Abstract
Objectives
1) To confirm the correlation of GnRH receptor (GnRHR) activating autoantibody (AAb) activity with polycystic ovary syndrome (PCOS) diagnosis in large well defined cohorts; and 2) to evaluate suppression of AAb activity with GnRH antagonist medication in transfected GnRHR cells exposed to serum of PCOS patients.
Design
Cross-sectional matched case-control study.
Setting
University-based research facility.
Patient(s)
Sera from 200 patients with PCOS from the Pregnancy in Polycystic Ovary Syndrome II (PPCOS II) trial and from 200 race, parity-, age-, and body mass index (BMI)–matched ovulatory unexplained infertile control patients from the Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS) trial were obtained and used for this study.
Intervention(s)
GnRHR AAb activity was determined with the use of the GeneBlazer cell-based fluorescence resonance energy transfer assay with and without cetrorelix, a GnRH antagonist.
Main Outcome Measure(s)
1) GnRHR AAb activity in PCOS patients compared with control subjects; and 2) effectiveness of GnRH antagonist in suppressing GnRHR AAb activity.
Result(s)
GnRHR AAb activity levels in the PCOS group were significantly higher than in the control group. With cetrorelix, GnRHR AAb activity was largely suppressed in the PCOS group but not in the control group. These differences remained significant after adjusting for within-pair differences in age, BMI, and antimüllerian hormone (AMH) levels.
Conclusion(s)
We confirmed higher GnRHR AAb activity levels in the sera of a large cohort of PCOS patients compared with unexplained infertile control subjects. Addition of cetrorelix resulted in significant suppression of AAb activity levels in PCOS patients as a group whereas control subjects were unaffected. GnRHR AAb, along with patient age and AMH level, may provide a promising future diagnostic test for PCOS.
Key Words: Polycystic ovarian syndrome (PCOS), activating autoantibodies (AAbs), antimüllerian hormone (AMH), GnRHR-cell activity, unexplained infertility
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-20-00113
Polycystic ovary syndrome (PCOS) is a systemic disease of unknown etiology that frequently displays a classic phenotype, affects 6%–10% of all reproductive-age women (1), and represents one of the most common endocrine disorders of women. The diagnosis is one of exclusion, and the disease is characterized by multiple small ovarian cysts or follicles on transvaginal ultrasound, ovulatory dysfunction, metabolic abnormalities including insulin resistance and type II diabetes mellitus, and abnormal sex hormone function (2). In addition, patients with PCOS also have higher rates of depression and anxiety and report decreased quality of life (2). The impacts of this disorder and its potential long-term sequelae are far reaching, with health consequences affecting the reproductive years and beyond.
Despite the significant personal and population-level health care impacts of this highly prevalent disease, PCOS unfortunately remains a diagnosis of exclusion. The leading diagnostic criterion used for PCOS are the Rotterdam criteria (3, 4), which are assessed only after all other mimicking diagnoses, such as thyroid disease, hyperprolactinemia, androgen-secreting tumors, and nonclassical congenital adrenal hyperplasia, are excluded. Two out of three criteria are required to make the diagnosis of PCOS in adults; they include oligoovulation or anovulation, clinical or biochemical signs of hyperandrogenism, and polycystic ovarian morphology as assessed by ultrasound. The absence of a simple diagnostic test for PCOS frequently results in multiple specialist visits that, in turn, increase the cost of health care, delay diagnosis and treatment, and add additional emotional and psychologic stress for the those affected by the disease (5).
Currently, PCOS is considered to be a multifactorial disease, with a complex etiology likely involving genetic, hormonal, and environmental factors (6, 7). Metabolic syndrome is frequently associated with PCOS and includes hypertension and cardiovascular disease, obesity, type II diabetes mellitus, and hyperandrogenism. Much research is ongoing to investigate the etiology of PCOS with various twin- and gene-targeting studies evaluating inheritance (6, 7, 8), as well as animal models exploring the prenatal influence of antimüllerian hormone (AMH) on PCOS development in adulthood (9).
Central to the known PCOS phenotype is dysregulation of GnRH, which is released from the hypothalamus and acts at the level of the pituitary (10). In normal menstrual cycle physiology, the resulting pattern of LH and FSH release results in ovulation. Abnormal pulsatility of GnRH, favoring LH release over FSH, triggers a series of hormonal effects resulting in increased androgen production and impaired follicular development. Together, these pathologic processes result in the defining characteristics of PCOS: hirsutism, increased insulin resistance, and oligoanovulation with resultant infertility and increased risk of endometrial hyperplasia. Although the concept of PCOS as an autoimmune condition is novel, it is certainly plausible when considering this dysregulation beginning at the hypothalamic level.
A well known example of a medical condition in which activating autoantibodies result in disease is Graves disease (11). Our laboratory has identified additional conditions in which activating autoantibodies have been detected, including postural orthostatic tachycardia syndrome, resulting from an activating autoantibody (AAb) to the second extracellular loop of G-protein receptors (12). Because the GnRH receptor (GnRHR) is also a member of the family of seven transmembrane G-protein–coupled receptors (GPCRs) and is expressed primarily on the surface of the pituitary gonadotrope cells (as well as lymphocytes, breast, ovary, and prostate), it is certainly reasonable to consider an autoimmune process resulting in dysregulation of GnRH release from the hypothalamus. We have identified such an AAb, which was found to be markedly higher in patients with PCOS than in normal control subjects (13). Furthermore, we observed a stimulatory activity of this AAb in patients with PCOS as displayed by increasing the activation of transfected GnRHR cells (13). This effect was significantly suppressed by the GnRHR antagonist cetrorelix.
We have also demonstrated that elevated GnRHR AAbs could induce changes in reproductive cyclicity in GnRHR-ECL2 AAb–immunized animals (14). Briefly, 16 Sprague-Dawley rats were randomly divided into two groups: a GnRHR group (n = 8) and a control group (n = 8). Rats in the GnRHR group were immunized with GnRHR ECL2 peptide. The GnRHR AAb titers and activity in the GnRHR group were significantly higher than in the control group, and the immunized group demonstrated increased atretic follicles, decreased corpora lutea, loosely packed granulosa cells, and thecal cell hyperplasia in ovarian tissue, as well as elevated numbers of LH pulses and concentration of testosterone compared with the control group (14).
The objective of the present study was to validate our previous findings of increased GnRHR AAb activity levels and the ability to subsequently decrease those levels through cetrorelix blockade in PCOS patients compared with unexplained infertile control subjects in a large national cohort.
Materials and Methods
This investigation was quantitative in design and included evaluation of deidentified stored sera from the Reproductive Medicine Network (RMN). The RMN is funded through the National Institute of Child Health and Human Development’s Fertility and Infertility branch and is composed of multiple research sites (seven primary and seven ancillary), with our fertility practice being one of the primary research sites. Through this collaborative effort, the RMN conducted two concurrent multicenter randomized controlled trials: Pregnancy in Polycystic Ovary Syndrome II (PPCOS II), with results published in 2014 (15), and Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation (AMIGOS), with results published in 2015 (16). From each of these databases, we requested a convenience sample of deidentified sera of 200 subjects with PCOS from PPCOS II and 200 matched ovulatory control subjects from AMIGOS.
In the PPCOS II trial, women with PCOS and their partners (n = 750 women) were randomized to clomiphene or letrozole treatment for ovulation induction for five cycles with timed intercourse. In the AMIGOS trial, ovulatory women without PCOS and their partners diagnosed with unexplained infertility (n = 900 women) were randomized to clomiphene, letrozole, or gonadotropins for controlled superovulation for four cycles with intrauterine insemination. The full details of both trials are published elsewhere (15, 16). The present study used banked serum from the screening visit (before treatment initiation) of the female subjects in both trials. Demographic data including AMH levels (Beckman-Coulter AMH ELISA assay; coefficient of variation <10%), metabolic parameters, pregnancy rates, and outcome information (miscarriage, clinical pregnancy with fetal heart beat, delivery) were available for all participants and reviewed.
Following institutional review board approval, a pairwise matched case-control sample comprising 200 women with PCOS (case subjects) from PPCOS II and 200 infertile ovulatory women (control subjects) from AMIGOS was used. Case subjects were selected based on the availability of a matched control. Control subjects were matched to cases based on (in order of priority) race/ethnicity (exact match), parity (exact match: nulliparous/parous), body mass index (BMI; ±3 kg/m2), and age (±5 years). The 200 closest matches were selected. For brevity, this sample of 400 women will be referred to hereafter as the RMN cohort.
Cell activity assays were then performed to assess GnRHR AAb activity in all samples. Serum activation of GnRHR in GnRHR-NFAT-bla CHO-K1 cells was measured with the use of the GeneBlazer fluorescence resonance energy transfer (FRET)–based β-lactamase reporter assay (Invitrogen) according to the manufacturer’s instructions. Briefly, cells were plated in 384-well plates (∼15,000 cells/well) and incubated overnight. The individual serum samples (1:20), in the absence and presence of a 30-minute preincubation with cetrorelix (0.1 mmol/L in phosphate-buffered saline solution), were then added and incubated for 4 hours, followed by incubation with the β-lactamase substrate CCF4-AM (LiveBlazer-FRET B/G Loading Kit; Invitrogen) for 2 hours. The plates were read with the use of a Hidex Sense fluorescence microplate reader (Hidex). All samples were tested in triplicate. Negative (buffer) and positive (GnRH) control samples were included in each assay. Data were calculated as the ratio of the emissions at 460/530 nm (blue/green) after subtraction of the background values and expressed as fold increase over buffer baseline to normalize the individual values. A value of 1 would be similar to buffer activity. To reduce the risk of measurement bias, the same research team performed all experiments.
Data are expressed as mean ± SD for continuous measures (e.g., age, GnRH activity) or as n (%) for categoric measures (e.g., race, parity). Appropriateness of assumption of normal distributions for the continuous measures was confirmed by d’Agostino-Pearson omnibus normality test; none were found to have sampling distributions significantly disparate from normal. Initially, paired (e.g., case vs. control) and unpaired (e.g., parous vs. nulliparous) Student t tests, as appropriate, were used for between-group comparisons of continuous measures; Pearson chi-square tests were used for categoric measures. To include adjustment for covariates, logistic regression within a generalized linear model framework to account for matching was used. Statistical analyses were performed in IBM SPSS Statistics for Windows (version 20.0; IBM Corp.) and R (version 3.5.1, gmodels package version 2.18.1). The threshold for statistical significance was set at P<.05.
Results
The clinical characteristics for the 200 PCOS patients and 200 control subjects are presented in Table 1. Although the intention was to match on race/ethnicity, parity, BMI, and age, the limitations of the PCOSII and AMIGOS cohorts and attrition of biosamples meant that this was not fully achieved. The two groups were 100% pair matched on race, but patients with PCOS had lower parity (0.2 vs. 0.4, with an 84% exact pair match), had higher BMI (31.5 vs. 28.9 kg/m2), and were younger (29 vs. 31 years) than their matched control subjects. Consequently, logistic regression analyses included adjusting for within-pair variation in age and BMI. Patients with PCOS had significantly higher levels of AMH, total T levels, E2, LH, A, and triglycerides, as well as a higher prevalence of metabolic syndrome than patients with unexplained infertility, even after using a Bonferroni adjustment for multiple hypothesis testing (P<.0025). They had lower levels of SHBG than patients with unexplained infertility.
Table 1.
Variables assessed for polycystic ovary syndrome (PCOS) and control patients.
| Measure (unit) | PCOS | Control | P value |
|---|---|---|---|
| Match variables | |||
| Race | – | ||
| White | 136 (90%) | 136 (90%) | |
| Black | 11 (7%) | 11 (7%) | |
| Asian | 4 (3%) | 4 (3%) | |
| Parity | <.0001 | ||
| 0 | 117 (78%) | 101 (67%) | |
| 1 | 28 (18%) | 43 (28%) | |
| 2 | 5 (3%) | 6 (4%) | |
| 3 | 1 (1%) | 1 (1%) | |
| Age (y) | 29.2 ± 4 | 30.8 ± 4 | <.0001 |
| BMI (kg/m2) | 31.5 ± 8 | 28.9 ± 7.4 | <.0001 |
| Other variables | |||
| AMH (ng/mL) | 9.3 ± 8.8 | 2.9 ± 2.1 | <.0001 |
| T (ng/dL) | 51 ± 25 | 26 ± 19 | <.0001 |
| E2 (pg/mL) | 53.2 ± 30.0 | 34.5 ± 33.1 | <.0001 |
| Glucose (mg/dL) | 84 ± 10 | 85 ± 11 | .35 |
| Insulin (mIU/L) | 15.0 ± 19.2 | 12.7 ± 22 | .11 |
| SHBG (nmol/L) | 39.2 ± 25.3 | 57.9 ± 29.4 | <.0001 |
| P (ng/mL) | 1.5 ± 2.9 | 0.8 ± 0.65 | .0044 |
| FSH (mIU/mL) | 6.5 ± 5.0 | 7.1 ± 3.2 | .22 |
| LH (IU/L) | 11.7 ± 8.9 | 5.3 ± 3.0 | <.0001 |
| TSH (mIU/L) | 1.9 ± 1.0 | 2.0 ± 1.0 | .15 |
| hsCRP (mg/L) | 4.7 ± 5.4 | 3.8 ± 4.8 | .15 |
| PRL (ng/mL) | 10.4 ± 5.8 | 10.9 ± 6.4 | .54 |
| Proinsulin (pmol/L) | 14.6 ± 10.7 | 13.1 ± 7.4 | .021 |
| A (ng/dL) | 4.0 ± 1.6 | 2.6 ± 0.9 | <.0001 |
| Total cholesterol (mg/dL) | 178 ± 36 | 168 ± 30 | .032 |
| Triglycerides (mg/dL) | 113 ± 59 | 98 ± 46 | .0020 |
| Systolic BP (mm Hg) | 117 ± 13 | 119 ± 12 | .056 |
| Diastolic BP (mm Hg) | 75 ± 9 | 75 ± 11 | .55 |
| Metabolic syndrome | .002 | ||
| Yes | 52 (30) | 36 (20) | |
| No | 119 (70) | 140 (80) | |
Note: Continuous variables are presented as mean ± SD, categoric variables as n (%). AMH = antimüllerian hormone; BMI = body mass index; BP = blood pressure; hsCRP = high sensitivity C-reactive protein; TSH = thyroid-stimulating hormone.
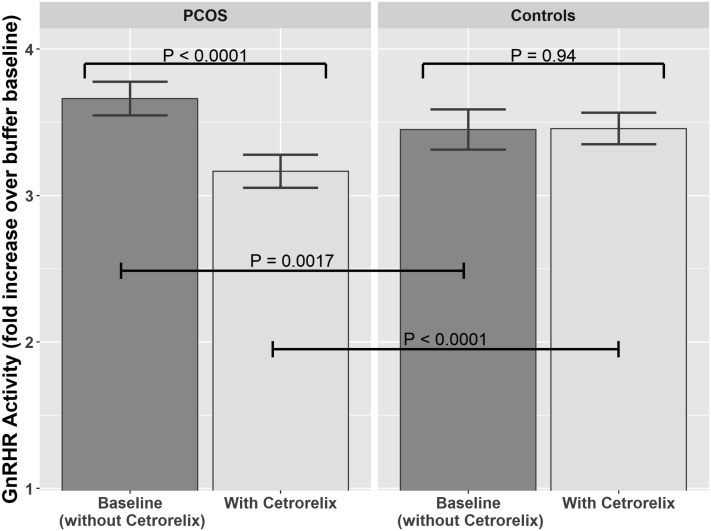
Logistic regressions comparing cell activity levels as measured by means of FRET, expressed as mean ± SD of the fold increase over buffer baseline, in patients with PCOS and patients with unexplained infertility are provided in Figure 1 and Table 2. The baseline GnRHR AAb activity level was significantly higher in patients with PCOS than in control subjects (3.66 vs. 3.45; P=.0017). This difference remained significant (P=.0029) after adjusting for pair differences in age, BMI, and AMH level. With addition of the GnRH antagonist cetrorelix, GnRHR AAb activity levels decreased significantly in patients with PCOS, from 3.66 to 3.17 (P<.0001). In contrast, GnRHR AAb activity levels in control subjects remained essentially unchanged from baseline after the addition of cetrorelix (from 3.45 to 3.46; P=.94; Table 2). Median change (with cetrorelix) in patients with PCOS was −0.51 with an interquartile range (IRQ) of −1.12 to 0.12, and in control subjects it was 0.05 with an IRQ of −0.61 to 0.79, showing that the majority (72%) of patients with PCOS had decreased activity with the use of cetrorelix compared with control subjects (random 50/50 whether decreased or increased). Finally, when the AAb activity levels following the addition of cetrorelix were compared between PCOS and control subjects the AAb activity results remained statistically significantly different between the two groups, with P<.0001 in both unadjusted and adjusted (for within-pair differences in age, BMI, and AMH level) analyses.
Figure 1.
GnRH receptor (GnRHR) activating autoantibody activity levels at baseline (without cetrorelix) and after addition of cetrorelix for all 200 patients with PCOS and their 200 matched infertile control subjects. Pairwise comparisons used paired t tests.
Table 2.
GnRH receptor (GnRHR) activating autoantibody (AAb) activity levels at baseline and after cetrorelix in polycystic ovary syndrome (PCOS) and control patients.
| Measure | PCOS | Control | Unadjusted P value | Adjusted P valuea |
|---|---|---|---|---|
| GnRHR AAb activity level | ||||
| Baseline (without cetrorelix) | 3.66 ± 0.84 | 3.45 ± 0.99 | .0017 | .0029 |
| With cetrorelix | 3.17 ± 0.82 | 3.46 ± 0.78 | <.0001 | <.0001 |
| P value, with vs. without cetrorelix | <.0001 | .94 | ||
| Δ GnRHR AAb activity level | −0.50 ± 0.97 | 0.01 ± 1.08 | <.0001 | <.0001 |
| Number with a decrease in GnRHR AAb activity level with cetrorelix | 144 (72%) | 104 (52%) | .0001 | |
Adjusted for pair differences in age, body mass index, and antimüllerian hormone.
Discussion
In this study, we demonstrated that our activity assay measures the direct (orthosteric) effect of AAbs and confirmed higher GnRHR AAb activity levels in the sera of a large independent cohort of patients with PCOS compared with patients with unexplained infertility. Furthermore, the addition of the specific GnRH antagonist cetrorelix resulted in significant suppression of GnRHR AAb activity levels in the sera of PCOS patients but not in infertile control sera.
This study confirms our previous findings. In our previous prospective case-control study of 40 infertile patients with PCOS, identified according to Rotterdam criteria, compared with 40 age- and BMI-matched infertile ovulatory controls, we identified the standardized optical density with the use of ELISA of GnRHR AAb levels in PCOS patients to be significantly higher than in ovulatory control subjects (P=.0032) and in normal control subjects (P=.0001) (13). With a subset of patient sera from that original study, we performed cell activity assays using transfected GnRHR cells with the addition of the GnRH antagonist cetrorelix to assess activity in both control samples (n = 7) and those with PCOS (n = 7). Similarly to the present study, we found statistically significantly higher GnRHR AAb activity level in patients with PCOS, but also significant suppression of activity level in the same group after addition of cetrorelix to the level of the control subjects. This suppression, specific to the patients with PCOS, is reflective of the activating nature of the GnRHR AAbs in this cohort.
We have previously demonstrated that the elevated levels of GnRHR AAbs in patients with PCOS can be suppressed with addition of cetrorelix to the cell assay (13). We have also demonstrated that the presence of the AAbs indirectly, in an allosteric fashion, enhances the effect of the natural ligand (GnRH) on the receptor activity (13). We think that this indirect effect may have significance along with the direct effect, although it is more difficult to measure and apply to the large number of samples in the present study. We think that these data, once available, will further enhance the accuracy of these computations.
One of the major strengths of the present study is the patient population, specifically the overall sample size and the use of two well characterized, generalizable, infertility populations. The deidentified sera from 200 patients with PCOS and 200 ovulatory control subjects represent participants from two major randomized controlled trials completed within the past 5 years in the United States: AMIGOS (16) and PPCOSII (15). These study groups were well matched on race and age but did display anticipated differences, including BMI and AMH level, among others.
The cell activity assay results from the present study provide promise in explaining the dysregulation of GnRH signaling associated with PCOS. While we originally examined the activating nature of the GnRHR AAbs in a small sample of patients (13), the expansion to include sera from 400 patients in the present study lends great strength to that finding.
Among both study groups, GnRHR AAb activity levels were examined with consideration of selected demographic and metabolic factors. Interestingly, statistically significant differences were observed only when baseline GnRHR AAb activity levels were compared with AMH. This finding suggests that there is an association of AMH with GnRHR AAb levels in those patients with unexplained infertility, but when PCOS patient serum is examined, the effect is no longer apparent. This lends support to our intention to use cell activity assay results in combination with AMH in development of an eventual screening/diagnostic test for PCOS.
The GnRHR AAbs that we identified may provide a future screening/diagnostic test for PCOS or a target for treatment. The development of an assay to detect such GnRHR AAbs and diagnose PCOS would alter and improve the clinical care of patients with PCOS by decreasing time to diagnosis, decreasing comorbidity by allowing earlier patient-physician relationships to develop, and providing an avenue for therapeutic investigation. Future directions for our research include examining adolescents at risk for PCOS, among many others. Identification and characterization of these AAbs will ultimately transform PCOS from a disease of unknown etiology to an autoimmune-linked disease and facilitate early preventative management. If these data are confirmed, agents to block these antibodies could then be used in the treatment of PCOS and possible restoration of normal GnRH pulsatility, leading to ovulation with the goal of streamlining and improving efficiency of diagnosis for patients with PCOS.
Acknowledgements
The authors acknowledge the Reproductive Medicine Network, Brendon Hines, Marci Beel, and Hayley Fischer for their contributions to the development and execution of this project.
Footnotes
E.A.W. has nothing to disclose. H.R.B. has nothing to disclose. X.Y. has nothing to disclose. H.L.L. has nothing to disclose. C.E.A. reports grants from NIH both for the submitted work and outside of the submitted work. D.C.K. reports a patent pending through the University of Oklahoma Health Sciences Center. L.B.C. reports travel support from Ferring Pharmaceuticals and a grant from University of Oklahoma College of Medicine Alumni Association.
Supported by the University of Oklahoma College of Medicine Alumni Association, Oklahoma Center for Advancement of Science and Technology, and University of Oklahoma Foundation, as well as the Oklahoma Shared Clinical and Translational Resources (grant no. U54GM104938 to A.E.S.) with an Institutional Development Award from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.March W.A., Moore V.M., Willson K.J., Phillips D.I., Norman R.J., Davies M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 2.Brady C., Mousa S.S., Mousa S.A. Polycystic ovary syndrome and its impact on women’s quality of life: more than just an endocrine disorder. Drug Healthc Patient Saf. 2009;1:9–15. doi: 10.2147/dhps.s4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teede H.J., Misso M.L., Costello M.F., Dokras A., Laven J., Moran L. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364–379. doi: 10.1016/j.fertnstert.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legro R.S., Arslanian S.A., Ehrmann D.A., Hoeger K.M., Murad M.H., Pasquali R. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson-Helm M., Teede H., Dunaif A., Dokras A. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604–612. doi: 10.1210/jc.2016-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legro R.S., Driscoll D., Strauss J.F., 3rd, Fox J., Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998;95:14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahsar-Miller M.D., Nixon C., Boots L.R., Go R.C., Azziz R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril. 2001;75:53–58. doi: 10.1016/s0015-0282(00)01662-9. [DOI] [PubMed] [Google Scholar]
- 8.Simonis-Bik A.M., Nijpels G., van Haeften T.W., Houwing-Duistermaat J.J., Boomsma D.I., Reiling E. Gene variants in the novel type 2 diabetes loci CDC123/CAMK1D, THADA, ADAMTS9, BCL11A, and MTNR1B affect different aspects of pancreatic beta-cell function. Diabetes. 2010;59:293–301. doi: 10.2337/db09-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tata B., Mimouni N.E.H., Barbotin A.L., Malone S.A., Loyens A., Pigny P. Elevated prenatal anti-müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24:834–846. doi: 10.1038/s41591-018-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speroff L., Fritz M.A. 7th ed. Lippincott Williams & Wilkins; Philadelphia: 2005. Clinical Gynecologic Endocrinology and Infertility. [Google Scholar]
- 11.Chazenbalk G.D., Pichurin P., Chen C.R., Latrofa F., Johnstone A.P., McLachlan S.M. Thyroid-stimulating autoantibodies in Graves disease preferentially recognize the free A subunit, not the thyrotropin holoreceptor. J Clin Invest. 2002;110:209–217. doi: 10.1172/JCI15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H., Yu X., Liles C., Khan M., Vanderlinde-Wood M., Galloway A. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kem D.C., Li H., Yu X., Weedin E., Reynolds A.C., Forsythe E. The role of GnRH receptor autoantibodies in polycystic ovary syndrome. J Endocr Soc. 2020;4 doi: 10.1210/jendso/bvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H., Guo Y., Zhang G., Deng J., Fischer H., Craig L.B. The effect of GNRHR autoantibody on reproduction function and insulin signaling intermediates in a new animal model of polycystic ovary syndrome. J Endocr Soc. 2020;4(Suppl 1) MON-002. [Google Scholar]
- 15.Legro R.S., Brzyski R.G., Diamond M.P., Coutifaris C., Schlaff W.D., Alvero R. The Pregnancy in Polycystic Ovary Syndrome II study: baseline characteristics and effects of obesity from a multicenter randomized clinical trial. Fertil Steril. 2014;101:258–269.e8. doi: 10.1016/j.fertnstert.2013.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diamond M.P., Legro R.S., Coutifaris C., Alvero R., Robinson R.D., Casson P. Letrozole, gonadotropin, or clomiphene for unexplained infertility. N Engl J Med. 2015;373:1230–1240. doi: 10.1056/NEJMoa1414827. [DOI] [PMC free article] [PubMed] [Google Scholar]