Abstract
Objective
To report a rare case of schistosomiasis observed during semen evaluation.
Design
Case report.
Setting
University hospital.
Patient(s)
A 30-year-old man referred for semen analysis.
Interventions(s)
None.
Main Outcome Measures(s)
Poor sperm motility and viability.
Result(s)
The patient produced 9.8 mL of brown colored semen with a bad odor. Total and progressive sperm motility were 9% and 2%, respectively. Sperm concentration was 112 million/mL. Microscopic semen evaluation showed slight sperm agglutination, a large number of Schistosoma haematobium ova, extensive debris, and a large numberot of amorphous cells. Approximately 20 million/mL of neutrophils were observed in the ejaculate. The sperm viability was extremely low (13%). Sperm morphology was 6% normal, and most abnormal sperm had coiled tails in addition to other abnormalities.
Conclusion(s)
A microscopic examination of semen from suspected Schistosoma haematobium–infected patients may not only help in confirming diagnosis but may also highlight the underlying infertility due to this infestation. Such cases are rarely observed in andrology laboratories; therefore, it is important to train all testing staff on rare semen samples.
Key Words: Schistosoma, ova, male, semen
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-20-00155
Introduction
Ejaculate nature and semen parameters are frequently observed in reference ranges that most testing personnel in andrology laboratories are familiar with. We report an unusual ejaculate with changes in semen quality due to Schistosoma haematobium infestation. Schistosomiasis is endemic in areas of Southeast Asia, Africa, and South America. Migration from endemic countries to the developed world and frequent traveling or tourism to these regions may introduce this disease to new regions. Therefore, health care workers in the developed world should be aware of these unseen conditions. Schistosomiasis is a snail-borne parasitic disease prevalent in poor communities with inadequate sanitation and unsafe waters (1). Schistosome ova are released in the feces or urine of a schistosomiasis-infected person. On contact with fresh water, the ova hatch and release larvae called miracidia that infest snails, which are an intermediate host. Miracidia multiply in snails and produce thousands of cercariae, which penetrate the human skin during farming, wading, swimming, or washing in the contaminated water. After penetrating the intact skin, cercariae reach the blood stream, and over the next 4–6 weeks, the parasite matures into a long worm, either male or female, which ultimately resides in the veins of the gastrointestinal (mesenteric and portal veins) or genitourinary (perivesical) tract. At their final location, the females lay between 200 and 2,000 ova per day over an average of 5 years. Only half of the ova that are laid are excreted in urine or feces, whereas the rest are trapped in tissues, which stimulates an intense immune reaction with inflammation and granuloma formation that causes major and progressive tissue damage (2). Schistosomiasis has various clinical manifestations caused by different Schistosoma species. Schistosoma mansoni usually affects the gastrointestinal tract, and Schistosoma haematobium predominantly affects the genitourinary tract. Genital schistosomiasis leads to infertility in both men and women (3, 4). In men, seminal vesicles, prostate, epididymis, and vas deferens are mainly affected (5); however, severe manifestation of schistosomiasis can cause intense granulomatous epididymitis and inhibition of spermatogenesis, causing male factor infertility (6). Schistosomiasis has been recently associated with the onset of prostatic adenocarcinoma (7). In addition, schistosomiasis patients are at higher risk of HIV infection (8).
Case report
A 30-year-old, nonsmoking, migrant male was referred for semen evaluation with a history of drinking and swimming in river water from a Schistosoma haematobium endemic area in Africa. He reported intermittent gross hematuria and pain with urination from the age of 14–15 years. He also noted that his semen was sometimes bloody. He was concerned about fertility due to medical history and hematospermia. He had unprotected intercourse without any known pregnancies. A semen analysis after 7 days of sexual abstinence was performed 45 minutes after collection in the laboratory according to World Health Organization criteria (9). Institutional Review Board approval was sought, and the necessary documentation was filed. No patient identity information has been linked to this case report.
The patient produced a 9.8 mL sample of nonviscous brown colored semen with a bad odor. The total and progressive sperm motility was 9% and 2%, respectively (reference range, ≥40% and ≥32%, respectively). The sperm concentration was 112 million/mL (reference, ≥15 million/mL). Microscopic semen evaluation showed slight sperm agglutination, a large number of Schistosoma haematobium ova, extensive debris, and a lot of amorphous cells under high power field (×200). Peroxidase staining revealed the presence of approximately 20 million/mL of neutrophils in the ejaculate, indicating an inflammatory response. The sperm viability was extremely low, and only 13% of the sperm were viable under supravital stain (reference, ≥58%). The sperm morphology was 6% normal (reference, ≥4% normal), and most abnormal sperm had coiled tails in addition to other abnormalities. The patient was referred to our infectious disease department and was treated with two courses of praziquantel. Unfortunately, he moved after this point, and further information regarding his outcome is not known.
Discussion
Claude Barlow (10) was the first to report hematospermia and the presence of ova in his semen after he voluntarily infected himself with the larvae of Schistosoma haematobium in 1949. Several studies have shown that ejaculate quality changes when Schistosoma haematobium ova primarily infest the male genital tract. A large autopsy study on 300 cadavers revealed that the ova of Schistosoma haematobium were mainly present in the urinary bladder, seminal vesicles, and vas deferens in 55%, 54%, and 39% of cases, respectively, whereas the prostate was affected in only 20% of cases (11). It has been reported that seminal vesicles and prostate are the main sites of infestation (5, 12), and the testicles are not a prime target of Schistosoma haematobium due to differences in anatomy and vasculature. Despite this, testicular tissue damage has been reported in 34.4% of 110 genital schistosomiasis autopsies (13). Once the testicular disease progresses to the granuloma stage, the damage is irreversible (6). Although vascular supply is generally not affected, occlusion of the spermatic venous plexus by Schistosoma haematobium ova has been reported. Venous obstruction with subsequent granuloma formation may result in testicular infarction (5, 6, 14, 15). Testicular schistosomiasis is a very rare presentation; however, several reports illustrate this as an incidental finding during postmortem or as a testicular biopsy finding (15, 16).
Infection often affects ejaculate gross appearance and volume. Alterations in ejaculate color in Schistosoma haematobium patients vary among reports, ranging from no color change to yellow or brown in appearance (17, 18, 19). A reduction in ejaculate volume has been also reported (17, 20). The seminal vesicles and prostate are frequently affected by egg-induced inflammation in Schistosoma haematobium infection, resulting in sperm apoptosis and reduction in seminal fluid (21). In this case, we found brown colored semen (Fig. 1) with no reduction in ejaculate volume. This suggests that the seminal vesicles and prostate gland, which produce the bulk of seminal plasma, may not have been affected.
Figure 2.
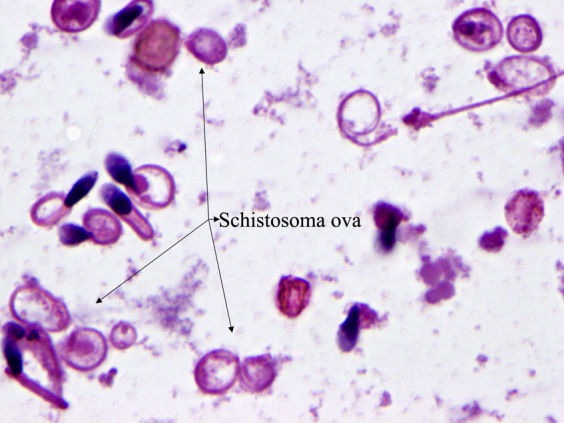
Multiple ova of Schistosoma haematobium with surrounding sperm with coiled tails (×1,000; hematoxylin-eosin stain).
Figure 1.

Brown color ejaculate from patient infected with Schistosoma haematobium.
Sperm concentration, motility, and morphology can also be altered by the presence of infection. The sperm concentration was not affected in this case, which agrees with previous studies (18, 22). This finding suggests that the testicular function was not negatively affected in this patient. Testicular venous obstruction can result in oligo- or azoospermia with a chronic inflammatory eosinophilic infiltrate in the interstitium of the testes, resulting in infertility (5, 6, 14, 15). A significant drop in total and progressive motility was observed in this case, which contrasts with previous findings where no decline in sperm motility was observed (17, 18, 22). The presence of debris, neutrophils, and Schistosoma ova in the ejaculate indicate an inflammatory condition, which possibly resulted in reduced sperm motility and viability and which can explain this case (Fig. 2). Most sperm tails were coiled in this patient, indicating a possible hypoosmotic stress response due to the parasitic infection. The sperm cells are very sensitive to hypoosmotic or hyperosmotic conditions, which can cause membrane damage and irreversible loss of motility. The hypoosmotic swelling test was inconclusive in this patient due to preexisting sperm with coiled tails in response to infection or hypoosmotic stress. The low sperm viability (13%) evaluated using supravital stain along with the poor sperm motility observed in this case supports our hypothesis. Overall, multiple semen parameters can be affected by chronic Schistosoma infection.
Conclusions
Change in ejaculate quality is a common presentation with schistosomal infection. Diagnostic tools such as microscopic examination of urine, feces, and rectal biopsy are widely used as standard methods to diagnose schistosomiasis, but sometimes they are unsuccessful (23). The case presented here emphasizes the importance of adding a semen analysis in addition to other diagnostic work in male genital schistosomiasis especially when there is a history of migration or travel to the endemic regions or when fertility is a concern. Evaluation of semen in male genital schistosomiasis could not only lead to a definitive diagnosis but could also help prevent infertility and provide information about the involvement of reproductive organs. Not all andrology testing personnel may be able to identify the ova of Schistosoma haematobium or similar rare findings in the ejaculates; therefore, it is very important to train all testing staff on rare semen samples. This will be educational for the laboratory staff and rewarding for the referring physician and more importantly the patient.
Footnotes
K.R.C. has nothing to disclose. C.A.K. has nothing to disclose. T.K.B. has nothing to disclose.
References
- 1.World Health Organization . World Health Organization; Geneva: 2018. Schistosomiasis, Fact Sheet No. 115. [Google Scholar]
- 2.World Health Organization Weekly epidemiological record. 2010;18:157–164. [Google Scholar]
- 3.Adisa J., Egbujo E.M., Yahaya B.A., Echejoh G. Primary infertility associated with schitosoma mansoni: a case report from the Jos plateau, north central Nigeria. Afr Health Sci. 2012;12:563–565. doi: 10.4314/ahs.v12i4.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodall P.A., Kramer M.R. Schistosomiasis and infertility in East Africa. Am J Trop Med Hyg. 2018;98:1137–1144. doi: 10.4269/ajtmh.17-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldmeier H., Leutscher P., Poggensee G., Harms G. Male genital schistosomiasis and haemospermia. Trop Med Int Health. 1999;4:791–793. doi: 10.1046/j.1365-3156.1999.00511.x. [DOI] [PubMed] [Google Scholar]
- 6.Kini S., Dayoub N., Raja A., Pickering S., Thong J. Schistosomiasis-induced male infertility. Br Med J Case Rep. 2009 doi: 10.1136/bcr.01.2009.1481. bcr01.2009.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figueiredo J.C., Richter J., Borja N., Balaca A., Costa S., Belo S. Prostate adenocarcinoma associated with prostatic infection due to Schistosoma haematobium. Case report and systematic review. Parasitol Res. 2015;114:351–358. doi: 10.1007/s00436-014-4250-9. [DOI] [PubMed] [Google Scholar]
- 8.Leutscher P., Ramarokoto C.E., Reimert C., Feldmeier H., Esterre P., Vennervald B.J. Community based study of genital schistosomiasis in men from Madagascar. Lancet. 2000;355:117–118. doi: 10.1016/S0140-6736(99)04856-4. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . 5th ed. World Health Organization; Geneva: 2010. WHO laboratory manual for the examination and processing of human semen. [Google Scholar]
- 10.Barlow B.A., Meleney H.E. A voluntary infection with S. haematobium. Am J Trop Med Hyg. 1949;29:79–87. doi: 10.4269/ajtmh.1949.s1-29.79. [DOI] [PubMed] [Google Scholar]
- 11.Gelfand M., Ross C.M., Blair D.M., Castle W.M., Weber M.C. Schistosomiasis of the male pelvic organs: severity of infection as determined by digestion of tissue and histological methods in 300 cadavers. Am J Trop Med Hyg. 1970;19:779–784. [PubMed] [Google Scholar]
- 12.Patil P.S., Elem B. Schistosomiasis of the prostate and the seminal vesicles: observations in Zambia. J Trop Med Hyg. 1988;91:245–248. [PubMed] [Google Scholar]
- 13.Gelfand M., Ross W.F. The distribution of Schistosoma ova in the genitourinary tract in subjects of Bilharziasis. Trans Roy Soc Trop Med Hyg. 1953;47:218–220. doi: 10.1016/0035-9203(53)90006-6. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Naser M.B., Altenburg A., Zouboulis C.C., Wollina U. Schistosomiasis (bilharziasis) and male infertility. Andrologia. 2018;51 doi: 10.1111/and.13165. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Naser M.B., Wollina U., Lohan M., Zouboulis C.C., Altenburg A. Schistosomiasis (bilharziasis) ova: an incidental finding in testicular tissue of an obstructive azoospermic man. Andrologia. 2018;50 doi: 10.1111/and.13131. [DOI] [PubMed] [Google Scholar]
- 16.El-Hawary A.K., Foda A.A. Incidentally detected schistosomiasis in male genital organs: case reports and review of literature. Am J Cancer Case Rep. 2016;4:25–30. [Google Scholar]
- 17.McKenna G., Schousboe M., Paltridge G. Subjective change in ejaculate as symptom of infection with Schistosoma haematobium in travellers. Br Med J. 1997;315:1000–1001. doi: 10.1136/bmj.315.7114.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torresi J., Sheori H., Ryan N., Yung A. Usefulness of semen microscopy in the diagnosis of a difficult case of Schistosoma haematobium infection in a returned traveller. J Travel Med. 1997;4:46–47. doi: 10.1111/j.1708-8305.1997.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 19.Hawary A., Taylor R., McEwans A., Napier-Hemy R. Change of semen quality after foreign travel: a rare presentation of genital schistosomiasis. Int Urol Nephrol. 2012;44:51–53. doi: 10.1007/s11255-010-9816-6. [DOI] [PubMed] [Google Scholar]
- 20.Leutscher P.D.C., Pedersen M., Raharisolo C., Jensen J.S., Hoffmann S., Lisse I. Increased prevalence of leukocytes and elevated cytokine levels in semen from Schistosoma haematobium–infected individuals. J Infect Dis. 2005;191:1639–1647. doi: 10.1086/429334. [DOI] [PubMed] [Google Scholar]
- 21.Leutscher P.D.C., Høst E., Reimert C.M. Semen quality in Schistosoma haematobium infected men in Madagascar. Acta Trop. 2009;109:41–44. doi: 10.1016/j.actatropica.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 22.van Delft F., Visser L., Polderman A., van Lieshout L. Cough and alterations in semen after a tropical swim. Neth J Med. 2007;65:304–306. [PubMed] [Google Scholar]
- 23.Obel N., Black F.T. Microscopic examination of sperm as the diagnostic clue in a case of Schistosoma haematobium infection. Scand J Infect Dis. 1994;26:117–118. doi: 10.3109/00365549409008602. [DOI] [PubMed] [Google Scholar]


