Abstract
Objective
To determine if there has been a change in empirical medical therapy (EMT) practices since a 2010 American Urological Association survey reported that 25% of urologists treated infertile men who were pursuing a pregnancy with testosterone (T).
Design
Survey-based cohort study of AUA members.
Setting
Practice patterns were evaluated of urologists in academic and nonacademic hospital centers.
Patient(s)
Practice patterns were evaluated in the treatment of men with idiopathic infertility.
Interventions(s)
None.
Main Outcome Measure(s)
Subgroup analysis by means of univariate analysis between means (Fisher exact test) and descriptive proportions was used to compare male infertility fellowship–trained urologists (RUs) to general urologists (non-RUs).
Result(s)
A total of 191 urologists responded (4.7%). Excluding trainees, 164 responses (85.9%) were analyzed: 134 (82.3%) were from non-RUs and 29 from (17.7%) RUs. Over all, 65.9% treated male infertility with a combination of EMT and surgery (93.1% of RU vs. 60.4% of non-RUs). The most common medications used by RUs were clomiphene (100%), anastrozole (85.7%), and hCG/LH (82.1%). Non-RUs used these less frequently. Overall, 24.4% of the urologists reported that they would use T to treat male infertility: 14.4% (n = 4) of RUs and 24.4% (n = 30) of non-RUs.
Conclusion(s)
A total of 65.9% of urologists would treat male infertility with the use of EMT and surgery. The most common EMTs were clomiphene, anastrozole, and hCG/LH. Of concern, 24.4% of urologists considered T to treat male infertility, a medication with known contraceptive potential. This is unchanged from the 2010 survey, and confirms the need for reproductive medicine guidelines that include the topic of EMT use in infertile men.
Key Words: Male infertility, testosterone, empirical medical therapy, clomiphene citrate, sperm analysis
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/users/16110-fertility-and-sterility/posts/65475-xfre00007
In 2010, an online survey by Ko et al. was emailed to members of the American Urological Association (AUA) to assess practice patterns, treatment trends, and attitudes toward empirical medical therapy (EMT) for male infertility (1). Among other findings, the survey reported that 25% of urologists would treat infertile males with testosterone (T), even while they were actively pursuing pregnancy. This fundamental misunderstanding regarding the effects of T suppression on the hypothalamic-pituitary-gonadal axis was a major concern. Regarding EMT, significant differences were also seen among urologists in the choice of medications, and trends were seen in the identification of EMT candidates, minimum sperm count needed to initiate therapy, treatment duration, predictors of outcome, and the use of EMT for nonobese patients.
Since then, educational efforts have been implemented, including a 2011 Optimal Evaluation of the Infertile Male Best Practice Statement, a 2018 Testosterone Deficiency AUA Guideline (2), and several AUA Update Series lessons. For EMT specifically, however, no consensus statements, clinical algorithms, or treatment recommendations have been established. In the present study, we aimed to determine if there has been a change since 2010 in urologists’ practices regarding the use of EMT and T for idiopathic male infertility.
Materials and methods
An electronic survey was sent to 4,000 randomly selected practicing urologists in 2018 via the AUA Office of Education and Inquisium/Cvent feedback management program. This served as a representative sample of the AUA membership. The survey closed on June 12, 2018; respondents were entered into a random drawing for a $150 Visa gift card. This study was exempt from institutional review board approval owing to its anonymous survey–based nature.
The survey was similar to the 2010 survey on the use of EMT for male infertility. In brief, there were three sections. This included a demographics section (9 questions), questions regarding EMT practice habits (11 questions), and infertility case scenarios (three cases with 11 questions). The questions in each section are reflected in Supplemental Table 1 (available online at www.fertstert.org) and Table 1, Table 2, Table 3. Demographics included gender, number of years beyond residency, patient volume, primary specialty focus, urban, metropolitan, or rural practice locations, size and type of practice group, and the degree of involvement in male infertility patients. Residents and fellows were excluded from the analysis. Of note, respondents could select more than one specialty focus area.
Table 1.
Summary of empirical medical therapy (EMT) survey results for reproductive urologists (RUs) versus nonreproductive urologists (non-RUs).
| EMT practice pattern | RUs | Non-RUs | P value |
|---|---|---|---|
| Idiopathic infertility can be treated with both EMT and surgery | 93.1% | 60.4% | .0004 |
| EMT will increase sperm counts | 52.5% | 84.3% | .0337 |
| EMT will increase pregnancy rates | 48.3% | 78.9% | .0031 |
| Clomiphene is best medication for nonobese male with idiopathic oligospermia | 100% | 94.8% | .3543 |
| Clomiphene is best medication for obese male with idiopathic oligospermia | 62.1% | 67.9% | .6639 |
| Anastrozole is best medication for obese male with idiopathic oligospermia | 37.9% | 26.1% | .2547 |
| Endorsed a baseline sperm concentration threshold for starting EMT | 52.9% | 74.6% | .8378 |
| Would use testosterone to treat male infertility | 13.8% | 22.2% | .3217 |
Table 2.
Factors used to determine ideal empirical medical therapy (EMT) candidate and ideal medication use, ranked by order of importance by each respondent group.
| Rank order | Ideal EMT candidate |
Ideal EMT medication |
||
|---|---|---|---|---|
| RUs | Non-RUs | RUs | Non-RUs | |
| 1 | Sperm conc. | Sperm conc. | T level | T level |
| 2 | FSH level | FSH level | T/E ratio | FSH level |
| 3 | Testis size | LH level | E2 level | T/E ratio |
| 4 | T/E ratio | T level | FSH level | BMI |
| 5 | T level | Testis size | BMI | E2 level |
| 6 | LH | Age of partner | Testis size | Testis size |
| 7 | Age of partner | T/E ratio | Age of partner | Age of partner |
Note: Non-RUs, nonreproductive urologists; RUs, reproductive urologists.
Table 3.
Patient scenarios.
| Scenario | RUs |
Non-RUs |
P value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| 1. 27-year-old male with a 3-year history of primary infertility. Sperm concentration is 25 million/mL, with normal motility and morphology, normal hormone studies, and a normal physical examination. Would you consider this patient to be an EMT candidate? | .1579 | ||||
| Yes | 4 | 13.8 | 37 | 27.6 | |
| No | 25 | 86.2 | 97 | 72.4 | |
| 2. 27-year-old male with a 3-year history of secondary infertility. Sperm concentration is 10 million/mL, with normal motility and morphology, normal hormone studies, and a normal physical examination. Would you consider this patient to be an EMT candidate? | .0252 | ||||
| Yes | 15 | 51.7 | 99 | 73.9 | |
| No | 14 | 48.3 | 35 | 26.1 | |
| 3. 33-year-old male with primary infertility. Sperm concentration is 7 million/mL, with a normal motility and morphology. Hormone studies are LH 3 IU/L (normal 2–9 IU/L), FSH 10 IU/L (normal 1–8 IU/L), T 400 ng/dL (normal >220 ng/dL). His physical examination is normal. Is he an appropriate candidate for EMT? | .2090 | ||||
| Yes | 14 | 48.3 | 84 | 62.7 | |
| No | 15 | 51.7 | 50 | 37.3 | |
| How much would you expect patient 3’s total sperm count to improve after a course of EMT?a | .6241 | ||||
| 0%–20% | 7 | 50.0 | 33 | 39.3 | |
| 21%–60% | 7 | 50.0 | 50 | 59.5 | |
| 60%–100% | 0 | 0 | 1 | 1.2 | |
| If patient 3 had an FSH of 7 IU/L, are you more or less likely to consider this patient for EMT? | .0365 | ||||
| More likely | 23 | 79.3 | 78 | 58.2 | |
| Less likely | 6 | 20.7 | 56 | 41.8 | |
| How much would you expect this patient’s sperm count to improve after a course of EMT (with FSH of 7 IU/L)? | .3767 | ||||
| 0%–20% | 16 | 58.1 | 60 | 44.7 | |
| 21%–60% | 13 | 41.9 | 73 | 54.5 | |
| 60%–100% | 0 | 0 | 1 | 0.7 | |
| If patient 3 had an FSH of 3 IU/L, are you more or less likely to consider this patient for EMT? | .0074 | ||||
| More likely | 26 | 89.6 | 86 | 64.2 | |
| Less likely | 3 | 10.4 | 48 | 35.8 | |
| How much would you expect this patient’s sperm count to improve after a course of EMT (with FSH of 3 IU/L)? | .2700 | ||||
| 0%–20% | 10 | 34.5 | 67 | 50.0 | |
| 21%–60% | 18 | 62.1 | 61 | 45.5 | |
| 60%–100% | 1 | 3.5 | 6 | 4.5 | |
| Patient 3 now has a left varicocele. Six months after varicocele repair, his sperm concentration improves to 10 million/mL. With normal hormone studies and an otherwise normal examination, is he a candidate for EMT? | .5198 | ||||
| Yes | 17 | 58.6 | 89 | 66.4 | |
| No | 12 | 41.4 | 45 | 33.6 | |
| How much would you expect this patient’s sperm count to improve after a course of EMT?a | .8781 | ||||
| 0%–20% | 9 | 52.9 | 41 | 46.1 | |
| 21%–60% | 8 | 47.1 | 45 | 50.5 | |
| 60%–100% | 0 | 0 | 3 | 3.4 | |
Note: EMT, empirical medical therapy; non-RUs, nonreproductive urologists; RUs, reproductive urologists.
Incomplete responses in both groups.
Additional questions were posed regarding EMT practice patterns. These included questions about the use of surgery versus EMT for idiopathic infertility, the medications used to treat male infertility, and the respondents’ expected outcomes. In deciding between EMT and surgery, respondents were not offered to specify surgical procedure. Infertility case scenarios also were presented, each with different semen parameters, laboratory results, and examination findings.
Demographic and statistical comparisons were made between the responses from general urologists (non-RUs) and male infertility and andrology fellowship–trained urologists (RUs). Univariate analysis between means to compare the two cohorts was conducted with the use of chi-square analysis and Fisher exact test. Statistical significance was indicated by a P value of <.05. Statistical analysis was performed with the use of SAS software, version 9.0.
Results
Respondent Characteristics
A total of 191 urologists responded (4.7%). Excluding trainees (residents and fellows), 164 (85.9%) responses were analyzed: 134 (82.3%) were non-RUs and 29 (17.7%) were RUs. Non-RU respondents listed their focus area as general urologists (79.1%), urologic oncologists (35.1%), stone specialists (29.8%), incontinence specialists (11.2%), obstructive disease specialists (11.9%), erectile dysfunction (ED) specialists (17.9%), pediatric urologists (3.0%), renal transplant (0.7%), and trauma (2.2%), with the remainder listing no special area of interest (2.9%). Of note, six respondents (4.5%) in the non-RU group noted infertility as a special interest area, although they were not infertility or andrology fellowship trained. Similarly, of the RU cohort, some respondents also included other aspects of urology as special interest areas. These included general urology (35.5%), oncology (10.3%), calculus disease (20.7%), incontinence (13.8%), ED (69.0%), and obstructive disease (6.9%).
Demographics between the cohorts are described in Supplemental Table 1. Overall, 41.7% of the respondents were in practice for >21 years (34.5% of RUs and 43.2% of non-RUs), and 25.2% were in practice for 1–5 years (37.9% of RUs and 22.4% of non-RUs; P=.1911). There were no differences seen between the two groups for practice location, time out of residency or fellowship, or gender, whereas more RUs were associated with academic centers (P=.0001). There were no RUs based in rural centers, all 29 of them being in urban and metropolitan areas. Of the RU respondents, 72.4% were in a urology practice with >30% infertility patients, whereas 80.6% of non-RUs had patient populations with <5% presenting for infertility (P<.0001). Similarly, RUs attended infertility continuing medical education (CME) programs more frequently than non-RUs (P<.0001), with 51.5% of non-RUs never attending any infertility-related CME.
Treatment of idiopathic infertility differed between the two groups (Table 1). The majority of RUs (93.0%) stated that idiopathic infertility patients can be treated with both surgery and EMT, although this distinction was less profound with non-RUs (60.4%; P=.0004). Non-RUs believed at a higher proportion than RUs that EMT would increase sperm counts (84.3% vs. 52.5%; P=.0337). The remainder of the respondents answered “no” or “unknown” in terms of effect of EMT on sperm counts. In addition, non-RUs stated more frequently that EMT would lead to increased pregnancy rates (78.9% vs. 48.3%; P=.0031).
Specific Medications
Clomiphene citrate (at 25 mg daily or 50 mg every other day) was the preferred medication across both groups for a nonobese (body mass index [BMI] <30 kg/m2; P=.3543) or obese (BMI >30 kg/m2; P=.6639) man with idiopathic oligospermia. All of the RUs selected clomiphene citrate for EMT for nonobese men, but in obese men some chose between clomiphene citrate and anastrozole. No differences were seen between groups when asked if anastrozole instead of clomiphene citrate was the best medication for an obese man with idiopathic oligospermia (37.9% vs. 26.1%; P=.2547).
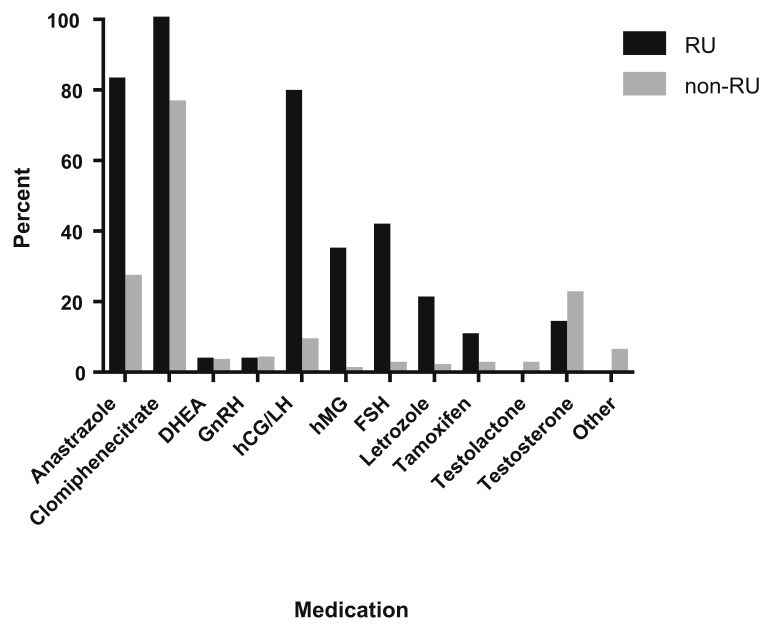
Figure 1 illustrates the proportion of RU and non-RU practitioners using various EMT medications: 82.8% of RUs prescribe anastrozole, 100% use clomiphene citrate, 79.3% use hCG/LH, and smaller percentages use hMG, FSH, letrozole, tamoxifen, and T. Non-RUs also used anastrozole (26.7%), clomiphene citrate (76.3%), and hCG/LH (8.9%), but at much lower numbers. Importantly, 22.2% (n = 30) of non-RUs opted for T in the idiopathic infertile man, compared with 13.8% (n = 4) of RUs (P=.3217).
Figure 1.
Proportion of reproductive urologists (RUs) and nonreproductive urologists (non-RUs) using specified empirical medical therapy medications.
Semen Parameters
Non-RU respondents more often than RUs thought that a minimal sperm concentration threshold was needed before initiating EMT, although the difference was not significant (74.6% vs. 52.9%; P=.8378). The majority of RUs selected 5–10 million/mL (normal >20 million/mL) as the minimum sperm concentration to start EMT (72.7%), whereas non-RUs did not display a strong preference for any concentration <20 million/mL. However, of those who did indicate a minimal sperm concentration to initiate therapy, there was no significant difference between the groups (P=.0937). No differences were seen between duration of EMT (P=.4689), with the majority of respondents in both groups opting for 3–6 months as an optimal course (65.5% of RUs vs. 55.9% of non-RUs).
Table 2 outlines respondents’ use of clinical and laboratory factors to determine the ideal EMT candidate and the factors used to select the choice of EMT medication. In general, similarities were seen between both groups in identifying the ideal EMT candidate (prioritizing sperm concentration and FSH level over other parameters). RUs considered testis size as an important consideration for EMT candidacy, which was not seen with non-RUs. Both groups deemed the age of the partner as a low priority for EMT candidacy. When identifying patient factors in selection of EMT medications, less agreement existed between groups. Interestingly, both groups considered testis size and partner age to be unimportant factors. Across each patient scenario, similar opinions were voiced that oligospermia was a big driver in establishing EMT candidacy compared with a patient with normal examination and hormone and sperm parameters. There was a consensus for 3–6 months as the optimal duration of EMT treatment, which was similar to the 2010 survey results.
Clinical Scenarios
Several clinical scenarios were suggested, revealing treatment decision differences between the two groups (Table 3). For the male patient with no examination or laboratory abnormality and with normal sperm concentration, 86.2% of RUs and 72.4% of non-RUs did not consider this patient to be an EMT candidate (P=.1579). Interestingly, non-RUs were more likely to treat this patient with EMT (27.6% vs. 13.8%).
For the oligospermic patient (sperm concentration of 10 million/mL) with no laboratory or examination abnormality, both groups treated this patient more often, with the majority of non-RUs now opting for EMT owing to the oligospermia (51.7% of RUs vs. 73.9% for non-RUs; P=.0252).
The patient in the third scenario had oligospermia (7 million/mL), elevated FSH to 10 IU/L (normal 1–8 IU/l), and normal T of 300 ng/dL; 62.7% of non-RUs considered him to be an EMT candidate, whereas only 48.3% of RUs did (P=.2090). When the FSH level was adjusted to 7 IU/L or 3 IU/L, RUs were more likely to consider this patient for EMT (79.3% and 89.6%, respectively, for each FSH value), whereas there was little change to the proportions among non-RUs.
The last scenario included a varicocele, with improved sperm concentration to 7 million/mL after repair. With normal hormone studies and examination otherwise, 66.4% of non-RUs considered him to be an EMT candidate at this point, versus 58.6% of RUs (P=.5198). No differences were seen between the groups on the expected improvement of sperm count after a course of EMT (P=.8781).
Discussion
This survey is a follow-up of the 2010 poll of the AUA on EMT practice patterns. There are currently no consensus statements or treatment algorithms for the management of idiopathic male infertility or the use of EMT from major organizations such as the AUA, American Society of Reproductive Medicine, or European Association of Urology. The challenge of addressing the needs of infertile men, particularly in the 25% of cases where no hormonal or examination findings exist, remains a pressing issue (3).
Our main finding demonstrates that T administration in the idiopathic male infertility patient still exists as a possibility among urologists, despite its contraceptive effect. This is unchanged from the 2010 AUA survey. The survey also illustrates that non-RUs are overall less familiar with EMT compared with RUs, which is understandable given differences in training, but non-RUs are using EMT medications more often than in 2010. Clomiphene citrate is by far the most common medication used by non-RUs. The ideal EMT candidate, or distinguishing clinical parameters, remains unclear among all urologists surveyed.
Comparisons between the respondents of the 2010 and the 2018 surveys show that they are similar in terms of characteristics. Most respondents were in practice for >5 years (74.8% in the 2010 survey compared with 74.2% presently), although in the 2018 study 41.7% of respondents were >21 years out of residency. RUs represented 17.7% of our cohort, and in 2010 study 16% were fellowship-trained urologists. RUs with >30% of their practice being infertility patients represented 74.2% of our RU cohort, compared with 66.7% in the 2010 cohort. Similarly, 80.6% of the current non-RU cohort had <5% of their practice being infertility, compared with 95.3% of the 2010 cohort. Attendance at infertility-related CME events was expectedly low for non-RUs, also unchanged from the previous study.
A combination of surgery and EMT was used by 93.1% of RUs for idiopathic male infertility, which was significantly higher than for non-RUs (P=.0004). It is unclear whether the surgical and medical aspect of idiopathic infertility accounts for this difference. Both groups also differed in the expectation that EMT would increase sperm counts and pregnancy rates, with non-RUs being overall more encouraging. Compared with the 2010 survey, the current expectations are much higher. In 2010, only 41% expected EMT to increase sperm concentration, and merely 9.2% of respondents thought that there would be an improvement in pregnancy rate.
EMT has been in use for decades, despite no Food and Drug Administration approval for men or clear evidence-based support. In 2013, Attia et al. reviewed six randomized controlled trials with 456 patients receiving gonadotropin treatment (hCG or recombinant or purified FSH) for male-factor subfertility (4). Spontaneous pregnancy rates were higher with the use of gonadotropins compared with placebo or no treatment (16% vs. 7%, OR 4.94, 95% CI 2.13–11.44). A similar result was seen in work from Santi et al. (5). While encouraging for injectable gonadotropins, the data supporting clomiphene citrate and other oral hormonal medications are less clear.
Clomiphene citrate is a selective estrogen receptor modulator that inhibits feedback of estrogen on the hypothalamus, thereby potentially promoting spermatogenesis and testosterone production. It was originally used to induce follicular development and ovulation in women with polycystic ovarian syndrome (PCOS). Its effects on improving testosterone and symptoms of hypogonadism, with minimal side-effects, is well established (6, 7, 8). Sperm concentration and total motile count have also been confirmed to improve in some studies (9, 10, 11, 12, 13). Work from Helo et al., however, did not confirm these findings. In a randomized prospective double-blind comparison trial of clomiphene and anastrozole for hypogonadal infertile men, neither sperm parameters nor pregnancy rates improved (14). Similar findings regarding a failure of sperm parameter or pregnancy rate improvement were seen in a much-cited 1992 study from the World Health Organization (15).
Reversible aromatase inhibitors, such as anastrozole and letrozole, increase intratesticular T levels and potentially support spermatogenesis. Aromatase is highly expressed in adipose tissue, making obese males who have elevated 17β-E2 levels particularly suitable for therapy. Subfertile hypoandrogenic men with oligospermia have been shown to respond to anastrozole with improvement in sperm concentration and total motile counts (16, 17). Combination therapy with clomiphene citrate and anastrozole is also safe and effective (18), but no long-term effects on sperm parameters or pregnancy have yet to be reported.
We show that clomiphene citrate is the medication preferred by RUs for nonobese patients, and largely by non-RUs as well (100% and 94.8%, respectively). For the obese man, anastrozole was, maybe unexpectedly, not preferred by either group (37.9% for RUs vs. 26.1% for non-RUs). In the 2010 study, fellowship-trained urologists administered anastrozole significantly more frequently than general urologists (P<.001); non-RUs may be more comfortable prescribing anastrozole at present. As shown in Figure 1, however, there remains a discrepancy in anastrozole use between the two groups. Clomiphene citrate use, chosen most often in both groups, is less discrepant (Table 1). All other medications were largely unfamiliar to non-RUs, with hCG/LH being used third most commonly. In addition, the order of factors used to determine the ideal EMT candidate or medication choice was largely similar in both groups (Table 2). Sperm and serum values focused on the male partner, with all groups ranking age of female partner last or nearly last. Although the present study polled only urologists, it highlights the lack of importance placed on female partner age. Yet a 3–6-month course of EMT may not provide any pregnancy outcome benefit, particularly for men with older female partners. In fact, older women or those with diminished ovarian reserve may be negatively affected by a 3–6-month delay for EMT and should be considered for up-front assisted reproductive technologies.
Importantly, exogenous T was chosen by respondents in both groups to treat idiopathic male infertility (13.8% of RUs and 22.2% of non-RUs). In the 2010 survey also, general urologists were more likely to give T than fellowship-trained urologists (P=.001). It is known that T suppresses the hypothalamic-pituitary-gonadal axis, reducing intratesticular T and spermatogenesis. This inappropriate practice pattern should be highlighted among urologists and hopefully abandoned. We acknowledge, however, that some RU practices may allow exogenous T with concomitant hCG for hypogonadal patients, but that scenario was not considered in the survey questions (19).
There are several limitations to this survey. First, the response rate was 4.7% and so the responses may not entirely reflect the practice patterns across all AUA members. In addition, many respondents were >21 years out of residency or fellowship, which might impart selection bias. Despite small response numbers, the 2010 survey also had a 5% response rate. Understandably, urologists who are not involved in infertility cases may not have had an interest to respond at all. Furthermore, this survey addressed only pharmaceutical options for EMT. Nutritional and antioxidant supplements are a large part of EMT (20), but in an effort to maintain consistency with the 2010 survey, use of those entities was not queried. For similar reasons, financial influences on EMT practice patterns, whether surgical or pharmaceutical, also were not queried.
Conclusion
In this follow-up survey, we illustrate current practice patterns and trends for EMT for idiopathic male infertility. Of concern, many urologists reported that they would use T to treat male infertility, a medication with known contraceptive potential. The percentage answering in this fashion is essentially from the 2010 survey. Although some agreement was discovered for identifying the ideal EMT candidate and the duration of EMT, there was less concordance between RUs and non-RUs in the selection of medications and familiarity with different medication options. The most common EMTs were clomiphene, anastrozole, and hCG/LH. RUs were more likely to use EMT than non-RUs. Most urologists thought that EMT increases sperm counts and pregnancy rates, more so than in 2010, despite a lack of evidence to that effect. These findings reiterate the need for guidelines and treatment algorithms that address the use of EMT in male infertility, as well as continuing educational effort of urologists regarding male reproductive physiology and pharmacology. Given the increasing subspecialization trend in urology, subfertile men may best achieve their reproductive goals with care delivered by high-volume fellowship-trained RUs.
Acknowledgments
The authors acknowledge the support of the AUA Office of Education, specifically Dr. Victor W. Nitti and Todd A. Carrick.
Footnotes
H.T. has nothing to disclose. E.Y.K. has nothing to disclose. E.S.S. has nothing to disclose. R.E.B. has nothing to disclose. J.P.A. has nothing to disclose. M.K.S. has nothing to disclose.
Supplementary data
References
- 1.Ko E.Y., Siddiqi K., Brannigan R.E., Sabanegh E.S. Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol. 2012;187:973–978. doi: 10.1016/j.juro.2011.10.137. [DOI] [PubMed] [Google Scholar]
- 2.Mulhall J.P., Trost L.W., Brannigan R.E., Kurtz E.G., Redmon J.B., Chiles K.A. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423–432. doi: 10.1016/j.juro.2018.03.115. [DOI] [PubMed] [Google Scholar]
- 3.Ross L.S. Selective estrogen receptor modulators, male hypogonadism, and infertility. Fertil Steril. 2014;102:687–688. doi: 10.1016/j.fertnstert.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Attia A.M., Abou-Setta A.M., Al-Inany H.G. Gonadotrophins for idiopathic male factor subfertility. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD005071.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santi D., Granata A.R., Simoni M. FSH treatment of male idiopathic infertility improves pregnancy rate: a meta-analysis. Endocr Connect. 2015;4:R46–R58. doi: 10.1530/EC-15-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krzastek S.C., Sharma D., Abdullah N., Sultan M., Machen G.L., Wenzel J.L. Long term safety and efficacy of clomiphene citrate for the treatment of hypogonadism. J Urol. 2019 doi: 10.1097/JU.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 7.Moskovic D.J., Katz D.J., Akhavan A., Park K., Mulhall J.P. Clomiphene citrate is safe and effective for long-term management of hypogonadism. BJU Int. 2012;110:1524–1528. doi: 10.1111/j.1464-410X.2012.10968.x. [DOI] [PubMed] [Google Scholar]
- 8.Chandrapal J.C., Nielson S., Patel D.P., Zhang C., Presson A.P., Brant W.O. Characterising the safety of clomiphene citrate in male patients through prostate-specific antigen, haematocrit, and testosterone levels. BJU Int. 2016;118:994–1000. doi: 10.1111/bju.13546. [DOI] [PubMed] [Google Scholar]
- 9.Sharma D., Zillioux J., Khourdaji I., Reines K., Wheeler K., Costabile R. Improvements in semen parameters in men treated with clomiphene citrate—a retrospective analysis. Andrologia. 2019;51:e13257. doi: 10.1111/and.13257. [DOI] [PubMed] [Google Scholar]
- 10.Patel D.P., Brant W.O., Myers J.B., Presson A.P., Johnstone E.B., Dorais J.A. The safety and efficacy of clomiphene citrate in hypoandrogenic and subfertile men. Int J Impot Res. 2015;27:221–224. doi: 10.1038/ijir.2015.21. [DOI] [PubMed] [Google Scholar]
- 11.Surbone A., Vaucher L., Primi M.P., Leyvraz C., Pitteloud N., Ballabeni P. Clomiphene citrate effect on testosterone level and semen parameters in 18 infertile men with low testosterone level and normal/low gonadotropines level. Eur J Obstet Gynecol Reprod Biol. 2019;238:104–109. doi: 10.1016/j.ejogrb.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Moradi M., Moradi A., Alemi M., Ahmadnia H., Abdi H., Ahmadi A. Safety and efficacy of clomiphene citrate and L-carnitine in idiopathic male infertility: a comparative study. Urol J. 2010;7:188–193. [PubMed] [Google Scholar]
- 13.Ghanem H., Shaeer O., El-Segini A. Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial. Fertil Steril. 2010;93:2232–2235. doi: 10.1016/j.fertnstert.2009.01.117. [DOI] [PubMed] [Google Scholar]
- 14.Helo S., Ellen J., Mechlin C., Feustel P., Grossman M., Ditkoff E. A randomized prospective double-blind comparison trial of clomiphene citrate and anastrozole in raising testosterone in hypogonadal infertile men. J Sex Med. 2015;12:1761–1769. doi: 10.1111/jsm.12944. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization A double-blind trial of clomiphene citrate for the treatment of idiopathic male infertility. Int J Androl. 1992;15:299–307. doi: 10.1111/j.1365-2605.1992.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 16.Shoshany O., Abhyankar N., Mufarreh N., Daniel G., Niederberger C. Outcomes of anastrozole in oligozoospermic hypoandrogenic subfertile men. Fertil Steril. 2017;107:589–594. doi: 10.1016/j.fertnstert.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro M.A., Gameiro L.F., Scarano W.R., Briton-Jones C., Kapoor A., Rosa M.B. Aromatase inhibitors in the treatment of oligozoospermic or azoospermic men: a systematic review of randomized controlled trials. JBRA Assist Reprod. 2016;20:82–88. doi: 10.5935/1518-0557.20160019. [DOI] [PubMed] [Google Scholar]
- 18.Alder N.J., Keihani S., Stoddard G.J., Myers J.B., Hotaling J.M. Combination therapy with clomiphene citrate and anastrozole is a safe and effective alternative for hypoandrogenic subfertile men. BJU Int. 2018;122:688–694. doi: 10.1111/bju.14390. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh T.C., Pastuszak A.W., Hwang K., Lipshultz L.I. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol. 2013;189:647–650. doi: 10.1016/j.juro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 20.Omar M.I., Pal R.P., Kelly B.D., Bruins H.M., Yuan Y., Diemer T. Benefits of empiric nutritional and medical therapy for semen parameters and pregnancy and live birth rates in couples with idiopathic infertility: a systematic review and meta-analysis. Eur Urol. 2019;75:615–625. doi: 10.1016/j.eururo.2018.12.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.