Abstract
Despite substantial grounds for such research, the role of chronic exposure to stressors in the onset and aggravation of learning disabilities (LDs) is largely unexplored. In this review, we first consider the hormonal, (epi)genetic, and neurobiological mechanisms that might underlie the impact of adverse childhood experiences, a form of chronic stressors, on the onset of LDs. We then found that stress factors combined with feelings of inferiority, low self-esteem, and peer victimization could potentially further aggravate academic failures in children with LDs. Since effective evidence-based interventions for reducing chronic stress in children with LDs could improve their academic performance, consideration of the role of exposure to stressors in children with LDs has both theoretical and practical importance, especially when delivered in combination with academic interventions.
Keywords: learning disabilities, children, adverse childhood experiences, HPA axis, cortisol, gene-environment interaction
Learning disabilities (LDs), referred to in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) as a family of specific learning disorders, are diagnosed when “there are specific deficits in an individual’s ability to perceive or process information efficiently and accurately. This neurodevelopmental disorder first manifests during the years of formal schooling and is characterized by persistent and impairing difficulties in learning foundational academic skills in reading, writing, and/or math” (American Psychiatric Association, 2016, p.3). LDs affect between 5.3% to 11.8% of schoolchildren (Katusic et al., 2001). However, despite numerous wide-ranging studies of the mechanisms of LDs (Fletcher & Grigorenko, 2017; Plomin & Kovas, 2005; Pugh et al., 2001), the understanding of their etiology is still far from comprehensive.
LDs are diverse in their presentation and etiology. Their presentation is multidimentional and they engage in numerous cognitive processes, including working memory, attention, and speed of processing, among others (Elliott & Grigorenko, 2014). Given this multidimentionality, variability of presentation, and a variety of theoretical perspectives on LDs, there are multiple criteria that are used to diagnose LDs (Fletcher et al., 2019). In addition, the etiology of LDs is complex. In general, it is assumed that these disorders are familial in nature, as the deficits, although they do not breed true, typically aggregate in families. Yet, there are sporadic cases of LDs related to the presence of both genetic (e.g., chromosomal aberrations) and environmental (e.g., exposure to adverse childhood experiences, ACEs) risk factors.
ACEs are a potential trigger for the onset of LDs as evidenced by greater likelihood of LDs associated with higher incidence of ACEs (Turney, 2020) and, to some extent, by the negative relationship between exposure to chronic stressors and academic performance observed in typically developing children1 (Bethell et al., 2014; Blodgett & Lanigan, 2018; Burke et al., 2011; Delaney-Black et al., 2002). However, no direct evidence is available concerning the effect of exposure to chronic stressors on the onset of LDs. In this review, we argue that there are substantial grounds to conduct studies that can secure such data.
Regardless of the etiology of LDs, their perpetuation and even aggravation might be related to the ongoing stress of living with LDs, especially if they are not remediated. Similar to stress’ contribution to the LDs onset, the contribution of stress to the aggravation of LDs is not well studied. Nonetheless, it is known that stress caused by academic struggles or failure is associated with negative outcomes, such as school anxiety (McClain, 1997) and low self-esteem (Alexander-Passe, 2008). Based on the relevant literature, we argue that systematic research into stress as an aggravator of LDs is both warranted and necessary.
To substantiate our argument, we structure this article as follows. First, we review insights on the hormonal, (epi)genetic, and neurobiological mechanisms that underlie the effects of exposure to chronic stressors associated with ACEs on academic performance and related cognitive processes. We explore to what extent these mechanisms may be involved in the etiology of heterogeneous disorders such as LDs. Second, we discuss the exposure to ACEs together or separately with stressors caused by poor academic performance in children with LDs of any etiology. Here, we assume that such exposures might lead to the aggravation of LDs. Importantly, whether resulting in the onset or aggravation of LDs, we argue that the underlying mechanisms are the same. Thus, the purpose of this review is to describe these mechanisms and consider their role in the effects of ACEs on the onset of LDs, and the effects of ACEs together or separately with academic-failure stress on the aggravation of LDs.
PubMed was searched to identify relevant publications. We also examined relevant references in the identified articles. Selected studies considered the relationships between the exposure to chronic stressors, academic performance, and LDs, as well as the neurobiological, hormonal, and (epi)genetic mechanisms of these relationships. The search results were limited to full-text peer-reviewed articles written in English and representing original empirical studies. All differences discussed in this article are statistically significant at p < 0.05, unless otherwise stated. The effect sizes (ES) presented here are Cohen’s d, a widely used indicator of effect size. When Cohen’s d was stated in the original articles, it was extracted and presented here. In other cases, Cohen’s d was calculated based on the data available in the original publications (e.g., mean values with standard deviations, correlation coefficients, odds ratios) using standard formulas (Borenstein et al., 2009; Cohen, 1988; Rosenthal & Rosnow, 2008). When Cohen’s d was impossible to calculate, the unstandardized beta (B) or standardized beta (ß) values were presented when available.
Chronic stress, academic performance, and LDs
Stress and learning and cognition
The stress response, or just stress, is an alteration in the biologic equilibrium of the organism in reaction to events is referred as stressors. Exposure to isolated short-term stressors leads to an acute stress response, which mobilizes the body’s defense mechanisms and is therefore beneficial, or adaptive. Prolonged or repeatedly occurring (chronic) stressors, or the sense of danger that may precede or follow a stressogenic episode, in the absence of habituation or coping with that stressor, leads to a chronic stress response, or chronic stress, that is maladaptive and correspondingly detrimental to the organism (Shonkoff et al., 2012). Among others, ACEs are often considered to be chronic stressors.
An ACE refers to the exposure of children to potentially traumatic events that may have short- or long-term impacts on their mental and physical health (Felitti et al., 1998). Such events can include (1) child maltreatment (psychological, physical, or sexual abuse), (2) household dysfunction (lack of parental care, experiencing domestic violence, or living with household members who are incarcerated or substance-abusing, mentally or physically ill), (3) community violence (homicides, sexual assaults, robberies, and weapons attacks), and (4) wars and natural disasters (Felitti et al., 1998; van der Kolk, 2005). These experiences may or may not cause chronic stress and the attendant negative consequences, depending on the existing balance between risk and protective factors.
Among such factors are the intensity and/or duration of the child’s exposure to a traumatic event and the presence of the buffering protection of significant others (Brown & Shillington, 2017), which, in turn, can create the prerequisites for children’s emotional coping (Dennis, 2006), a factor that can promote children’s resilience to chronic stressors. Such resilience has been illustrated by the fact that a child’s emotional reactivity moderates the relationship between risk factors and child chronic physiological dysregulation. This can be indexed by hair cortisol levels: for children who showed more emotional reactivity, a higher level of risk factors (low socioeconomic status and low parental sensitivity) was related to higher child cortisol levels (Kao et al., 2019). However, there was no relationship between risk factors and child cortisol levels for children who were less emotionally reactive (Kao et al., 2019).
The literature regarding the relationship between ACEs and academic performance in typically developing children is limited. In one of the available studies, Delaney-Black et al. (2002) found that first graders (n = 299) with higher ACEs exposure had lower reading performance (assessed by the Test of Early Reading Ability, ES = 1.25), and lower intellectual functioning (assessed by the Wechsler Primary and Preschool Scale of Intelligence–Revised, ES = 1.09). A related large-scale study of kindergarten to sixth-graders (n = 2,101) showed the association between higher number of ACEs and higher risk of behavioral/learning problems rated by school staff or documented in medical charts by pediatricians (academic failure, attendance concerns, and school behavior problems, ES = 1.07) (Blodgett & Lanigan, 2018). Another study (n = 701, mean age 8.13 years) demonstrated similar results with ES = 1.92 (Burke et al., 2011)). A higher incidence of ACEs was associated with a higher risk of poor school attendance with ES = 0.88 (Blodgett & Lanigan, 2018) and failure to meet grade-level standards in mathematics, reading, or writing ES = 0.68 (Blodgett & Lanigan, 2018). Also, it has been shown that the number of problems in school increases with the number of ACEs. ACE scores ≥ 1 were associated with increased odds of reporting learning/behavior problems as documented in medical charts by pediatricians as compared to an ACE score of 0, ES = 1.29, while an ES at ACE scores ≥ 4 was 1.92 (Burke et al., 2011). An ACE score of 1 was associated with increased odds of reporting school behavior problems (externalizing, internalizing, or both), as compared to an ACE score of 0 with an ES = 0.48, while an ES in cases of ACE score ≥ 4 was 1.07 (Blodgett & Lanigan, 2018). Similarly, an ACE score of 1 was associated with increased odds of reported academic failure, defined as not meeting grade-level standards in reading, writing, or math, as compared to an ACE score of 0, ES = 0.26, while an ES in cases of ACE scores ≥ 4 was 0.68 (Blodgett & Lanigan, 2018). In a nationally representative sample of US children ages 0–17 (n = 95,677), aimed to assess the prevalence of adverse childhood experiences and associations between them and factors affecting children’s development and lifelong health using the 2011–12 National Survey of Children’s Health, children with two or more ACEs were reported to have a 2.67 times higher risk of repeating a grade than children without any such experiences (Bethell et al., 2014).
Regarding children with LDs, we identified one study that investigated the association between ACEs incidence and the likelihood of LDs (Turney, 2020). In a nationally representative sample of US children ages 0–17 (n = 71,811), aimed to estimate the association between number of ACEs and children’s health using the 2016–17 National Survey of Children’s Health, exposure to three or more ACEs, compared to exposure to no ACEs, was associated with a greater likelihood of LDs (β = 0.96). Reduction of risks was found from the age of 6 to the age of 17.
Importantly, although there are a number of studies similar to those described above, their explanatory power is limited because of their correlational nature. This is because causation research requires designs (e.g., randomization to having or not having ACEs) that are simply not appropriate for this research. Prospective cohort studies satisfy one essential criterion of a cause-effect relationship, temporal precedence of cause over effect, but they hardly meet the other criterion, the absence of a “third variable”, i.e. an unobservable variable that might account for the observed intergroup difference in the absence of randomization. In light of this limitation, several strategies have been suggested (McLanahan et al., 2013). Taking into account available observational studies, authors reviewed strategies for deriving causal effects of traumatic events, such as a father’s absence, on various outcomes including reading and math ability. Most of these strategies include various mathematical modelling approaches (e.g., lagged dependent variable model, growth curve model, individual fixed effects model, sibling fixed effects model, propensity score matching), each with its own advantages and disadvantages, yet none provide a satisfactory substitution for the experimental approach.
To some extent, data obtained using animal experimental approaches can be informative in the context of this review, given that the molecular and cellular substrates of cognitive processes and the stress response are similar in animals and humans. For example, it was shown that chronically stressed rats exhibit profound changes in the morphology of the hippocampus accompanied by learning deficits (Sousa et al., 2000). Such data are of importance in this context since, for example, the hippocampal-dependent learning system plays a role in math learning and disruptions in the functioning of this system, regardless of their origin, could contribute to persistent low-achievement in math (Qin et al., 2014). The relevant animal literature is large, but it is outside the scope of this review. However, we will sample this literature again when causal relationships between exposure to chronic stressors and learning are considered below.
For now, based on this brief summary of the limited literature available, it appears that evidence for the causal connection between ACEs and academic performance in the general population is not sufficiently established, although there is circumstantial evidence (e.g., data from animal studies modeling the impact of ACEs on development and data from correlational studies capturing the associations between exposure to ACEs and academic performance).
If ACEs are associated with an onset of learning difficulties (defined as consistent difficulties related to the mastery of basic concepts, underlying the foundation of reading, mathematics, and other academic skills) or a decline in academic performance, the next question is whether there is a certain “threshold” of exposure to chronic stressors, the crossing of which could not only lead to a decline in academic performance, but also contribute to the onset of LDs. As mentioned above, no direct evidence is available concerning the effect of exposure to chronic stressors on the onset of LDs. However, at least three lines of evidence seem to indirectly support the significance of exposure to such stressors, which we will consider in greater detail later in this review. Briefly, they are: (1) the data on the contribution of stress system dysregulation to physical pathologies and cognitive decline, (2) the data on genetic vulnerability factors that make the carriers of certain genetic variants particularly susceptible to exposure to stressors, and (3) the data on the neurobiological bases that potentially underlie these effects of exposure to stressors.
Learning difficulties as sources of stress
The next question is if LDs can be aggravated by exposure to chronic stressors. In children with LDs, regardless of their etiology, feelings of inferiority and low self-esteem together or separately with ACEs might further compromise their academic performance. Several studies have reported that students with LDs are bullied more than other students, with ES = 0.65 (Nabuzoka & Smith, 1993) and 1.03 (Sabornie, 1994). Twenty-five to 30% of children with LDs have been reported to be socially rejected, compared with 8 to 16% of their typically-achieving peers (Greenham, 1999). In comparison with typically developing children in grades 3 to 5, children who demonstrate poor reading performance experience more sources of school stress, such as negative interactions with teachers (ES = 0.30) and peers (ES = 0.51), poor academic self-esteem (ES = 0.93), and a higher level of manifested school stress, both through emotional (fear, shyness, and loneliness, ES = 0.31) and physiological (nausea, tremors, and rapid heartbeat, ES = 0.26) manifestations (Alexander-Passe, 2008). Middle-school boys with LDs had less academic self-esteem (ES = 1.44) and experienced more anxiety (ES = 0.66 according to self-report, 1.06 as per parental report, and 1.29 according to teacher report) and depression (ES = 0.94, according to self-report, 1.02 as per parental report, and 1.02 according to teacher report) compared to the general education group (McClain, 1997). In addition to these studies, there are books that are partially or wholly devoted to describing the stressors that children with LDs experience (Eaude, 1999; Edwards, 1994; Miles, 2004). Again, all these studies are descriptive in nature and do not elucidate the underlying mechanisms of the observed associations.
Even though the effects of exposure to chronic stressors on the onset and aggravation of LDs have not yet been registered unequivocally, this issue permits and requires thorough investigation. In this review, we will consider biological mechanisms that could potentially underlie these effects. First, we briefly describe the organization of the stress system. Then, we will describe how chronic exposure to stressors is associated with alterations in brain structure and function, which might lead to both the onset and aggravation of LDs. Finally, we will review the genetic and epigenetic mechanisms that could, together with environmental factors, determine or alter stress vulnerability.
Hormonal mechanisms of exposure to stressors on cognition and LDs in children
General mechanisms of stress response
The stress response involves fast sympathetic nervous system activation followed by activation of the hypothalamic-pituitary-adrenal (HPA) axis – the neuroendocrine system crucial for the stress response (Gunnar & Quevedo, 2007). Despite the fact that genes and systems involved in stress resilience/vulnerability go beyond the HPA axis (Feder et al., 2009), the HPA axis is the main focus of our review, since its relationship with the cognitive processes, which are of particular interest to us in the context of LDs, is the most studied. The HPA system is hierarchically organized and very complex, with multiple feedback paths (Figure 1). Exposure to stressors activates the limbic system and the prefrontal cortex, triggering the release of corticotropin-releasing hormone (CRH) by the hypothalamus. CRH binds to CRH receptors in the anterior pituitary gland, leading to the release of adrenocorticotropic hormone (ACTH). ACTH binds to receptors in the adrenal cortex and this leads to the release of glucocorticoids (cortisol in humans and primates; corticosterone in rats and mice) from the adrenal cortex. To protect the body against further activation of the HPA axis, and therefore against excessive secretion of cortisol in response to stressors, negative feedback is activated. Glucocorticoids bind to their receptors in the hypothalamus, the anterior pituitary gland, and the hippocampus. This dampens the release of CRH by the hypothalamus and ACTH by the anterior pituitary gland, inhibiting HPA axis activity. Another negative feedback mechanism is the binding of co-chaperone FKBP5 (FK506-binding protein 51) to the glucocorticoid receptors (GRs). This binding lowers the affinity of cortisol to these receptors.
Figure 1. Scheme of the Hypothalamic-Pituitary-Adrenal (HPA) Axis.
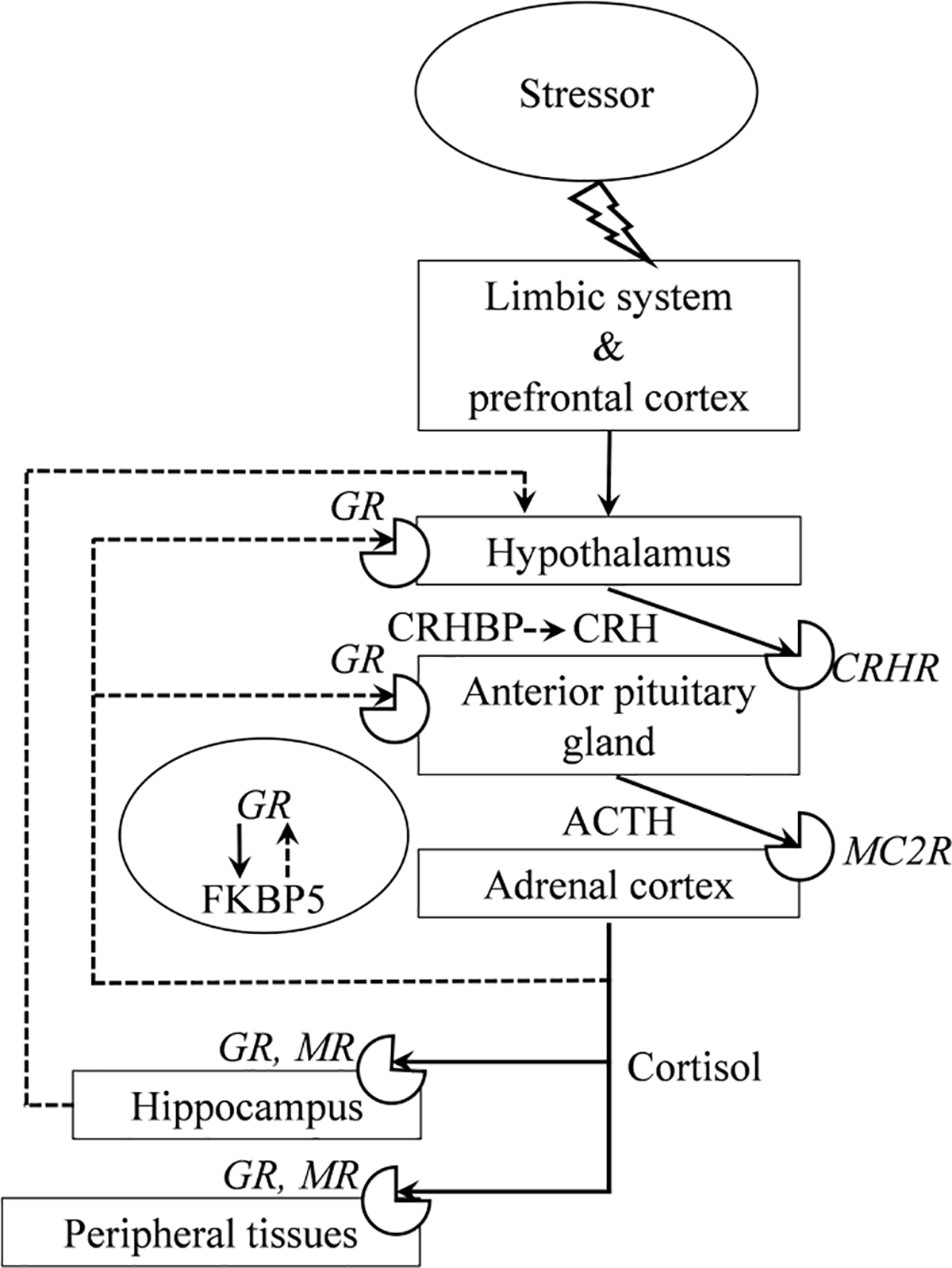
Note. Activating pathways are indicated by solid lines, inhibitory – with dashed ones; circular sector icons represent receptors. ACTH – adrenocorticotropic hormone, CRH – corticotropin-releasing hormone, CRHBP – corticosterone-releasing hormone binding protein, CRHR – corticotropin-releasing factor receptor, FKBP5 – FK506-binding protein 51, GR – glucocorticoid receptor, MC2R – melanocortin-2 receptor, and MR – mineralocorticoid receptor.
Cortisol, the major end product of the HPA axis, is the most researched among the hormones of the HPA axis. This attention stems from its widespread regulatory influences and well-proven role in the stress response, demonstrated in numerous studies and highlighted in meta-analyses that consider cortisol a reliable biomarker of response to stressors. Human studies have indicated that the average effect size of the cortisol response is medium to large (Michaud et al., 2008).
Adaptive responses of the HPA axis to stressors are manifested in the elevation of cortisol levels. This elevation allows the organism to deal with real and perceived threats through the mobilization of energy stores, heightened vigilance, and the activation of learning and memory processes (Sapolsky et al., 2000). However, not every stress response is adaptive. In children, situations in which intense and/or prolonged activations of stress response systems may take place, especially in the absence of support by a caring adult, can be associated with the occurrence of chronic stress response, one of the manifestations of which is HPA axis dysregulation. Such dysregulation may be expressed in both the hyper- (Azar et al., 2007) or hypo-activation (Ouellet-Morin et al., 2011) of this axis in response to exposure to stressors, and thus could be associated with negative outcomes for academic performance, which we will consider further. In addition, cortisol secretion has a diurnal rhythm: its level peaks in the morning to prepare the organism for the beginning of the day, then slowly decreases, plateauing in the middle of the day and reaching its lowest level in the evening. Deviations from this pattern of cortisol release can be also considered HPA axis dysregulation: both reduced morning baseline cortisol levels (Bernard et al., 2017) or increased morning and afternoon cortisol levels (Cicchetti & Rogosch, 2001) are observed during the day in children subjected to ACEs. As revealed in some cases of chronic exposure to stressors, reduced cortisol levels could reflect the phenomenon of hypocortisolism, a form of HPA axis dysfunction. Hypocortisolism is presumably associated with the overactivation of the negative feedback pathways to protect the organism from the persistent elevation of cortisol levels in response to chronic exposure to stressors. However, such mechanisms are not yet well understood.
One of the mechanisms highly involved in the regulation of the HPA axis activity is the epigenetic regulation of glucocorticoid receptor gene expression in the hippocampus (McGowan et al., 2009; Turecki & Meaney, 2016). At the molecular level, epigenetic processes are those that regulate gene expression without altering the DNA sequence (Ptashne, 2007). DNA methylation comprises one of the most studied epigenetic mechanisms (Boyes & Bird, 1992; Hsieh, 1994) by means of which adversity gets under the skin (Hyman, 2009). One example is association between early-life adversity and an increase in the DNA methylation level of the GR gene in the hippocampus, and hence a decrease in its expression (McGowan et al., 2009; Turecki & Meaney, 2016). A decrease in the number of GRs in the hippocampus leads to an attenuation of the negative feedback of the HPA axis, which, in turn, results in an increase of cortisol level when an individual is exposed to stressors.
Cortisol as a marker of chronic exposure to stressors in academic performance and LDs
Multiple research designs have been used to investigate the connection between HPA axis dysregulation and developmental outcomes. Prospective cohort studies in older persons have shown that HPA axis dysregulation could predict an increased risk of negative outcomes. For example, elevated cortisol levels predict an increased risk of dying from cardiovascular disease across six years of follow-up, ES = 0.89 (Vogelzangs et al., 2010). Higher cortisol reactivity to a psychosocial stressor (assessed by the Trier Social Stress Test, TSST2) was associated with the heightened probability (B = 0.01, p = 0.023, n = 129) of developing cognitive impairment without dementia five years later (de Souza-Talarico et al., 2020). Elevated morning cortisol levels predicted an increased risk of deteriorated cognitive status (ES = 0.26) across one year of follow-up (Zhong et al., 2018). Therefore, HPA axis dysregulation may contribute to the pathology of physical and cognitive decline in older persons. In children, the only prospective cohort study we could find revealed that higher basal salivary cortisol levels at 7, 15, and 24 months of life were predictors of a lower level of academic performance at 60 months of life (ß = −0.07, p = 0.003, n = 1,292), but only for children with concurrently moderate to high levels of salivary alpha-amylase, a marker of autonomic nervous system (ANS) activity (Berry et al., 2012). Since the ANS and HPA axis systems function in a coordinated manner to regulate stress physiology (Granger et al., 2007), it is not surprising that behavioral effects are influenced by both of them, such that both high cortisol and high alpha-amylase concentrations are associated with the most pronounced behavior problems.
Most of the studies on the role of cortisol in academic performance are cross-sectional (or cross-sectional with a delayed follow-up). It has been demonstrated (Jimerson et al., 2006) that a higher salivary cortisol response to school settings (school relative to home cortisol levels) was correlated with academic failure in 5 to 12 year old children (ES = 0.49 for language scores, ES = 0.52 for social studies scores, ES = 0.47 for science scores). Children aged 8 to 10 years who were given the TSST performed more poorly in the delayed memory retrieval test than children from the non-stressed group (ES = 0.65) (Quesada et al., 2012). Furthermore, in this study, the participants characterized by a higher cortisol response committed more errors (ES = 0.80) during the delayed retrieval than those who showed a lower cortisol response. No statistically significant association was found between cortisol levels during school standardized math testing and academic test performance, although an increase in cortisol level was observed almost exclusively (87.5%) in students with low test performance (Lindahl et al., 2005). Interestingly, this work demonstrated that the lack of an effective coping strategy (reported children’s reactions to performance demands, “I get worried and will have problems solving other tasks too”) was accompanied by an increase in cortisol levels during the test situation. Higher levels of salivary cortisol reactivity to the testing of various cognitive processes were associated with poorer working memory for numbers (ES = 0.66), weaker working memory for words (ES = 0.88), and lower performance on quantitative concepts tasks in grade 1 children (ES = 0.55) (MacKinnon McQuarrie et al., 2014).
Together, these studies show a rather straightforward relationship of high cortisol levels with low levels of academic performance and memory retrieval, with ESs ranging from small to large. These associations may either indicate a more pronounced stress reaction in low achieving students, a negative impact of strong cortisol response on academic performance, or both. Given that HPA axis dysregulation may contribute to the pathology of physical and cognitive decline in older persons, its contribution to the development of cognitive deficits in children, particularly LDs, is quite possible.
It is notable that studies on the association of ACEs and cortisol level with academic performance have been conducted separately, and there is a lack of research examining cortisol level as a mediator of the associations between ACEs and academic performance. Importantly, however, there is evidence that previously institutionalized children exhibited hypocortisolism at ages 1.5–4.5 years, and this predicted problems with attention and externalizing behaviors during kindergarten (Koss et al., 2016). These early deficits might form the foundation for subsequent weaknesses in academic performance.
Regarding children with LDs, we found three studies that investigated their HPA axis activity. In the first study, children with one type of LD, specific reading disability (SRD), and mean age of 12.61 years showed lower baseline cortisol levels and hypo-activation of the HPA axis in response to an acute psychosocial stressor (TSST). Specifically, more children who were non-reactive to the stressor were found in the group of children with SRD (64.7%; n = 11) than in the group of typically developing children (27.3%, n = 6), with no statistically significant differences in cortisol reactivity between these groups (Espin et al., 2019). In the second study (Huang et al., 2020), the basal level of cortisol in children with SRD (average age of 10.09 years), remained consistently low throughout the day without any signs of the diurnal rhythm normally seen in typically developing children (Z = −7.65, −6.97, and −6.77 for morning, afternoon, and night cortisol, correspondingly; all p < 0.001, n = 144). Finally, a third study investigated the associations between HPA axis activity and academic performance. Children at the mean age of 6.5 years with math disabilities had significantly lower scores on a working memory task involving numbers and letters (letter-number sequence task) when they had higher cortisol reactivity to the testing procedures, ES = 1.28 (MacKinnon McQuarrie et al., 2014). Unfortunately, these studies lack data on the children’s levels of chronic exposure to stressors. Taking into account the fact that children with LDs are often bullied (Greenham, 1999; Nabuzoka & Smith, 1993; Sabornie, 1994), and the evidence that bullying as a form of psychological maltreatment heightens cortisol response to acute psychosocial stressors (Chen et al., 2018), studying the role of stress in their academic performance is of particular importance.
Thus, currently available data indicate that ACEs are associated with the dysregulation of the HPA axis, expressed in both its hyper- and hypoactivity. It has also been shown that elevated cortisol levels are associated with lower academic performance. Since cortisol plays a significant role in memory processes (de Kloet et al., 1999; Oitzl & de Kloet, 1992; Roozendaal et al., 2010), its reduction can also negatively affect them (Marin et al., 2011). An essential link between the components of the HPA axis and behavioral outcomes, including academic performance, is brain morphophysiology. In the next section, we will discuss the role of stress on the brain’s morphophysiology and the potential role of these effects in the onset and aggravation of LDs.
Neurobiological mechanisms of effects of exposure to stressors on cognition and LDs in children
Memory is one of the most intensively studied processes in research on the role of exposure to stressors on cognitive processes. Cortisol levels are related to memory and learning, but not in a straightforward way. Moderate cortisol levels positively affect memory formation and modulation (de Kloet et al., 1999; Oitzl & de Kloet, 1992; Roozendaal et al., 2010), playing an adaptive role, since long-term memories allow one to remember and effectively cope with dangerous conditions upon repeated encounters with them. However, exposure to chronic stressors could potentially lead to a number of negative consequences for structural and functional brain organization, which may potentially underlie deleterious effects on cognitive processes, including low academic performance. Of note, in addition to glucocorticoids, a number of molecular mediators of the negative neurophysiological effects of chronic exposure to stressors have been revealed (Cameron et al., 1997; McEwen et al., 2016; Sandi, 2004; Smith et al., 1995).
Data on cellular alterations in brain structure under the influence of chronic stressors come from animal studies. In rats, chronic exposure to stressors leads to: the reduction of dendritic spine density (Radley et al., 2006) and dendritic length (Radley et al., 2004) in pyramidal neurons of the medial prefrontal cortex, the reduction in the dendritic length of hippocampal pyramidal neurons (Watanabe et al., 1992), the suppression of neurogenesis in the hippocampus (Pham et al., 2003), and an increase in neuronal death in the cerebral cortex (Bachis et al., 2008). Since these changes are accompanied by learning deficits (Sousa et al., 2000), it is likely that these same mechanisms, at least partially, cause the associations between children’s exposure to stressors and low academic performance.
Numerous studies have shown a correlation between children’s brain structural outcomes and exposure to ACEs. The severity of exposure to stressors during early childhood (through 5 years of age) was negatively associated with the left (ES = 0.59) and right (ES = 0.67) hippocampal volumes in 9–13-year-old children (Humphreys et al., 2019). Post-institutionalized children with median age at adoption of 12 months had smaller left hippocampal volumes than non-adopted children at the age of 12–14 years with ES = 0.46 (Hodel et al., 2015). It has been demonstrated (Tyborowska et al., 2018) that children who experienced more negative ACEs before age 5 showed the largest grey matter volume decrease of the prefrontal cortex at the age of 12–14 years (p < 0.05, n = 37). The data on the relationship between the volume of the amygdala and the ACEs exposure are contradictory and revealed both an increase (Mehta et al., 2009; Tottenham et al., 2010) and a decrease (Hodel et al., 2015; Tyborowska et al., 2018) in its volume.
It can be seen from the above examples that the hippocampus, the prefrontal cortex, and the amygdala are the main targets of exposure to stressors among the brain structures, which has been partly explained by the presence of the high number of GRs (Sanchez et al., 2000). A decrease in the number of neurons and the volume of brain areas enriched with GRs, along with the changes in cell morphology described above, can potentially lead to a corresponding reduction in the number of GRs. This decrease can attenuate the negative feedback pathways (Figure 2), which, in turn, can increase stress hyperactivity. Furthermore, exposure to chronic stressors downregulates the expression of GRs (at both the mRNA and protein levels) in the hippocampus and the prefrontal cortex. This can also diminish the effectiveness of the negative feedback in the HPA axis (Chiba et al., 2012; Mizoguchi et al., 2003), reflecting HPA axis dysregulation associated with various negative outcomes in academic performance, as we described earlier.
Figure 2.
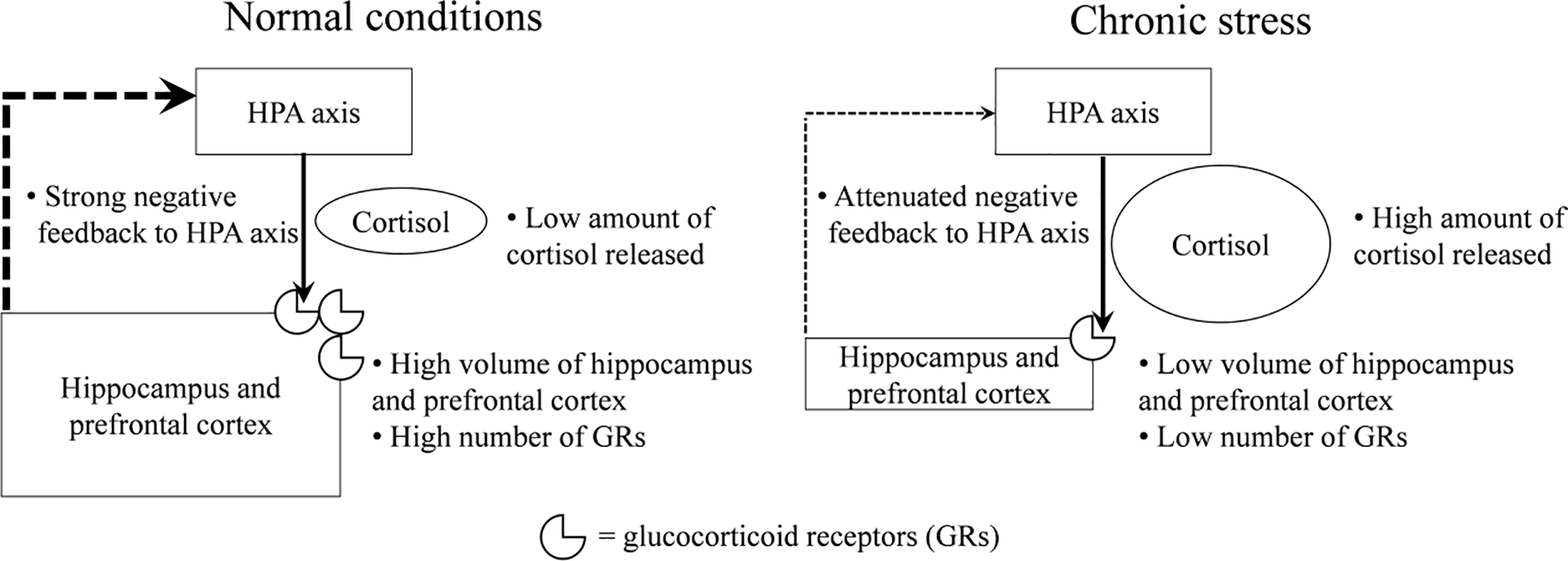
The negative feedback mechanism in the HPA axis under normal conditions and under chronic stress
In addition to enabling negative feedback in the regulation of HPA axis activity, these brain structures are involved in cognitive functioning: the hippocampus plays a critical role in memory consolidation and retrieval (Lisman et al., 2017), and the prefrontal cortex is involved in executive functions, memory, and verbal fluency (Siddiqui et al., 2008). These brain areas also play a role in mastering reading and math skills (Grigorenko et al., 2019) and, therefore, may be relevant in the pathogenesis of LDs, not least due to the fact that children with LDs are characterized by the disruption of memory processes and executive functioning (Alloway, 2018). Of note, the list of brain areas involved in reading- and math-related networks is much more extensive than, but not limited to, the hippocampus and the prefrontal cortex (Grigorenko et al., 2019; Pugh et al., 2001).
Taking into account the aforementioned associations of exposure to stressors and cognitive functions, and given the widespread distribution of GRs in the brain beyond the hippocampus and the prefrontal cortex (Siamatras & Stratakis, 2017), it could be said that this exposure may also affect the structure and the functioning of other brain areas, at both macro and micro levels. It should be also noted that the parameters commonly used in studies of the negative impact of exposure to stressors on the brain – specifically brain surface area, volume, and cortical thickness – are very general characteristics of the brain. Therefore, even in the absence of changes in these indicators, it is impossible to exclude alterations at the microstructural cellular and molecular levels of the brain, whose study is limited in humans. As has been concluded, structural and functional brain connectivity changes are also among the neurophysiological consequences of chronic exposure to stressors (Teicher et al., 2016).
The associations between this exposure and macrostructural deficits described in the human brain, as well as the causal relationships between chronic exposure to stressors and microstructural deficits in the animal brain, suggest that exposure to chronic stressors can potentially lead to changes in the neuronal substrates. These substrates enable the emergence of academic skills, such as reading, writing, and math, therefore changes in these substrates may compromise this functioning, causing the onset and aggravation of LDs. We assume that stress affects these two processes in a spiral cyclic manner: stress caused by ACEs exposure leads to or is a factor in the macro- and microstructural changes in the brain that can provoke the emergence of academic failures that qualify as LDs. The manifestation of LDs, regardless of their etiology, in turn, can provoke bullying, feelings of inferiority, and low self-esteem in a school context that continuously demands high levels of performance from struggling students. These additional stressors could cause further macro- and microstructural changes in the brain, resulting in further deterioration of academic performance, that is, the aggravation of LDs. As children grow older, this spiral can potentially lead to deeper and broader consequences, as illustrated by data on elevated rates of LDs among youth involved with the Juvenile Justice System (Grigorenko, 2006).
An essential modulator of the association between ACEs and the development of cognitive functions could be a genetic predisposition to stress vulnerability. In the next section, we will consider the origins of children’s resilience or vulnerability to stress, as determined by genetic and epigenetic mechanisms that control HPA axis functioning, which could play a role in the onset and aggravation of LDs.
Genetic and epigenetic factors in the vulnerability and resilience to exposure to stressors
Stress vulnerability and resilience are usually understood as the inability or ability to cope with exposure to stressors, respectively. Coping efficiency could be assessed by the level of hormonal response to a stressor, by a circadian pattern of cortisol secretion, or by the incidence of pathological outcomes of stress. Stress vulnerability can manifest as neuroendocrine hypo- and hyperactivity of the HPA axis in response to a stressor, an abnormal change in the circadian pattern of cortisol secretion, or the induction of stress outcomes and stress-related psychopathologies, such as depression, anxiety, post-traumatic stress disorder (PTSD), or attention-deficit/hyperactivity disorder (ADHD). Correspondingly, the adaptive reactivity of the HPA axis to stressors, the normal circadian pattern of cortisol secretion, or the minimal incidence of stress outcomes can be regarded as stress resilience.
One of the key factors influencing children’s stress coping strategies, and therefore their stress resilience, is their relationships with attachment figures. Secure attachment has been shown to be effective in the reduction or prevention of cortisol increases to exposure to stressors at the age 12–18 months old (Luijk et al., 2010; Nachmias et al., 1996; Spangler & Grossmann, 1993; Spangler & Schieche, 1998). Less information is available for older ages. In girls aged 7– 12 years, contact with mothers after taking the TSST almost eliminated the cortisol response, which was expressed in more rapidly decreasing and lower levels of peak cortisol, compared to situations with no maternal contact (Seltzer et al., 2010). Of note, preparing for a public speech together with the parent at ages 9–16 becomes a less potent buffer of HPA axis activation in response to stress as pubertal stage increases (Doom et al., 2015; Hostinar et al., 2015). Taken together, these data indicate the importance of the social regulation of stress resilience.
Regarding the genetic basis of stress vulnerability/resilience, we will provide some examples of the role of the polymorphisms in HPA axis genes in stress reactivity, which can constitute either protective or risk factors. The role of genetic factors in non-HPA systems involved in stress resilience/vulnerability, specifically SNP alleles and genotypes3, has been previously discussed (Feder et al., 2009). In addition, we will review the data on the impact of genome-environment interactions on stress vulnerability/resilience.
The variety of genetic variants in the HPA-axis genes underlies the interindividual variability in HPA axis activity and, eventually, stress vulnerability or resilience. Such variants are called “risk” or “protective” alleles, correspondingly. Generally, the HPA axis genes’ polymorphisms can moderate cortisol reactivity and recovery and the presence of certain genetic variants can lead to the prolongation of the HPA axis activation, which can result in chronically elevated cortisol levels after repeated exposure to stressors. This has been revealed in the relevant genetic association studies, and these results are summarized in Table S1. It is important to note that the literature on the role of genetic variability on stress vulnerability/resilience is primarily based on studies in adults with a minor proportion of research conducted with children.
Notwithstanding, there is evidence of gene-environment interactions4: ACEs can modulate the associations between genetic polymorphisms and stress vulnerability/resilience, potentially presenting a double risk/benefit to the allele carrier (for review, see Normann & Buttenschøn, 2020). An important outcome of stress vulnerability/resilience is academic performance in children, but we found only one relevant study (Halldorsdottir et al., 2019), Table S1. It reported that children with genetic risk who were prenatally exposed to stress via violence from the mother’s partner, are characterized by high, prolonged stress-induced cortisol reactivity in test situations designed to induce fear and frustration (mask presentation, barrier procedure, arm restraint procedure, and toy removal procedures) at the age of 15 (ES = 0.13) and 24 months (ES = 0.20). Furthermore, reading ability in primary school grades (1, 2, and 5) was lower in these children (ES = 0.39). Thus, both genetic and environmental factors determine stress resistance and vulnerability, and the HPA axis genes’ polymorphisms moderate the risk for negative outcomes after ACEs.
The mechanisms modulating the associations of ACEs with HPA axis activation can include epigenetic mechanisms, particularly DNA methylation (for review, see Krause et al., 2020). In addition to the sole involvement of epigenetic mechanisms, their interactions with genetic polymorphisms can also take place. For example, the carriers of the risk alleles of HPA axis genes with specific DNA demethylation levels in these genes are characterized by more exaggerated or prolonged cortisol reactivity to stressors (Table S1).
Data on the role of polymorphisms in HPA axis genes and epigenetic regulation of HPA axis activity in modulating reactivity to stressors in children with LDs are currently not available and remain to be explored. However, based on the data described above, it may be that polymorphisms of the HPA axis genes and the epigenetic statuses of these genes might play an essential role in the influence of exposure to stressors on the onset and aggravation of LDs. Currently, there are no data showing that all children who are exposed to ACEs will develop LDs in the future, and this seems unlikely. It is more probable that children who later develop LDs could have some kind of innate vulnerability to LDs and, when exposed to stressors, LDs may be expressed. Among the candidate mechanisms of this vulnerability, there are undoubtedly genetic mechanisms that are far from being limited to only the HPA axis genes (Fletcher & Grigorenko, 2017).
Conclusions
In this review, we hypothesized that many associations between chronic exposure to stressors and low academic performance could be at least in part caused by dysregulation of the HPA axis. We discussed a number of sources of indirect evidence of this causal pathway and also examined genetic factors that could have an effect on these processes. A theoretical framework of factors that potentially contribute to the onset and aggravation of LDs is summarized in the diagram (Figure 3).
Figure 3. A Theoretical Framework of Factors Potentially Contributing to the Onset (A) and Aggravation (B) of LDs.
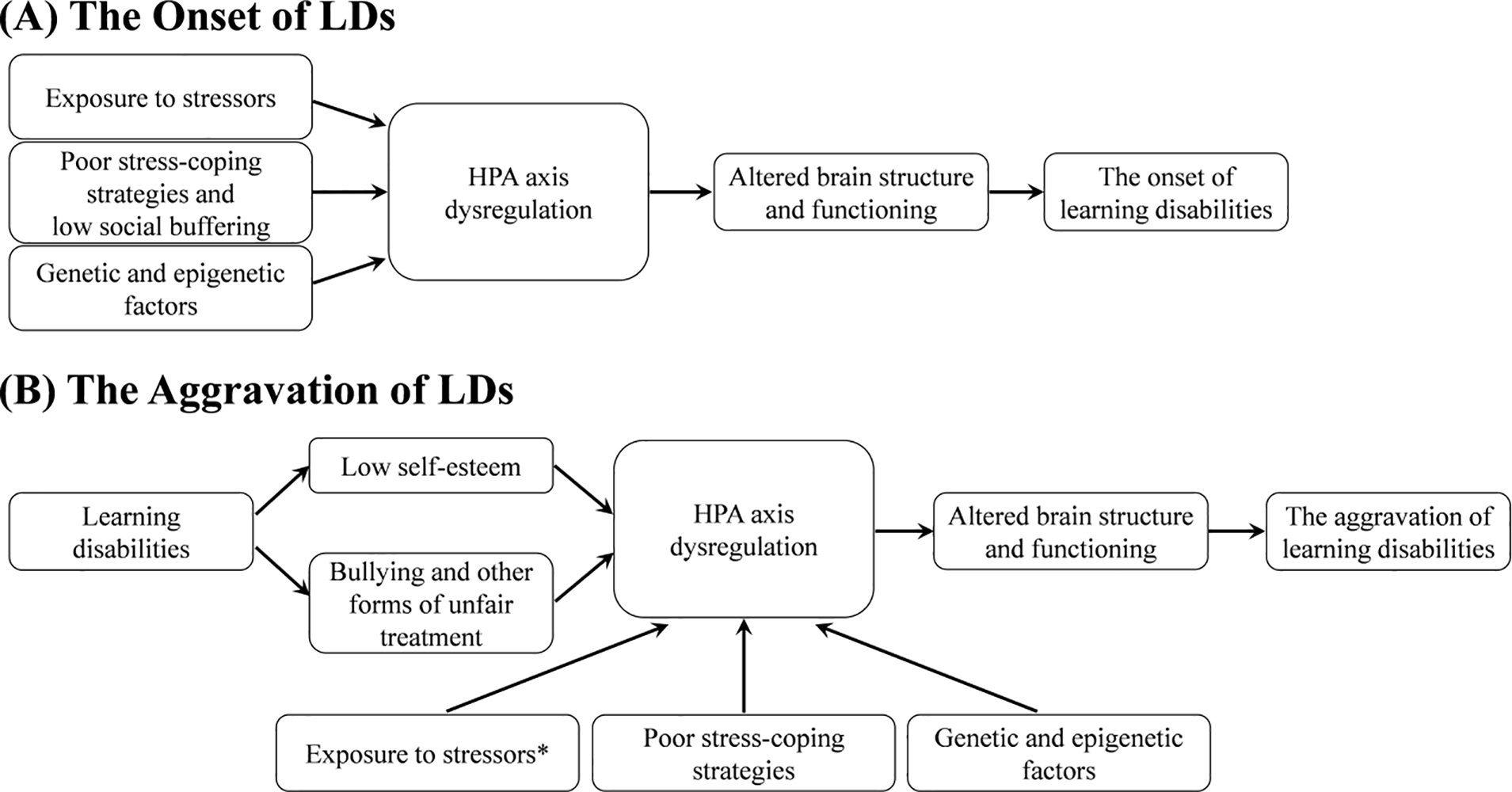
Note. *Exposure to stressors may or may not occur in parallel with bullying and low self-esteem.
Genetic and epigenetic factors, exposure to stressors and poor stress-coping strategies may moderate cortisol reactivity and recovery and determine stress vulnerability, expressed in dysregulation of the HPA axis. Dysregulation of the HPA axis influences brain structure and functioning, which, in parallel, are guided by a wide range of genes (Lenroot & Giedd, 2011). Altered brain structure, function, and connectivity induced by ACEs could influence a decline in academic performance that ultimately, at a certain threshold, may become classified as LDs. Children with LDs may then be subjected to even higher exposure to stressors as their learning difficulties are associated with more frequent experiences of failure, lower self-esteem, and increased peer victimization, thus possibly leading to further dysregulation of the HPA axis, and, ultimately, to aggravation of LDs.
Considering all above-mentioned prerequisites, future research directed towards testing our hypothesis on the influence of exposure to chronic stressors on the onset and further aggravation of LDs seems promising in terms of both its theoretical and practical importance. To study the role of exposure to chronic stressors in the onset of LDs, prospective cohort studies could be performed with children without LDs, who are naturally subjected to different levels of chronic stressors, while subsequently monitoring the incidence of LDs depending on children’s chronic stress response. Our hypothesis is that children showing the most pronounced chronic stress response will have a higher incidence of LDs. To reveal the role of stress in the aggravation of LDs, a prospective cohort design should also be utilized to measure the level of exposure to chronic stressors, level of chronic stress response, and the severity of LDs at the beginning of the study, with subsequent measurements of these three indicators longitudinally. We hypothesize that in children with LDs who are showing the most pronounced chronic stress response to chronic stressors, the severity of LDs will increase over time.
In both cases, cortisol levels, particularly within hair, appear to be a well-established and a reliable marker of chronic stress response. Thus, among the sources of cortisol to be analyzed (e.g., saliva, blood, urine, cerebrospinal fluid, and hair), hair cortisol concentration is the most cumulative measure, meaning it is not subjected to diurnal fluctuations seen in blood and saliva, and can be used as a retrospective measure of cortisol secretion for periods up to 6 months (Kirschbaum et al., 2009). Also, hair cortisol level has been positively associated with ACEs (Simmons et al., 2016).
The assumption that chronic stress response induced by chronic exposure to stressors may be involved in the onset and aggravation of LDs can lead to the following conclusion: effective science-based interventions for reducing chronic stress levels, thus improving the academic trajectories of children at risk for LDs, need to be created and implemented. Current educational interventions are mainly aimed at the cognitive aspects of LDs, namely instruction and teaching of the affected skills. These programs could become more effective if they included elements of intervention aimed at stress management and coping. 15-minute relaxation exercises, given once a week for a school year, raised reading performance and lowered anxiety among readers with disabilities (Frey, 1980). Mindfulness-based interventions show promise in relieving stress, anxiety, and depression and improving academic performance in children with LDs (Beauchemin et al., 2008; Peddigrew & McNamara, 2019). Taken together, these approaches might have long-term benefits for at-risk children.
There are a number of limitations to the current review. First, we did not consider the role of children’s gender and age. Gender and age differences in HPA axis activity take place in childhood, but the evidence is not unequivocal (Hollanders et al., 2017; van der Voorn et al., 2017). Given the existence of sensitive periods – times of increased brain plasticity when the brain may be particularly susceptible to negative influences – the timing of chronic exposure to stressors could influence its effects on children’s cognitive development (Teicher et al., 2016). Second, we did not take into account the role of children’s temperaments in vulnerability to stress, despite the availability of relevant data (Burenkova & Podturkin, 2020). Third, the impact of stress on various forms of LDs deserves separate consideration, since certain forms of LDs can potentially be more or less sensitive to the effects of stress, both in their onset and aggravation. Yet, although the remediation of these limitations requires empirical data and more theoretical work, we think that the main objective of this article has been accomplished: we have outlined possible mechanisms that could underlie the assumed effects of exposure to stressors on the onset and aggravation of LDs. Therefore, the link between ACEs and LDs should be studied in both children who have reported ACEs and may be at risk for the development of LDs, and in children with LDs, whose adverse experience might result in the aggravation of their LDs.
Supplementary Material
Highlights.
Stress plays a role in the onset and aggravation of learning disabilities (LDs).
The relevant possible mechanisms involve hormonal, (epi)genetic, and neurobiological pathways.
Stress-reducing interventions could improve academic performance in children with LDs.
Acknowledgments
This work was supported by the award P50HD052117 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to the University of Houston, for the Texas Center for Learning Disabilities (PI: J. Fletcher), P20HD091005 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to Baylor College of Medicine (PI: E. Grigorenko), and by the Government of the Russian Federation, grant number 14.Z50.31.0027 (PI: E. Grigorenko). We are grateful to Dr. Aleksei Podturkin for discussions and critical review of the manuscript and Mr. Rammy Allouche, Ms. Nicole Guha and Ms. Mei Tan for their editorial support.
Footnotes
Here, we define “child” as a person 0–18 years of age in accordance with the Convention on the Rights of the Child UNICEF (1989).
The Trier Social Stress Test (TSST) is a well-known, standardized laboratory psychosocial stress task that elicits a profound HPA axis activation Kirschbaum, C., Pirke, K. M., & Hellhammer, D. H. (1993). The Trier Social Stress Test - a Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology, 28(1–2), 76–81. https://doi.org/Doi10.1159/000119004. It consists of three stages: preparation for giving a speech, giving a free speech for a mock job interview, and the math task (orally performed serial subtraction), the last two of which occur in front of an audience of evaluative experts and are videotaped. The experience of being exposed to potentially negative judgment by experts, and of anticipating an uncontrollable performance outcome is associated with the largest and the most reliable cortisol responses compared to other laboratory stressors Campbell, J., & Ehlert, U. (2012). Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology, 37(8), 1111–1134. https://doi.org/10.1016/j.psyneuen.2011.12.010
SNP (single nucleotide polymorphism) is a variation of a single base pair at a specific position in DNA sequence (due to substitution, deletion or insertion) found in a population. A particular single nucleotide variant (SNV) is called an allele, and a group of SNVs that are inherited together is called a haplotype. The combination of the gene alleles in a diploid individual genome is called a genotype.
Gene–environment interaction is defined as “a different effect of an environmental exposure on disease risk in persons with different genotypes,” or, alternatively, “a different effect of a genotype on disease risk in persons with different environmental exposures.” Ottman, R. (1996). Gene–environment interaction: definitions and study design. Preventive Medicine, 25(6), 764–770. https://doi.org/10.1006/pmed.1996.0117
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander-Passe N (2008). The sources and manifestations of stress amongst school-aged dyslexics, compared with sibling controls. Dyslexia, 14(4), 291–313. 10.1002/dys.351 [DOI] [PubMed] [Google Scholar]
- Alloway TP (2018). Working Memory and Clinical Developmental Disorders: Theories, Debates and Interventions. Routledge. [Google Scholar]
- American Psychiatric Association. (2016). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). American Psychiatric Publishing. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Azar R, Paquette D, Zoccolillo M, Baltzer F, & Tremblay RE (2007). The association of major depression, conduct disorder, and maternal overcontrol with a failure to show a cortisol buffered response in 4-month-old infants of teenage mothers. Biological Psychiatry, 62(6), 573–579. 10.1016/j.biopsych.2006.11.009 [DOI] [PubMed] [Google Scholar]
- Bachis A, Cruz MI, Nosheny RL, & Mocchetti I (2008). Chronic unpredictable stress promotes neuronal apoptosis in the cerebral cortex. Neuroscience Letters, 442(2), 104–108. 10.1016/j.neulet.2008.06.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin J, Hutchins TL, & Patterson F (2008). Mindfulness meditation may lessen anxiety, promote social skills, and improve academic performance among adolescents with learning disabilities. Complementary Health Practice Review, 13(1), 34–45. 10.1177/1533210107311624 [DOI] [Google Scholar]
- Bernard K, Frost A, Bennett CB, & Lindhiem O (2017). Maltreatment and diurnal cortisol regulation: A meta-analysis. Psychoneuroendocrinology, 78, 57–67. 10.1016/j.psyneuen.2017.01.005 [DOI] [PubMed] [Google Scholar]
- Berry D, Blair C, Willoughby M, Granger DA, & Family Life Project Key, I. (2012). Salivary alpha-amylase and cortisol in infancy and toddlerhood: direct and indirect relations with executive functioning and academic ability in childhood. Psychoneuroendocrinology, 37(10), 1700–1711. 10.1016/j.psyneuen.2012.03.002 [DOI] [PubMed] [Google Scholar]
- Bethell CD, Newacheck P, Hawes E, & Halfon N (2014). Adverse childhood experiences: assessing the impact on health and school engagement and the mitigating role of resilience. Health Affairs, 33(12), 2106–2115. 10.1377/hlthaff.2014.0914 [DOI] [PubMed] [Google Scholar]
- Blodgett C, & Lanigan JD (2018). The Association Between Adverse Childhood Experience (ACE) and School Success in Elementary School Children. School Psychology Quarterly, 33(1), 137–146. 10.1037/spq0000256 [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges LV, Higgins JP, & Rothstein HR (2009). Introduction to meta-analysis. John Wiley & Sons, Ltd. [Google Scholar]
- Boyes J, & Bird A (1992). Repression of genes by DNA methylation depends on CpG density and promoter strength: evidence for involvement of a methyl-CpG binding protein. The EMBO Journal, 11(1), 327–333. 10.1002/j.1460-2075.1992.tb05055.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, & Shillington AM (2017). Childhood adversity and the risk of substance use and delinquency: The role of protective adult relationships. Child Abuse & Neglect, 63, 211–221. 10.1016/j.chiabu.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Burenkova OV, & Podturkin AA (2020). Objective Assessment of Temperament in Temperamentally Vulnerable Children: Role in the Studies on Their Stress Levels. New Directions for Child and Adolescent Development, 169, 1–19. 10.1002/cad.20330 [DOI] [PubMed] [Google Scholar]
- Burke NJ, Hellman JL, Scott BG, Weems CF, & Carrion VG (2011). The impact of adverse childhood experiences on an urban pediatric population. Child Abuse & Neglect, 35(6), 408–413. 10.1016/j.chiabu.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Tanapat P, & Gould E (1997). Adrenal steroids and N-methyl-D-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience, 82(2), 349–354. 10.1016/s0306-4522(97)00303-5 [DOI] [PubMed] [Google Scholar]
- Campbell J, & Ehlert U (2012). Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology, 37(8), 1111–1134. 10.1016/j.psyneuen.2011.12.010 [DOI] [PubMed] [Google Scholar]
- Chen G, Kong Y, Deater-Deckard K, & Zhang W (2018). Bullying Victimization Heightens Cortisol Response to Psychosocial Stress in Chinese Children. Journal of Abnormal Child Psychology, 46(5), 1051–1059. 10.1007/s10802-017-0366-6 [DOI] [PubMed] [Google Scholar]
- Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, & Kunugi H (2012). Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 39(1), 112–119. 10.1016/j.pnpbp.2012.05.018 [DOI] [PubMed] [Google Scholar]
- Cicchetti D, & Rogosch FA (2001). Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology, 13(3), 677–693. 10.1017/s0954579401003145 [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioural sciences. (2nd ed.). Laurence Erlbaum Associates. [Google Scholar]
- de Kloet ER, Oitzl MS, & Joels M (1999). Stress and cognition: are corticosteroids good or bad guys? Trends in Neurosciences, 22(10), 422–426. 10.1016/s0166-2236(99)01438-1 [DOI] [PubMed] [Google Scholar]
- de Souza-Talarico JN, Alves AR, Brucki SMD, Nitrini R, Lupien SJ, & Suchecki D (2020). Cortisol reactivity to a psychosocial stressor significantly increases the risk of developing Cognitive Impairment no Dementia five years later. Psychoneuroendocrinology, 115, 104601. 10.1016/j.psyneuen.2020.104601 [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Ondersma SJ, Nordstrom-Klee B, Templin T, Ager J, Janisse J, & Sokol RJ (2002). Violence exposure, trauma, and IQ and/or reading deficits among urban children. Archives of Pediatrics & Adolescent Medicine, 156(3), 280–285. 10.1001/archpedi.156.3.280 [DOI] [PubMed] [Google Scholar]
- Dennis T (2006). Emotional self-regulation in preschoolers: The interplay of child approach reactivity, parenting, and control capacities. Developmental Psychology, 42(1), 84–97. 10.1037/0012-1649.42.1.84 [DOI] [PubMed] [Google Scholar]
- Doom JR, Hostinar CE, VanZomeren-Dohm AA, & Gunnar MR (2015). The roles of puberty and age in explaining the diminished effectiveness of parental buffering of HPA reactivity and recovery in adolescence. Psychoneuroendocrinology, 59, 102–111. 10.1016/j.psyneuen.2015.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaude T (1999). Learning difficulties. Dyslexia, bullying and other issues. London: Letts Educational. [Google Scholar]
- Edwards J (1994). The scars of dyslexia: Eight case studies in emotional reactions. Continuum International Publishing Group. [Google Scholar]
- Elliott JG, & Grigorenko EL (2014). The dyslexia debate. Cambridge University Press. [Google Scholar]
- Espin L, Garcia I, Del Pino Sanchez M, Roman F, & Salvador A (2019). Effects of psychosocial stress on the hormonal and affective response in children with dyslexia. Trends in Neuroscience and Education, 15, 1–9. 10.1016/j.tine.2019.03.001 [DOI] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, & Charney DS (2009). Psychobiology and molecular genetics of resilience. Nature Reviews Neuroscience, 10(6), 446–457. 10.1038/nrn2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, & Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14(4), 245–258. 10.1016/s0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- Fletcher JM, & Grigorenko EL (2017). Neuropsychology of Learning Disabilities: The Past and the Future. Journal of the International Neuropsychological Society, 23(9–10), 930–940. 10.1017/S1355617717001084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Lyon GR, Fuchs LS, & Barnes MA (2019). Learning disabilities: From identification to intervention. Learning disabilities: From identification to intervention (2nd ed.). The Guilford Press. [Google Scholar]
- Frey H (1980). Improving the Performance of Poor Readers through Autogenic Relaxation Training. The Reading Teacher, 33(8), 928–932. https://www.jstor.org/stable/20195149 [Google Scholar]
- Granger DA, Kivlighan KT, el-Sheikh M, Gordis EB, & Stroud LR (2007). Salivary alpha-amylase in biobehavioral research: recent developments and applications. Annals of the New York Academy of Sciences, 1098(1), 122–144. 10.1196/annals.1384.008 [DOI] [PubMed] [Google Scholar]
- Greenham SL (1999). Learning disabilities and psychosocial adjustment: A critical review. Child Neuropsychology, 5(3), 171–196. 10.1076/chin.5.3.171.7335 [DOI] [Google Scholar]
- Grigorenko EL (2006). Learning disabilities in juvenile offenders. Child and Adolescent Psychiatric Clinics, 15(2), 353–371. 10.1016/j.chc.2005.11.001 [DOI] [PubMed] [Google Scholar]
- Grigorenko EL, Compton DL, Fuchs LS, Wagner RK, Willcutt EG, & Fletcher JM (2019). Understanding, educating, and supporting children with specific learning disabilities: 50 years of science and practice. American Psychologist. 10.1037/amp0000452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, & Quevedo K (2007). The neurobiology of stress and development. Annual Review of Psychology, 58, 145–173. 10.1146/annurev.psych.58.110405.085605 [DOI] [PubMed] [Google Scholar]
- Halldorsdottir T, Kurtoic D, Muller-Myhsok B, Binder EB, & Blair C (2019). Neurobiology of Self-Regulation: Longitudinal Influence of FKBP5 and Intimate Partner Violence on Emotional and Cognitive Development in Childhood. American Journal of Psychiatry, 176(8), 626–634. 10.1176/appi.ajp.2019.18091018 [DOI] [PubMed] [Google Scholar]
- Hodel AS, Hunt RH, Cowell RA, Van Den Heuvel SE, Gunnar MR, & Thomas KM (2015). Duration of early adversity and structural brain development in post-institutionalized adolescents. Neuroimage, 105, 112–119. 10.1016/j.neuroimage.2014.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollanders JJ, van der Voorn B, Rotteveel J, & Finken MJJ (2017). Is HPA axis reactivity in childhood gender-specific? A systematic review. Biology of Sex Differences, 8(1), 23. 10.1186/s13293-017-0144-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, & Gunnar MR (2015). Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Developmental Science, 18(2), 281–297. 10.1111/desc.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CL (1994). Dependence of transcriptional repression on CpG methylation density. Molecular and Cellular Biology, 14(8), 5487–5494. 10.1128/mcb.14.8.5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Xu C, He M, Huang W, & Wu K (2020). Saliva cortisol, melatonin levels and circadian rhythm alterations in Chinese primary school children with dyslexia. Medicine (Baltimore), 99(6), e19098. 10.1097/MD.0000000000019098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, King LS, Sacchet MD, Camacho MC, Colich NL, Ordaz SJ, Ho TC, & Gotlib IH (2019). Evidence for a sensitive period in the effects of early life stress on hippocampal volume. Developmental Science, 22(3), e12775. 10.1111/desc.12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE (2009). How adversity gets under the skin. Nature Neuroscience, 12(3), 241–243. 10.1038/nn0309-241 [DOI] [PubMed] [Google Scholar]
- Jimerson SR, Durbrow EH, Adam E, Gunnar MR, & Bozoky IK (2006). Associations Among Academic Achievement, Attention, and Adrenocortical Reactivity in Caribbean Village Children. Canadian Journal of School Psychology, 21(1–2), 120–138. 10.1177/0829573506298899 [DOI] [Google Scholar]
- Kao K, Tuladhar CT, Meyer JS, & Tarullo AR (2019). Emotion regulation moderates the association between parent and child hair cortisol concentrations. Developmental Psychobiology, 61(7), 1064–1078. 10.1002/dev.21850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, & Jacobsen SJ (2001). Incidence of reading disability in a population-based birth cohort, 1976–1982, Rochester, Minn. Mayo Clinic Proceedings, 76(11), 1081–1092. 10.4065/76.11.1081 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer DH (1993). The Trier Social Stress Test - a Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology, 28(1–2), 76–81. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, & Dettenborn L (2009). Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology, 34(1), 32–37. 10.1016/j.psyneuen.2008.08.024 [DOI] [PubMed] [Google Scholar]
- Koss KJ, Mliner SB, Donzella B, & Gunnar MR (2016). Early adversity, hypocortisolism, and behavior problems at school entry: A study of internationally adopted children. Psychoneuroendocrinology, 66, 31–38. 10.1016/j.psyneuen.2015.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause BJ, Artigas R, Sciolla AF, & Hamilton J (2020). Epigenetic mechanisms activated by childhood adversity. Epigenomics, 12(14), 1239–1255. 10.2217/epi-2020-0042 [DOI] [PubMed] [Google Scholar]
- Lenroot RK, & Giedd JN (2011). Annual Research Review: Developmental considerations of gene by environment interactions. Journal of Child Psychology and Psychiatry, 52(4), 429–441. 10.1111/j.1469-7610.2011.02381.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M, Theorell T, & Lindblad F (2005). Test performance and self-esteem in relation to experienced stress in Swedish sixth and ninth graders - saliva cortisol levels and psychological reactions to demands. Acta Paediatrica, 94(4), 489–495. 10.1080/08035250410025131 [DOI] [PubMed] [Google Scholar]
- Lisman J, Buzsaki G, Eichenbaum H, Nadel L, Ranganath C, & Redish AD (2017). Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nature Neuroscience, 20(11), 1434–1447. 10.1038/nn.4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijk MP, Velders FP, Tharner A, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Jaddoe VW, Hofman A, Verhulst FC, & Tiemeier H (2010). FKBP5 and resistant attachment predict cortisol reactivity in infants: gene-environment interaction. Psychoneuroendocrinology, 35(10), 1454–1461. 10.1016/j.psyneuen.2010.04.012 [DOI] [PubMed] [Google Scholar]
- MacKinnon McQuarrie MA, Siegel LS, Perry NE, & Weinberg J (2014). Reactivity to stress and the cognitive components of math disability in grade 1 children. Journal of Learning Disabilities, 47(4), 349–365. 10.1177/0022219412463436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin MF, Hupbach A, Maheu FS, Nader K, & Lupien SJ (2011). Metyrapone administration reduces the strength of an emotional memory trace in a long-lasting manner. The Journal of Clinical Endocrinology & Metabolism, 96(8), E1221–1227. 10.1210/jc.2011-0226 [DOI] [PubMed] [Google Scholar]
- McClain GA (1997). Success/failure attributions, academic self-concept, and the internalizing patterns of anxiety and depression in middle school males with learning disabilities [Dissertation, University of Detroit Mercy. [Google Scholar]
- McEwen BS, Nasca C, & Gray JD (2016). Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology, 41(1), 3–23. 10.1038/npp.2015.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, & Meaney MJ (2009). Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience, 12(3), 342–348. 10.1038/nn.2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLanahan S, Tach L, & Schneider D (2013). The Causal Effects of Father Absence. Annual Review of Sociology, 39, 399–427. 10.1146/annurev-soc-071312-145704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, Rutter M, & Sonuga-Barke EJ (2009). Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. Journal of Child Psychology and Psychiatry, 50(8), 943–951. 10.1111/j.1469-7610.2009.02084.x [DOI] [PubMed] [Google Scholar]
- Michaud K, Matheson K, Kelly O, & Anisman H (2008). Impact of stressors in a natural context on release of cortisol in healthy adult humans: a meta-analysis. Stress, 11(3), 177–197. 10.1080/10253890701727874 [DOI] [PubMed] [Google Scholar]
- Miles TRE (2004). Dyslexia and Stress. John Wiley & Sons. [Google Scholar]
- Mizoguchi K, Ishige A, Aburada M, & Tabira T (2003). Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience, 119(3), 887–897. 10.1016/s0306-4522(03)00105-2 [DOI] [PubMed] [Google Scholar]
- Nabuzoka D, & Smith PK (1993). Sociometric status and social behaviour of children with and without learning difficulties. Journal of Child Psychology and Psychiatry, 34(8), 1435–1448. 10.1111/j.1469-7610.1993.tb02101.x [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, & Buss K (1996). Behavioral inhibition and stress reactivity: the moderating role of attachment security. Child Development, 67(2), 508–522. 10.1111/j.1467-8624.1996.tb01748.x [DOI] [PubMed] [Google Scholar]
- Normann C, & Buttenschøn HN (2020). Gene–environment interactions between HPA-axis genes and childhood maltreatment in depression: A systematic review. Acta Neuropsychiatrica, 32(3), 111–121. 10.1017/neu.2020.1 [DOI] [PubMed] [Google Scholar]
- Oitzl MS, & de Kloet ER (1992). Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behavioral Neuroscience, 106(1), 62–71. 10.1037//0735-7044.106.1.62 [DOI] [PubMed] [Google Scholar]
- Ottman R (1996). Gene–environment interaction: definitions and study design. Preventive Medicine, 25(6), 764–770. 10.1006/pmed.1996.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS, Caspi A, Moffitt TE, & Arseneault L (2011). Blunted Cortisol Responses to Stress Signal Social and Behavioral Problems Among Maltreated/Bullied 12-Year-Old Children. Biological Psychiatry, 70(11), 1016–1023. 10.1016/j.biopsych.2011.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peddigrew E, & McNamara J (2019). From Medication to Meditation: A Critical Disability Studies Analysis of Mindfulness-Based Practices for Children With Learning Disabilities. Journal of Education and Development, 3(3), 1. 10.20849/jed.v3i3.623 [DOI] [Google Scholar]
- Pham K, Nacher J, Hof PR, & McEwen BS (2003). Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. European Journal of Neuroscience, 17(4), 879–886. 10.1046/j.1460-9568.2003.02513.x [DOI] [PubMed] [Google Scholar]
- Plomin R, & Kovas Y (2005). Generalist genes and learning disabilities. Psychological Bulletin, 131(4), 592–617. 10.1037/0033-2909.131.4.592 [DOI] [PubMed] [Google Scholar]
- Ptashne M (2007). On the use of the word ‘epigenetic’. Current Biology, 17(7), R233–R236. 10.1016/j.cub.2007.02.030 [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, & Shaywitz BA (2001). Neurobiological studies of reading and reading disability. Journal of Communication Disorders, 34(6), 479–492. 10.1016/s0021-9924(01)00060-0 [DOI] [PubMed] [Google Scholar]
- Qin S, Cho S, Chen T, Rosenberg-Lee M, Geary DC, & Menon V (2014). Hippocampal-neocortical functional reorganization underlies children’s cognitive development. Nature Neuroscience, 17(9), 1263–1269. 10.1038/nn.3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada AA, Wiemers US, Schoofs D, & Wolf OT (2012). Psychosocial stress exposure impairs memory retrieval in children. Psychoneuroendocrinology, 37(1), 125–136. 10.1016/j.psyneuen.2011.05.013 [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, & Morrison JH (2006). Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral Cortex, 16(3), 313–320. 10.1093/cercor/bhi104 [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, & Morrison JH (2004). Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience, 125(1), 1–6. 10.1016/j.neuroscience.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, & Wood MA (2010). Membrane-Associated Glucocorticoid Activity Is Necessary for Modulation of Long-Term Memory via Chromatin Modification. Journal of Neuroscience, 30(14), 5037–5046. 10.1523/Jneurosci.5717-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, & Rosnow RL (2008). Essentials of behavioral research: Methods and data analysis (3rd ed.). McGraw-Hill. [Google Scholar]
- Sabornie EJ (1994). Social-Affective Characteristics in Early Adolescents Identified as Learning-Disabled and Nondisabled. Learning Disability Quarterly, 17(4), 268–279. 10.2307/1511124 [DOI] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, & Insel TR (2000). Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. Journal of Neuroscience, 20(12), 4657–4668. 10.1523/JNEUROSCI.20-12-04657.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C (2004). Stress, cognitive impairment and cell adhesion molecules. Nature Reviews Neuroscience, 5(12), 917–930. 10.1038/nrn1555 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, & Munck AU (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21(1), 55–89. 10.1210/edrv.21.1.0389 [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, & Pollak SD (2010). Social vocalizations can release oxytocin in humans. Proceedings of the Royal Society B: Biological Sciences, 277(1694), 2661–2666. 10.1098/rspb.2010.0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, Committee on Psychosocial Aspects of, C., Family H, Committee on Early Childhood, A., Dependent, C., Section on, D., & Behavioral P (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics, 129(1), e232–246. 10.1542/peds.2011-2663 [DOI] [PubMed] [Google Scholar]
- Siamatras TD, & Stratakis CA (2017). NR3C1 (nuclear receptor subfamily 3, group C, member 1/glucocorticoid receptor). Atlas of Genetics and Cytogenetics in Oncology and Haematology(3). 10.4267/2042/62525 [DOI] [Google Scholar]
- Siddiqui SV, Chatterjee U, Kumar D, Siddiqui A, & Goyal N (2008). Neuropsychology of prefrontal cortex. Indian Journal of Psychiatry, 50(3), 202–208. 10.4103/0019-5545.43634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JG, Badcock PB, Whittle SL, Byrne ML, Mundy L, Patton GC, Olsson CA, & Allen NB (2016). The lifetime experience of traumatic events is associated with hair cortisol concentrations in community-based children. Psychoneuroendocrinology, 63, 276–281. 10.1016/j.psyneuen.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, & Post RM (1995). Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. Journal of Neuroscience, 15(3), 1768–1777. 10.1523/JNEUROSCI.15-03-01768.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov N, Madeira M, Almeida O, & Paula-Barbosa M (2000). Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience, 97(2), 253–266. 10.1016/S0306-4522(00)00050-6 [DOI] [PubMed] [Google Scholar]
- Spangler G, & Grossmann KE (1993). Biobehavioral organization in securely and insecurely attached infants. Child Development, 64(5), 1439–1450. 10.1111/j.1467-8624.1993.tb02962.x [DOI] [PubMed] [Google Scholar]
- Spangler G, & Schieche M (1998). Emotional and adrenocortical responses of infants to the strange situation: The differential function of emotional expression. International Journal of Behavioral Development, 22(4), 681–706. 10.1080/016502598384126 [DOI] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, & Ohashi K (2016). The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience, 17(10), 652–666. 10.1038/nrn.2016.111 [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, & Eigsti IM (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science, 13(1), 46–61. 10.1111/j.1467-7687.2009.00852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecki G, & Meaney MJ (2016). Effects of the Social Environment and Stress on Glucocorticoid Receptor Gene Methylation: A Systematic Review. Biological Psychiatry, 79(2), 87–96. 10.1016/j.biopsych.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turney K (2020). Cumulative adverse childhood experiences and children’s health. Children and Youth Services Review, 119, 105538. 10.1016/j.childyouth.2020.105538 [DOI] [Google Scholar]
- Tyborowska A, Volman I, Niermann HCM, Pouwels JL, Smeekens S, Cillessen AHN, Toni I, & Roelofs K (2018). Early-life and pubertal stress differentially modulate grey matter development in human adolescents. Scientific Reports, 8(1), 9201. 10.1038/s41598-018-27439-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unicef. (1989). Convention on the Rights of the Child.
- van der Kolk BA (2005). Developmental Trauma Disorder: Toward a rational diagnosis for children with complex trauma histories. Psychiatric Annals, 35(5), 401–408. 10.3928/00485713-20050501-06 [DOI] [Google Scholar]
- van der Voorn B, Hollanders JJ, Ket JCF, Rotteveel J, & Finken MJJ (2017). Gender-specific differences in hypothalamus-pituitary-adrenal axis activity during childhood: a systematic review and meta-analysis. Biology of Sex Differences, 8(1), 3. 10.1186/s13293-016-0123-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman AT, Milaneschi Y, Bandinelli S, Ferrucci L, & Penninx BW (2010). Urinary cortisol and six-year risk of all-cause and cardiovascular mortality. The Journal of Clinical Endocrinology & Metabolism, 95(11), 4959–4964. 10.1210/jc.2010-0192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, & McEwen BS (1992). Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Research, 588(2), 341–345. 10.1016/0006-8993(92)91597-8 [DOI] [PubMed] [Google Scholar]
- Zhong XM, Ning YP, Gu Y, Wu ZY, Ouyang C, Liang WY, Chen B, Peng Q, Mai NK, Wu YJ, Chen XR, Huang XB, & Pan SY (2018). A reliable global cognitive decline and cortisol as an associated risk factor for patients with late-life depression in the short term: A 1-year prospective study. Journal of Affective Disorders, 240, 214–219. 10.1016/j.jad.2018.07.052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


