Abstract
Originally referred to as ‘muscle sense’, the notion that skeletal muscle held a peripheral sensory function was first described early in the 19th century. Foundational experiments by Sherrington in the early 20th century definitively demonstrated that proprioceptors contained within skeletal muscle, tendons, and joints are innervated by sensory neurons and play an important role in the control of movement. In this review, we will highlight several recent advances in the ongoing effort to further define the molecular diversity underlying the proprioceptive sensorimotor system. Together, the work summarized here represents our current understanding of sensorimotor circuit formation during development and the mechanisms that regulate the integration of proprioceptive feedback into the spinal circuits that control locomotion in both normal and diseased states.
MAIN
In their simplest form, locomotor behaviors are controlled by spinal interneuron networks called central pattern generators (CPGs). This is best exemplified in ‘fictive locomotion’ where, in the absence of sensory or descending input, local spinal circuits generate patterned flexor-extensor and left-right alternating motor activity (Kiehn, 2016; Rossignol et al., 2006). However, skilled motor tasks and coordinated movement require additional input from sensory feedback systems (Akay, 2020; Kiehn, 2016; Rossignol et al., 2006; Tuthill and Azim, 2018). Proprioceptive systems thus have a critical role in motor behavior. In this review we will discuss current knowledge and integrate recent findings relating to proprioceptive sensory neuron development, circuit formation and function.
Components of the proprioceptive system that underlie locomotion
Diversity and development of primary sensory neurons
The dorsal root ganglia (DRG) contain a mosaic of primary sensory neuron classes. These pseudounipolar neurons relay sensory information to the spinal cord through a characteristic bifurcating axon, which sends one projection to the periphery and the other into the spinal cord (Figure 1). During locomotion, sensory neurons provide important information about limb location and movement in space. In this review we will focus on a subset of sensory neurons that provide proprioceptive feedback through their innervation of specialized sensory end-organs within the skeletal muscle. While these proprioceptive sensory neurons (pSNs) provide the majority of sensory feedback during locomotion, it is noteworthy that other sensory modalities including joint mechanoreceptors and cutaneous receptors also contribute to motor behaviors and reflexes (Gatto et al., 2021; MacKinnon, 2018; Tuthill and Azim, 2018).
Figure 1. Sensorimotor circuits involved in locomotion.
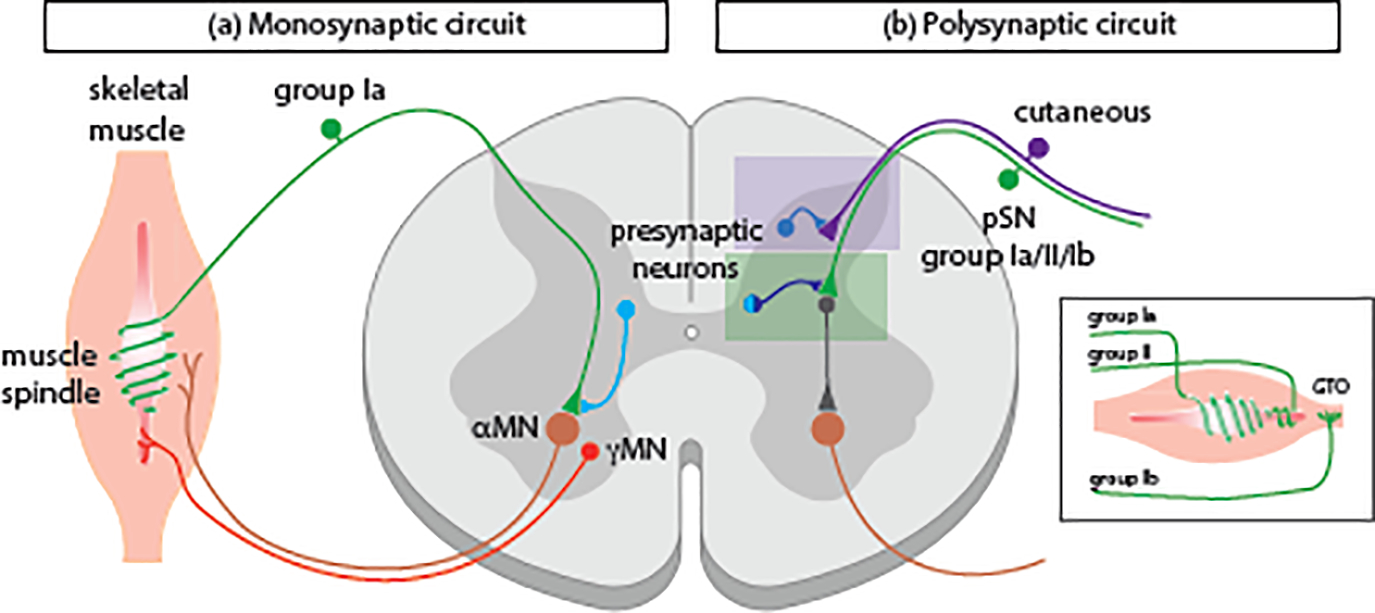
(a) Changes in muscle length are detected by group Ia sensory afferents (green) coiled around the non-contractile region of muscle spindles. γMNs (red) innervate intrafusal muscle fibers to regulate the sensitivity of stretch-sensitive muscle spindles. In the monosynaptic stretch reflex circuit, lengthening of skeletal muscle induces a deformation stimulus that activates group Ia sensory afferents, which form direct synaptic contacts on synergistic motor neurons to promote muscle contraction. Ptf1a-lineage GABApre interneurons (light blue) presynaptically inhibit monosynaptic proprioceptive feedback. (b) pSN afferents form more complex, polysynaptic connections with motor neurons. The box on the far right illustrates the innervation pattern of pSN afferents in skeletal muscle, including muscle spindle group Ia/II afferents and GTO Ib afferents. Different classes of sensory neurons exhibit different central termination patterns with cutaneous afferents ending in more dorsally (purple box) and proprioceptive afferents ending in the intermediate spinal cord (green box). Like monosynaptic circuits, polysynaptic proprioceptive and cutaneous sensory inputs are subject to presynaptic inhibition from different classes of GABApre neurons. More specifically, dorsal Ptf1a-lineage GABApre neurons (blue) target cutaneous afferents while Pax2+/Rorβ+ GABApre neurons (light/dark blue) target proprioceptive afferents that synapse on premotor interneurons.
pSNs can be subclassified into three subtypes that exhibit characteristic innervation patterns and physiological properties (Figure 1b). Muscle spindles that detect the amount and rate of muscle stretch are innervated at equatorial regions by group Ia afferents, and at polar regions by group II afferents. The third class, group Ib afferents, respond to changes in muscle load through innervation of Golgi tendon organs (GTOs) localized at skeletal muscle myotendinous junctions (Brown, 1981). Given that pSN neurons only represent 10–20% of all DRG neurons, large pan-sensory screens have been limited in their ability to assess the molecular diversity among distinct pSN subsets. The resultant lack of molecular markers for specific pSN groups has hampered investigation into their development and function. To address this challenge, bulk RNA sequencing (RNAseq) and extensive in situ RNA hybridization were used to identify genes unique to pSNs. This characterization suggested that pSN subtypes could be delineated by combinatorial gene expression (Wu et al., 2019). Further investigating the genetic diversity of pSN subsets, a recent single-cell RNAseq (scRNAseq) analysis used an intersectional genetic labeling strategy to enrich for pSNs from adult DRG and identified five distinct clusters of pSN neurons. Interestingly, group Ia and group Ib afferents were represented in two unique clusters, whereas group II afferents were proposed to segregate into the three remaining clusters, suggesting greater molecular diversity than has previously been described (Oliver et al., 2021).
Mechanisms that control the specification of sensory neurons including pSNs have been a topic of dedicated study for quite some time (reviewed in Lallemend and Ernfors, 2012). Building upon previous knowledge, two recent scRNAseq studies have provided new insights into the stepwise manner by which sensory neurons acquire their fate. Analysis of DRG neurons isolated from timepoints spanning early development through adulthood demonstrated that nascent sensory neurons are unspecialized and broadly co-express transcription factors that later mark specific sensory classes in the adult (Sharma et al., 2020). In a separate but complementary study, gene modules corresponding to proprioceptive and mechanoreceptive fate choice were used to examine the bifurcation between pSN and mechanoreceptor lineages. This revealed both gene modules were initially expressed in single cells, but gradually became more exclusive to one of the two developing classes leading up to the bifurcation point (Faure et al., 2020).
In addition to intrinsic drivers of cell fate, the developmental regulation of sensory neurons relies on extrinsic cues from the local environment and peripheral target tissues (reviewed in Lallemend and Ernfors, 2012). Though the focus of Faure et al. centered around the transcriptional state of cells during lineage birfurcation, the early gene modules used to examine proprioceptive and mechanoreceptive fate choice were enriched for genes related to membrane and cytoskeletal remodeling, potentially allowing environmentally-derived cues to influence specification. Recent studies have further emphasized the importance of target-derived cues for the generation of the full complement of nociceptive and pSN subtypes. In the absence of target-derived nerve growth factor (NGF), Sharma et al. demonstrated that nociceptive DRG neurons formed fewer transcriptionally distinct neuron classes, while NGF-independent mechanoreceptive and pSN diversification was unaffected. Similarly, examination of pSN gene expression at embryonic and postnatal stages supported the idea that genetic diversity among pSN subtypes only occurs post-target innervation (Oliver et al., 2021; Wu et al., 2019).
Together, these studies provide a transcriptional roadmap of sensory neurons spanning both embryonic and adult stages. The molecular signatures of diverse sensory and pSN subtypes identified in these studies open new possibilities for future genetic targeting of specific sensory and proprioceptive neurons.
Integration of proprioceptive information into sensorimotor circuits
Motor neurons that reside in the ventral spinal cord provide efferent control of skeletal muscle and can be divided into three populations based on the type of muscle fiber they innervate (Figure 1a). Alpha motor neurons (αMNs) at the neuromuscular junction innervate extrafusal muscle fibers that generate force-producing contractions, whereas gamma motor neurons (γMNs) innervate intrafusal muscle fibers to regulate muscle spindle sensitivity and beta motor neurons (βMNs) innervate both intra- and extrafusal muscle fibers. While pSNs do not form direct synaptic contacts onto γMNs, they make both direct monosynaptic (group Ia afferents, Figure 1a) and indirect polysynaptic (all afferents, Figure 1b) connections with αMNs (Burke, 2004; Eccles et al., 1957).
The simplicity and experimentally tractable nature of the monosynaptic sensorimotor circuit have made it an ideal model system to better understand the mechanisms regulating axon guidance, synaptogenesis, and circuit formation (Arber, 2012; Imai and Yoshida, 2018). In this circuit, group Ia afferents preferentially connect to motor neurons innervating the same (homonymous) and synergistic (heteronymous) muscles, while connections between antagonistic sensorimotor pairs are strictly avoided (Eccles et al., 1957). Positional programs, molecular guidance cues, and neuronal activity have all been investigated as strategies that mediate the developmental specificity of sensorimotor connections.
αMN diversification and motor pool formation are part of a highly organized process that requires the cooperation of extrinsic inductive signals and Hox transcription-factor based programs (Dasen, 2017; Jessell et al., 2011; Stifani, 2014). Loss of Foxp1, an essential Hox cofactor, scrambles motor neuron position (Dasen et al., 2008; Rousso et al., 2008; Sürmeli et al., 2011), but does not affect pSN termination zones, thereby resulting in the formation of synaptic connections between inappropriate sensorimotor pairs (Sürmeli et al., 2011). More recently, sensorimotor synapse formation was found to correlate with the axo-dendritic angle formed between motor neuron dendrites and approaching pSN afferents, providing further insight into how specificity within sensory termination zones may be achieved, even in regions containing dendritic branches corresponding to antagonistic motor pools (Balaskas et al., 2019). Augmenting these positional strategies, motor neuron intrinsic signals (Baek et al., 2017) and molecular recognition-based cues on sensory neurons and their specific target motor neurons (Fukuhara et al., 2013; Pecho-Vrieseling et al., 2009) have been shown to participate in functional circuit formation. As for the role of neuronal activity, although early studies established the formation of sensorimotor synapses as largely activity-independent (Mears and Frank, 1997), heteronymous sensorimotor connections are refined through activity-driven processes (Mendelsohn et al., 2015).
Mirroring motor neurons, pSNs were recently found to express Hox proteins at particular rostral-caudal levels of the spinal cord, independent of target limb signals (Shin et al., 2020). Hoxc8 is expressed preferentially in pSNs of distal extensor forelimb muscles, and in the absence of Hoxc8, pSNs erroneously target antagonistic forelimb MN pools (Shin et al., 2020). Altogether, complex and layered mechanisms utilize a combination of intrinsic genetic programs and extrinsic target-derived signaling to generate sensorimotor connections with great specificity.
Integration and regulation of the proprioceptive circuit during locomotion
In the study of sensory feedback in locomotion, cats have been historically favored as the animal model of choice. Compared with rodent models, the larger size and higher center of mass in cats better approximates human locomotion (Akay, 2020). However, as molecular studies in mouse models advance our understanding of the development of neural circuits, coupling behavioral and electrophysiological techniques with mouse genetics can provide an important platform to interrogate the role of proprioception in locomotion. In this section we will discuss recent studies that have used genetic perturbations in sensory feedback systems to infer how distinct pSN subtypes regulate gait during locomotion, and we will further explore the way in which proprioceptive feedback is dynamically integrated into neural circuits.
The role of proprioception in locomotion
Early studies on muscle spindle development and function utilized a mutation in the early growth response 3 (Egr3) gene to disrupt proprioceptive feedback from group Ia/II pSN afferents. Egr3-deficient mice exhibited ataxic gait, demonstrating that locomotion is affected by the absence of sensory feedback (Tourtellotte and Milbrandt, 1998). More recent investigations have utilized kinematic and electromyogram (EMG) analysis of Egr3-deficient mice to directly examine the contribution of pSNs to locomotion (Akay et al., 2014; Mayer et al., 2018; Takeoka et al., 2014). These studies demonstrate that feedback from muscle spindles controls the timing of ankle flexion for proper foot placement (Akay et al., 2014; Takeoka et al., 2014) and allows for the correct matching of extensor activity to different locomotive speeds (Mayer et al., 2018). Interestingly, a more pronounced degradation of locomotor patterns was observed both in Egr3-deficient mice during swimming, which does not require significant GTO activation, and in mouse models that lack all pSNs (Akay et al., 2014; Takeoka et al., 2014). Accordingly, these results suggest GTO compensation from Group Ib afferents that are unaffected in Egr3-deficient mice may compensate for the loss of muscle spindle feedback. The ongoing efforts detailed in the previous section to define the molecular signature of different pSN subsets will enable future experiments to tease apart the distinct contributions of these different populations during locomotion.
Presynaptic inhibition regulates the flow of proprioceptive feedback
A common mechanism used to gate sensory information throughout the nervous system is presynaptic inhibition (PSI). Within the spinal cord, PSI derives from a population of interneurons that express gamma-aminobutyric acid (GABA) and form specialized axo-axonic synapses with sensory afferent terminals (Eccles et al., 1963; Rudomin and Schmidt, 1999). Presynaptic GABAergic (GABApre) neurons arise from Ptf1a-lineage dorsal interneuron progenitors (Betley et al., 2009; Hughes et al., 2005). Several studies have described distinct classes of GABApre neurons that settle in different locations and target different subsets of sensory afferents (Figure 1). GABApre neurons that reside in the dorsal horn are directed towards cutaneous afferents where they mediate PSI of tactile and noxious stimuli (Betley et al., 2009; Boyle et al., 2019; Comitato and Bardoni, 2021; Cui et al., 2016; Zhang et al., 2017). Meanwhile, those in the intermediate spinal cord are segregated into at least two populations that target proprioceptive afferents: a Ptf1a+ population that projects to group Ia pSN terminals in the monosynaptic stretch reflex (Betley et al., 2009; Zhang et al., 2017) and a Pax2+/Rorβ+ population that provides PSI to group Ib/II pSN terminals, which form polysynaptic connections with motor neurons (Koch et al., 2017).
How do GABApre neurons inhibit sensory afferent terminals? Presynaptic inhibition of glutamate release from sensory afferents is dependent on GABAA-receptor (GABAAR) mediated afferent depolarization (PAD), resulting from the high intracellular chloride concentration in sensory terminals (Rudomin and Schmidt, 1999). In addition to GABAARs, slow-acting GABABRs, NMDARs and extrasynaptic GABAARs have all been implicated in PSI (Rudomin, 2009; Russo et al., 2000; Zimmerman et al., 2019). Downstream of PAD, proposed mechanisms leading to reduced glutamate release include the inactivation of voltage gated sodium and/or calcium channels and the increased chloride conductance impairing action potential amplitude through shunting inhibition (Rudomin and Schmidt, 1999). In turn, signals released from sensory afferent terminals have been shown to direct the efficacy of PSI in GABApre neurons. The GABA-synthesizing enzymes glutamic acid decarboxylase (GAD)65 and GAD67 are co-expressed in GABApre terminals (Betley et al., 2009; Hughes et al., 2005). Impaired glutamate release from pSN afferents reduces the expression of both GADs in GABApre terminals. The expression of GAD65 is indirectly regulated by sensory glutamate through autocrine regulation of brain derived neurotropic factor (BDNF) release, which acts through GABApre TrkB receptors to influence GAD65 synaptic localization (Betley et al., 2009; Mende et al., 2016). In contrast, the levels of GAD67 and the efficacy of PSI appear to be directly regulated through pSN glutamate release via the activation of GABApre metabotropic glutamate receptor 1β (mGluR1β) (Mende et al., 2016).
Though sensory feedback is highly regulated by PSI, interrogating the functional consequence of disrupting PSI has been challenging. On one hand, elimination of GAD65+ GABApre neurons from the cervical spinal cord impaired skilled forelimb reaching without affecting locomotor control (Fink et al., 2014). On the other hand, in the lumbar spinal cord, loss of Pax2+/Rorβ+ neurons that inhibit polysynaptic proprioceptive afferents resulted in a ‘duck-like’ gait due to inappropriate hyperflexion during locomotion (Koch et al., 2017). Thus, motor circuits along the rostrocaudal extent of the spinal cord may utilize PSI in different ways to produce specific motor behaviors. Future studies into the functional specificity of GABApre populations and the mechanisms by which PSI influences the integration of proprioceptive feedback will lead to a better understanding of the spinal circuity that underlies locomotion.
Proprioception and neurodegeneration in sensorimotor circuits
Increasingly, dysfunction of pSNs and other neurons in the sensorimotor circuit has been implicated in neurodegenerative disease progression. Spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS) are neurodegenerative disorders characterized by dysfunction and death of spinal motor neurons. SMA is caused by loss of survival motor neuron (SMN) protein (Burghes and Beattie, 2009), whereas more than 20 genes in different molecular pathways have been implicated in ALS pathogenesis (Harris, 2014). Preceding motor neuron cell death, SMA results in a reduction of pSN synapses on αMNs (Fletcher et al., 2017), which are vulnerable to synaptic pruning due to aberrantly upregulated complement protein C1q (Vukojicic et al., 2019). Furthermore, rescue of SMN1 in pSNs or viral delivery of its downstream target protein stasimon is sufficient to increase the number of pSN synapses on αMNs and improve motor outcomes (Fletcher et al., 2017; Simon et al., 2019). Alterations in pSN activity may also contribute to the loss of αMNs characteristic of ALS. Degeneration of peripheral pSN endings has been observed in early pre-symptomatic stages, followed by a decrease in pSN central synapses on αMNs (Vaughan et al., 2015). αMNs susceptible to ALS lose the ability to fire repetitively and become hypoexcitable prior to their degeneration (Martínez-Silva et al., 2018). As removing pSN input on αMNs improves motor neuron survival, and reducing pSN activity through the elimination of γMNs delays disease onset (Lalancette-Hebert et al., 2016), these studies suggest αMN hypoexcitability may result from over-taxing proprioceptive input. In addition to changes in proprioceptive input, other alterations in premotor input have been reported in ALS (Chang and Martin, 2009; Konsolaki et al., 2020; Landoni et al., 2019; Pullen and Athanasiou, 2009; Salamatina et al., 2020). Taken together, these studies suggest that pSN activity and synaptic excitation contribute to αMN degeneration in neurodegenerative disease and that restructuring of premotor contacts may play an important compensatory role during disease progression. These examples underscore the importance of investigating how proprioceptive subsets are entangled in pathologies of the sensorimotor circuit.
Conclusions and future perspectives
Understanding the development, organization and function of the proprioceptive system is critical for our knowledge of both basic motor functioning and the pathophysiology of neurodegenerative disease. This review highlights the importance of ongoing efforts to clarify the diversity of pSN subsets, which contribute diverse proprioceptive feedback from the periphery, and GABApre neurons that modulate how that information is integrated via presynaptic inhibition. Future work leveraging new markers that have been recently identified and the power of intersectional mouse genetics will help to advance our understanding of the complex and diverse roles these populations have within the sensorimotor circuitry, both in normal locomotion and during neurodegenerative disease progression.
ACKNOWLEDGEMENTS
We thank Turgay Akay, Joriene DeNooij, Lori Dershowitz, Jacob Hull, and Peter van Roessel for helpful comments on the manuscript. Artwork was created with Biorender.com and Adobe Illustrator. This work was funded by NIH grant R01 NS083998.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- Akay T (2020). Sensory Feedback Control of Locomotor Pattern Generation in Cats and Mice. Neuroscience 450, 161–167.*In the absence of muscle spindle feedback, Egr3-deficient mice exhibit an altered locomotor pattern, suggesting that proprioceptive feedback is important for the generation of coordinated movements.
- Akay T, Tourtellotte WG, Arber S, and Jessell TM (2014). Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proc. Natl. Acad. Sci 111, 16877–16882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S (2012). Motor Circuits in Action: Specification, Connectivity, and Function. Neuron 74, 975–989. [DOI] [PubMed] [Google Scholar]
- Baek M, Pivetta C, Liu J-P, Arber S, and Dasen JS (2017). Columnar-Intrinsic Cues Shape Premotor Input Specificity in Locomotor Circuits. Cell Rep 21, 867–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaskas N, Abbott LF, Jessell TM, and Ng D (2019). Positional Strategies for Connection Specificity and Synaptic Organization in Spinal Sensory-Motor Circuits. Neuron 102, 1143–1156.e4.**The axo-dendritic angle of approaching proprioceptive sensory afferents and motor neuron bodies infers additional specificity in sensorimotor connections.
- Betley JN, Wright CVE, Kawaguchi Y, Erdélyi F, Szabó G, Jessell TM, and Kaltschmidt JA (2009). Stringent Specificity in the Construction of a GABAergic Presynaptic Inhibitory Circuit. Cell 139, 161–174.**Ptf1a-lineage GABAergic neurons target monosynaptic sensory afferent terminals with high specificity. Retrograde BDNF from sensory afferents induces expression of GAD65, a marker specific to presynaptic GABAergic inputs.
- Boyle KA, Gradwell MA, Yasaka T, Dickie AC, Polgár E, Ganley RP, Orr DPH, Watanabe M, Abraira VE, Kuehn ED, et al. (2019). Defining a Spinal Microcircuit that Gates Myelinated Afferent Input: Implications for Tactile Allodynia. Cell Rep 28, 526–540.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG (1981). Organization in the Spinal Cord (London: Springer London; ). [Google Scholar]
- Burghes AHM, and Beattie CE (2009). Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat. Rev. Neurosci 10, 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE (2004). Spinal Cord: Ventral Horn. In The Synaptic Organization of the Brain, (Oxford University Press; ), pp. 79–124. [Google Scholar]
- Chang Q, and Martin LJ (2009). Glycinergic Innervation of Motoneurons Is Deficient in Amyotrophic Lateral Sclerosis Mice. Am. J. Pathol 174, 574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comitato A, and Bardoni R (2021). Presynaptic Inhibition of Pain and Touch in the Spinal Cord: From Receptors to Circuits. Int. J. Mol. Sci 22, 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Miao X, Liang L, Abdus-Saboor I, Olson W, Fleming MS, Ma M, Tao Y-X, and Luo W (2016). Identification of Early RET+ Deep Dorsal Spinal Cord Interneurons in Gating Pain. Neuron 91, 1137–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS (2017). Master or servant? emerging roles for motor neuron subtypes in the construction and evolution of locomotor circuits. Curr. Opin. Neurobiol 42, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS, De Camilli A, Wang B, Tucker PW, and Jessell TM (2008). Hox Repertoires for Motor Neuron Diversity and Connectivity Gated by a Single Accessory Factor, FoxP1. Cell 134, 304–316. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, and Lundberg A (1957). The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J. Physiol 137, 22–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Schmidt R, and Willis WD (1963). Pharmacological studies on presynaptic inhibition. J. Physiol 168, 500–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure L, Wang Y, Kastriti ME, Fontanet P, Cheung KKY, Petitpré C, Wu H, Sun LL, Runge K, Croci L, et al. (2020). Single cell RNA sequencing identifies early diversity of sensory neurons forming via bi-potential intermediates. Nat. Commun 11, 4175.*Deep sequencing single-cell analysis of the bifurcation of proprioceptive and mechanoreceptive sensory neuron lineages. Cell fate determination is demonstrated to involve gene modules that are initially broadly co-expressed 405 but become exclusive to differentiating sensory neuron subtypes.
- Fink AJP, Croce KR, Huang ZJ, Abbott LF, Jessell TM, and Azim E (2014). Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 509, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EV, Simon CM, Pagiazitis JG, Chalif JI, Vukojicic A, Drobac E, Wang X, and Mentis GZ (2017). Reduced sensory synaptic excitation impairs motor neuron function via Kv2.1 in spinal muscular atrophy. Nat. Neurosci 20, 905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara K, Imai F, Ladle DR, Katayama K, Leslie JR, Arber S, Jessell TM, and Yoshida Y (2013). Specificity of Monosynaptic Sensory-Motor Connections Imposed by Repellent Sema3E-PlexinD1 Signaling. Cell Rep 5, 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto G, Bourane S, Ren X, Di Costanzo S, Fenton PK, Halder P, Seal RP, and Goulding MD (2021). A Functional Topographic Map for Spinal Sensorimotor Reflexes. Neuron 109, 91–104.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BT (2014). Amyotrophic Lateral Sclerosis. In Pathobiology of Human Disease, (Elsevier; ), pp. 2036–2044. [Google Scholar]
- Hughes DI, Mackie M, Nagy GG, Riddell JS, Maxwell DJ, Szabo G, Erdelyi F, Veress G, Szucs P, Antal M, et al. (2005). P boutons in lamina IX of the rodent spinal cord express high levels of glutamic acid decarboxylase-65 and originate from cells in deep medial dorsal horn. Proc. Natl. Acad. Sci 102, 9038–9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai F, and Yoshida Y (2018). Molecular mechanisms underlying monosynaptic sensory-motor circuit development in the spinal cord. Dev. Dyn 247, 581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM, Sürmeli G, and Kelly JS (2011). Motor Neurons and the Sense of Place. Neuron 72, 419–424. [DOI] [PubMed] [Google Scholar]
- Kiehn O (2016). Decoding the organization of spinal circuits that control locomotion. Nat. Rev. Neurosci 17, 224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SC, Del Barrio MG, Dalet A, Gatto G, Günther T, Zhang J, Seidler B, Saur D, Schüle R, and Goulding M (2017). RORβ Spinal Interneurons Gate Sensory Transmission during Locomotion to Secure a Fluid Walking Gait. Neuron 96, 1419–1431.e5.*A population of inhibitory Pax2+/Rorβ+ interneurons in the intermediate spinal cord provides presynaptic inhibition to sensory afferents that form polysynaptic connections with motor neurons. Loss of this population results in a ‘hopping gait’ due to pronounced hyperflexion.
- Konsolaki E, Koropouli E, Tsape E, Pothakos K, and Zagoraiou L (2020). Genetic Inactivation of Cholinergic C Bouton Output Improves Motor Performance but not Survival in a Mouse Model of Amyotrophic Lateral Sclerosis. Neuroscience 450, 71–80. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Sharma A, Lyashchenko AK, and Shneider NA (2016). Gamma motor neurons survive and exacerbate alpha motor neuron degeneration in ALS. Proc. Natl. Acad. Sci 113, E8316–E8325.*Gamma motor neurons, a class of motor neurons that do not receive monosynaptic input from proprioceptive sensory neurons, are resistant to neurodegenerative disease. Removal of excitatory proprioceptive inputs to alpha motor neurons is protective in mouse models of ALS neurodegeneration.
- Lallemend F, and Ernfors P (2012). Molecular interactions underlying the specification of sensory neurons. Trends Neurosci 35, 373–381. [DOI] [PubMed] [Google Scholar]
- Landoni LM, Myles JR, Wells TL, Mayer WP, and Akay T (2019). Cholinergic modulation of motor neurons through the C-boutons are necessary for the locomotor compensation for severe motor neuron loss during amyotrophic lateral sclerosis disease progression. Behav. Brain Res. 369, 111914. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD (2018). Sensorimotor anatomy of gait, balance, and falls. In Handbook of Clinical Neurology, pp. 3–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Silva ML, Imhoff-Manuel RD, Sharma A, Heckman C, Shneider NA, Roselli F, Zytnicki D, and Manuel M (2018). Hypoexcitability precedes denervation in the large fast-contracting motor units in two unrelated mouse models of ALS. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer WP, Murray AJ, Brenner-Morton S, Jessell TM, Tourtellotte WG, and Akay T (2018). Role of muscle spindle feedback in regulating muscle activity strength during walking at different speed in mice. J. Neurophysiol 120, 2484–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears SC, and Frank E (1997). Formation of Specific Monosynaptic Connections between Muscle Spindle Afferents and Motoneurons in the Mouse. J. Neurosci 17, 3128–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende M, Fletcher EV, Belluardo JL, Pierce JP, Bommareddy PK, Weinrich JA, Kabir ZD, Schierberl KC, Pagiazitis JG, Mendelsohn AI, et al. (2016). Sensory-Derived Glutamate Regulates Presynaptic Inhibitory Terminals in Mouse Spinal Cord. Neuron 90, 1189–1202.*Glutamate released from sensory afferent terminals regulates the effectiveness of presynaptic inhibition at monosynaptic sensorimotor connections.
- Mendelsohn AI, Simon CM, Abbott LF, Mentis GZ, and Jessell TM (2015). Activity Regulates the Incidence of Heteronymous Sensory-Motor Connections. Neuron 87, 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver K, Florez-Paz D, Badea T, Mentis G, Menon V, and de Nooij J (2021). Molecular correlates of muscle spindle and Golgi tendon organ afferents. Nat Commun 12, 1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, and Arber S (2009). Specificity of sensory–motor connections encoded by Sema3e–Plxnd1 recognition. Nature 459, 842–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen AH, and Athanasiou D (2009). Increase in presynaptic territory of C-terminals on lumbar motoneurons of G93A SOD1 mice during disease progression. Eur. J. Neurosci 29, 551–561. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, and Gossard J-P (2006). Dynamic Sensorimotor Interactions in Locomotion. Physiol. Rev 86, 89–154. [DOI] [PubMed] [Google Scholar]
- Rousso DL, Gaber ZB, Wellik D, Morrisey EE, and Novitch BG (2008). Coordinated Actions of the Forkhead Protein Foxp1 and Hox Proteins in the Columnar Organization of Spinal Motor Neurons. Neuron 59, 226–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P (2009). In search of lost presynaptic inhibition. Exp. Brain Res 196, 139–151. [DOI] [PubMed] [Google Scholar]
- Rudomin P, and Schmidt RF (1999). Presynaptic inhibition in the vertebrate spinal cord revisited. Exp. Brain Res 129, 1–37. [DOI] [PubMed] [Google Scholar]
- Russo RE, Delgado-Lezama R, and Hounsgaard J (2000). Dorsal root potential produced by a TTX-insensitive micro-circuitry in the turtle spinal cord. J. Physiol 528, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamatina A, Yang JH, Brenner-Morton S, Bikoff JB, Fang L, Kintner CR, Jessell TM, and Sweeney LB (2020). Differential Loss of Spinal Interneurons in a Mouse Model of ALS. Neuroscience 450, 81–95. [DOI] [PubMed] [Google Scholar]
- Sharma N, Flaherty K, Lezgiyeva K, Wagner DE, Klein AM, and Ginty DD (2020). The emergence of transcriptional identity in somatosensory neurons. Nature 577, 392–398.**Single-cell profiling of dorsal root ganglia cell types demonstrates that somatosensory neurons progress from an unspecialized state to distinct sensory neuron classes. Target-derived factors play an important role in this process.
- Shin MM, Catela C, and Dasen J (2020). Intrinsic control of neuronal diversity and synaptic specificity in a proprioceptive circuit. Elife 9.*Intrinsic Hox transcription factors in proprioceptive sensory neurons are involved in rostrocaudal patterning of sensorimotor circuits.
- Simon CM, Van Alstyne M, Lotti F, Bianchetti E, Tisdale S, Watterson DM, Mentis GZ, and Pellizzoni L (2019). Stasimon Contributes to the Loss of Sensory Synapses and Motor Neuron Death in a Mouse Model of Spinal Muscular Atrophy. Cell Rep 29, 3885–3901.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifani N (2014). Motor neurons and the generation of spinal motor neuron diversity. Front. Cell. Neurosci 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sürmeli G, Akay T, Ippolito GC, Tucker PW, and Jessell TM (2011). Patterns of Spinal Sensory-Motor Connectivity Prescribed by a Dorsoventral Positional Template. Cell 147, 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeoka A, Vollenweider I, Courtine G, and Arber S (2014). Muscle Spindle Feedback Directs Locomotor Recovery and Circuit Reorganization after Spinal Cord Injury. Cell 159, 1626–1639. [DOI] [PubMed] [Google Scholar]
- Tourtellotte WG, and Milbrandt J (1998). Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat. Genet 20, 87–91. [DOI] [PubMed] [Google Scholar]
- Tuthill JC, and Azim E (2018). Proprioception. Curr. Biol 28, R194–R203. [DOI] [PubMed] [Google Scholar]
- Vaughan SK, Kemp Z, Hatzipetros T, Vieira F, and Valdez G (2015). Degeneration of proprioceptive sensory nerve endings in mice harboring amyotrophic lateral sclerosis-causing mutations. J. Comp. Neurol 523, 2477–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukojicic A, Delestrée N, Fletcher EV, Pagiazitis JG, Sankaranarayanan S, Yednock TA, Barres BA, and Mentis GZ (2019). The Classical Complement Pathway Mediates Microglia-Dependent Remodeling of Spinal Motor Circuits during Development and in SMA. Cell Rep 29, 3087–3100.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Schieren I, Qian Y, Zhang C, Jessell TM, and de Nooij JC (2019). A Role for Sensory end Organ-Derived Signals in Regulating Muscle Spindle Proprioceptor Phenotype. J. Neurosci 39, 4252–4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Weinrich JAP, Russ JB, Comer JD, Bommareddy PK, DiCasoli RJ, Wright CVE, Li Y, van Roessel PJ, and Kaltschmidt JA (2017). A Role for Dystonia-Associated Genes in Spinal GABAergic Interneuron Circuitry. Cell Rep 21, 666–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AL, Kovatsis EM, Pozsgai RY, Tasnim A, Zhang Q, and Ginty DD (2019). Distinct Modes of Presynaptic Inhibition of Cutaneous Afferents and Their Functions in Behavior. Neuron 102, 420–434.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]


