Abstract
Objective
To describe the current practice indications, methodology, and outcomes from a real-world experience of intravaginal culture (IVC) using INVOCELL.
Design
A descriptive study outlining real-world experience with INVOCELL that addresses patient selection, ovarian stimulation, embryology laboratory practices, and outcomes.
Setting
Five fertility centers in Missouri, Texas, North Carolina, South Carolina, and Virginia.
Patients
Four hundred sixty-three patients undergoing 526 cycles.
Intervention
IVC using INVOCELL.
Main Outcome Measures
Cumulative pregnancy rate and live births. Secondary outcomes of interest included percent good quality embryos.
Results
IVC with INVOCELL was primarily used in women <38 years with anti-Mullerian hormone level >0.8 ng/mL. The mean numbers of retrieved oocytes ranged from 9.2 to 16. Mean numbers of oocytes and sperm-injected oocytes loaded per INVOCELL ranged from a mean of 6.4–9.5 with a reported maximum of 34 oocytes loaded into the device. Most (95%) of the embryos were transferred on day 5. The mean blastocyst recovery per oocyte loaded into the device ranged from 19% to 34%; mean cumulative live birth plus ongoing pregnancy rates ranged from 29% to 53% per cycle start and 40% to 61% per transfer.
Conclusions
This study of IVC using INVOCELL as an alternative model for infertility treatment confirms its utility as a viable alternative to standard incubator-based in vitro fertilization. The technology is compatible within the current framework of practice patterns and, when appropriately used, results in acceptable blastocyst recovery and live birth rates. Further use of INVOCELL in other clinical situations is warranted.
Key words: INVOCELL, intravaginal culture, infertility, IUI
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/posts/xfre-d-20-00156
Infertility affects 6.7% of women of reproductive age according to the Centers for Disease Control and Prevention, 2011–2015 data (1). However, in a more recent study, this number was found to be nearly twice as high, at 12.5% in women aged 20–44 years in the United States (2). Despite its common prevalence and designation as a “disease” by the World Health Organization, a significant proportion of patients does not or cannot access treatment (3). Cost is a major factor that limits access to care; a reduction in treatment cost tantamount to a 1% savings in disposable income has been shown to increase assisted reproductive technology (ART) use by 3.2% (4). In the United States, one fresh in vitro fertilization (IVF) cycle costs 52% of the average annual household disposable income in nonmandated states compared with 13% in states with insurance mandates; in most other comparably developed countries, this proportion is <10% (4). In addition to cost, however, geographic, educational, religious, cultural and emotional factors also impair use and compound diminished ART use rates (5, 6). Unlike other diseases, the complex medical, social, geographic, and financial implications that surround infertility need to be comprehensively addressed to improve the overall access to care.
From a patient’s perspective, current fertility treatment paradigms are polarized in terms of costs and outcomes. Lower priced treatment options like ovulation induction with or without intrauterine insemination (IUI) are associated with low success rates (7, 8). In contrast, the high success rates from currently offered ARTs can only be achieved at a concomitant high cost. In the current therapeutic paradigm, when patients fail therapy with oral ovulation induction and IUI, they may be offered treatment with gonadotropins and IUI, but unfortunately, this can be associated with a higher risk of multiple pregnancies, as well as success rates that are marginally incremental compared with less aggressive options and at a much higher cost (9). Therefore, many patients discontinue treatment after repeated treatment failures before ever undertaking ART (10). There is a critical unmet need for therapeutic options that offer higher success rates at a more reasonable cost, as well as address other access barriers, to optimize the options of most patients seeking infertility treatment. The need for an alternative model for treatment has only been amplified in the current pandemic crisis in which economic considerations have caused patients to seek lower cost options, and clinics and laboratories strive to ensure that treatments are offered safely while carefully considering efficient and practical optimization of their resources.
In 2015, the American Society for Reproductive Medicine hosted an “Access to Care Summit,” with the goal of identifying strategies to increase access to fertility care throughout the world. Intravaginal culture (IVC) was highlighted as a possible solution (11). Intravaginal culture spans the dyads of low cost/low success rate treatment options against those that have high costs/high success rates. In contrast to IUI, higher success rates with IVC are primarily achieved by ensuring gamete availability and proximity; however, the rates, nevertheless, are tempered by lack of sophisticated technology associated with conventional ART such as real-time embryo monitoring, genetic screening before implantation, and other manipulations that optimize sperm, oocyte, and embryo physiology (12, 13, 14, 15, 16, 17, 18, 19).
IVC originated in France and consisted of coincubation of oocytes with sperm within a hermetically sealed tube that was placed in the patient’s vagina for a period of 44 to 50 hours (19). After initial success reports, it was offered by clinical centers in 6 countries including several in Western Europe, the United States, and Japan with an overall clinical pregnancy rate of 19.6% after fresh transfer of day-3 embryos (20, 21). Extensive design modifications to the hermetically sealed tube to optimize efficacy and safety led to the development of INVOCELL (INVO Bioscience Inc., Lakewood Ranch, FL), the current and only Food and Drug Administration (FDA) cleared device used for IVC. Since the development of INVOCELL, studies evaluating its efficacy and safety have been done in several countries; live birth rates up to 55% after transfer of day 5 vaginally incubated embryos were shown from a single United States center (22, 23, 24).
Although IVC with INVOCELL is currently used at 65 centers in the United States, published data on contemporary usage of this relatively novel technology are limited (24). In this retrospective, multicenter cohort study, we present the combined experience of 5 centers with 526 cycles of IVF with IVC using INVOCELL as an alternative therapeutic option for patients with infertility. This article highlights the versatility and adaptability of this technology in contemporary ART practice.
Materials and methods
This was a descriptive study that outlined the real-world experience of 5 fertility centers in the United States offering INVOCELL through May 31, 2019. Seven centers with high usage of INVOCELL were invited to take part in the study, and 5 centers (located in Missouri, Texas, North Carolina, South Carolina, and Virginia) agreed to participate. The study protocol was reviewed by a central institutional review board (IRB) (Advarra; Pro00043808) as none of the sites were under the purview of a local IRB. The study was deemed exempt from IRB oversight because the information was recorded by the investigators in such a manner that the identity of the human subjects could not be readily ascertained.
Deidentified cohort data were collected from all centers as an intent-to-treat analysis for all started cycles that planned for IVC using INVOCELL. Data collection/analysis was performed by the embryologist and verified by the clinician. Site 5 predominantly performed split cycles, in which a portion of retrieved oocytes underwent IVC using INVOCELL and the remainder underwent intracytoplasmic sperm injection (ICSI) with traditional laboratory incubation. For this site, the results were still analyzed as an intent-to-treat approach for any INVOCELL initiated cycle.
This is the first study that provides a real-world perspective on current clinical practices of INVOCELL users. The primary outcomes of interest were cumulative pregnancy (cumulative pregnancy defined as any ongoing pregnancy/live birth within 6 months of ovarian stimulation) and live-birth rates (25). Clinical variables of interest included patient selection, stimulation protocols, and embryology laboratory-related procedures (culture media used, number of oocytes loaded/device, insemination method and timing). The secondary outcome of interest was the percentage of good quality embryos (defined as ≥3BB grade as defined by Gardner and Schoolcraft classification) calculated as the number of embryos ≥3BB/total embryos incubated.
Results
The data presented here represent 526 cycles from 463 patients, which were collected from 5 centers that have integrated IVC using INVOCELL into their practice for ≥1 year (Table 1). Most commonly, IVC was offered to young patients (<38 years) with a body mass index (BMI) <35 kg/m2 and an adequate ovarian reserve (Table 2). With increased comfort in using IVC, each site, acting in response to the specific population of patients served, has expanded the scope of indications both medically, such as polycystic ovary syndrome (PCOS), and socially, such as same-sex female couples and others with religious reservations, over time. However, most centers still excluded older patients (>38 years), with diminished ovarian reserve (anti-Mullerian hormone < 0.8 ng/mL), BMI >35 kg/m2, and moderate-to-severe male factor infertility, except when using donor sperm (Table 2).
Table 1.
INVOCELL cycles at 5 United States centers. Five centers performed intravaginal culture with INVOCELL; 526 cycles were performed in 463 patients.
| Site | Number of Patients Treated | Number of Cycles |
|---|---|---|
| 1 | 72 | 73 |
| 2 | 120 | 165 |
| 3 | 53 | 53 |
| 4 | 185 | 201 |
| 5 | 33 | 34 |
| Total | 463 | 526 |
Table 2.
The most common demographics/characteristics for INVOCELL patients across the 5 sites.
| Patient Characteristics | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age (y) | 35 (26–48) | 33.9 (23–50) | 32.5 (24–44) | 34.4 (24.5–43.5) | 33.7 (26–40) |
| BMI (kg/m2) | 29.9 (18.9–45.4) | 31 (19.6–43.1) | 25.8 (18.3–38.3) | 26.2 (17.5–36.0) | 30.02 (18.33–41.53) |
| Ovarian reserve (AMH) | 2.74 (0.28–8.08) | 3.9 (0.01–22.9) | 5.0 (0.67–45.6) | 3.03 (0.23–17.5) | 4.83 (0.32–13.24) |
| Infertility diagnosis (%) | |||||
| Tubal factor | 56.2% | 17.6% | 31% | 28.4% | 4% |
| Unexplained | 20.5% | 15.2% | 13% | 24.9% | N/A |
| Diminished ovarian reserve | 2.7% | 10.3% | 4% | 4.5% | 24% |
| PCOS/ovulatory dysfunction | 5.5% | 21.8% | 19% | 18.9% | 36% |
| Endometriosis | N/A | 2% | N/A | 6.5% | 4% |
| Other | N/A | 1% | N/A | 1.5% | 8% |
| Male factor | 15% | 32% | 33% | 6.5% | 24% |
| Donor sperm | |||||
| Donor sperm: absence of male partner | 100% | 73% | 78% | 84% | 80% |
| Donor sperm: abnormal sperm parameters | 0% | 27% | 22% | 16% | 20% |
Note: Inclusion criteria for use of INVOCELL typically included young patients (<38 years) with a BMI <35 kg/m2 and an adequate ovarian reserve. Exclusion criteria typically included older patients (age ≥ 38 years), with diminished ovarian reserve (AMH < 0.8 ng/mL), BMI >35 kg/m2, and moderate-to-severe male factor infertility, except when using donor sperm. With increasing comfort using intravaginal culture, some sites expanded the scope of indications to include PCOS and social and religious indications. Percentages represent primary diagnosis. Donor sperm was used primarily in cases because of absence of a male partner. AMH = anti-Mullerian hormone; BMI = body mass index; N/A = not available/not applicable; PCOS = polycystic ovary syndrome.
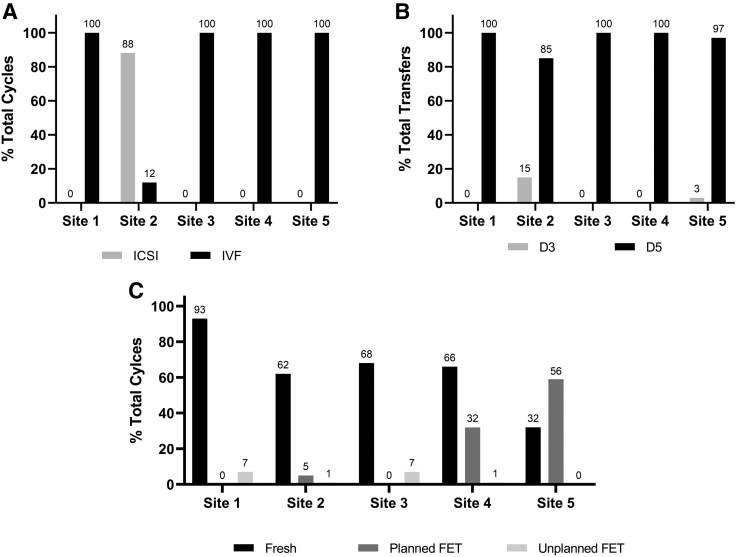
Ovarian stimulation protocols for IVC using INVOCELL vary greatly between centers. In general, protocols tend to be milder (≤225 IU/day, sustained) to limit the number of monitoring visits and intracycle testing as well as to safely be able to offer fresh transfers with a low risk of ovarian hyperstimulation (Supplemental Table 1, available online). Stimulation protocols at all sites included the use of gonadotropins with or without concomitant use of oral agents. Whereas 4 of the 5 sites routinely offered cryopreservation of untransferred embryos and/or oocytes by vitrification, 1 site also triggered some patients with gonadotropin-releasing hormone (GnRH) agonist with no fresh transfer, cryopreservation of all embryos and subsequent frozen embryo transfer(s) (Site 4) (Fig. 1C). The mean numbers of oocytes retrieved ranged from 9.2 to 16; whereas all sites loaded greater than the FDA suggested limit of 7 inseminated oocyte cumulus complexes, the number of injected metaphase II oocytes loaded in ICSI cycles was lower (Table 3).
Figure 1.
Details of INVOCELL cycles performed at each center. (A) Percentage of cycles where ICSI or standard IVF was performed by the clinic. (B) Percentage of total transfers where day-3 or day-5 transfer was performed by the clinic. (C) Percentage of total cycles that resulted in a fresh or frozen (planned or unplanned) transfer. FET = frozen embryo transfer; ICSI = intracytoplasmic sperm injection; IVF = in vitro fertilization.
Table 3.
Stimulation results and live birth outcomes for INVOCELL cycles at the 5 centers.
| Stimulation Results | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| OHSS | 0% | 1.2% | 1.9% | 1.0% | 0% |
| Mean number of oocytes retrieved | 9.5 | 9.2 | 9.9 | 10.4 | 16 |
| Mean number of oocytes loaded into the INVOCELL device standard Insemination (min–max) | 9.4 (1–30) | 8.4 (2–27) | 8.7 (1–30) | 9.5 (1–34) | 8 (2–21) |
| Mean number of oocytes loaded into the INVOCELL device ICSI (min–max) | N/A | 6.4 (1–25) | N/A | N/A | N/A |
| % Top quality (≥3BB) embryos recovered | 85% | 38% | 77% | 25% | 40% |
| % Blastocysts recovered per oocyte loaded into the INVOCELL device | 19% | 27% | 20% | 34% | 20% |
| Mean number of blastocysts frozen | 0.82 | 1.4 | 1.2 | 2.4 | 3.76 |
| % of cycles that had supernumerary blastocysts to cryopreserve | 32.9% | 23% | 36.5% | 58.7% | 70% |
| Number and percentage of day 5 transfers in which embryos other than blastocysts were transferred | 0; 0% | 9; 8% | 0; 0% | 4; 1.8% | 0; 0% |
| Single embryo transfer | 60% | 71% | 97% | 81% | 87% |
| Implantation rate | 38.8% | 47.0% | 52.9% | 48.2% | 51.4% |
| Multiple pregnancies | 6.8% | 4.2% | 1.9% | 4.3% | 3.2% |
| Live Birth Rates | |||||
| Live birth rate: fresh transfer | |||||
| Per cycle start | 23% (15/64) | 41% (34/83) | 42% (14/33) | 28% (43/153) | 15% (5/34) |
| Per transfer | 35% (15/43) | 50% (34/68) | 64% (14/22) | 54% (43/80) | 45% (5/11) |
| Live birth rate: frozen transfer | |||||
| Per cycle start | 50% (5/10) | 56% (5/9) | 29% (2/7) | 46% (38/83) | 21% (7/34) |
| Per transfer | 50% (5/10) | 56% (5/9) | 29% (2/7) | 46% (38/82) | 37% (7/19) |
| Cumulative Pregnancy Rate (Live Birth and Ongoing Pregnancy) | |||||
| Cumulative pregnancy rate | |||||
| Per cycle start | 29% (21/73) | 32% (52/165) | 46% (30/66) | 33% (101/302) | 53% (18/34) |
| Per transfer | 42% (21/50) | 40% (52/130) | 61% (30/49) | 49% (101/207) | 60% (18/30) |
Note: Single embryo transfer rate is shown as the percentage of total transfers. OHSS rate and multiple pregnancy rate are shown as the percentage of total cycles. Mean number of oocytes retrieved ranged from 9.5 to 16 and mean number of oocyte/sperm complexes loaded in the INVOCELL varied from 8 to 9.5 with a broad range of 1–30 loaded per device for standard insemination and 6.4 with a range of 1–25 for ICSI oocytes. Cycles with fresh transfer or frozen transfer resulting in a live birth (shown as the number of live births per started cycle and per transfer) are indicated for cycles started prior to October 31, 2018. Cumulative pregnancy rate is defined as live birth resulting from fresh and frozen transfers (for cycles started prior to October 31, 2018) plus ongoing pregnancy (for cycles performed between November 1, 2018, and May 31, 2019). For site 5, live birth rate and cumulative pregnancy rate data are shown for the entire time period. ICSI = intracytoplasmic sperm injection; N/A = not available; OHSS = ovarian hyperstimulation syndrome.
IVC using INVOCELL is FDA cleared for use with both insemination and ICSI. In this dataset, 4 of 5 sites performed standard insemination and 1 center predominantly performed ICSI. Insemination was therefore performed in 380 of 526 (72%) and ICSI in 146 of 526 (28%) of the cycles reported here (Fig. 1A). Sperm for insemination was prepared by gradient separation to a final total motile sperm concentration that ranged from 200,000 to 400,000/mL; prepared sperm and partially stripped oocytes were coincubated for 5–15 minutes (Supplemental Table 2, available online). Immediately after retrieval, ICSI was performed followed by the placement of the injected oocytes into the INVOCELL for subsequent intravaginal culture. Key center-specific gamete handling parameters are outlined in Supplemental Table 2.
Although the FDA has cleared incubation periods of 72 hours, 95% of reported transfers were performed using extended culture and blastocyst transfer (Fig. 1B). In the centers surveyed, fresh transfers were performed more frequently than frozen transfers for IVC using INVOCELL cycles. The rate of fresh transfers ranged from 32% to 93%/cycle start. Frozen transfer rates ranged from 0% to 56%/cycle start for planned frozen transfers and 0% to 7%/cycle start for unplanned frozen transfers (Fig. 1C). Embryo quality at most sites was assessed using a modified Gardner and Schoolcraft grading system. Most sites reported obtaining embryos of grade ≥3BB in >25% of blastocysts (Table 3), and 4 of the 5 centers reported obtaining grade 5 or 6 embryos in IVC cycles. The blastocyst conversion rate per number of inseminated oocyte/cumulus complexes loaded varied from 19% to 34%. The mean number of blastocysts transferred varied from 1 to 1.4, with a 60%–97% single embryo transfer rate resulting in an implantation rate ranging from 38.8% to 51.4%. The percentage of cycles that had supernumerary blastocysts to cryopreserve ranged from 23% to 70%, with an average of 0.82–3.76 embryos frozen per cycle. In only a small percentage, nine transfers from a single clinic, was there a transfer of an embryo other than a blastocyst on day 5 (Table 3).
The IVC paradigm allows neither a fertilization check after oocyte or sperm coincubation or injection nor determination of the rate and status of early embryo development. The cancelation of an embryo transfer could result from either failed fertilization (implied from recovery of oocytes at the end of the incubation period) or from a general arrest of embryo development at the end of the predetermined period of intravaginal culture and embryo development. Of the 526 reported cycles, 410 cycles progressed to transfer (78%) with cycle cancelation in 23 (4.3%) cycles because of no fertilized oocytes observed upon removal of the device, in 66 (12.5%) cycles because of arrested embryonic development, and in the remaining 27 (5.1%) cycles, which were canceled prior to insertion of the INVOCELL device, because of causes unrelated to the IVC procedure, such as poor response to stimulation.
IVC using INVOCELL has only been implemented relatively recently. Of the 5 sites included in these data, 4 have used it for ≥2 years and one has used it for 18 months. Cycle outcomes per started cycle and per transfer by the center are thus chronologically differentiated and classified as live birth data for all IVC cycles started and fresh embryo transfers completed prior to October 31, 2018, as well as for all IVC cycles started with resulting frozen embryos and frozen embryo transfer completed prior to October 31, 2018. Finally, cumulative live birth (for cycles started prior to October 31, 2018) plus ongoing pregnancy (for cycles performed between November 1, 2019, and May 31, 2019) rates are shown in Table 3. Also shown by center are the incidences of ovarian hyperstimulation syndrome as well as multiple pregnancies (Table 3).
Discussion
This analysis summarized the data from 526 INVOCELL cycles conducted at 5 centers across the United States through May 31, 2019. These 5 centers represent most of INVOCELL usage during the defined time period, and use of INVOCELL by these sites is representative of current operative practice trends. Two other centers that offer INVOCELL at a comparable volume as the 5 centers declined involvement in this study. Across all sites, IVC was generally offered to patients <38 years, in the absence of male factor infertility, unless donor sperm was used. All sites used gonadotropins either exclusively or in combination with oral agents for controlled ovarian stimulation. Most IVC cycles involved fresh embryo transfers with cryopreservation of untransferred embryos, although some sites offered freeze-all with subsequent elective frozen transfer, indicating the compatibility of adapting IVC to this frequently employed approach in contemporary ART cycles. INVOCELL has been cleared for use with insemination as well as ICSI; it was found that both approaches were being employed.
Historical Data vs. Current Practice
IVC using INVOCELL was cleared for use in the United States by the FDA in 2016 (26). The device label recommends an intravaginal incubation period ≤72 hours based on the data that were submitted in support of the application. These data were accrued from 2 non-US studies involving day 3 transfers, one of which also included ICSI (22, 23). The practice in the United States has steadily evolved in the interim, in tandem with improved embryo culture media, toward performing day 5 embryo transfers. The only published study from the United States demonstrated the feasibility, efficacy, and safety of extended intravaginal culture for 5 days in 20 patients treated at a single center with IVC using INVOCELL (24). In keeping with current United States practice patterns favoring blastocyst transfer, 95% of transfers were accomplished with extended intravaginal culture to day 5; mean cumulative live birth or ongoing pregnancy rates (dependent on availability of data) ranged from 29% to 53% per cycle start and 40% to 61% per transfer across all centers. The FDA also cleared the use of INVOCELL for up to 7 oocytes based on submitted data; Doody et al loaded the INVOCELL with up to 10 oocytes in their study (24). The 5 centers reported a mean number of oocytes loaded into the device ranging from 6.4 to 9.5, with minimum and maximum numbers being 1 and 34, respectively. Despite the fact that the data are limited to one prospective study describing a protocol of longer incubation and greater number of oocytes (24), the practice of IVC using INVOCELL in the United States is performed mostly off-label.
Patient Access
The authors mainly cited “expanded access” as their prime reason for opting to offer IVC using INVOCELL. Facilitation of access to care is even more critical in these current times with the coronavirus disease 2019 pandemic as patients dealing with financial insecurities seek lower cost treatments and as providers cope with the limitations inherent to, and imposed by, incorporating social distancing, decreased monitoring, diminished staff, and laboratory burden into clinical practice. Access limitations arise from financial, geographic, cultural, and religious impediments to treatment use. The centers included in this analysis have identified the use of INVOCELL to address one or more of these needs in their patient cohort. Furthermore, providers treat an evolving demographic of patients who seek information through peer groups as well as social and educational electronic media. Informed patients proactively identify the type and terms of care they would like to receive, expecting not only efficacy and safety but also value in the therapeutic experience: time to pregnancy, cost of treatment, and alignment with social, cultural, and religious values. One example is same-sex female couples in which both partners, rather than just the gestational carrier, can contribute to the growth of their offspring.
Cost Considerations
IVC has been offered as an intermediate cost option between IUI and IVF using diverse approaches such as low monitoring frequency and/or stimulation protocols that involve oral agents supplemented by low gonadotropin doses. As expected, milder stimulation protocols are associated with lower oocyte and embryo yields. Although treatment costs are reduced, there is also a reduced likelihood of having frozen embryos available to provide additional opportunities to become pregnant. However, fertilization rates in the face of low oocyte yields can be optimized by ICSI as was done at 2 of the centers included here. Because IVC is currently offered at a lower cost than other ART options, there is a need for formal cost-efficacy evaluation in a prospective study using a standardized mild stimulation protocol with minimal monitoring. Furthermore, such a trial can only include patients in whom such a practice paradigm is likely to yield maximal efficacy and safety. However, such a trial design would necessarily exclude most of the successfully implemented, real-world practice paradigms described here. A cost-efficacy analysis would therefore not be applicable to the current dataset. We plan to conduct a prospective study using standardized dosing, monitoring, and interventions at 5 centers that will directly evaluate the cost of treatment.
Considerations for Adoption
As with any novel technology, there is a learning curve that must be overcome prior to seamless integration into existing practice. There is limited information to guide appropriate patient selection, protocol, and use of clinic resources. In addition, there are key logistical considerations that must be resolved, including procedural order at the time of oocyte retrieval and embryo transfer to accommodate INVOCELL loading and unloading, respectively, as well as timing of semen sample collection. In the laboratory, workflows need to be developed around rapid processing of retrieved cumulus/oocyte complexes, limited cumulus oophorus trimming, earlier/expedited sperm preparation, insemination or immediate ICSI, and embryo retrieval from the INVOCELL on completion of intravaginal incubation. This analysis addresses some of these issues, whereas others may be learned through experienced peer-to-peer interactions. We found that most centers initiated the use of IVC with INVOCELL in normal responder patients with a good prognosis; with increased skill and comfort, many have progressively expanded their profile of indications while also adapting protocols and ancillary procedures as needed to enhance patient options to access care.
Future Research
The aim of this analysis was to provide insights on the diverse modalities of practice patterns that are compatible with IVC. This report is based on the largest cohort of patients treated with IVC from multiple centers yet evaluated. We acknowledge that the data presented here are retrospective and representative of diverse protocols that could affect outcomes. We therefore have not formally compared safety and efficacy outcomes of cycles using INVOCELL relative to other options used in the treatment of infertility. Given the limited dataset from Doody et al, which showed inferior blastocyst development using IVC when compared with IVF (24), one interesting finding across the majority of sites was the recovery of a high percentage of embryos of grade ≥3BB, the mechanism of which could potentially be evaluated in a future prospective study. We also acknowledge that differences in patient selection among sites were likely to have influenced the outcomes; although this would be a major limitation in a randomized clinical trial comparing different treatment options, in the real world we would expect this to be a key operational driver that guides individualized treatment selection based on specific patient characteristics. As such, it provides useful information that could allow improved access to infertility treatment.
In conclusion, the FDA recently cleared IVC using INVOCELL as a therapeutic option for patients with infertility. This descriptive real-world study provides a clinical perspective of the indications, patient profile, lab protocols, procedures, and clinical outcomes from 5 clinical sites across the United States that currently offer this technology. The IVC procedure provides an alternative treatment model with satisfactory outcomes for patients seeking a different path of treatment. The sites participating in this study used IVC to address a diversity of medical, financial, and social factors that determine treatment access.
Acknowledgments
We would like to thank Julia Butler, Martin Langley, Caitlin Williams, and Shawn Zimmerman for their assistance in data extraction and compilation. All authors contributed to the study conduct, data acquisition, analysis, and interpretation, manuscript development, and have read and approved the final manuscript for submission.
Footnotes
T.J.-N. is a full-time employee of Ferring Pharmaceuticals Inc. A.R.C. has received payment for lectures from Ferring Pharmaceuticals Inc, Celmatix, and Progenity. K.J.D is a paid consultant for Ferring Pharmaceuticals Inc. J.E.N. has nothing to disclose. J.K.P. is a paid consultant for Ferring Pharmaceuticals Inc. and Natera and has received payment for lectures from Ferring Pharmaceuticals Inc. R.L.P.-Z. is a paid consultant for Ferring Pharmaceuticals Inc. A.F.K. is a full-time employee of Ferring Pharmaceuticals Inc. L.M.S. has nothing to disclose. R.J.P. has nothing to disclose. G.S.D. is a full-time employee of Ferring Pharmaceuticals Inc.
Supported by Ferring Pharmaceuticals Inc., Parsippany, New Jersey.
Supplementary data
References
- 1.CDC Key Statistics from the National Survey of Family Growth. https://www.cdc.gov/nchs/nsfg/key_statistics/i.htm#infertility Available at:
- 2.Kelley A.S., Qin Y., Marsh E.E., Dupree J.M. Disparities in accessing infertility care in the United States: results from the National Health and Nutrition Examination Survey, 2013-16. Fertil Steril. 2019;112:562–568. doi: 10.1016/j.fertnstert.2019.04.044. [DOI] [PubMed] [Google Scholar]
- 3.Zegers-Hochschild F., Adamson G.D., de Mouzon J., Ishihara O., Mansour R., Nygren K. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92:1520–1524. doi: 10.1016/j.fertnstert.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Chambers G.M., Hoang V.P., Sullivan E.A., Chapman M.G., Ishihara O., Zegers-Hochschild F. The impact of consumer affordability on access to assisted reproductive technologies and embryo transfer practices: an international analysis. Fertil Steril. 2014;101:191–198.e4. doi: 10.1016/j.fertnstert.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Rich C.W., Domar A.D. Addressing the emotional barriers to access to reproductive care. Fertil Steril. 2016;105:1124–1127. doi: 10.1016/j.fertnstert.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Paulson R.J., Fauser B.C.J.M., Vuong L.T.N., Doody K. Can we modify assisted reproductive technology practice to broaden reproductive care access? Fertil Steril. 2016;105:1138–1143. doi: 10.1016/j.fertnstert.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 7.ASRM Practice Committee. Effectiveness and treatment for unexplained infertility Fert Steril 2006;86:s111–s114. [DOI] [PubMed]
- 8.Huang L.N., Tan J., Hitkari J., Dahan M.H. Should IVF be used as first-line treatment or as a last resort? A debate presented at the 2013 Canadian Fertility and Andrology Society meeting. Reprod Biomed Online. 2015;30:128–136. doi: 10.1016/j.rbmo.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Reindollar R.H., Regan M.M., Neumann P.J., Levine B.S., Thornton K.L., Alper M.M. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertil Steril. 2010;94:888–899. doi: 10.1016/j.fertnstert.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Gameiro S., Boivin J., Peronace L., Verhaak C.M. Why do patients discontinue fertility treatment? A systematic review of reasons and predictors of discontinuation in fertility treatment. Hum Reprod Update. 2012;18:652–669. doi: 10.1093/humupd/dms031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Society of Reproductive Medicine Disparities in access to effective treatment for infertility in the United States: an ethics committee opinion. Fertil Steril. 2015;104:1104–1110. doi: 10.1016/j.fertnstert.2015.07.1139. [DOI] [PubMed] [Google Scholar]
- 12.Dolinko A.V., Farland L.V., Kaser D.J., Missmer S.A., Racowsky C. National survey on use of time-lapse imaging systems in IVF laboratories. J Assist Reprod Genet. 2017;34:1167–1172. doi: 10.1007/s10815-017-0964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neal S.A., Morin S.J., Franasiak J.M., Goodman L.R., Juneau C.R., Forman E.J. Preimplantation genetic testing for aneuploidy is cost-effective, shortens treatment time, and reduces the risk of failed embryo transfer and clinical miscarriage. Fertil Steril. 2018;110:896–904. doi: 10.1016/j.fertnstert.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Gode F., Bodur T., Gunturkun F., Gurbuz A.S., Tamer B., Pala I. Comparison of microfluid sperm sorting chip and density gradient methods for use in intrauterine insemination cycles. Fertil Steril. 2019;112:842–848.e1. doi: 10.1016/j.fertnstert.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 15.McQueen D.B., Zhang J., Robins J.C. Sperm DNA fragmentation and recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2019;112:54–60.e3. doi: 10.1016/j.fertnstert.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Alegre L., Del Gallego R., Arrones S., Hernández P., Muñoz M., Meseguer M. Novel noninvasive embryo selection algorithm combining time-lapse morphokinetics and oxidative status of the spent embryo culture medium. Fertil Steril. 2019;111:918–927.e3. doi: 10.1016/j.fertnstert.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Lepine S., McDowell S., Searle L.M., Kroon B., Glujovsky D., Yazdani A. Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst Rev. 2019;7 doi: 10.1002/14651858.CD010461.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreno J.M., Núñez M.J., Quiñonero A., Martínez S., de la Orden M., Simón C. Follicular fluid and mural granulosa cells microRNA profiles vary in in vitro fertilization patients depending on their age and oocyte maturation stage. Fertil Steril. 2015;104:1037–1046.e1. doi: 10.1016/j.fertnstert.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Ranoux C., Aubriot F.X., Dubuisson J.B., Cardone V., Foulot H., Poirot C. A new in vitro fertilization technique: intravaginal culture. Fertil Steril. 1988;49:654–657. doi: 10.1016/s0015-0282(16)59835-5. [DOI] [PubMed] [Google Scholar]
- 20.Frydman R., Ranoux C., editors. INVO: a simple, low cost effective assisted reproductive technology. ESHRE Monographs: Oxford University Press; 2008. pp. 85–89. [Google Scholar]
- 21.Taymor M.L., Ranoux C.J., Gross G.L. Natural oocyte retrieval with intravaginal fertilization: a simplified approach to in vitro fertilization. Obstet Gynecol. 1992;80:888–891. [PubMed] [Google Scholar]
- 22.Lucena E., Saa A.M., Navarro D.E., Pulido C., Lombana O., Moran A. INVO procedure: minimally invasive IVF as an alternative treatment option for infertile couples. Sci World J. 2012;2012 doi: 10.1100/2012/571596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.García-Ferreyra J., Hilario R., Luna D., Villegas L., Romero R., Zavala P. In vivo culture system using the INVOcell device shows similar pregnancy and implantation rates to those obtained from in vivo culture system in ICSI procedures. Clin Med Insights Reprod Health. 2015;9:7–11. doi: 10.4137/CMRH.S25494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doody K.J., Broome E.J., Doody K.M. Comparing blastocyst quality and live birth rates of intravaginal culture using INVOcell™ to traditional in vitro incubation in a randomized open-label prospective controlled trial. J Assist Reprod Genet. 2016;33:495–500. doi: 10.1007/s10815-016-0661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner D.K., Schoolcraft W.B. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11:307–311. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 26.De Novo classification request for INVOCELL Intravaginal Culture System. Available at: https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN150008.pdf. Accessed February 23, 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.