Abstract
Objectives
Diffuse large B-cell lymphoma (DLBCL) is an aggressive non-Hodgkin lymphoma with a heterogenous genetic landscape that can require multiple assays to characterize. We reviewed a 1-step RNA-based assay to determine cell of origin (COO), detect translocations, and identify mutations and to assess the role of the assay in diagnosis.
Methods
Using a single custom Archer FusionPlex Lymphoma panel, we performed anchored multiplex polymerase chain reaction–based RNA sequencing on 41 cases of de novo DLBCL. Each case was subclassified by COO, and gene fusions and hotspot mutations were identified. The findings were then compared with COO classification by the Hans immunohistochemical algorithm and NanoString technology, cytogenetics, and fluorescence in situ hybridization results.
Results
Concordant COO classification by the FusionPlex panel and NanoString was observed in 35 of 41 cases (85.3%), with NanoString and Hans concordant in 33 of 41 cases (80.5%) and FusionPlex and Hans concordant in 33 of 41 cases (80.5%). The FusionPlex assay also detected 6 of 11 BCL6 translocations (4 cryptic), 2 of 3 BCL2 translocations, and 2 of 4 MYC translocations. Mutations were detected in lymphoma-related genes in 24 of 41 cases.
Conclusion
This FusionPlex assay offers a single method for COO classification, mutation detection, and identification of important translocations in DLBCL. Although not replacing traditional testing, it could offer useful data when limited tissue is available
Keywords: Diffuse large B-cell lymphoma, ell of origin, ranslocations, utations, RNA sequencing
Key Points.
• We evaluate an anchored multiplex polymerase chain reaction–based RNA-sequencing assay for classifying de novo diffuse large B-cell lymphoma by cell of origin and detecting important hotspot mutations and translocations.
• We showed that this assay delivers equivalent cell of origin classification to NanoString and the Hans algorithm and successfully identifies translocations and important hotspot mutations.
• Although not replacing traditional testing methods, this assay could play a useful role in certain situations, such as when limited specimens are available for testing.
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma, accounting for approximately 25% to 35% of adult non-Hodgkin lymphoma cases in developed countries.1 DLBCL may affect patients of any age, although it is more common in elderly patients. It is slightly more common in men than in women and does not display significant differences in incidence among ethnicities.
Much work has been done elucidating the genetic landscape of DLBCL. The normal counterpart of the DLBCL cell is postulated to be a B cell that is either located within the germinal center or that has recently exited the germinal center.1,2 Although several distinct clinicopathologic subtypes of DLBCL are recognized, most cases are classified as DLBCL, not otherwise specified (NOS).1 Gene expression profiling has revealed that most cases of DLBCL NOS can be categorized within one of 2 distinct categories of cell of origin (COO): the germinal center B-cell type (GCB) and activated B-cell or post–germinal center B-cell type (ABC).3-5 In addition to the 2 well-described GCB and ABC groups, approximately 10% of cases cannot be definitively assigned a COO category and remain unclassified.
The underlying biology associated with each COO category is highly divergent, as reflected in the differing gene mutations, chromosomal abnormalities, and gene expression patterns.1 For example, mutations in EZH2 are more common in GCB cases, and mutations in CARD11, CD79B, and MYD88 are more common in ABC cases.6-8 The fundamental differences in the underlying biology of the COO subtypes also correlate with different responses to chemotherapy and targeted agents and with differences in overall survival.9-11
In addition to gene expression profiling, alternative test modalities such as NanoString RNA-based technology can achieve accuracy comparable to that of microarray in classification of COO subtype.12 However, because sophisticated molecular testing for DLBCL is not currently widely available, pathologists typically use the Hans immunohistochemical algorithm, which relies on assessing the immunohistochemical staining pattern of tumor cells for CD10, MUM1, and BCL6 to predict COO subtype.13 Although the Hans algorithm is effective and widely utilized, it may deliver imperfect results.14 As such, there is a gap in clinical practice where targeted molecular assays could help to improve the accuracy of classification.12
The Archer FusionPlex Lymphoma panel is an RNA-based assay using anchored multiplex polymerase chain reaction–based enrichment technology to evaluate 125 lymphoma-associated genes for a combination of gene expression and specific point mutations and to use these data to classify DLBCL cases by COO category. In addition, the RNA-based nature of the assay allows for identification of translocations, without prior knowledge of breakpoints or fusion partners, through detection of chimeric mRNA transcripts. We sought to evaluate the utility of a customized FusionPlex Lymphoma panel in predicting COO and identifying common translocations and gene mutations in a series of DLBCL cases. Finally, we further aimed to assess the likely role this panel could play in the diagnostic process of DLBCL, by leveraging simultaneous assessment of these 3 modalities, particularly for triaging specimens, and in circumstances in which limited tissue is available for evaluation.
Materials and Methods
Forty-one cases of de novo DLBCL, NOS, diagnosed between 2010 and 2016 were selected from the archives based on adequate availability of formalin-fixed, paraffin-embedded material and clinical follow-up. Diagnostic biopsies obtained before initiation of therapy and subsequently treated with rituximab-containing therapies were included. Standard H&E-stained sections and immunohistochemical stains for CD20, CD10, BCL6, and MUM1 were performed for each case.
For each case, 5 unstained 5-µm slides were cut from formalin-fixed, paraffin-embedded tissue. These samples then underwent RNA extraction, followed by anchored multiplex polymerase chain reaction–based enrichment RNA sequencing via the Archer FusionPlex Lymphoma platform. Although we did not set minimum specimen requirements, we included a mixture of lymph node resections and smaller biopsies, with a target of obtaining at least 200 ng of RNA, based on our experience with our institution’s clinical next-generation sequencing assays. The smallest tissue sample tested was 6 × 4 mm in cross-sectional area. Each case was also evaluated for translocations involving the BCL2, BCL6, and MYC genes through detection of chimeric mRNA transcripts. The FusionPlex Lymphoma platform evaluated a panel of 125 genes with a combination of gene expression analysis and evaluation for translocations and mutations (see Supplementary Table 1 for a full list of targets; all supplementary material can be found at American Journal of Clinical Pathology online). The panel then classified each case by COO and reported detected translocations and mutations. Mutations were considered significant if they had a variant allele frequency of 10% or more with 10 or more alternative reads. Mutations previously reported in large population databases such as gnomAD were considered single-nucleotide variants and excluded.
Each case underwent orthogonal testing by NanoString technology. The cohort was analyzed using the RUO version of the NanoString Lymphoma Subtyping Test algorithm to determine the Cell-of-Origin molecular subtype of each sample. The algorithm measures the geometric mean of 5 housekeeping genes to ensure RNA quality based on a predefined threshold. Each sample meeting the threshold was reported as GCB subtype, ABC subtype, or unclassified if within an equivocal zone. Sufficient material was available for NanoString testing in each case.
A subset of cases underwent cytogenetic testing at the time of diagnosis. Cytogenetic analysis was performed on metaphases from 2 cultures using G-banding at or below the 450-band resolution level. All cases with diagnostic material available underwent fluorescence in situ hybridization (FISH) analysis for rearrangements in BCL2, BCL6, and/or MYC (BCL2 and BCL6: Kreatech break-apart probes [Leica Biosystems] or Vysis LSI Dual Color, Dual Fusion Translocation Probes [Abbott Laboratories]; MYC: Vysis LSI MYC Dual Color, Break Apart Rearrangement Probe [Abbott Laboratories]).
Results
Patient Demographics
Among the 41 cases of de novo DLBCL, NOS, the male-to-female ratio was 27:14. Patient ages at diagnosis ranged from 21 to 86 years (median, 61 years). See Table 1 for demographic data by case.
Table 1.
List of Cases Examined, Comparing Cell of Origin Classification by Different Methods, and Reporting Mutations Detected by FusionPlex Lymphoma Panel by Casea
| Case | Age (y)/Sex | FPL | Hans | NS | Gene | Mutation | VAF, % |
|---|---|---|---|---|---|---|---|
| 1 | 59 F | ABC | ABC | ABC | |||
| 2 | 57 M | GCB | GCB | GCB | |||
| 3 | 52 M | ABC | ABC | ABC | MYC | p.Gln52del | 10.5 |
| 4 | 69 M | ABC | ABC | ABC | |||
| 5 | 86 F | GCB | GCB | GCB | TNFRSF13B | p.Cys104Tyr | 75.0 |
| BRAF | p.Lys601Glu | 48.0 | |||||
| 6 | 60 M | ABC | ABC | ABC | MYD88 | p.Val217Phe | 30.1 |
| 7 | 53 M | GCB | GCB | GCB | BLNK | p.Glu60Lys | 38.1 |
| 8 | 75 M | GCB | GCB | GCB | |||
| 9 | 76 F | ABC | ABC | ABC | TCF3 | p.Gly362Asp | 59.5 |
| ALK | p.Thr1026Pro | 22.0 | |||||
| 10 | 52 F | ABC | ABC | ABC | XPO1 | p.Glu571Lys | 31.8 |
| 11 | 31 M | GCB | GCB | GCB | MYC | p.Pro233Thr | 89.7 |
| 12 | 83 M | GCB | GCB | GCB | |||
| 13 | 34 M | GCB | GCB | GCB | |||
| 14 | 44 F | ABC | ABC | ABC | KRAS | p.Gly12Cys | 23.1 |
| AICDA | p.Arg25His | 29.1 | |||||
| 15 | 39 F | GCB | GCB | GCB | |||
| 16 | 43 M | GCB | GCB | GCB | MYC | p.Asp2His | 51.1 |
| SYNE1 | p.Leu3057Val | 52.5 | |||||
| 17 | 60 M | ABC | ABC | ABC | |||
| 18 | 27 F | GCB | GCB | GCB | XPO1 | p.Glu571Lys | 36.0 |
| 19 | 62 M | ABC | ABC | ABC | MUC1 | p.Arg99Cys | 49.1 |
| 20 | 74 F | GCB | GCB | GCB | BLNK | p.Gly30Arg | 48.2 |
| 21 | 39 M | GCB | GCB | GCB | |||
| 22 | 62 M | GCB | GCB | GCB | PIM1 | p.Glu170Asp | 41.5 |
| ALK | p.Thr1026Pro | 37.1 | |||||
| 23 | 84 F | GCB | GCB | GCB | |||
| 24 | 61 M | ABC | ABC | ABC | BCL3 | p.Ala68Val | 48.8 |
| 25 | 70 M | GCB | GCB | GCB | BCL2 | p.Pro233Thr | 89.7 |
| 26 | 71 M | ABC | ABC | ABC | PIM1 | p.Ala113Thr | 74.2 |
| PIM1 | p.Gly141Asp | 73.8 | |||||
| PIM1 | p.Gly119Asp | 47.8 | |||||
| PIM1 | p.Asn269Ser | 18.5 | |||||
| MYD88 | p.Leu265Pro | 33.8 | |||||
| 27 | 61 F | GCB | GCB | GCB | PDCD1LG2 | p.Glu11AsnfsTer7 | 66.7 |
| 28 | 35 M | GCB | ABC | GCB | |||
| 29 | 65 F | GCB | ABC | GCB | ALK | p.Thr1026Pro | 28.6 |
| 30 | 74 M | GCB | ABC | GCB | ALK | p.Thr1026Pro | 25.8 |
| 31 | 72 F | UNC | ABC | UNC | TCF3 | p.Gly362Asp | 57.4 |
| KMT2A | p.Leu3614Pro | 52.9 | |||||
| 32 | 62 M | GCB | ABC | GCB | BCL2 | p.Ser87Arg | 25.2 |
| 33 | 21 M | GCB | ABC | GCB | |||
| 34 | 66 M | UNC | ABC | UNC | |||
| 35 | 84 F | GCB | ABC | ABC | |||
| 36 | 42 M | UNC | ABC | ABC | |||
| 37 | 31 M | GCB | ABC | ABC | |||
| 38 | 71 M | ABC | ABC | GCB | |||
| 39 | 59 M | GCB | ABC | ABC | ETV6 | p.Lys11Asn | 34.8 |
| MYD88 | p.Leu265Pro | 51.9 | |||||
| ALK1 | p.Thr1026Pro | 53.3 | |||||
| 40 | 66 M | GCB | GCB | UNC | BMP7 | p.Pro106Ala | 92.9 |
| PIM1 | p.Val90Leu | 51.8 | |||||
| PIM1 | p.Val90Ile | 41.0 | |||||
| 41 | 83 F | ABC | ABC | GCB | LRMP | p.Ile102Val | 46.6 |
ABC, activated B-cell subtype; FPL, Archer FusionPlex Lymphoma assay; GCB, germinal center B-cell subtype; NS, NanoString; UNC, unclassified; VAF, variant allele fraction.
aFor the purposes of comparison between methods, cases classified as ABC and UNC categories by the FusionPlex Lymphoma panel and/or NS were considered non-GCB.
COO Classification
Each case was evaluated with immunohistochemical stains for CD20, CD10, BCL6, and MUM1. COO assignments for each case were then determined based on immunohistochemical stains in accordance with the Hans algorithm.13 By this method, 17 of 41 cases (41.4%) were GCB subtype, and 24 of 41 cases (58.5%) were non-GBC subtype.
Each case then underwent testing by the FusionPlex Lymphoma assay. The FusionPlex assay classified 25 of 41 cases (61.0%) as GCB subtype and 13 of 41 (31.7%) as ABC subtype. Three of 41 cases (7.3%) were unclassified. Finally, each case was subsequently analyzed by NanoString technology, which classified 23 of 41 cases (56.1%) as GCB subtype and 15 of 41 (36.6%) as ABC subtype, with 3 of 41 (7.3%) unclassified (see Table 1 for case-by-case breakdown of COO calls by method).
Comparison of COO Calls by Method
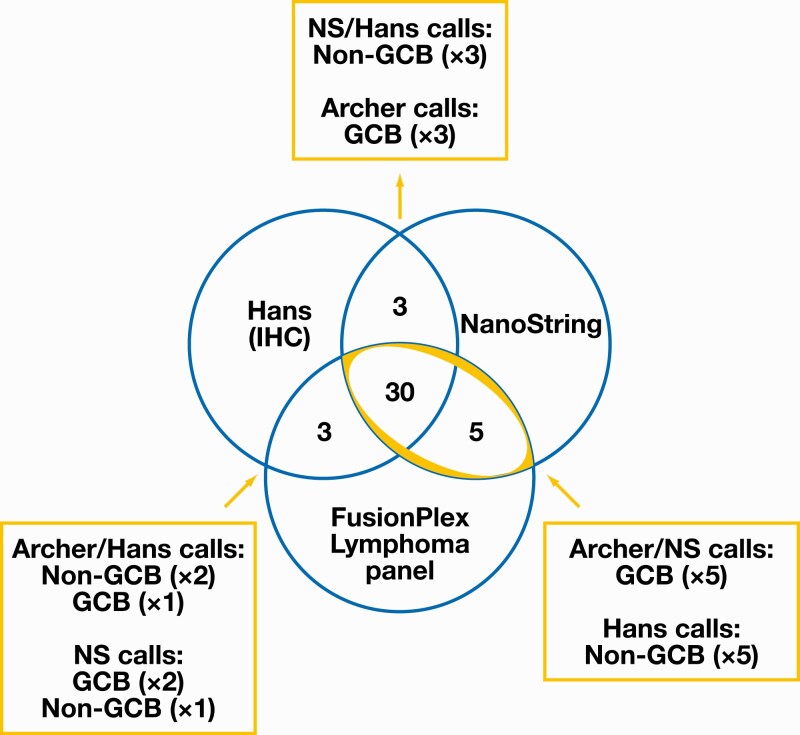
When assessing the concordance of COO calls between methods, cases assigned as ABC or unclassified by the FusionPlex and NanoString methods were categorized as non-GCB to allow for comparison with the Hans method. Of the 41 examined cases, 30 were fully concordant by all 3 methods (73.2%), of which 16 (53.3%) were classified as GCB type and 14 (46.7%) were classified as non-GCB type.
In 11 of 41 cases (26.8%), there was discordance between at least 2 of the 3 methods Figure 1. Of these 11 cases, NanoString and FusionPlex were concordant in 5 cases (all GCB) that were classified as non-GCB by the Hans algorithm. In the remaining 6 discordant cases, which were characterized by discordance between NanoString and FusionPlex, the FusionPlex assay was concordant with Hans in 3 cases and discordant with both Hans and NanoString in the remaining 3 cases.
Figure 1.
Distribution of concordant and discordant cases, with further detail on classification of discordant calls by method. Non-GCB includes cases classified as activated B-cell subtype and unclassified subtype by NS and/or FusionPlex methods. Highlighted cases demonstrated concordance between FusionPlex Lymphoma panel and NS. ABC, activated B-cell type (equivalent to non-GCB designation by Hans); GCB, germinal center B-cell subtype; IHC, immunohistochemistry; NS, NanoString.
In total, the FusionPlex Lymphoma panel was concordant with the NanoString gold standard in 35 of 41 cases, corresponding to a concordance of 85.3% (95% CI, 71%-94%). By comparison, the Hans algorithm was concordant with NanoString in 33 of 41 cases, representing concordance of 80.5% (95% CI, 65%-91%) (Supplementary Table 2). The FusionPlex panel was concordant with the Hans algorithm in 33 of 41 cases, for concordance of 80.5% (95% CI, 65%-91%).
FusionPlex Detection of Mutations
The FusionPlex Lymphoma assay evaluated 35 genes for hotspot mutations Table 1. After applying the restriction criteria previously described (in the Materials and Methods), a total of 38 mutations were identified across 24 of 41 cases (58.5%). No reportable mutations were detected in the remaining 17 cases. Fifteen cases had 1 mutation detected, 6 cases had 2 mutations detected, 2 cases had 3 mutations detected, and 1 case had 5 mutations detected (overall median: 1 mutation per case). See Table 1 for a full list of mutations identified.
Some recurrent DLBCL mutations are not evenly distributed across COO categories and occur more frequently in either ABC or GCB DLBCL. On review of the mutations called by FusionPlex, 3 cases (cases 6, 26, and 39) were found to have MYD88 mutations, which occur mostly in ABC DLBCL. Of these 3 cases, 2 (cases 6 and 26) were classified as ABC/non-GBC type by all 3 assays, and the remaining case (case 39) was classified as ABC/non-GCB type by both NanoString and Hans algorithm. One case (case 5) was found to have a BRAF mutation, which occurs mostly in GCB DLBCL. This case was classified as GCB type by all 3 assays.
FusionPlex Detection of BCL2, BCL6, and MYC Gene Rearrangements
Our analysis of gene fusions detected by the FusionPlex Lymphoma panel focused on rearrangements involving BCL2, BCL6, and MYC, which have significant implications for disease classification and patient management. The FusionPlex assay identified 2 cases with BCL2 rearrangements (4.9%), 6 cases with BCL6 rearrangements (14.6%), and 2 cases with MYC rearrangement (4.9%). See Table 2 for a list of BCL2, BCL6, and MYC rearrangements detected, including partner genes.
Table 2.
Overview of BCL2, BCL6, and MYC Translocations Detected by Various Methods
| Case | Gene | Partner Gene | COO (FPL) | Detected by FPL | Detected by CG | Detected by FISH |
|---|---|---|---|---|---|---|
| 1 | BCL6 | IGH | ABC | N | Y | Y |
| 2 | BCL6 | UD | GCB | N | Y | — |
| 3Aa | BCL6 | IGH | ABC | Y | N | Y |
| 3B | BCL2 | UD | ABC | N | Y | — |
| 4a | BCL6 | IGH | ABC | Y | N | Y |
| 6 | MYC | IGH | ABC | Y | Y | — |
| 10 | BCL6 | IGH | ABC | Y | — | Y |
| 11a | MYC | IGH | GCB | Y | N | Y |
| 14 | BCL6 | IGH | ABC | Y | N | N |
| 16 | MYC | UD | GCB | N | Y | Y |
| 17A | BCL6 | IGH | ABC | Y | Y | Y |
| 17B | MYC | UD | ABC | N | Y | Y |
| 28 | BCL2 | IGH | GCB | Y | — | Y |
| 30A | BCL2 | IGH | GCB | Y | Y | — |
| 30B | BCL6 | UD | GCB | N | Y | — |
| 32 | BCL6 | UD | GCB | N | Y | Y |
| 34a | BCL6 | RCC1 | UNC | Y | N | Y |
| 37 | BCL6 | UD | GCB | N | Y | Y |
ABC, activated B-cell subtype; CG, cytogenetics; COO, cell of origin (by FusionPlex Lymphoma assay); FISH, fluorescence in situ hybridization; FPL, FusionPlex Lymphoma assay; GCB, germinal center B-cell subtype; N, no; UD, undetermined; UNC, unclassified; Y, yes; —, test not performed.
aTranslocations detected by FPL and missed by CG.
Correlation of BCL2, BCL6, and MYC Rearrangement Calls With Cytogenetics and/or FISH
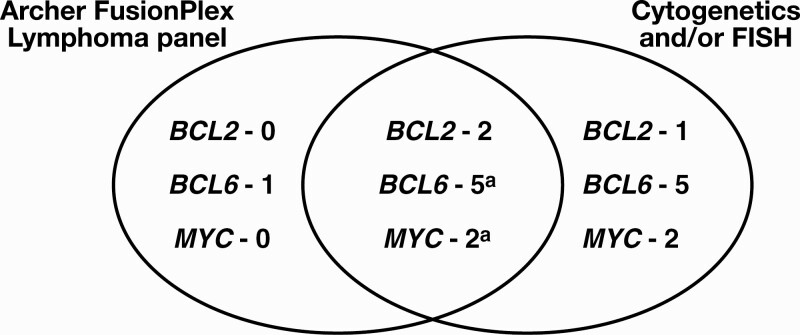
If available, translocations identified by cytogenetics and FISH were compared with fusions identified by FusionPlex. Cytogenetic testing was performed at diagnosis in 28 of 41 cases, with karyotypes successfully obtained for 27 of these 28 cases. FISH was also performed in all cases for which material was available (23/41 cases). Together, cytogenetics and FISH identified the following gene rearrangements: BCL6 (11/41 cases), BCL2 (3/41 cases), and MYC (4/41 cases). There was concordance on 4 of the 5 BCL6 rearrangements and 2 of the 2 MYC rearrangements identified in cases for which both assays were performed.
The FusionPlex Lymphoma panel detected 6 of 11 BCL6 translocations, 2 of 3 BCL2 translocations, and 2 of 4 MYC translocations detected by cytogenetics and/or FISH (Table 2, Figure 2). The FusionPlex Lymphoma panel identified additional BCL6 rearrangements not identified by cytogenetics in 3 cases (cases 3, 4, and 34), an additional MYC rearrangement not identified by cytogenetics in 1 case (case 11), and an additional BCL6 rearrangement not identified by either cytogenetics or FISH in 1 case (case 14). The sequencing data for this particular BCL6 rearrangement (BCL6-RCC1) was reviewed and demonstrated an in-frame fusion with multiple reads across the breakpoint by at least 2 independent primers, mapping to exons 1 and 2 of RCC1. Furthermore, this translocation has not been previously detected in other cases run on this platform, suggesting that the likelihood of cross-contamination is minimal. These findings are highly supportive of a true BCL6 translocation.
Figure 2.
Comparison of number of BCL2, BCL6, and MYC rearrangements detected by the FusionPlex Lymphoma panel and by traditional methods (cytogenetics and/or FISH). aThree of 5 BCL6 translocations and 1 of 2 MYC translocations were missed by cytogenetic analysis and were identified using the FusionPlex Lymphoma panel and FISH only. See Table 2 for further details. FISH, fluorescence in situ hybridization.
Discussion
DLBCL is a clinically and biologically heterogenous disease. Correct classification of disease by COO and identification of important translocations are crucial for understanding the biology and prognosis of a patient’s disease. Furthermore, detection of driver mutations is likely to play an increasingly critical role in the era of targeted therapies.15,16 However, in today’s clinical practice, multiple distinct tests and steps are necessary to acquire these data, and those increase complexity, costs, turnaround time, and specimen requirements.
In this study, we evaluated the utility of a single, custom, RNA-based sequencing assay for simultaneously classifying COO; identifying translocations in BCL2, BCL6, and MYC; and identifying mutations in certain genes using RNA extracted from formalin-fixed, paraffin-embedded tissue (5 slides cut at 5-µm thickness, ideally capable of yielding at least 200 ng of RNA). This reduces by about half the amount of material required to perform these assays independently (3 sections for COO classification by Hans, 3 sections needed for FISH, and a further 5 sections required for mutational analysis). We sought to evaluate not just the performance of the assay against standard testing modalities but also what role it could play in the diagnostic process.
Using NanoString as the gold standard, we showed that FusionPlex is at least equally accurate to the widely used Hans immunohistochemical algorithm at predicting COO. FusionPlex can also identify a subset of clinically relevant translocations, such as those involving BCL2, BCL6, and MYC, which may lead to a diagnosis of double-hit or triple-hit lymphoma, requiring significantly different patient management.17 Because this technology is driven by detection of chimeric mRNA transcripts, it can detect rearrangements involving immunoglobulin genes in cases in which a chimeric transcript is produced, although rearrangements that do not lead to a chimeric transcript (eg, translocations involving uncovered regions of the 5′ untranslated region or upstream of the 5′ untranslated region) are not detected by the assay. Of note, a subset of the translocations detected by the assay in this cohort had not been detected previously by cytogenetic analysis, as confirmed by subsequent FISH testing. Interestingly, case 34, unclassified by both FPL and NanoString, demonstrated a BCL6 rearrangement, suggesting that this case may belong to a recently described genetic subgroup of DLBCL distinct from the common ABC and GCB subgroups. Cases in this subgroup are enriched for BCL6 rearrangements and frequently have an unclassified gene expression profile.5,10
Finally, the FusionPlex assay can also be used to detect mutations that may be important for prognosis in DLBCL. The overall prevalence of mutations detected in this cohort by the FusionPlex assay is broadly in line with those identified in recent studies of large DLBCL populations (see Supplementary Table 3). One limitation of the currently reported assay is that a limited number of mutational hotspots were included. Certain genes were not included in the initial target panel due to limitations of coverage by RNA-based methods. For example, TP53 was excluded because the lack of mutational hotspots in TP53 (as in many other tumor-suppressor genes) means that the entire coding sequence would need to be evaluated by the panel to be of meaningful value. Losses of TP53 are also common and can be detected only by DNA-based methods. We plan to expand the panel of gene targets in subsequent versions of the assay to allow for detection of a more comprehensive panel of mutations that may help in classification, prognostication, and response and potential resistance to novel targeted therapies.
We believe that the limitations described suggest that this assay should not replace traditional testing methods; however, compensating advantages point to a different role for the FusionPlex assay in the diagnostic process. The assay’s main strength is that it allows several diagnostic analyses to be performed simultaneously on the same material and offers a significantly simplified workflow, resulting in decreased testing burden on laboratories. In addition to delivering similar COO classification to immunohistochemistry, this assay can detect important translocations that may be missed by routine cytogenetic analysis and can identify mutations with very reasonable accuracy.
Although we do not advocate that this assay be a replacement for traditional cytogenetic analysis or next-generation sequencing, we believe that it could act as a useful complement to or substitute for current methodologies in certain situations, such as cases in which limited tissue is available for ancillary studies.
Supplementary Material
This study was supported by funding to Dr Louissaint from an anonymous donor.
References
- 1. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shaffer AL III, Young RM, Staudt LM. Pathogenesis of human B cell lymphomas. Annu Rev Immunol. 2012;30:565-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503-511. [DOI] [PubMed] [Google Scholar]
- 4. Lenz G, Wright G, Dave SS, et al. Lymphoma/Leukemia Molecular Profiling Project . Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morin RD, Mungall K, Pleasance E, et al. Mutational and structural analysis of diffuse large B-cell lymphoma using whole-genome sequencing. Blood. 2013;122:1256-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378:1396-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morin RD, Johnson NA, Severson TM, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010;42:181-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rutherford SC, Leonard JP. DLBCL cell of origin: what role should it play in care today? Oncology (Williston Park). 2018;32:445-449. [PubMed] [Google Scholar]
- 10. Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24:679-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenwald A, Wright G, Chan WC, et al. Lymphoma/Leukemia Molecular Profiling Project . The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937-1947. [DOI] [PubMed] [Google Scholar]
- 12. Scott DW, Wright GW, Williams PM, et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood. 2014;123:1214-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275-282. [DOI] [PubMed] [Google Scholar]
- 14. Choi WW, Weisenburger DD, Greiner TC, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009;15:5494-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brach D, Johnston-Blackwell D, Drew A, et al. EZH2 inhibition by tazemetostat results in altered dependency on B-cell activation signaling in DLBCL. Mol Cancer Ther. 2017;16:2586-2597. [DOI] [PubMed] [Google Scholar]
- 17. Rosenthal A, Younes A. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev. 2017;31:37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.