Abstract
Objective
To evaluate the quantity and use of embryos cryopreserved at assisted reproductive technology (ART) clinics in the United States from 2004 through 2013 and to characterize trends in ART cycles in which all embryos were cryopreserved.
Design
Retrospective analysis.
Setting
Not applicable.
Patient(s)
Registry data from the Society for Assisted Reproductive Technology.
Intervention(s)
Historical cohort of U.S. ART cycles reported to the Society for Assisted Reproductive Technology Clinical Outcomes Reporting System between 2004 and 2013.
Main Outcome Measure(s)
Number of embryos cryopreserved and factors associated with having cryopreserved embryos.
Result(s)
The percentage of fresh cycles in which all embryos were frozen increased dramatically each year after 2010: 15.6% (2010), 19.9% (2011), 30.7% (2012), and 40.7% (2013). During 10 years, 1,954,548 embryos were cryopreserved and 717,345 embryos were transferred. In freeze-only cycles from 2004 to 2013, there was a significant increase in the percentage of women with diminished ovarian reserve (19.9% to 34.1%) and in those who used preimplantation genetic testing (3.2% to 6.9%). During the 10-year period, there were 294,575 fresh cycles with embryo transfer and at least one embryo cryopreserved. Overall, 52.5% (n = 154,543) did not undergo a subsequent frozen embryo transfer, 29.5% (n = 40,462) were left with no frozen embryos, 50.4% (n = 68,875) had one–five embryos, and 20.0% (n = 27,396) had ≥six. Factors associated with having excess embryos included donor oocyte cycles and increased antimüllerian hormone levels.
Conclusion(s)
There has been a sharp increase in U.S. ART cycles in which all embryos are frozen and this may result in more embryos in storage.
Key Words: Embryo cryopreservation, embryo disposition, in vitro fertilization
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/users/16110-fertility-and-sterility/posts/xfre19-00001
With the evolution of techniques in human assisted reproductive technology (ART), many more fertilized eggs and early embryos are created than can be safely transferred to a woman’s uterus. To avoid the morbidity of multiple gestation, embryo cryopreservation has developed as a routine practice among U.S. ART clinics (1). Despite the clinical advantages that embryo cryopreservation provides, it also presents new ethical, legal, and healthcare policy challenges. As a result of the prevalent practice of embryo cryopreservation, in 2003 it was estimated that at least 400,000 cryopreserved embryos were in storage at that time in the United States (2). Couples primarily have five choices regarding disposition of supernumerary embryos: save the embryos for a future embryo transfer cycle, donate embryos for research, thaw and discard, donate their embryos to another intended parent, or continue to store embryos. Evidence suggests, however, that many embryos remain in storage with no specific plan for future use because the majority of patients delay the final disposition decision (3).
Previous research has demonstrated that the disposition of cryopreserved embryos in storage, once family-building has been completed, often presents a decision-making dilemma for patients (4). Qualitative studies demonstrate that patients feel a significant amount of emotional distress when deciding what to do with spare embryos (5). One nationwide survey of patients who underwent fertility treatment found that 40% of those who completed childbearing could not identify a preferred disposition option for excess embryos and nearly 20% indicated they were likely to delay the decision indefinitely (3). Additionally, another national survey of 1,005 patients with cryopreserved embryos found that 39% reported high decisional conflict regarding disposition of their spare embryos (6). Identifying patients at risk for having spare embryos could allow fertility centers to target those who would benefit from counseling regarding the implications of embryo cryopreservation and spare embryos. Interviews of women and/or couples with cryopreserved embryos demonstrated that counseling regarding cryopreservation options decreased emotional distress (7).
Until recently, fresh embryo transfer historically had been the norm during ART cycles. Cycles in which embryos are cryopreserved until a subsequent cycle, “freeze-only” or “freeze-all” cycles, have increased in use to reduce risk of ovarian hyperstimulation (OHSS) and for preimplantation genetic testing (PGT) cycles. Also, freeze-only cycles have been used to allow poor-responder patients to bank embryos (8). Additionally, there have been recent concerns that controlled OHSS could have adverse effects on the endometrium and decrease pregnancy rates in fresh embryo transfer cycles (8). Due to initial data that suggested improved pregnancy rates, placentation, and possibly even improved fetal outcomes with frozen embryo transfer (FET) compared with fresh embryo transfer, some U.S. clinics have promoted freezing all embryos in a cycle followed by subsequent FET for all cycles, regardless of patient characteristics (9). The impact of the evolution of freeze-only cycles on embryo cryopreservation storage patterns has not been fully elucidated.
Since publication of the article by Hoffman et al. (2) in 2003, there have been no additional studies evaluating the number of cryopreserved embryos in the United States or that have accumulated since that time. Therefore, a current estimate of the number of cryopreserved embryos in the United States is not available. In this study, we sought to investigate the current quantity and use of embryos cryopreserved at ART clinics in the United States from 2004 through 2013. We also aimed to characterize patient trends in fresh ART cycles in which all embryos were cryopreserved. Finally, we sought to estimate the number of patients with excess cryopreserved embryos after a fresh embryo transfer and to determine factors associated with having “leftover embryos” in storage after subsequent transfers.
Materials and methods
The data for this study were obtained from the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS), which contains comprehensive data from more than 90% of all clinics performing ART in the United States. Data were collected and verified by SART and reported to the Centers for Disease Control and Prevention. The SART maintains Health Insurance Portability and Accountability Act–compliant business associates agreements with reporting clinics. In 2004, after a contract change with the Centers for Disease Control and Prevention, SART gained access to the SART CORS data system for the purposes of conducting research, which is the earliest year for which we were able to evaluate cycles for this study. The study was reviewed and approved by the SART Research Committee before provision of data. The study was reviewed by the Johns Hopkins University institutional review board and was considered exempt.
This study included U.S. ART cycles reported to the SART CORS from January 1, 2004 through December 31, 2013. The study was limited to cycles in which an oocyte retrieval and embryo cryopreservation took place followed by any FET cycles that made use of those cryopreserved embryos. Frozen oocyte thaw cycles were excluded. FET cycles performed in 2014 or later using embryos created in our data set were not included in the study. Also, FET cycles performed in 2004–2013 using embryos created in 2003 or earlier were not included in the study. During the 10-year period, there were a total of 411,811 autologous fresh ART retrievals from women in whom at least one embryo was cryopreserved. Cycles were categorized by year and by whether they resulted in one of the following: embryo transfer and no pregnancy, embryo transfer and clinical pregnancy, embryo transfer and live birth, or cryopreservation of all embryos with no embryo transfer. Based on the date from each category per year, the total number of embryos during the 10-year period both cryopreserved and transferred were calculated.
To evaluate freeze-only cycles, during the 10-year period we evaluated a total of 79,360 autologous fresh ART retrievals from women in whom all embryos were cryopreserved. Cycles were categorized by year and analyzed based on age, body mass index (BMI), reason for ART, number of oocytes retrieved, number of embryos cryopreserved, whether PGT was performed, and geographic region. Cochran-Armitage trend test or Spearman correlation measure was performed to determine if there was an increasing or decreasing trend from the years of 2004–2013 depending on the variable data type.
Finally, we examined all cycles during the 10-year period in which oocyte retrieval and embryo transfer took place with at least one embryo cryopreserved. We used patient identification and ART cycle sequence to link the fresh cycle and subsequent FETs for each patient. Any fresh ART cycles after a subsequent fresh cycle were excluded; we only followed the first fresh cycle for each patient in which at least one embryo was cryopreserved. Patients were categorized by remaining embryos after FET as zero, one–five, or ≥six embryos and compared for age, BMI, antimüllerian hormone (AMH), and infertility diagnosis. Kruskal-Wallis and Chi-square tests were used for continuous and categorical variables, as appropriate. All analyses were performed with SAS, (version X4; SAS Institute). Statistical significance was defined as a two-sided P <.05.
Results
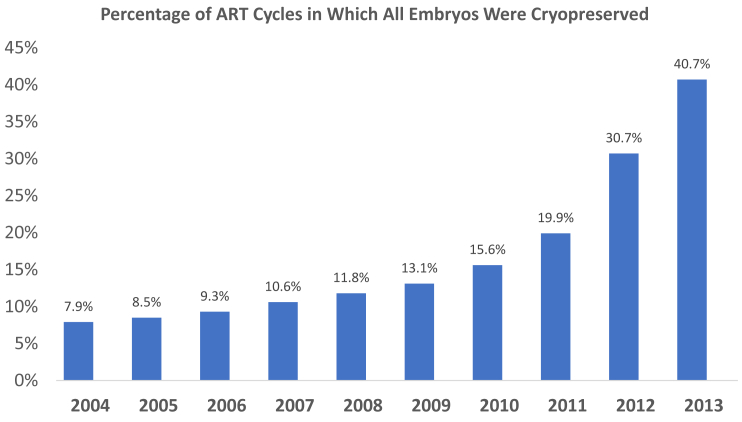
Of 411,811 fresh ART cycles during the 10-year period in which at least one embryo was cryopreserved, 31.5% resulted in no pregnancy, 48.6% resulted in a clinical intrauterine pregnancy, 41.5% resulted in a live birth, and 19.2% had all embryos cryopreserved (results are not cumulative but for the initial fresh cycle). The percentage of fresh cycles in which all embryos were frozen increased significantly each year after 2010 with the following percentages of freeze-only cycles: 15.6% (2010), 19.9% (2011), 30.7% (2012), and 40.7% (2013); the trend was significant with P<.0001 (Fig. 1). The mean number of embryos cryopreserved per cycle was 4.75. The number of embryos cryopreserved per year steadily increased from 158,383 in 2004 to 303,203 in 2013 (trend analysis significant; P<.0019). During the 10-year period, 1,954,548 embryos were cryopreserved and 717,345 embryos were subsequently thawed and transferred. In total, 1,237,203 embryos were cryopreserved and are potentially still in storage, although we cannot account for embryos that were discarded, donated, or did not survive the thaw.
Figure 1.
Trend in assisted reproductive technology (ART) cycles in which all embryos were cryopreserved. The percentage of fresh cycles in which all embryos were frozen increased significantly each year after 2010.
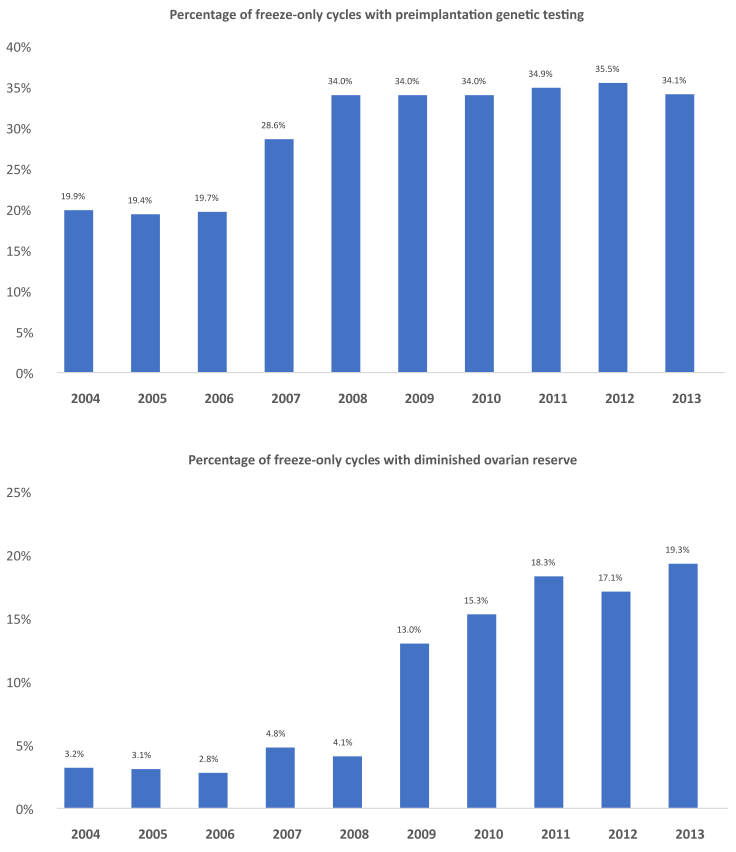
As shown in Table 1, among fresh ART cycles in which all embryos were cryopreserved, the mean patient age was 36.3 years old and the mean number of embryos cryopreserved per cycle was 6.3. As Figure 2 shows, in freeze-only cycles from 2004–2013, there was a significant increase in the percentage of women with diminished ovarian reserve (19.9% to 34.1%; P=.001) and in the percentage who used PGT (3.2%–6.9%; P=.017). During the 10-year period, the proportion of freeze-only cycles for women with polycystic ovary syndrome (PCOS) decreased significantly from 18.3% per year to 10.3% (P<.001), and the proportion of cycles for those with endometriosis decreased from 9.9% per year to 5.6% (P<.001). The mean number of oocytes retrieved among freeze-only cycles decreased from 17.8–14.0 (P=.002) and the mean number of embryos cryopreserved per cycle decreased from 8.9–6.0 (P<.001). There was no significant difference in patient age or BMI among freeze-only cycles. The proportion of freeze-only cycles among geographic regions did not change significantly during the 10-year period: Northeast (38.6%), West (31.7%), South (19.4%), and Midwest (10.3%).
Table 1.
Patient characteristics in freeze-only cycles from 2004–2013.
| Variable | 2004 | 2008 | 2013 |
|---|---|---|---|
| Total freeze-only cycles (n) | 2,325 | 4,442 | 25,346 |
| Mean age (y) | 35.2 | 36.2 | 36.5 |
| Mean BMI (kg/m2) | Not reported | 24.5 | 24.3 |
| Percent nulliparous, % (N) | 49.4 (1,162) | 46.4 (2,063) | 50.7 (12,844) |
| Percent donor egg cycles, % (N) | 12.2 (284) | 8.9 (395) | 6.2 (1,575) |
| Mean eggs retrieved | 18.9 | 13.6 | 14.3 |
| Mean embryos frozen (n) | 9.1 | 6.2 | 6.1 |
| Diagnosis, % (N) | |||
| Other reason for ART | 24.9 (578) | 27.2 (1,206) | 39.5 (10,011) |
| Diminished ovarian reserve | 19.9 (463) | 34.0 (1,509) | 34.1 (8,637) |
| Male infertility | 28.4 (660) | 24.8 (1,101) | 23.2 (5,871) |
| Preimplantation genetic testing | 3.2 (74) | 4.1 (182) | 19.3 (4,891) |
| Polycystic ovary syndrome | 18.3 (425) | 13.0 (579) | 10.3 (2,617) |
| Tubal factor | 15.7 (365) | 13.3 (591) | 7.3 (1,850) |
| Unexplained | 8.6 (200) | 6.1 (272) | 8.9 (2,245) |
| Endometriosis | 9.9 (231) | 7.3 (326) | 5.6 (1,424) |
| Uterine | 8.9 (206) | 6.2 (277) | 5.7 (1,436) |
| Other, noninfertile | 0.0 (0) | 0.7 (29) | 6.3 (1,590) |
Note: Data presented as % (N), unless noted otherwise. ART = assisted reproductive technology; BMI = body mass index.
Figure 2.
Percentage of freeze-only cycles with preimplantation genetic testing (PGT) and diminished ovarian reserve. In freeze-only cycles from 2004–2013, there was a significant increase in the percentage of women with diminished ovarian reserve and in the percentage who used PGT.
During the 10-year period, there were 294,575 fresh cycles with embryo transfer with at least one embryo cryopreserved and these were linked to subsequent cycles during the 10-year period. Table 2 demonstrates characteristics of these cycles and how many subsequent FET cycles that patients underwent during the 2004–2013 time period. Overall, 52.5% (n = 154,543) did not undergo a subsequent FET, 35.4% underwent one FET, 9.0% (n = 26,570) underwent two FETs, 2.3% (n = 6,707) underwent three FETs, and the remainder, 0.9% (n = 2,505), underwent four or more FETs. Among the cohort, 29.5% (n = 40,462) had no additional frozen embryos, 50.4% (n = 68,875) had one–five embryos, and 20.0% (n = 27,396) had ≥six. Patients with six or greater remaining frozen embryos had a significantly higher AMH level than those with no embryos or one–five embryos (4.0 vs. 2.5 and 3.0; P<.001) and were more likely to have used donor oocytes (25.2% vs. 13.7% and 17.2%; P<.001). There was a higher percentage of patients with PCOS among those with ≥six frozen embryos remaining (21.2% vs. 16.9% and 14.4%; P<.001).
Table 2.
Distribution of subsequent FET cycles after fresh embryo transfer cycles with at least one additional embryo frozen.
| A. Disposition of subsequent FET cycles | |
|---|---|
| FET | % (N) |
| 0 | 52.5 (154,543) |
| 1 | 35.4 (104,250) |
| 2 | 9.0 (26,570) |
| 3 | 2.3 (6,707) |
| ≥4 | 0.9 (2,505) |
| B. Remaining embryos for patients who froze embryos initially after 1 fresh cycle | |
|---|---|
| Embryos cryopreserved per patient, n | % (N) |
| 0 | 29.5 (40,462) |
| 1–5 | 50.4 (68,875) |
| ≥6 | 20.0 (27,396) |
| C. Characteristics more likely in patients with ≥6 remaining frozen embryos | ||||
|---|---|---|---|---|
| Characteristic | ≥6 Embryos | 1–5 Embryos | 0 Embryos | P value |
| Mean AMH level (pg/mL) | 4.0 | 3.0 | 2.5 | <.001 |
| Donor oocytes, % | 25.2 | 17.2 | 2.5 | <.001 |
| PCOS diagnosis, % | 21.2 | 16.9 | 13.7 | <.001 |
Note: AMH = antimüllerian hormone; FET = frozen embryo transfer; PCOS = polycystic ovary syndrome.
Discussion
The number of ART cycles has increased significantly since the birth of the first in vitro fertilization baby over 40 years ago, and with these advancements new issues have emerged. This is the first article in recent years to evaluate embryo cryopreservation trends in the United States. Our analysis demonstrates that there has been a sharp increase in the number of U.S. ART cycles in which all embryos are frozen, and this has resulted in more embryos in storage. Additionally, the upsurge in the number of patients with cryopreserved embryos results in a subsequent increase in disposition decisions and counseling that will be required by patients and clinics.
A notable finding in our analysis was the increase in freeze-only cycles. Underlying factors associated with freeze-only ART cycles have changed significantly during the past 10 years. Although an increased proportion of PGT among freeze-only cycles is not unexpected, the trend of an increased proportion of patients with diminished ovarian reserve among freeze-only cycles reflects a noteworthy change in practice pattern. Indeed, it is well known that in recent years freezing all embryos has been recommended in patients at risk for OHSS. However, fertility specialists are now often recommending freezing of all available good-quality embryos and then planning for delayed embryo transfer in a subsequent cycle, and the benefit of this is still in question (10). Our data demonstrate the trend to shift from fresh to FETs in many programs for reasons beyond OHSS prevention and PGT cycles. It should be noted that a higher percentage of freeze-only cycles were in the “other reasons for ART” category. High rates of “other reason for ART” are an expected finding for freeze-only cycles. Rather than undergoing ART for specific infertility issues, freeze-only cycles are often are for such things as cancer or other medical conditions requiring preservation of embryos. They also include those banking embryos for social reasons. Although today SART is more specific with fertility preservation diagnoses, in the years 2004–2013 specific information was not collected.
Multiple studies have compared fresh embryo transfer with FET, with inconsistent results. Two recent meta-analyses showed that freeze-only cycles are associated with a lower risk of low birthweight and preterm delivery compared with fresh embryo transfers, whereas FET may be associated with hypertensive disorders of pregnancy and large-for-gestational age neonates (11, 12). Another recent population-based database study also demonstrated that compared with fresh transfer, FET is associated with higher birth weight infants with increased risk of complications during the neonatal period (13). A Cochrane review and meta-analysis that combined four randomized controlled trials comparing fresh versus FETs found no significant difference in cumulative live birth rates (14). In another meta-analysis evaluating 2,728 cycles with fresh versus FET, a subanalysis of the number of eggs retrieved found that the freeze-only strategy was beneficial when 15 or more eggs were retrieved but not when lower numbers of oocytes were retrieved (15).
Two large randomized controlled trials in China compared fresh embryo transfers versus FETs in two populations, respectively, women with PCOS and ovulatory women (16, 17). Among women with PCOS, FET demonstrated a higher live birth rate and lower pregnancy loss rate compared with fresh embryo transfer (16). In contrast, in ovulatory women who had a more moderate response to controlled ovarian hyperstimulation, the live birth rate did not differ significantly between the frozen embryo group and the fresh embryo group (17). A recent SART analysis of more than 80,000 autologous first, fresh ART cycles reported that freeze-only with FET cycles have higher pregnancy rates than fresh transfers in high responders (with 15 or more oocytes retrieved) who are undergoing their first ART transfer. However, FET was not beneficial and indeed was associated with lower clinical pregnancy and live birth rates in intermediate and low responders (18).
In our 10-year analysis of linked fresh and frozen ART cycles, more than half of cryopreserved embryos were not subsequently transferred. Factors associated with ≥six embryos remaining after subsequent FET included increased AMH, PCOS diagnosis, and use of donor eggs. Given the large number of cryopreserved embryos potentially stored indefinitely, attention should be directed to creating a taskforce to address the issue of excess cryopreserved embryos after family-building goals have been achieved. As embryo cryopreservation continues and surplus embryos become more frequent, assisting patients in making embryo disposition decisions will become even more critical. Additionally, excess cryopreserved embryos provide fertility centers and embryology laboratories with administrative, financial, legal, and ethical dilemmas (3, 19, 20, 21).
Embryo cryopreservation may be initially reassuring to infertile patients as it represents the promise of family building (4). But once family building is completed, embryo disposition presents a major decision-making dilemma that can be difficult (6). In fact, many patients report planning to delay the decision as long as possible (22). It is estimated that approximately one third of patients will not return to provide medical directives for their embryos (23). For many couples, it is difficult to commit to a disposition decision so decisional regret is avoided by continuing to store embryos (24, 25). Counseling for patients with cryopreserved embryos thus far have been overlooked at most centers (26).
Factors that influence embryo disposition decisions fall into four categories: personal beliefs and values, life circumstances, embryo quality, and level of clinical support and information (24). The factor that has been identified as playing the largest role in determining the ultimate embryo disposition decision is the way people conceptualize the embryo (4, 5, 27, 28, 29). One study showed that nearly 90% of patients with cryopreserved embryos who had children perceived the embryos to be siblings of their existing children (22). One survey of 1,020 patients with cryopreserved embryos at nine centers in the United States reported that the perceived moral status of the embryo correlated with options for disposition with “future child” more likely to be used for future pregnancy attempts whereas “biologic material” was more likely to be chosen for donation to research or discarding (3). Additionally, couples frequently change their minds regarding embryo disposition from the option first chosen (19, 30, 31). Although some studies report patients’ decisions to donate excess embryos to research are increasing (32, 33, 34), other studies report that most couples will decide ultimately to discard their embryos (20, 23, 35, 36, 37).
Our study demonstrates that many couples are cryopreserving embryos and will need to make an embryo disposition decision. This establishes the need for fertility centers to counsel patients upfront regarding the potential need to make a disposition decision prior to starting ART treatment (5, 27). One cross-sectional study surveyed patients with embryos cryopreserved at a fertility center for more than 2 years. They found that while the majority of patients reported that prior to treatment the information provided by clinics was adequate, they also reported that after treatment the education received was not adequate to assist them in making embryo disposition decisions (38). One multicenter, U.S.-based study interviewed families with embryos cryopreserved at least 6 years. The majority of families with cryopreserved embryos perceived their fertility center team as the primary source of information and felt that clinics were under an obligation to help them with embryo disposition decisions (7). A survey of patients who underwent fertility treatment found that 40% of patients who had completed childbearing could not identify a preferred disposition option for their excess embryos; one in five of those individuals indicated they were likely to put off the decision indefinitely (3). Additionally, research has demonstrated that patients’ intentions regarding spare embryos change after ART treatment and also can be predicted by ART cycle outcomes (23, 31, 39).
A notable strength of our study is that this is the largest study to date examining trends in embryo cryopreservation among U.S. ART cycles. Our study limitations include its retrospective nature with a lag in data reporting, therefore, only the available parameters could be included. Additionally, this was a very heterogeneous cohort of cycles, with overall results including both cleavage-stage and blastocyst embryos. Another limitation is that for the time frame of this study, from 2004–2013, it was not possible to identify the percentage of cycles that were done for fertility preservation indications only. Additionally, due to the lag in reporting cycles that is associated with the SART CORS data, we cannot account for embryos created and cryopreserved during that study period that may have been transferred in 2014 or later. We also cannot account for “compassionate embryo transfers,” FETs performed during a suboptimal time during the cycle as a disposition alternative to discarding embryos. However, the largest limitation of this study is that SART CORS cannot account for embryos that were discarded, donated to research, or donated to other couples. For instance, it was not possible to know from the SART data available whether embryos that underwent preimplantation genetic testing were acceptable to be transferred, both by aneuploid status and/or if affected for a genetic mutation or structural abnormality. These affected or abnormal embryos presumably would not be transferred in most cases.
Conclusions
In conclusion, we found that embryo cryopreservation has increased sharply in the United States in a 10-year period from 2003–2013. There has been a dramatic increase in the United States in the number of cycles in which all embryos are frozen. Both the increase in embryo cryopreservation with fresh cycles in which fresh embryo transfer takes place as well as the increase in freeze-only cycles likely are resulting in more embryos in storage and a subsequent increase in disposition decisions required by patients and clinics. In our 10-year analysis of linked fresh and frozen ART cycles, more than half of cryopreserved embryos were not subsequently transferred, further demonstrating the importance of counseling patients regarding accumulation of excess embryos after family-building goals are made. A taskforce is needed to address the issue of excess embryo accumulation.
Acknowledgments
We thank all SART members for providing clinical information to the SART CORS database for use by patients and researchers. Without the efforts of SART members, this research would not have been possible.
Footnotes
M.S.C. has nothing to disclose. J.E.S. has nothing to disclose. F.S. has nothing to disclose. H.Z. has reported grants from the National Institutes of Health during the conduct of the study. A.K.S. has nothing to disclose. W.V. has nothing to disclose. A.J.P. has nothing to disclose.
Supported by the Clinical Research Scientist Training Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development (R25 HD 075737).
References
- 1.Cedars M.I. Embryo cryopreservation. Semin Reprod Endocrinol. 1998;16:183–195. doi: 10.1055/s-2007-1016277. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman D.I., Zellman G.L., Fair C.C., Mayer J.F., Zeitz J.G., Gibbons W.E. Cryopreserved embryos in the United States and their availability for research. Fertil Steril. 2003;79:1063–1069. doi: 10.1016/s0015-0282(03)00172-9. [DOI] [PubMed] [Google Scholar]
- 3.Lyerly A.D., Steinhauser K., Voils C., Namey E., Alexander C., Bankowski B. Fertility patients' views about frozen embryo disposition: results of a multi-institutional U.S. survey. Fertil Steril. 2010;93:499–509. doi: 10.1016/j.fertnstert.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachtigall R.D., Becker G., Friese C., Butler A., MacDougall K. Parents' conceptualization of their frozen embryos complicates the disposition decision. Fertil Steril. 2005;84:431–434. doi: 10.1016/j.fertnstert.2005.01.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyerly A.D., Steinhauser K., Namey E., Tulsky J.A., Cook-Deegan R., Sugarman J. Factors that affect infertility patients' decisions about disposition of frozen embryos. Fertil Steril. 2006;85:1623–1630. doi: 10.1016/j.fertnstert.2005.11.056. [DOI] [PubMed] [Google Scholar]
- 6.Lyerly A.D., Nakagawa S., Kuppermann M. Decisional conflict and the disposition of frozen embryos: implications for informed consent. Hum Reprod. 2011;26:646–654. doi: 10.1093/humrep/deq368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nachtigall R.D., Mac Dougall K., Lee M., Harrington J., Becker G. What do patients want? Expectations and perceptions of IVF clinic information and support regarding frozen embryo disposition. Fertil Steril. 2010;94:2069–2072. doi: 10.1016/j.fertnstert.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doody K.J. Cryopreservation and delayed embryo transfer-assisted reproductive technology registry and reporting implications. Fertil Steril. 2014;102:27–31. doi: 10.1016/j.fertnstert.2014.04.048. [DOI] [PubMed] [Google Scholar]
- 9.Cedars M.I. Fresh versus frozen: initial transfer or cumulative cycle results: how do we interpret results and design studies? Fertil Steril. 2016;106:251–256. doi: 10.1016/j.fertnstert.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Coutifaris C. Freeze-only in vitro fertilization cycles for all? Fertil Steril. 2017;108:233–234. doi: 10.1016/j.fertnstert.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Sha T., Yin X., Cheng W., Massey I.Y. Pregnancy-related complications and perinatal outcomes resulting from transfer of cryopreserved versus fresh embryos in vitro fertilization: a meta-analysis. Fertil Steril. 2018;109:330–342.e9. doi: 10.1016/j.fertnstert.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Maheshwari A., Pandey S., Amalraj Raja E., Shetty A., Hamilton M., Bhattacharya S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update. 2018;24:35–58. doi: 10.1093/humupd/dmx031. [DOI] [PubMed] [Google Scholar]
- 13.Hwang S.S., Dukhovny D., Gopal D., Cabral H., Diop H., Coddington C.C. Health outcomes for Massachusetts infants after fresh versus frozen embryo transfer. Fertil Steril. 2019;112:900–907. doi: 10.1016/j.fertnstert.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong K.M., van Wely M., Mol F., Repping S., Mastenbroek S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev. 2017;3 doi: 10.1002/14651858.CD011184.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieamant F.C., Petersen C.G., Mauri A.L., Comar V., Mattila M., Vagnini L.D. Fresh embryos versus freeze-all embryos - transfer strategies: nuances of a meta-analysis. JBRA Assist Reprod. 2017;21:260–272. doi: 10.5935/1518-0557.20170048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z.J., Shi Y., Sun Y., Zhang B., Liang X., Cao Y. Fresh versus frozen embryos for Infertility in the polycystic ovary syndrome. N Engl J Med. 2016;375:523–533. doi: 10.1056/NEJMoa1513873. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y., Sun Y., Hao C., Zhang H., Wei D., Zhang Y. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. 2018;378:126–136. doi: 10.1056/NEJMoa1705334. [DOI] [PubMed] [Google Scholar]
- 18.Acharya K.S., Acharya C.R., Bishop K., Harris B., Raburn D., Muasher S.J. Freezing of all embryos in in vitro fertilization is beneficial in high responders, but not intermediate and low responders: an analysis of 82,935 cycles from the Society for Assisted Reproductive Technology registry. Fertil Steril. 2018;110:880–887. doi: 10.1016/j.fertnstert.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Bankowski B.J., Lyerly A.D., Faden R.R., Wallach E.E. The social implications of embryo cryopreservation. Fertil Steril. 2005;84:823–832. doi: 10.1016/j.fertnstert.2005.02.057. [DOI] [PubMed] [Google Scholar]
- 20.Klock S.C., Sheinin S., Kazer R.R. The disposition of unused frozen embryos. N Engl J Med. 2001;345:69–70. doi: 10.1056/NEJM200107053450118. [DOI] [PubMed] [Google Scholar]
- 21.Goedeke S., Daniels K., Thorpe M., du Preez E. The fate of unused embryos: discourses, action possibilities, and subject positions. Qual Health Res. 2017;27:1529–1540. doi: 10.1177/1049732316686759. [DOI] [PubMed] [Google Scholar]
- 22.Stiel M., McMahon C.A., Elwyn G., Boivin J. Pre-birth characteristics and 5-year follow-up of women with cryopreserved embryos after successful in vitro fertilisation treatment. J Psychosom Obstet Gynaecol. 2010;31:32–39. doi: 10.3109/01674820903537081. [DOI] [PubMed] [Google Scholar]
- 23.Newton C.R., Fisher J., Feyles V., Tekpetey F., Hughes L., Isacsson D. Changes in patient preferences in the disposal of cryopreserved embryos. Hum Reprod. 2007;22:3124–3128. doi: 10.1093/humrep/dem287. [DOI] [PubMed] [Google Scholar]
- 24.Nachtigall R.D., Mac Dougall K., Harrington J., Duff J., Lee M., Becker G. How couples who have undergone in vitro fertilization decide what to do with surplus frozen embryos. Fertil Steril. 2009;92:2094–2096. doi: 10.1016/j.fertnstert.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Provoost V., Pennings G., De Sutter P., Gerris J., Van de Velde A., Dhont M. Patients' conceptualization of cryopreserved embryos used in their fertility treatment. Hum Reprod. 2010;25:705–713. doi: 10.1093/humrep/dep387. [DOI] [PubMed] [Google Scholar]
- 26.Machin L. A hierarchy of needs? Embryo donation, in vitro fertilisation and the provision of infertility counselling. Patient Educ Couns. 2011;85:264–268. doi: 10.1016/j.pec.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Lacey S. Parent identity and 'virtual' children: why patients discard rather than donate unused embryos. Hum Reprod. 2005;20:1661–1669. doi: 10.1093/humrep/deh831. [DOI] [PubMed] [Google Scholar]
- 28.de Lacey S. Decisions for the fate of frozen embryos: fresh insights into patients' thinking and their rationales for donating or discarding embryos. Hum Reprod. 2007;22:1751–1758. doi: 10.1093/humrep/dem056. [DOI] [PubMed] [Google Scholar]
- 29.Provoost V., Pennings G., De Sutter P., Dhont M. Decisions on embryo disposition in cross-border reproductive care: differences between Belgian and Dutch patients at a Belgian fertility center. Facts Views Vis Obgyn. 2011;3:293–301. [PMC free article] [PubMed] [Google Scholar]
- 30.Blyth E. Guidelines for infertility counselling in different countries: is there an emerging trend? Hum Reprod. 2012;27:2046–2057. doi: 10.1093/humrep/des112. [DOI] [PubMed] [Google Scholar]
- 31.Lornage J., Chorier H., Boulieu D., Mathieu C., Czyba J.C. Six year follow-up of cryopreserved human embryos. Hum Reprod. 1995;10:2610–2616. doi: 10.1093/oxfordjournals.humrep.a135755. [DOI] [PubMed] [Google Scholar]
- 32.Lanzendorf S., Ratts V., Keller S., Odem R. Disposition of cryopreserved embryos by infertility patients desiring to discontinue storage. Fertil Steril. 2010;93:486–489. doi: 10.1016/j.fertnstert.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Hammarberg K., Tinney L. Deciding the fate of supernumerary frozen embryos: a survey of couples' decisions and the factors influencing their choice. Fertil Steril. 2006;86:86–91. doi: 10.1016/j.fertnstert.2005.11.071. [DOI] [PubMed] [Google Scholar]
- 34.Provoost V., Pennings G., De Sutter P., Van de Velde A., Dhont M. Trends in embryo disposition decisions: patients' responses to a 15-year mailing program. Hum Reprod. 2012;27:506–514. doi: 10.1093/humrep/der419. [DOI] [PubMed] [Google Scholar]
- 35.Cattoli M., Borini A., Bonu M.A. Fate of stored embryos: our 10 years experience. Eur J Obstet Gynecol Reprod Biol. 2004;115:S16–S18. doi: 10.1016/j.ejogrb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Hill G.A., Freeman M.R. Embryo disposition: choices made by patients and donor oocyte recipients. Fertil Steril. 2011;95:940–943. doi: 10.1016/j.fertnstert.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Jin X., Wang G., Liu S., Liu M., Zhang J., Shi Y. Patients' attitudes towards the surplus frozen embryos in China. Biomed Res Int. 2013;2013 doi: 10.1155/2013/934567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deniz S.G., Hughes E.G., Neal M.S., Faghih M., Amin S., Karnis M.F. Are health care providers adequately educating couples for embryo disposition decisions? Fertil Steril. 2016;105:684–689. doi: 10.1016/j.fertnstert.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 39.Hounshell C.V., Chetkowski R.J. Donation of frozen embryos after in vitro fertilization is uncommon. Fertil Steril. 1996;66:837–838. [PubMed] [Google Scholar]