Abstract
Aim
Extracorporeal cardiopulmonary resuscitation (ECPR) can treat cardiac arrest refractory to conventional therapies. Our goal was to identify the best protocol for survival with good neurological outcome through the evaluation of current inclusion criteria, exclusion criteria, cannulation strategies and additional therapeutic measures.
Methods
A systematic literature search was used to identify eligible publications from PubMed, Embase, Web of Science and Cochrane for articles published from 29 June 2009 until 29 June 2019.
Results
The selection process led to a total of 24 eligible articles, considering 1723 patients in total. A good neurological outcome at hospital discharge was found in 21.3% of all patients. The most consistent criterion for inclusion was refractory cardiac arrest (RCA), used in 21/25 (84%) of the protocols. The preferred cannulation method was the percutaneous Seldinger technique (44%).
Conclusion
ECPR is a feasible option for cardiac arrest and should already be considered in an early stage of CPR. One of the key findings is that time-to-ECPR seems to be correlated with good neurological survival. An important contributing factor is the definition of RCA. Protocols defining RCA as >10 min had a mean good neurological survival of 26.7%. Protocols with a higher cut-off, between 15 and 30 min, had a mean good neurological survival of 14.5%. Another factor contributing to the time-to-ECPR is the preferred access technique. A percutaneous Seldinger technique combined with ultrasonography and fluoroscopic guidance leads to a reduced cannulation time and complication rate. Conclusive research around prehospital cannulation still needs to be conducted.
Keywords: Extracorporeal cardiopulmonary resuscitation, Cardiac arrest, Guidelines, Cannulation technique
Introduction
Survival rates for in-hospital and especially out-of-hospital cardiac arrest (OHCA) remain low.1,2 The optimization of the chain-of-survival is vital in improving the survival rates of OHCA. Early CPR could double or quadruple survival rates and early defibrillation in shockable rhythms can result in survival rates up to 40%.3,4 Despite these efforts, the survival of OHCA remains quite poor. This has sparked interest in the development of new methods and techniques to improve the survival of patients with OHCA. One of these relatively new techniques to resolve prolonged CPR is extracorporeal cardiopulmonary resuscitation (ECPR).5,6 By maintaining vital organ perfusion, it gives more essential time for the emergency physician to find the underlying cause of the cardiac arrest. As such, ECPR could be a very useful tool in the emergency setting. (see Fig. 1)
Fig. 1.
Interrater reliability
As the interest in ECPR grows, different centres across multiple countries have made their own protocols for the use of ECPR, a highly specialized and resource-intensive technique.7 The protocols differ in their inclusion criteria as well as in the techniques used by the healthcare professional to obtain veno-arterial access. Inclusion and exclusion criteria which are most commonly used are: age, comorbidity, witnessed arrest with bystander CPR and time to refractory cardiac arrest.
The ELSO-ECPR guidelines of 2013 recommend central or peripheral cannulation at the discretion of the surgical team, and a percutaneous approach should only be used if previous access to the vessels exist.8 However, a percutaneous approach with ultrasound guidance has been reported to improve time-to-cannulation and could be beneficial to survival.9
These protocols are necessary to limit the use of ECPR to patients who might benefit the most from this technique. In limited populations, observational studies have shown an association with improved survival using ECPR compared to conventional CPR.10 Furthermore, ECPR for refractory cardiac arrest has been shown to be cost-effective.11 This adds a positive financial aspect to ECPR and may prompt further interest in the development and implementation of this technique. Yet while there are guidelines for ECPR suggesting limiting the use of this technique to selected patients with cardiac arrest of potentially reversible aetiology, there is no unified protocol concerning the selection of patients.12 This has caused heterogeneity in articles concerning this topic due to the difference in eligibility, making a combined analysis impossible.13, 14, 15
The aim of this systematic review is to compare the ECPR protocols used by different centres for adults with cardiac arrest and search for protocols with the best neurological outcome. This review thus offers the most recent evidence concerning ECPR protocols and their inclusion/exclusion criteria. This analysis could then be used to aid the formulation of a unified protocol mainly considering the inclusion/exclusion criteria, approach and additional therapies.
Methods
Protocol and registration
The methodology and report of the present systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.16 Before starting selection of the articles a request to register the protocol of this review was sent to the International Prospective Register of Systematic Reviews (PROSPERO). The registration number is CRD42020139103. No ethical approval and patient consent were required, as all analyses were based on previous published studies.
Study design and database search
A systematic search was performed using PubMed, Embase, Web of Science and Cochrane Library to identify all eligible studies. An experienced research librarian was consulted for the development and execution of the search strategy. The PICO format was used to develop a well-formulated research question; P: Heart Arrest (and synonyms), I: ECPR (and synonyms), O: primary outcome cerebral performance, there was no comparative therapy.
All four databases were searched electronically from 29 June 2009 until 29 June 2019. References of all relevant articles were searched for additional studies. No language restrictions were applied. Unpublished studies were not included. The search strategy combined Medical Search Headings and Subheadings (MeSH) terms (in PubMed) with Boolean operators ‘AND’ and ‘OR’ to capture all relevant article suggestions. Appendix I shows the search terms.
Studies lacking empirical data, and abstracts without full-text articles were excluded. Paediatric, animal, case studies and systematic reviews were also excluded.
Study selection and data extraction
Two independent reviewers (KTJ, NT) screened all titles and abstracts for inclusion using predetermined criteria. Eligibility criteria used in the selection procedure were: 1/ECMO use in case of cardiac arrest, 2/the use of a clear ECPR protocol without physician-based decision and 3/a patient follow up with the inclusion of the neurological outcome.
The RAYYAN platform, owned by the Qatar Computing Research Institute, was used as a validated screening tool. Afterwards, the full texts of potentially eligible studies were independently screened by the same reviewers for final inclusion. Disagreements between the reviewers were resolved by consensus.
The two reviewers independently extracted the following data from the included studies: year of publication, characteristics and number of participants, study design, initial shockable rhythm, cannulation procedure, additional therapies and outcome. The data was entered in a pre-developed and piloted data collection form. Only explicitly reported data were abstracted; no data was inputted.
Outcome measures
The primary outcome was the neurological status, measured with the CPC, mRS, GOS or GOSE score. There was no chronological cut-off because of a lack of homogeneity.
Risk of bias
Two independent reviewers (KTJ, NT) performed a risk of bias assessment of the included studies using the Medical Education Research Study Quality Instrument (MERSQI).17,18 This instrument allows assessing of the methodological rigour of articles, includes a comprehensive list of review items, and has a growing body of validity evidence.19 The included studies were assessed for study design, the number of sampling institutions, type of data, validity evidence for evaluation instrument scores, sophistication and appropriateness of data analysis, and outcome. The response rate was not applicable to our selected studies. Each domain was assessed as low, unclear, or high risk of bias. All discrepancies were resolved by consensus.
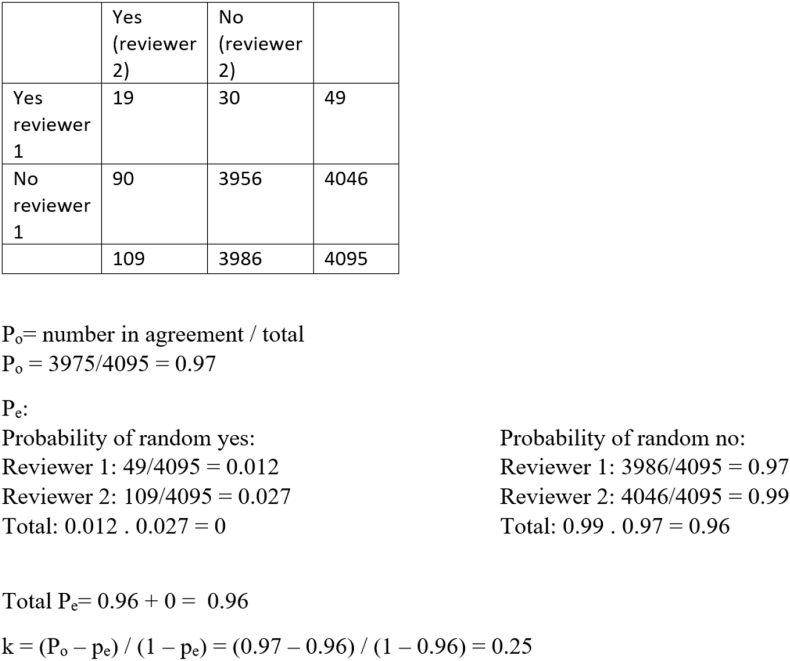
The interrater reliability between the reviewers was calculated using the Cohen’s Kappa Statistic.
Data synthesis and statistical analysis
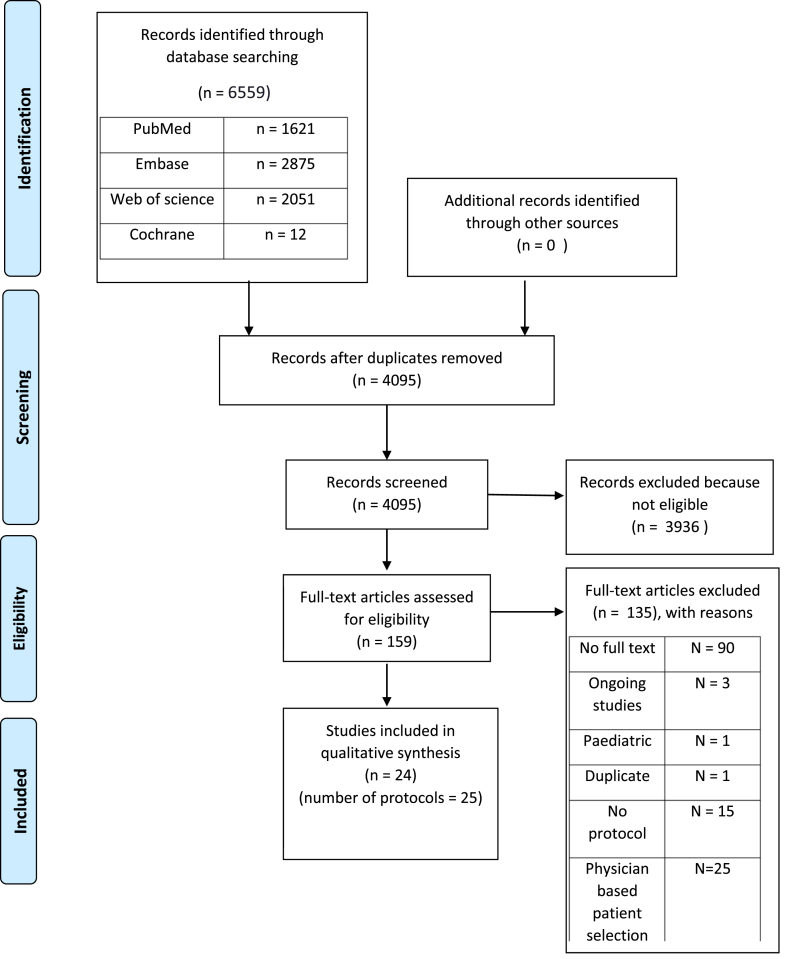
The inclusion of all articles was summarized in the PRISMA flow chart (Table 1).The used data analysis was descriptive, considering a meta-analysis and any meaningful statistical analysis are impossible because of heterogeneity. This heterogeneity is due to differences in sample size, inclusion criteria, definition of refractory cardiac arrest, interventions and post-resuscitation care. There is also heterogeneity in the time of outcome measurement.
Table 1.
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. https://doi.org/10.1371/journal.pmed1000097. For more information, visitwww.prisma-statement.org.
Results
Study selection
The total amount of hits was 6559 articles. After removing duplicates with endnote, 4797 hits remained. An additional 702 duplicates were removed by RAYYAN leaving 4095 articles for selection. Both programs were incomplete in removing all the duplicates as we encountered more duplicates later in our search. These were treated as unique articles. One duplicate was removed in our final selection, leaving us with 24 selected articles and 25 protocols as 1 article contained 2 protocols.20 Conflicts in selection were resolved by discussion between the two review authors. Details of our selection process can be found in Table 1.Data extraction was done with an extraction table (Table 4), ranked by MERSQI score from the highest to lowest.
Table 4.
Overview of the used inclusion and exclusion criteria.
| Inclusion | Exclusion |
|---|---|
|
Can be used both as inclusion or exclusion criterion.
Study characteristics
Our selected studies consisted mainly of retrospective studies, 18 in total (75%). There was one pre-post-test study21 (4,2%) and five prospective studies (20,8%).20,22, 23, 24, 25
There were no randomized controlled trials (RCT) available in our selection, although we found four RCT’s that are still ongoing (ClinicalTrails.gov Identifier: NCT03101787, NCT03065647, NCT01511666, NCT02527031).
Risk of bias
The mean MERSQI score of our selected articles was 13.6, meaning a low to moderate risk of bias. Individual MERSQI assessments and scores are described in appendix II. The response rate scoring was not applicable to our selected articles.
The interrater reliability was calculated to be 0.25, meaning there is a fair agreement between the two reviewers. Considering our Po was 0.97 and the largest part of the articles were excluded, the value of 0.25 might give a skewed vision. It is debatable if the large amount of rejected articles (‘no’) was due to randomised events or due to good inclusion criteria.
Patient characteristics
The total amount of included patients is 1723. Of this total amount, 812 cases had an IHCA and 843 cases had an OHCA, 68 cases were unspecified. The aetiology of cardiac arrests can be subdivided into different categories (Table 2). Acute coronary syndrome was the most prevalent aetiology, representing 41.15% of all cases. Unfortunately, there was a high number of unspecified aetiologies in 19% of all cases. (see Table 3)
Table 2.
Data extraction table.
| Author | Year | Study design | # ECPR patients | OHCA/IHCA | Inclusion criteria | Exclusion criteria | Aetiology | Initial shockable rhythm | How and where cannulation | Additional therapy | Outcome: |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sakamoto et al22 | 2014 | Prospective trial | 260 | OHCA |
|
|
ACS (165), Arrhythmia (42) Myocarditis (2), Myopathy (17), Unknown (27), NA (7) |
260 |
How:percutaneous/surgical Where:femoral Location:NR |
TH, PCI, IABP | 32/260 (12.3%) At 30 days |
| Blumenstein et al39 | 2016 | Retrospective analysis | 52 | IHCA |
|
|
AMI (15), CAD (non-AMI) (8), Valvular heart disease (16) Dilated cardiomyopathy (6),PE (3), Heart Tx waiting list (1), Other (3 |
52 |
How:Percutaneous or surgical cut-down Where:femoral Location:In ICU, CathLab, OR, Other |
CAG±PCI, TH, CABG, pulmonary endarterectomy |
11/52 (21.2%) 30d survival |
| Hashiba et al43 | 2012 | Retrospective analysis | 28 | OHCA |
|
|
PE: 12 AMI: 16 |
PE: 0, AMI: 8 |
How:percutaneous with Seldinger Where:femoral Location:NR |
Thrombolysis, catheter embolectomy, PCI |
PE: 7/12 (58.3%) AMI: 1/16 (6.3%) |
| Kim S.J. et al33 | 2014 | Retrospective analysis | 55 | OHCA |
|
|
Cardiac (49) Non-cardiac (6) |
31 |
How:percutaneous with Seldinger Where:femoral Location:ED, CathLab |
TH, CAG, PCI | 08/55 (14.5%) |
| Kim Y.S. et al40 | 2018 | Retrospective analysis | 101 | IHCA (79) OHCA (22) |
|
|
Cardiac (84) Non cardiac (17) |
45 |
How: percutaneous with Seldinger, surgical cannulation if failure Where: femoral Location: NR |
TH, PCI | Non-TH: 26/76 (34.2%) TH: 8/25 (32%) |
| Kuroki et al36 | 2017 | Retrospective analysis | 119 | OHCA (37) IHCA (82) |
|
|
ACS | NR |
How: Seldinger technique or cut down technique Where: Femoral Location: NR |
PCI, TH | 38/119 (32%) At 30 days |
| Mandigers et al27 | 2019 | Retrospective analysis | 19 | OHCA (13) IHCA (6) |
|
|
PE | NR |
How: NR Where: femoral Location: ICU, CathLab, OR |
Thrombolysis | 4/19 (21%) |
| Pozzi et ala37 | 2016 | Retrospective analysis | 68 | OHCA IHCA |
|
|
ACS (27), Aortic dissection (5), PE (4) Pre-existing cardiomyopathy (4) Various (9), Unknown (19) |
19 |
How:surgical with modified Seldinger Where:femoral Location:OR |
CAG±PCI, mCPR, TH |
3/68 (4.4%) |
| Pozzi et ala34 | 2019 | Retrospective analysis | 131 | OHCA (86) IHCA (45) |
|
|
ACS (38), cardiomyopathy (18) PE (5), Intoxication (4), Aortic dissection (6), post cardiotomy (9), Various (15), Unknown (36) |
38 |
How:surgical with modified Seldinger Where:femoral Location:OR |
CAG±PCI, mCPR, TH |
IHCA 3/45 (6.7%) OHCA 5/86 (5.8%) |
| Grunau et al21 | 2017 | Before/After cohort | 4 (pre-protocol) 9 (post-protocol) |
OHCA |
|
|
Pre: Hypothermia (2), ACS (1), unknown (1) Post: Hypothermia (2), ACS (3), unknown (1), Aortic dissection (2), electrolyte (1) |
Pre: 3 Post: 5 |
How:percutaneous Where:femoral Location:ED |
PCI, CABG, mCPR, TH |
Pre: 1/4 (25%) Post: 2/7 (29%) |
| Lamhaut et al20 | 2017 | Observational prospective study |
114 | OHCA |
|
|
NR | 56 |
How:Surgical with Seldinger Where:femoral Location:pre-hospital and in-hospital |
PCI, TH, mCPR | 9/114 (7.9%) At ICU discharge/28 days |
| Observational prospective study |
42 | OHCA |
|
|
NR | 25 |
How:Surgical with Seldinger Where:femoral Location:pre-hospital and in-hospital |
PCI, TH, mCPR | 12/42 (28.6%) At ICU discharge/28 days |
||
| Bednarczyk et al28 | 2014 | Retrospective analysis | 22 | IHCA |
|
|
ACS (17), Valvular (2), Arrhythmia (2), constrictive pericarditis (1) |
15 |
How:Percutaneous Where:femoral Location:NR |
TH, PCI, CABG | 10/22 (45%) 30d survival |
| Fagnoul et al23 | 2013 | Prospective analysis | 24 | IHCA (10) OHCA (14) |
|
|
ACS (7), Arrhythmia (5), PE (3), Trauma (2) Intoxication (2), Hypothermia (3), Other (2) |
10 |
How:Surgical Where:femoral Location:shock-lab |
TH, mCPR, PCI | 6/24 (25%) At ICU discharge |
| Haneya et al41 | 2012 | Retrospective analysis | 85 | OHCA (26) IHCA (59) |
|
|
Cardiac: 54 Non-cardiac: 31 |
25 |
How:percutaneous with Seldinger Where:femoral Location:ED, ICU, OR, Cathlab, Ward |
PCI, cardio-surgery, TH, embolectomy |
27/85 (31.8%) |
| Komeyama et al44 | 2019 | Retrospective analysis | 67 | OHCA (16) IHCA (51) |
|
|
ACS (57), VF (non-ACS) (10) | 40 |
How:percutaneous with Seldinger Where:femoral Location:NR |
PCI | 20/67 (29.8%) |
| Le Guen et al24 | 2011 | Observational prospective study | 51 | OHCA |
|
|
Cardiac (44), trauma (3), drug overdose (2), respiratory (1), electrocution (1) |
32 (63%) |
How:Surgical cannulation Where:femoral Location:ICU |
TH, mCPR, PCI | GOS 4–5: 2/51 (4%) At 28 days |
| Pang et al31 | 2017 | Retrospective analysis | 79 | OHCA (6) IHCA (73) |
|
|
AMI (62), myocarditis (7), cardiomyopathy (6) PE (2), Tamponade (2) |
33 (41.8%) |
How:Percutaneous with Seldinger Where:femoral Location:NR |
TH | Total: 16/79 (20.3%) TTM: 6/14 (42.9%) No TTM: 10/65 (15.4%) |
| Peigh et al32 | 2015 | Retrospective analysis | 23 | IHCA |
|
|
AMI (9), tachyarrhythmia (5), myocarditis (2), PE (2), hypothermia (2), acute rejection (1), drug-induced CA (1), post cardiotomy failure (1) |
8 |
How:percutaneous/surgical Where:femoral/centrally Location:ICU, Cathlab, ED, OR |
TH | 07/23 (30.4%) |
| Wang et al38 | 2014 | Prospective observational analysis |
230 | OHCA (31) IHCA (199) |
|
|
Cardiomyopathy (31), ACS (104), Hypovolemia (11), myocarditis (12), PE (6), Drug/electrolyte effect (8), Post-HTx (18), Others (37), Electric shock/CO poisoning (3) |
IHCA (91) OHCA (15) |
How:NR Where:NR Location:ER, ICU, OR, Ward, Cathlab |
TH, PCI, CABG Etiological approach |
IHCA 50/199 (25.1%) OHCA 8/31 (25.8%) |
| Bellezo et al35 | 2012 | Retrospective analysis | 18 | OHCA |
|
|
Coronary artery disease (11), cardiomyopathy (1), iatrogenic ventricular laceration (1), Tracheal obstruction (1), Drug overdose (1), Aortic dissection (2), Hypothermia/VF arrest (1) |
NR |
How:Percutaneous Where:femoral Location:ED |
TH, PCI | 5/18 (28%) |
| Jo et al45 | 2011 | Retrospective analysis | 83 | IHCA |
|
|
ACS (40), aggravation HF (16), myocarditis (2) PE (4), arrhythmia (1), circulatory obstruction (5) Unknown (15) |
39 |
How:percutaneous with Seldinger, surgical if difficult Where:femoral Location: ED, ICU, Cathlab, Ward |
PCI, CABG | 29/83 (34.9%) |
| Khorsandi et al42 | 2017 | Retrospective analysis | 11 | OHCA |
|
|
Hypothermia | 6 |
How:Surgical Where:femoral Location:OR |
mCPR | 06/11 (54.5%) |
| Pozzi et al29 | 2017 | Retrospective analysis | 12 | IHCA |
|
|
Drug intoxication | NR |
How:surgical with modified Seldinger Where:femoral Location:OR |
NR | 9/12° (75%) |
| Lazzeri et al30 | 2013 | Retrospective analysis | 16 | IHCA |
|
|
ACS (10), Takotsubo (1), dilated CM (4), PE (1) |
NR |
How:percutaneous Where:femoral Location:NR |
PCI | 02/16 (12.5%) |
| Total | 1723 | 812 IHCA 843 OHCA 68 non specified |
367/1723 (21.3%) |
Abbreviations:IHCA: in-hospital cardiac arrest, OHCA: out-of- hospital cardiac arrest, CPC: cerebral performance category, RCA: refractory cardiac arrest, NR: Not reported, ACS: acute coronary syndrome, TH: therapeutic hypothermia, PCI: percutaneous coronary intervention, mCPR: mechanical cardiopulmonary resuscitation, CABG: coronary artery bypass graft, PE: pulmonary embolism, ICU: intensive care unit, ED: emergency department, VF: ventricular fibrillation, EMS: emergency medical services.
Severe neurological damage includes both pre-existing diseases and acute irreversible damage.
Outcome is defined as CPC 1–2 at hospital discharge unless otherwise specified.
Change of protocol mid-study, °: ECPR and shock not reported separately.
Table 3.
Overview Aetiologies of the cardiac arrest.
| Aetiology | Total n = 1723 | % | Aetiology | Total n = 1723 | % |
|---|---|---|---|---|---|
| Cardiac origin | 1157 | 67,15% | Non-Cardiac origin | 238 | 13,81% |
| ACS | 709 | 41,15% | Intoxication/electrolyte effect | 31 | 1,80% |
| Valvular failure | 18 | 1% | Aorta dissection | 15 | 0,87% |
| Arrhythmia | 65 | 37,72% | Hypothermia | 21 | 1,22% |
| Cardiomyopathy | 87 | 5,05% | Pulmonary embolism (PE) | 61 | 3,54% |
| Heart failure | 18 | 1.04% | Postsurgical | 29 | 1,68% |
| Myocarditis | 25 | 1,45% | Hypovolemia | 11 | 0,64% |
| Othera | 4 | 0,058% | Non specified non-cardiac origins | 54 | 3, 13% |
| Non specified cardiac origin | 231 | 13.40% | Otherb | 16 | 0,23% |
| Non categorized | 328 | 19% |
Other: Iatrogenic laceration of the ventricle (1), constrictive pericarditis (1), tamponade (2).
Other: Electrocution/CO poisoning (4), Respiratory (2), Trauma (5), Circulatory obstruction (5).
Inclusion/exclusion criteria
The inclusion criteria for ECPR differed among the included protocols. The most consistent criterion for inclusion was refractory cardiac arrest (RCA) used in 21/25 (84%) of the protocols. The most widely used definition of RCA was the absence of sustained ROSC after 10 min of conventional CPR (8/25). A cut-off of 15, 20 and 30 min was also used (3/25,22,27,28 1/25,22, 27, 28 4/25 20,24,29,30 respectively). Another frequent criterion was the age of ECPR candidates and was utilised in 15/25 (60%) articles. This could be formulated both as an inclusion or exclusion criterion. The exact age varied between 65 and 80 as the upper limit, with 70 being the most used (4/15 24,27,31,32), and 16 to 20 as the lower limit, with 18 the most frequent (2/15 33,34).
Other important inclusion criteria were: a witnessed cardiac arrest (14/25), no-flow time less than 5 or 10 min (10/25) and low-flow time (10/25). The definition of acceptable low-flow time varied between 20 min (Peigh et al.32) and 120 min (Fagnoul et al.23) with 60 min being the most frequently used cut off (3/10).20,27,35 An initial (non-)shockable rhythm could also be used as an inclusion or exclusion criterion. Six of the 25 protocols used VT and/or VF as an inclusion criterion or asystole and/or PEA as an exclusion criterion.20,22,34, 35, 36 Several articles (11/25) report a specific aetiology for the cardiac arrest as an inclusion criterion such as hypothermia, intoxication, pulmonary embolism or a presumed cardiac cause.
The most frequently used exclusion criterion was the presence of major comorbidity (15/25). The definition of major comorbidity varied among the analysed protocols and included among others: coronary artery disease (CAD), multi-organ failure, aortic dissection, BMI >40, poor level of ADL and life expectancy <1y. Advanced malignancy (10/25), active haemorrhage (8/25) and severe neurological damage (8/25) were other frequently used exclusion criteria. Fewer protocols mentioned the following exclusion criteria: a signed DNR form, no informed consent from family or a representative, sepsis/uncontrolled infection or traumatic cardiac arrest.
Cannulation
Different cannulation strategies were used. The most utilised method of cannulation was a percutaneous approach, 11/25 (44%) of the protocols. Seldinger was the most frequently used technique. The second most reported technique was a surgical approach in 8/25 (32%) of the protocols. Four out of the 25 protocols (16%) used the hybrid method or used a surgical approach as salvage when percutaneous cannulation was difficult or impossible.29,32,34,37 A small number of protocols (2/25 (8%)) did not describe their access strategy.25,27
The most frequently used anatomical location for arteriovenous access was the femoral region, in 24/25 protocols. One other reported method of gaining access was central cannulation between the aorta and right atrium with an open sternotomy in two patients.32 One study did not report its cannulation location.38
The main setting for cannulation was in-hospital with mostly the operating room, emergency department, intensive care unit and catheterization lab. Only the two protocols in the study of Lamhaut et al. (2017) described an out-of-hospital setting.20
Additional therapies
Therapeutic hypothermia (TH) was used in 18/25 of the protocols. Four protocols used 33 °C for 24h as their target.25,32,34,37 Other protocols used therapeutic hypothermia targets ranging between 32 °C and 36 °C.21,24,28,31,39, 40, 41 Unfortunately, 5 protocols did not report their specific hypothermia targets.22,23,33,35,36 Etiological based therapy such as percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG) and thrombolysis were used in 21/25 of the protocols. Four protocols did not describe any form of etiological based therapy.29,31,32,42
Eight protocols used a mechanical CPR (mCPR) device (LUCAS, Autopulse) during transport to the hospital.20,21,23,24,34,37,42
Outcome
The aggregate survival rate with good neurological outcome (CPC score of 1–2) in this systematic review is 21.3%. When only considering the protocols that analysed the CPC score at hospital discharge, the aggregate survival rate is 247/1037 (23.8%). Lamhaut et al. analysed the CPC score either at ICU discharge or at 28 days. Their first protocol yielded an outcome of 9/114 (7.9%) while their second protocol produced a good neurological survival of 12/42 (28.6%).20 Fagnoul et al. also analysed CPC score at ICU discharge: 6/24 (25%).23 Mean time to ICU discharge was 12.5 days. Five protocols reported the neurological survival at 1 month: 93/504 (18.5%).22,24,28,36,39 Kim S.J. et al. reported the CPC score at 3 months: 8/55.33 Two studies had a follow-up time longer than 1 year. Blumenstein et al. reported a good neurological survival of 10/52 (19.2%).39 Pozzi et al. improved their initial outcome of 3/68 to 4/68 (5.9%) owing to the complete recovery of 1 patient after hospital discharge.37
Discussion
The definition of refractory cardiac arrest may be an important inclusion criterion contributing to a good neurological outcome. Our findings show that protocols where cardiac arrest was deemed refractory after 10 min had a better neurological survival rate (mean = 26.7%) compared to those that defined it as more than 15–30 min (mean = 14.5%). Future protocols should consider using a lower cut-off time in their definition of a refractory cardiac arrest. An explanation for this better outcome may be that by using a stricter definition for refractory cardiac arrest the low-flow time could be reduced, which is known to improve survival.46 Furthermore, a no-flow time less than 5 min and a low-flow time less than 60 min also seem to follow this association. This was illustrated most remarkably in the study by Lamhaut et al..20 Two protocols were compared against each other: the first had a good neurological survival rate of 7.9% whereas the second protocol reported a rate of 28.6%. The most important difference in inclusion criteria was the reduction of low-flow time from 100 to 60 min, and RCA from 30 to 20 min. Using a short no- and low-flow time as inclusion criteria should thus be considered when designing an ECPR protocol.
The aetiology of the cardiac arrest seemed to be an important factor for survival as well. The selected protocols only including non-cardiac aetiologies (4/25) had a remarkably higher survival rate than those that included cardiac aetiologies. This would suggest that non-cardiac aetiologies for cardiac arrest should not be excluded from ECPR protocols. However, in our analysis most cardiac arrests had a cardiac aetiology.
Advanced age is another exclusion criterion for many protocols in this review (15/25). But the cut-off for advanced age varied amongst the protocols. In a recent meta-analysis survival was not associated with increasing age.46 Other authors contradict this, although they do acknowledge that age by itself is not a predictor.47,48 A possible explanation might be that the comorbidities associated with old age lead to a declining prognosis and not old age in itself. This would suggest that old age should not necessarily be used as an exclusion criterion for ECPR.
Percutaneous cannulation was the most frequently used method. This method combined with ultrasonography and fluoroscopic guidance is beneficial for shortening the cannulation time and reducing the complication rate.9,49 So, evidence suggests that this method should be the first choice when cannulating a patient for ECPR. .In consideration of our findings, cannulation of the femoral vessels should remain the standard. Taking into account the time to cannulation, the idea for a stay and play method is motivated by the fact that if ROSC is not achieved within 15 min the chance of a good neurological outcome drops to 10–15%. The use of a pre-hospital cannulation approach as opposed to a scoop and run approach needs further investigation.50 Using a pre-hospital approach could be feasible in lowering the time to ECMO, although no time should be wasted on cannulating in bad conditions. The APPACAR2 trail investigating this question will hopefully give an evidence-based answer (NCT02527031).
Eight protocols used a mCPR device. When we look at the latest evidence on these devices, we find that there is no significant benefit towards neurological survival of using them in a CPR protocol.51 Yet these devices could still be useful in transport from an OHCA towards the hospital so quality of compressions can be guaranteed, and the health care providers can concentrate more on the advanced life support protocol. However, evidence in these settings is inconclusive.
An etiological based approach post cannulation was utilised frequently (12/25). This approach is probably the most important of all considering it is the only way to save the patient. ECPR is a bridge to a means, it should not be considered as salvation in itself. An ACS is a frequent aetiology (41.15%) of cardiac arrest. Thus when the cause of the CA is unclear at first evaluation, a CAG could win time.52
The majority of our selected protocols use therapeutic hypothermia (TH) (18/25). A systematic review investigating the use of TH at 32–36 °C in the first 18–24h, considering OHCA CPR protocols with an initial shockable rhythm, has shown that there is low-quality evidence TH improves the neurological outcome. However, they reported this was not beneficial in OHCA survivors with an initial non-shockable rhythm, nor amongst IHCA survivors.53 Furthermore, an older systematic review shows on the contrary that there is a beneficial trend in the usage of TH for patients resuscitated from non-shockable rhythms. However the incidence of infectious complications increased for patients treated with hypothermia.54 Given the small benefit in using this technique in CPR protocols, it could be worth implementing in an ECPR protocol, although there is currently no clear evidence which supports the systematic use of TH in an ECPR protocol. Further investigation of the effects of TH in these situations is needed.
In total 24 articles with 25 protocols were included with a population of 1723 patients. The aggregate survival at hospital discharge with good neurological outcome was 21.3%. The studies reporting more long-term outcomes had similar results. The protocol with the highest survival rate was reported by Pozzi et al., but this was a protocol restricted to cardiac arrest caused by drug intoxication.29 The protocol with the best survival rate (45%), without including a single aetiology of cardiac arrests, was reported by Bednarczyk et al.28 This survival rate could be caused by their population (only IHCA) or by the inclusion criteria (RCA after 15 min) which could both lead to a lower time-to-ECPR. Yet the small sample size (n = 22), could indicate that their sample was not representative of the general outcome of ECPR recipients. When comparing to the protocol of Sakamoto et al. with the greatest sample size (n = 260), the survival rate with good neurological outcome falls to 12.3%.22 These results might be explained by different factors. First, their population was purely OHCA, which may cause longer no- and low-flow times. Second, no-flow time <5 min or witnessed cardiac arrest were not used as inclusion criteria. This decision could mean inclusion of ECPR recipients with long no-flow times, which is known to reduce survival rates.55 Adding a no-flow time <5 min as inclusion criteria should be considered in future protocols.
This review focused on a good neurological outcome. Other outcomes such as organ donation after circulatory death were not considered, but can be important in the clinical practice nonetheless. Another aspect of ECPR is its cost-effectiveness. A recent cost-effectiveness analysis study showed a favourable comparison to the current established cost effectiveness thresholds in different countries.11
A previous systematic review concerning ECPR reported a good neurological survival rate of 13%.15 However, studies were not excluded when physician-based decision-making was present or when no ECPR protocol was used. In our selection process, we encountered multiple articles describing their ECPR protocol, but where ultimately deciding whether to include a patient was based on the attending physician’s decision. In our review, these articles were excluded because of the selection bias this physician-based decision introduced, although we recognize that in the clinical practice this kind of bias cannot be avoided. An important difference between our review and Ortega-Deballon et al. is that they only included OHCA studies, which might explain our better outcome.15 Other significant factors cannot be excluded.
Limitations
Because our selected studies only consisted of observational studies, we should take into account that these results were not controlled. Thus, possible selection bias cannot be ruled out. There are 4 RCT studies that still need to be finished. A new analysis with the results of these could bring another view of our findings here. Due to the heterogeneity of the different protocols, a meta-analysis was not possible. A unified protocol amongst the different ECPR centres could resolve this issue. Furthermore, statistical analysis for significance of different low- and no-flow time, use of mCPR and TH was not possible due to low power. The analysis for significance of RCA <10 vs < 15–30 min was not controlled for possible underlying factors. Looking at the outcome CPC score 1–2 we found that the selected articles used different points in time when measuring their CPC score, ranging from a CPC at hospital discharge to CPC at one year. A time dependent evolution of the CPC score cannot be excluded.
Conclusion
This systematic review compares the different ECPR protocols and their neurological outcome. The aggregate survival rate with good neurological outcome at hospital discharge is 21.5%. A key finding was that a shorter definition of refractory cardiac arrest as inclusion criterion, seems to be associated with a better neurological outcome. Furthermore, using a no-flow time of less than 5 min and a low-flow time as low as possible in the inclusion criteria, also seems to raise survival rates. This implicates that ECPR should already be considered in an early stage of CPR. A percutaneous approach to cannulation reduces time-to-ECPR and the complication rate. Applying this technique to further shorten the low-flow time is advised. Although the evidence to implement techniques like a mechanical CPR device and therapeutic hypothermia in an ECPR protocol is still inconclusive, they are frequently used as additional therapies. An aetiological approach during and after ECPR is necessary. As the majority of cardiac arrests have a coronary aetiology, a CAG should be employed in unclear circumstances. To conclude, ECPR is a feasible strategy for refractory cardiac arrest and should be considered to heighten the survival rate in this critical population. Further research to work towards a unified and optimised protocol is warranted.
Funding
The authors have not received any funding for this research from either public, commercial or not-for-profit sectors.
Author contributions
Both N. Thelinge and K. ‘T Joncke contributed equally to this article.
Declaration of competing interest
The authors declare they have no conflicting interests.
Acknowledgements
We thank Thomas Vandendriessche, Magdalena Jans and Krizia Tuand, the biomedical reference librarians of the KU Leuven Libraries – 2Bergen – Learning Centre Désiré Collen (Leuven, Belgium), for their advice before conducting the systematic literature search and Niels Vanhasbroeck for his insight.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2020.100018.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gräsner J.T., Lefering R., Koster R.W. EuReCa ONE—27 Nations, ONE Europe, ONE Registry: a prospective one month analysis of out-of-hospital cardiac arrest outcomes in 27 countries in Europe. Resuscitation. 2016;105:188–195. doi: 10.1016/j.resuscitation.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Qvick A., Radif M., Brever C., Myrvik J.O., Schenk Gustafsson K., Djärv T. Survival of in-hospital cardiac arrest in men and women in a large Swedish cohort. Scand J Trauma Resuscitation Emerg Med. 2018;26:1–6. doi: 10.1186/s13049-018-0576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wissenberg M., Lippert F.K., Folke F. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out-of-hospital cardiac arrest. JAMA, J Am Med Assoc. 2013;310:1377–1384. doi: 10.1001/jama.2013.278483. [DOI] [PubMed] [Google Scholar]
- 4.Blom M.T., Beesems S.G., Homma P.C.M. Improved survival after out-of-hospital cardiac arrest and use of automated external defibrillators. Circulation. 2014;130:1868–1875. doi: 10.1161/CIRCULATIONAHA.114.010905. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy J.H. The role of assisted circulation in cardiac resuscitation. JAMA, J Am Med Assoc. 1966;197:615–618. doi: 10.1001/jama.1966.03110080055017. [DOI] [PubMed] [Google Scholar]
- 6.Vandervelden S., Sabbe M., Dewolf P., Prolonged C.P.R. Trends Anaesth Crit Care. 2016;9:13–19. doi: 10.1016/J.TACC.2016.05.007. [DOI] [Google Scholar]
- 7.Abrams D., Garan A.R., Abdelbary A. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med. 2018;44:717–729. doi: 10.1007/s00134-018-5064-5. [DOI] [PubMed] [Google Scholar]
- 8.Extracorporeal Life Support Organization ( ELSO ) Guidelines for ECPR Cases. 2013. pp. 1–4. [Google Scholar]
- 9.Voicu S., Henry P., Malissin I. Improving cannulation time for extracorporeal life support in refractory cardiac arrest of presumed cardiac cause – comparison of two percutaneous cannulation techniques in the catheterization laboratory in a center without on-site cardiovascular surgery. Resuscitation. 2018;122:69–75. doi: 10.1016/J.RESUSCITATION.2017.11.057. [DOI] [PubMed] [Google Scholar]
- 10.Twohig C.J., Singer B., Grier G., Finney S.J. A systematic literature review and meta-analysis of the effectiveness of extracorporeal-CPR versus conventional-CPR for adult patients in cardiac arrest. J Intensive Care Soc. November. 2019:347–357. doi: 10.1177/1751143719832162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennis M., Zmudzki F., Burns B. Cost effectiveness and quality of life analysis of extracorporeal cardiopulmonary resuscitation (ECPR) for refractory cardiac arrest. Resuscitation. 2019;139:49–56. doi: 10.1016/J.RESUSCITATION.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Link M.S., Berkow L.C., Kudenchuk P.J. Part 7: adult advanced cardiovascular life support: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S444–S464. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 13.Debaty G., Babaz V., Durand M. Prognostic factors for extracorporeal cardiopulmonary resuscitation recipients following out-of-hospital refractory cardiac arrest. A systematic review and meta-analysis. Resuscitation. 2017;112:1–10. doi: 10.1016/j.resuscitation.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Tramm R., Ilic D., Ar D., Va P., Romero L., Hodgson C. 2015. Cochrane Database of Systematic Reviews Extracorporeal Membrane Oxygenation for Critically Ill Adults (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortega-Deballon I., Hornby L., Shemie S.D., Bhanji F., Guadagno E. Extracorporeal resuscitation for refractory out-of-hospital cardiac arrest in adults: a systematic review of international practices and outcomes. Resuscitation. 2016;101:12–20. doi: 10.1016/j.resuscitation.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed D.A., Beckman T.J., Wright S.M., Levine R.B., Kern D.E., Cook D.A. Predictive validity evidence for medical education research study quality instrument scores: quality of submissions to JGIM’s medical education special issue. J Gen Intern Med. 2008;23:903–907. doi: 10.1007/s11606-008-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed D.A., Beckman T.J., Wright S.M. An assessment of the methodologic quality of medical education research studies published in the American Journal of Surgery. Am J Surg. 2009;198:442–444. doi: 10.1016/j.amjsurg.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan G.M. Deconstructing quality in education research. J Grad Med Educ. 2011;3:121–124. doi: 10.4300/JGME-D-11-00083.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamhaut L., Hutin A., Puymirat E. A Pre-Hospital Extracorporeal Cardio Pulmonary Resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: an observational study and propensity analysis. Resuscitation. 2017;117:109–117. doi: 10.1016/J.RESUSCITATION.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Grunau B., Carrier S., Bashir J. A comprehensive regional clinical and educational ECPR protocol decreases time to ECMO in patients with refractory out-of-hospital cardiac arrest. Can J Emerg Med. 2017;19:424–433. doi: 10.1017/cem.2017.376. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto T., Morimura N., Nagao K. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85:762–768. doi: 10.1016/J.RESUSCITATION.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Fagnoul D., Taccone F.S., Belhaj A. Extracorporeal life support associated with hypothermia and normoxemia in refractory cardiac arrest. Resuscitation. 2013;84:1519–1524. doi: 10.1016/J.RESUSCITATION.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Le Guen M., Nicolas-Robin A., Carreira S. Extracorporeal life support following out-of-hospital refractory cardiac arrest. Crit Care. 2011;15:1–9. doi: 10.1186/cc9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C.-H., Chou N.-K., Becker L.B. Improved outcome of extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest – a comparison with that for extracorporeal rescue for in-hospital cardiac arrest. Resuscitation. 2014;85:1219–1224. doi: 10.1016/J.RESUSCITATION.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Mandigers L., Scholten E., Rietdijk W.J.R. Survival and neurological outcome with extracorporeal cardiopulmonary resuscitation for refractory cardiac arrest caused by massive pulmonary embolism: a two center observational study. Resuscitation. 2019;136:8–13. doi: 10.1016/j.resuscitation.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Bednarczyk J.M., White C.W., Ducas R.A. Resuscitative extracorporeal membrane oxygenation for in hospital cardiac arrest: a Canadian observational experience. Resuscitation. 2014;85:1713–1719. doi: 10.1016/J.RESUSCITATION.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Pozzi M., Koffel C., Djaref C. High rate of arterial complications in patients supported with extracorporeal life support for drug intoxication-induced refractory cardiogenic shock or cardiac arrest. J Thorac Dis. 2017;9:1988–1996. doi: 10.21037/jtd.2017.06.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazzeri C., Sori A., Bernardo P., Picariello C., Gensini G.F., Valente S. In-hospital refractory cardiac arrest treated with extracorporeal membrane oxygenation: a tertiary single center experience. Acute Card Care. 2013;15:47–51. doi: 10.3109/17482941.2013.796385. [DOI] [PubMed] [Google Scholar]
- 31.Pang P.Y.K., Wee G.H.L., Huang M.J. Therapeutic hypothermia may improve neurological outcomes in extracorporeal life support for adult cardiac arrest. Hear Lung Circ. 2017;26:817–824. doi: 10.1016/J.HLC.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 32.Peigh G., Cavarocchi N., Hirose H. Saving life and brain with extracorporeal cardiopulmonary resuscitation: a single-center analysis of in-hospital cardiac arrests. J Thorac Cardiovasc Surg. 2015;150:1344–1349. doi: 10.1016/J.JTCVS.2015.07.061. [DOI] [PubMed] [Google Scholar]
- 33.Kim S.J., Jung J.S., Park J.H., Park J.S., Hong Y.S., Lee S.W. An optimal transition time to extracorporeal cardiopulmonary resuscitation for predicting good neurological outcome in patients with out-of-hospital cardiac arrest: a propensity-matched study. Crit Care. 2014;18:1–15. doi: 10.1186/s13054-014-0535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pozzi M., Armoiry X., Achana F. Extracorporeal life support for refractory cardiac arrest: a 10-year comparative analysis. Ann Thorac Surg. 2019;107:809–816. doi: 10.1016/J.ATHORACSUR.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Bellezzo J.M., Shinar Z., Davis D.P. Emergency physician-initiated extracorporeal cardiopulmonary resuscitation. Resuscitation. 2012;83:966–970. doi: 10.1016/J.RESUSCITATION.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 36.Kuroki N., Abe D., Iwama T. Association between delay to coronary reperfusion and outcome in patients with acute coronary syndrome undergoing extracorporeal cardiopulmonary resuscitation. Resuscitation. 2017;114:1–6. doi: 10.1016/J.RESUSCITATION.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Pozzi M., Koffel C., Armoiry X. Extracorporeal life support for refractory out-of-hospital cardiac arrest: should we still fight for? A single-centre, 5-year experience. Int J Cardiol. 2016;204:70–76. doi: 10.1016/J.IJCARD.2015.11.165. [DOI] [PubMed] [Google Scholar]
- 38.Wang C.-H., Chou N.-K., Becker L.B. Improved outcome of extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest – a comparison with that for extracorporeal rescue for in-hospital cardiac arrest. Resuscitation. 2014;81:1219–1224. doi: 10.1016/J.RESUSCITATION.2014.06.022. Wang C. [DOI] [PubMed] [Google Scholar]
- 39.Blumenstein J., Leick J., Liebetrau C. Extracorporeal life support in cardiovascular patients with observed refractory in-hospital cardiac arrest is associated with favourable short and long-term outcomes: a propensity-matched analysis. Eur Hear J Acute Cardiovasc Care. 2016;5:13–22. doi: 10.1177/2048872615612454. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y.S., Cho Y.H., Sung K. Target temperature management may not improve clinical outcomes of extracorporeal cardiopulmonary resuscitation. J Intensive Care Med. 2018;34:790–796. doi: 10.1177/0885066618801269. [DOI] [PubMed] [Google Scholar]
- 41.Haneya A., Philipp A., Diez C. A 5-year experience with cardiopulmonary resuscitation using extracorporeal life support in non-postcardiotomy patients with cardiac arrest. Resuscitation. 2012;83:1331–1337. doi: 10.1016/J.RESUSCITATION.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Khorsandi M., Dougherty S., Young N. Extracorporeal life support for refractory cardiac arrest from accidental hypothermia: a 10-year experience in edinburgh. J Emerg Med. 2017;52:160–168. doi: 10.1016/J.JEMERMED.2016.10.043. [DOI] [PubMed] [Google Scholar]
- 43.Wang J., Ma Q., Zhang H., Liu S., Zheng Y. Predictors of survival and neurologic outcome for adults with extracorporeal cardiopulmonary resuscitation: a systemic review and meta-analysis. Med (United States) 2018;97 doi: 10.1097/MD.0000000000013257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersen L.W., Bivens M.J., Giberson T. The relationship between age and outcome in out-of-hospital cardiac arrest patients. Resuscitation. 2015;94:49–54. doi: 10.1016/J.RESUSCITATION.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Cardarelli M.G., Young A.J., Griffith B. 2009. Use of Extracorporeal Membrane Oxygenation for Adults in Cardiac Arrest ( E-CPR ): A Meta-Analysis of Observational Studies. July. [DOI] [PubMed] [Google Scholar]
- 46.Kashiura M., Sugiyama K., Tanabe T., Akashi A., Hamabe Y. Effect of ultrasonography and fluoroscopic guidance on the incidence of complications of cannulation in extracorporeal cardiopulmonary resuscitation in out-of-hospital cardiac arrest: a retrospective observational study. BMC Anesthesiol. 2017;17:1–7. doi: 10.1186/s12871-016-0293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer B., Reynolds J.C., Lockey D.J., O’Brien B. Pre-hospital extra-corporeal cardiopulmonary resuscitation. Scand J Trauma Resuscitation Emerg Med. 2018;26:1–8. doi: 10.1186/s13049-018-0489-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pl W., Sc B., Pl W., Sc B. 2018. Mechanical versus Manual Chest Compressions for Cardiac Arrest (Review)www.cochranelibrary.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larsen J.M., Ravkilde J. Acute coronary angiography in patients resuscitated from out-of-hospital cardiac arrest-A systematic review and meta-analysis. Resuscitation. 2012;83:1427–1433. doi: 10.1016/j.resuscitation.2012.08.337. [DOI] [PubMed] [Google Scholar]
- 50.Stanger D., Mihajlovic V., Singer J., Desai S., El-sayegh R., Wong G.C. s Choice-Effects of Targeted Temperature Management on Mortality and Neurological Outcome : A Systematic Review and Meta-Analysis. 2018. [DOI] [PubMed] [Google Scholar]
- 51.Song L., Wei L., Zhang L., Lu Y., Wang K., Li Y. 2016. The Role of Targeted Temperature Management in Adult Patients Resuscitated from Nonshockable Cardiac Arrests : An Updated Systematic Review and Meta-Analysis. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koike S., Ogawa T., Tanabe S. Collapse-to-emergency medical service cardiopulmonary resuscitation interval and outcomes of out-of-hospital cardiopulmonary arrest: a nationwide observational study. Crit Care. 2011;15:R120. doi: 10.1186/cc10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.