Abstract
Objective
To study decidualization-associated endometrial factors.
Design
Retrospective cohort study to compare endometrial gene expression patterns in women experiencing reproductive failure including recurrent pregnancy loss or unexplained infertility versus fertile controls.
Setting
University Reproductive Medicine Center.
Patient(s)
Women experiencing recurrent reproductive failure including recurrent pregnancy loss or unexplained infertility (n = 42) and fertile controls (n = 18).
Intervention(s)
Endometrial biopsy samples were analyzed with targeted ribonucleic acid sequencing via next-generation sequencing.
Main Outcome Measure(s)
The primary end point measurements were the expression of genes important for endometrial transformation during decidualization measured singly and in a combined/cumulative score approach. The secondary end point measurements were receiver operating curve analysis and comparisons between the specific biomarkers.
Result(s)
The comparison revealed differential expression of factors associated with decidualization, tissue homeostasis, and immune regulation: FOXO1, GZMB, IL15, SCNN1A, SGK1, and SLC2A1. A combined evaluation of these 6 signature factors was designated as a decidualization score in which the maximal score was “6” and the minimal was “0”. Among controls, 89% of the samples had a score ≥5 and 11% had a score of “4”. A total of 76% of samples in the patient group had scores ≤4 and 19% had the lowest score of “0”. A decidualization score <4 provided evidence of abnormality in the decidualization process with a sensitivity of 76% (95% CI 61%-88%) and specificity of 89% (95% CI 65%-99%).
Conclusion(s)
Decidualization scoring can determine whether the endometrial molecular profile is implantation-friendly. Further validation of this testing approach is necessary to determine a particular patient population in whom it could be used for selecting patients that require therapeutic actions to improve endometrial conditions prior to the in vitro fertilization procedure
Key Words: Endometrium, gene expression, recurrent pregnancy loss, infertility, decidualization
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-20-00075
Embryo implantation followed by a crucial period of placenta formation are the main limiting factors to a successful pregnancy. Despite extensive research, an etiology behind repeated implantation failures after euploid embryo transfers in otherwise healthy women often remains unknown (1). Embryo implantation as such also cannot guarantee a successful pregnancy, as approximately 15% of all clinically recognized pregnancies result in miscarriages (2). When 2 consecutive miscarriages occur before 20 weeks of gestation, it is defined as a disease, recurrent pregnancy loss (RPL) (3). In about 50% of RPL, the cause still remains unknown when the possible etiological factors, such as ovulatory dysfunction, diminished ovarian reserve, endometriosis, anatomic factors, antiphospholipid syndrome, and thrombophilias were excluded (4). It becomes apparent that the endometrial condition is a key element and emerging studies are showing various new factors being dysregulated in the endometrium from women with reproductive failures (1, 5, 6, 7, 8, 9).
The endometrial lining of the uterus undergoes extensive proliferation, secretion, and regression in a cyclic manner with peak receptivity occurring for 2-4 days during the mid-luteal phase of each cycle (10). This preparation, termed decidualization, involves differentiation of the human endometrial stromal cells into decidual cells that form a receptive tissue suitable for implantation (11). This process depends primarily on the effect of progesterone on the estradiol-primed progesterone receptors on the endometrial stromal cells (12). Among the important progesterone signaling factors, Forkhead box O1 (FOXO1) is known to play a central role in the induction of senescence in a subpopulation of decidualized stromal cells, a process that is essential for tissue remodeling in preparation for embryo implantation (13). Changes associated with decidualization also include increased expression of tissue and cellular homeostatic factors. Thus, a key regulator of sodium homeostasis, Serum- and glucocorticoid-inducible Kinase 1 (SGK1), was shown to be induced rapidly by a rise in the levels of progesterone (14) and activated by cyclic adenosine monophosphate (15). The glucose transport molecules (Glut) that expressed in human endometrium, Glut1 and Glut3, are known to peak in the mid-luteal endometrium (16) to ensure a nutritional and receptive medium for embryo implantation and growth.
In parallel with an increase in metabolism, the mid-luteal endometrium is characterized by a sharp rise in the number of uterine natural killer (uNK) cells (17). Because of the secretion of growth-promoting, angiogenic, chemotactic, and immunoregulatory factors, uNK cells play an important role in the regulation of angiogenesis, placental growth, and control of trophoblast invasion (18, 19). The endometrial levels of interleukin (IL) 15, which is essential for NK cell proliferation and survival, also vary within a cycle. They are low in the menstrual and proliferative phases, but increased expression is characteristic of the luteal endometrium (20, 21).
Clearly, the endometrial decidualization stands on a complex crosstalk between all cellular players to generate an implantation-friendly environment. In this study, we performed a targeted ribonucleic acid (RNA) sequencing analysis using next-generation sequencing (NGS) of endometrial biopsy samples from women with reproductive failures of unknown etiologies and compared the results with those of healthy fertile controls. A significant difference in the expression of factors involved in regulation of progesterone signaling, tissue homeostasis, and uNK cell proliferation was revealed. A combined evaluation approach was used to develop a decidualization score, which has a potential to be advantageous in selecting samples with abnormalities in the process of decidualization. Further study is important to validate the effectiveness and utilization of this score. Here we discuss its potential implementation in standard infertility evaluation and its use for determining possible treatment strategies.
Materials and methods
Study Samples
Approval was obtained from the Institutional Review Board at Rosalind Franklin University of Medicine and Science, North Chicago, IL and Clinica Las Condes, Santiago, Chile. Informed consent was obtained from all participants prior to the study. The patient group (n = 42) consisted of women with RPL or unexplained infertility (UI). RPL was defined as ≥2 failed clinical pregnancies, confirmed by ultrasound; infertility was defined as failure to achieve a clinical pregnancy after >12 months. Out of 26 RPL patients, 18 had 2-4 losses and 8 had >5 losses. In the RPL group, 6 patients had secondary infertility recognized because of a history of a successful pregnancy. The UI group consisted of 10 patients with no record of pregnancy, 4 patients with a record of chemical pregnancy/pregnancies and 2 patients with a history of 1 loss and failure to get pregnant for >12 months. No causes of reproductive failure such as chromosomal abnormalities or anatomic defects were identified on initial screen. Patients with known endometriosis, adenomyosis, endocrine disorders (polycystic ovary syndrome), autoimmune diseases, or thrombophilia (inherited or acquired) were excluded from the study. Workup included pelvic ultrasonography, screening for metabolic abnormalities, auto-antibodies (Abs), and mutations known as risk factors for thrombophilia. Following laboratory tests were evaluated: fasting free insulin, prolactin, testosterone total and free, homocysteine, dehydroepiandrosterone (DHEA), DHEA sulfate, anti-nuclear, -double-stranded deoxyribonucleic acid (DNA), single-stranded DNA, -Histone, -Scl70, -thyroglobulin, -thyroid peroxidase, -ovarian, -phospholipid Abs, factor V Leiden mutation, factor VH1299R (HR2) mutation, MTHFR C677T and A1298C mutations, and PAI-1 4G/5G mutation. Healthy fertile women (n = 18) were included as controls. Among the controls,3 had a history of 1 prior spontaneous abortion, but otherwise they did conceive naturally and had live birth/births. There was no significant difference in age between the patients (mean ± SD, 37.7 ± 3.8 years, 95% confidence interval (CI) 36.5-38.9) and controls (38.1 ± 5.0 years, 95% CI 35.3- 40.9). A summary of the obstetrical histories of the study participants is presented in Supplemental Table 1 (available online).
Sample Collection and RNA Extraction
Endometrial biopsy sampling was performed 7-9 days after the luteinizing hormone surge detected using urine luteinizing hormone tests. Endometrial tissue was obtained by Pipelle catheter and placed into a container with 5 mL of RNAlater solution (Invitrogen, Life Technologies). Samples were delivered to the laboratory at ambient temperature within 1 week and stored at −80°C until used.
The tissue was mixed with RLT lysis buffer (Qiagen) with 2-mercaptoethanol (Sigma), processed on TissueLyser LT homogenizer (Qiagen) and spun at 3,000 rpm for 3 minutes. The supernatant was mixed with 250 μL of ethyl alcohol and transferred to Qiagen’s micro RNeasy columns for RNA extraction per manufacturer’s recommendations.
Targeted RNAseq via Next-Generation Sequencing
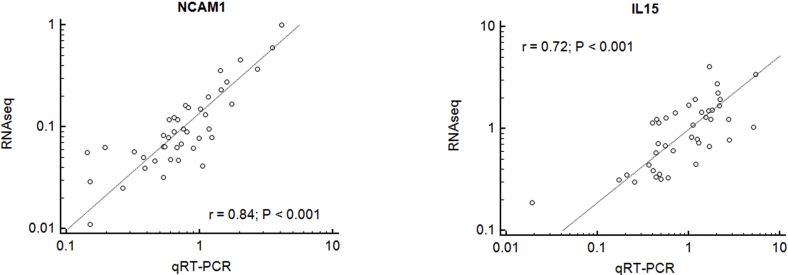
The complementary deoxyribonucleic acid (cDNA) synthesis and NGS library preparation were performed according to Qiagen’s custom targeted ribonucleic acid sequencing (RNAseq) panel (Cat. No. 333025, Supplemental Table 2, available online). Briefly, cDNA was made from 25 ng of RNA and unique 12 nucleotide tags were incorporated via multiplexed gene-specific primer extension. The reaction was purified using Qiagen’s size-selection magnetic beads. The barcoded cDNA was amplified using polymerase chain reaction with gene-specific primers. The completed library was loaded into a V3 reagent cartridge (Illumina) with a standard flow cell and custom sequencing primers (Qiagen). NGS was performed on the MiSeq (Illumina) per manufacturer’s instructions. The data were exported into a format that provides the total unique molecular barcode reads per gene. All sequencing reads were normalized to 10 internal control housekeeping genes. The obtained data (mRNA relative expression in molecular units) was used for statistical analysis. The RNAseq data was also verified by quantitative reverse transcriptase–polymerase chain reaction (Supplemental Fig. 1, available online).
Statistical Analysis
The statistical analysis was performed using MedCalc for Windows, v13.04.0 (MedCalc Software, Ostend, Belgium). Spearman’s rho rank correlation coefficient was used to assess the degree of association in the expression of selected genes. The Kruskal-Wallis (with the Conover’s post-hoc test) or the analysis of variance (ANOVA) test (with the Student-Newman-Keuls post-hoc test) depending on the Levene's test outcome were used for statistical analysis between the groups. Receiver Operating Characteristic (ROC) curve analysis was used to obtain the area under the ROC curve (AUC) value, its P value, the criterion, and the associated sensitivity and specificity values. Sample size calculation was performed to target AUC value ≥0.750, with alpha significance for type 1 error as 0.05 to ensure the sample was sufficient for the analysis. Welch’s adjusted t-test and Kruskal-Wallis test were used for a comparison of decidualization scores between groups. P value <.05 was considered statistically significant.
Results
Targeted RNAseq Analysis of Endometrial Biopsy Samples
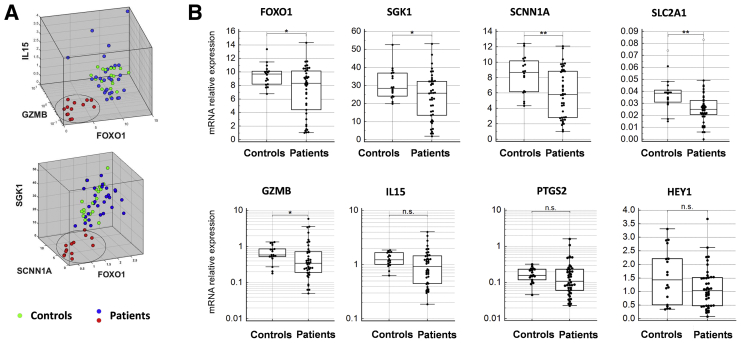
Endometrial tissue samples from 42 patients and 18 controls were analyzed with targeted RNA sequencing. The panel was designed to include various markers involved in the process of tissue modification during decidualization and included genes coding proteins associated with decidualization, inflammation, angiogenesis, metabolism, and NK cell-related markers (Supplemental Table 2). Differential expression of several genes between patients and controls was revealed by the analysis (Fig. 1). The gene expression profiles for IL15, GZMB, FOXO1, SGK1, and SCNN1A showed heterogeneity among samples, and a clustering of samples with a decreased expression of the indicated genes was observed (Fig. 1A). Strong association between the expression of FOXO1 with SGK1, SCNN1A, SLC2A1, GZMB and IL15 was revealed (Supplemental Table 3). A further comparison of the mRNA expression data between patients and controls confirmed significant differences in the expression of FOXO1, SGK1, SCNN1A, SLC2A1 and GZMB levels (Fig. 1B, Supplemental Table 4). However, no significant difference was found when 2 groups of patients, RPL and UI, were compared. The characteristics of patients whose samples had low FOXO1 levels were compared with those of patients whose samples had normal FOXO1 levels (Supplemental Table 5). No significant differences in the age, gravidity, parity, and number of spontaneous miscarriages were observed when all patients (Supplemental Table 5A), RPL patients (Supplemental Table 5B), or UI patients (Supplemental Table 5C) were compared.
Figure 1.
Analysis of targeted RNA sequencing data revealed differentially expressed genes in the endometrium from women with reproductive failures compared with those of healthy fertile controls. (A) Representative three-dimensional plot demonstrating patients’ samples clustering in the analysis of genes that expressed differently between patients and controls. (B) Comparison of endometrial gene expression for selected markers between women with reproductive failures (Patients, n = 42) and healthy controls (Controls, n = 18). Panels show relative mRNA expression levels. Based on the outcome of Levene's test for equality of variances, the Kruskal-Wallis test was used for statistical analysis of FOXO1, SGK1, SCNN1A, GZMB, IL15, and PTGS2, and the ANOVA test was used for the analysis of SLC2A1 and HEY1. ∗P<.05, ∗∗P<.01. ANOVA = analysis of variance; mRNA = messenger ribonucleic acid; RNA = ribonucleic acid.
Expression of NK Cell-Associated Markers in the Endometrium
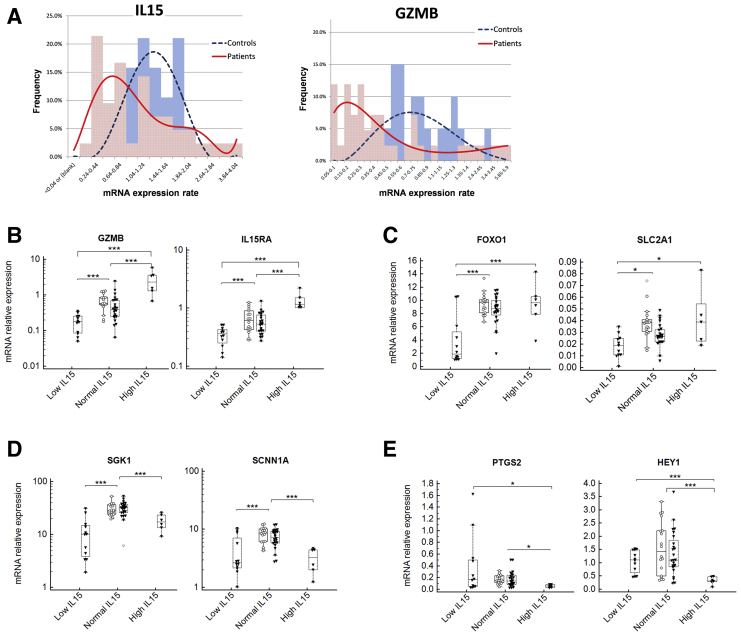
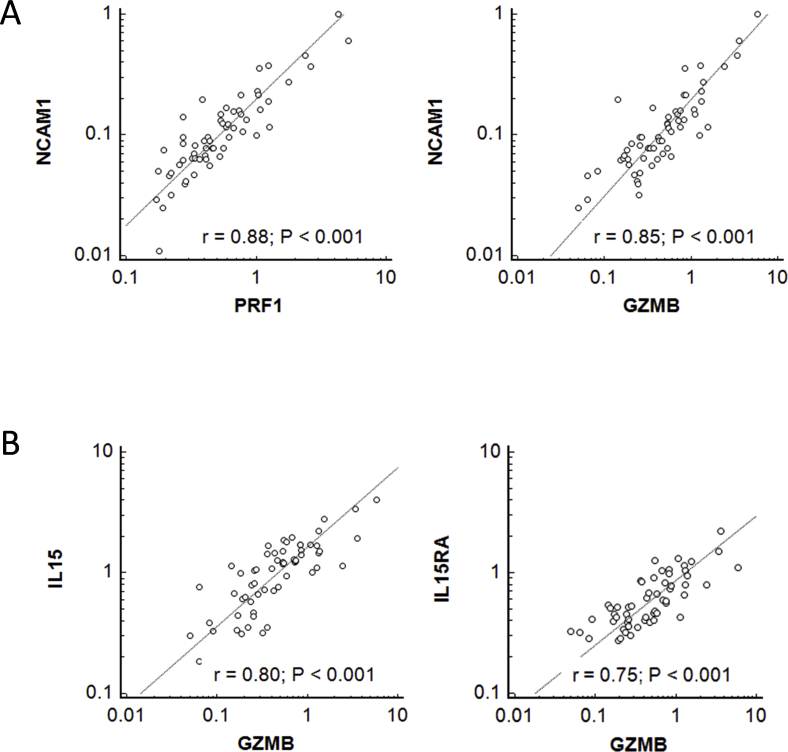
CD56-expressing NK cells are abundant in the mid-luteal endometrium. Indeed, the RNAseq analysis revealed substantial levels of NCAM1 transcripts (mRNA for CD56) in the studied samples. NCAM1 levels were correlated highly with other NK cell-related markers, such as GZMB (encodes granzyme B) and PRF1 (perforin) (Supplemental Fig. 2A). The expression of IL15 and its receptor IL15RA, which are important for NK cell proliferation, differentiation, and survival, also were correlated strongly with the transcript levels of NK cell markers (Supplemental Fig. 2B). A substantially wider range of GZMB and IL15 expression was seen in the patient group when compared with that of the controls. A normal (bell-shaped) distribution was characteristic of the control group and a double-peaked contour (bimodal distribution) was revealed for the patient samples (Fig. 2A).
Figure 2.
Expression of IL15 and GZMB in the endometrium. (A) Histograms showing the distribution of the endometrial biopsy samples based on IL15 and GZMB gene expression levels. The distribution of the healthy controls is shown in blue and that of the samples from the patient group in red. A normal bell-shaped distribution is characteristic of the healthy control group (– – –), in contrast to the double-peaked contour that emerged when the patients’ samples were analyzed (——). The X-axis shows relative mRNA expression levels (IL15 or GZMB). The Y-axis shows the percent of samples with specified levels of IL15 or GZMB. (B-E) Endometrial biopsy samples with abnormal levels of IL15 (either low or high) were characterized by distinct molecular profiles. All samples were grouped into IL15 low, IL15 normal, and IL15 high samples according to the levels of IL15 transcripts in the sample. Filled triangles represent samples from the women with reproductive failures, open circles represent samples from the healthy controls. (B) The IL15 low samples revealed the lowest levels of GZMB and IL15RA. The IL15 high samples had the highest levels of GZMB and IL15RA. (C) The IL15 low samples had significantly lower levels of expression of FOXO1 and SLC2A1 in comparison with those of the IL15 normal and high samples. (D) Both the low and the high IL15 samples revealed decreased expression of SGK1 and SCNN1A. (E) The samples with high IL15 were marked with the lowest levels of PTGS2 and HEY1. ANOVA or Kruskal-Wallis test was used for statistical analysis between the IL15 low, normal, and high groups. ∗P<.05, ∗∗P<.01, ∗∗∗P<.001.
To determine the difference between samples with high and low levels of IL15, we divided all samples according to the levels of IL15 transcripts and compared them for the expression of other markers. The samples with abnormal levels of IL15 (either low or high) were found to have distinct molecular profiles (Fig. 2B-E). In agreement with the correlation data described previously, the IL15-low samples had significantly lower expression of GZMB and IL15RA, whereas the IL15-high samples revealed a significantly higher expression of these genes (Fig. 2B). Moreover, IL15-low samples were found to have significantly lower levels of FOXO1 and SLC2A1 when compared with IL15-normal or IL15-high samples (Fig. 2C). Both IL15-low and IL15-high samples showed significantly decreased levels of SGK1 and SCNN1A (Fig. 2D). Finally, Fig. 2E demonstrates that IL15-high samples exhibited the lowest expression of PTGS2 (encodes COX2) and HEY1 (mediator of Notch signaling).
However, no significant difference in patient characteristics (age, gravidity, parity, and number of spontaneous miscarriages) were detected among samples with low, normal, or high IL15 levels (Supplemental Table 6).
Receiver Operating Characteristic Analysis and Criterions for a Decidualization Score
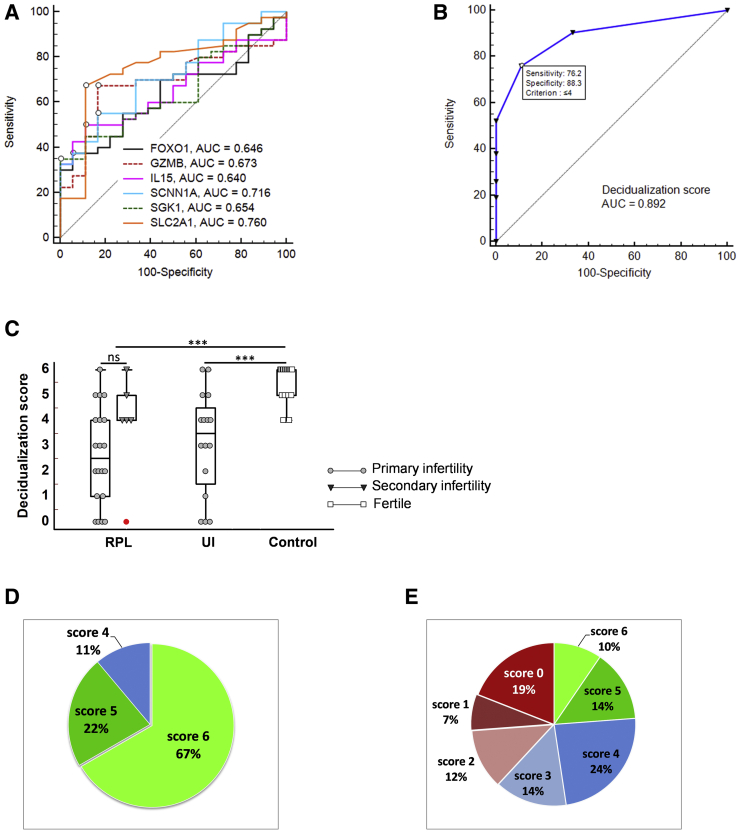
ROC analysis was performed to evaluate the differential ability of the RNAseq gene panel to identify endometrial samples that had a strong association with reproductive failures. As a result, markers with the AUC values significantly >0.5 were selected. They included 6 signature genes—FOXO1, SGK1, SCNN1A, SLC2A1, GZMB, and IL15 (Fig. 3A). The AUC, 95% CI, the significance level P, Youden’s index, and the associated criterion for these 6 markers are presented in Table 1. Each criterion indicates a point in the AUC curve with the best separation between the groups and was used to establish a cutoff value for each marker. To determine a combined effect of the selected markers, a cumulative ROC analysis was performed. The markers with values that were greater than or equal to the cutoff point were considered normal and received a score of 1. The markers with values that were below the cutoff point received a score of 0. Additionally, IL15 and GZMB upper-level cutoff points were introduced because of their bimodal distribution in the patient samples. A mean +2SD value in the control group was used as the upper cutoff point. The GZMB and IL15 values that were greater than the upper cutoff were considered out of range and received a score of 0. The cumulative score model, which was called the decidualization score, reflected how many markers out of 6 signature genes were expressed at normal levels in a tested sample.
Figure 3.
Receiver operating characteristic curve analysis. (A) Comparison of ROC curves for FOXO1, GZMB, IL15, SCNN1A, SGK1, and SLC2A1. The corresponding AUC values of the ROC curves are shown for each gene. (B) ROC curve analysis for the cumulative score model (decidualization score) determined a cutoff criterion to identify compromised samples. The AUC, its P value (the probability that the AUC is different from the null hypothesis: AUC = 0.5), the cutoff point with the best separation between the 2 groups (the criterion), and the associated sensitivity and specificity values are presented on the graph. Kruskal-Wallis test. ∗∗∗ P<.001 for RPL or UI versus Control; ns = nonsignificant difference. (C) Comparison of decidualization scores between women with RPL, UI, and healthy controls. The RPL group included cases of primary and secondary infertility. Statistical analysis was performed with Welch’s adjusted t-test and the Kruskal-Wallis test. ∗∗∗ P<.001 for RPL versus Control, and UI versus Control; ns = nonsignificant difference. (D, E). Proportion of decidualization scores in the healthy control group (D) and in the patient group (E).
Table 1.
Summary of the receiver operating characteristic curve analysis for individual genes and cumulative model.
| Marker name | AUC (95% CI) | Significance level Pa | Youden’s index | Associated criterion |
|---|---|---|---|---|
| FOXO1 | 0.646 (0.505 to 0.787) | .041 | 0.33 | >7.47 |
| SGK1 | 0.654 (0.519 to 0.774) | .031 | 0.37 | <18.97 |
| SCNN1A | 0.716 (0.584 to 0.825) | .001 | 0.38 | <4.02 |
| SCL2A1 | 0.760 (0.630 to 0.863) | <.001 | 0.56 | <0.028 |
| GZMB | 0.673 (0.538 to 0.789) | .016 | 0.49 | <0.46 |
| IL15 | 0.640 (0.504 to 0.761) | .049 | 0.38 | <0.82 |
| Cumulative model (decidualization score) | 0.892 (0.780 to 0.956) | <.001 | 0.65 | <4.0 |
Note: AUC = area under the receiver operating characteristic curve; 95% CI = 95% confidence interval.
Only markers with P<.05 for AUC >0.5 are shown in the table.
The ROC analysis for the cumulative score model revealed a substantially improved AUC value (AUC = 0.892, P<.001, Fig. 3B). The decidualization score ≤4 corresponded to an optimal criterion that allowed the best separation between patients and controls. The coordinates of the ROC curve associated with this criterion were as follows: sensitivity 76.2% (95% CI 60.5-87.9), specificity 88.9 (95% CI 65.3-98.6), positive likelihood ratio 6.86 (95% CI 1.8-25.6), positive predictive value 94.1 (95% CI 80.0-99.3), and negative predictive value 61.5 (95% CI 40.6-79.8).
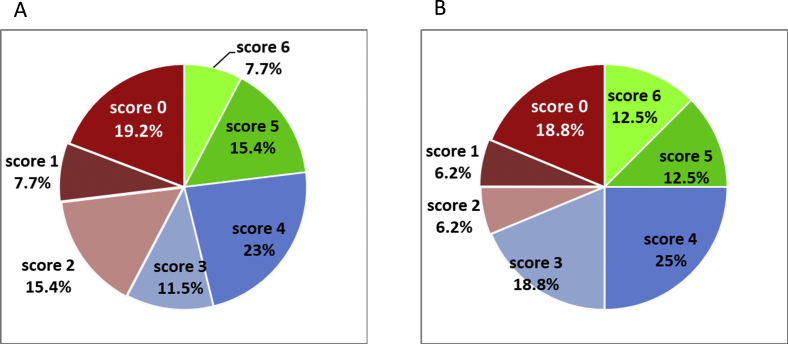
A comparison of the decidualization scores between groups is shown in Figure 3C. In the control group, 89% of samples had scores of 5 or 6 (Fig. 3D). In contrast, only 24% of samples in the patient group had scores that were >4 (Fig. 3E). A significant proportion of samples in the patient group had very low scores. Score 0, which indicated that none of the 6 signature genes were expressed at the level of the controls, was found in 19% of samples. Score 4 was detected in 24% of patients as well as in 11% of controls; therefore, it was considered as borderline normal. Post-hoc analysis of the decidualization score for the control and patient groups showed 100% power. The decidualization score was not significantly different between the RPL and UI patients (Fig. 3C, Supplemental Fig. 3). The median score was 3.0 (95% CI 2–4) for the RPL group and 3.5 (95% CI 1.6-4.4) for the UI group. Interestingly, the secondary infertility subgroup revealed mostly high scores (median 4.0, 95% CI 0.8-5.8) with the exception of 1 patient with ta score of 0. However, the difference between the primary and secondary infertility subgroups did not reach statistical significance.
Discussion
Endometrial targeted RNA sequencing analysis revealed a set of markers that were differentially expressed between women with reproductive failures and healthy fertile controls. They included molecules involved in progesterone signaling and decidualization (FOXO1), tissue and cellular homeostasis (SGK1, SCNN1A, SLC2A1), as well as immunoregulatory and tissue remodeling factors (IL15, GZMB). The regulatory roles for these markers in infertility or their importance in the endometrium and pregnancy have been reported (6, 8, 13, 22, 23). Yet, we found that combined evaluation of these genes was valuable and important for identifying reproductive failures associated with abnormal endometrium conditions.
The FOXO1 gene encodes a transcription factor that is involved in the binding of a progesterone receptor to its genomic targets and is considered as a marker for the window of implantation (22, 24, 25, 26, 27). Our analysis revealed a significantly lower expression of FOXO1 among the patients than that in the controls. Interestingly, the activity of FOXO1 is known to be regulated by SGK1 (28, 29), which was another marker that we revealed was differentially expressed between patients and controls. SGK1 expression can be triggered in vitro by serum and glucocorticoids and in vivo by various stimuli, including growth factors and cytokines (granulocyte-macrophage colony-stimulating factor, transforming growth factor beta, IL-6) (30, 31). Dysregulated endometrial expression of SGK1 has been reported previously in women with reproductive disorders (8, 14). Both the mRNA and protein of SGK1 have a short half-life, 20 and 60 minutes, respectively (30). It seems that a multitude of stimuli are present in the mid-luteal endometrium as our RNAseq assay revealed relatively high levels of SGK1 in comparison with other analytes. Its expression among patients varied significantly with approximately 33% of patients having SGK1 levels below the ranges seen among fertile controls. A strong correlation between SGK1 and its direct target, epithelial sodium channel (ENaC), confirmed further the finding of dysregulated SGK1 expression in the patent group. It was reported previously that protein levels of SCNN1a and SCNN1b (2 subunits of the ENaC complex) in endometrial samples obtained before in vitro fertilization (IVF) treatment were significantly lower in women who failed the subsequent IVF (23). Similarly, our analysis revealed significantly lower SCNN1A levels in the patients than that in the healthy fertile controls.
Corticosteroid treatment is often taken into consideration for the management of recurrent reproductive failure cases with unknown etiology when no other options are left (31, 32, 33). However, inconsistent results for the use of corticosteroids have been reported (34). Among the markers we found were expressed differentially in women with reproductive failures and healthy controls, SGK1 is a well-known target for glucocorticoids (35). An application of this information on the expression of prednisolone responsive genes in the endometrium potentially could help in the selection of patients who will benefit from corticosteroids and avoid unnecessary risk for patients with normal levels of FOXO1, SGK1, and SCNN1A.
An important component of endometrial decidualization, which provides a nutritional and receptive medium for embryo implantation and growth, involves glucose transport molecules. A suppression of Glut1 was reported previously in patients with idiopathic infertility (6). Here we revealed significantly lower levels of Glut1 in patients than that in healthy controls. Decreased endometrial expression of glucose transporters is associated with PCOS and obesity (36). Interestingly, women for whom lifestyle interventions led to weight loss and improved menstrual function had a significant upregulation of Glut1 in the endometrium at the mRNA and protein level (36).
In our study, we found a substantially wider range of IL15 expression in the patient group than that in the controls. IL15 levels also correlated strongly with the expression of NK cell markers, namely with GZMB, which encodes granzyme B, a serine protease found in NK cell granules. Granzyme B is essential in the process of senescent decidual cell clearance during tissue remodeling for incoming blastocysts (13). The degranulation of uNK cells needs to be well balanced, as too much activity might lead to a miscarriage and insufficient activity might lead to implantation failure (13). The samples with either low or high levels of IL15 were further compared. A decreased expression of SGK1 and SCNN1A was a common characteristic for all samples with abnormal IL15 (either low or high). However, we observed low levels of transcript for PTGS2 (prostaglandin G/H synthase 2, encodes COX2) and HEY1 (Hes-related family BHLH transcription factor) only in the samples with high IL15. Administration of nonsteroidal anti-inflammatory drugs in patients with high IL15 possibly could lead to an additional inhibition of COX2, which might affect prostaglandin synthesis and impact implantation. HEY1 is known as a repressor for the androgen receptor (AR); it blocks transcription of AR-dependent target genes (37). AR is known to be expressed in the endometrium (38). It is possible that in women with a low endometrial expression of HEY1 and elevated levels of androgens, signaling from the AR takes place and interrupts the estrogen- and progesterone-based regulation in the endometrium. Further study with a focus on these cases might shed a new light on the etiology of RPL and infertility.
The study had several limitations including the small sample size and the heterogeneity of the patient group. The targeted RNA sequencing data were compared between RPL and UI; however, no significant differences were observed. It was reported recently (39) that undiagnosed endometriosis was a common finding in both populations, and it was associated with increased BCL6 protein detection in the endometrium. However, our data were not able to confirm this at the RNA level. Another limitation of our study was its cross-sectional nature, as it was based only on observed findings. Further study involving an in vitro cell culture model or an animal model would be necessary to confirm the combination of decidualization score factors was involved in reproductive failures.
The difference in the expression pattern of progesterone signaling (FOXO1) and tissue homeostasis molecules (SGK1, SCNN1A, SLC2A1) among the IL15 low, high, and normal samples indicated that immune factors were among the key factors involved in the endometrium decidualization. All samples with abnormal IL15 revealed some deficiency in the expression of other decidualization score factors as well as factors associated with prostaglandin synthesis (PTGS2) and androgen signaling (HEY1). Using the decidualization score algorithm would help to identify a subset of patients with endometrial dysregulation. Further study is highly important to validate our initial findings and determine if this algorithm could direct therapy selection. For example, the levels of expression of corticosteroid targets (FOXO1, SGK1, and SCNN1A) should be taken into account if prednisolone treatment is considered. Or the use of nonsteroidal anti-inflammatory drugs in patients with abnormally high IL-15, which is associated with very low COX2 levels and potentially decreased prostaglandin synthesis.
In the population of women who suffer from recurrent reproductive failures, an endometrial biopsy sample could be taken for evaluation and certain tests including an endometrial receptivity array, endometrial immune profile, and endometrial function test could be included in the workup of infertility (5, 40, 41). We implemented a combined 6 gene signature approach to evaluate the endometrium. The molecular testing of endometrial tissue provided important information on the expression of genes associated with decidualization, tissue homeostasis, and immune regulation. The decidualization score reflects whether these factors are expressed at normal ranges and helps to determine if the molecular profile in the endometrium is implantation-friendly. This data could be applied for selecting patients that require therapeutic actions to improve endometrial conditions before IVF/embryo transfer procedures. Further study is needed to confirm this finding.
In conclusion, the decidualization score approach provided combined evaluation of endometrial expression of immune regulatory factors, such as IL15 and GZMB, and factors directly related to progesterone signaling and tissue homeostasis (FOXO1, SCL2A1, SGK1, SCNN1A). The results of this preliminary study showed that a low decidualization score was a frequent finding in patients with reproductive failure. Further validation of this testing approach is necessary to determine a particular patient population for whom this testing approach will be the most beneficial and its use for therapy application.
Acknowledgments
The authors thank all the personnel and clinical fellows at the Reproductive Medicine and Immunology, Health Clinics and Clinical Immunology Laboratory at Rosalind Franklin University of Medicine and Science for their passionate work, and Azib Shahid at Internal Medicine, Clinical Sciences Department, Chicago Medical School, Rosalind Franklin University for writing assistance.
Footnotes
S.D. has nothing to disclose. M.B. has nothing to disclose. S.S. has nothing to disclose. A.G. has nothing to disclose. E.F. has nothing to disclose. J.K.-K. has nothing to disclose. K.B. has nothing to disclose. C.C. has nothing to disclose.
Supplementary data
Supplemental Figure 1.
The data obtained using the targeted RNAseq method was validated with quantitative reverse transcriptase–polymerase chain reaction (RT-PCR) method. Representative plots demonstrating rank correlation analysis with Spearman's rho rank correlation coefficient and P value are shown. Quantitative RT-PCR method: cDNA synthesis was performed using SuperScript II First-Strand Synthesis System for RT-PCR (Invitrogen; Thermo Fisher Scientific, Inc.). Quantitative RT-PCR assays were performed using TaqMan gene expression assay (Applied Biosystems, Thermo Fischer Scientific) using the StepOnePlus real-time PCR system (Applied Biosystems, Thermo Fischer Scientific). Specific gene CT values were normalized to internal B2M controls and then quantified for relative gene expression utilizing the 2ˆ−ΔCT method. Following pre-validated TaqMan gene expression assays were used: NCAM1 (Hs00941833_m1), PRF1 (xxx), GZMB (xxx), IL15 (Hs01003715_m1), IL15RA (xxx), SCNN1A (Hs00168906_m1), FOXO1 (Hs00231106_m1), SGK1 (Hs00985033_g1), SLC2A1 (Hs00892681_m1) and endogenous control B2M (all from Applied Biosystems, Foster City, CA).
Supplemental Figure 2.
NK cell’s specific gene expression in the endometrium. A. The endometrial expression of NCAM1 (encodes CD56) strongly correlates with other NK cell specific markers: PRF1 (perforin) and GZMB (granzyme B). B. The expression of NK cell specific GZMB in the endometrium strongly correlates with the expression of IL15 and IL15RA.
Supplemental Figure 3.
Comparison of decidualization scores between RPL (A) and UI (B). Diagrams demonstrate proportion of each score for each patient population.
References
- 1.Macklon N. Recurrent implantation failure is a pathology with a specific transcriptomic signature. Fertil Steril. 2017;108(1):9–14. doi: 10.1016/j.fertnstert.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 2.Ford H.B., Schust D.J. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009;2(2):76–83. [PMC free article] [PubMed] [Google Scholar]
- 3.Practice Committee of the American Society for Reproductive M Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103–1111. doi: 10.1016/j.fertnstert.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 4.Rai R., Regan L. Recurrent miscarriage. Lancet. 2006;368(9535):601–611. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- 5.Ledee N., Petitbarat M., Chevrier L., Vitoux D., Vezmar K., Rahmati M. The uterine immune profile may help women with repeated unexplained embryo implantation failure after in vitro fertilization. Am J Reprod Immunol. 2016;75(3):388–401. doi: 10.1111/aji.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Wolff M., Ursel S., Hahn U., Steldinger R., Strowitzki T. Glucose transporter proteins (GLUT) in human endometrium: expression, regulation, and function throughout the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab. 2003;88(8):3885–3892. doi: 10.1210/jc.2002-021890. [DOI] [PubMed] [Google Scholar]
- 7.Salker M.S., Singh Y., Zeng N., Chen H., Zhang S., Umbach A.T. Loss of endometrial sodium glucose cotransporter SGLT1 is detrimental to embryo survival and fetal growth in pregnancy. Sci Rep. 2017;7(1):12612. doi: 10.1038/s41598-017-11674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salker M.S., Christian M., Steel J.H., Nautiyal J., Lavery S., Trew G. Deregulation of the serum- and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat Med. 2011;17(11):1509–1513. doi: 10.1038/nm.2498. [DOI] [PubMed] [Google Scholar]
- 9.Gellersen B., Brosens J.J. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851–905. doi: 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]
- 10.de Ziegler D., Bergeron C., Cornel C., Medalie D.A., Massai M.R., Milgrom E. Effects of luteal estradiol on the secretory transformation of human endometrium and plasma gonadotropins. J Clin Endocrinol Metab. 1992;74(2):322–331. doi: 10.1210/jcem.74.2.1730810. [DOI] [PubMed] [Google Scholar]
- 11.Okada H., Tsuzuki T., Murata H. Decidualization of the human endometrium. Reprod Med Biol. 2018;17(3):220–227. doi: 10.1002/rmb2.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brar A.K., Frank G.R., Kessler C.A., Cedars M.I., Handwerger S. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine. 1997;6(3):301–307. doi: 10.1007/BF02820507. [DOI] [PubMed] [Google Scholar]
- 13.Brighton P.J., Maruyama Y., Fishwick K., Vrljicak P., Tewary S., Fujihara R. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. Elife. 2017;6 doi: 10.7554/eLife.31274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feroze-Zaidi F., Fusi L., Takano M., Higham J., Salker M.S., Goto T. Role and regulation of the serum- and glucocorticoid-regulated kinase 1 in fertile and infertile human endometrium. Endocrinology. 2007;148(10):5020–5029. doi: 10.1210/en.2007-0659. [DOI] [PubMed] [Google Scholar]
- 15.Perrotti N., He R.A., Phillips S.A., Haft C.R., Taylor S.I. Activation of serum- and glucocorticoid-induced protein kinase (Sgk) by cyclic AMP and insulin. J Biol Chem. 2001;276(12):9406–9412. doi: 10.1074/jbc.M007052200. [DOI] [PubMed] [Google Scholar]
- 16.Thorens B., Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298(2):E141–E145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell P., Sacks G., Tremellen K., Gee A. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. III: Further observations and reference ranges. Pathology. 2013;45(4):393–401. doi: 10.1097/PAT.0b013e328361429b. [DOI] [PubMed] [Google Scholar]
- 18.Ratsep M.T., Felker A.M., Kay V.R., Tolusso L., Hofmann A.P., Croy B.A. Uterine natural killer cells: supervisors of vasculature construction in early decidua basalis. Reproduction. 2015;149(2):R91–R102. doi: 10.1530/REP-14-0271. [DOI] [PubMed] [Google Scholar]
- 19.Gaynor L.M., Colucci F. Uterine natural killer cells: functional distinctions and influence on pregnancy in humans and mice. Front Immunol. 2017;8:467. doi: 10.3389/fimmu.2017.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitaya K., Yasuda J., Yagi I., Tada Y., Fushiki S., Honjo H. IL-15 expression at human endometrium and decidua. Biol Reprod. 2000;63(3):683–687. doi: 10.1095/biolreprod63.3.683. [DOI] [PubMed] [Google Scholar]
- 21.Okada S., Okada H., Sanezumi M., Nakajima T., Yasuda K., Kanzaki H. Expression of interleukin-15 in human endometrium and decidua. Mol Hum Reprod. 2000;6(1):75–80. doi: 10.1093/molehr/6.1.75. [DOI] [PubMed] [Google Scholar]
- 22.Kajihara T., Brosens J.J., Ishihara O. The role of FOXO1 in the decidual transformation of the endometrium and early pregnancy. Med Mol Morphol. 2013;46(2):61–68. doi: 10.1007/s00795-013-0018-z. [DOI] [PubMed] [Google Scholar]
- 23.Ruan Y.C., Guo J.H., Liu X., Zhang R., Tsang L.L., Dong J.D. Activation of the epithelial Na+ channel triggers prostaglandin E(2) release and production required for embryo implantation. Nat Med. 2012;18(7):1112–1117. doi: 10.1038/nm.2771. [DOI] [PubMed] [Google Scholar]
- 24.Vasquez Y.M., Mazur E.C., Li X., Kommagani R., Jiang L., Chen R. FOXO1 is required for binding of PR on IRF4, novel transcriptional regulator of endometrial stromal decidualization. Mol Endocrinol. 2015;29(3):421–433. doi: 10.1210/me.2014-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grinius L., Kessler C., Schroeder J., Handwerger S. Forkhead transcription factor FOXO1A is critical for induction of human decidualization. J Endocrinol. 2006;189(1):179–187. doi: 10.1677/joe.1.06451. [DOI] [PubMed] [Google Scholar]
- 26.Labied S., Kajihara T., Madureira P.A., Fusi L., Jones M.C., Higham J.M. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol. 2006;20(1):35–44. doi: 10.1210/me.2005-0275. [DOI] [PubMed] [Google Scholar]
- 27.Gellersen B., Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178(3):357–372. doi: 10.1677/joe.0.1780357. [DOI] [PubMed] [Google Scholar]
- 28.Di Pietro N., Panel V., Hayes S., Bagattin A., Meruvu S., Pandolfi A. Serum- and glucocorticoid-inducible kinase 1 (SGK1) regulates adipocyte differentiation via forkhead box O1. Mol Endocrinol. 2010;24(2):370–380. doi: 10.1210/me.2009-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu C., Yosef N., Thalhamer T., Zhu C., Xiao S., Kishi Y. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Cristofano A. SGK1: the dark side of PI3K signaling. Curr Top Dev Biol. 2017;123:49–71. doi: 10.1016/bs.ctdb.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michael A.E., Papageorghiou A.T. Potential significance of physiological and pharmacological glucocorticoids in early pregnancy. Hum Reprod Update. 2008;14(5):497–517. doi: 10.1093/humupd/dmn021. [DOI] [PubMed] [Google Scholar]
- 32.Bansal A.S., Bajardeen B., Thum M.Y. The basis and value of currently used immunomodulatory therapies in recurrent miscarriage. J Reprod Immunol. 2012;93(1):41–51. doi: 10.1016/j.jri.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Mekinian A., Cohen J., Alijotas-Reig J., Carbillon L., Nicaise-Roland P., Kayem G. Unexplained recurrent miscarriage and recurrent implantation failure: is there a place for immunomodulation? Am J Reprod Immunol. 2016;86(1):8–82. doi: 10.1111/aji.12493. [DOI] [PubMed] [Google Scholar]
- 34.Homer H.A. Modern management of recurrent miscarriage. Aust N Z J Obstet Gynaecol. 2019;59(1):36–44. doi: 10.1111/ajo.12920. [DOI] [PubMed] [Google Scholar]
- 35.Lang F., Stournaras C., Zacharopoulou N., Voelkl J., Alesutan I. Serum- and glucocorticoid-inducible kinase 1 and the response to cell stress. Cell Stress. 2018;3 (1):1–8. doi: 10.15698/cst2019.01.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ujvari D., Hulchiy M., Calaby A., Nybacka A., Bystrom B., Hirschberg A.L. Lifestyle intervention up-regulates gene and protein levels of molecules involved in insulin signaling in the endometrium of overweight/obese women with polycystic ovary syndrome. Hum Reprod. 2014;29 (7):1526–1535. doi: 10.1093/humrep/deu114. [DOI] [PubMed] [Google Scholar]
- 37.Belandia B., Powell S.M., Garcia-Pedrero J.M., Walker M.M., Bevan C.L., Parker M.G. Hey1, a mediator of notch signaling, is an androgen receptor corepressor. Mol Cell Biol. 2005;25 (4):1425–1436. doi: 10.1128/MCB.25.4.1425-1436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibson D.A., Simitsidellis I., Saunders P.T. Regulation of androgen action during establishment of pregnancy. J Mol Endocrinol. 2016;57 (1):R35–R47. doi: 10.1530/JME-16-0027. [DOI] [PubMed] [Google Scholar]
- 39.Fox C.W., Savaris R.F., Jeong J.W., Kim T.H., Miller P.B., Likes C.E., Schammel D.P., Young S.L., Lessey B.A. Unexplained recurrent pregnancy loss and unexplained infertility: twins in disguise. Hum Reprod Open. 2019;2020 (1):1–8. doi: 10.1093/hropen/hoz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubowy R.L., Feinberg R.F., Keefe D.L., Doncel G.F., Williams S.C., McSweet J.C. Improved endometrial assessment using cyclin E and p27. Fertil Steril. 2003;80 (1):146–156. doi: 10.1016/s0015-0282(03)00573-9. [DOI] [PubMed] [Google Scholar]
- 41.Diaz-Gimeno P., Horcajadas J.A., Martinez-Conejero J.A., Esteban F.J., Alama P., Pellicer A. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011;95 (1) doi: 10.1016/j.fertnstert.2010.04.063. 50-60,e1-e15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.