Abstract
Objective
To determine the proportion of men presenting for fertility evaluation who reported having an established primary care physician (PCP).
Design
Retrospective, observational study.
Setting
Academic health center.
Patient(s)
All men presenting for initial male factor infertility consultation with a single reproductive urologist between 2002 and 2018.
Intervention(s)
Men were asked to provide the name of their PCP at the time of initial visit.
Main Outcome Measure(s)
Descriptive statistics characterized the proportion of men with a PCP at the time of evaluation and associations between PCP status and clinical characteristics.
Result(s)
Among 4,127 men presenting for initial fertility consultation, 844 (20.5%) reported having an established PCP, 480 (11.6%) reported no PCP, and 2,803 (67.9%) did not have data available. Among 1,302 men who had a prior primary care visit within our healthcare system, 414 (31.8%) had been seen within 1 year before their fertility evaluation. Men with an established PCP were slightly older than those without a PCP, with higher body mass index, and lower systolic blood pressure. Hormonal profiles were similar across groups, but men with an established PCP had a significantly higher total motile sperm count than those without a PCP, median 53 (interquartile range, 11–109) versus 35 (interquartile range, 8–98).
Conclusion(s)
More than one third of men presenting for fertility evaluation did not have an established PCP. Reproductive urologists are uniquely positioned to facilitate the critical relationship between young men and PCPs, which should be a key component of the male fertility treatment paradigm.
Key Words: Male factor infertility, primary care physicians, primary health care, men’s health
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/users/16110-fertility-and-sterility/posts/64894-xfre00029
There is increasing evidence that male factor infertility is strongly associated with medical comorbidity. Early studies found that men presenting for fertility evaluation were at increased risk for testicular cancer and endocrine abnormalities (1, 2). More recently, many investigators have demonstrated a correlation between male fertility and a wide range of medical conditions. For example, Cazzaniga et al. (3) reported higher blood pressure among men with primary infertility compared with fertile controls. Likewise, there are strong associations between male factor infertility and obesity, testicular cancer, lymphoma, and other conditions (4, 5). Men with abnormal semen parameters may even have a higher risk of mortality compared with those with normal semen parameters (6).
The prevalence of medical comorbidity in subfertile men has profound implications for men’s health and access to preventative care. Although there are limited data regarding the utilization of primary care by male patients, one study (7) found that nearly one third of men did not have a regular primary care physician (PCP), whereas only 17% of women did not have a PCP. Many women have an established relationship with their gynecologist with whom they schedule regular visits and who can fulfill the role of a PCP (8). In addition, gynecologists are often involved in the initial evaluation and diagnosis of infertility, positioning these physicians to serve a dual role as PCP and member of the fertility care team, thereby ensuring that they remain involved in a woman’s healthcare after initial diagnosis and treatment. In contrast, young men are probably less likely to have an established urologist, as there are no recommended urologic screening tests for young men and the typical onset of many urologic issues occurs later in life. Furthermore, there may be little role for the urologist to remain involved in a man’s care after the diagnosis and treatment of infertility. As such, there appears to be an opportunity for reproductive urologists to serve as gatekeepers for men’s health, helping subfertile men at high risk for comorbidity to establish care with a PCP and obtain appropriate preventative healthcare.
We sought to characterize the proportion of men presenting for fertility evaluation without an established PCP to determine the extent to which reproductive urologists can play a role in assisting men to establish routine primary care. We hypothesized that a large proportion of men presenting for fertility evaluation did not have an established PCP or had not seen a PCP in the prior year.
Materials and methods
Patient Cohort
We performed a retrospective review of all men presenting for initial fertility evaluation at a tertiary care center between 2002 and 2018, during which time an electronic medical record was active. We queried the Northwestern Medicine Enterprise Data Warehouse to identify all men with a new patient visit to a reproductive urologist during the study period. Patient demographics and clinical characteristics were abstracted including body mass index (BMI) and elevated systolic or diastolic blood pressure (SBP, DBP), when available. Elevated blood pressure was defined as SBP >130 mmHg or DBP >80 mmHg, according to the American College of Cardiology/American Heart Association guidelines. The BMI was recorded at the point of care, and blood pressure abstracted from the most recent clinical encounter within 1 year before the initial visit. Initial serum hormone levels and semen parameters were also recorded.
Primary Outcome
The primary outcome for the study was the presence or absence of an established PCP. At the time of initial evaluation, all men were asked to provide the name of a PCP. These data were obtained from any one of multiple members of the care team including the receptionist, medical assistant, registered nurse, or physician. Any man who provided the name of a physician was considered to have an established PCP, irrespective of that physician’s specialty. Only men who explicitly reported not having a physician were considered not to have a PCP, whereas men who did not answer the question or for whom data were not available were categorized separately.
Statistical Analysis
The proportion of men reporting an established PCP was examined over time using univariable logistic regression. Descriptive statistics were used to compare men with an established PCP, men without a PCP, and men who did not answer the question. Given the large proportion of missing data, sensitivity analysis was performed with exclusion of all men who did not answer the question. The χ2 and Kruskall-Wallis tests were used, where appropriate. Statistical significance was determined at the level of P<.05. All statistical analysis was performed using Stata SE 16.0 (StataCorp). The study was approved by the local institutional review board.
Results
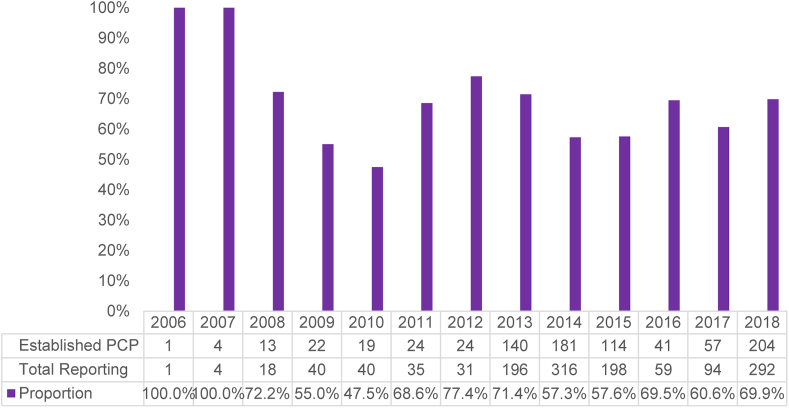
A total of 4,127 men presented for fertility evaluation during the study period of whom 844 (20.5%) reported having an established PCP, 480 (11.6%) reported no PCP, and 2,803 (67.9%) did not answer the question or did not have data available. Among all men reporting an established PCP, 409 (48.5%) had at least one prior internal medicine or primary care encounter within our healthcare system. In contrast, among all men who did not answer the question, 789 (28.2%) had at least one prior internal medicine or primary care encounter within our healthcare system (P<.001). When considering only those men who responded to the question, there was no change in the proportion of men reporting an established PCP during the study period (Fig. 1; P = .261).
Figure 1.
Change over time in the proportion of men presenting for fertility evaluation reporting an established primary care physician (PCP).
Among 1,302 men who had an internal medicine or primary care visit within our healthcare system, only 414 (31.8%) had been seen within 1 year before their fertility evaluation. Demographic and clinical characteristics are presented in Table 1. Men with an established PCP were slightly older than those without a PCP, median 35 years (interquartile range [IQR], 32–40) vs. 34 years (IQR, 31–38; P<.001 [sensitivity analysis, P<.001]). Among men for whom BMI was available (N = 2,027; 49.1%), median BMI was higher among men with an established PCP (median, 26.5; IQR, 24.3–29.5) versus those without a PCP (median, 25.8; IQR, 23.7–29.8; P = .015 [sensitivity analysis, P = .240]). A similar proportion of men met criteria for obesity in both groups, 17.6% and 17.5%, respectively; however, the overall distribution of obesity (yes, no, unknown) was statistically significant across groups (P = .001), driven by a difference in the proportion of men with unknown obesity status in each group. Among men for whom blood pressure was available (N = 1,370; 33.2%), SBP was lower among men with an established PCP (median, 120; IQR, 112–130) versus those without a PCP (median, 125; IQR, 116–134; P = .001 [sensitivity analysis, P = .007]). There was no difference in DBP across groups (P = .236; sensitivity analysis, P = .109). However, the proportion of men meeting criteria for elevated blood pressure was higher among men with an established PCP (22.3%) compared with those without a PCP (15.6%) (P<.001; sensitivity analysis, P<.001).
Table 1.
Demographic and clinic characteristics of men according to primary care physician (PCP) status.
| Characteristics | No PCP | Established PCP | No response | P value |
|---|---|---|---|---|
| N (%) | 480 (11.6%) | 844 (20.5%) | 2,803 (67.9%) | |
| Age, y, median (IQR) | 34 (31–38) | 35 (32–40) | 34 (31–39) | <.001 |
| Age, y, mean (SD) | 34.7 (6.6) | 36.2 (7.3) | 35.4 (6.7) | <.001 |
| SBP, mmHg, median (IQR) | 125 (116–134) | 120 (112–130) | 120 (110–130) | .001 |
| DBP, mmHg, median (IQR) | 77 (70–82) | 76 (70–80) | 76 (70–80) | .236 |
| High blood pressure | <.001 | |||
| No | 57 (11.9%) | 219 (25.9%) | 430 (15.3%) | |
| Yes | 75 (15.6%) | 188 (22.3%) | 401 (14.3%) | |
| Unknown | 348 (72.5%) | 437 (51.8%) | 1972 (70.4%) | |
| BMI, median (IQR) | 25.8 (23.7–29.8) | 26.4 (24.3–29.4) | 26.6 (24.4–30.3) | .015 |
| Obesity | .001 | |||
| No | 258 (53.8%) | 533 (63.2%) | 742 (26.5%) | |
| Yes | 84 (17.5%) | 148 (17.6%) | 261 (9.3%) | |
| Unknown | 138 (28.8%) | 163 (19.3%) | 1799 (64.2%) |
Note: BMI = body mass index; DBP = diastolic blood pressure; IQR = interquartile range; SBP = systolic blood pressure; SD = standard deviation.
Hormonal profiles were similar across groups (Table 2). Complete hormone levels were available for 2,003 (48.5%) men. There was no difference in serum testosterone (P = .191), follicle stimulating hormone (P = .068), luteinizing hormone (P = .206), and prolactin (P = .244) across groups and on sensitivity analysis. Serum estradiol was higher among men with an established PCP (median, 30; IQR, 23–37) versus men without a PCP (median, 27; IQR, 21–35; P<.001 [sensitivity analysis, P = .007]).
Table 2.
Hormonal profile of 2,003 men for whom complete hormonal parameters were available.
| Characteristics | No PCP | Established PCP | No response | P value |
|---|---|---|---|---|
| Testosterone (ng/dL) | 299 (241–394) | 314 (245–378) | 316 (249–395) | .191 |
| FSH (mIU/mL) | 4.6 (3.0–7.2) | 4.6 (3.2–7.4) | 5.0 (3.3–8.3) | .068 |
| LH (mIU/mL) | 3.6 (2.6–5.4) | 3.8 (2.7–5.5) | 3.6 (2.6–5.2) | .206 |
| Estradiol (pg/mL) | 27 (21–35) | 30 (23–37) | 32 (26–40) | <.001 |
| Prolactin (ng/mL) | 7.8 (5.9–9.9) | 7.5 (5.7–9.6) | 7.8 (6.0–10.0) | .244 |
Note: All data presented as median (interquartile range).
FSH = follicle-stimulating hormone; LH = luteinizing hormone; PCP = primary care physician.
Semen parameters are presented in Table 3. Complete semen analysis data were available for 2,012 (48.8%) men. Men with an established PCP had a significantly higher total motile sperm count (TMSC) than those without a PCP (median, 53; IQR, 11–109 vs. median, 35; IQR, 8–98; P = .001 [sensitivity analysis, P = .126]). A smaller proportion of men with an established PCP had oligospermia compared with those without a PCP (30.0% vs. 34.3%; P = .003 [sensitivity analysis, P = .272]). Likewise, a smaller proportion of men with an established PCP had severe oligospermia compared with those without a PCP (16.7.0% vs. 20.6%; P = .001 [sensitivity analysis, P = .232]).
Table 3.
Semen parameters among 2,012 men for whom semen analysis data were available.
| Characteristics | No PCP | Established PCP | No response | P value |
|---|---|---|---|---|
| N | 204 (42.5%) | 414 (49.1%) | 1394 (49.7%) | |
| Volume, mL, median (IQR) | 2.8 (1.9–3.9) | 2.8 (1.9–3.8) | 2.8 (1.8–3.8) | .371 |
| Concentration, median (IQR) | 25 (6–59) | 32 (9–64) | 24 (5–64) | .017 |
| % motility, median (IQR) | 58 (46–66) | 58 (50–68) | 56 (33–66) | .001 |
| TMSC, median (IQR) | 35 (8–98) | 53 (11–109) | 30 (5–97) | .001 |
| Oligospermia, N (%) | 70 (34.3%) | 124 (30.0%) | 542 (38.9%) | .003 |
| Severe oligospermia, N (%) | 42 (20.6%) | 69 (16.7%) | 356 (25.5%) | .001 |
Note: IQR = interquartile range; PCP = primary care physician; TMSC = total motile sperm count.
When excluding all men with missing data for any of the variables of interest, a total of 341 men remained (Supplemental Table 1, available online). In this highly selected cohort, there were no significant differences in patient demographics and clinical parameters between men with and without an established PCP (all P>.05). However, there were persistent differences in semen parameters, as men with an established PCP had higher motility (P<.001), higher TMSC (P = .064), and lower likelihood of severe oligospermia (P = .074).
Discussion
This is the first study to characterize the proportion of men presenting for male fertility evaluation who report having an established PCP. Only 20.5% of all men confirmed having an established PCP. Although when accounting for missing data (i.e., considering only those men who responded to the question), one third of men did not have an established PCP. These findings are consistent with population-based data suggesting that one third of all men, irrespective of fertility evaluation, do not have a PCP (7). Furthermore, when examining men who did have a primary care visit within our healthcare system, one third had not been seen within a year of the fertility evaluation.
Male fertility is a window to a man’s overall health (9). There is a growing body of evidence suggesting that men with subfertility are at increased risk of metabolic syndrome, cancer, and even mortality (4, 10, 11, 12). In the present study, a significant proportion of all men had elevated BMI meeting criteria for obesity and elevated blood pressure. Although these cross-sectional data are insufficient for a diagnosis of metabolic syndrome, they are indicative of significant morbidity in the population of men presenting for fertility evaluation. There were small, statistically significant differences in the prevalence of BMI and blood pressure abnormalities according to PCP status, but these differences are of questionable clinical significance compared with the larger finding of substantial comorbidity in this group of young men.
Young men are at particularly high risk of not engaging with the healthcare system for routine medical care. The median age (years) of men presenting for fertility evaluation was mid-30s. Although young men are, by and large, healthier than older men, population-level data suggest that men between the ages of 25 and 44 years have an average of 0.5 major comorbidities, and 11% of these men have multimorbidity, indicating a significant burden of medical disease (13). However, young men are less likely to see a physician and establish routine medical care compared with older adults (14). Transitions from pediatric to adult care can be challenging, and many pediatricians do not have clear policies for ensuring that young men establish care with an adult PCP upon leaving a pediatric practice (15, 16). These young men, newly responsible for their own medical decision making, may have little impetus or sense of urgency to establish routine medical care as an independent adult, particularly if they do not suffer from pre-existing chronic conditions. This is perhaps best captured by the title of a recent article in the Washington Post, “For millennials, a regular visit to the doctor’s office is not a primary concern” (17).
There are also cultural and sociodemographic factors that contribute to underutilization of healthcare among young men (18, 19, 20). Qualitative studies have demonstrated that many men consider regular health care use as antithetical to a masculine self-identity (19, 21). Lifestyle choices also contribute to health engagement, and men with unhealthy lifestyles (smoking, alcohol consumption, poor diet) are even less likely to see a primary care physician, rendering it particularly important to help these men establish routine preventative medical care (22).
Men without an established PCP will miss screening opportunities for multiple significant medical conditions. The United States Preventative Services Task Force recommends that men under age 35 years undergo routine screening for alcohol misuse, tobacco use, depression, hypertension, obesity, sexually transmitted infections, and lipid disorders (23). Furthermore, men engaging with a PCP will receive additional counseling on important preventative health issues such as safe sex practices, diet and exercise recommendations, and skin cancer prevention.
Fertility evaluation is an opportunity to help men establish a primary and preventative healthcare relationship, and there are a number of approaches that can help reproductive urologists integrate this into clinical practice. First, a simple screening question for all new patients can help determine which patients do not have PCPs. Second, reproductive urologists should be familiar with multiple primary care physicians either at their institution or in the geographic region who accept new patients. A prepared referral list could be provided to all men in need of a new physician. Third, any strategy that facilitates easy appointment scheduling will lower the barrier to entry. For example, our institution has recently implemented an online system enabling patients to schedule new primary care appointments, thereby helping patients to find appointments at a convenient time and location across a large number of primary care providers. Fourth, the emerging paradigm of a men’s health center, wherein a combination of primary medical and urologic care is provided, may offer synergy and expedited access to care (24). To this point, most of these centers have targeted urologic patients presenting for urinary or sexual dysfunction. However, the current data provide a rationale for incorporation of reproductive urology into the men’s health center umbrella. This is particularly important, as prior studies have demonstrated that men are more likely to engage with the healthcare system when offered the opportunity for treatment at a dedicated men’s health clinic (25).
We also found that semen parameters, including TMSC, were slightly higher among men reporting an established PCP, although the statistical significance of this comparison decreased upon sensitivity analysis. There are a number of potential explanations for this finding. Although men with an established PCP did have slightly higher BMI in our study, these men may be generally healthier with regard to the presence and management of comorbidities, such as hyperlipidemia, hypertension, and diabetes, due to their regular medical care. Given the potential effects of these comorbidities on semen parameters, appropriate management and preventative care could partially explain differences between the groups (3, 26). Alternatively, men with an established PCP may be more likely to engage with the healthcare system and be more proactive about their general health. By extension, it is possible that these men more often sought fertility evaluation in the absence of risk factors or after a shorter period of attempting to conceive, thus skewing their semen parameters toward a more normal range.
The current findings must be interpreted within the context of certain limitations of the study design. First, due to the retrospective nature of the study, a substantial proportion of men had missing data for the primary outcome of PCP status. We treated these men separately in the analysis. Although some of the men with missing data may not have been asked the question altogether, it is likely that a substantial proportion of these men, if not the majority, did not have an established PCP, as men with an established PCP would have likely answered the question and provided data. As such, the results presented likely underestimate the proportion of men without a PCP, which could be much higher. Second, clinical characteristics, such as vital signs, hormone profile, and semen parameters, were not available for all men. Thus, there may inherent bias in these comparisons due to missing data. Third, blood pressure was abstracted from a single reading in a physician’s office, which is subject to recording error and not necessarily diagnostic of hypertension (27).
In conclusion, more than one third of men presenting for fertility evaluation did not have an established PCP, and among those who did, a sizable proportion had not seen their PCP in the previous year. Given the strong link between male factor infertility and medical comorbidity, reproductive urologists are uniquely positioned to facilitate the critical relationship between young men and PCPs, which should be a key component of the male fertility treatment paradigm.
Footnotes
J.A.H. has nothing to disclose. A.L.D.-B. has nothing to disclose. R.J.F. has nothing to disclose. M.K.K. has nothing to disclose. J.W. has nothing to disclose. N.E.B. has nothing to disclose. R.E.B. has nothing to disclose.
Supplementary data
References
- 1.Honig S.C., Lipshultz L.I., Jarow J. Significant medical pathology uncovered by a comprehensive male infertility evaluation. Fertil Steril. 1994;62:1028–1034. [PubMed] [Google Scholar]
- 2.Kolettis P.N., Sabanegh E.S. Significant medical pathology discovered during a male infertility evaluation. J Urol. 2001;166:178–180. [PubMed] [Google Scholar]
- 3.Cazzaniga W., Capogrosso P., Ventimiglia E., Pederzoli F., Boeri L., Frego N. High blood pressure is a highly prevalent but unrecognised condition in primary infertile men: results of a cross-sectional study. Eur Urol Focus. 2020;6:178–183. doi: 10.1016/j.euf.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg M.L., Li S., Brooks J.D., Cullen M.R., Baker L.C. Increased risk of cancer in infertile men: analysis of U.S. claims data. J Urol. 2015;193:1596–1601. doi: 10.1016/j.juro.2014.11.080. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg M.L., Li S., Cullen M.R., Baker L.C. Increased risk of incident chronic medical conditions in infertile men: analysis of United States claims data. Fertil Steril. 2016;105:629–636. doi: 10.1016/j.fertnstert.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg M.L., Li S., Behr B., Cullen M.R., Galusha D., Lamb D.J. Semen quality, infertility and mortality in the USA. Hum Reprod. 2014;29:1567–1574. doi: 10.1093/humrep/deu106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Henry J., Kaiser Family Foundation Percent of men who report having no personal doctor/health care provider, by race/ethnicity. https://www.kff.org/disparities-policy/state-indicator/percent-of-men-who-report-having-no-personal-doctorhealth-care-provider-by-raceethnicity/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D Available at:
- 8.Mazzoni S., Brewer S., Durfee J., Pyrzanowski J., Barnard J., Dempsey A.F. Patient perspectives of obstetrician-gynecologists as primary care providers. J Reprod Med. 2017;62:3–8. [PubMed] [Google Scholar]
- 9.Choy J.T., Eisenberg M.L. Male infertility as a window to health. Fertil Steril. 2018;110:810–814. doi: 10.1016/j.fertnstert.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg M.L., Sundaram R., Maisog J., Buck Louis G.M. Diabetes, medical comorbidities and couple fecundity. Hum Reprod. 2016;31:2369–2376. doi: 10.1093/humrep/dew200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenberg M.L., Li S., Behr B., Pera R.R., Cullen M.R. Relationship between semen production and medical comorbidity. Fertil Steril. 2015;103:66–71. doi: 10.1016/j.fertnstert.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Eisenberg M.L., Kim S., Chen Z., Sundaram R., Schisterman E.F., Buck Louis G.M. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod. 2014;29:193–200. doi: 10.1093/humrep/det428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnett K., Mercer S.W., Norbury M., Watt G., Wyke S., Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Centers for Disease Control and Prevention; Atlanta: 2018. Summary Health Statistics: National Health Interview Survey, 2018. [Google Scholar]
- 15.Maddux M.H., Ricks S., Bass J. Preparing patients for transfer of care: practices of primary care pediatricians. J Community Health. 2015;40:750–755. doi: 10.1007/s10900-015-9994-3. [DOI] [PubMed] [Google Scholar]
- 16.Burke R., Spoerri M., Price A., Cardosi A.-M., Flanagan P. Survey of primary care pediatricians on the transition and transfer of adolescents to adult health care. Clin Pediatr (Phila) 2008;47:347–354. doi: 10.1177/0009922807310938. [DOI] [PubMed] [Google Scholar]
- 17.The Washington Post Primary care doctors aren’t so important to millennials. https://www.washingtonpost.com/national/health-science/for-millennials-a-regular-visit-to-the-doctors-office-is-not-a-primary-concern/2018/10/05/6b17c71a-aef3-11e8-9a6a-565d92a3585d_story.html?noredirect=on&utm_term=.1c6d896a1a94 Available at:
- 18.Schlichthorst M., Sanci L.A., Pirkis J., Spittal M.J., Hocking J.S. Why do men go to the doctor? Socio-demographic and lifestyle factors associated with healthcare utilisation among a cohort of Australian men. BMC Public Health. 2016;16:1028. doi: 10.1186/s12889-016-3706-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeffries M., Grogan S. Oh, I“m just, you know, a little bit weak because I”m going to the doctor’s': young men's talk of self-referral to primary healthcare services. Psychol Health. 2012;27:898–915. doi: 10.1080/08870446.2011.631542. [DOI] [PubMed] [Google Scholar]
- 20.Knight R., Shoveller J.A., Oliffe J.L., Gilbert M., Frank B., Ogilvie G. Masculinities, “guy talk” and “manning up”: a discourse analysis of how young men talk about sexual health. Sociol Health Illn. 2012;34:1246–1261. doi: 10.1111/j.1467-9566.2012.01471.x. [DOI] [PubMed] [Google Scholar]
- 21.Noone J.H., Stephens C. Men, masculine identities, and health care utilisation. Sociol Health Illn. 2008;30:711–725. doi: 10.1111/j.1467-9566.2008.01095.x. [DOI] [PubMed] [Google Scholar]
- 22.Feng X., Girosi F., McRae I.S. People with multiple unhealthy lifestyles are less likely to consult primary healthcare. BMC Fam Pract. 2014;15:126. doi: 10.1186/1471-2296-15-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swenson P.F., Ebell M.H. Introducing a one-page adult preventive health care schedule: USPSTF recommendations at a glance. Am Fam Physician. 2016;93:738–740. [PubMed] [Google Scholar]
- 24.Miner M.M., Heidelbaugh J., Paulos M., Seftel A.D., Jameson J., Kaplan S.A. The intersection of medicine and urology: an emerging paradigm of sexual function, cardiometabolic risk, bone health, and men’s health centers. Med Clin North Am. 2018;102:399–415. doi: 10.1016/j.mcna.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Vincent A.D., Drioli-Phillips P.G., Le J., Cusack L., Schultz T.J., McGee M.A. Health behaviours of Australian men and the likelihood of attending a dedicated men’s health service. BMC Public Health. 2018;18:1078. doi: 10.1186/s12889-018-5992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiraishi K., Matsuyama H. Effects of medical comorbidity on male infertility and comorbidity treatment on spermatogenesis. Fertil Steril. 2018;110:1006–1011.e2. doi: 10.1016/j.fertnstert.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Collins K.J., Dennison Himmelfarb C. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2018;138:e484–e594. doi: 10.1161/CIR.0000000000000596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.