Abstract
Objective
To evaluate the impact of paternal age on embryology and pregnancy outcomes in the setting of a euploid single-embryo transfer.
Design
Retrospective cohort study.
Setting
Not applicable.
Patient(s)
Couples undergoing a first in vitro fertilization cycle with fresh ejaculated sperm who used intracytoplasmic sperm injection for fertilization followed by preimplantation genetic testing for aneuploidy and single-embryo transfer of a euploid embryo between January 2012 and December 2018.
Intervention(s)
Not applicable.
Main Outcome Measure(s)
Embryology outcomes assessed were fertilization rate, blastulation rate, and euploid rate. Pregnancy outcomes assessed included positive human chorionic gonadotropin rate, delivery rate, biochemical loss rate, and clinical loss rate.
Results
A total of 4,058 patients were assessed. After adjusting for female age, increased paternal age in the setting of fresh ejaculated sperm use was associated with decreased blastulation and decreased euploid rate using 40 years as an age cutoff.
Conclusion(s)
In this study, advancing paternal age appears to have a detrimental impact on rates of blastocyst formation and euploid status. However, if a euploid embryo is achieved, older paternal age does not appear to affect negatively pregnancy outcomes.
Key Words: Andrology, ART, IVF, male factor, reproductive aging
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/users/16110-fertility-and-sterility/posts/xfre00043
Over the last several decades, the trend toward delayed fatherhood has become increasingly common. It has been reported that mean paternal age is increasing in the United States within all regions, races, and education levels. In fact, between 1972 and 2015, the mean paternal age increased from 27.4 to 30.9 years (1). As higher numbers of older men pursue fatherhood, it becomes increasingly important to understand whether advanced paternal age has a detrimental effect on embryology and pregnancy outcomes. Despite numerous studies evaluating the impact of older paternal age on reproductive outcomes, the true effect of paternal age remains controversial (2, 3, 4, 5, 6).
Several studies have associated older male partner age with diminished reproductive potential. Specifically, paternal age >40 years has been associated with higher rates of failure to achieve spontaneous conception (7). With the use of assisted reproductive technology, older paternal age also has been associated with decreased blastocyst formation (8, 9, 10), higher rates of pregnancy loss (8), and lower live birth rates (8, 11). However, other studies have demonstrated no significant differences in reproductive outcomes with advancing age of the male partner (2, 4, 12, 13). The controversial nature of the current literature likely stems from methodological challenges in separating the paternal and maternal contributions to reproduction.
To control for the negative effects of advanced maternal age on embryo ploidy and quality, many clinical research studies have used oocyte donor models or preimplantation genetic testing for aneuploidy (PGT-A) protocols to standardize the population of interest (2, 6). By controlling for maternal factors, researchers can determine more accurately the paternal role in early embryo development and pregnancy. However, oocyte donor models may be insufficient to evaluate the male contribution to fertility because younger oocytes potentially can compensate for the lower reproductive potential of poor quality sperm (4). Additionally, although the incorporation of PGT-A into a study design appropriately controls for altered pregnancy outcomes related to aneuploidy, PGT-A alone does not address the issue of embryo and endometrial synchrony. If various maternal factors lead to delayed embryo development, this could impact negatively an embryo’s ability to implant during a fresh embryo transfer cycle. Therefore, the issue of synchrony is best addressed through the use of frozen embryo transfers and is a factor that must be controlled. The use of cryopreserved embryo transfers, which are temporally separate from the ovarian stimulation cycle, can normalize the hormonal milieu at the time of embryo transfer and enhance synchrony between the embryo and the endometrium (14).
The current study investigates the relationship between paternal age and reproductive outcomes in the setting of a single-embryo transfer (SET). This study is unique from previous publications in that it controls for issues related to transfer order, evaluates only embryos deemed to be euploid based on PGT-A, controls for embryo and endometrial synchrony through the use of frozen embryo transfers, uses intracytoplasmic sperm injection (ICSI) for fertilization in all cases, and controls for female age and oocyte yield through a statistical adjustment. The transfer of aneuploid embryos and embryo-endometrial dyssynchrony are known to worsen clinical outcomes, so controlling for these factors is a key component in the evaluation of reproductive outcomes. Specifically, this study evaluates the relationship between paternal age and fertilization rate, blastulation rate, euploid rate, positive human chorionic gonadotropin (hCG) rate, delivery rate, biochemical loss rate, and clinical loss rate.
Materials and methods
Study Subjects, Design, and Eligibility
This retrospective cohort study evaluates patients who had a frozen SET performed between January 1, 2012 and December 31, 2018. This timeframe was selected because all embryos in the study center were cultured to the blastocyst stage during this period. The decision to evaluate frozen embryo transfers ensured embryonic and endometrial synchrony at the time of transfer. Institutional review board approval was obtained to evaluate retrospective data (Reproductive Medicine Associates institutional review board number CR00109375).
A chart review was performed using a secure electronic medical record to identify patients who had undergone a first cycle of in vitro fertilization (IVF) with fresh ejaculated sperm. Patients were included if they had fertilization achieved via ICSI, underwent PGT-A by means of a trophectoderm (TE) biopsy with a subsequent euploid biopsy result, and then had frozen SET of a euploid embryo performed. Inclusion was limited to euploid SET to standardize pregnancy outcomes and minimize the detrimental effect of aneuploidy. Embryos with PGT-A results classified as mosaic, duplications/deletions, nonconcurrent, or amplification failures were excluded. Donor oocyte cycles also were excluded.
Sperm Collection
Sperm samples were collected via ejaculation. Ejaculated semen samples were obtained into sterile, labeled containers after 2 to 5 days of abstinence. All ejaculated semen samples were incubated at 37⁰C for 30 minutes and then were used subsequently for ICSI. A sperm gradient was used in all cases. Surgically extracted sperm and cryopreserved sperm were excluded from the analysis to limit potential confounders arising from the male partner.
Ovarian Stimulation, ICSI, and Embryo Transfer
Controlled ovarian hyperstimulation cycles were conducted using a gonadotropin-releasing hormone (GnRH)-antagonist, long GnRH-agonist, or GnRH-microflare protocol. Each participant’s IVF cycle was managed by the patient’s physician following standard clinic practice. Decisions regarding which IVF stimulation protocol was used and doses of gonadotropins were based on clinical judgment as well as the results of the patient’s ovarian reserve testing. Administration of hCG and/or a GnRH-agonist trigger for final oocyte maturation occurred at the discretion of the patient’s primary physician, generally when it was felt that the center of the follicular cohort was between 15 and 20 mm in diameter. Oocyte retrieval took place 36 hours after administration of the trigger injection via ultrasound-guided aspiration.
Cumulus stripping with hyaluronidase was then performed approximately 3.5 hours after oocyte retrieval. Consistent with study inclusion criteria, ICSI was performed in all cases regardless of semen parameters approximately 1 hour later. This was done to reduce DNA contamination at the time of PGT-A and to standardize the fertilization method for all participants. A fertilization check was performed approximately 18 hours after ICSI. Embryo development progressed using sequential culture media. Laser-assisted hatching was performed on day 3 of development, and all embryos were placed in extended culture. Assessment for cryopreservation was made on day 5, 6, or 7. Only embryos achieving a 4CC grade or better based on modified Gardner scoring were considered eligible for vitrification (15).
All patients included in the current study underwent TE biopsy on day 5, 6, or 7 for PGT-A. Timing of the TE biopsy varied based on each embryo’s progression to the blastocyst stage. During the study period, the two genetic testing platforms used by the institution were quantitative polymerase chain reaction and targeted next-generation sequencing. Embryos were then vitrified for transfer in a future cycle to ensure embryo-endometrial synchrony.
After confirmation of an embryo’s euploid status, an embryo transfer cycle was performed with the goal of obtaining a trilaminar endometrial lining measuring at least 7 mm in thickness. Endometrial preparation protocols consisted primarily of oral estrogen followed by intramuscular injections of progesterone in oil. However, alternative programmed regimens as well as natural transfer cycle protocols were considered and used on an individual basis. For patients included in the analysis, embryos were warmed on the day of replacement and transferred under ultrasound-confirmed intrauterine placement of the catheter. Pregnancy outcomes then were assessed according to standard institutional protocols.
Data Collection
The outcome measures of interest were divided into two categories: embryology outcomes and pregnancy outcomes. Only a couple’s first embryo transfer was considered in the data analysis to avoid previous failure bias. Embryology outcomes included fertilization rate, blastulation rate, and euploid rate. Fertilization rate was defined as the number of 2 pronuclear embryos divided by the number of metaphase II oocytes injected via ICSI. Blastulation rate was defined as the number of clinically usable blastocysts (those that were vitrified) divided by the number of successfully fertilized 2 pronuclear embryos. Euploid rate was defined as the number of euploid embryos based on PGT-A divided by the total number of embryos biopsied with adequate tissue to obtain a result.
The pregnancy outcomes assessed included positive hCG rate, delivery rate, biochemical loss rate, and clinical loss rate. Positive hCG rate was defined as a positive serum beta hCG after SET. Delivery rate was defined as the number of viable deliveries per embryo transferred. A biochemical loss was defined as miscarriage after a positive serum beta hCG but prior to visualization of a gestational sac on ultrasound. A clinical loss was defined as the loss of pregnancy and subsequent decrease in beta hCG after visualization of at least an intrauterine gestational sac on ultrasound.
To assess the quality of sperm obtained in the study population, sperm concentration and total motile sperm count (TMSC) were analyzed. Sperm concentration was defined as the number of sperm per milliliter of ejaculate, and TMSC was defined as the total number of motile sperm within the entire ejaculated sample. These parameters were assessed in aggregate for the study population as well as based on specific paternal age cohorts. A comparison of sperm quality between older and younger men was performed.
Statistical Analysis
Patients were divided into two categories: couples with a male partner <40 years and those with a male partner ≥40 years of age. Linear regression models were used to examine the association between paternal age and fertilization rate, blastulation rate, and euploid rate after adjusting for female age. A second analysis was performed adjusting for both female age and oocyte yield. Logistic regression models were applied to examine the association between paternal age and positive hCG rate, delivery rate, biochemical loss rate, and clinical loss rate after adjusting for female age. Again, a second analysis was performed adjusting for both female age and oocyte yield.
The category of patients with a male partner age of <40 years was used as the statistical reference group for the analyses. To analyze the baseline characteristics of the study population, a Wilcoxon rank sum test was applied for numeric outcomes, and a chi-square test was used for categorical outcomes. P<.05 was considered statistically significant for all analyses.
Results
A total of 4,058 patients were included in the study. Patient and cycle-specific descriptive statistics are provided in Table 1. As noted previously, if a patient underwent multiple embryo transfers during the study time period, only the first transfer was included in the data analysis. The average paternal age was mean ± standard deviation, 37.2 ± 5.3 years among couples using ejaculated sperm. Male partner age was slightly older than female partner age, with female partner age noted to be 35.1 ± 3.8 years overall.
Table 1.
Baseline characteristics and cycle outcomes for patients undergoing euploid single embryo transfer after PGT-A, divided by age.
| Characteristic | Male age category, y |
P value | ||
|---|---|---|---|---|
| All subjects | Age <40 | Age ≥40 | ||
| n | 4,058 | 3,021 | 1,037 | |
| Male age (y) | 37.2 ± 5.3 | 34.8 ± 3.1 | 44.2 ± 4.2 | |
| Female partner age (y) | 35.1 ± 3.8 | 34.0 ± 3.5 | 38.1 ± 3.1 | |
| TMSC (in millions), median (IQR) | 56.4 (23.2, 105.4) | 59.9 (24.4, 110.5) | 48.1 (19.7, 93.6) | <.01 |
| Sperm concentration (M/mL), median (IQR) | 48.0 (28.0, 74.0) | 49.0 (28.0, 75.0) | 45.0 (27.0, 72.5) | .11 |
| Fertilization rate (%) | 85.1 ± 13.7 | 85.2 ± 13.8 | 85.0 ± 13.6 | .58 |
| Blastulation rate (%) | 54.9 ± 22.6 | 56.4 ± 22.2 | 50.6 ± 23.2 | <.01 |
| Euploid rate (%) | 69.0 ± 23.9 | 71.1 ± 22.6 | 62.9 ± 26.5 | .03 |
| Positive hCG rate | 3,321 (81.8) | 2,484 (82.2) | 837 (80.7) | .27 |
| Delivery ratea | 2,290 (60.8) | 1,713 (61.1) | 577 (59.7) | .42 |
| Biochemical loss rateb | 362 (10.9) | 283 (11.4) | 79 (9.4) | .11 |
| Clinical loss ratec | 294 (9.9) | 208 (9.5) | 86 (11.3) | .13 |
Note: Data presented as mean ± standard deviation or n (%), unless stated otherwise. hCG = human chorionic gonadotropin; IQR = interquartile range; PGT-A = preimplantation genetic testing for aneuploidy; TMSC = total motile sperm count.
Delivery data available for 3,769 patients.
Biochemical loss data available for 3,321 patients.
Clinical loss data available for 2,959 patients.
The median TMSC for the entire study population was 56.4 million (interquartile range [IQR] 23.2, 105.4). Male partner age <40 years was associated with a higher TMSC than age ≥40 years, corresponding with median values of 59.9 million (IQR 24.4, 110.5) and 48.1 million (IQR 19.7, 93.6), respectively (P<.01). However, the percentage of men with a TMSC <10 million was equivalent in both age categories, with 12.7% of men <40 years and 14.7% of men ≥40 years in this category (P=.09). Additionally, sperm concentration was not statistically different between the younger and older age groups, with men <40 years exhibiting a median sperm concentration of 49.0 million/mL (28.0, 75.0) and men ≥40 years demonstrating a median sperm concentration of 45.0 million/mL (27.0, 72.5; P=.11). Overall, 494 of the 4,058 participants (12.2%) had a sperm concentration <15 million/mL, which is the lower reference limit of normal based on the 2010 WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th Edition (16). There was no statistically significant difference in the percentage of men with an abnormal sperm concentration based on age (12.6% for men <40 years and 12.1% for men ≥40 years; P=.63).
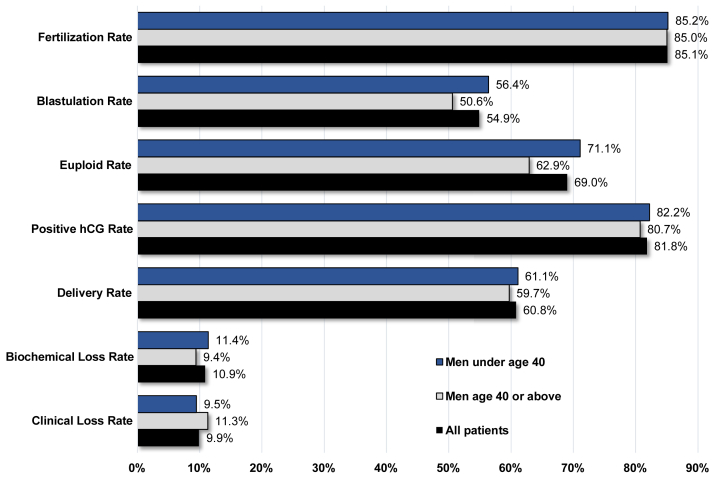
Overall fertilization rate was 85.1 ± 13.7%, blastulation rate was 54.9 ± 22.6%, euploid rate was 69.0 ± 23.9%, positive hCG rate was 81.8% (3,321/4,058), delivery rate was 60.8% (2,290/3,769), biochemical loss rate was 10.9% (362/3,321), and clinical loss rate was 9.9% (294/2,959). The specific outcomes stratified by male partner age are detailed in Table 1. Graphical depictions comparing the embryology and pregnancy outcomes based on each paternal age group can be found in Figure 1.
Figure 1.
A comparison of embryology and pregnancy outcomes based on paternal age following intracytoplasmic sperm injection (ICSI), preimplantation genetic testing for aneuploidy (PGT-A), and frozen single-embryo transfer (SET) of a euploid embryo with ejaculated sperm. hCG = human chorionic gonadotropin.
When comparing embryology and pregnancy outcomes, significantly lower rates of blastulation were obtained with increasing paternal age after controlling for maternal age (P<.01 for patients with a male partner aged ≥40 years). Statistically significant decreases in euploid embryo rates also were observed with increasing paternal age (P=.03 for male partners ≥40 years). There were no associations noted between paternal age and positive hCG rate, delivery rate, biochemical loss rate, clinical loss rate, or fertilization rate (Table 2).
Table 2.
Association between paternal age and reproductive outcomes after adjusting for female age.
| Variable | Male age category, y |
||
|---|---|---|---|
| Age <40 | Age ≥40 | P value | |
| Positive hCG rate | 1.06 (0.86–1.30) | .57 | |
| Delivery rate | 1.12 (0.94–1.32) | .19 | |
| Biochemical loss rate | Reference | 0.74 (0.55–1.00) | .05 |
| Clinical loss rate | 1.08 (0.80–1.46) | .61 | |
| Fertilization rate | 0.21 (0.56) | .70 | |
| Blastulation rate | −3.01 (0.91) | <.01 | |
| Euploid rate | −2.08 (0.94) | .03 | |
Note: Data presented as odds ratio (95% confidence interval) or beta value (standard error), unless stated otherwise. Statistical analysis was performed using logistic and linear regression models. hCG = human chorionic gonadotropin.
These findings also were consistent when an analysis was performed controlling for both maternal age and number of mature oocytes retrieved. Applying linear and logistic regression models that considered both female partner age and mature oocyte yield, a male partner age of ≥40 years was associated with worsened rates of blastocyst formation (P<.01) and decreased rates of euploid embryos (P=.02) but was not associated with changes in positive hCG rate, delivery rate, biochemical loss rate, clinical loss rate, or fertilization rate (P=.55, .19, .05, .64, and .70, respectively) when compared with male partners <40 years of age.
Secondary Analyses
To further assess the impact of paternal age on rates of blastocyst development and euploid embryo rates based on semen analysis parameters, a subgroup analysis was performed that stratified older and younger men based on sperm concentration. For the entire study population, 36 participants (0.9%) had a sperm concentration <1 million/mL. This included 27 of 3,021 (0.9%) subjects with a male partner <40 years and 9 of 1,037 (0.9%) subjects with a male partner ≥40 years. One hundred thirty-eight participants (3.5%) had a sperm concentration between 1 million/mL and 4.9 million/mL (n = 107 or 3.6% for the <40 years group and n = 31 or 3.1% for the ≥40 years group). Three hundred twenty participants (8.1%) had a sperm concentration between 5 million/mL and 14.9 million/mL (n = 238 or 8.1% for the <40 years group and n = 82 or 8.1% for the ≥40 years group). The remaining 3,463 participants (87.5%) had a normal sperm concentration of ≥15 million/mL. In this study, 2,573 subjects (87.4%) in the <40 years group and 890 subjects (87.9%) in the ≥40 years group demonstrated a normal sperm concentration. There were no statistically significant differences found in stratified sperm concentration between the younger and older age cohorts (P=.86).
When comparing participants with a male partner age ≥40 years to the <40 years age group as a reference, stratified sperm concentration did not affect fertilization rate in a statistically significant manner (P=.88 for concentration < 1 million/mL, P=.29 for concentration 1–4.9 million/mL, P=.91 for concentration 5–14.9 million/mL, and P=.49 for concentration ≥15 million/mL). Older men with a sperm concentration <1 million/mL, 1–4.9 million/mL, and 5–14.9 million/mL did not appear to have a worsened blastulation rate when compared with younger men with similar sperm concentrations (P=.05, .79, and .38, respectively). However, the total number of older men in these categories was only 122, which led to a lack of statistical power for this analysis. Older men with a sperm concentration of ≥15 million/mL (n = 890) demonstrated a worsened blastulation rate when compared with younger men with a similar sperm concentration (P<.01), in line with the findings observed in the primary analysis. Similarly, older men with a sperm concentration <1 million/mL, 1–4.9 million/mL, and 5–14.9 million/mL did not appear to have worsened euploid rates when compared with younger men with similar sperm concentrations (P=.84, .45, and .15, respectively). Older men with a sperm concentration of ≥15 million/mL demonstrated worsened euploid rates than younger men with similar sperm concentrations (P=.02). The negative impact of paternal age on euploid rates was observed in the group that was adequately powered (concentration ≥15 million/mL), confirming the findings of the primary analysis.
It is important to note that all cycles included in the primary analysis had at least one euploid blastocyst available for embryo transfer. In addition to these 4,058 included cycles, another 3,945 cycles that otherwise met inclusion criteria were excluded from the primary analysis because no euploid blastocyst was generated. Among couples with a male partner aged ≥40 years, approximately 50.9% of initiated cycles failed to generate a euploid blastocyst. After adjusting for female age, however, advancing paternal age was not associated with a statistically significant elevation in the risk of cycle cancellation due to the lack of a euploid blastocyst.
Discussion
Although increasing paternal age was associated with decreased blastulation and euploid rates among patients using ejaculated sperm, there was no effect on pregnancy outcomes or delivery rates after the transfer of a single euploid embryo in this study population. The relationships between older paternal age and the diminished embryology outcomes seen in the current study lack clear mechanistic explanations. The current findings are consistent with a small number of prior studies that have observed a detrimental effect on the number of high-quality or usable embryos achieved with advanced paternal age (8, 9, 10, 17, 18). Specifically, it previously has been reported that advanced paternal age may compromise fertilization and blastulation rates, but pregnancy outcomes after successful implantation do not appear to be impacted negatively by older paternal age (19). However, these findings are not consistent across the literature, making it difficult to attribute confidently certain laboratory outcomes to older male partners (4, 20).
One possible explanation for the link between advanced paternal age and poorer embryology outcomes relates to levels of sperm DNA fragmentation. At relatively low levels, it is theorized that sperm DNA strand breaks can be repaired within the oocyte after fertilization. With significantly elevated sperm DNA fragmentation, however, the developing embryo may be unable to compensate for the diminished DNA quality, potentially resulting in poorer embryo quality (5, 21). Because sperm DNA fragmentation appears to worsen with age, it is possible that the decreased rates of blastulation and decreased euploid rates noted in the current study are the result of higher rates of sperm DNA damage seen in older men (22). However, this study does not include data regarding sperm DNA fragmentation, and the relationship between sperm DNA damage and embryology outcomes is a putative association lacking confirmation.
In the data presented, sperm quality as determined based on sperm concentration was equivalent between the older and younger male age groups. Although TMSC was noted to be higher in the group with younger male partners, the universal use of ICSI in the current study likely corrects for any detrimental effects of lower TMSC. Although some controversy exists, prior research has indicated that when ICSI is used differences in TMSC have no impact on rates of blastocyst formation, euploid rates, positive hCG rates, clinical pregnancy rate, and live birth rate (23, 24, 25). Additionally, the proportions of men with a sperm concentration of <15 million or a TMSC of <10 million were equivalent between the two age groups. Based on these findings, truly significant differences attributed to either sperm concentration or TMSC between the two age categories are unlikely.
This study is not without limitations. Issues related to the retrospective nature of the study may have affected the findings. Although this study attempts to correct for certain confounders, factors such as body mass index, smoking, drug use, and alcohol use in male participants were not assessed. In the future, controlling for the many factors that have been associated with elevated rates of sperm DNA fragmentation and decreased live birth rates would add to the strength of findings related to this topic (26, 27, 28, 29). Additionally, diagnoses associated with male factor infertility such as varicocele that often require evaluation or treatment by a urologist were not evaluated. If an individual had been seen by a urologist before undergoing IVF or underwent IVF and then subsequently had evaluation or treatment performed by a urologist, this information was not captured in the data. Furthermore, no differences were observed in rates of fertilization based on paternal age. This may be related to the fact that ICSI was used for all participants because prior studies have noted differences in fertilization rates when conventional insemination was used for fertilization (2, 5).
The current study used a paternal age of 40 as a cutoff for statistical analysis. This decision was made based on prior reports of increased rates of de novo genetic mutations when paternal age exceeds 40 years as well as the ability to achieve adequate statistical power (30). Although there is no universally accepted definition for advanced paternal age, if differences in embryology or pregnancy outcomes begin to arise at an age significantly older than 40 years, the current study would not have identified these differences. The retrospective nature of the study also eliminated the ability to assess for levels of sperm DNA fragmentation directly. Although elevated sperm DNA fragmentation serves as one potential explanation for the findings observed in this study, it cannot be known with certainty whether the older patients had higher levels of sperm DNA damage because this was not assessed.
Finally, it must be recognized that selection bias was introduced through the study design. By requiring couples to undergo a euploid SET to be included in the primary analysis, all patients who were unable to generate a euploid embryo were excluded. The relationship between increased paternal age and adverse outcomes for patients who failed to create successfully a euploid blastocyst, therefore, was not evaluated in the primary analysis. Future studies are necessary to determine whether the outcomes observed in the current study hold true when additional confounding variables are considered, when surgically extracted or cryopreserved sperm samples are used, and when different age cutoffs are applied.
Conclusions
The current study links advancing paternal age with poorer rates of blastocyst formation and euploid status among men using ejaculated sperm. It is reassuring to note that if a euploid embryo is achieved, older paternal age does not appear to affect negatively pregnancy outcomes. A clear mechanistic explanation for these findings fully has not been elucidated. However, sperm DNA fragmentation or other factors related to the functional characteristics of spermatozoa within aging men may provide plausible explanations.
Comprehensive data sets linking paternal age to levels of sperm DNA fragmentation may prove to be useful in the future. Additionally, generating a standard definition for advanced paternal age that correlates to specific embryologic, pregnancy, or developmental outcomes would allow for consistency in data collection for future studies. Going forward, a thorough investigation into potential interventions that may protect against the harmful effects of paternal age on male fertility is warranted.
Footnotes
B.M.H. has nothing to disclose. J.G.K. has nothing to disclose. E.K.O. has nothing to disclose. A.W.T. has nothing to disclose. R.B.L. has nothing to disclose. P.J.C. has nothing to disclose. R.T.S. has nothing to disclose. J.M.F. has nothing to disclose.
References
- 1.Khandwala Y., Zhang C., Lu Y., Eisenberg M. The age of fathers in the USA is rising: an analysis of 168,867,480 births from 1972 to 2015. Hum Reprod. 2017;32:2110–2116. doi: 10.1093/humrep/dex267. [DOI] [PubMed] [Google Scholar]
- 2.Tiegs A., Sachdev N., Grifo J., McCullon D., Licciardi F. Paternal age is not associated with pregnancy outcomes after single thawed euploid blastocyst transfer. Reprod Sci. 2017;24:1319–1324. doi: 10.1177/1933719116687660. [DOI] [PubMed] [Google Scholar]
- 3.Sharma R., Agarwal A., Rohra V., Assidi M., Abu-Elmagd M., Turki R. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol Endocrinol. 2015;13:1–20. doi: 10.1186/s12958-015-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begueria R., Garcia D., Obradors A., Poisot F., Bassena R., Vernaeve V. Paternal age and assisted reproductive outcomes in ICSI donor oocytes: is there an effect of older fathers? Hum Reprod. 2014;29:2114–2122. doi: 10.1093/humrep/deu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapuis A., Gala A., Ferrieres-Hoa A., Mullet T., Bringer-Deutsch S., Vintejoux E. Sperm quality and paternal age: effect on blastocyst formation and pregnancy rates. Basic Clin Androl. 2017;27 doi: 10.1186/s12610-016-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagi-Dain L., Sagi S., Dirnfeld M. Effect of paternal age on reproductive outcomes in oocyte donation model: a systematic review. Fertil Steril. 2015;104:857–865. doi: 10.1016/j.fertnstert.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 7.DeLaRochebrochard E., DeMouzon J., Thepot F., Thonneau P., FIVNAT Fathers over 40 and increased failure to conceive: the lessons of in vitro fertilization in France. Fertil Steril. 2006;85:1420–1424. doi: 10.1016/j.fertnstert.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 8.Frattarelli J., Miller K., Miller B., Elkind-Hirsch K., Scott R. Male age negatively impacts embryo development and reproductive outcome in donor oocyte assisted reproductive technology cycles. Fertil Steril. 2008;90:97–103. doi: 10.1016/j.fertnstert.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Ferreyra J., Luna D., Villegas L., Romero R., Zavala P., Hilario R. High aneuploidy rates observed in embryos derived from donated oocytes are related to male aging and high percentages of sperm DNA fragmentation. Clin Med Insights Reprod Health. 2015;9:21–27. doi: 10.4137/CMRH.S32769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris I., Ilott S., Dixon L., Brison D. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum Reprod. 2002;17:990–998. doi: 10.1093/humrep/17.4.990. [DOI] [PubMed] [Google Scholar]
- 11.Robertshaw I., Khoury J., Abdallah M., Warikoo P., Hofmann G. The effect of paternal age on outcome in assisted reproductive technology using the ovum donation model. Reprod Sci. 2014;21:590–593. doi: 10.1177/1933719113506497. [DOI] [PubMed] [Google Scholar]
- 12.Bellver J., Garrido N., Remohi J., Pellicer A., Meseguer M. Influence of paternal age on assisted reproduction outcome. Reprod Biomed Online. 2008;17:595–604. doi: 10.1016/s1472-6483(10)60305-7. [DOI] [PubMed] [Google Scholar]
- 13.Paulson R., Milligan R., Sokol R. The lack of influence of age on male fertility. Am J Obstet Gynecol. 2001;184:818–822. doi: 10.1067/mob.2001.113852. [DOI] [PubMed] [Google Scholar]
- 14.Franasiak J., Forman E., Patounakis G., Hong K., Werner M., Upham K. Investigating the impact of the timing of blastulation on implantation: management of embryo-endometrial synchrony improves outcomes. Hum Reprod Open. 2018;2018:1–6. doi: 10.1093/hropen/hoy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner D., Lane M., Stevens J., Schlenker T., Schoolcraft W. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–1158. doi: 10.1016/s0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 16.WHO . 5th ed. WHO Press; Geneva: 2010. WHO laboratory manual for the examination and processing of human semen. [Google Scholar]
- 17.Wu Y., Kang X., Zheng H., Liu H., Huang Q., Liu J. Effect of paternal age on reproductive outcomes of intracytoplasmic sperm injection. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luna M., Finkler E., Barritt J., Bar-Chama N., Sandler B., Copperman A. Paternal age and assisted reproductive technology outcome in ovum recipients. Fertil Steril. 2009;92:1772–1775. doi: 10.1016/j.fertnstert.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 19.Bartolacci A., Pagliardini L., Makieva S., Salonia A., Papaleo E., Vigano P. Abnormal sperm concentration and motility as well as advanced paternal age compromise early embryonic development but not pregnancy outcomes: a retrospective study of 1266 ICSI cycles. J Assist Reprod Genet. 2018;35:1897–1903. doi: 10.1007/s10815-018-1256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai Y., Lan K., Kung F., Lin P., Chiang H., Lin Y. The effect of advanced paternal age on the outcomes of assisted reproductive techniques among patients with azoospermia using cryopreserved testicular spermatozoa. Taiwan J Obstet Gynecol. 2013;52:351–355. doi: 10.1016/j.tjog.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Yang H., Li G., Jin H., Guo Y., Sun Y. The effect of sperm DNA fragementation index on assisted reproductive technology outcomes and its relationship with semen parameters and lifestyle. Trans Androl Urol. 2019;8:356–365. doi: 10.21037/tau.2019.06.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen C., Mauri A., Vagnini L., Renzi A., Petersen B., Mattila M. The effects of male age on sperm DNA damage: an evaluation of 2,178 semen samples. JBRA Assist Reprod. 2018;22:323–330. doi: 10.5935/1518-0557.20180047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanVoorhis B., Barnett M., Sparks A., Syrop C., Rosenthal G., Dawson J. Effect of the total motile sperm count on the efficacy and cost-effectiveness of intrauterine insemination and in vitro fertilization. Fertil Steril. 2001;75:661–668. doi: 10.1016/s0015-0282(00)01783-0. [DOI] [PubMed] [Google Scholar]
- 24.Morin S., Juneau C., Neal S., Scott R., Hotaling J. Total motile sperm count is negatively correlated with fertilization rate but not blastulation, euploidy, or implantation in ICSI cycles. Fertil Steril. 2017;108:Se127. [Google Scholar]
- 25.Borges E., Setti A., Braga D., Figueira R., Iaconelli A. Total motile sperm count has a superior predictive value over the WHO 2010 cut-off values for the outcomes of intracytoplasmic sperm injection cycles. Andrology. 2016;4:880–886. doi: 10.1111/andr.12199. [DOI] [PubMed] [Google Scholar]
- 26.Sepidarkish M., Maleki-Hajiagha A., Maroufizadeh S., Rezaeinejad M., Almasi-Hashiani A., Razavi M. The effect of body mass index on sperm DNA fragmentation: a systematic review and meta-analysis. Int J Obes (Lond) 2020;44:549–558. doi: 10.1038/s41366-020-0524-8. [DOI] [PubMed] [Google Scholar]
- 27.Cui X., Jing X., Wu X., Wang Z., Li Q. Potential effect of smoking on semen quality through DNA damage and the downregulation of Chk1 in sperm. Mol Med Rep. 2016;14:753–761. doi: 10.3892/mmr.2016.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pourmasumi S., Sabeti P., Rahiminia T., Mangoli E., Tabibnejad N., Reza-Talebi A. The etiologies of DNA abnormalities in male infertility: an assessment and review. Int J Reprod Biomed (Yazd) 2017;15:331–344. [PMC free article] [PubMed] [Google Scholar]
- 29.Colaci D., Afeiche M., Gaskins A., Wright D., Toth T., Tanrikut C. Men's body mass index in relation to embryo quality and clinical outcomes in couples undergoing in vitro fertilization. Fertil Steril. 2012;98:1193–1199. doi: 10.1016/j.fertnstert.2012.07.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toriello H., Meck J. Statement on guidance for genetic counseling in advanced paternal age. Genet Med. 2008;10:457–460. doi: 10.1097/GIM.0b013e318176fabb. [DOI] [PMC free article] [PubMed] [Google Scholar]