Abstract
Objective
To determine whether in vitro fertilization (IVF) with preimplantation genetic testing for aneuploidy (PGT-A) is cost effective to achieve a live birth compared with IVF alone in fresh donor oocyte cycles.
Design
Theoretical cost-effectiveness study.
Setting
Not applicable.
Patient(s)
None.
Intervention(s)
Comparison between the cost of IVF with PGT-A vs. IVF alone to achieve a live birth. The model analyzed a hypothetical single fresh oocyte donor IVF cycle with PGT-A vs. IVF alone and followed the progression of a single embryo through the different decision nodes. Cost estimates assigned to each clinical event were based on data obtained from the literature and institutional costs.
Main Outcome Measure(s)
Cost per live birth.
Result(s)
In the base-case analysis, IVF with PGT-A was not cost effective in fresh donor oocyte cycles when compared with IVF alone to achieve a live birth. The cycles using PGT-A cost an additional $6,018.66. The incremental cost-effectiveness ratio was found to be $119,606.59 per additional live birth achieved with IVF with PGT-A. Monte Carlo simulations demonstrated that IVF with PGT-A was not cost effective in nearly all iterations.
Conclusion(s)
PGT-A in fresh donor oocyte IVF cycles is not cost effective compared with IVF alone over a wide range of probabilities and costs.
Key Words: Oocyte donor, cost effective, preimplantation genetic testing
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-20-00206
Advances in assisted reproductive technologies have made the use of donor oocytes in in vitro fertilization (IVF) cycles an increasingly viable option for patients with age-related infertility, premature ovarian insufficiency, and multiple failed IVF attempts using autologous oocytes. Consequently, the use of donor oocytes has increased and today accounts for 10% of all assisted reproductive technology cycles in the United States (1). The highest pregnancy and live birth rates from any IVF procedure have resulted from cycles utilizing donor oocytes. Due to the highly selective characteristics of donors to optimize the oocyte quality, some studies have shown a live birth rate as high as 50%–58% per transfer in donor oocyte cycles (2, 3).
Despite the high live birth rates and increasing usage rates, there are still some patients who fail to conceive after using donor oocytes. Aneuploidy, although less prevalent in the oocyte donor population, can still be a cause of treatment failure. A study looking at aneuploidy rates in donor oocyte cycles in 42 clinics in the United States showed that the mean expected rate of aneuploidy per cycle was 31.6%. Although the rates were treatment center-dependent, the study findings highlighted that the increase in chromosome abnormalities observed with increasing maternal age is already seen in this group of young oocyte donors (4). Therefore, it can be theorized that screening for chromosomal abnormalities through preimplantation genetic testing for aneuploidy (PGT-A) could potentially increase the efficacy and efficiency of donor egg cycles.
The question of whether PGT-A in donor oocyte recipient cycles improves IVF outcomes has been debated in the literature. Some studies have argued that genetic testing might improve the IVF outcomes with embryos derived from donor oocyte cycles (5, 6); conversely, other studies have revealed either the absence of clinical benefit (3, 7) or even a negative impact on IVF outcomes (2). Although there is no clear consensus on the use of PGT-A for donor oocyte cycles, patients may inquire about its use, especially after failed cycles with untested embryos.
Many patients may perceive that using PGT-A would have the implied benefit of excluding abnormal embryos and thus aid in decreasing the cost of donor oocyte cycles, which can be financially overwhelming for patients undergoing fertility treatment and is usually not covered by insurance. In the absence of definitive clinical guidance, cost may be an important deciding factor for patient use of PGT-A.
As the cost of a donor IVF cycle can exceed tens of thousands of dollars, in addition to the thousands of dollars required per transfer cycle, it is imperative to financially, clinically, and in practice maximize every transfer cycle. To date, there is no published study reporting on the cost-effectiveness of using PGT-A in donor oocyte cycles. This study sought to assess whether IVF with PGT-A to achieve a live birth is cost effective compared with IVF alone in fresh donor oocyte cycles. The objective of this study was to determine if it is cost effective to genetically test blastocyst embryos in donor oocyte IVF cycles.
Materials and methods
This study received approval from the Johns Hopkins Institutional Review Board.
Decision model
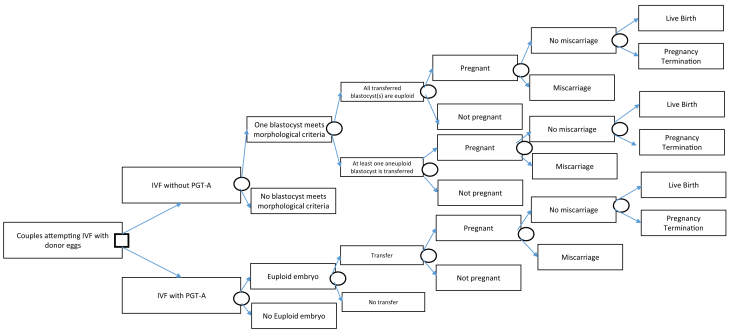
We modified and adapted the decision analytic model developed by Collins et al. (8) to study the cost-effectiveness of PGT-A for blastocyst embryos in donor oocyte IVF cycles. The model was constructed using TreeAge Pro Suite 2016 (TreeAge Software Inc., Williamstown, MA), and it outlined a hypothetical donor oocyte IVF cycle with PGT-A vs. IVF alone. The model included important clinical events such as development of a blastocyst embryo, PGT-A testing, blastocyst transfer, clinical pregnancy, miscarriage, pregnancy termination, and live birth rate. Figure 1 outlines the decision tree model. Several assumptions were made before constructing the decision model. It was assumed that all oocyte donors were between 21 and 34 years of age. Other assumptions regarding the donors included that they had laboratory evidence of normal ovarian reserve, no indication of impaired fertility, were tested for communicable infectious diseases, and received preconception genetic carrier screening testing. These characteristics of the donor were based on the American Society for Reproductive Medicine recommendations for oocyte donation (9). The recipient age was assumed to be 42 years old or younger. This assumption was on the basis of the mean age of recipients from the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS) database (2, 6). The recipient was also assumed to have had a normal uterine cavity evaluation before the embryo transfer as that is the standard of care for patients undergoing IVF cycles. We also assumed that all fresh donor oocytes were obtained from controlled ovarian hyperstimulation cycles, because the outcomes obtained from the literature were on the basis of fresh cycles. We also assumed that IVF PGT-A cycles in which at least one euploid embryo was obtained would proceed to transfer of that embryo. Additionally, we assumed that in both IVF alone cycles and IVF with PGT-A cycles, only a single embryo was transferred as per American Society for Reproductive Medicine recommendations (10).
Figure 1.
The decision tree model generated on the basis of study assumptions analyzing two treatment strategies of in vitro fertilization with donor oocytes with and without the use of preimplantation genetic testing for aneuploidy (PGT-A).
The decision model started at the time point at which the recipient had at least one blastocyst embryo from a fresh donor oocyte cycle. It is routine clinical practice today that embryo biopsies for PGT-A are performed on blastocyst embryos. Hence, having one blastocyst embryo was a requirement for proceeding with PGT-A. The decision to proceed with PGT-A testing thus was dependent on whether there are blastocyst embryos available for testing. Furthermore, the costs and probabilities were the same between the two different groups up until this time point and did not affect the calculation of the cost-effectiveness of PGT-A in donor oocyte cycles. For this reason, we decided to start the decision model at the time of the creation of a blastocyst embryo from a fresh donor oocyte cycle. In IVF alone cases, we further bifurcated our model to incorporate the probability of having either an aneuploid or a euploid blastocyte transferred. Furthermore, consistent with the literature and clinical guidelines, we assumed that all pregnant patients who did not miscarry would undergo amniocentesis, and that all patients with evidence of aneuploidy would receive genetic counseling. The patients with aneuploid embryos would be assumed to be the same as those with a euploid pregnancy for the remainder of their gestational period and would receive the normal prenatal care as they progressed toward live birth.
Parameter Estimates and Analysis
The analysis was conducted from a health care payer perspective and included costs related to the clinically relevant events in the study. The effectiveness of each decision tree was measured by the live birth rate. Base case estimates and probabilities for the main clinical events in our model were obtained through a thorough review of the literature. Most probabilities were obtained from a study using the SART CORS database that included outcomes from fresh donor IVF cycles from 2005–2013 (2) and are included in Table 1. This study is the largest study to date that included the use of PGT-A in fresh donor oocyte cycles and compared it with donor oocyte cycles in which PGT-A was not used.
Table 1.
The cost and probability input parameters for the decision model of in vitro fertilization with preimplantation genetic testing for aneuploidy vs. in vitro fertilization without preimplantation genetic testing for aneuploidy for donor egg cycles.
| Event Probabilities | Base Case | Minimum | Maximum |
|---|---|---|---|
| IVF success rate (number of cycles to successful blastocyst formation) (2, 11, 12) | 0.667 | 0.56 | 0.87 |
| Rate for performing PGT-A (2) | 0.06 | 0.045 | 0.06 |
| Non PGT-A Arm | |||
| At least one blastocyst meets the morphological criteria (7, 13) | 0.667 | 0.56 | 0.87 |
| All transferred blastocytes are euploid (5, 12) | 0.7 | 0.45 | 0.81 |
| Pregnancy confirmed—for euploid blastocyst (2, 7) | 0.6 | ||
| Pregnancy confirmed—for aneuploid blastocyst (14) | 0.3 | ||
| Miscarriage in the euploid arm (2, 7, 15) | 0.12 | 0.06 | 0.17 |
| Miscarriage in the aneuploid arm (16) | 1 | ||
| Live birth in the euploid arm (2, 7, 15) | 0.6 | 0.54 | 0.62 |
| Live birth in the aneuploid arm (16) | 0 | ||
| PGT-A Arm | |||
| Having a euploid embryo (2, 11, 12) | 0.7 | 0.395 | 0.875 |
| Pregnancy confirmed (2, 12, 17) | 0.65 | 0.62 | 0.69 |
| Miscarriage (2, 15, 17) | 0.14 | 0.14 | 0.152 |
| Live birth (2, 12, 17) | 0.57 | 0.56 | 0.62 |
| Cost parameters | Base Case | Minimum | Maximum |
|---|---|---|---|
| Cost of basic IVF on average—including multiple cycles (does not include cost of donor eggs and donor sperm) (18, 19) | $18,277 | $6,920 | $27,685 |
| Cost of donor eggs (19) | $9,150 | $6,742 | $15,142 |
| Cost of donor sperm (19) | $450 | $300 | $1600 |
| Cost of embryo transfer (19) | $6,395 | $3,155 | $12,626 |
| Cost of PGT-A (20, 21) | $4,268 | $3,155 | $12,626 |
| Cost of miscarriage management (21, 22, 23) | $1,304 | $517 | $2,058 |
| Cost of prenatal care—average (22, 24) | $1,590 | $761 | $3,696 |
| Cost of amniocentesis (22, 25) | $1,397 | $699 | $2,794 |
| Cost of genetic counseling (22) | $146 | $109 | $182 |
| Cost of live birth (22, 24) | $15,385 | $2,700 | $20,629 |
| Utility | Base Case | Minimum | Maximum |
|---|---|---|---|
| Live birth rate IVF—non PGT-A (2) | 0.56 | 0.472 | 0.59 |
| Live birth rate IVF—PGT-A (2) | 0.6 | 0.53 | 0.71 |
Note: IVF = in vitro fertilization; PGT-A = preimplantation genetic testing for aneuploidy.
The cost estimates assigned to each clinical event were generated on the basis of the average of the data obtained from the literature and the costs at our institution. An average of the direct cost for each clinical event can be seen in Table 1. Even though our model started at the development of blastocyte embryo stage, because PGT-A was performed before embryo transfer, the cost of the IVF cycle using donor oocytes was included separate from the cost of the embryo transfer. The cost of PGT-A included both the biopsy and testing costs. We included the cost of IVF cycles in our study despite it having no impact on the cost-effectiveness to assess the total cost associated with the process. The cost of miscarriage management was considered an average cost incurred for management of miscarriage, which incorporated medical, expected, and surgical management options weighted by patient preferences. All cost parameters were inflation adjusted to 2018 US dollars using the medical care component of the consumer price index (26). (Table 1)
We also conducted sensitivity analyses to assess the model’s robustness and account for uncertainties in probabilities and cost estimates. One-way deterministic sensitivity analysis was performed for all variables in our model by varying the values across plausible ranges. The lower and upper plausible ranges were determined a priori and derived by either using the lower and upper limits of reported values or using standing deviations or by adding or subtracting 25% from the base-case value in cases where standard deviations and lower and upper limits were not reported. A probabilistic sensitivity analysis was conducted using a Monte Carlo simulation (MCS) in which all parameters were varied simultaneously with either a triangular distribution bounded by defined minimum and maximum values or a beta distribution around a mean and standard deviation. Ten thousand iterations of the simulation were performed to adjust for the second order uncertainty by randomly sampling parameters for each variable. Results of the MCS are provided as the percentage iterations that were considered cost effective, assuming a willingness-to-pay (WTP) threshold of $50,000 per quality-adjusted life year gained. The distribution of these results against different WTP thresholds have been plotted on the acceptability curve. A scatterplot (Supplemental Fig. 1, available online) was plotted to visualize the distribution of the incremental cost-effectiveness ratios (ICERs) computed using the MCS on a cost-effectiveness plane. The WTP threshold was determined based on the World Health Organization guidelines of using one to three times the gross domestic product per capita and represented an estimate of what a consumer of health care might be prepared to pay for the health benefit (gross domestic product per capita) (27).
Results
Base-case analysis results of IVF without PGT-A vs. IVF with PGT-A showed that IVF with PGT-A in donor oocyte IVF cycles was associated with an additional total cost of $6,018.66 when compared with IVF without PGT-A ($41,613.26 vs. $35,594.60, respectively). The decision model calculated that IVF with PGT-A was associated with a 5% greater probability of live birth rate when compared with IVF without PGT-A (13% vs. 8%, respectively). The ICER thus calculated, as a ratio of the incremental costs versus the incremental effectiveness, was found to be $119,606.59 per additional live birth achieved with IVF with PGT-A. This means that IVF with PGT-A was associated with an additional cost of $119,606.59 per additional live birth achieved. Thus, at the WTP threshold of $50,000 per additional live birth achieved, IVF with PGT-A was not a cost-effective option for couples undergoing IVF with donor oocytes.
The results of the one-way sensitivity analysis for the five highest impact parameters for IVF with PGT-A compared with IVF without PGT-A demonstrated that the cost of PGT-A had the most impact on the estimated ICER (Table 2). This was interpreted by observing the highest spread of $188,213.67 per additional live birth achieved between the ICERs obtained using the highest and the lowest values for the cost of PGT-A (ICERs $97,488.36 to $285,702.03).
Table 2.
The one-way sensitivity analysis for the five highest impact parameters for in vitro fertilization with preimplantation genetic testing for aneuploidy compared with in vitro fertilization without preimplantation genetic testing for aneuploidy.
| Variable name | Low ICER ($/additional live birth) | High ICER ($/additional live birth) | ICER Spread ($/additional live birth) |
|---|---|---|---|
| Cost of PGT-A ($3,155–$12,626) | 97,488.36 | 28,5702 | 188,213.7 |
| Live birth rate for IVF with PGT-A for a euploid embryo (0.53–0.71) | 80,404.27 | 17,3410.4 | 93,006.17 |
| Confirmed euploid pregnancy in the non PGT-A arm (0.45–0.75) | 96,109.58 | 17,5966.4 | 79,856.82 |
| Probability of having a euploid embryo in the non PGT-A arm (0.45–0.81) | 88,083.3 | 14,8913.9 | 60,830.63 |
| Live birth rate for IVF without PGT-A for a euploid embryo (0.472–0.59) | 94,867.88 | 13,1277 | 36,409.1 |
Note: ICER = incremental cost-effectiveness ratio; IVF = in vitro fertilization; PGT-A = preimplantation genetic testing for aneuploidy.
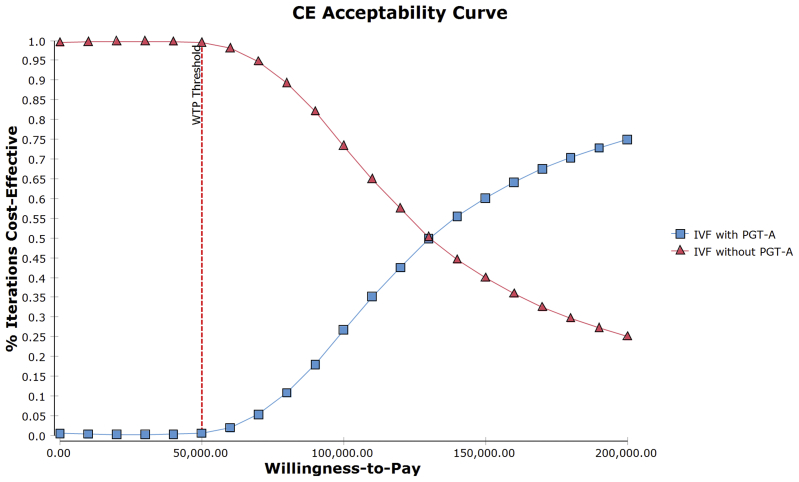
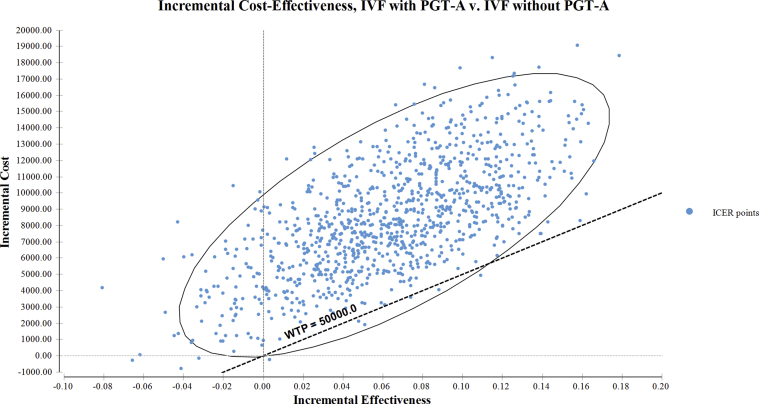
Our results from the probabilistic sensitivity analysis for IVF with PGT-A versus IVF without PGT-A that used 10,000 iterations of the MCS showed that <1% of the iterations were cost effective at the WTP of $50,000 per additional live birth achieved. We further plotted the incremental costs and the incremental effectiveness on a scatterplot to visualize the spread of all the individual ICERs on the cost-effectiveness plane (Supplemental Fig. 1). We observed that most of the ICER distribution fell above the WTP threshold of $50,000 and was spread in the northeast, northwest, and southwest quadrants, with the majority of the spread located in the northeast quadrant. The minimal ICER spread below the threshold was only distributed in the northeast quadrant. We also plotted the acceptability curve (Fig. 2), which showed that a crossover between the two strategies only happened beyond the WTP threshold of $120,000.
Figure 2.
The cost-effectiveness acceptability curve for in vitro fertilization (IVF) with preimplantation genetic testing for aneuploidy (PGT-A) compared with IVF without PGT-A for donor oocyte cycles. The X-axis denotes the different thresholds for willingness to pay in US dollars; the Y-axis denotes the percent iterations of the simulation that were cost effective; Blue square denotes the percent of iterations for IVF with PGT-A that were cost effective; Red triangle denotes the percent of iterations for IVF without PGT-A that were cost effective.
Discussion
Using a base-case cost-effectiveness analysis, our study showed that PGT-A is not cost effective in IVF cycles using fresh donor oocytes. To our knowledge, this is the first cost-effectiveness study comparing IVF with PGT-A with IVF without PGT-A in donor oocyte cycles. On the basis of our findings, patients who used PGT-A in fresh donor oocytes cycles would have an additional total cost of $6,018.66 when compared with IVF without PGT-A. The ICER was found to be $119,606.59 per additional live birth achieved with IVF with PGT-A. This amount was much higher than the assumed willingness to pay, which was set to $50,000. The results from the probabilistic sensitivity analysis using MCS showed that the scatterplot of the ICERs on the cost-effectiveness plane had most of the iterations of the ICER above the WTP line and that they seemed to be congregated in the northeast quadrant with some spillover in the northwest quadrant. This indicated that in all of the MCS scenarios, IVF with PGT-A was associated with a higher cost and that in most cases it was associated with an additional gain in effectiveness. In the cost-effectiveness acceptability curve, IVF with PGT-A only surpassed IVF alone at WTP values above $120,000, indicating the lack of cost-effectiveness of IVF with PGT-A at any threshold below that. Furthermore, our one-way sensitivity analysis showed that the additional cost of PGT-A was the major driving factor on the incremental ICER. These findings suggest that IVF with PGT-A was not cost effective for couples undergoing IVF with donor oocytes.
With the improvement of cryopreservation techniques, oocyte donation has become a viable option for those seeking fertility treatment who have age-related infertility, primary ovarian insufficiency, or those with multiple failed IVF attempts using autologous oocytes. Data from the Centers for Disease Control suggest that 10% of all IVF cycles performed annually in the United States use donor oocytes (1). Women who are recruited to be oocyte donors are typically young and do not have infertility. These characteristics translate to higher pregnancy rates in donor oocyte cycles compared with those in cycles using autologous oocytes (28).
Although PGT-A has been shown to improve outcomes in certain groups of infertile patients using autologous oocytes (15, 29, 30), the full impact and utility of PGT-A on donor oocyte cycles is still unclear. In a large retrospective cross-sectional study performed using data from SART CORS from 2005 to 2013, live birth rates were significantly lower for PGT-A (51.8%) than for control cycles (59.9%, P<.001). For donor oocyte-recipient cycles, the adjusted odds of a live birth were reduced by 35% (P<.001)(2). Another smaller retrospective study showed a higher live birth rate in donor recipients when euploid embryos were tested compared with when they were not tested; however, the results did not reach statistical significance because of the small sample size (5).
Despite the potential improvement in outcomes with the use of PGT-A in donor oocyte cycles, patients should also be counseled about whether it is cost effective to proceed with PGT-A when using donor oocytes. Such counseling is important as most of these cycles are not covered by insurance and thus can be a huge financial burden for patients. Our findings that PGT-A in donor oocyte cycles is not cost effective seem to be in line with other studies looking at PGT-A in other patient populations. Somigliana et al. (20) showed that PGT-A only became cost effective in 38–40-year-old infertile women who have more than one blastocyst for biopsy. The procedure did not seem to be cost effective for infertile women who were younger than 35 years old. Another study looking at over 153,865 autologous IVF cycles showed similar results (31). Furthermore, Murugappan et al. (21) showed that IVF with PGT-A was not a cost-effective strategy for increasing live births compared with expectant management in patients with recurrent pregnancy loss.
The major strength of our study was the fact that the probabilities used in the decision model were taken from one of the largest studies to date regarding the use of PGT-A in donor oocyte cycles (2). That study used the SART CORS database, which makes the results generalizable. We also used Monte Carlos simulations, which allowed for thousands of cost simulations for each of the data points, making the cost-effective analysis even more comprehensive.
However, there are several limitations to our study. First, we constructed a theoretical model on the basis of parameters estimated from the published literature rather than on a randomized controlled trial. To reliably answer our study question, a large, probably unachievable, randomized controlled study would have needed to be conducted, and such study has not been done to date. Our study tried to circumvent the lack of prospective data by using data from large retrospective studies analyzing the impact of PGT-A in donor oocyte IVF cycles. Because of the largely theoretical nature of our model, some assumptions listed in Table 1 may lack robust scientific support. These uncertainties were addressed with the sensitivity analyses, which further confirmed our findings and showed that the incremental cost seen in IVF cycles with PGT-A was largely driven by the cost of PGT-A alone.
Second, our study delineated extremely specific cycle parameters for the construction of our model and thus our results can only be applied to the clinical scenarios outlined in our assumptions. We assumed that all donor oocytes originated from fresh donor IVF cycles and only one embryo was transferred to the recipient. These assumptions were based on what data was available and in accordance with American Society for Reproductive Medicine guidelines. We also did not include cumulative live birth data as it was not available. Inferences to other scenarios such as frozen donor oocyte cycles cannot be extrapolated from our findings. Specific cost-effective analyses using those parameters would be required to evaluate the cost-effectiveness of PGT-A in all types of donor oocyte IVF cycles. In addition, indirect costs in donor oocyte cycles were not included; however, those would be comparable between PGT-A and non PGT-A cycles and therefore would not change the study findings. Lastly, our study mostly focused on the monetary implications of using PGT-A in donor oocyte cycles and thus could not truly estimate the emotional cost for couples after a miscarriage that might happen with an untested embryo from a donor oocyte cycle.
In conclusion, as health care providers, we should always consider the cost-effectiveness of expensive healthcare technologies when counseling our patients. Our decision analytic model showed that based on the current data, PGT-A is not cost effective to achieve a live birth in patients using fresh donor oocytes. These results may aid providers when it comes to counseling patients about using PGT-A in fresh donor oocyte cycles. Despite these results, further research into the appropriate threshold values to use in evaluating cost-effectiveness of infertility treatments as well as additional studies to refine the probability parameters would help improve future assessments of the cost-effectiveness of PGT-A in all donor oocyte cycles.
Acknowledgments
The authors thank Dr. Edward Wallach, whose grant dedicated to help fund fellows research projects was key in allowing this study to be conducted.
Footnotes
All authors have nothing to disclose.
Supported by the Edward Wallach Fellow research grant, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Supplementary data
Supplemental Figure 1.
Scatter plot of distribution of ICER values on the cost-effectiveness plane comparing ICER distribution for IVF with PGT-A versus IVF without PGT-A for donor eggs. X-axis denotes the incremental effectiveness in live birth rate; Y-axis denotes the incremental cost in USD; - Blue circle denotes the ICER values.
References
- 1.Centers for Disease Control and Prevention, American Society for Reproductive Medicine, Society for Assisted Reproductive Technology. 2015. 2015. https://www.cdc.gov/art/reports/2014/national-summary-figures.html Available at: Accessed September 15, 2020.
- 2.Barad D.H., Darmon S.K., Kushnir V.A., Albertini D.F., Gleicher N. Impact of preimplantation genetic screening on donor oocyte-recipient cycles in the United States. Am J Obstet Gynecol. 2017;217:576.e1–576.e8. doi: 10.1016/j.ajog.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Doyle N., Gainty M., Eubanks A., Doyle J., Hayes H., Tucker M. Donor oocyte recipients do not benefit from preimplantation genetic testing for aneuploidy to improve pregnancy outcomes. Hum Reprod. 2020;35:2548–2555. doi: 10.1093/humrep/deaa219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munné S., Alikani M., Ribustello L., Colls P., Martínez-Ortiz P.A., McCulloh D.H. Euploidy rates in donor egg cycles significantly differ between fertility centers. Hum Reprod. 2017;32:743–749. doi: 10.1093/humrep/dex031. [DOI] [PubMed] [Google Scholar]
- 5.Coates A., Bankowski B.J., Kung A., Griffin D.K., Munne S. Differences in pregnancy outcomes in donor egg frozen embryo transfer (FET) cycles following preimplantation genetic screening (PGS): a single center retrospective study. J Assist Reprod Genet. 2017;34:71–78. doi: 10.1007/s10815-016-0832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roeca C., Johnson R.L., Carlson N., Polotsky A.J. Preimplantation genetic testing (PGT) is associated with higher odds of a healthy livebirth among donor oocyte recipients in the United States: a 2013–2015 national study. Fertil Steril. 2019;112:e31–e32. [Google Scholar]
- 7.Haddad G., Deng M., Wang C.T., Witz C., Williams D., Griffith J. Assessment of aneuploidy formation in human blastocysts resulting from donated eggs and the necessity of the embryos for aneuploidy screening. J Assist Reprod Genet. 2015;32:999–1006. doi: 10.1007/s10815-015-0492-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins S.C., Xu X., Mak W. Cost-effectiveness of preimplantation genetic screening for women older than 37 undergoing in vitro fertilization. J Assist Reprod Genet. 2017;34:1515–1522. doi: 10.1007/s10815-017-1001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Society for Reproductive Medicine Guidelines for oocyte donation. Fertil Steril. 2004;82(Suppl 1):S13–S15. doi: 10.1016/j.fertnstert.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Practice Committee of the American Society for Reproductive Medicine Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril. 2017;107:901–903. [Google Scholar]
- 11.Hoyos L.R., Cheng C.Y., Brennan K., Hubert G., Wang B., Buyalos R.P. Euploid rates among oocyte donors: is there an optimal age for donation? J Assist Reprod Genet. 2020;37:589–594. doi: 10.1007/s10815-020-01694-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masbou A.K., Friedenthal J.B., McCulloh D.H., McCaffrey C., Fino M.E., Grifo J.A. A comparison of pregnancy outcomes in patients undergoing donor egg single embryo transfers with and without preimplantation genetic testing. Reprod Sci. 2019;26:1661–1665. doi: 10.1177/1933719118820474. [DOI] [PubMed] [Google Scholar]
- 13.Yang L., Cai S., Zhang S., Kong X., Gu Y., Lu C. Single embryo transfer by Day 3 time-lapse selection versus Day 5 conventional morphological selection: a randomized, open-label, non-inferiority trial. Hum Reprod. 2018;33:869–876. doi: 10.1093/humrep/dey047. [DOI] [PubMed] [Google Scholar]
- 14.Fragouli E., Alfarawati S., Spath K., Babariya D., Tarozzi N., Borini A. Analysis of implantation and ongoing pregnancy rates following the transfer of mosaic diploid-aneuploid blastocysts. Hum Genet. 2017;136:805–819. doi: 10.1007/s00439-017-1797-4. [DOI] [PubMed] [Google Scholar]
- 15.Forman E.J., Tao X., Ferry K.M., Taylor D., Treff N.R., Scott R.T., Jr. Single embryo transfer with comprehensive chromosome screening results in improved ongoing pregnancy rates and decreased miscarriage rates. Hum Reprod. 2012;27:1217–1222. doi: 10.1093/humrep/des020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott R.T., Miller K.A., Olivares R., Su J., Fratterelli J.L., Treff N.R. Microarray based 24 chromosome preimplantation genetic diagnosis (mPGD) is highly predictive of the reproductive potential of human embryos: a prospective blinded non-selection trial. Fertil Steril. 2008;90:S22–S23. [Google Scholar]
- 17.Klenov V.E., Boulet S.L., Mejia R.B., Kissin D.M., Munch E., Mancuso A. Live birth and multiple birth rates in US in vitro fertilization treatment using donor oocytes: a comparison of single-embryo transfer and double-embryo transfer. J Assist Reprod Genet. 2018;35:1657–1664. doi: 10.1007/s10815-018-1243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Fertility Association The costs of infertility treatment: the Resolve Study. http://familybuilding.resolve.org/site/PageServer/application/PageServer?pagename=lrn_mta_cost Available at:
- 19.Internal Data Johns Hopkins Fertility Center 2019.
- 20.Somigliana E., Busnelli A., Paffoni A., Vigano P., Riccaboni A., Rubio C. Cost-effectiveness of preimplantation genetic testing for aneuploidies. Fertil Steril. 2019;111:1169–1176. doi: 10.1016/j.fertnstert.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Murugappan G., Ohno M.S., Lathi R.B. Cost-effectiveness analysis of preimplantation genetic screening and in vitro fertilization versus expectant management in patients with unexplained recurrent pregnancy loss. Fertil Steril. 2015;103:1215–1220. doi: 10.1016/j.fertnstert.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Evans M.I., Sonek J.D., Hallahan T.W., Krantz D.A. Cell-free fetal DNA screening in the USA: a cost analysis of screening strategies. Ultrasound Obstet Gynecol. 2015;45:74–83. doi: 10.1002/uog.14693. [DOI] [PubMed] [Google Scholar]
- 23.Dalton V.K., Liang A., Hutton D.W., Zochowski M.K., Fendrick A.M. Beyond usual care: the economic consequences of expanding treatment options in early pregnancy loss. Am J Obstet Gynecol. 2015;212:177.e1–177.e6. doi: 10.1016/j.ajog.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 24.Ohno M., Caughey A. The role of noninvasive prenatal testing as a diagnostic versus a screening tool—a cost-effectiveness analysis. Prenat Diagn. 2013;33:630–635. doi: 10.1002/pd.4156. [DOI] [PubMed] [Google Scholar]
- 25.Mersereau J.E., Plunkett B.A., Cedars M.I. Preimplantation genetic screening in older women: a cost-effectiveness analysis. Fertil Steril. 2008;90:592–598. doi: 10.1016/j.fertnstert.2007.07.1307. [DOI] [PubMed] [Google Scholar]
- 26.CPI Inflation Calculator 2020. Available at: https://www.bls.gov/data/inflation_calculator.htm. Accessed September 15, 2020.
- 27.Adam T., Murray C. World Health Organization; 2003. Making choices in health: WHO guide to cost-effectiveness analysis. [Google Scholar]
- 28.Yeh J.S., Steward R.G., Dude A.M., Shah A.A., Goldfarb J.M., Muasher S.J. Pregnancy rates in donor oocyte cycles compared to similar autologous in vitro fertilization cycles: an analysis of 26,457 fresh cycles from the Society for Assisted Reproductive Technology. Fertil Steril. 2014;102:399–404. doi: 10.1016/j.fertnstert.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 29.Kang H.J., Melnick A.P., Stewart J.D., Xu K., Rosenwaks Z. Preimplantation genetic screening: who benefits? Fertil Steril. 2016;106:597–602. doi: 10.1016/j.fertnstert.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Lee H.-L., McCulloh D.H., Hodes-Wertz B., Adler A., McCaffrey C., Grifo J.A. In vitro fertilization with preimplantation genetic screening improves implantation and live birth in women age 40 through 43. J Assist Reprod Genet. 2015;32:435–444. doi: 10.1007/s10815-014-0417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee M.S., Lofgren K.T., Thomas A.M., Lanes A., Goldman R.H., Ginsburg E.S. The cost-effectiveness of preimplantation genetic testing for aneuploidy (PGT-A): an analysis of 153,865 SART cycles. Fertil Steril. 2019;112:e234. [Google Scholar]