Abstract
Background
Candida pelliculosa is an ecological fungal species that can cause infections in immunocompromised individuals. Numerous studies globally have shown that C. pelliculosa infects neonates. An outbreak recently occurred in our neonatal intensive care unit; therefore, we aimed to evaluate the risk factors in this hospital-acquired fungal infection.
Methods
We performed a case-control study, analysing the potential risk factors for neonatal infections of C. pelliculosa so that infection prevention and control could be implemented in our units. Isolated strains were tested for drug resistance and biofilm formation, important factors for fungal transmission that give rise to hospital-acquired infections.
Results
The use of three or more broad-spectrum antimicrobials or long hospital stays were associated with higher likelihoods of infection with C. pelliculosa. The fungus was not identified on the hands of healthcare workers or in the environment. All fungal isolates were susceptible to anti-fungal medications, and after anti-fungal treatment, all infected patients recovered. Strict infection prevention and control procedures efficiently suppressed infection transmission. Intact adhesin-encoding genes, shown by genome analysis, indicated possible routes for fungal transmission.
Conclusions
The use of three or more broad-spectrum antimicrobials or a lengthy hospital stay is theoretically associated with the risk of infection with C. pelliculosa. Strains that we isolated are susceptible to anti-fungal medications, and these were eliminated by treating all patients with an antifungal. Transmission is likely via adhesion to the cell surface and biofilm formation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-021-06295-1.
Keywords: Candida pelliculosa, Fungemia, Antifungal drugs, Infection control, Biofilm
Background
Candida pelliculosa, also known as Pichia anomala or Hansenula anomala, is an environmental fungal species frequently isolated from soil, plants, fruits, or organic compounds [1–3]. It is considered a rare fungal pathogen compared with other fungal species such as Candida albicans and Candida parapsilosis. Clinical analyses reveal that infants, children, and individuals with compromised immune systems, including those from hematologic units, surgical ICUs, and neonatal intensive care units, are more susceptible to bloodstream infections of C. pelliculosa that often lead to high morbidity and mortality [4–8]. A recent study found a 41.2% mortality rate in a pediatric ICU [9].
Since the first identified case of infection in the 1950s [8], few outbreaks of fungemia from C. pelliculosa have been reported worldwide [10]. To adhere to and transmit between patients, Candida species have evolved into sophisticated machineries. For instance, C. albicans is capable of forming a biofilm structure by adhering to solid non-biological surfaces such as medical devices. C. parapsilosis is frequently found on the hands of healthcare workers. Studies have extensively shown that the expression of adhesion proteins, along with morphological transitions, govern the colonization of Candida species [11–17]. The biofilm formed by C. albicans has been shown to occur in several stages, including adherence to surfaces followed by hyphal structure formation and the production of an extracellular polysaccharide matrix layer. To initiate the adherence step, C. albicans ubiquitously expresses an adhesion molecule known as agglutinin-like sequence 3 (Als3) on the cell surface; this plays an essential role in biofilm formation. The biofilm structure plays crucial roles in fungal adhesion and drug resistance. One study demonstrated that the C. pelliculosa biofilm consists primarily of yeast-forming cells [18]; however, very little is known about the molecular nature of its biofilm formation process.
In this work, we report our investigation of an epidemiological outbreak of 21 cases of neonatal fungemia caused by C. pelliculosa in the neonatal intensive care unit of a tertiary hospital in northeast China. We systematically analyzed risk factors for C. pelliculosa infection, and we phenotypically characterized isolates recovered from the bloodstream. We further analyzed the presence of important genes encoding adhesion proteins, showing that C. pelliculosa is capable of forming a biofilm structure on medical devices.
Materials and methods
Hospital
Shengjing Hospital of China Medical University is a 6700-bed teaching hospital located in Shenyang in northeast China. It has three neonatal wards in which 140 beds are priority-managed, and tertiary care for patients younger than 28 days of age is delivered. They are open wards with high-frequency doctor and nurse rotations and constant interactions between members of the medical staff. From October 21 to December 142,017, C. pelliculosa strains were isolated from blood sampled from 21 newborns on the first and second neonatal wards.
Clinical characteristics and risk factor analysis
Using these infected newborns, a case-control study was performed to identify the risk factors for C. pelliculosa fungemia, enrolling all neonates without fungemia who were born from November 3 to 262,017 as controls. Information was collected from both the treatment and control groups. Data included sex, age at onset of infection (same as hospital day at onset of candidemia), ward, birth weight, gestational age, age of the mother, premature-birth-related problems, delivery type, Apgar scores (at 1 and 5 min), glucose at admission, and length of hospital stay. Also noted were whether any of the following applied: peripherally inserted central catheter (PICC), endotracheal tube, tracheostomy, bladder catheter, umbilical catheter, parenteral nutrition (lipid solution), surgical procedure, broad-spectrum antibiotic use (BSAU), previous or concomitant bacteremic infection (PCBI), three or more broad-spectrum antimicrobials, or a nasogastric tube.
The date of candidemia onset, hospital day (time since admission) of candidemia onset, results of a catheter tip culture, antifungal drug used, and patient outcome were also collected for the study group to reflect clinical characteristics. In addition to the growth of fungi in the blood, the diagnosis of fungal infection was established based on the presence of the following findings: temperature instability (hypothermia, hyperthermia), respiratory issues (grunting, intercostal-subcostal retractions, apnea, tachypnea, cyanosis), cardiovascular disorders (bradycardia, tachycardia, poor perfusion, hypotension), neurological problems (hypotonia, lethargy, seizures), gastrointestinal problems (feeding intolerance, abdominal distension), and laboratory findings, which may indicate an infection, including leukopenia (leukocyte count < 5000/mm3), leukocytosis (leukocyte count > 22,000/mm3), immature to total neutrophil ratio < 0.2, C-reaction protein (> 8 mg/L), and procalcitonin (> 0.5 ng/mL) [19].
Outbreak investigation and infection prevention and control
To identify the origin of C. pelliculosa in our two neonatal departments, epidemiological investigations were performed from November 25 to 30, 2017. Environmental samples were obtained from air conditioners, sinks, refrigerators, tables, patient beds, incubators, trolleys, and respiratory care equipment. Samples from the hands of doctors and nurses were obtained at the same time, resulting in 143 swabs collected for fungus-culturing tests. Outbreak investigations were accompanied by strict infection prevention and control in all wards, and these measures were continued after completion of the investigations. Infected patients were isolated, and uninfected patients were given preventive treatments of fluconazole. Environmental disinfection procedures were carried out three times a day using gamma wipes (Clinell Universal Wipes), air purifiers, and oxyacetic acid fumigation. Furthermore, hand hygiene was practiced before and after each patient contact and before donning or doffing personal protection equipment, and sterilization and daily disinfection of personal protection equipment were employed. Only three cases occurred after these measures were established, all in the first ward and in 2018. No cases occurred in 2019.
Identification and antifungal susceptibility testing of strains
In vitro cell growth and a mass spectrometer (VITEK MS; bioMerieux, Inc., France) were used to identify cultures isolated from blood samples. If C. pelliculosa was identified, an antimicrobial susceptibility test was performed using Fungus 3 (bioMerieux, Inc., France). C. pelliculosa drug resistance profiles was performed using the guidelines of the Clinical and Laboratory Standards Institute. Fluconazole, voriconazole, itraconazole, amphotericin B, and flucytosine were selected as antifungal drugs. Candida parapsilosis ATCC 22019, Candida krusei ATCC 6258, and C. albicans ATCC90028 were selected as reference strains. Fluconazole susceptibility (susceptible ≤2, resistant > 4 for non-species related breakpoints for Candida) was determined using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoint [20].
Pathogenicity investigation
Hyphae formation ability
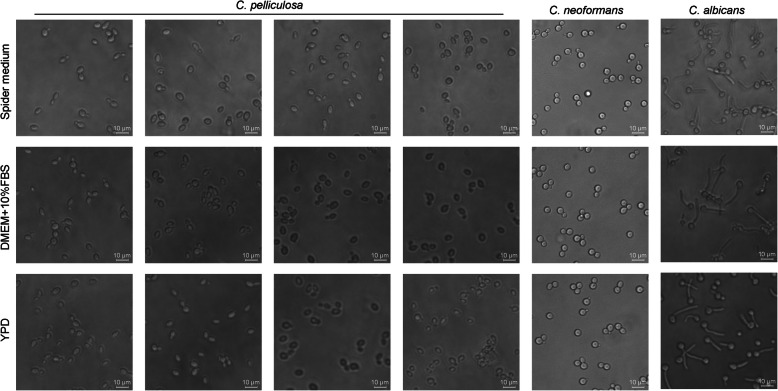
The clinical isolates of C. pelliculosa and the reference strains C. albicans SC5314 and Cryptococcus neoformans H99 were grown in either Spider medium (1% nutrient broth, 1% mannitol, and 0.2% K2PO4) [11, 12], yeast extract peptone dextrose medium (1% yeast extract, 2% peptone, and 2% dextrose), or Dulbecco’s modified Eagle’s medium (DMEM; supplemented with 10% fetal bovine serum) at 37 °C. After 1 h incubation, fungal cell morphology was examined using a Leica DMI3000 B scanning microscope. Cells with filamentous morphologies are hyphal cells, and those with budding or round morphologies are yeast cells [21].
Biofilm formation ability
Biofilm formation assays were performed as described elsewhere [17, 22]. Fungal cells were grown at 30 °C in an orbital shaker in liquid yeast extract peptone dextrose medium for 16 h. The fungal cells were washed twice with equivalent volumes of phosphate-buffered saline (PBS; 0.15 M NaCl and 0.03 M phosphate [pH 7.2]) and then diluted to 100 μL with 0.02 of Optical Density (Absorbance = 600 nm) in Spider medium and placed in each well of a 96-well plate. The plates were placed on a rotor and agitated at 100 rpm and 37 °C for 2 h (initial adhesion phase). The supernatant cells (planktonic and non-adherent cells) were then removed, and the adherent cells were washed three times with 200 μL PBS. The wells were then filled with 200 μL fresh Spider medium and incubated at 37 °C. After incubating for 48 h, the plates were washed three times with 200 μL PBS. Fungal biofilms were stained by adding 100 μL 0.2% aqueous crystal violet, and the plates were then incubated at room temperature for 15 min and de-stained with 100 μL glacial acetic acid. The adherent cells were analyzed by measuring the crystal violet solution spectrophotometrically at 570 nm [23]. For each clinical isolate of C. pelliculosa, five biological replicates were performed. For each biological replicate, five technical replicates were performed.
Als sequence analysis
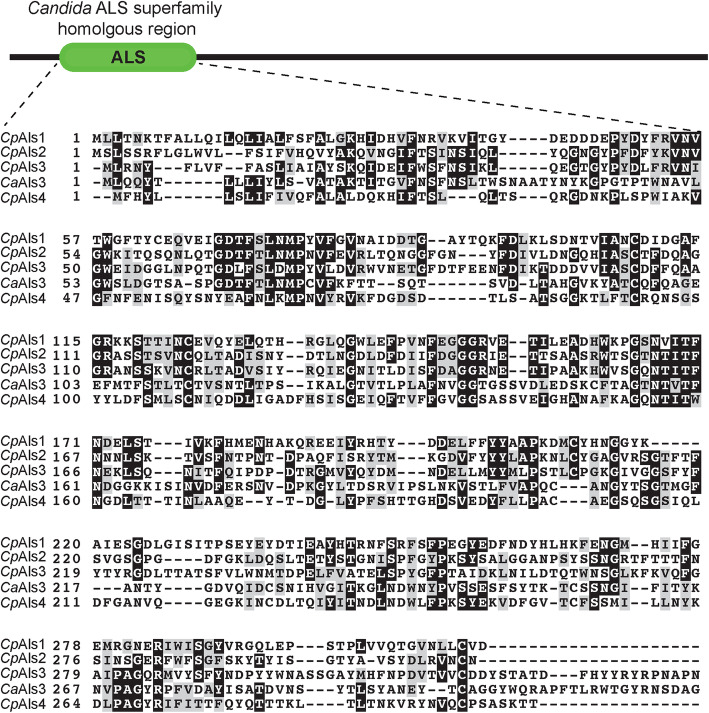
The biofilm-related proteins (Als proteins) were identified using protein BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastp&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome), applying the C. albicans Als3 protein as the query sequence. They were further determined using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) for multiple sequence alignment analyses to the C. pelliculosa genome. The homologous region was highlighted using BoxShade (https://embnet.vital-it.ch/software/BOX_form.html), and a phylogenetic tree was constructed as described elsewhere [24].
Total RNA isolation and qRT-PCR
To determine adhesin gene expression, total RNA samples were isolated from C. pelliculosa clinical strains followed by DNase treatment to eliminate genomic DNA. This was verified using RT-PCR. One microgram of total RNA was used for each strain, then cDNA was synthesized using SuperScript™ III First-Strand Synthesis System kit (ThermoFisher) according to manufacturer instructions and as described elsewhere [15]. Quantitative PCR was performed as described elsewhere [15], and the expressions of the adhesin gene was measured using oligo pairs CpAls4F (gttcagttgttgtctcctcagaa) and CpAls4R (ctctggagttgatgggtattgag), and CaAls3F (atcgcctatcatttcttctagtgct) and CaAls3R (gggtatcagaattggattgcga). ACT1 were measured using oligo pair ActF (tgctgaacgtatgcaaaagg) and ActR (atccacatttgttggaaagt). Relative expression was calculated using the 2-ΔΔCt method. Standard deviations were calculated using three replicates.
Ethics approval and consent to participate
All information was anonymized before being made available for research. This study was approved by the Shengjing Hospital of China Medical University Ethics Committee (Approval No. 201PS049K). All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was documented from parents and/or legal guardians.
Statistical analysis
Statistical analysis was performed using the SPSS 22.0 software package. The χ2 test or Fisher’s exact probability method was used for comparing count data between groups. Normally distributed data were represented using the mean (± standard deviation; []) or the median (interquartile range). Groups were compared using the t test or the Wilcoxon rank sum test. Multivariate analyses of the risk factors for fungemia were performed using unconditional logistic regression, finding statistical significance when P<0.05.
Results
Clinical characteristics of the patients
Twenty-one infected patients experienced fungemia onset from October 21 to December 14, 2017. Ten were from the second neonatal ward (accounting for 67% of the patients infected in that time frame). The hospital day at fungemia onset ranged from 10 to 42 (Table S1). All patients received interventional procedures: either a PICC, endotracheal tube, nasogastric tube, tracheostomy (without tracheal cut), bladder catheter, or umbilical catheter (Table 1). The catheter tip culture tests from 7 patients were positive for C. pelliculosa infection (accounting for 33% of the study group), and the results were consistent with the blood culture tests. Eighteen of the 21 neonates (86%) were treated with broad-spectrum antibiotics (piperacillin/tazobactam, cefoperazone/sulbactam, or meropenem) for documented or suspected bacterial infections prior to fungemia diagnoses. A large majority (20/21, 95%) were treated with fluconazole, and the other was treated with voriconazole (Table S1).
Table 1.
Univariate analyses of factors associated with C. pelliculosa fungemia
| Factor | Fungemia | No fungemia | χ2/t/Z | P |
|---|---|---|---|---|
| Sex | 0.015 | 0.904 | ||
| Male | 13 | 81 | ||
| Female | 8 | 47 | ||
| Age admitted in hospital (day) | 1(1,1) | 1(1,1) | 0.741 | 0.642 |
| Ward | 1.014 | 0.314 | ||
| Neonatal ward 1 | 9 | 70 | ||
| Neonatal ward 2 | 12 | 58 | ||
| Age of the mother (year) | 9.826 | 0.007 | ||
| ≥ 35 | 13 | 39 | ||
| 30–34 | 7 | 49 | ||
| ≤ 29 | 1 | 40 | ||
| Gestational age (WK) | 28.837 | < 0.001 | ||
| ≥ 36 | 0 | 27 | ||
| 30–35 | 6 | 83 | ||
| ≤ 29 | 15 | 18 | ||
| PBRP | 1.961 | 0.744 | ||
| PRM | 3 | 31 | ||
| PHSP | 7 | 35 | ||
| Gestational diabetes | 0 | 3 | ||
| Twins preterm birth | 3 | 11 | ||
| Other | 8 | 48 | ||
| Type of delivery | 0.121 | 0.728 | ||
| Cesarean | 15 | 96 | ||
| Natural labor | 6 | 32 | ||
| Birth weight | 35.849 | < 0.001 | ||
| ELBW (≤1000 g) | 8 | 4 | ||
| VLBW (> 1000 g, ≤1500 g) | 11 | 32 | ||
| LBW (> 1500, ≤2500 g) | 1 | 60 | ||
| NBW (> 2500) | 1 | 32 | ||
| Apgar (1 min) | 27.137 | < 0.001 | ||
| ≥ 7 | 6 | 105 | ||
| < 7 | 15 | 23 | ||
| Apgar (5 min) | 0.000 | 1.000 | ||
| ≥ 7 | 21 | 125 | ||
| < 7 | 0 | 3 | ||
| Glucose at admitted (mmol/l) | 4.076 ± 1.685 | 3.681 ± 1.175 | −1.567 | 0.119 |
| PICC | 71.406 | < 0.001 | ||
| No | 0 | 111 | ||
| Yes | 21 | 17 | ||
| Endotracheal tube | 22.252 | < 0.001 | ||
| No | 0 | 71 | ||
| Yes | 21 | 57 | ||
| Nasogastric tube | 1.781 | 0.182 | ||
| No | 0 | 16 | ||
| Yes | 21 | 112 | ||
| Parenteral nutrition (lipid solution) | 8.667 | 0.003 | ||
| No | 0 | 39 | ||
| Yes | 21 | 89 | ||
| PCBI | 1.454 | 0.228 | ||
| No | 20 | 105 | ||
| Yes | 1 | 23 | ||
| BSAU | 17.421 | < 0.001 | ||
| No | 0 | 62 | ||
| Yes | 21 | 66 | ||
| TBSA | 4.582 | 0.032 | ||
| No | 15 | 116 | ||
| Yes | 6 | 12 | ||
| Surgical procedure | 0.000 | 1.000 | ||
| No | 20 | 121 | ||
| Yes | 1 | 7 | ||
| Time hospitalization | 59.790 ± 21.423 | 25.190 ± 16.185 | −10.146 | < 0.001 |
PBRP premature birth related problems, PRM Premature rupture of membranes, PHSP pregnancy-induced hypertension syndrome or preeclampsia, ELBW extremely low birth weight, VLBW very low birth weight, LBW low birth weight, NBW normal birth weight, PICC peripherally inserted central catheter, PCBI previous or concomitant bacteremic infection, BSAU broad-spectrum antibiotics use before fungemia, TBSA three or more broad-spectrum antimicrobials
In 76% (16/21) of the study group, white blood counts were significantly below the reference range, and in 14% (3/21), they were above the reference range. Similarly, in 71% (15/21), values for C-reactive protein were above normal levels. Finally, 8 of the 9 patients (89%) tested for procalcitonin showed abnormally high values (greater than 0.5 ng/mL; Table S1).
Risk factor analysis
To analyze potential risk factors for C. pelliculosa infection and transmission in our neonatal wards, patient characteristics were compared between the study and control groups. As shown in Table 1, univariate analysis demonstrated that 10 factors were significant: age of the mother, gestational age, birth weight, 1-min Apgar score, use of a PICC, use of an endotracheal tube, use of parenteral nutrition (lipid solution), use of a broad-spectrum antibiotic, use of three or more broad-spectrum antimicrobials, and hospitalization time (Table 1). Further analysis using multivariate analysis revealed that only three or more broad-spectrum antimicrobials and hospitalization time were statistically significant (P values = 0.024 and P = 0.023, respectively; Table 2).
Table 2.
Multivariate analyses of factors associated with C. pelliculosa fungemia
| Factor | OR | 95%CI | P |
|---|---|---|---|
| Age of the mother (year) | |||
| ≥ 35 | 1.000 | ||
| 30–34 | 0.842 | 0.084 ~ 8.440 | 0.884 |
| ≤ 29 | 1.610 | 0.022 ~ 118.431 | 0.828 |
| Gestational age (WK) | |||
| ≥ 36 | 1.000 | ||
| 30–35 | 1166.210 | 0.000 ~ +∞ | 0.999 |
| ≤ 29 | 262.736 | 0.000 ~ +∞ | 1.000 |
| Birth weight | |||
| ELBW(≤1000 g) | 1.000 | ||
| VLBW (> 1000 g,≤1500 g) | 4.232 | 0.222 ~ 80.831 | 0.338 |
| LBW (> 1500, ≤2500 g) | 0.681 | 0.012 ~ 38.272 | 0.852 |
| NBW(> 2500) | 2,141,908.204 | 0.000 ~ +∞ | 0.998 |
| Apgar (1 min) | |||
| ≥ 7 | 1.000 | ||
| < 7 | 2.831 | 0.297 ~ 26.981 | 0.366 |
| PICC | |||
| No | 1.000 | ||
| Yes | 2.319E+ 10 | 0.000 ~ +∞ | 0.997 |
| Endotracheal tube | |||
| No | 1.000 | ||
| Yes | 60,507,419.70 | 0.000 ~ +∞ | 0.997 |
| Parenteral nutrition (lipid solution) | |||
| No | 1.000 | ||
| Yes | 204.134 | 0.000 ~ +∞ | 1.000 |
| BSAU | |||
| No | 1.000 | ||
| Yes | 0.835 | 0.000 ~ +∞ | 1.000 |
| TBSA | |||
| No | 1.000 | ||
| Yes | 0.043 | 0.003 ~ 0.656 | 0.024 |
| Time hospitalization | 1.138 | 1.018 ~ 1.272 | 0.023 |
ELBW Extremely low birth weight, VLBW Very low birth weight, LBW Low birth weight, NBW Normal birth weight, PICC Peripherally inserted central catheter, BSAU Broad spectrum antibiotics use before fungemia, TBSA Three or more broad-spectrum antimicrobials
Outbreak investigation
No C. pelliculosa was detected in 143 swab samples from the environment, doctors, and nurses. The outbreak investigation failed to produce significant results due to the open ward settings and the high-frequency rotation of doctors and nurses as well as regular contact between medical personnel in both wards.
Antifungal susceptibility testing and clinical therapy
Antifungal susceptibility analyses showed that across the 21 isolates of C. pelliculosa, the minimum inhibitory concentrations of fluconazole, voriconazole, itraconazole, flucytosine, and amphotericin B were 2 to 4 μg/mL, 0.125 to 0.25 μg/mL, 0.125 to 0.25 μg/mL, no more than 4 μg/mL, and no more than 0.5 μg/mL, respectively (Table S1). Sixteen fungal isolates were susceptible to fluconazole (MIC ≤2), while the remaining five isolates were susceptible dose-dependently. Fluconazole was used to treat 20 patients and significantly improved symptoms after treatment for 10 to 21 days. Voriconazole was used to treat the other patient, and recovery occurred after treatment for 3 weeks. Treatment with azole drugs cleared all infections, and all neonates survived.
Identification of molecular factors for transmission in C. pelliculosa
Numerous studies have extensively demonstrated that biofilm structures facilitate microbial colonization and transmission in the environment. Biofilm formation by C. pelliculosa initiates with the adhesion of yeast cells to various surfaces and the subsequent generation of a hyphal structure. Although other fungal species such as C. parapsilosis and C. glabrata are unable to produce hyphal structures, they can form biofilms. To analyze potential transmission factors during our outbreak, we examined cell morphological switching and biofilm formation in C. pelliculosa. A poor biofilm-producing fungal species, C. neoformans, was used as a negative control. Differing from the hyphal-forming C. albicans SC5314, C. pelliculosa and C. neoformans H99 showed yeasts and a non-hyphal-cell morphologies, in spite of being cultured in hyphal-inducing media (Spider medium and DMEM; Fig. 1). Biofilm analyses using 96-well plates demonstrated that all C. pelliculosa strains were able to produce biofilms, but they were weaker in C. pelliculosa compared to C. albicans SC5314. The trend was to form 2- to 3-fold more biofilm in 18 isolates (not in isolates 6, 9, or 14) than in C. neoformans H99 strain (Fig. 2).
Fig. 1.
Cell morphological analysis in C. albicans, C. neoformans, and C. pelliculosa. Overnight fungal cell cultures of C. albicans, C. neoformans, and C. pelliculosa were diluted and subcultured in either yeast extract peptone dextrose medium, Spider medium, or Dulbecco’s modified Eagle’s medium (10% fetal bovine serum). Cultures were incubated at 37 °C for 1 h followed by microscopic analysis. Scale bars represent 10 μm
Fig. 2.
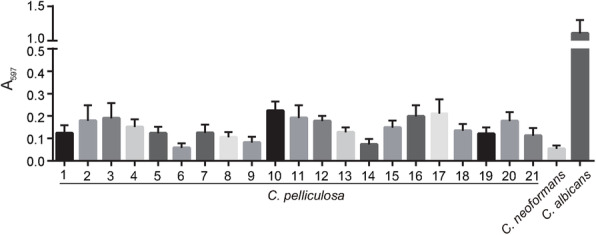
Biofilm assays of C. albicans, C. neoformans, and C. pelliculosa. Overnight cultures of C. albicans, C. neoformans, and C. pelliculosa were washed, diluted, and subcultured in Spider medium. The biofilm structures were developed by agitating on a rotor (100 rpm) at 37 °C for 48 h. Biofilm cells were then washed and stained using crystal violet, then quantified at 597 nm The 21 isolates of clinical C. pelliculosa and the references strains C. neoformans H99 and C. albicans SC5314 were tested. Five biological and five technical replicates were performed for each isolate
The genome of C. pelliculosa was searched and analyzed for the presence of homologs to C. albicans Als3 (a major biofilm adhesion molecules family). The protein sequence of C. albicans Als3 (CaAls3) was used to BLAST the C. pelliculosa genome, and four proteins were found to share significant Als3 similarity: XP_019040826 (CpAls4), XP_019038428.1 (CpAls3), XP_019037149 (CpAls2), and XP_019036962.1 (CpAls1; Fig. 3). Using phylogenic relationship analysis, XP_019040826 (CpAls4) was shown to be the closest homolog to C. albicans Als3 (31% similarity; Fig. 4). To confirm the expression of Als4 in C. pelliculosa, RT-PCR was performed, showing that all isolates express ALS4 (Fig. 5). The biofilm data and genome analysis demonstrated the presence of functional cell surface adhesion proteins, and the ability to produce biofilm structures contributed to the high rate of transmission of C. pelliculosa in our neonatal intensive care unit.
Fig. 3.
Multiple alignment of Als proteins from C. albicans and C. pelliculosa. The C. albicans Als3 protein is labelled CaAls3, whereas the C. pelliculosa Als homologs are labelled CpAls1–4. The National Center for Biotechnology Information accession numbers are: CpAls1, XP_019036962.1; CpAls2, XP_019037149; CpAls3, XP_019038428.1; and CpAls4, XP_019040826
Fig. 4.
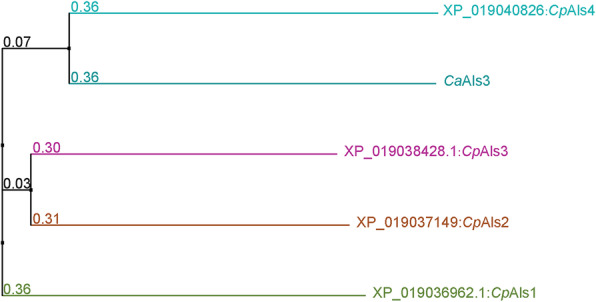
Phylogenic analysis of the Als family in C. albicans and C. pelliculosa. Numbers indicate the level of bootstrap resampling. Candida albicans Als3 protein is labelled CaAls3. Homologs of C. pelliculosa Als are labelled with their National Center for Biotechnology Information accession numbers and serial numbers
Fig. 5.
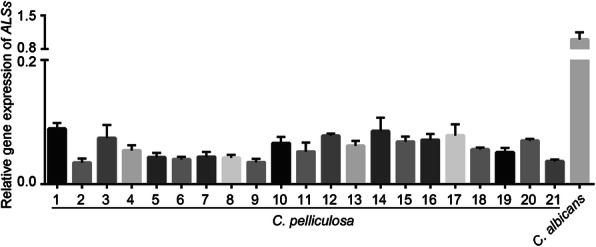
Results of RT-PCR analysis of the expression of ALS4 in C. pelliculosa. The gene expression of ALS4 in C. pelliculosa was confirmed using RT-PCR, isolating mRNA samples from 21 isolates of C. pelliculosa. cDNA was synthesized as described in Material and Methods. The endogenous control was ACT1. The CT values were calculated using the 2-ΔΔCt methods
Discussion
Candida species are life-threatening fungal species that cause bloodstream infections worldwide. An opportunistic fungus belonging to the Candida clade, C. pelliculosa is evolutionarily related to the deadliest fungus, C. albicans [4, 6, 25–30]. Recent studies have demonstrated that the infection caused by C. pelliculosa is very common in neonates [31, 32].
In this study, we extensively investigated potential risk factors for infection, using univariate analysis to find a significant correlation between 10 individual factors with fungemia, including gestational age, birth weight [31, 32], use of a dwelling central venous catheter [4, 9, 31], use of parenteral nutrition [4, 31], and use of an endotracheal tube [4]. Studies have shown that lower gestational age and lower birth weight are important risk factors for C. pelliculosa fungemia [31, 32], and our study supports that. Because neonates with these conditions are often accompanied by impaired or immature immune systems, they are easily invaded by various infectious fungal species [32]. Invasive treatments are considered critical risk factors for Candida infections. Studies have shown that use of a dwelling central venous catheter [4, 9, 31], parenteral receipt of complete nutrition [4, 31], and mechanical ventilation [4] present essential risks for C. pelliculosa infection. Our data support this: the use of an endotracheal tube or a PICC were significantly correlated with C. pelliculosa infection; however, no significant difference was found with the use of a nasogastric tube.
In addition, multivariate analysis found that the use of three or more broad-spectrum antimicrobials or hospitalization time played critical roles in infection acquisition, demonstrating the significance of evaluating these factors for acquired hospitalized infection. Although we were unable to identify the origin of the outbreak in our facility (the open ward environment and frequent contact with individuals), infection was significantly controlled using strict infection prevention and control procedures.
We failed to identify breakpoints in C. pelliculosa drug resistance profiles using the guidelines of the Clinical and Laboratory Standards Institute, and this led to ineffective identification of drug sensitivities in this fungus. The breakpoint for C. pelliculosa has not been demonstrated by CLSI or EUCAST, so non-species related breakpoints for Candida were chosen as a guide (from https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/AFST_BP_v10.0_200204_updated_links_200924_final.xlsx) [20]. All tested strains demonstrated fair sensitivities to the anti-fungal agents tested (MIC < 4), and no drug-resistant isolates were identified. By treating with either fluconazole or voriconazole, all our patients survived. Similar survival rates have been reported: all patients survived the outbreaks in Brazil [8, 31, 33] and Turkey [29]. However, high mortality rates have also been reported, ranging from 16.7 to 42.2% in India [32], Croatia [5], Taiwan [6], South Korea [4], and the 2005 outbreak in Brazil [9]. The fluconazole-tolerant concentration reported in Taiwan and South Korea was approximately 2 μg/mL, consistent with our data (2–4 μg/mL). We therefore speculate that the reported high mortality rates could have been attributable to fungal pathogenicity, care protocols, or patient immunity.
Others have demonstrated that fungi, Candida species in particular, are capable of expressing adhesion proteins to form strong and sticky biofilm structures which become infection sources for transmission and fungemia [34–36]. C. albicans cells can generate hyphal structures upon incubation in hyphal-inducing media, wherein hyphae-specific adhesion proteins, including the major biofilm regulators Als3 and hyphal wall protein 1 are expressed on the hyphal cell wall [11, 12, 37]. These proteins play critical functions in fixing fungal cells on various surfaces such as medical devices and mucosal surfaces. Most importantly, C. albicans Als3 is strongly associated with tissue invasion, which leads to candidiasis [37].
Similar to C. albicans, C. pelliculosa is capable of expressing cell surface adhesin and producing a biofilm structure. Despite the phylogenic analysis of the Als family demonstrating weak support as a result of the limited number of genes characterized, C. pelliculosa Als4 indeed showed significant motif conservation in the Als domain. Therefore, the adhesion of C. pelliculosa to surfaces is similarly achieved through cell surface adhesins such as the Als3 homologs of C. albicans.
In summary, we employed two analyses strategies in this study, univariate and multivariate, to uncover the possible risk factors for hospital-acquired C. pelliculosis infection in our neonatal intensive care unit. We were able to successfully identify two essential factors relevant to fungal transmission. However, the research has some limitations. For example, due to the complexity of the environment and the open ward layout settings in our unit, the outbreak investigation failed to yield concrete results. The gene expression of many adhesins in C. pelliculosa was discovered through genomic research. As a result, C. pelliculosa transmission is most likely similar to that of other Candida species. We are presently focusing on a gene disruption method in C. pelliculosa to better understand how these adhesive genes manipulate biofilm formation and transmission.
Conclusion
This study showed the use of three or more broad-spectrum antimicrobial agents, as well as hospitalization time, were critical risk factors in an outbreak of C. pelliculosa in our neonatal intensive care unit. Infection prevention and control measures, as well as antifungal treatment, greatly slowed the infection’s spread in the environment and among patients. C. pelliculosa has an intact capacity for biofilm formation and adhesive gene expression suggesting potential routes of transfer. More research into the function of adhesive genes is required to better understand of C. pelliculosa transmission and develop possible preventive strategies. Our research identified important risk factors for C. pelliculosa infection in a neonatal intensive care unit, highlighting the significance of evaluating factors for fungal transmission.
Supplementary Information
Additional file 1: Table S1. Clinical characteristics of patients with C. pelliculosa fungemia.
Acknowledgements
The authors thank all our colleagues from the Clinical Microbiology Group in the Department of Laboratory Medicine and the Department of Hospital Infection at Shengjing Hospital. We also acknowledge the graduate students from the College of Life and Health Sciences at Northeastern University for their contributions to the experimental design and data analyses.
Authors’ contributions
Z.Z., Y.C. and Yong Liu. collected and identified strains. Yanjian Li, X.C. and C.D. performed experiments and analyzed the data. Z.Z., C.D. and Yong Liu wrote the manuscript. The authors read and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (31870140 to C.D.), the Fundamental Research Funds for Central Universities of China (N142005001 and N172002001 to C.D.), Liaoning Revitalization Talents Program (XLYC1807001), Liaoning Natural Science Funds (2020-MS-10 to Z.Z.), Ministry of Science and Technology Basic Resources Investigation (SQ2019FY010016 and 2019FY101213 to Yong Liu).
Availability of data and materials
The datasets used and /or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All information was anonymized before being made available for research. This study was approved by the Shengjing Hospital of China Medical University Ethics Committee. All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was documented from parent and/or legal guardian.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abouloifa H, Rokni Y, Bellaouchi R, Ghabbour N, Karboune S, Brasca M, Ben Salah R, Chihib NE, Saalaoui E, Asehraou A. Characterization of probiotic properties of antifungal Lactobacillus strains isolated from traditional fermenting green olives. Probiotics Antimicrob Proteins. 2020;12(2):683–696. doi: 10.1007/s12602-019-09543-8. [DOI] [PubMed] [Google Scholar]
- 2.Campioni TS, Soccol CR, Libardi Junior N, Rodrigues C, Woiciechowski AL, Letti LAJ, Vandenberghe LPS. Sequential chemical and enzymatic pretreatment of palm empty fruit bunches for Candida pelliculosa bioethanol production. Biotechnol Appl Biochem. 2020;67(5):723–731. doi: 10.1002/bab.1826. [DOI] [PubMed] [Google Scholar]
- 3.Mujdeci G, Arevalo-Villena M, Ozbas ZY, Briones Perez A. Yeast identification during fermentation of Turkish Gemlik olives. J Food Sci. 2018;83(5):1321–1325. doi: 10.1111/1750-3841.14124. [DOI] [PubMed] [Google Scholar]
- 4.Jung J, Moon YS, Yoo JA, Lim JH, Jeong J, Jun JB. Investigation of a nosocomial outbreak of fungemia caused by Candida pelliculosa (Pichia anomala) in a Korean tertiary care center. J Microbiol Immunol Infect. 2018;51(6):794–801. doi: 10.1016/j.jmii.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Kalenic S, Jandrlic M, Vegar V, Zuech N, Sekulic A, Mlinaric-Missoni E. Hansenula anomala outbreak at a surgical intensive care unit: a search for risk factors. Eur J Epidemiol. 2001;17(5):491–496. doi: 10.1023/A:1013739802940. [DOI] [PubMed] [Google Scholar]
- 6.Lin HC, Lin HY, Su BH, Ho MW, Ho CM, Lee CY, Lin MH, Hsieh HY, Lin HC, Li TC, Hwang KP, Lu JJ. Reporting an outbreak of Candida pelliculosa fungemia in a neonatal intensive care unit. J Microbiol Immunol Infect. 2013;46(6):456–462. doi: 10.1016/j.jmii.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Sardana V, Pandey A, Madan M, Goel SP, Asthana AK. Neonatal candidemia: a changing trend. Indian J Pathol Microbiol. 2012;55(1):132–133. doi: 10.4103/0377-4929.94900. [DOI] [PubMed] [Google Scholar]
- 8.Thuler LCS, Faivichenco S, Velasco E, Martins CA, Nascimento CRG, Castilho IAMA. Fungaemia caused by Hansenula anomala - an outbreak in a cancer hospital. Mycoses. 1997;40(5–6):193–196. doi: 10.1111/j.1439-0507.1997.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 9.Pasqualotto AC, Sukiennik TC, Severo LC, de Amorim CS, Colombo AL. An outbreak of Pichia anomala fungemia in a Brazilian pediatric intensive care unit. Infect Control Hosp Epidemiol. 2005;26(6):553–558. doi: 10.1086/502583. [DOI] [PubMed] [Google Scholar]
- 10.Murphy N, Buchanan CR, Damjanovic V, Whitaker R, Hart CA, Cooke RW. Infection and colonisation of neonates by Hansenula anomala. Lancet. 1986;1(8476):291–293. doi: 10.1016/s0140-6736(86)90827-5. [DOI] [PubMed] [Google Scholar]
- 11.Nobile CJ, Nett JE, Andes DR, Mitchell AP. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot Cell. 2006;5(10):1604–1610. doi: 10.1128/EC.00194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nobile CJ, Schneider HA, Nett JE, Sheppard DC, Filler SG, Andes DR, Mitchell AP. Complementary adhesin function in C. albicans biofilm formation. Curr Biol. 2008;18(14):1017–1024. doi: 10.1016/j.cub.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding C, Butler G. Development of a gene knockout system in Candida parapsilosis reveals a conserved role for BCR1 in biofilm formation. Eukaryot Cell. 2007;6(8):1310–1319. doi: 10.1128/EC.00136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding C, Vidanes GM, Maguire SL, Guida A, Synnott JM, Andes DR, Butler G. Conserved and divergent roles of Bcr1 and CFEM proteins in Candida parapsilosis and Candida albicans. PLoS One. 2011;6(12):e28151. doi: 10.1371/journal.pone.0028151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossignol T, Ding C, Guida A, d'Enfert C, Higgins DG, Butler G. Correlation between biofilm formation and the hypoxic response in Candida parapsilosis. Eukaryot Cell. 2009;8(4):550–559. doi: 10.1128/EC.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly LA, Riccombeni A, Grozer Z, Holland LM, Lynch DB, Andes DR, Gacser A, Butler G. The APSES transcription factor Efg1 is a global regulator that controls morphogenesis and biofilm formation in Candida parapsilosis. Mol Microbiol. 2013;90(1):36–53. doi: 10.1111/mmi.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laffey SF, Butler G. Phenotype switching affects biofilm formation by Candida parapsilosis. Microbiology (Reading) 2005;151(Pt 4):1073–1081. doi: 10.1099/mic.0.27739-0. [DOI] [PubMed] [Google Scholar]
- 18.Leite de Andrade MC, Soares de Oliveira MA, Santos F, Ximenes Vilela PB, da Silva MN, DPC M, de Lima Neto RG, HJP N, ISL B, Chaves GM, et al. A new approach by optical coherence tomography for elucidating biofilm formation by emergent Candida species. PLoS One. 2017;12(11):e0188020. doi: 10.1371/journal.pone.0188020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuming W, Guangfeng S, Ning Q, Gang L. Infectious diseases. Beijing: People’s Medical Publishing House; 2014. [Google Scholar]
- 20.Arendrup MC, Friberg N, Mares M, Kahlmeter G, Meletiadis J, Guinea J, Subcommittee on Antifungal Susceptibility Testing of the EECfAST How to interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST) Clin Microbiol Infect. 2020;26(11):1464–1472. doi: 10.1016/j.cmi.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Enjalbert B, Whiteway M. Release from quorum-sensing molecules triggers hyphal formation during Candida albicans resumption of growth. Eukaryot Cell. 2005;4(7):1203–1210. doi: 10.1128/EC.4.7.1203-1210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramage G, Saville SP, Wickes BL, Lopez-Ribot JL. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol. 2002;68(11):5459–5463. doi: 10.1128/AEM.68.11.5459-5463.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Djordjevic D, Wiedmann M, McLandsborough LA. Microtiter plate assay for assessment of listeria monocytogenes biofilm formation. Appl Environ Microbiol. 2002;68(6):2950–2958. doi: 10.1128/AEM.68.6.2950-2958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding C, Yin J, Tovar EM, Fitzpatrick DA, Higgins DG, Thiele DJ. The copper regulon of the human fungal pathogen Cryptococcus neoformans H99. Mol Microbiol. 2011;81(6):1560–1576. doi: 10.1111/j.1365-2958.2011.07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan AW, Cartwright EJ, Reddy SC, Kraft CS, Wang YF. Pichia anomala (Candida pelliculosa) fungemia in a patient with sickle cell disease. Mycopathologia. 2013;176(3–4):273–277. doi: 10.1007/s11046-013-9677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chitasombat MN, Kofteridis DP, Jiang Y, Tarrand J, Lewis RE, Kontoyiannis DP. Rare opportunistic (non-Candida, non-Cryptococcus) yeast bloodstream infections in patients with cancer. J Inf Secur. 2012;64(1):68–75. doi: 10.1016/j.jinf.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi SW, Lee TJ, Kim MK, Lee M, Jung JH. A case of fungal arthritis caused by Hansenula anomala. Clin Orthop Surg. 2010;2(1):59–62. doi: 10.4055/cios.2010.2.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esgin H, Bulut E, Orum C. Candida pelliculosa endophthalmitis after cataract surgery: a case report. BMC Res Notes. 2014;7(1):169. doi: 10.1186/1756-0500-7-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalkanci A, Dizbay M, Turan O, Fidan I, Yalcin B, Hirfanoglu I, Kustimur S, Aktas F, Sugita T. Nosocomial transmission of Candida pelliculosa fungemia in a pediatric intensive care unit and review of the literature. Turk J Pediatr. 2010;52(1):42–49. [PubMed] [Google Scholar]
- 30.Ratcliffe L, Davies J, Anson J, Hales S, Beeching NJ, Beadsworth MBJ. Candida pelliculosa meningitis as an opportunistic infection in HIV: the first reported case. Int J STD AIDS. 2011;22(1):54–56. doi: 10.1258/ijsa.2010.010113. [DOI] [PubMed] [Google Scholar]
- 31.Aragao PA, Oshiro IC, Manrique EI, Gomes CC, Matsuo LL, Leone C, Moretti-Branchini ML, Levin AS, Group IS Pichia anomala outbreak in a nursery: exogenous source? Pediatr Infect Dis J. 2001;20(9):843–848. doi: 10.1097/00006454-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Chakrabarti A, Singh K, Narang A, Singhi S, Batra R, Rao KL, Ray P, Gopalan S, Das S, Gupta V, et al. Outbreak of Pichia anomala infection in the pediatric service of a tertiary-care center in northern India. J Clin Microbiol. 2001;39(5):1702–1706. doi: 10.1128/JCM.39.5.1702-1706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.da Silva CM, de Carvalho Parahym AM, Leao MP, de Oliveira NT, de Jesus Machado Amorim R, Neves RP. Fungemia by Candida pelliculosa (Pichia anomala) in a neonatal intensive care unit: a possible clonal origin. Mycopathologia. 2013;175(1–2):175–179. doi: 10.1007/s11046-012-9605-0. [DOI] [PubMed] [Google Scholar]
- 34.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183(18):5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox EP, Nobile CJ. A sticky situation: untangling the transcriptional network controlling biofilm development in Candida albicans. Transcription. 2012;3(6):315–322. doi: 10.4161/trns.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. Our current understanding of fungal biofilms. Crit Rev Microbiol. 2009;35(4):340–355. doi: 10.3109/10408410903241436. [DOI] [PubMed] [Google Scholar]
- 37.Bruder-Nascimento A, Camargo CH, Mondelli AL, Sugizaki MF, Sadatsune T, Bagagli E. Candida species biofilm and Candida albicans ALS3 polymorphisms in clinical isolates. Braz J Microbiol. 2014;45(4):1371–1377. doi: 10.1590/S1517-83822014000400030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Clinical characteristics of patients with C. pelliculosa fungemia.
Data Availability Statement
The datasets used and /or analyzed during the current study are available from the corresponding author on reasonable request.