Abstract
Objective
To investigate the effects of oocyte donor and recipient body mass index (BMI) on outcomes of vitrified donor oocyte assisted reproductive technology (ART).
Design
Retrospective cohort study.
Setting
Private fertility center.
Patient(s)
A total of 338 oocyte donors and 932 recipients who underwent 1,651 embryo transfer cycles in 2008–2015.
Intervention(s)
Multivariable log binomial regression models with cluster-weighted generalized estimating equations were used to estimate the adjusted risk ratios.
Main Outcome Measure(s)
Live birth, defined as the delivery of at least one live-born infant, including all embryo transfer cycles. Secondary outcomes included birth weight and gestational length only among singleton live births.
Results
The mean ± SD body mass indexes (BMIs) of donors and recipients were 22.6 ± 2.5 kg/m2 and 24.6 ± 4.8 kg/m2, respectively. There were no significant associations between donor BMI and probability of live birth. Recipients with BMI ≥35 kg/m2 had a significantly higher probability of live birth compared with normal-weight recipients. Among singleton live births, recipients with BMI <18.5 kg/m2 had a lower risk whereas women with BMI ≥35 kg/m2 had a higher risk of delivery in an earlier gestational week compared with normal weight women. Recipients with a BMI ≥35 kg/m2 also had a higher risk of having a low birth weight infant compared with normal-weight women.
Conclusions
In the setting of vitrified donor oocyte ART, recipient BMI was positively associated with probability of live birth but negatively associated with gestational length and birth weight among singleton births.
Key Words: Donor oocyte, body mass index, in vitro fertilization, assisted reproductive technology, pregnancy, fertility, live birth, gestational age, birthweight
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-20-00186
Infertility, the failure to conceive after 1 year of regular unprotected sexual intercourse, affects 10%–25% of couples in Western countries (1, 2). Assisted reproductive technologies (ART) have become one of the main treatment modalities for couples facing fertility problems. Over the past two decades, an increasing number of ART cycles have been performed yearly and are responsible for ∼2% of live births in the United States (3, 4, 5). Excess body weight is one of the most consistent factors that has been related to impaired fertility in women conceiving both with and without medical assistance (6, 7, 8). However, there is still controversy over whether excess body weight negatively influences female fertility at the level of the oocyte, embryo, and/or uterine environment.
Studies among women undergoing ART with oocyte donation represent a unique population in which these questions can be further assessed. There have been a handful of previous studies focusing on obese donor oocyte recipients, but they have come to disparate conclusions regarding the effects of recipient obesity on ART outcomes (9, 10, 11, 12, 13, 14, 15, 16). A systematic review and meta-analysis conducted by Jungheim et al. in 2013 reported that, across six studies including a total of 4,758 women using donor oocytes, no significant associations were observed between recipient obesity and likelihood of implantation, pregnancy, miscarriage, or live birth (17). This suggests that female obesity may affect oocyte quality to a greater extent than the endometrium or uterine environment, and may be the driving factor underlying the observed associations between female obesity and reduced fertility. To date, only two studies—one from Spain (n = 1092 cycles) and one from the U.S. (n = 235 cycles)—have investigated the influence of donor body mass index (BMI) on ART outcomes following oocyte donation, with conflicting findings (18, 19). The Spanish study found no associations between BMI in the donor or recipient and ART outcomes; the American study reported a trend toward reduced odds of clinical pregnancy and live birth with increasing donor BMI, after accounting for recipient BMI.
To expand on this existing literature, we used information from a large cohort of vitrified donor oocytes to investigate the influence of both oocyte donor and recipient BMI on outcomes of ART. Because vitrified oocytes are obtained from anonymous, young, healthy female donors, there is little to no correlation in BMI between the donor and the female recipient, which allows for the independent investigation of how excess body weight affects oocyte quality and endometrial function and receptivity. Moreover, the standardized ovarian stimulation and endometrial preparation protocols used in vitrified donor oocyte ART cycles limits confounding by clinical procedures.
Materials and methods
Study Design
This study involved a retrospective cohort of vitrified donor oocyte ART cycles at Reproductive Biology Associates in Sandy Springs, Georgia, from 2008 to 2015. We included cycles in which all donor oocytes were cryopreserved via vitrification for storage in an oocyte bank and later warmed in separate cohorts for recipient use. Cycles in which gestational carriers were used or no embryos were transferred were excluded. After further excluding donors (n = 65) and recipients (n = 129) who were missing data on BMI (the primary exposure of interest), we had 338 oocyte donors and 932 recipients who underwent a total of 1,651 embryo transfer cycles. This study was approved by the Emory Institutional Review Board before study initiation (IRB no. 80463).
Body Weight Assessment and Covariate Information
At the donor’s and recipient’s first clinic visit, height and weight were measured with a standardized scale and stadiometer. BMI was calculated as weight in kg divided by height in meters squared. As expected, the range of BMIs among donors was much smaller than among recipients, owing to the strict inclusion criteria for becoming a donor. Therefore, donor BMI was divided into a three-level categoric variable (≤21, 21.1−24.9, and ≥25 kg/m2) based on established cutoffs and the distribution in our cohort. Recipient BMI was categorized in 5 groups (<18.5, 18.5−24.9, 25−29.9, 30−34.9, and ≥35 kg/m2) based on World Health Organization guidelines. At the initial patient visit, patients completed an intake form concerning their demographics (e.g., age, race/ethnicity, smoking status) and reproductive history (e.g., gravidity, parity). Information on infertility diagnoses among the recipients was abstracted from the medical record and classified according to Society for Assisted Reproductive Technology (SART) guidelines. For each retrieval that the donor underwent, we collected ovarian reserve data (e.g., bilateral antral follicle count and antimüllerian hormone) and ovarian stimulation data (e.g., gonadotropin dose, number of days of stimulation, peak E2 level, number of follicles >14 mm at time of trigger, and trigger type). Among recipients, we collected information on number of warmed donor oocytes, number of embryos transferred, and embryo stage at transfer for each ART cycle.
Recipient Preparation and Outcome Assessment
In advance of oocyte warming, recipients were given a standard endometrial preparation of leuprolide acetate, estrogen, and progesterone. After sufficient endometrial development, the donor oocytes were warmed and, 2–3 hours later, fertilized via intracytoplasmic sperm injection (20). The resulting embryos were then cultured in the laboratory until cleavage (day 3) or blastocyst stage (day 5 or 6). None of the embryos underwent preimplantation genetic testing. Embryo transfer was performed in a standard fashion, with the highest-quality embryo(s) transferred first and the remaining embryos cryopreserved for future use. Many recipients had one or more frozen embryo transfers from their initial cohort of warmed oocytes.
Live birth, defined as the delivery of at least one live-born infant in a given embryo transfer cycle, was our primary outcome. Secondary outcomes included positive pregnancy test (PPT; defined as serum β-hCG level >6 mIU/mL), pregnancy loss (defined as all positive pregnancy tests lost before 20 weeks of gestation), and the proportion of warmed oocytes that survived warming, fertilized normally, and subsequently developed into usable embryos. We also abstracted information on gestational age and birth weight among the ART cycles resulting in live birth. Preterm delivery was defined as gestational age <37 weeks, and low birth weight was defined as a birth weight <2,500 g.
Statistical Analysis
Descriptive statistics were calculated across BMI categories for demographic, reproductive, and clinical characteristics of the donor and recipient. We tested for differences across BMI categories with the use of chi-square tests for categoric variables and Kruskal-Wallis tests for continuous variables. Log binomial regression with cluster-weighted generalized estimating equations (GEEs) was used to analyze the association between donor/recipient BMI and probability of live birth. Our weight was equal to the inverse of the cluster size (number of embryo transfer cycles), and was chosen to account for the fact that women with more severe infertility likely had a greater number of cycles. When cluster size is informative, using an unweighted approach in marginal analyses will overweigh couples with the most severe infertility, leading to biased estimates. Donor BMI and recipient BMI were analyzed as both continuous and categoric variables. Risk ratios (RRs) were calculated comparing the risk of live birth in a specific BMI category compared with the risk in the reference category (e.g., 21.1−24.9 kg/m2 in donors and 18.5–24.9 kg/m2 in recipients). Tests for linear trends were conducted using the median values of each BMI category as continuous variables. We also examined the joint effect of donor and recipient BMI on the probability of live birth by cross-classifying donor/recipient pairs into BMI categories. Sensitivity analyses were conducted by restricting to only single-embryo transfers, only blastocyst transfers, only first embryo transfers, and only recipients without uterine-factor infertility or polycystic ovary syndrome (PCOS).
To explore the associations between donor/recipient BMI and risk of PPT and risk of pregnancy loss, we used cluster-weighted GEE models; however, for the outcome of pregnancy loss, we restricted the analysis to only cycles in which a pregnancy was achieved. The association between donor BMI and secondary outcomes following oocyte warming (e.g., % survived, % fertilized, % usable embryos) were analyzed with the use of GEEs with binomial distribution. Data are presented as back-transformed marginal percentages and 95% confidence intervals (CIs) at the mean level of continuous covariates and most common level of categoric covariates. Among singleton live births (n = 670), we analyzed the association between BMI and length of gestation with the use of a cluster-weighted Cox proportional hazards model with a robust sandwich covariance estimate. A cluster-weighted GEE with normal distribution and identity link function was used for birth weight analysis. For pre-term birth and low birth weight, a cluster weighted GEE with binomial distribution and logit link function was specified to calculate the odds ratios (ORs) and 95% CIs. All of these models accounted for the multiple live births that a woman could contribute to the analysis and the presence of nonignorable cluster size by weighting each recipient inversely according to the number of live births they achieved.
Confounding factors were selected based on previous studies, a priori knowledge, and descriptive statistics from our cohort through the use of directed acyclic graphs. The final model retained the following variables: donor and recipient age, donor or recipient race (white, black, or other), retrieval year (2008–2009, 2010–2011, 2012–2013, or 2014–2015), and recipient diagnoses of uterine-factor infertility or PCOS. Because race was highly correlated between donors and recipients, both variables could not be included in the final multivariable model. Therefore, donor race was considered as a confounder when the exposure was donor BMI, and recipient race was considered as a confounder when the exposure was recipient BMI. All tests of statistical significance were two sided and a significance level of 0.05 was used. All data were analyzed with the use of SAS 9.4 (SAS Institute).
Results
Our study population was composed of 338 oocyte donors and 932 recipients. The median (range) number of recipients per oocyte donor was 2 (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15). The mean (range) BMIs of donors and recipients were 22.6 kg/m2 (17.1–33.5 kg/m2) and 24.6 kg/m2 (16.1–45.5 kg/m2), respectively. The prevalence of overweight (BMI >25 kg/m2) was 17.8% in donors and 36.5% in recipients (Tables 1 and 2). The average age of donors at their first retrieval was 25.5 years, and the majority of donors were White (75%) and nulliparous (78%). There were no statistically significant differences in demographic, reproductive history, or ovarian stimulation parameters across the donor BMI categories. The majority of recipients were >40 years of age (68.5%), White (74%), and nulliparous (73%). Recipients with higher BMIs tended, on average, to be older, Black, and more likely to be diagnosed with uterine-factor infertility. Among the 1,651 embryo transfer cycles, 1,160 resulted in PPTs (70%) and 813 (49%) resulted in live births.
Table 1.
Characteristics of oocyte donors by body mass index (BMI) category, 2008–2015.
| Characteristic | Total | Donor BMI category, kg/m2 |
P valuea | ||
|---|---|---|---|---|---|
| ≤21 | 21.1–24.9 | ≥25 | |||
| No. of women | 338 | 97 | 181 | 60 | |
| Age at first retrieval, y | 25.5 (23.0–28.0) | 25.0 (23.0–28.0) | 26.0 (23.0–28.0) | 26.0 (23.0–28.0) | .79 |
| Year of retrieval | .15 | ||||
| 2008–2009 | 88 (26.0) | 25 (25.8) | 41 (22.7) | 22 (36.7) | |
| 2010–2011 | 107 (31.7) | 31 (32.0) | 57 (31.5) | 19 (31.7) | |
| 2012–2013 | 102 (30.2) | 34 (35.1) | 56 (30.9) | 12 (20.0) | |
| 2014–2015 | 41 (12.1) | 7 (7.2) | 27 (14.9) | 7 (11.7) | |
| Race | .12 | ||||
| White | 245 (74.7) | 71 (75.5) | 136 (76.8) | 38 (66.7) | |
| Black | 36 (11.0) | 6 (6.4) | 19 (10.7) | 11 (19.3) | |
| Other | 47 (14.3) | 17 (18.1) | 22 (12.4) | 8 (14.0) | |
| No. of previous births | .76 | ||||
| 0 | 263 (77.8) | 74 (76.3) | 144 (79.6) | 45 (75.0) | |
| 1 | 36 (10.7) | 9 (9.3) | 19 (10.5) | 8 (13.3) | |
| ≥2 | 39 (11.5) | 14 (14.4) | 18 (9.9) | 7 (11.67) | |
| Antimullerian hormone, ng/mL | 4.4 (3.0–6.6) | 4.6 (3.4–8.2) | 3.9 (2.8–6.5) | 4.8 (2.7–5.7) | .26 |
| Antral follicle count, n | 33.0 (25.0–41.5) | 32.0 (25.0–40.0) | 33.0 (26.0–41.5) | 34.0 (23.5–44.0) | .72 |
| Gonadotropin total dose, IU | 2,400.0 (1,950.0–2,850.0) | 2,250.0 (1,875.0–2,925.0) | 2,400.0 (2,025.0–2,850.0) | 2,400.0 (1,987.5–2,850.0) | .87 |
| Days of stimulation | .78 | ||||
| 8–9 | 88 (26.0) | 23 (23.7) | 47 (26.0) | 18 (30.0) | |
| 10–11 | 207 (61.2) | 60 (61.9) | 110 (60.8) | 37 (61.7) | |
| 12–13 | 43 (12.7) | 14 (14.4) | 24 (13.3) | 5 (8.3) | |
| Follicles >14 mm at trigger, n | 20.0 (16.0–25.0) | 20.0 (16.0–24.0) | 20.0 (16.0–25.0) | 20.0 (16.0–25.0) | .99 |
| Peak E2, pg/mL | 2,849.5 (1,882.0–4,548.0) | 2,979.0 (2,009.0–4,479.0) | 2,698.0 (1,842.0–4,197.0) | 3,247.5 (1,950.5–4,749.0) | .48 |
| Maturation trigger type | .70 | ||||
| hCG | 125 (37.1) | 36 (37.1) | 64 (35.6) | 25 (41.7) | |
| GnRH agonist (Lupron) | 212 (62.9) | 61 (62.9) | 116 (64.4) | 35 (58.3) | |
Note: Data are presented as median (interquartile range) or n (%). Amount of women with missing data: 10 for race, 203 for antimüllerian hormone (which was not routinely measured before 2012), 2 for antral follicle count, and 1 for maturation trigger type.
P values for differences across donor BMI categories were calculated with the use of chi-square tests for categoric variables and Kruskal-Wallis tests for continuous variables.
Table 2.
Characteristics of oocyte recipients by body mass index (BMI) category, 2008–2015.
| Characteristic | Total | Recipient BMI category, kg/m2 |
P valuea | ||||
|---|---|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25–29.9 | 30–34.9 | ≥35 | |||
| No. of women | 932 | 15 | 577 | 218 | 82 | 40 | |
| Age, y | 42.0 (38.0–44.0) | 39.0 (37.0–41.0) | 42.0 (39.0–44.0) | 41.0 (38.0–44.0) | 42.0 (38.0–44.0) | 42.0 (40.0–45.0) | .02 |
| Year of embryo transfer | .46 | ||||||
| 2008–2009 | 257 (27.6) | 5 (33.3) | 155 (26.9) | 59 (27.1) | 25 (30.5) | 13 (32.5) | |
| 2010–2011 | 298 (32.0) | 4 (26.7) | 188 (32.6) | 67 (30.7) | 25 (30.5) | 14 (35.0) | |
| 2012–2013 | 294 (31.6) | 5 (33.3) | 175 (30.3) | 82 (37.6) | 23 (28.1) | 9 (22.5) | |
| 2014–2015 | 83 (8.9) | 1 (6.7) | 59 (10.2) | 10 (4.6) | 9 (11.0) | 4 (10.0) | |
| Race | <.001 | ||||||
| White | 683 (73.6) | 11 (73.3) | 438 (76.4) | 152 (69.7) | 56 (68.4) | 26 (65.0) | |
| Black | 121 (13.0) | 0 (0.0) | 45 (7.9) | 45 (20.6) | 20 (24.4) | 11 (27.5) | |
| Other | 124 (13.4) | 4 (26.7) | 90 (15.7) | 21 (9.6) | 6 (7.3) | 3 (7.5) | |
| Recent tobacco use | .13 | ||||||
| Yes | 29 (3.1) | 0 (0.0) | 20 (3.5) | 2 (0.9) | 5 (6.1) | 2 (5.0) | |
| No | 903 (96.9) | 15 (100.0) | 557 (96.5) | 216 (99.1) | 77 (93.9) | 38 (95.0) | |
| No. of previous births | .50 | ||||||
| 0 | 676 (72.5) | 11 (73.3) | 415 (71.9) | 163 (74.8) | 57 (69.5) | 30 (75.0) | |
| 1 | 179 (19.2) | 3 (20.0) | 115 (19.9) | 39 (17.9) | 13 (15.9) | 9 (22.5) | |
| ≥2 | 77 (8.3) | 1 (6.7) | 47 (8.2) | 16 (7.3) | 12 (14.6) | 1 (2.5) | |
| Previous autologous IVF transfers | .12 | ||||||
| 0 | 485 (52.2) | 3 (20.0) | 295 (51.3) | 127 (58.3) | 43 (52.4) | 17 (42.5) | |
| 1 | 173 (18.6) | 3 (20.0) | 112 (19.5) | 35 (16.1) | 18 (22.0) | 5 (12.5) | |
| 2 | 113 (12.2) | 4 (26.7) | 68 (11.8) | 24 (11.0) | 10 (12.2) | 7 (17.5) | |
| ≥3 | 159 (17.1) | 5 (33.3) | 100 (17.4) | 32 (14.7) | 11 (13.4) | 11 (27.5) | |
| Previous donor IVF transfers | .88 | ||||||
| 0 | 781 (84.0) | 15 (100.0) | 483 (84.0) | 182 (83.4) | 68 (82.9) | 33 (82.5) | |
| 1 | 94 (10.1) | 0 (0.0) | 61 (10.4) | 21(10.1) | 8 (9.8) | 4 (10.0) | |
| ≥2 | 55 (5.9) | 0 (0.0) | 32 (5.6) | 14 (6.5) | 6 (7.3) | 3 (7.5) | |
| Uterine-factor infertility | .01 | ||||||
| Yes | 151(16.2) | 1 (6.7) | 78 (13.5) | 46 (21.1) | 14 (17.1) | 12 (30.0) | |
| No | 781 (83.8) | 14 (93.3) | 499 (86.5) | 172 (78.9) | 68 (82.9) | 28 (70.0) | |
| Recurrent pregnancy loss | .59 | ||||||
| Yes | 60 (6.4) | 0 (0.0) | 37 (6.4) | 17 (7.8) | 3 (3.7) | 3 (7.5) | |
| No | 872 (93.6) | 15 (100.0) | 540 (93.6) | 201 (92.2) | 79 (96.3) | 37 (92.5) | |
| PCOS or other ovulatory dysfunction | <.001 | ||||||
| Yes | 27 (2.9) | 1 (6.7) | 14 (2.4) | 2 (0.9) | 5 (6.1) | 5 (12.5) | |
| No | 905 (97.1) | 14 (93.3) | 563 (97.6) | 216 (99.1) | 77 (93.9) | 35 (87.5) | |
| No. of oocytes thawed | .17 | ||||||
| ≤5 | 158 (16.9) | 6 (40.0) | 95 (16.5) | 36 (16.5) | 14 (17.1) | 7 (17.5) | |
| 6 | 555 (59.6) | 7 (46.7) | 337 (58.4) | 130 (59.6) | 52 (63.4) | 29 (72.5) | |
| ≥7 | 219 (23.5) | 2 (13.3) | 145 (25.1) | 52 (23.9) | 16 (19.5) | 4 (10.0) | |
| No. of embryos transferred | .71 | ||||||
| 1 | 567 (60.8) | 12 (80.0) | 354 (61.4) | 125 (57.3) | 50 (61.0) | 26 (65.0) | |
| 2 | 356 (38.2) | 3 (20.0) | 216 (37.4) | 91 (41.7) | 32 (39.0) | 14 (35.0) | |
| 3 | 9 (1.0) | 0 (0.0) | 7 (1.2) | 2 (0.9) | 0 (0.0) | 0 (0.0) | |
| Embryo stage at transfer | .49 | ||||||
| Day 3 | 95 (10.2) | 2 (13.3) | 66 (11.4) | 16 (7.3) | 8 (9.8) | 3 (7.5) | |
| Day 5 | 837 (89.8) | 13 (86.7) | 511 (88.6) | 202 (92.7) | 74 (90.2) | 37 (92.5) | |
Note: Data are presented as median (interquartile range) or n (%). Amount of women with missing data: four for race, two for previous autologous IVF cycles, and two for donor IVF cycles. IVF = in vitro fertilization; PCOS = polycystic ovary syndrome.
P values for differences across recipient BMI categories were calculated with the use of chi-square tests for categoric variables and Kruskal-Wallis tests for continuous variables.
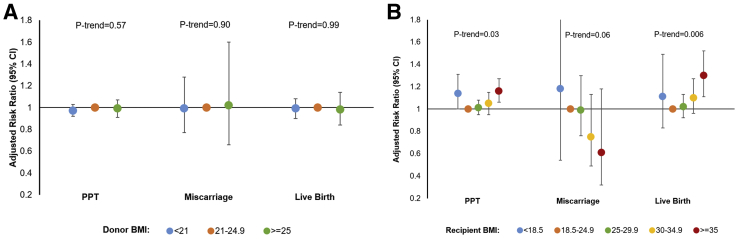
There were no significant differences in the percentage of oocytes surviving warming, percentage of oocytes that fertilized, and percentage of usable embryos across donor BMI categories (Supplemental Table 1, available online at www.fertstert.org). There was also no significant effect of donor BMI on the probability of PPT, miscarriage, or live birth, regardless of whether donor BMI was considered as a categoric or continuous variable (Fig. 1). In contrast, there was a statistically significant positive association between recipient BMI and probability of live birth (adjusted RR [aRR] 1.02 per 2 kg/m2 increase in BMI, 95% CI 1.00–1.04). Recipients with a BMI ≥35 kg/m2 had a significantly higher risk of PPT (aRR 1.16, 95% CI 1.06–1.27) and live birth (aRR 1.30, 95% CI 1.11–1.52) compared with normal-weight recipients. Underweight recipients had a higher risk of PPT (aRR 1.14, 95% CI 1.00–1.31) compared with normal-weight recipients; however, associations with live birth were attenuated. Results were similar when analyses were restricted to single-embryo transfers, blastocyst transfers, first embryo transfers, recipients without uterine-factor infertility, and recipients without PCOS or other ovulatory disorders (Supplemental Table 2, available online at www.fertstert.org). There was no indication of an interaction between donor and recipient BMI on probability of live birth (Supplemental Table 3, available online at www.fertstert.org).
Figure 1.
Association between (A) donor and (B) recipient body mass index (BMI) and probability of positive pregnancy test (PPT), miscarriage, and live birth (among 338 donors and 932 recipients who had a total of 1,651 embryo transfer cycles). Log binomial regression with cluster-weighted generalized estimating equations was used to analyze the association between donor/recipient BMI and probability of PPT, miscarriage, and live birth. The weight was equal to the inverse of the number of embryo transfer cycles. Models for donor BMI were adjusted for donor’s age and race, recipient’s BMI and age, uterine-factor infertility, polycystic ovary syndrome (PCOS), and retrieval year. Models for recipient BMI were adjusted for donor’s age and BMI, recipient’s age and race, uterine-factor infertility, PCOS, and retrieval year. Ptrend was calculated with the median values of each category of BMI as continuous variables.
In total, there were 670 singleton infants born among all embryo transfer cycles. After multivariable adjustment, there were no significant associations between continuous or categorical BMI among donors and length of gestation or birth weight (Table 3). Among recipients, there was an inverse association between BMI and gestational length (Ptrend=.13). Compared with women of a normal weight, women with BMI <18.5 kg/m2 had a lower hazard of delivering at an earlier gestational week (aHR 0.64 95% CI 0.43–0.95), while women with BMI ≥35 kg/m2 had a higher hazard of delivering at an earlier gestational week (aHR 1.43 95% CI 0.94–2.22). Associations between recipient BMI and risk of preterm delivery were in a similar direction, though nonsignificant. Women with BMI ≥35 kg/m2 had 1.76 times (95% CI 1.02–3.02) the risk of delivering a low-birth-weight infant compared with normal-weight women. These associations persisted after excluding women with uterine-factor infertility (data not presented).
Table 3.
Association between donor and recipient body mass index (BMI) and length of gestation and birthweight among donor oocyte recipient singleton live births.
| No. of live births | Length of gestation |
Birth weight |
|||||
|---|---|---|---|---|---|---|---|
| Mean, wk/% <37 wk | Adjusted HR (95% CI)a | Adjusted OR of preterm (95% CI)b | Mean, g/% <2,500 g | Adjusted β (95% CI)c | Adjusted RR of low birth weight (95% CI)d | ||
| Donor BMI, per 2 kg/m2 | 0.99 (0.92–1.06) | 1.05 (0.90–1.22) | 22.8 (−26.3 to 72.0) | 0.98 (0.85–1.14) | |||
| Donor BMI | |||||||
| ≤21 | 203 | 38.3/16.3 | 1.05 (0.88–1.26) | 0.84 (0.55–1.28) | 3197.7/11.3 | −24.0 (−153.6 to 105.5) | 0.96 (0.65–1.41) |
| 21.1–24.9 | 384 | 38.4/15.6 | 1.00 (ref.) | 1.00 (ref.) | 3279.0/8.9 | 0 (ref.) | 1.00 (ref) |
| ≥25 | 78 | 38.4/11.5 | 0.99 (0.76–1.30) | 0.79 (0.42–1.48) | 3394.1/9.0 | 60.7 (−131.9 to 253.4) | 0.94 (0.55–1.60) |
| p-trend4 | 0.61 | 0.91 | 0.44 | 0.99 | |||
| Recipient BMI, per 2 kg/m2 | 1.04 (0.96–1.12) | 1.04 (0.96–1.12) | −12.0 (−34.8 to 10.8) | 1.04 (0.99–1.11) | |||
| Recipient BMI | |||||||
| <18.5 | 11 | 39.5/9.1 | 0.64 (0.43–0.95) | 0.90 (0.28–2.92) | 3395.6/0.0 | 137.2 (−234.6 to 509.0) | 1.14 (0.41–3.16) |
| 18.5–24.9 | 409 | 38.4/15.7 | 1.00 (ref.) | 1.00 (ref.) | 3257.0/9.5 | 0 (ref.) | 1.00 (ref) |
| 25–29.9 | 158 | 38.3/12.0 | 1.13 (0.90–1.41) | 0.79 (0.52–1.19) | 3309.3/10.8 | 11.4 (−136.5–159.2) | 0.98 (0.64–1.48) |
| 30–34.9 | 63 | 38.0/20.3 | 1.01 (0.75–1.35) | 1.02 (0.57–1.83) | 3173.5/11.1 | −89.0 (−302.2 to 124.1) | 1.11 (0.63–1.94) |
| ≥35 | 25 | 38.0/20.8 | 1.43 (0.94–2.22) | 1.93 (0.78–4.75) | 3373.2/4.0 | −138.2 (−409.3 to 133.0) | 1.76 (1.02–3.04) |
| Ptrend | .13 | .37 | .23 | .17 | |||
Note: All models for donor BMI were adjusted for donor’s age and race, recipient’s BMI and age, uterine-factor infertility, polycystic ovary syndrome (PCOS), and retrieval year. All models for recipient BMI were adjusted for donor’s age and BMI, recipient’s age and race, uterine-factor infertility, PCOS, and retrieval year. CI = confidence interval; HR = hazard ratio; OR = odds ratio.
Analyses for gestational length were conducted using cluster-weighted Cox proportional hazard and a robust sandwich covariance estimate to account for the multiple live births per woman in the presence of nonignorable cluster size. Each observation was weighted inversely to the number of live births they contributed to the analysis.
Analyses for preterm birth and low birth weight were conducted using cluster-weighted generalized estimating equations with binomial distribution and logit link function to account for within-person correlations in the presence of nonignorable cluster size. Each observation was weighted inversely to the number of live births they contributed to the analysis.
Analyses for birth weight were conducted using cluster-weighted generalized estimating equations with normal distribution and identity link function to account for within-person correlations in the presence of nonignorable cluster size. Each observation was weighted inversely to the number of live births they contributed to the analysis.
Ptrend was calculated using the median values of each category of BMI as continuous variables.
Discussion
In this large cohort of women donating and receiving vitrified oocytes from a national oocyte bank, we found no association between donor BMI and ART outcomes. However, counter to our initial hypothesis, recipient BMI was positively associated with the likelihood of PPT and live birth. The positive association between recipient BMI and live birth persisted after further adjustment and stratification for various demographic and reproductive characteristics, namely, number of embryos transferred, stage of embryos transferred, and diagnoses of uterine-factor infertility and PCOS. While they were more likely to conceive with ART, recipients with a higher BMI had significantly shorter gestations and higher odds of having a low-birth-weight infant.
To date, numerous individual studies, reviews, and meta-analyses have documented an adverse effect of female overweight and obesity on outcomes of autologous ART (8, 21, 22, 23). In the most recent meta-analysis, which included 49 studies, the authors demonstrated that overweight (OR 0.92, 95% CI 0.86–0.97) and obese (OR 0.81, 95% CI 0.79–0.82) women undergoing autologous ART had reduced live birth rates compared with women of normal BMI (<25 kg/m2) (23). Yet, whether this association is due to an adverse effect of high BMI on oocyte quality, on the endometrium, or perhaps on both is still debated. In an attempt to disentangle this question, recent studies have focused on a donor oocyte model, because it is possible that the negative outcomes in autologous cycles are primarily due to the effect of obesity on the oocyte, rather than on the endometrium. In a 2013 meta-analysis of six studies evaluating ART outcomes among obese donor oocyte recipients, Jungheim et al. found no association between recipient obesity and clinical pregnancy (RR 0.98, 95% CI 0.83–1.15) or live birth rate (RR 0.91, 95% CI 0.6–1.27) (17). However, the authors noted that there was a high degree of heterogeneity across studies: Of the individual studies included in the meta-analysis, two found a mild negative effect (10, 12), two found no effect (11, 13), and one found a positive effect of recipient obesity on ART outcomes following oocyte donation (17). There have been two large studies using the national SART Clinical Outcomes Reporting System that were not included in the meta-analysis. The first study, using data from 2007 and focusing on oocyte donation outcomes among women aged ≥35 years, concluded that there was no significant association between increased recipient BMI and failure to achieve a clinical intrauterine pregnancy (16). However, the second study, which included 22,317 donor/recipient oocyte cycles from 2008–2010, found lower live birth rates in obese donor oocyte recipients (9). Since the meta-analysis was published, there has also been an expanded study (n = 9,587 cycles) by Bellver et al. including data from 2000–2011 which found that obese recipients had a significantly lower live birth rate compared with normal-weight women (15).
Based on this literature, our finding of a significant positive association between recipient BMI and live birth rate was unexpected and remains difficult to explain. While Jungheim et al. also found a positive association between recipient obesity and likelihood of live birth following oocyte donation (OR 1.43 95% CI 1.04–1.97) in their data from their fertility clinic in Missouri, the results were published only as a part of the meta-analysis (17). Of note, many of the previously cited studies are older; for example, in the two SART studies, the majority of cycles were fresh donor oocyte cycles, often with transfer of cleavage-stage embryos and an average of two embryos transferred per cycle. Similarly, in the Bellver et al. study from 2013, the embryos were predominantly cleavage-stage with more than one transferred (15). It is possible that changes in practice patterns, with a focus on extended culture and elective single-embryo transfer mitigates some of the negative effects of obesity. Interestingly, a recent publication focused on frozen embryo transfers in autologous oocyte cycles with predominantly blastocyst elective single-embryo transfers did not find obesity to have an impact on outcomes (24).
Two recent studies have documented increased endometrial thickness among obese women undergoing frozen embryo transfer compared with normal-weight women, which suggests that obese women may respond better to endometrial preparation protocols owing to their hyperestrogenic state (24, 25). Yet whether small gains in endometrial thickness translate into clinical differences is less clear. The unexpected association between recipient obesity and higher probability of live birth could also be attributable to a type of selection bias due to clinic restrictions imposed on donor egg recipients. For example, obese women with comorbidities (e.g., prediabetes, prior poor obstetrical history) may be more likely to forgo treatment and be underrepresented in our data, which could lead to inflated success rates among the selected group of included obese recipients. That theory, however, does not coincide with the worse pregnancy and birth outcomes we observed in our obese recipients. The shorter gestational ages and lower birth weights that we observed among obese recipients are consistent with findings in spontaneous conceptions and is likely due to an increased risk of gestational hypertension, gestational diabetes, and preeclampsia—all disorders that can lead to maternal-fetal distress (and medically indicated early delivery), an increased inflammatory response (which may predispose obese women to spontaneous early delivery), and alterations in placental development and perfusion (which may restrict fetal growth) (26).
Only two studies to date have considered the effect of donor BMI on live birth following oocyte donation ART, and the results have been conflicting (18, 19). Similarly to our results, a retrospective cohort study in Spain (n = 1,092 embryo transfer cycles), which included oocyte donors with BMIs from 16 to 42 kg/m2, found that donor BMI was not associated with likelihood of positive pregnancy (19). In contrast, a retrospective cohort study from Massachusetts (n = 235 fresh donor oocyte ART cycles), which included donors with BMIs from 17.1 to 33.5 kg/m2, found that oocyte donors with higher BMIs had reduced clinical pregnancy and live birth rates. Moreover, this association persisted even after excluding known donors and those with BMI ≥25 kg/m2. These disparate findings may be due to differences in the criteria used to screen donors, which is difficult to directly assess because most studies provide only broad descriptions of their donor-screening methods.
Our study had several strengths. By using data from a solitary large vitrified donor oocyte bank, we were able to ensure that all of our donors were anonymous, all underwent similar oocyte stimulation protocols, and all their oocytes underwent a standard process of vitrification and later warming. This allowed us to control for multiple clinical factors by design. The fertility center is located in Atlanta, Georgia, which is racially diverse and makes it possible for the clinic to screen a relatively large proportion of non-White donors. The ability of the clinic to be highly selective within each racial and ethnic group also makes our comparative findings more generalizable. Because we had comprehensive information on the BMI of donors and recipients, we were able to consider the joint effect of both donor and recipient BMI on ART outcomes, unlike many previous studies. We were also able to mutually adjust our statistical models for both donor and recipient BMI, which helped us to delineate the independent impact of donor and recipient BMIs on ART outcomes.
There were, however, several limitations. Because we used data from a donor oocyte bank with strict eligibility criteria for their donors, we had a limited range of donor BMIs. Moreover, because of the extensive donor exclusion criteria, the overweight donors we included in our analysis are most likely a highly selected sample of reproductive-age women with a BMI ≥25 kg/m2. Therefore, it is possible that the effect of higher donor BMI on ART outcomes is underestimated. Similarly, owing to the inclusion of only young oocyte donors with high-quality oocytes, it was impossible for us to test for effect modification by donor age. Future studies are needed to evaluate whether BMI might have a stronger impact on fertility among older women with lower-quality oocytes. Although our sample size was large relative to many previous analyses, we included only 383 donors, which limited our power to discern small but potentially meaningful effects. Because it was a retrospective cohort study, we also lacked information on potential confounders of interest, including socioeconomic status (SES). Among our oocyte recipients, SES is controlled for, in part, by design, because the ability to afford this type of infertility procedure (which is not often covered by insurance and costs a minimum of $19,000 per cycle) is limited. Among donors, however, there is likely more variation in SES, which may be related to BMI and fertility outcomes.
In conclusion, this study found that donor BMI had no significant impact on ART outcomes, whereas recipient BMI had a positive relationship with likelihood of PPT and live birth. The results support the conclusion that oocyte quality rather than endometrial receptivity may be the overriding factor influencing ART outcomes in obese women using autologous oocytes, although further studies are warranted to further bolster this finding.
Acknowledgments
The authors acknowledge the members of the clinical and administrative staff at Reproductive Biology Associates for their support. For additional aid in completing chart review, the authors thank Hannah Marcovitch, Alexandrea Ramsey, Sydney Archer, and Deandrea Ellis. The authors also thank Dr. Michael Heard for financial support.
Footnotes
J.X. has nothing to disclose. H.S.H. has nothing to disclose. S.M.C. has nothing to disclose. Z.P.N. is a member of the Origio/Cooper-Surgical Scientific Advisory Board a stock owner of Prelude Fertility. D.B.S. is a stock owner of Prelude Fertility. J.B.S. has nothing to disclose. A.J.G. has nothing to disclose.
A.J.G. was supported by a career development grant, R00ES026648, from the National Institute of Environmental Health Sciences. REDCap support was provided by UL1 TR000424 at Emory University. The funding sources had no involvement in the study design, collection, analysis, or interpretation of the data; in the writing of the report; and in the decision to submit the article for publication.
Supplementary data
References
- 1.Thoma M.E., McLain A.C., Louis J.F., King R.B., Trumble A.C., Sundaram R. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–1331.e1. doi: 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slama R., Hansen O.K., Ducot B., Bohet A., Sorensen D., Giorgis Allemand L. Estimation of the frequency of involuntary infertility on a nation-wide basis. Hum Reprod. 2012;27:1489–1498. doi: 10.1093/humrep/des070. [DOI] [PubMed] [Google Scholar]
- 3.Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion; American Society for Reproductive Medicine; Society for Assisted Reproductive Technology. 2007 assisted reproductive technology success rates: national summary and fertility clinic reports. Atlanta: Centers for Disease Control and Prevention, 2009.
- 4.Sunderam S., Kissin D.M., Crawford S.B., Folger S.G., Boulet S.L., Warner L. Assisted reproductive technology surveillance—United States, 2015. MMWR Surveill Summ. 2018;67:1–28. doi: 10.15585/mmwr.ss6703a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion . Centers for Disease Control and Prevention; Atlanta: 2019. 2017 ART fertility clinic success rates report. [Google Scholar]
- 6.Boots C., Stephenson M.D. Does obesity increase the risk of miscarriage in spontaneous conception: a systematic review. Semin Reprod Med. 2011;29:507–513. doi: 10.1055/s-0031-1293204. [DOI] [PubMed] [Google Scholar]
- 7.Kawwass J.F., Kulkarni A.D., Hipp H.S., Crawford S., Kissin D.M., Jamieson D.J. Extremities of body mass index and their association with pregnancy outcomes in women undergoing in vitro fertilization in the United States. Fertil Steril. 2016;106:1742–1750. doi: 10.1016/j.fertnstert.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sermondade N., Huberlant S., Bourhis-Lefebvre V., Arbo E., Gallot V., Colombani M. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update. 2019;25:439–451. doi: 10.1093/humupd/dmz011. [DOI] [PubMed] [Google Scholar]
- 9.Provost M.P., Acharya K.S., Acharya C.R., Yeh J.S., Steward R.G., Eaton J.L. Pregnancy outcomes decline with increasing recipient body mass index: an analysis of 22,317 fresh donor/recipient cycles from the 2008–2010 Society for Assisted Reproductive Technology Clinic Outcome Reporting System registry. Fertil Steril. 2016;105:364–368. doi: 10.1016/j.fertnstert.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Bellver J., Melo M.A., Bosch E., Serra V., Remohi J., Pellicer A. Obesity and poor reproductive outcome: the potential role of the endometrium. Fertil Steril. 2007;88:446–451. doi: 10.1016/j.fertnstert.2006.11.162. [DOI] [PubMed] [Google Scholar]
- 11.Bodri D., Guillen J.J., Lopez M., Vernaeve V., Coll O. Racial disparity in oocyte donation outcome: a multiethnic, matched cohort study. Hum Reprod. 2010;25:436–442. doi: 10.1093/humrep/dep414. [DOI] [PubMed] [Google Scholar]
- 12.DeUgarte D.A., DeUgarte C.M., Sahakian V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil Steril. 2010;93:1008–1010. doi: 10.1016/j.fertnstert.2009.07.1005. [DOI] [PubMed] [Google Scholar]
- 13.Styne-Gross A., Elkind-Hirsch K., Scott R.T., Jr. Obesity does not impact implantation rates or pregnancy outcome in women attempting conception through oocyte donation. Fertil Steril. 2005;83:1629–1634. doi: 10.1016/j.fertnstert.2005.01.099. [DOI] [PubMed] [Google Scholar]
- 14.Wattanakumtornkul S., Damario M.A., Stevens Hall S.A., Thornhill A.R., Tummon I.S. Body mass index and uterine receptivity in the oocyte donation model. Fertil Steril. 2003;80:336–340. doi: 10.1016/s0015-0282(03)00595-8. [DOI] [PubMed] [Google Scholar]
- 15.Bellver J., Pellicer A., Garcia-Velasco J.A., Ballesteros A., Remohi J., Meseguer M. Obesity reduces uterine receptivity: clinical experience from 9,587 first cycles of ovum donation with normal weight donors. Fertil Steril. 2013;100:1050–1058. doi: 10.1016/j.fertnstert.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Luke B., Brown M.B., Stern J.E., Missmer S.A., Fujimoto V.Y., Leach R. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod. 2011;26:245–252. doi: 10.1093/humrep/deq306. [DOI] [PubMed] [Google Scholar]
- 17.Jungheim E.S., Schon S.B., Schulte M.B., DeUgarte D.A., Fowler S.A., Tuuli M.G. IVF outcomes in obese donor oocyte recipients: a systematic review and meta-analysis. Hum Reprod. 2013;28:2720–2727. doi: 10.1093/humrep/det292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubio C., Vassena R., Garcia D., Vernaeve V., Madero J.I. Influence of donor, recipient, and male partner body mass index on pregnancy rates in oocyte donation cycles. JBRA Assist Reprod. 2015;19:53–58. doi: 10.5935/1518-0557.20150013. [DOI] [PubMed] [Google Scholar]
- 19.Cardozo E.R., Karmon A.E., Gold J., Petrozza J.C., Styer A.K. Reproductive outcomes in oocyte donation cycles are associated with donor BMI. Hum Reprod. 2016;31:385–392. doi: 10.1093/humrep/dev298. [DOI] [PubMed] [Google Scholar]
- 20.Nagy Z.P., Liu J., Joris H., Bocken G., Desmet B., van Ranst H. The influence of the site of sperm deposition and mode of oolemma breakage at intracytoplasmic sperm injection on fertilization and embryo development rates. Hum Reprod. 1995;10:3171–3177. doi: 10.1093/oxfordjournals.humrep.a135881. [DOI] [PubMed] [Google Scholar]
- 21.Rittenberg V., Seshadri S., Sunkara S.K., Sobaleva S., Oteng-Ntim E., El-Toukhy T. Effect of body mass index on IVF treatment outcome: an updated systematic review and meta-analysis. Reprod Biomed Online. 2011;23:421–439. doi: 10.1016/j.rbmo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Jungheim E.S., Travieso J.L., Hopeman M.M. Weighing the impact of obesity on female reproductive function and fertility. Nutr Rev. 2013;71(Suppl 1):S3–S8. doi: 10.1111/nure.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Supramaniam P.R., Mittal M., McVeigh E., Lim L.N. The correlation between raised body mass index and assisted reproductive treatment outcomes: a systematic review and meta-analysis of the evidence. Reprod Health. 2018;15:34. doi: 10.1186/s12978-018-0481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prost E., Reignier A., Leperlier F., Caillet P., Barriere P., Freour T., Lefebvre T. Female obesity does not impact live birth rate after frozen-thawed blastocyst transfer. Hum Reprod. 2020;35:859–865. doi: 10.1093/humrep/deaa010. [DOI] [PubMed] [Google Scholar]
- 25.Crosby D., O’Brien Y., Glover L., Martyn F., Wingfield M. Influence of body mass index on the relationship between endometrial thickness and pregnancy outcome in single blastocyst frozen embryo transfer cycles. Hum Fertil (Camb) 2018:1–6. doi: 10.1080/14647273.2018.1504324. [DOI] [PubMed] [Google Scholar]
- 26.Stang J., Huffman L.G. Position of the Academy of Nutrition and Dietetics: obesity, reproduction, and pregnancy outcomes. J Acad Nutr Diet. 2016;116:677–691. doi: 10.1016/j.jand.2016.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.