycemic clamp studies [39] to assess insulin secretion from β-cell in subjects treated with either EX or MET.
Figure 3B showed the average relative insulin concentrations (insulin concentration at time 0 of clamp study was set to 100) at each time points during the 2-step clamp for both groups. Asterisks indicate time points when insulin concentrations were statistically different between the 2 groups. The step 1 data showed that the serum glucose-induced insulin secretion was slightly enhanced by EX at 40 minutes, 70 minutes, and 90 minutes points, but was mostly comparable at the remainder of the 11 time points between the 2 groups. In contrast, the post oral glucose challenge, hence incretin-enhanced insulin secretion, assessed during step 2, was significantly higher in EX group than in the MET group at most time points.
AUC of phase 1 (0-10 minutes) and phase 2 (10-90 minutes) insulin secretion of both steps were further analyzed. Consistent with the above results, AUC of phase 1 insulin secretion during step 1 clamp was not significantly different between EX (1649.21 ± 366.32) and MET (917.09 ± 277.55, P = .13) groups. AUCs of phase 2 insulin secretion during step 1 clamp and both phases 1 and 2 during step 2 clamp were all significantly higher in patients treated with EX, suggesting a preferred enhancement of postprandial insulin secretion by EX among PCOS patients with prediabetes (Fig. 3C).
Hormonal levels, lipid profiles, and anthropometric measures
Table 2 summarizes the hormonal levels, lipid profiles, and anthropometric measures of the 3 treatment groups.
Table 2.
Hormonal, metabolic, and anthropometric changes at the completion of 12 weeks of treatment
| Parameters | MET (n = 50) | EX (n = 50) | COM (n = 50) | P value | |||
|---|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | ||
| Hormonal levels | |||||||
| LH, IU/L | 7.81 (4.46, 12.00) | 6.16 (4.49, 8.09)** | 7.59 (4.59, 9.22) | 6.86 (4.15, 9.13) | 7.49 (5.17, 10.18) | 6.50 (4.03, 7.07)* | .193 |
| FSH, IU/L | 6.32 (5.51, 7.64) | 6.26 (4.67, 7.19) | 5.69 (4.38, 6.54) | 6.08 (4.31, 6.76) | 6.46 (5.30, 7.88) | 6.51 (5.55, 6.94) | .211 |
| DHEAS, ng/ml | 255.07 (189.50, 339.00) | 246.00 (230.00, 302.25) | 225.50 (181.25, 276.50) | 211.00 (160.75, 266.25) | 248.31 (214.40, 324.25) | 234.50 (201.00, 291.25) | .684 |
| T, nmol/L | 2.21 ± 0.97 | 1.86 ± 0.82* | 2.26 (1.91, 2.68) | 2.03 (1.72, 2.45)* | 2.33 (1.84, 2.85) | 1.89 (1.39, 2.69)** | .627 |
| FAI | 10.75 (7.17, 17.00) | 8.50 (3.89, 12.61)** | 10.17 (7.22, 16.09) | 7.74 (4.90, 12.60)** | 12.83 (8.67, 20.66) | 10.44 (6.41, 13.87)** | .873 |
| SHBG, nmol/L | 19.85 (13.23, 29.80) | 23.15 (15.88, 35.28)** | 22.80 (13.48, 32.90) | 27.20 (19.40, 35.50)** | 14.95 (10.28, 27.28) | 24.07 (18.10, 29.70)* | .94 |
| A2, µg/dL | 4.29 (2.84, 5.61) | 3.31 (2.61, 3.37)* | 4.05 (3.19, 4.53) | 3.28 (2.66, 3.71)** | 4.35 (2.61, 5.10) | 2.66 (2.23, 3.76)** | .204 |
| Lipid levels | |||||||
| TG, mmol/L | 1.55 (1.06, 2.55) | 1.38 (1.26, 1.44)* | 1.84 (1.20, 2.20) | 1.26 (0.93, 1.52)** | 1.99 (1.48, 2.39) | 1.19 (1.04, 1.80)** | .505 |
| TC, mmol/L | 4.59 (4.02, 5.14) | 4.66 (4.49, 4.76) | 4.82 (4.28, 5.39) | 4.70 (4.34, 4.94) | 4.71 (4.19, 5.22) | 4.60 (4.31, 5.21) | .714 |
| HDL, mmol/L | 1.17 (1.08, 1.29) | 1.24 (1.18, 1.28) | 1.18 (1.03, 1.35) | 1.19 (1.03, 1.27) | 1.13 (1.04, 1.26) | 1.12 (0.99, 1.24) | .31 |
| LDL, mmol/L | 3.02 (2.55, 3.44) | 2.52 (2.21, 2.59)** | 2.98 (2.40, 3.33) | 2.71 (2.33, 3.03) | 3.36 (2.82, 3.81) | 2.81 (2.51, 3.21)* | <.05 |
| Anthropometric characteristics | |||||||
| Weight, kg | 80.00 (74.70, 86.00) | 76.00 (68.50, 82.00)** | 80.00 (72.88, 92.75) | 74.55 (68.00, 85.25)** | 83.17 ± 12.98 | 76.65 ± 13.18** | .203 |
| BMI, kg/m2 | 29.64 (28.23, 33.14) | 28.19 (25.91, 30.86)** | 30.99 (27.84, 33.34) | 28.46 (25.69, 31.37)** | 31.66 ± 4.67 | 29.17 ± 4.80** | .158 |
Variables with normal distribution in both Before and After treatment are presented as Mean ± SD, otherwise are presented as median (Q1, Q3).
Abbreviations: COM, combination; EX, exenatide; MET, metformin; LH, luteinizing hormone; FSH, follicle stimulating hormone; DHEAS, dehydroepiandrosterone; T, total testosterone; FT, free testosterone; FAI, free androgen index; SHBG, sex hormone binding globulin; A2, androstenedione; TG, triglyceride; TC, total cholesterol, HDL, high density lipoprotein; LDL, low density lipoprotein; BMI, body mass index. * P < 0.05 and ** P < 0.01 compared with baseline (before treatment);
At completion of 12 weeks of therapy (ie, week 12 of the study), a significant decrease in LH level from baseline was observed in the MET (P < .001) and COM (P < .05) groups but not in the EX group (P = .521). T, SHBG, and A2 levels were all significantly decreased from baseline in each treatment group (P < .05). The delta values of the decreases were however not significantly different between groups (data not shown). FSH and DHEAS levels did not change significantly.
TG levels significantly decreased from baseline in all 3 groups. LDL levels declined in MET and COM groups, but not in the EX group. The LDL decrease was greater in the MET group (by average of 0.5 mmol/L) than in the COM group (by average of 0.28 mmol/L, P = .008). TC and HDL lipid levels did not change from baseline in any treatment group.
Both weight and BMI were lowered from baseline in each treatment group (P < .001), but the delta values were not different among the 3 groups.
Discussion
The clinical value of MET for prediabetes was most strongly established by the Diabetes Prevention Program trial [12], which demonstrated that MET could reduce incidence of T2DM by 31% among people with prediabetes. While an intensive lifestyle intervention could reduce the incidence by 58% over the initial 3 years, this difference between groups diminished over time, as shown in the Diabetes Prevention Program Outcomes Study (DPPOS) [43]. MET is a drug of choice for prediabetes with the strongest evidence and long-term safety data [44]. While medications such as GLP-1RA including EX [45] and liraglutide [46], α-glucosidase inhibitors, thiazolidinediones, and several agents approved for weight loss have been shown in research studies to reduce the incidence of diabetes in those with prediabetes [46-50], MET is the only medication approved by the US Food and Drug Administration specifically for diabetes prevention.
Among many possible mechanisms, MET is known to suppress hepatic gluconeogenesis and improve FBG [24]. It is interesting to notice that at the time of the 15-year follow-up (DPPOS), exploratory analyses have demonstrated that participants with a higher baseline FBG (≥110 mg/dL vs 95-109 mg/dL) experienced higher risk reductions with MET than with placebo [51], suggesting MET’s preferred effect on FBG. While there is no large-scale clinical study on prediabetes among PCOS patients, MET is the recommended medication for prediabetes in PCOS patients by most professional societies [15-17]. Consistent with the DPPOS [51] findings, our current data showed that MET can substantially improve FBG level in PCOS patients with prediabetes. However, this beneficial effect on FBG was not proportionally translated to the prediabetes remission rate as one would otherwise expect.
It is impressive to notice that postprandial hyperglycemia or IGT, rather than IFG, is the dominant and almost exclusive type of glucose metabolism abnormality in PCOS patients with prediabetes. In addition to the above data, a separate study involving 1787 consecutive cases of PCOS showed that 26% of them had prediabetes based on OGTT. Among those with prediabetes, 95.90% were isolated IGT and 2.81% were IFG + IGT. Isolated IFG was only 1.30% (our unpublished data). The GLP-1RA EX is clearly superior in addressing IGT in prediabetic PCOS patients. The 2-hour glucose increments of OGTT were significantly lower in patients treated with EX than with MET. The high prevalence of IGT and dominant EX effect on 2-hour glucose increment has led to the most striking finding of the present study, which is that 56% (28/50) of patients in the EX group and 64% (32/50) in the COM group achieved sustained remission of prediabetes whereas the remission rates achieved by MET monotherapy were significantly lower at 32% (or 16/50).
While it is widely assumed that insulin resistance is the underlying pathophysiology of prediabetes in PCOS, insulin sensitivities both at steady fasting state and during OGTT were comparable between MET and EX groups. The difference in remission rate must therefore reside in postprandial insulin secretion. In line with this expectation, serum insulin levels at 2-hour post oral glucose challenge during OGTT was found to be significantly higher in patients treated with EX than those treated with MET. These findings are consistent with a previous report by Anwar et al. wherein it was suggested that abnormal glucose metabolism in PCOS patients was associated with pancreatic β-cell dysfunction [52].
The idea that EX, compared with MET, results in higher prediabetes remission rate in prediabetic PCOS patients by enhancing postprandial insulin section was further clarified by a two-step hyperglycemic clamp, a technique that allowed us to quantitatively distinguish insulin secretion mediated purely by circulating blood glucose from that enhanced by incretin which in turn was induced by oral glucose.
GLP-1RAs activate the GLP-1 receptor on β-cells and hence mimic endogenous GLP-1 stimulating glucose-dependent phases 1 and 2 insulin secretion, contributing to reductions in fasting and more so in postprandial glycemia [24, 53]. Introduced in 2005, EX was the first GLP-1RA approved for T2DM [24]. The present study shows that EX is an effective agent for prediabetes in patients with PCOS by enhancing postprandial insulin secretion and flattening the 2-hour glucose increment, thus specifically addressing IGT, the predominant type of glucose metabolism abnormality in this group of people.
It is noteworthy that GLP-1 deficiency has been described in patients with T2DM [54]. Given clinical efficacy of EX reported by the present study, one might wonder if deficiency of endogenous GLP-1 is part of the underlying pathophysiology of prediabetes in PCOS, a valid question that warrants further investigation.
It is also important to notice that EX’s clinical efficacy persisted after 12 weeks of drug washout, suggesting possible biological effects beyond insulin secretion directly enhanced by administered medication per se. In this regard, GLP-1RAs including EX are known to be β-cell protective by reducing apoptosis and improving the ultrastructure of β-cells, which ultimately leads to increased insulin production by endogenous GLP-1 [55]. Recent studies have suggested possible involvement of miRNA in these protective effects [56]. In addition, GLP-1RAs have been shown to protect β-cells from lipotoxicity in cell lines and animal models [57]. Reduced lipotoxicity can then promote the reverse differentiation of dedifferentiated β-cells into mature insulin-secretory β-cells, thus increasing insulin synthesis [58]. Finally, GLP-1 receptor activation has been shown to directly modulate the endoplasmic reticulum stress response, leading to the promotion of β-cell adaptation and survival [59]. Collectively, these mechanisms may explain the sustained effect of EX observed in our study.
In addition to glucose metabolism, recent studies have shown that EX can improve the hypothalamic–pituitary–gonad axis function and ovarian reserve in animal models of PCOS [60, 61]. These findings, if proven true in human, will be relevant for EX as a treatment option for PCOS patients with prediabetes.
While an oral formula of GLP-1RA (semaglutide [62]) is now available for type 2 DM, most GLP-1RAs including EX require subcutaneous injection, which represents a practical burden for applying this group of medications to the prediabetics including those with PCOS. A logical alternative is DPP4 inhibitors, which, by blocking degradation of endogenous GLP-1, augment glucose-stimulated insulin secretion. One of our ongoing studies is actively testing the clinical efficacy of DPP4 inhibitors, alone or in combination with MET, in PCOS patients with prediabetes.
It is interesting to notice that LH levels were significantly decreased in the MET and COM groups, but not in the EX group. In mice GLP-1RAs do not affect circulating LH levels [63]. Gain or loss of function mutations of GLP-1 receptor do not alter the concentration or distribution of gonadotropins [64]. While a reduced LH level associated with MET treatment might be beneficial for PCOS, the significance of this finding remains to be elucidated. The endocrine society recommends MET as a second-line therapy for women with PCOS with menstrual irregularity who cannot take or do not tolerate hormonal contracepts [15]. It is however noteworthy that hyperandrogenism was improved to a similar extent by EX and MET in our study populations.
The principal limitations of our study are single-center design, the relatively small sample size, and short duration of drug treatment. The 24-week period was too short to compare the sustainability of the benefits from these regimens. Although EX, alone or in combination with MET, demonstrated superior efficacy in reversing prediabetes in PCOS patients in a Chinese population, future trials of a longer duration, larger scale, and multicenter/ethnic groups would be helpful to confirm our findings. A second shortfall is that an OGTT was not conducted in all participants at completion of 12 weeks of treatment. While we believed that normalization of OGTT immediately after treatment would not represent the true remission, lack of these data at week 12 did deprive us of an opportunity to identify potential dynamic changes of glucose metabolism. Finally, a relatively high dropout rate (11 out of 61, or 18%), while within the generally accepted threshold of 20%, does pose potential associated bias.
Conclusion
To the best of our knowledge, this is the first randomized controlled trial to compare the clinical efficacy of EX, MET, or combination of the 2 in treating prediabetes in PCOS patients. Our data have shown that, compared with MET monotherapy, EX alone or in combination with MET is associated with higher remission rate of prediabetes among patients with PCOS by improving postprandial insulin secretion. The study suggests potential clinic values of GLP-1RAs for PCOS patients with prediabetes.
Acknowledgments
We thank the patients, and the investigators and their staff, for participating in this study. Thank professors Wei Qing Wang and Yi Fei Zhang in Shanghai RuiJin Hospital for their supports regarding the 2-step hyperglycemic clamp technique. We also highly appreciate the critical review and invaluable comments by Dr. Deborah Wexler from the Diabetes Center, Massachusetts General Hospital. We also highly appreciate the critical review of statistical analysis methods and invaluable comments by Professor Xiao Fei Ye from the Department of Health Statistics, Second Military Medical University.
Financial Support: This study was supported by the Medical Guidance Science and Technology Support Projects of Shanghai Municipal Science and Technology Commission (18411968700); Young Scientists Fund of the National Natural Science Foundation of China (81200628); and the Natural Science Foundation Project of Shanghai (12ZR1417800). The funders had no role in the design of the study, the collection, analysis, and interpretation of data, or in writing the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Clinical Trials Information: Registration no. NCT03352869 (registered September 11, 2017).
Glossary
Abbreviations
- A2
androstenedione
- AUC
area under curve
- BMI
body mass index
- COM
combination
- DHEAS
dehydroepiandrosterone
- EX
exenatide
- FAI
free androgen index
- FBG
fasting plasma glucose
- FSH
follicle stimulating hormone
- GLP
glucagon-like peptide
- HDL
high-density lipoprotein
- HOMA-IR
homeostasis model assessment of insulin resistance
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- LDL
low-density lipoprotein
- LH
luteinizing hormone
- MET
metformin
- NGT
normal glucose tolerance
- OGTT
oral glucose tolerance test
- PCOS
polycystic ovary syndrome
- PPG
postprandial glucose
- SHBG
sex hormone binding globulin
- T
testosterone
- T2DM
type 2 diabetes
- TC
total cholesterol
- TG
triglyceride
- VAI
visceral adiposity index
- WC
waist circumference
Additional Information
Disclosure Summary: The authors declare no conflicts of interest.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685-697. [DOI] [PubMed] [Google Scholar]
- 2. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elkind-Hirsch KE, Paterson MS, Seidemann EL, Gutowski HC. Short-term therapy with combination dipeptidyl peptidase-4 inhibitor saxagliptin/metformin extended release (XR) is superior to saxagliptin or metformin XR monotherapy in prediabetic women with polycystic ovary syndrome: a single-blind, randomized, pilot study. Fertil Steril. 2017;107(1):253-260.e1. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14-S31. [DOI] [PubMed] [Google Scholar]
- 5. Franks S. Polycystic ovary syndrome in adolescents. Int J Obes (Lond). 2008;32(7):1035-1041. [DOI] [PubMed] [Google Scholar]
- 6. Nathan DM, Davidson MB, DeFronzo RA, et al. ; American Diabetes Association . Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753-759. [DOI] [PubMed] [Google Scholar]
- 7. Gong Q, Zhang P, Wang J, et al. ; Da Qing Diabetes Prevention Study Group . Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019;7(6):452-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dmitrovic R, Katcher HI, Kunselman AR, Legro RS. Continuous glucose monitoring during pregnancy in women with polycystic ovary syndrome. Obstet Gynecol. 2011;118(4):878-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lundblad D, Eliasson M. Silent myocardial infarction in women with impaired glucose tolerance: the Northern Sweden MONICA study. Cardiovasc Diabetol. 2003;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brohall G, Schmidt C, Behre CJ, Hulthe J, Wikstrand J, Fagerberg B. Association between impaired glucose tolerance and carotid atherosclerosis: a study in 64-year-old women and a meta-analysis. Nutr Metab Cardiovasc Dis. 2009;19(5):327-333. [DOI] [PubMed] [Google Scholar]
- 11. Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90(6):3236-3242. [DOI] [PubMed] [Google Scholar]
- 12. Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hostalek U, Gwilt M, Hildemann S. Therapeutic use of metformin in prediabetes and diabetes prevention. Drugs. 2015;75(10):1071-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merlotti C, Morabito A, Pontiroli AE. Prevention of type 2 diabetes; a systematic review and meta-analysis of different intervention strategies. Diabetes Obes Metab. 2014;16(8):719-727. [DOI] [PubMed] [Google Scholar]
- 15. Legro RS, Arslanian SA, Ehrmann DA, et al. ; Endocrine Society . Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amsterdam EA-SrPCWG. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS). Hum Reprod. 2012;27(1):14-24. [DOI] [PubMed] [Google Scholar]
- 17. Kubota T. Update in polycystic ovary syndrome: new criteria of diagnosis and treatment in Japan. Reprod Med Biol. 2013;12(3):71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Endocrinology Subgroup and Expert Panel Chines Society of Obstetrics and Gynecology, Chinese Medical Association. [Chinese guideline for diagnosis and management of polycystic ovary syndrome]. Zhonghua Fu Chan Ke Za Zhi. 2018;53(1):2-6. [DOI] [PubMed] [Google Scholar]
- 19. Teede HJ, Misso ML, Costello MF, et al. ; International PCOS Network . Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dunaif A, Finegood DT. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1996;81(3):942-947. [DOI] [PubMed] [Google Scholar]
- 21. Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest. 1995;96(1):520-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim JY, Tfayli H, Michaliszyn SF, Lee S, Arslanian S. Distinguishing characteristics of metabolically healthy versus metabolically unhealthy obese adolescent girls with polycystic ovary syndrome. Fertil Steril. 2016;105(6):1603-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tao T, Li S, Zhao A, Mao X, Liu W. Early impaired β-cell function in Chinese women with polycystic ovary syndrome. Int J Clin Exp Pathol. 2012;5(8):777-786. [PMC free article] [PubMed] [Google Scholar]
- 24. Tahrani AA, Barnett AH, Bailey CJ. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat Rev Endocrinol. 2016;12(10):566-592. [DOI] [PubMed] [Google Scholar]
- 25. Syed YY, McCormack PL. Exenatide extended-release: an updated review of its use in Type 2 diabetes mellitus. Drugs. 2015;75(10):1141-1152. [DOI] [PubMed] [Google Scholar]
- 26. Davidson MB, Bate G, Kirkpatrick P. Exenatide. Nat Rev Drug Discov. 2005;4(9):713-714. [DOI] [PubMed] [Google Scholar]
- 27. Derosa G, Franzetti IG, Querci F, et al. Exenatide plus metformin compared with metformin alone on β-cell function in patients with Type 2 diabetes. Diabet Med. 2012;29(12):1515-1523. [DOI] [PubMed] [Google Scholar]
- 28. Derosa G, Carbone A, Franzetti I, et al. Effects of a combination of sitagliptin plus metformin vs metformin monotherapy on glycemic control, β-cell function and insulin resistance in type 2 diabetic patients. Diabetes Res Clin Pract. 2012;98(1):51-60. [DOI] [PubMed] [Google Scholar]
- 29. Derosa G, Franzetti IG, Querci F, et al. Variation in inflammatory markers and glycemic parameters after 12 months of exenatide plus metformin treatment compared with metformin alone: a randomized placebo-controlled trial. Pharmacotherapy. 2013;33(8):817-826. [DOI] [PubMed] [Google Scholar]
- 30. Derosa G, Cicero AF, Franzetti IG, et al. Effects of exenatide and metformin in combination on some adipocytokine levels: a comparison with metformin monotherapy. Can J Physiol Pharmacol. 2013;91(9):724-732. [DOI] [PubMed] [Google Scholar]
- 31. Rotterdam EA-SPcwg. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47. [DOI] [PubMed] [Google Scholar]
- 32. WHO Regional Office for the Western Pacific. The Asia-Pacific Perspective: Redefining Obesity and its Treatment. Sydney: Health Communications Australia; 2000. [Google Scholar]
- 33. Amato MC, Giordano C, Pitrone M, Galluzzo A. Cut-off points of the visceral adiposity index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian Sicilian population. Lipids Health Dis. 2011;10:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89(2):534-543. [DOI] [PubMed] [Google Scholar]
- 35. Mathur RS, Moody LO, Landgrebe S, Williamson HO. Plasma androgens and sex hormone-binding globulin in the evaluation of hirsute females. Fertil Steril. 1981;35(1):29-35. [DOI] [PubMed] [Google Scholar]
- 36. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 37. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462-1470. [DOI] [PubMed] [Google Scholar]
- 38. Albareda M, Rodríguez-Espinosa J, Murugo M, de Leiva A, Corcoy R. Assessment of insulin sensitivity and beta-cell function from measurements in the fasting state and during an oral glucose tolerance test. Diabetologia. 2000;43(12):1507-1511. [DOI] [PubMed] [Google Scholar]
- 39. Zhang Y, Chi J, Wang W, et al. Different effects of two dipeptidyl peptidase-4 inhibitors and glimepiride on β-cell function in a newly designed two-step hyperglycemic clamp. J Diabetes. 2015;7(2):213-221. [DOI] [PubMed] [Google Scholar]
- 40. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391(10120):541-551. [DOI] [PubMed] [Google Scholar]
- 41. Armato J, DeFronzo RA, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: targeting insulin resistance and β-cell dysfunction. Endocr Pract. 2012;18(3):342-350. [DOI] [PubMed] [Google Scholar]
- 42. Tao T, Yi Z, Yuchen Z, et al. Exenatide, metformin, or both for prediabetes in PCOS: a randomized, open-labeled, parallel-group controlled study. Figshare 2020. ProMED-mail website. Deposited September 19, 2020. 10.6084/m9.figshare.12899960.v5. Accessed 1 September 2020. [DOI]
- 43. Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):866-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moin T, Schmittdiel JA, Flory JH, et al. Review of metformin use for Type 2 diabetes prevention. Am J Prev Med. 2018;55(4):565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rosenstock J, Klaff LJ, Schwartz S, et al. Effects of exenatide and lifestyle modification on body weight and glucose tolerance in obese subjects with and without pre-diabetes. Diabetes Care. 2010;33(6):1173-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. le Roux CW, Astrup A, Fujioka K, et al. ; SCALE Obesity Prediabetes NN8022-1839 Study Group . 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399-1409. [DOI] [PubMed] [Google Scholar]
- 47. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M; STOP-NIDDM Trail Research Group . Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072-2077. [DOI] [PubMed] [Google Scholar]
- 48. Gerstein HC, Yusuf S, Bosch J, et al. ; DT Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368(9541): 1096-1105. [DOI] [PubMed] [Google Scholar]
- 49. DeFronzo RA, Tripathy D, Schwenke DC, et al. ; ACT NOW Study . Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364(12):1104-1115. [DOI] [PubMed] [Google Scholar]
- 50. Garvey WT, Ryan DH, Henry R, et al. Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care. 2014;37(4):912-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Diabetes Prevention Program Research Group. Long-term effects of metformin on diabetes prevention: identification of subgroups that benefited most in the diabetes prevention program and diabetes prevention program outcomes study. Diabetes Care. 2019;42(4):601-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Anwar S, Shikalgar N. Prevention of type 2 diabetes mellitus in polycystic ovary syndrome: a review. Diabetes Metab Syndr. 2017;11(Suppl 2):S913-S917. [DOI] [PubMed] [Google Scholar]
- 53. Chon S, Gautier JF. An update on the effect of incretin-based therapies on β-cell function and mass. Diabetes Metab J. 2016;40(2):99-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia. 2011;54(1):10-18. [DOI] [PubMed] [Google Scholar]
- 55. Orskov C, Wettergren A, Holst JJ. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol. 1996;31(7):665-670. [DOI] [PubMed] [Google Scholar]
- 56. Shang J, Li J, Keller MP, et al. Induction of miR-132 and miR-212 Expression by glucagon-like peptide 1 (GLP-1) in rodent and human pancreatic β-cells. Mol Endocrinol. 2015;29(9):1243-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huang C, Yuan L, Cao S. Endogenous GLP-1 as a key self-defense molecule against lipotoxicity in pancreatic islets. Int J Mol Med. 2015;36(1):173-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang Z, York NW, Nichols CG, Remedi MS. Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 2014;19(5):872-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yusta B, Baggio LL, Estall JL, et al. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4(5): 391-406. [DOI] [PubMed] [Google Scholar]
- 60. Artunc-Ulkumen B, Pala HG, Pala EE, Yavasoglu A, Yigitturk G, Erbas O. Exenatide improves ovarian and endometrial injury and preserves ovarian reserve in streptozocin induced diabetic rats. Gynecol Endocrinol. 2015;31(3): 196-201. [DOI] [PubMed] [Google Scholar]
- 61. Jensterle M, Janez A, Fliers E, DeVries JH, Vrtacnik-Bokal E, Siegelaar SE. The role of glucagon-like peptide-1 in reproduction: from physiology to therapeutic perspective. Hum Reprod Update. 2019;25(4):504-517. [DOI] [PubMed] [Google Scholar]
- 62. Husain M, Birkenfeld AL, Donsmark M, et al. ; PIONEER 6 Investigators . Oral semaglutide and cardiovascular outcomes in patients with Type 2 diabetes. N Engl J Med. 2019;381(9):841-851. [DOI] [PubMed] [Google Scholar]
- 63. Pujadas G, Drucker DJ. Vascular biology of glucagon receptor superfamily peptides: mechanistic and clinical relevance. Endocr Rev. 2016;37(6):554-583. [DOI] [PubMed] [Google Scholar]
- 64. MacLusky NJ, Cook S, Scrocchi L, et al. Neuroendocrine function and response to stress in mice with complete disruption of glucagon-like peptide-1 receptor signaling. Endocrinology. 2000;141(2):752-762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.