Abstract
Objective
To investigate if there are any obvious clinical factors associated with delayed blastulation at day 6 (D6) compared with day 5 (D5).
Design
Monocentric observational cohort study from November 2012 to December 2018.
Setting
Tertiary-care academic medical center.
Patient(s)
A total of 941 women with an entire cohort of exclusively D5 blastocysts compared with 162 patients with a cohort of exclusively D6 blastocysts.
Intervention(s)
None.
Main Outcome Measure(s)
Clinical characteristics and data related to the ovarian stimulation protocols.
Result(s)
After univariate analysis, a significantly higher proportion of women who were active smokers was found in the D6 group compared with the D5 group (n = 22/162 [13.6%] vs. n = 82/941 [8.7%]). In addition, the women in the D6 group had a higher rank number of assisted reproductive technology (ART; total no. of ART cycles performed: 2.1 ± 1.4 vs. 1.6 ± 1.1) and a lower antral follicle count (AFC; 18.7 ± 11.3 vs. 22.2 ± 12.8). Moreover, fertilization with the use of intracytoplasmic sperm injection was used more frequently in the D6 group compared with the D5 group. Logistic regression analysis adjusted for confounders highlighted several independent predictors for reaching blastocyst stage at D6 rather than D5: being an active smoker, previous ART cycles, and a lower AFC.
Conclusion(s)
Obtaining an exclusively D6 blastocyst cohort is independently associated with women who are active smokers, previous ART cycles, and a lower AFC. These findings provide evidence, to be confirmed by further studies, that women who are active smokers could greatly benefit from smoking cessation before undergoing ART.
Key Words: Assisted reproductive technology, blastocyst, day 5, day 6
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/users/16110-fertility-and-sterility/posts/xfre00002
Embryo transfer at the blastocyst stage is increasingly accepted and frequently preferred in assisted reproductive technology (ART) centers worldwide, especially in the context of a single-embryo transfer strategy (1, 2). Thanks to a better embryo selection, an increase in the implantation rate has been observed in blastocyst compared to cleavage-stage (day-2 to day-3) embryo transfers (3, 4, 5). At the same time, improvement in and the development of blastocyst culture conditions, including efficient media systems, have resulted in wide utilization of extended culture through to the blastocyst stage. Moreover, blastocyst culture enables better embryo self-selection of a viable embryo owing to embryonic genome activation (6). In most cases, embryos reach the blastocyst stage 5 days after fertilization, although slower embryos can reach expanded blastocele formation on day 6 (D6) or even later under the same culture conditions (7, 8).
The currently available data in the literature data indicates that there is a difference in ART outcomes between blastocysts developing on day-5 (D5) and slower-developing D6 blastocysts. The latter appear to be associated with lower pregnancy rates (9, 10, 11, 12). In addition to the suboptimal synchrony between the endometrium and the blastocyst in case of fresh D6 blastocyst transfer, it has been hypothesized that slower D6 blastocysts may also suffer from an intrinsic impairment of their implantation potential compared with D5 blastocysts. Indeed, in the frozen-thawed blastocyst transfer model, which allows for correction of the duration of the exposure to progesterone for an optimal implantation window, the live birth rate is significantly lower with D6 compared with D5 blastocysts, regardless of the embryo quality (11, 12). These data indirectly suggest that the better performance of D5 blastocysts is not only due to better synchrony between these faster-growing embryos and the advanced endometrium in frozen autologous cycles, but also to impairment of the embryo implantation potential in slower D6 blastocysts. Thus, the day of blastocyst expansion is an important parameter that can be used during the blastocyst selection procedure before elective single-embryo transfer. Therefore, based on the latest data, to increase pregnancy chances in clinical practice, it would appear that, if a choice is possible, it would be preferable to prioritize blastocysts developing on D5 rather than slower-developing D6 blastocysts (12). However, for a number of women, none of their embryos reach the blastocyst stage at D5 and all of the embryos reach the expanded blastocele stage at D6 or later. The question that remains is whether embryo quality is reduced with an increased duration of embryo culture and what factors are involved in this process. Kirillova et al. (13) reported similar clinical factors in women achieving pregnancy after the transfer of poor-quality compared with good- and fair-quality embryos, such as female age or ovarian stimulation protocols, whereas the number of mature oocytes retrieved was significantly different according to the embryo quality. Regarding ploidy, Kimelman et al. showed similar rates of euploidy in D5 and D6 embryos that underwent preimplantation genetic testing for aneuploidy, suggesting that the speed of embryo development can be due to other factors than aneuploidy (14). Factors that affect embryo quality between D5 and D6 is deserving of further study. Moreover, the identification of deleterious factors that lead to delayed blastocyst expansion at D6 rather than D5 is crucial to improving knowledge in the field of prolonged embryo blastocyst culture. This is particularly the case when these factors may be preventable or reversible, because this would allow the ultimate goal of improving ART outcomes to be reached. To the best of our knowledge, the factors acting on delayed blastocyst development are still mostly unknown and there are no data in the literature regarding this specific topic. The aim of the present study was therefore to identify clinical predictive factors related to the timing of blastocele expansion, with embryos reaching blastocyst stage at D5 versus D6 blastocysts as the desired end point.
Materials and methods
Study Population
The study was conducted from January 2012 to December 2018 in the ART unit at the reproductive medicine center of our university-based institution. Infertile patients who underwent in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycles in a freeze-all strategy (15, 16) in our center with a prolonged embryo culture leading to at least one elective blastocyst viable for implantation were considered. Each woman was included only once (first ART cycle performed in our center).
The data had been collected and recorded in a registered database. The inclusion criteria were as follows: 1) a requirement for ART with IVF or ICSI; 2) age ≤42 years at the time of the oocyte retrieval; 3) at least one expanded blastocyst electively vitrified at D5 or D6. The exclusion criteria were: 1) having both D5 and D6 blastocysts vitrified; and 2) having been included previously.
Two groups were compared: patients with an entire cohort of blastocysts that reached blastocyst stage at D5 (the D5 group) and those with an entire cohort of blastocysts that reached blastocyst stage at D6 (the D6 group). This study was reviewed and approved by the Ethics Committee of Cochin Hospital (research license AAA-2019-08015) on June 27, 2019.
Fertilization Methods and Blastocyst Culture and Scoring
Conventional IVF or ICSI was performed based on the presence or absence of male-factor infertility and ovarian response to stimulation as appropriate. In case of non–male-factor infertility, another indication for ICSI was low oocyte yield (≤4 oocytes) (17). The embryos were cultured individually in 50 μL Global medium droplets (LifeGlobal) under mineral oil (Origio). The dishes were prepared 24 hours in advance and left in the incubator to equilibrate in an atmosphere of 5.5% CO2 and 5% O2 at 37°C. Fertilization was assessed 16–18 hours after insemination or injection, and the embryos were maintained in extended culture in tri-gas incubators. Quality assessment after blastocyst culture was performed on the morning of D5 and on D6 if the blastocyst expansion had not occurred by D5.
The blastocysts were graded according to the Gardner criteria (3). At D5, only expanded blastocysts were retained. Nonexpanded blastocysts on D5 (blastocele expansion less than grade B3, including morula stage, B1, and B2) were cultured for an additional day (i.e., until D6), and only expanded blastocysts were retained at D6 (i.e., exceeding or equal to grade B3).
Data Analysis and Statistics
The general characteristics of the patients in both of the groups were recorded prospectively during their medical consultations (Tables 1 and 2). All of the data were compiled into a digital database and analyzed with the use of SPSS software. A P value of <.05 was considered to be statistically significant. For univariate statistical analysis, we used the Pearson χ2 test or Fisher exact test for qualitative variables and the Mann-Whitney test for quantitative variables. To identify factors associated with having an entire cohort of D6 blastocysts, we performed a logistic regression analysis. The factors were tested by means of univariate analysis and added in a multiple logistic regression model. We included all of the available factors that could be linked to our outcome in the multiple logistic regression model. The degree of correlation between variables was tested, and when two variables were highly correlated, we introduced only one of them and suppressed the other in the model, such as for the number of metaphase II oocytes and the number of oocytes retrieved (Spearman correlation 0.930; P<.001; the last parameter was excluded). In case of significant differences, odds ratios (ORs) and their 95% confidence intervals (95% CIs) were calculated.
Table 1.
Patient characteristics.
| Characteristic | Overall (n = 1,103) | Day-5 blastocyst group (n = 941) | Day-6 blastocyst group (n = 162) | P value |
|---|---|---|---|---|
| Female | ||||
| Age at retrieval, y | 34.7 ± 4.3 | 34.6 ± 4.4 | 34.9 ± 3.6 | .447c |
| BMI, kg/m2 | 23.3 ± 4.4 | 23.3 ± 4.3 | 23.6 ± 4.8 | .582c |
| Women actively smokinga | 104 (9.4) | 82 (8.7) | 22 (13.6) | .050d |
| Parity | 0.2 ± 0.5 | 0.2 ± 0.5 | 0.2 ± 0.4 | .755c |
| Previous history of miscarriage | 205 (18.6) | 172 (18.3) | 33 (20.4) | .527d |
| Ovarian reserve at day 3 | ||||
| AFC | 21.7 ± 12.6 | 22.2 ± 12.8 | 18.7 ± 11.3 | <.001c |
| AMH (ng/mL) | 4.1 ± 4.2 | 4.2 ± 4.4 | 3.4 ± 2.6 | .020c |
| FSH (IU/L) | 6.8 ± 2.2 | 6.8 ± 2.2 | 6.9 ± 2.2 | .725c |
| Male | ||||
| Age, y | 38.8 ± 6.9 | 38.8 ± 6.8 | 38.7 ± 7.4 | .735c |
| BMI, kg/m2 | 24.7 ± 3.9 | 24.7 ± 3.9 | 24.5 ± 4.0 | .369c |
| Couple | ||||
| Type of infertility | .348d | |||
| Primary | 790 (71.6) | 669 (71.1) | 121 (74.7) | |
| Secondary | 313 (28.4) | 272 (28.9) | 41 (25.3) | |
| Cause of infertility | .774d | |||
| Tubal factor | 101 (9.2) | 88 (9.4) | 13 (8.0) | |
| Male factor | 335 (30.3) | 281 (29.8) | 54 (33.3) | |
| Endometriosis | 292 (26.5) | 253 (26.9) | 39 (24.1) | |
| Ovulatory factor | 73 (6.6) | 65 (6.9) | 8 (4.9) | |
| Idiopathic | 117 (10.6) | 97 (10.3) | 20 (12.3) | |
| Diminished ovarian reserve | 42 (3.8) | 34 (3.6) | 8 (4.9) | |
| More than one etiology | 143 (13.0) | 123 (13.1) | 20 (12.3) | |
| ART cycle rank,b n | 1.7 ± 1.1 | 1.6 ± 1.1 | 2.1 ± 1.4 | <.001c |
Note: Data are presented as mean ± standard error of the mean or n (%). AFC = antral follicle count; AMH = antimüllerian hormone; ART = assisted reproductive technology; BMI = body mass index.
Women actively smoking (19, 20): defined as a patient who answered “yes” to the following question: “Did you smoke a cigarette during the past 30 days?” (18).
Cycle rank: total number of ART cycles performed per woman.
Mann-Whitney test.
Pearson chi-square test.
Table 2.
Ovarian stimulation characteristics.
| Characteristic | Overall (n = 1,103) | Day-5 blastocyst group (n = 941) | Day-6 blastocyst group (n = 162) | P value |
|---|---|---|---|---|
| Stimulation protocol | .653a | |||
| Antagonist protocol | 912 (82.7) | 782 (83.1) | 130 (80.2) | |
| Long agonist protocol | 74 (6.7) | 61 (6.5) | 13 (8.1) | |
| Short agonist protocol | 117 (10.6) | 98 (10.4) | 19 (11.7) | |
| Total dose of gonadotropins, IU | 2,336.8 ± 945.1 | 2,290.7 ± 929.4 | 2,604.7 ± 992.6 | <.001b |
| Type of triggering | .356a | |||
| hCG | 281 (25.5) | 235 (25.0) | 46 (28.4) | |
| GnRH agonist | 822 (74.5) | 706 (75.0) | 116 (71.6) | |
| ART procedure | .006a | |||
| IVF | 311 (28.2) | 280 (29.8) | 31 (19.1) | |
| ICSI | 792 (71.8) | 661 (70.2) | 131 (80.9) | |
| Sperm origin | .218a | |||
| Sperm donation | 35 (3.2) | 31 (3.3) | 4 (2.5) | |
| Frozen testicular or epididymal | 53 (4.8) | 41 (4.4) | 12 (7.4) | |
| Ejaculated sperm from partner | 1015 (92.0) | 869 (92.3) | 146 (90.1) | |
| Estradiol at ovulation induction, pg/mL | 2,169.1 ± 1,295.9 | 2,197.6 ± 1,306.4 | 2,001.9 ± 1,223.8 | .034b |
| No. of retrieved oocytes | 11.4 ± 6.8 | 11.7 ± 6.8 | 9.3 ± 5.9 | <.001b |
| Mean no. of MII | 9.1 ± 5.5 | 9.4 ± 5.6 | 7.5 ± 4.8 | <.001b |
| Mean no. of blastocysts | 2.8 ± 2.3 | 3.0 ± 2.4 | 1.4 ± 1.0 | <.001b |
| No. of expanded blastocysts | <.001a | |||
| 1 | 407 (36.9) | 279 (29.6) | 128 (79.0) | |
| 2 | 255 (23.1) | 237 (25.2) | 18 (11.1) | |
| ≥3 | 441 (40.0) | 425 (45.2) | 16 (9.9) | |
Note: Data are presented as mean ± standard error of the mean or n (%). ART = assisted reproductive technology; ICSI = intracytoplasmic sperm injection; IVF = in vitro fertilization; MII = metaphase II oocytes.
Pearson chi-square test.
Mann-Whitney test.
Results
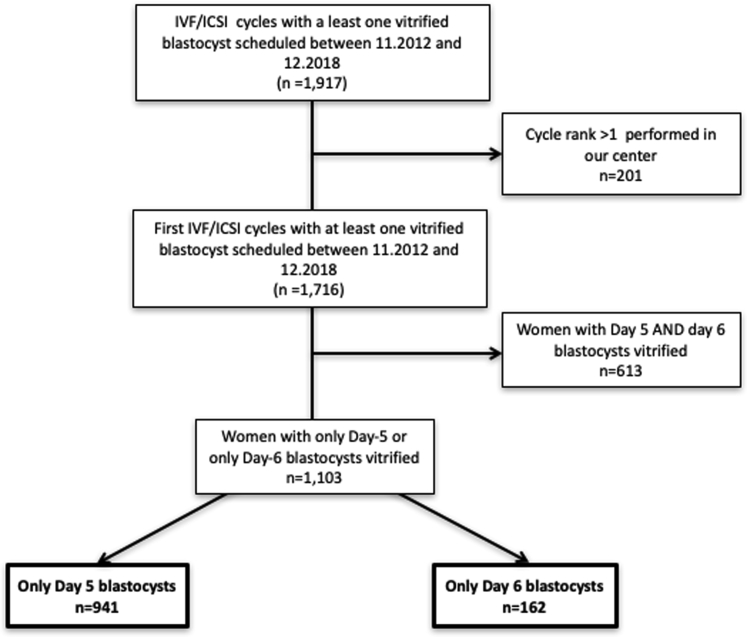
A total of 1,103 women were included during the study period. They underwent combined totals of 941 cycles (85.3%) that led to at least one blastocyst at D5 exclusively and 162 (14.7%) that exclusively obtained blastocysts at D6 after prolonged embryo culture. A flowchart (Fig. 1) depicts the number of cycles with at least one expanded blastocyst at D5 compared with cycles with at least one blastocyst electively expanded at D-6.
Figure 1.
Patient inclusion flowchart. ICSI = intracytoplasmic sperm injection; IVF = in vitro fertilization.
Both the women’s ages and their BMIs were similar between the D5 and D6 groups, as were the men’s ages and their BMIs (Table 1). The type and cause of infertility, parity, and history of miscarriage were not significantly different between the D5 group and the D6 group. Conversely, a significantly higher mean number of previous ART cycles (ART cycle rank 2.1 ± 1.4 vs. 1.6 ± 1.1; P<.001) and a significantly higher proportion of women who actively smoked (18, 19, 20) (n = 22/162 [13.6%] vs. n = 82/941 [8.7%]; P=.05) were encountered in the D6 group compared with the D5 group. Regarding ovarian reserve markers, the serum antimüllerian hormone (AMH) concentration was significantly lower in the D6 group compared with the D5 group (3.4 ± 2.6 ng/mL vs. 4.2 ± 4.4 ng/mL; P=.02), as was the antral follicle count (AFC; 18.7 ± 11.3 vs. 22.2 ± 12.8; P<.01), whereas the D3 FSH level and the proportion of women with AMH level <1 ng/mL (n = 15 [9.3%] vs. 71 [7.5%]; P=.45) were similar between the two groups.
ICSI was used more frequently in the D6 group for fertilization compared with the D5 group (n = 131/162 [80.9%] vs. n = 661/941 [70.2%]; P<.01), although the prevalence of male-related infertility was similar between the two groups. Regarding the ovarian response to stimulation, despite the use of a significantly higher dose of gonadotropins in the D6 group compared with the D5 group during the (P<.01), the E2 levels at triggering were significantly lower in the D6 group compared with the D5 group (P=.03), and the mean number of oocytes retrieved (P<.01) and consequently the mean number of blastocysts (P<.01) in the D6 group were lower than in the D5 group (Table 2).
A multivariate analysis was performed to adjust for potential confounding factors (Table 3). The following variables were found to be independently associated with the entire embryo cohort reaching delayed blastocyst expansion at D6 rather than D5: female active smoking during ART (OR 2.33,95% CI 1.16-4.70; P=.02); previous ART cycles (OR 1.40, 95% CI 1.13-1.72; P<.01); and lower AFC (OR 0.97, 95% CI 0.94-0.99; P=.03).
Table 3.
Risk factor for cohorts of exclusively day-6 embryos: multiple logistic regression model.
| Variablea | OR (95% CI) | P value |
|---|---|---|
| ART rankb | 1.40 (1.14-1.72) | <.01 |
| Active smoking | 2.33 (1.16-4.70) | .02 |
| AFC | 0.97 (0.94-0.99) | .03 |
Note: AFC = antral follicle count; ART = assisted reproductive technology; CI = confidence interval; OR = odds ratio.
Variables included in the model: female and male age.
ART rank: total number of ART cycles performed per woman, female and male body mass indexes, parity, previous miscarriage, type and cause of infertility, women's smoking habits, ovarian reserve parameters (FSH, antimüllerian hormone, AFC), type of ovarian stimulation protocol, type of triggering, total dose of gonadotropins used, E2 level at triggering, sperm origin (donor, partner, or frozen from epididymis or testis), technique of fertilization used, and number of metaphase II oocytes.
Discussion
Main Finding
This study demonstrates that, compared with a D5 cohort, obtaining an exclusively D6 blastocyst cohort was associated with active smoking, based on univariate or multivariate analysis. In addition, previous ART cycles and lower AFC were factors significantly associated with D6 cohorts.
Strengths and Limitations
To our knowledge, this study is the first to investigate the factors associated with the day of blastocyst expansion (i.e., D5 vs. D6), especially a negative impact of active smoking by women. Although there have been studies that investigated the link between ART characteristics and embryo development, most of them evaluated the influence of only one isolated parameter and generally focused on early embryo morphokinetic aspects rather than on features associated with the developmental capacity to the blastocyst stage. Moreover, this study is the first to show a negative impact of active smoking by women on the time to blastocyst expansion, although no previous study compared blast formation at D5 or D6, particularly in the context of cigarette smoking (21, 22, 23). According to a number of authors, the dose of recombinant FSH, the E2 level on the day of hCG trigger, and the type of GnRH analogue used during ovarian stimulation affect early embryo developmental kinetics (24, 25). Bodri et al. similarly reported a difference between standard IVF and ICSI techniques on late embryo development, with slightly faster embryo development following ICSI (26). Bellver et al. reported similar morphokinetic patterns in embryos regarding the women’s weight (27).
Another strength of the present study is that we performed a multivariate analysis after adjustment for several confounders to isolate independently associated factors related to the timing of blastocele expansion at D5 versus D6. Strength also lies in the methodologic design, with a large number of patients (1,103 women) included. Thus, our study’s sample size is likely to have minimized statistical errors. In addition, numerous epidemiologic variables were collected prospectively through face-to-face interviews before ART treatment (regarding infertility data and ovarian stimulation characteristics) to avoid memorization bias and to allow multivariate analysis for adjustment on several confounders.
Despite the precautions taken, our study may nonetheless be subject to certain shortcomings and biases. One limitation is the self-reporting of smoking (based on personal interviews) (19). Thus, there could be a risk of underestimation in this regard because we have no other information about the quantity of tobacco consumption (28). In addition, it cannot be ruled out that other female or male clinical predictive factors are involved. Other lifestyle factors (e.g., alcohol consumption, recreational drug use) could have influenced blastulation time. Unfortunately, those are not exhaustively available in our database. For example, the proportion of male smokers was not known. A male partner who smokes has been associated with detrimental effects on ART outcomes, although the results are still controversial (23, 29). Indeed, a meta-analysis did not report a significant effect of active smoking by male partners on ART outcomes (30).
A number of differences persisted between the groups. The ICSI procedure was performed more frequently in the D6 group compared with the D5 group, and a subsequent impact on blastulation cannot be ruled out. However, recent data in the literature suggest that ICSI techniques may be associated with a slightly faster embryonic development compared with standard IVF (26), although those authors embryonic development to D5 was assessed and not to D6. In our protocols, the ICSI technique is mainly used in case of male-associated infertility or in case a small cohort of oocytes is obtained after stimulation (17) . Given that the proportion of male-associated infertility was similar between the groups, the difference could be linked to a lower ovarian response in the women who exclusively obtained D6 embryos versus the women with a blastocyst cohort that was exclusively D5. In accordance with this hypothesis, we observed that our D6 group had a less efficient response to stimulation. Despite a higher mean dose of injected gonadotropins, the mean number of oocytes obtained in the D6 group was lower than that in the D5 group. In addition, after multivariate analysis, the fertilization method (ICSI vs. IVF) did not appear to be an independent predictive factor for slow-growing blastocysts. Finally, we chose to exclude women with a mixed cohort of D5 and D6 blastocysts to decipher clinical factors associated with delayed blastulation at D6 compared with D5. Therefore, it is likely that our results cannot be extrapolated to patients with mixed D5 and D6 blastocyst cohorts.
Interpretation
It is widely recognized in the literature that active smoking by women adversely affects fertility outcomes (31). Our results provide new data regarding a potential link between active smoking by women and obtaining slow-growing D6 blastocysts exclusively. It has been reported previously that smokers have a lower mean age of menopause and increased follicular loss leading to a diminished ovarian reserve (32, 33). Several authors have reported that female smoking is associated with a lower AFC (34), a lower number of oocytes retrieved (35), reduced fertilization rates (36, 37), and lower clinical pregnancy rates (30, 38). The effects of smoking are mainly mediated by the pharmacologic action of tobacco alkaloids (nicotine and its metabolite cotinine). Interestingly, using a time-lapse system, a study has reported impaired early embryonic development for women who smoke compared with women who do not smoke (22). The mechanisms leading to delayed embryonic development for women who actively smoked during ART treatment have not been evaluated, and this question remains to be addressed. The hypothesis of toxicity of a tobacco component on the ovaries, inducing disrupted oogenesis and impaired follicular development, has previously been reported in nonhuman animal models (34). Cigarette smoking acts on steroid hormone metabolism and secretion. Polycyclic aromatic hydrocarbons, which are present in cigarette smoke, may lead to up-regulation of a number of genes, including cytochrome P450, family 1, subfamily A. The latter enzyme catalyzes hydroxylation of E2 and reduces the more estrogenically potent 16α-hydroxylation to less available catechol estrogens (39). Some have postulated that the benefit of cessation of cigarette smoking is reached in 6 months. Like the olive tree surviving after serious cold, the ovary may resuscitate after tobacco cessation, growing follicles (“the leaves”) from a pool of resting follicles (“the trunk”) that resisted the toxic effects (40). In the present study, we observed a reduced response to ovarian stimulation in the D6 group compared with the D5 group. Despite a significantly higher total dose of gonadotropins administrated during the OS, the E2 levels at triggering as well as the number of oocytes retrieved were significantly lower in the D6 group. Although the lower AFC in the D6 group could partly explain these differences, a negative influence of smoking in women on estrogen metabolism could also be hypothesized, thereby resulting in less efficient ovarian stimulation. Moreover, cigarette smoking could initiate oxidative stress in granulosa cells, as indicated by statistically significant overexpression of superoxide dismutase 2 and catalase mRNA in smokers compared with nonsmokers (21). This is likely to then lead to reduced oocyte and embryo quality.
In the present study, we observed that having a cohort of exclusively D6 blastocysts was linked with a lower response to ovarian stimulation, and that this occurred in women with less favorable ART prognoses and with lower AFC. Indeed, after adjustment for the women’s age and ovarian reserve parameters, an increasing number of previous ART cycles was found to be an independent predictive factor of a cohort of exclusively D6 blastocysts (vs. D5). Along these lines, a previous study comparing D5, D6, and D7 blastocyst transfer cohorts, also found a lower AFC, a higher number of previous ART cycles, a higher dose of gonadotropins, and a lower E2 surge at triggering when the embryo growth was delayed (41). It could be hypothesized that women who obtained exclusively D6 blastocysts have a lower intrinsic oocyte quality, thereby leading to slower-growing embryos after oocyte fecundation, regardless of tobacco consumption. However, pregnancy and live birth rates appear to be lower after transfer of a D6 versus D5 blastocyst in fresh and frozen-thawed cycles, and this is so even with equal embryo qualities (according to the Gardner classification) or when only euploid embryos are compared (12, 41, 42). D6 blastocysts could exhibit an impairment of their intrinsic implantation potential that could lead to ART failures that stem from poor oocyte quality.
Conclusion
Active smoking in women undergoing ART is associated with an increased time frame to achieve blastocyst expansion after prolonged culture: Patients who smoked exhibited blastocyst expansion at D6 rather than D5. A cohort of exclusively D6 blastocyst was also associated with a higher number of previous ART cycles and lower AFC. The identification of deleterious components impairing embryo development to the blastocyst stage is of considerable relevance for improving ART outcomes. This is particularly so for preventable and reversible factors such as smoking. The present study shows a correlation between women actively smoking and a delay in blastocyst expansion. However, further studies could establish a causative impact by testing the effectiveness of smoking cessation on blastocyst development.
Acknowledgments
The authors thank the team members of the biology and clinical ART unit of the Cochin–Port Royal University Hospital for having provided assistance.
Footnotes
M.B. has nothing to disclose. L.F. has nothing to disclose. C.M. has nothing to disclose. C.P. has nothing to disclose. L.M. has nothing to disclose. K.P.-C. has nothing to disclose. C.C. has nothing to disclose. P.S. has nothing to disclose.
M.B. and L.F. should be considered similar in author order. C.C. and P.S. should be considered similar in author order.
References
- 1.Gardner D.K. Blastocyst culture: toward single embryo transfers. Hum Fertil (Camb) 2000;3:229–237. doi: 10.1080/1464727002000199051. [DOI] [PubMed] [Google Scholar]
- 2.Glujovsky D., Farquhar C., Quinteiro Retamar A.M., Alvarez Sedo C.R., Blake D. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD002118.pub5. [DOI] [PubMed] [Google Scholar]
- 3.Gardner D.K., Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3:367–382. [PubMed] [Google Scholar]
- 4.Wilson M., Hartke K., Kiehl M., Rodgers J., Brabec C., Lyles R. Integration of blastocyst transfer for all patients. Fertil Steril. 2002;77:693–696. doi: 10.1016/s0015-0282(01)03235-6. [DOI] [PubMed] [Google Scholar]
- 5.Voelker R. Researchers in Canada call for policy to mandate single-embryo transfer in IVF. JAMA. 2011;305:1848. doi: 10.1001/jama.2011.602. [DOI] [PubMed] [Google Scholar]
- 6.Magli M.C., Jones G.M., Gras L., Gianaroli L., Korman I., Trounson A.O. Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro. Hum Reprod. 2000;15:1781–1786. doi: 10.1093/humrep/15.8.1781. [DOI] [PubMed] [Google Scholar]
- 7.Ivec M., Kovacic B., Vlaisavljevic V. Prediction of human blastocyst development from morulas with delayed and/or incomplete compaction. Fertil Steril. 2011;96:1473–1478.e2. doi: 10.1016/j.fertnstert.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Hammond E.R., Cree L.M., Morbeck D.E. Should extended blastocyst culture include day 7? Hum Reprod. 2018;33:991–997. doi: 10.1093/humrep/dey091. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro B.S., Richter K.S., Harris D.C., Daneshmand S.T. A comparison of day 5 and day 6 blastocyst transfers. Fertil Steril. 2001;75:1126–1130. doi: 10.1016/s0015-0282(01)01771-x. [DOI] [PubMed] [Google Scholar]
- 10.Barrenetxea G., López de Larruzea A., Ganzabal T., Jiménez R., Carbonero K., Mandiola M. Blastocyst culture after repeated failure of cleavage-stage embryo transfers: a comparison of day 5 and day 6 transfers. Fertil Steril. 2005;83:49–53. doi: 10.1016/j.fertnstert.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 11.Ferreux L., Bourdon M., Sallem A., Santulli P., Barraud-Lange V., le Foll N. Live birth rate following frozen-thawed blastocyst transfer is higher with blastocysts expanded on day 5 than on day 6. Hum Reprod. 2018;33:390–398. doi: 10.1093/humrep/dey004. [DOI] [PubMed] [Google Scholar]
- 12.Bourdon M., Pocate-Cheriet K., Finet de Bantel A., Grzegorczyk-Martin V., Amar Hoffet A., Arbo E. Day 5 versus day 6 blastocyst transfers: a systematic review and meta-analysis of clinical outcomes. Hum Reprod. 2019;34:1948–1964. doi: 10.1093/humrep/dez163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirillova A., Lysenkov S., Farmakovskaya M., Kiseleva Y., Martazanova B., Mishieva N. Should we transfer poor quality embryos? Fertil Res Pract. 2020;6:2. doi: 10.1186/s40738-020-00072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimelman D., Confino R., Okeigwe I., Lambe-Steinmiller J., Confino E., Shulman L.P. Assessing the impact of delayed blastulation using time lapse morphokinetics and preimplantation genetic testing in an IVF patient population. J Assist Reprod Genet. 2019;36:1561–1569. doi: 10.1007/s10815-019-01501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourdon M., Santulli P., Gayet V., Maignien C., Marcellin L., Pocate-Cheriet K. Assisted reproduction technique outcomes for fresh versus deferred cryopreserved day-2 embryo transfer: a retrospective matched cohort study. Reprod Biomed Online. 2017;34:248–257. doi: 10.1016/j.rbmo.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Blockeel C., Campbell A., Coticchio G., Esler J., Garcia-Velasco J.A., Santulli P. Should we still perform fresh embryo transfers in ART? Hum Reprod. 2019;34:2319–2329. doi: 10.1093/humrep/dez233. [DOI] [PubMed] [Google Scholar]
- 17.Luna M., Bigelow C., Duke M., Ruman J., Sandler B., Grunfeld L. Should ICSI be recommended routinely in patients with four or fewer oocytes retrieved? J Assist Reprod Genet. 2011;28:911–915. doi: 10.1007/s10815-011-9614-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren C.W., Jones N.R., Eriksen M.P., Asma S., Global Tobacco Surveillance System Collaborative Group Patterns of global tobacco use in young people and implications for future chronic disease burden in adults. Lancet. 2006;367:749–753. doi: 10.1016/S0140-6736(06)68192-0. [DOI] [PubMed] [Google Scholar]
- 19.Bernaards C.M., Twisk J.W., Snel J., van Mechelen W., Kemper H.C. Is calculating pack-years retrospectively a valid method to estimate life-time tobacco smoking? A comparison between prospectively calculated pack-years and retrospectively calculated pack-years. Addiction. 2001;96:1653–1661. doi: 10.1046/j.1360-0443.2001.9611165311.x. [DOI] [PubMed] [Google Scholar]
- 20.Chapron C., Souza C., de Ziegler D., Lafay-Pillet M.-C., Ngô C., Bijaoui G. Smoking habits of 411 women with histologically proven endometriosis and 567 unaffected women. Fertil Steril. 2010;94:2353–2355. doi: 10.1016/j.fertnstert.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Budani M.C., Fensore S., Di Marzio M., Tiboni G.M. Cigarette smoking impairs clinical outcomes of assisted reproductive technologies: a meta-analysis of the literature. Reprod Toxicol. 2018;80:49–59. doi: 10.1016/j.reprotox.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Fréour T., Dessolle L., Lammers J., Lattes S., Barrière P. Comparison of embryo morphokinetics after in vitro fertilization-intracytoplasmic sperm injection in smoking and nonsmoking women. Fertil Steril. 2013;99:1944–1950. doi: 10.1016/j.fertnstert.2013.01.136. [DOI] [PubMed] [Google Scholar]
- 23.Fuentes A., Muñoz A., Barnhart K., Argüello B., Díaz M., Pommer R. Recent cigarette smoking and assisted reproductive technologies outcome. Fertil Steril. 2010;93:89–95. doi: 10.1016/j.fertnstert.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 24.Muñoz M., Cruz M., Humaidan P., Garrido N., Pérez-Cano I., Meseguer M. Dose of recombinant FSH and oestradiol concentration on day of HCG affect embryo development kinetics. Reprod Biomed Online. 2012;25:382–389. doi: 10.1016/j.rbmo.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Muñoz M., Cruz M., Humaidan P., Garrido N., Pérez-Cano I., Meseguer M. The type of GnRH analogue used during controlled ovarian stimulation influences early embryo developmental kinetics: a time-lapse study. Eur J Obstet Gynecol Reprod Biol. 2013;168:167–172. doi: 10.1016/j.ejogrb.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 26.Bodri D., Sugimoto T., Serna J.Y., Kondo M., Kato R., Kawachiya S. Influence of different oocyte insemination techniques on early and late morphokinetic parameters: retrospective analysis of 500 time-lapse monitored blastocysts. Fertil Steril. 2015;104:1175–1181.e2. doi: 10.1016/j.fertnstert.2015.07.1164. [DOI] [PubMed] [Google Scholar]
- 27.Bellver J., Mifsud A., Grau N., Privitera L., Meseguer M. Similar morphokinetic patterns in embryos derived from obese and normoweight infertile women: a time-lapse study. Hum Reprod. 2013;28:794–800. doi: 10.1093/humrep/des438. [DOI] [PubMed] [Google Scholar]
- 28.Connor Gorber S., Schofield-Hurwitz S., Hardt J., Levasseur G., Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11:12–24. doi: 10.1093/ntr/ntn010. [DOI] [PubMed] [Google Scholar]
- 29.Joesbury K.A., Edirisinghe W.R., Phillips M.R., Yovich J.L. Evidence that male smoking affects the likelihood of a pregnancy following IVF treatment: application of the modified cumulative embryo score. Hum Reprod. 1998;13:1506–1513. doi: 10.1093/humrep/13.6.1506. [DOI] [PubMed] [Google Scholar]
- 30.Waylen A.L., Metwally M., Jones G.L., Wilkinson A.J., Ledger W.L. Effects of cigarette smoking upon clinical outcomes of assisted reproduction: a meta-analysis. Hum Reprod Update. 2009;15:31–44. doi: 10.1093/humupd/dmn046. [DOI] [PubMed] [Google Scholar]
- 31.Wesselink A.K., Hatch E.E., Rothman K.J., Mikkelsen E.M., Aschengrau A., Wise L.A. Prospective study of cigarette smoking and fecundability. Hum Reprod. 2019;34:558–567. doi: 10.1093/humrep/dey372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freour T., Masson D., Mirallie S., Jean M., Bach K., Dejoie T. Active smoking compromises IVF outcome and affects ovarian reserve. Reprod Biomed Online. 2008;16:96–102. doi: 10.1016/s1472-6483(10)60561-5. [DOI] [PubMed] [Google Scholar]
- 33.Tuttle A.M., Stämpfli M., Foster W.G. Cigarette smoke causes follicle loss in mice ovaries at concentrations representative of human exposure. Hum Reprod. 2009;24:1452–1459. doi: 10.1093/humrep/dep023. [DOI] [PubMed] [Google Scholar]
- 34.Budani M.C., Tiboni G.M. Ovotoxicity of cigarette smoke: a systematic review of the literature. Reprod Toxicol. 2017;72:164–181. doi: 10.1016/j.reprotox.2017.06.184. [DOI] [PubMed] [Google Scholar]
- 35.Harrison K.L., Breen T.M., Hennessey J.F. The effect of patient smoking habit on the outcome of IVF and GIFT treatment. Aust N Z J Obstet Gynaecol. 1990;30:340–342. doi: 10.1111/j.1479-828x.1990.tb02024.x. [DOI] [PubMed] [Google Scholar]
- 36.Elenbogen A., Lipitz S., Mashiach S., Dor J., Levran D., Ben-Rafael Z. The effect of smoking on the outcome of in-vitro fertilization–embryo transfer. Hum Reprod. 1991;6:242–244. doi: 10.1093/oxfordjournals.humrep.a137314. [DOI] [PubMed] [Google Scholar]
- 37.Rosevear S.K., Holt D.W., Lee T.D., Ford W.C., Wardle P.G., Hull M.G. Smoking and decreased fertilisation rates in vitro. Lancet. 1992;340:1195–1196. doi: 10.1016/0140-6736(92)92895-m. [DOI] [PubMed] [Google Scholar]
- 38.Hughes E.G., Brennan B.G. Does cigarette smoking impair natural or assisted fecundity? Fertil Steril. 1996;66:679–689. doi: 10.1016/s0015-0282(16)58618-x. [DOI] [PubMed] [Google Scholar]
- 39.Marom-Haham L., Shulman A. Cigarette smoking and hormones. Curr Opin Obstet Gynecol. 2016;28:230–235. doi: 10.1097/GCO.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 40.de Ziegler D., Santulli P., Seroka A., Decanter C., Meldrum D.R., Chapron C. In women, the reproductive harm of toxins such as tobacco smoke is reversible in 6 months: basis for the “olive tree” hypothesis. Fertil Steril. 2013;100:927–928. doi: 10.1016/j.fertnstert.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 41.Hernandez-Nieto C., Lee J.A., Slifkin R., Sandler B., Copperman A.B., Flisser E. What is the reproductive potential of day 7 euploid embryos? Hum Reprod. 2019;34:1697–1706. doi: 10.1093/humrep/dez129. [DOI] [PubMed] [Google Scholar]
- 42.Irani M., O’Neill C., Palermo G.D., Xu K., Zhang C., Qin X. Blastocyst development rate influences implantation and live birth rates of similarly graded euploid blastocysts. Fertil Steril. 2018;110:95–102.e1. doi: 10.1016/j.fertnstert.2018.03.032. [DOI] [PubMed] [Google Scholar]