Abstract
Aims
Evaluate cerebral autoregulation (CAR) by intracranial pressure reactivity index (PRx) and cerebral blood flow reactivity index (CBFx) during the first four hours following return of spontaneous circulation (ROSC) in a porcine model of pediatric cardiac arrest. Determine whether impaired CAR is associated with neurologic outcome.
Methods
Four-week-old swine underwent seven minutes of asphyxia followed by ventricular fibrillation induction and hemodynamic-directed CPR. Those achieving ROSC had arterial blood pressure, intracranial pressure (ICP), and microvascular cerebral blood flow (CBF) monitored for 4 h. Animals were assigned an 8 -h post-ROSC swine cerebral performance category score (1 = normal; 2–4=abnormal neurologic function). In this secondary analytic study, we calculated PRx and CBFx using a continuous, moving correlation coefficient between mean arterial pressure (MAP) and ICP, and between MAP and CBF, respectively. Burden of impaired CAR was the area under the PRx or CBFx curve using a threshold of 0.3 and normalized as percentage of monitoring duration.
Results
Among 23 animals, median PRx was 0.14 [0.06,0.25] and CBFx was 0.36 [0.05,0.44]. Median burden of impaired CAR was 21% [18,27] with PRx and 30% [17,40] with CBFx. Neurologically abnormal animals (n = 10) did not differ from normal animals (n = 13) in post-ROSC MAP (63 vs. 61 mmHg, p = 0.74), ICP (15 vs. 14 mmHg, p = 0.78) or CBF (274 vs. 397 Perfusion Units, p = 0.12). CBFx burden was greater among abnormal than normal animals (45% vs. 24%, p = 0.001), but PRx burden was not (25% vs. 20%, p = 0.38).
Conclusion
CAR is impaired early after ROSC. A greater burden of CAR impairment measured by CBFx was associated with abnormal neurologic outcome.
CHOP Institutional Animal Care and Use Committee protocol 19-001327.
Keywords: Pediatric cardiac arrest, Cerebral autoregulation, Cerebral blood flow
Introduction
Brain injury is a leading cause of morbidity and mortality after pediatric cardiac arrest.[1], [2] Post-cardiac arrest neuronal injury starts immediately after return of spontaneous circulation (ROSC) and involves complex pathophysiological responses to ischemia and reperfusion.3 During this vulnerable period, secondary brain injury can occur due to impaired cerebrovascular autoregulation (CAR) that leads to compromised cerebral perfusion and oxygen delivery.[4], [5] CAR is a physiologic process by which cerebral blood vessels dilate or constrict to maintain appropriate cerebral blood flow (CBF) over a wide range of cerebral perfusion pressures (CPP). This innate process protects the brain from ischemia and hyperemia.
CAR integrity can be assessed by the relationship between spontaneous low frequency fluctuations in cerebral blood flow (CBF) and mean arterial pressure (MAP) as a proxy for CPP and the driving pressure for CBF.[6], [7] The most commonly used metric is the Pressure Reactivity Index (PRx) which quantifies this relationship between MAP and intracranial pressure (ICP), a surrogate for CBF, using a moving Pearson’s correlation coefficient. Other similar metrics are based on the same computations and use different surrogates for CBF like regional brain tissue oxygenation or Doppler flow velocity.[8], [9] When derived from a direct measure of CBF, the CAR metric is termed CBFx. Small studies have demonstrated that infants, children, and adults have impaired CAR after cardiac arrest and that impaired CAR is associated with worse outcomes.[10], [11], [12], [13], [14], [15], [16], [17] However, CAR assessment in these studies did not begin until many hours after ROSC, thus limiting the ability to determine CAR integrity early after ROSC.
The objective of this study was to determine the integrity of CAR using 1) intracranial pressure reactivity index (PRx) and 2) cerebral blood flow reactivity index (CBFx) in the first four hours after ROSC in a swine model of pediatric cardiac arrest and determine the association of CAR impairment with neurologic outcome. We hypothesized that CAR would be impaired after cardiac arrest and that the burden of impaired CAR would be associated with abnormal neurologic outcome.
Methods
Experimental overview
This was an analytical study using data from completed experiments in an ongoing pre-clinical trial studying hemodynamic targets during cardiopulmonary resuscitation (CPR) (NHLBI R01HL141386). The primary findings of this study have not yet been published. The Institutional Animal Care and Use Committee of the Children’s Hospital of Philadelphia approved the experimental protocol (19-001327), which was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animal preparation and experimental protocol
Full methods and model justification are detailed in previous publications.[18], [19], [20], [21] Briefly, healthy four-week-old Yorkshire domestic swine (approximately 10 kg) were anesthetized, intubated, and mechanically ventilated. Central venous and arterial access was established and invasive hemodynamic monitors were advanced to the aorta and right atrium. Through right frontal burr holes, a Millar Mikro-Tip pressure catheter (Millar Instruments, Houston, TX, USA) was advanced 5 mm into the cerebral cortex to measure ICP and a laser Doppler probe (Periflux; Perimed AB, Jarafalla, Sweden) was secured over the dura mater to measure local microcirculatory blood perfusion (CBF) in a similar location.
All swine underwent seven minutes of asphyxia via endotracheal tube clamping, followed by electrical induction of ventricular fibrillation and immediate commencement of hemodynamic-directed CPR with titration of chest compression force to achieve an a priori systolic blood pressure goal of either 90 mmHg or 110 mmHg and vasopressor administration to achieve a diastolic blood pressure goal of either 30 mmHg or 40 mm Hg. An initial defibrillation attempt was provided after 10 min of CPR. Animals without ROSC at that time continued to receive hemodynamic-directed CPR for up to 10 additional minutes with defibrillation attempts, when indicated by rhythm, every two minutes. The experiment ended after 20 min of CPR in animals not achieving ROSC.
Those achieving ROSC were maintained on a post-arrest protocol including the following: 1) titration of epinephrine infusion and crystalloid boluses to maintain MAP between 45 and 60 mmHg; 2) titration of minute ventilation to maintain end-tidal carbon dioxide between 38 and 42 mmHg; 3) titration of supplemental oxygen to maintain peripheral oxygen saturation between 92 and 98%; and 4) titration of inhaled isoflurane to maintain general anesthesia. Four hours post-ROSC, invasive monitors were removed and animals were extubated. Animals that did not survive to 4 h post-ROSC or did not have sufficient data from invasive monitors were excluded. Swine were serially assessed by trained investigators, who assigned an 8 -h post-ROSC swine cerebral performance category (CPC) score. This scale is a global assessment of neurologic function with a value of 1 representing normal or near-normal function and values of 2–4 representing substantial neurologic dysfunction.[19], [22]
Data acquisition and cerebral autoregulation monitoring
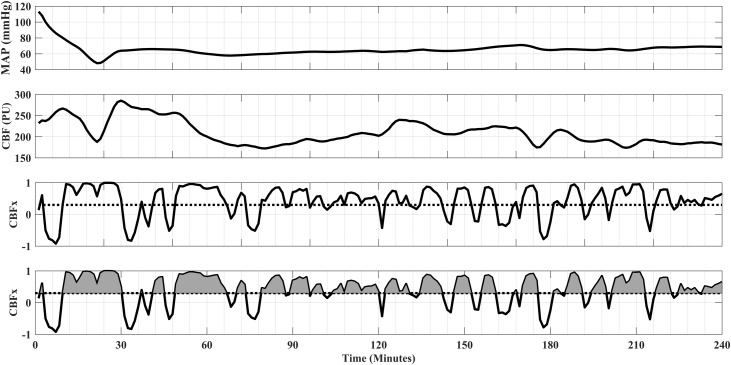
Aortic blood pressure, ICP, and CBF signals were sampled at 100 Hz (PowerLab; ADInstruments, Chelmsford, MA, USA) for the first 4 h after ROSC. We manually excluded periods of unusable data (e.g., sensor calibration, catheter obstruction) and applied an automated sliding-median filter with a variable window size to remove residual artifacts. With MATLAB (Mathworks, Natick, MA), we calculated PRx and CBFx using a continuous, moving correlation coefficient between MAP and ICP and between MAP and CBF, respectively.23 First, a 10-second average filter was applied to limit the influence of faster frequencies related to the pulse and respiration waveform components. Then, PRx and CBFx were calculated as Pearson’s correlation coefficient of 30 consecutive pairs of these 10-second averaged values (total duration of 300 s for each calculation). PRx and CBFx are indices that range from -1 to +1. Negative or near-zero values represent intact CAR because MAP and ICP (or MAP and CBF) are either negatively correlated or are not correlated. Whereas, when cerebral autoregulation is impaired, the indices are positive and approach +1 because MAP and ICP (or MAP and CBF) positively correlate. The indices were updated every 60 s using a moving window (80% overlap of data). To determine the total burden (combining the magnitude and duration) of impaired CAR, we calculated the area under the PRx and CBFx curves using a threshold of ≥0.3 and normalized as a percentage of the monitoring duration (Fig. 1).24 Using this method, if PRx (or CBFx) was <0.3 for the entire monitored duration, the percent burden would be 0%, whereas if PRx (or CBFx) was 1 for the entire monitored duration, the percent burden would be 100%.
Fig. 1.
Representative data from a single animal demonstrating the calculation for the burden of impaired autoregulation. The four graphs (top to bottom) represent mean arterial pressure (MAP), cerebral blood flow (CBF), cerebral autoregulation index (CBFx) correlating MAP with CBF, and the burden of CBFx >0.3 over the 4 -h post-ROSC monitoring period. The CBFx burden is calculated by the area under the curve (gray shaded areas) representing both the magnitude and duration of impaired cerebral autoregulation. The area under the curve is then normalized as a percentage of the monitoring duration for each animal.
Statistical analysis
Physiologic data were summarized as 1-minute mean values throughout the post-ROSC period. From these values, mean values for each animal were calculated for each hour post-ROSC as well as for the entire 4 -h post-ROSC period. Data were described as means with standard deviations (SD) or medians with interquartile ranges (IQR).
Each animal was classified as normal (8 -h CPC 1) or abnormal (8 -h CPC 2 or 3).19 Differences in physiologic parameters over the entire 4 -h period were compared between outcome groups using Wilcoxon rank-sum tests. Hourly mean values of systemic (MAP, PaCO2, PaO2) and cerebral (ICP, CBF) physiologic parameters, as well as CAR indices (PRx, CBFx, percent PRx and CBFx burden), were compared between outcome groups at each time point using Wilcoxon rank-sum with the Bonferroni correction. MAP, ICP, CBF were compared at 5 different time points; therefore, a significant p-value was <0.01. PaO2, PaCO2, PRx, CBFx and percent PRx and CBFx burden were compared at 4 time points; therefore, a significant p-value was considered < 0.0125. Analyses were performed using SAS v9.2 (SAS Institute Inc., Cary, NC, USA), GraphPad Prism (v5.03, GraphPad Software Inc., La Jolla, CA, USA), and Stata (Version 14.2; StataCorp, College Station, TX, USA).
Results
Twenty-eight animals completed the experimental protocol. Twenty-five of 28 remaining animals survived to 4 h post-ROSC and 23 had sufficient post-ROSC data for analysis. The median duration of post-ROSC monitoring was 3.9 [3.7, 4] hours. There were no adverse events. Among these 23 animals, the median 4 -h post-ROSC PRx was 0.14 [0.06, 0.25] and the median CBFx was 0.36 [0.05, 0.44]. The median percent burden of impaired CAR measured by PRx was 21% [18,27] and measured by CBFx was 30% [17,40].
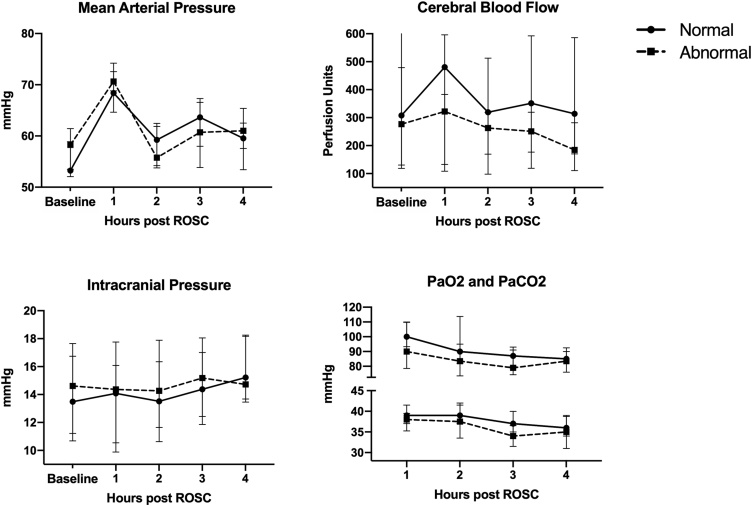
Thirteen (56%) animals had normal neurologic function at 8 h post-ROSC, and 10 (44%) had abnormal neurologic function, seven with CPC 2 and three with CPC 3. There were no differences in baseline or hourly values of MAP, ICP, CBF, PaO2, or PaCO2 between neurologic outcome groups (Fig. 2). Neurologically abnormal animals did not differ from normal animals in post-ROSC MAP (63 [59, 65] vs. 61 [60, 66] mmHg, p = 0.74), ICP (15 [12,17] vs. 14 [13,17] mmHg, p = 0.78) or CBF (274 [129, 315] vs. 397 [210, 486] Perfusion Units, p = 0.12).
Fig. 2.
Cerebral and systemic physiologic measurements at baseline (i.e. pre-arrest) and over the 4 -h post-ROSC recording period for normal and abnormal animals. Plots are medians with interquartile ranges. Baseline represents pre-arrest values. Values at each timepoint compared with Wilcoxon rank-sum test with Bonferroni correction (p < 0.01 considered significant for MAP, ICP, CBF; p < 0.0125 considered significant for PaO2, PaCO2).
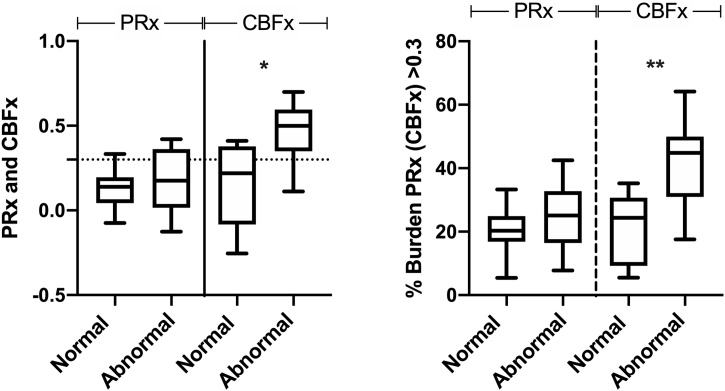
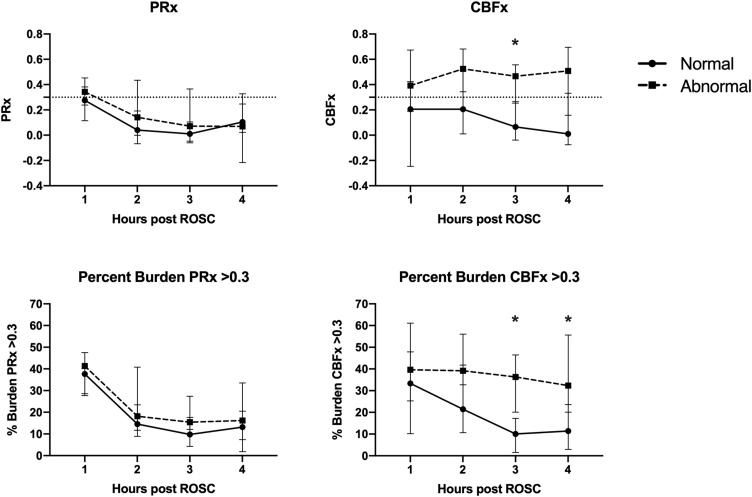
Median CBFx in the four hours post-ROSC was greater for animals with abnormal versus normal neurologic outcome (0.50 [0.37, 0.57] vs. 0.22 [-0.07, 0.36], p = 0.002, Fig. 3), and the percent burden of impaired CBFx over the 4 -h period was greater for abnormal animals (45% [32, 47] vs. 24% [10,27], p = 0.001, Fig. 3). In contrast, median PRx in the four hours post-ROSC was not different between the abnormal and normal neurologic outcome groups (0.14 [0.09, 0.19 vs. 0.18 [0.06, 0.34], p = 0.648), and the percent burden of impaired PRx was not different between these two outcome groups (25% [18,32] vs. 20% [18,25], p = 0.376). The hourly PRx and CBFx comparisons between the two outcome groups is shown in Fig. 4 which demonstrates that CBFx differed between groups in Hour 3 (0.47 [0.27, 0.53] vs. 0.07 [-0.02, 0.22], p = 0.0019), and the percent burden of impaired CBFx differed between groups in Hour 3 (36% [21, 44] vs. 10% [2,15], p = 0.0008) and Hour 4 (32% [26, 50] vs. 11% [4,22], p = 0.0056).
Fig. 3.
PRx, CBFx and percent PRx and CBFx burden in normal and abnormal outcome animals over the entire 4 -h post-ROSC recording period. Whiskers represent 5-95% percentile. Dotted line represents PRx (CBFx) = 0.3, the threshold for impaired autoregulation. Comparisons between outcome groups performed with Wilcoxon rank-sum test (p < 0.05 considered significant). * p = 0.002; **p = 0.001.
Fig. 4.
PRx, CBFx and percent PRx and CBFx burden >0.3 in normal and abnormal animals for each hour of the post-ROSC recording period. Dotted line represents PRx (CBFx) = 0.3, the threshold for impaired autoregulation. Plots are medians with interquartile ranges. Values at each timepoint compared with Wilcoxon rank-sum test with Bonferroni correction (*p < 0.0125 considered significant).
There was no difference between abnormal and normal outcome groups in either the amount of intravenous fluids (36 [13,40] vs. 40 [20, 50] ml/hour, p = 0.416) or epinephrine infusion rates (4 [0,25] vs. 0 [0,19] mcg/hour, p = 0.628) received during the four-hour post-ROSC period.
Discussion
This study demonstrated that CAR is impaired early after ROSC in a porcine model of pediatric cardiac arrest. A greater burden of impaired CAR, as determined by the cerebral blood flow reactivity index (CBFx), within the first 4 h following ROSC was associated with worse neurologic outcome. When derived from the pressure reactivity index (PRx), the burden of impaired CAR was not associated with neurologic outcome. These data from a translational animal model contribute to further understanding of the cerebral pathophysiologic consequences of cardiac arrest and suggest that altered CAR in the early post-arrest period may have a role in the pathogenesis of post-arrest brain injury.
A limited number of studies in adults, children, and neonates suggest that CAR may be impaired after cardiac arrest and associated with worse outcomes.[10], [11], [13], [14], [15], [25] Unfortunately, the techniques used to measure CAR varied widely between these studies, as did the timing and duration of monitoring. While some degree of impaired CAR was observed after cardiac arrest in all cohorts, the limited sample sizes and substantially variability in patient populations and severity of brain injury makes comparing the degree of impaired CAR between children and adults not feasible. None of these clinical studies, however, evaluated CAR early after ROSC. In our animal model, CAR was impaired within these early hours post-arrest. Interestingly, clinical investigations have shown that hypotension is also common early (0−6 hours) after ROSC and is associated with worse brain injury and outcomes, even after accounting for cardiac arrest characteristics.[26], [27], [28] Patients with impaired CAR and concomitant hypotension early after ROSC may have worse secondary brain injury due to critical alterations in CBF and cerebral metabolism that trigger a cascade of cellular damage.[29], [30] All of these data suggest that the first few hours post-ROSC may be a crucial time for rigorous systemic and cerebral hemodynamic monitoring and aggressive supportive care.
In our study, animals with a greater burden of impaired CAR using a direct measurement of CBF were more likely to have abnormal neurologic outcomes. We hypothesize that these animals had increased cerebral metabolic demand that was not met by their tightly controlled blood pressure. Alternatively, their impaired CAR may have led to hyperemic brain injury. Although while impaired CAR determined by CBFx burden was associated with worse neurologic outcomes, impaired CAR determined by PRx burden was not. Both CBFx and PRx are time domain-based relationships between low frequency fluctuations in MAP and CBF.[31], [32] However, CBFx directly assesses the effects of MAP fluctuations on CBF, whereas PRx measures the effects of MAP fluctuations on ICP, a surrogate marker of CBF. Notably, in both our study and in prior large animal and human studies, ICP is not elevated in the first few hours post-arrest.[7], [33] This may explain in part why there was no demonstrable difference in PRx between normal and abnormal animals.
Post-arrest care focuses on limiting secondary brain injury.2 The findings from this translational model suggest that avoiding hypotension and maintaining normoxia and normocapnia are not sufficient to limit secondary brain injury. Instead, our data suggest that rigorous continuous monitoring of cerebral hemodynamics and physiology, including assessments of CAR, may provide novel opportunities to uncover cerebral hemodynamic abnormalities and perhaps ultimately prevent or mitigate secondary brain injury. Although we measured cerebral hemodynamics with an invasive technique, promising non-invasive technologies have been successfully employed in translational swine studies,34 as well as clinical studies.[35], [36], [37] Utilizing non-invasive devices that directly measure CBF at the bedside may facilitate detection of CBFx-determined CAR impairments that could be used to individualize hemodynamic management during post-cardiac arrest care and potentially influence neurologic outcomes.[38], [39], [40]
This study has several limitations. We only evaluated CAR during the initial 4 h post-arrest. Longer monitoring periods would allow for the measurement of temporal trends in CAR. Increased PRx burden later in the process may have been associated with worse neurologic function, as has been shown in adult clinical studies.41 However, our data suggest that monitoring CBFx in the first hours post-ROSC may be a fertile approach to delineate a potential harbinger of poor neurologic outcome and perhaps ultimately mitigate the damage when effective interventions are developed. The lack of variability in blood pressures due to the strict post-cardiac arrest treatment protocol precluded determination the lower limit of autoregulation for each animal. Yet, this protocol allowed us to show that increased CBFx burden was associated with poor neurologic outcome even in the setting of tight blood pressure control and prevention of post-arrest hypotension. We were also unable to determine PRx- or CBFx-derived optimal blood pressure due to the limited duration of the experiment designed to investigate the first 4 h after ROSC.42 The CPC scale is a gross measure of neurologic outcome for swine and was only assessed at eight hours post-ROSC. It is unclear if the association between the burden of impaired CAR and abnormal neurologic outcome will persist at later time points. Lastly, the calculated burden of impaired CAR in this study combined both the duration and magnitude of impaired CAR. Larger studies with longer recording periods will be needed to determine if one of these components is more strongly associated with adverse outcomes.
Conclusions
In a large animal model of pediatric cardiac arrest, CAR was impaired in the first several hours after ROSC and a greater burden of CAR impairment when determined using a direct measurement of microvascular cerebral blood flow was associated with an abnormal neurologic outcome.
Conflicts of interest
None: Dr. Kirschen, Mr. Majmudar, Mr. Landis, Dr. Balu, Dr. Balasubramanian, Dr. Topjian, Dr. Berg.
Dr. Morgan – NIH NHLBI grant support
Dr. Ko - NIH NHLBI T32-HL007915 grant support
Dr. Sutton – NIH NHLBI grant support; additional NIH support in the area of hemodynamic-directed CPR; main author Pediatric Advanced Life Support Guidelines; Chair: American Heart Association Get with the Guidelines – Resuscitation Pediatric Research Task Force
Dr. Kilbaugh - NIH NHLBI grant support
CRediT authorship contribution statement
Matthew P Kirschen: Conceptualization, Methodology, Validation, Formal analysis, Writing - original draft. Ryan W. Morgan: Conceptualization, Methodology, Validation, Investigation, Resources, Data curation, Writing - review & editing, Funding acquisition. Tanmay Majmudar: Methodology, Software, Formal analysis, Writing - review & editing. William P. Landis: Methodology, Software, Investigation, Data curation, Writing - review & editing. Tiffany Ko: Methodology, Investigation, Data curation, Writing - review & editing. Ramani Balu: Conceptualization, Methodology, Writing - review & editing. Sriram Balasubramanian: Supervision, Formal analysis, Resources, Writing - review & editing. Alexis Topjian: Supervision, Conceptualization, Methodology, Writing - review & editing. Robert M. Sutton: Supervision, Conceptualization, Methodology, Investigation, Resources, Writing - review & editing. Robert A. Berg: Supervision, Conceptualization, Methodology, Investigation, Resources, Writing - review & editing. Todd J. Kilbaugh: Supervision, Conceptualization, Methodology, Validation, Investigation, Resources, Writing - review & editing, Funding acquisition.
Acknowledgements
The authors wish to thank Thomas Hallowell, Nile Delso, Kathryn Graham, Yuxi Lin, Sarah Morton, J. Laurenson Ward, Jonathan Starr, Norah Taraska, Anna Roberts, and Adam S. Himebauch for their work on this project. This study was funded by NIH NHLBI R01HL141386 (PI: Kilbaugh). Dr. Morgan’s effort on this study was supported by NIH NHLBI K23HL148541.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.resplu.2020.100051.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Neumar R.W., Nolan J.P., Adrie C. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 2.Topjian A.A., de Caen A., Wainwright M.S. Pediatric Post-Cardiac Arrest Care: A Scientific Statement From the American Heart Association. Circulation. 2019 doi: 10.1161/CIR.0000000000000697. CIR0000000000000697. [DOI] [PubMed] [Google Scholar]
- 3.Manole M.D., Kochanek P.M., Fink E.L., Clark R.S. Postcardiac arrest syndrome: focus on the brain. Curr Opin Pediatr. 2009;21:745–750. doi: 10.1097/MOP.0b013e328331e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekhon M.S., Ainslie P.N., Griesdale D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a "two-hit" model. Crit Care. 2017;21:90. doi: 10.1186/s13054-017-1670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishizawa H., Kudoh I. Cerebral autoregulation is impaired in patients resuscitated after cardiac arrest. Acta Anaesthesiol Scand. 1996;40:1149–1153. doi: 10.1111/j.1399-6576.1996.tb05579.x. [DOI] [PubMed] [Google Scholar]
- 6.Zweifel C., Dias C., Smielewski P., Czosnyka M. Continuous time-domain monitoring of cerebral autoregulation in neurocritical care. Med Eng Phys. 2014;36:638–645. doi: 10.1016/j.medengphy.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Lee J.K., Brady K.M., Mytar J.O. Cerebral blood flow and cerebrovascular autoregulation in a swine model of pediatric cardiac arrest and hypothermia. Crit Care Med. 2011;39:2337–2345. doi: 10.1097/CCM.0b013e318223b910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnelly J., Budohoski K.P., Smielewski P., Czosnyka M. Regulation of the cerebral circulation: bedside assessment and clinical implications. Crit Care. 2016;20:129. doi: 10.1186/s13054-016-1293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoiland R.L., Sekhon M.S., Cardim D. Lack of agreement between optimal mean arterial pressure determination using pressure reactivity index versus cerebral oximetry index in hypoxic ischemic brain injury after cardiac arrest. Resuscitation. 2020;152:184–191. doi: 10.1016/j.resuscitation.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Lee J.K., Brady K.M., Chung S.E. A pilot study of cerebrovascular reactivity autoregulation after pediatric cardiac arrest. Resuscitation. 2014;85:1387–1393. doi: 10.1016/j.resuscitation.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekhon M.S., Smielewski P., Bhate T.D. Using the relationship between brain tissue regional saturation of oxygen and mean arterial pressure to determine the optimal mean arterial pressure in patients following cardiac arrest: A pilot proof-of-concept study. Resuscitation. 2016;106:120–125. doi: 10.1016/j.resuscitation.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Sekhon M.S., Gooderham P., Menon D.K. The Burden of Brain Hypoxia and Optimal Mean Arterial Pressure in Patients With Hypoxic Ischemic Brain Injury After Cardiac Arrest. Crit Care Med. 2019;47:960–969. doi: 10.1097/CCM.0000000000003745. [DOI] [PubMed] [Google Scholar]
- 13.Pham P., Bindra J., Chuan A., Jaeger M., Aneman A. Are changes in cerebrovascular autoregulation following cardiac arrest associated with neurological outcome? Results of a pilot study. Resuscitation. 2015;96:192–198. doi: 10.1016/j.resuscitation.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Carrasco M., Perin J., Jennings J.M. Cerebral Autoregulation and Conventional and Diffusion Tensor Imaging Magnetic Resonance Imaging in Neonatal Hypoxic-Ischemic Encephalopathy. Pediatr Neurol. 2018;82:36–43. doi: 10.1016/j.pediatrneurol.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burton V.J., Gerner G., Cristofalo E. A pilot cohort study of cerebral autoregulation and 2-year neurodevelopmental outcomes in neonates with hypoxic-ischemic encephalopathy who received therapeutic hypothermia. BMC Neurol. 2015;15:209. doi: 10.1186/s12883-015-0464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundgreen C., Larsen F.S., Herzog T.M., Knudsen G.M., Boesgaard S., Aldershvile J. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke. 2001;32:128–132. doi: 10.1161/01.str.32.1.128. [DOI] [PubMed] [Google Scholar]
- 17.Ameloot K., Genbrugge C., Meex I. An observational near-infrared spectroscopy study on cerebral autoregulation in post-cardiac arrest patients: time to drop’ one-size-fits-all’ hemodynamic targets? Resuscitation. 2015;90:121–126. doi: 10.1016/j.resuscitation.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Morgan R.W., Kilbaugh T.J., Shoap W. A hemodynamic-directed approach to pediatric cardiopulmonary resuscitation (HD-CPR) improves survival. Resuscitation. 2017;111:41–47. doi: 10.1016/j.resuscitation.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lautz A.J., Morgan R.W., Karlsson M. Hemodynamic-Directed Cardiopulmonary Resuscitation Improves Neurologic Outcomes and Mitochondrial Function in the Heart and Brain. Crit Care Med. 2019;47 doi: 10.1097/CCM.0000000000003620. e241-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mavroudis C.D., Ko T.S., Morgan R.W. Epinephrine’s effects on cerebrovascular and systemic hemodynamics during cardiopulmonary resuscitation. Crit Care. 2020;24:583. doi: 10.1186/s13054-020-03297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marquez A.M., Morgan R.W., Ko T. Oxygen Exposure During Cardiopulmonary Resuscitation Is Associated With Cerebral Oxidative Injury in a Randomized, Blinded, Controlled, Preclinical Trial. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berg R.A., Chapman F.W., Berg M.D. Attenuated adult biphasic shocks compared with weight-based monophasic shocks in a swine model of prolonged pediatric ventricular fibrillation. Resuscitation. 2004;61:189–197. doi: 10.1016/j.resuscitation.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Czosnyka M., Smielewski P., Kirkpatrick P., Laing R.J., Menon D., Pickard J.D. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41:11–17. doi: 10.1097/00006123-199707000-00005. discussion 7-9. [DOI] [PubMed] [Google Scholar]
- 24.Ono M., Brady K., Easley R.B. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg. 2014;147:483–489. doi: 10.1016/j.jtcvs.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovett M.E., Maa T., Chung M.G., O’Brien N.F. Cerebral blood flow velocity and autoregulation in paediatric patients following a global hypoxic-ischaemic insult. Resuscitation. 2018;126:191–196. doi: 10.1016/j.resuscitation.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Laverriere E.K., Polansky M., French B., Nadkarni V.M., Berg R.A., Topjian A.A. Association of Duration of Hypotension With Survival After Pediatric Cardiac Arrest. Pediatr Crit Care Med. 2020;21:143–149. doi: 10.1097/PCC.0000000000002119. [DOI] [PubMed] [Google Scholar]
- 27.Topjian A.A., Telford R., Holubkov R. The association of early post-resuscitation hypotension with discharge survival following targeted temperature management for pediatric in-hospital cardiac arrest. Resuscitation. 2019;141:24–34. doi: 10.1016/j.resuscitation.2019.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Topjian A.A., Telford R., Holubkov R. Association of Early Postresuscitation Hypotension With Survival to Discharge After Targeted Temperature Management for Pediatric Out-of-Hospital Cardiac Arrest: Secondary Analysis of a Randomized Clinical Trial. JAMA Pediatr. 2018;172:143–153. doi: 10.1001/jamapediatrics.2017.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busl K.M., Greer D.M. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation. 2010;26:5–13. doi: 10.3233/NRE-2010-0531. [DOI] [PubMed] [Google Scholar]
- 30.Iordanova B., Li L., Clark R.S.B., Manole M.D. Alterations in Cerebral Blood Flow after Resuscitation from Cardiac Arrest. Front Pediatr. 2017;5:174. doi: 10.3389/fped.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dias C., Silva M.J., Pereira E. Optimal Cerebral Perfusion Pressure Management at Bedside: A Single-Center Pilot Study. Neurocrit Care. 2015;23:92–102. doi: 10.1007/s12028-014-0103-8. [DOI] [PubMed] [Google Scholar]
- 32.Zeiler F.A., Ercole A., Czosnyka M. Continuous cerebrovascular reactivity monitoring in moderate/severe traumatic brain injury: a narrative review of advances in neurocritical care. Br J Anaesth. 2020 doi: 10.1016/j.bja.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 33.Sekhon M.S., Griesdale D.E., Ainslie P.N. Intracranial pressure and compliance in hypoxic ischemic brain injury patients after cardiac arrest. Resuscitation. 2019;141:96–103. doi: 10.1016/j.resuscitation.2019.05.036. [DOI] [PubMed] [Google Scholar]
- 34.Ko T.S., Mavroudis C.D., Baker W.B. Non-invasive optical neuromonitoring of the temperature-dependence of cerebral oxygen metabolism during deep hypothermic cardiopulmonary bypass in neonatal swine. J Cereb Blood Flow Metab. 2018 doi: 10.1177/0271678X18809828. 271678X18809828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Favilla C.G., Mesquita R.C., Mullen M. Optical bedside monitoring of cerebral blood flow in acute ischemic stroke patients during head-of-bed manipulation. Stroke. 2014;45:1269–1274. doi: 10.1161/STROKEAHA.113.004116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lynch J.M., Ko T., Busch D.R. Preoperative cerebral hemodynamics from birth to surgery in neonates with critical congenital heart disease. J Thorac Cardiovasc Surg. 2018;156:1657–1664. doi: 10.1016/j.jtcvs.2018.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim M.N., Edlow B.L., Durduran T. Continuous optical monitoring of cerebral hemodynamics during head-of-bed manipulation in brain-injured adults. Neurocrit Care. 2014;20:443–453. doi: 10.1007/s12028-013-9849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim M.N., Durduran T., Frangos S. Noninvasive measurement of cerebral blood flow and blood oxygenation using near-infrared and diffuse correlation spectroscopies in critically brain-injured adults. Neurocrit Care. 2010;12:173–180. doi: 10.1007/s12028-009-9305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Busch D.R., Balu R., Baker W.B. Detection of Brain Hypoxia Based on Noninvasive Optical Monitoring of Cerebral Blood Flow with Diffuse Correlation Spectroscopy. Neurocrit Care. 2019;30:72–80. doi: 10.1007/s12028-018-0573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He L., Baker W.B., Milej D. Noninvasive continuous optical monitoring of absolute cerebral blood flow in critically ill adults. Neurophotonics. 2018;5 doi: 10.1117/1.NPh.5.4.045006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balu R., Baghshomali S., Abella B.S., Kofke A. Cerebrovascular pressure reactivity predicts outcome in diffuse hypoxic-ischemic brain injury. Circulation. 2018;138:A12. doi: 10.1016/j.resuscitation.2021.04.023. [DOI] [PubMed] [Google Scholar]
- 42.Donnelly J., Czosnyka M., Adams H. Individualizing Thresholds of Cerebral Perfusion Pressure Using Estimated Limits of Autoregulation. Crit Care Med. 2017;45:1464–1471. doi: 10.1097/CCM.0000000000002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.