Abstract
Objective
To investigate if patients with polycystic ovary syndrome (PCOS) have altered embryo morphokinetics when compared with controls.
Design
Retrospective cohort analysis.
Setting
Single academic fertility clinic in a tertiary hospital setting.
Patient(s)
Age- and body mass index–matched patients who underwent in vitro fertilization diagnosed with PCOS using the Rotterdam criteria. A subanalysis was performed on patients with PCOS with hyperandrogenemia. Sixty-four patients with PCOS were identified with 990 embryos that were matched with 64 control patients with 628 embryos.
Intervention(s)
None.
Main Outcome Measure(s)
Time to blastulation.
Result(s)
Embryos from women with PCOS displayed faster growth rate at t7, t8, and t9; all other morphokinetic points were similar. Patients with PCOS also had a higher number of oocytes retrieved. No differences were seen in the fertilization rate or blastulation rate. Patients with PCOS had a higher miscarriage rate (38.1% in PCOS vs. 18.8% in controls). Patients with hyperandrogenic PCOS showed a faster growth rate at t5, t6, t7, t8, t9, and morula.
Conclusion(s)
Embryos from women with PCOS grew faster until 9-cell stage and women with hyperandrogenic PCOS until morula. Patients with PCOS also showed a higher miscarriage rate. The alterations in early embryo development are consistent with altered fertility and obstetric outcomes in the population with PCOS and may be due to the hyperandrogenic microenvironment in the ovarian follicle.
Key Words: PCOS, morphokinetics, hyperandrogenemia, infertility
Discuss: You can discuss this article with its authors and other readers at https://www.fertstertdialog.com/users/16110-fertility-and-sterility/posts/xfre00031
Polycystic ovary syndrome (PCOS) is the most common ovulatory disorder in the world, affecting more than 100 million women worldwide (1, 2, 3). It most commonly is diagnosed using the Rotterdam criteria (oligomenorrhea, laboratory or clinical evidence of hyperandrogenemia, and polycystic ovarian morphology), provided that other common causes such as thyroid or prolactin disorders are excluded (4). PCOS is a complex disease composed of a broad and variable phenotype, with many associated comorbidities and complications (5, 6, 7, 8, 9, 10). Despite numerous studies over several decades on this disease, many questions remain unanswered (11). One of the challenges in the study of PCOS is the heterogeneous nature of the disease. PCOS represents the final common pathway from other metabolic disorders such as insulin resistance and glucose intolerance, obesity and the metabolic syndrome, overproduction of ovarian androgens, abnormal hypothalamic-pituitary signaling, environmental and genetic factors, and several others (1, 3, 5).
PCOS is also one of the most common causes of infertility, and thus it is imperative to understand the influence of this disease on the reproductive system, particularly at oocyte and subsequent embryo development. In patients with PCOS, adverse fertility outcomes have been observed during different stages of reproduction, including impaired oocyte maturation and decreased fertilization, blastulation and implantation, and higher miscarriage rates (9). Subsequently, patients with PCOS also have increased obstetric risks including pre-eclampsia, gestational diabetes, preterm delivery, and infant mortality (10). It is not understood clearly how PCOS affects oocyte and embryo quality (12). Interestingly, a study performed by Wood et al. (13) demonstrated abnormal gene expression in human PCOS oocytes, specifically relating to key biologic processes in early embryo development. These genes included processes such as maternal effect genes and mitotic cell cycle, which may be driven by the androgen (and insulin)–rich environment of a PCOS follicle.
Study of the preimplantation embryo has proven challenging, although several recent technologies have opened new and promising avenues for research. For instance, incubators fitted with time-lapse microscopy (TLM) have allowed for prospective, real-time analysis of the early cell divisions of the preimplantation embryo (14). Although the clinical utility of these data remains controversial (15, 16), these time points in embryo development can serve as markers of embryo quality and viability as shown in both in animal models (17, 18) and human studies (19, 20, 21, 22, 23). Four studies have been performed on patients with PCOS undergoing in vitro fertilization (IVF) using TLM reaching contradictory conclusions (24, 25, 26, 27). We undertook the present study to elucidate the effects of PCOS on embryo quality as it relates to preimplantation growth and pregnancy outcomes. We designed this study to investigate if the embryo morphokinetics of women with PCOS were different from the embryo morphokinetics of an age- and body mass index (BMI)–matched control population.
Materials and methods
Study Design
This is a retrospective cohort study. The analysis of embryos from patients with PCOS along with an age- and BMI-matched control population was approved by the Institutional Review Board of Baylor College of Medicine, Houston, Texas. All patients undergoing IVF via intracytoplasmic sperm injection (ICSI) at a single-site academic fertility clinic with embryos incubated using TLM from July 2014 through December of 2017 were screened for participation. Only ICSI cycles were included to ensure that time of fertilization was known for further assessment using TLM. The time-lapse system used was the Embryoscope (Vitrolife). PCOS was defined using the Rotterdam criteria, and subsequent controls were matched 1:1 by both age and BMI given that these are known confounders in fertility outcomes. Controls were chosen at random from a cohort of patients undergoing IVF with embryos incubated in the Embryoscope during the same time period, and were matched using both age and BMI.
Clinical Data and Reproductive Hormone Measurements
Demographic data were collected and included age, BMI, antimullerian hormone (AMH) levels, metformin use, total testosterone (T), total gonadotropin dose, peak estradiol, number of oocytes collected, number of mature oocytes, number fertilized, and number of blastocysts. The primary outcome was time to blastulation. Secondary outcomes included pregnancy, defined as positive cardiac activity noted on ultrasound, live birth rate, defined as live birth after 20 weeks gestation per embryo transferred, and miscarriage rate, defined as no fetal cardiac activity after positive serum human chorionic gonadotropin test.
To study the effects of increased androgen production on embryo quality, a secondary analysis was performed on patients with PCOS who were specifically hyperandrogenic by laboratory or clinical criteria (PCOS-HA), as well as patients with PCOS who had a normal androgen profile (PCOS-NA). Hyperandrogenism was determined using clinical evidence as noted in the patient’s history or using laboratory evidence of elevated T levels. Immunoassay was used for T measurement, where applicable, which had an inter- and intra-assay coefficient of variation of <6%. These patients were again compared with respective age- and BMI-matched controls in the same fashion as the initial analysis.
Embryo Morphokinetics
The primary outcome was time to blastulation (tB), with other morphokinetic parameters noted as follows: time to pronuclei appearance (tPNa), time to pronuclei fade (tPNf), time to two-cell division (t2), three-cell division (t3), four-cell division (t4), and so on to nine cells or greater (t9). The start of compaction of the morula was annotated as tM, the start of blastulation as tSB, and the formation of the blastocyst was defined as the point at which the blastocoel comprised 50% of the embryo and was annotated as tB.
To obtain the morphokinetic parameters, time-lapse images collected from all embryos of patients undergoing IVF via ICSI at the time of the study were analyzed retrospectively and annotated manually by two independent researchers. The embryoscope captured an image in seven focal planes every 10 minutes of each embryo from fertilization to transfer or cryopreservation at the blastocyst stage and thus allowed for a comprehensive analysis. Although the researchers were not blinded to the clinical characteristics of the study subjects, prior to the initiation of the study, all morphokinetic parameters objectively were agreed upon based on prior publications and morphokinetic annotations were reviewed by at least two researchers for agreement (14). Briefly, tPNa was considered to be the initial presentation of both pronuclei, and tPNf was determined as the point at which the pronuclei were no longer visible. All divisions of the cells were denoted at the point at which the cell membranes distinctly were formed. Compaction (tM) was annotated as the point in time in which the cell boundaries became indistinct. The tSB was considered to be the time point at which the first sign of a blastocoel cavity was apparent, and tB was the time at which 50% of the blastocyst was occupied by the blastocoel cavity.
Statistical Analysis
Statistical analysis of age was performed using Student t test. Wilcoxon rank sum test was used for analysis of BMI, AMH levels, total T, chi-square test for tobacco, and metformin use. All cycle characteristics and morphokinetic data were analyzed using generalized linear mixed model with random intercept (proc glimmix) or generalized linear model using maximum likelihood estimation (proc genmod) to account for multiple samples per patient. Because this was a retrospective cohort study, no power analysis was calculated. All analyses were performed using SAS statistical software (version 9.4).
Results
In total, 64 patients with PCOS were matched 1:1 using age and BMI to a control population undergoing ICSI during the same period. There were 990 embryos analyzed from the patients with PCOS, and 628 from the control population for a total of 1,618 embryos. For secondary analysis, there was a total of 47 patients with PCOS-HA, which included 715 embryos compared with 47 controls with 449 embryos, leaving 17 patients with PCOS who did not have signs or symptoms of androgen excess with 275 embryos and their respective controls.
Primary Analysis
In the primary analysis, the mean age was not different between groups (32 ± 3.8 years in PCOS vs. 32 ± 3.8 years in controls; P=.71), with a mean BMI of 26 (26.9 in PCOS ± 6.5 vs. 26.2 ± 5.9 in controls; P=.53), and no difference in parity (Table 1). There was a statistically significant difference between the PCOS group and the control group in AMH level (8 ± 6.2 ng/mL in PCOS vs. 3.0 ± 2.2 ng/mL in controls; P<.0001) and use of metformin (57.8% in PCOS vs. 9.4% in controls; P<.0001). There were differences in total T that approached significance (35.9 ± 15.3 ng/dL in PCOS vs. 26.5 ± 12.3 ng/dL in controls; P=.08). Additionally, as shown in Table 2, patients with PCOS required less gonadotropins (2,990 ± 167 IU in PCOS vs. 3,673 ± 167 IU; P=.014) and had a higher peak estradiol level (3,232.0 ± 186 pg/mL vs. 2,413.7 ± 183.7 pg/mL; P=.009). Regarding other IVF outcomes, patients with PCOS had a higher number of oocytes retrieved (22.1 ± 1.3 vs. 14.4 ± 1.2; P=.0009; Table 2). There were no differences in the percent of mature oocytes (84.5 ± 2% in PCOS vs. 81.7 ± 2% in controls; P=.29), fertilization rates (79.7 ± 2% in PCOS vs. 78.5 ± 2% in controls; P=.68), or blastulation rates (56.1 ± 2.9% in PCOS vs. 51.1 ± 2.85% in controls; P=.24). There was no difference in patients with PCOS versus controls regarding elective single embryo transfer (eSET), use of preimplantation genetic testing for aneuploidies, or euploid rate (Table 2), however, patients with PCOS did have a higher rate of frozen embryo transfers (FET; 90% in PCOS vs. 77% in controls; P=.03).
Table 1.
Demographics of control and polycystic ovary syndrome patients.
| Variable | PCOS (n = 64) | Control (n = 64) | P value | PCOS-HA (n = 47) | Control (n = 47) | P value | PCOS-NA (n = 17) | Control (n = 17) | P value |
|---|---|---|---|---|---|---|---|---|---|
| Age (y), mean (SD) | 32.2 (3.8) | 32.4 (3.8) | .71 | 32.0 (3.7) | 31.75 (3.7) | .70 | 33.29 (4.1) | 33.29 (3.9) | .95 |
| BMI (kg/m2) mean (SD) | 26.9 (6.5) | 26.2 (5.9) | .53 | 27.44 (6.3) | 26.4 (5.9) | .38 | 25.58 (7.1) | 25.59 (5.7) | .96 |
| Parous, n (%) | 4 (6.5) | 10 (15.9) | .15 | 2 (4.4) | 7 (15.2) | .16 | 2 (12.5) | 3 (17.7) | .99 |
| Tobacco, n (%) | 6 (9.4) | 2 (3.2) | .14 | 6 (12.8) | 1 (2.2) | .10 | 0 (0.0) | 1 (5.9) | .35 |
| AMH (ng/mL) mean (SD) | 8.0 (6.2) | 3.0 (2.2) | <.0001 | 7.77 (6.0) | 3.23 (2.4) | <.0001 | 8.75 (6.9) | 2.50 (1.5) | .0004 |
| Testosterone (ng/dL), mean (SD) | 35.9 (15.3) | 26.5 (12.3) | .08 | 39.07 (15.1) | 23.48 (11.1) | .02 | 24.38 (10.0) | 35.5 (14.8) | .23 |
| Metformin, n (%) | 37 (57.8) | 6 (9.4) | <.0001 | 31 (66.0) | 5 (10.6) | <.0001 | 6 (35.3) | 1 (5.9) | .09 |
Note: Patients are divided by overall cohort and patients with PCOS with HA or those with NA and their corresponding controls. AMH = antimullerian hormone; BMI = body mass index; HA = hyperandrogenemia; NA = normal androgens; PCOS = polycystic ovary syndrome; SD = standard deviation.
Table 2.
Cycle characteristics and birth outcomes.
| Variable | PCOS (n = 64) | Control (n = 64) | P value | PCOS-HA (n = 47) | Control (n = 47) | P value | PCOS-NA (n = 17) | Control (n = 17) | P value |
|---|---|---|---|---|---|---|---|---|---|
| Total GND, mean (SE) | 2,990.4 (167.2) | 3,673.0 (166.7) | .014 | 2,805.5 (196.5) | 3,644.1 (195.7) | .01 | 3,489.9 (303.2) | 3,748.6 (303.1) | .59 |
| Days stim, mean (SE) | 9.85 (0.19) | 9.65 (0.19) | .49 | 9.65 (0.24) | 9.69 (0.24) | .91 | 10.4 (0.33) | 9.6 (0.32) | .15 |
| Peak E2, mean (SE) | 3,232.0 (185.7) | 2,413.74 (183.7) | .009 | 3,078.2 (210.9) | 2,498.2 (207.8) | .08 | 3,676.11 (387.3) | 2,230.31 (386.5) | .08 |
| Oocytes, mean (SE) | 22.14 (1.25) | 14.42 (1.24) | .0009 | 21.66 (1.4) | 14.49 (1.4) | .006 | 23.56 (2.7) | 14.23 (2.7) | .09 |
| % Mature, mean (SE) | 84.46 (1.79) | 81.7 (1.77) | .29 | 84.81 (2.1) | 80.78 (2.1) | .20 | 83.75 (3.5) | 84.83 (3.5) | .84 |
| Fert rate (%), mean (SE) | 79.68 (1.98) | 78.53 (1.95) | .68 | 81.56 (2.10) | 76.299 (2.07) | .11 | 74.68 (4.4) | 84.86 (4.3) | .20 |
| Blast rate (%), mean (SE) | 56.06 (2.89) | 51.07 (2.80) | .24 | 55.43 (3.4) | 49.31 (3.31) | .23 | 57.94 (5.6) | 56.03 (5.3) | .82 |
| FET, n (%) | 63 (90) | 59 (77) | .03 | 51 (88) | 39 (75) | .08 | 12 (100) | 20 (80) | .15 |
| eSET n (%)a |
61 (88) | 73 (95) | .23 | 53 (93) | 49 (94) | .99 | 8 (66.7) | 24 (96) | .04 |
| PGT-A, n (%) | 38 (60) | 41 (59) | .85 | 26 (54) | 27 (55) | .99 | 12 (80) | 14 (70) | .45 |
| Euploid rate (%), mean (SE) | 49 (4) | 48 (4) | .79 | 53 (5) | 44 (4) | .26 | 41 (8) | 55 (7) | .33 |
| Cardiac activity, n (%) | 37 (54.4) | 44 (57.1) | .75 | 30 (52.6) | 30 (58.8) | .54 | 7 (63.6) | 14 (53.9) | .57 |
| Live birth, n (%) | 32 (45.7) | 42 (53.9) | .33 | 26 (44.8) | 28 (54.9) | .33 | 6 (50.0) | 14 (51.9) | .97 |
| Miscarriage, n (%) | 21 (29.6) | 11 (14.1) | .018 | 16 (27.6) | 8 (15.7) | .14 | 5 (38.5) | 3 (11.3) | .07 |
Note: Blast = blastulation; eSET = elective single embyro transfer; E2 = estradiol; Fert = fertilization; FET = frozen embryo transfer; GND = gonadotropin; PGT-A = preimplantation genetic testing for aneuploidies; SE = standard error; stim = stimulated.
All embryo transfers that we were not eSET were double embryo transfers.
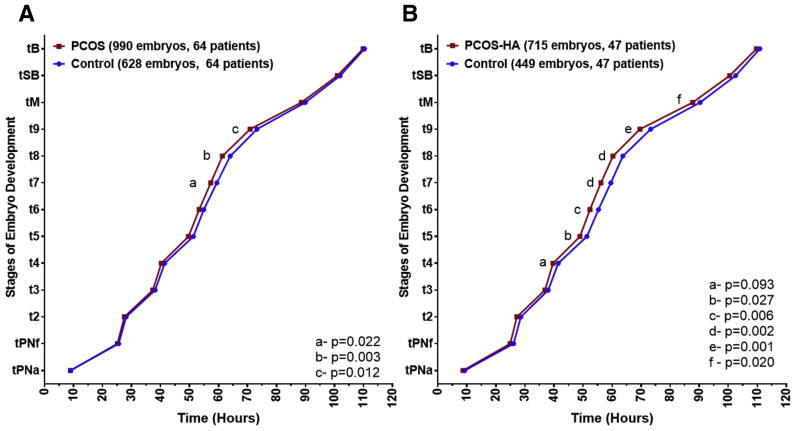
Regarding the primary outcome, there was no difference in time to blastulation (109.88 ± 0.65 hours vs. 110.36 ± 0.70 hours; P=.6); however, PCOS embryos overall displayed a faster time to t7 (57.33 ± 0.63 hours vs. 59.43 ± 0.67 hours [95% confidence interval (CI), 58.12–60.74]; P=.022), t8 (61.35 ± 0.63 hours vs. 64.06 ± 0.67 hours; P=.003), and t9 (70.90 ± 0.63 hours vs. 73.23 ± 0.68 hours; P=.012) compared with controls (Fig. 1A). There were no differences in any other morphokinetic parameters. With respect to secondary outcomes, there was no difference in live birth rate (45.7% in PCOS vs. 53.9% in controls; P=.33), however, patients with PCOS had a higher miscarriage rate (29.6% in PCOS vs. 14.1% in controls; P=.018; Table 2).
Figure 1.
Morphokinetics of patients with PCOS and patients with PCOS-HA compared with respective controls. (A) Patients with PCOS developed faster than controls with statistically significant different time points at t7, t8, and t9. (B) Patients with PCOS-HA developed to morula faster than controls with statistically significant different time points at t5, t6, t7, t8, t9, and tM. PCOS = polycystic ovary syndrome; PCOS-HA = polycystic ovary syndrome hyperandrogenic.
Subanalysis
In the analysis of the PCOS-HA group, there was no difference noted in age or BMI (31.8 ± 3.7 in PCOS-HA vs. 32.0 ± 3.7 years in controls and 27.4 ± 6.3 in PCOS-HA vs. 26.4 ± 5.9 in controls, respectively; P>.05). However, the PCOS-HA population had a higher AMH (7.8 ± 6.3 ng/mL in PCOS-HA vs. 3.2 ± 2.4 ng/mL in controls; P<.0001), a higher T (39.1 ± 15.1 ng/dL in PCOS-HA vs. 23.5 ± 11.1 ng/dL in controls; P=.02), and a higher use of metformin (66% in PCOS-HA vs 11% in controls; P<.0001; Table 1). Furthermore, PCOS-HA patients used less gonadotropins (2,805.5 ± 196.5 IU vs. 3,644.1 ± 195.7 IU; P=.01) and had more oocytes retrieved (21.7 ± 1.4 oocytes vs. 14.5 ± 1.4 oocytes; P=.006), which translated to more blastocysts at the end of the cycle (7.8 ± 0.6 blastocysts vs. 4.8 ± 0.6 blastocysts; P=.006). The percent of mature oocytes, fertilization rates, and blastulation rates were again similar between groups. There was no difference in patients with PCOS-HA versus controls regarding rate of FET, eSET, use of preimplantation genetic testing for aneuploidies, or euploid rate (Table 2).
Patients with PCOS-HA also showed no difference in time to blastulation (109.72 ± 0.76 hours vs. 110.88 ± 0.84 hours; P=.3). However, there were more differences noted in morphokinetics, with a faster time to t5 (48.96 ± 0.73 hours in PCOS-HA vs. 51.31 ± 0.78 hours in controls; P=.027), t6 (52.37 ± 0.73 hours in PCOS-HA vs. 55.34 ± 0.78 hours in controls; P=.006), t7 (56.18 ± 0.73 hours in PCOS vs. 59.59 ± 0.79 hours in controls; P=.002), t8 (60.32 ± 0.73 hours in PCOS-HA vs. 63.74 ± 0.79 hours in controls; P=.002), t9 (69.60 ± 0.74 hours in PCOS-HA vs. 73.30 ± 0.80 hours in controls; P=.001), and tM (87.77 ± 0.75 hours in PCOS-HA vs. 90.36 ± 0.81 hours in controls; P=.02), with a trend toward a faster time to tSB (100.49 ± 0.75 hours in PCOS-HA vs. 102.58 ± 0.83 hours [95% CI, 100.96–104.2] in controls; P=.06; Fig. 1B). However, in the secondary analysis, there were no differences in pregnancy rate, live birth rate, or miscarriage (Table 2). When analyzing the patients with PCOS-NA, similar results were seen for the demographic and clinical outcomes data except for a higher rate of eSET for the control population (96% vs. 66.7% in the patients with PCOS-NA; P=.04; Tables 1 and 2), however, there were no differences noted in morphokinetics across all time points analyzed (Supplemental Fig. 1, available online).
Discussion
PCOS is a complex and multifactorial disease with serious implications in fertility (9, 10, 11). Previous studies have shown that oocytes developing in a patient with PCOS have impaired oocyte competence due to abnormal follicle development due to hyperandrogenemia (2, 3, 28, 29, 30, 31, 32, 33). Furthermore, studies have demonstrated impaired gene expression in the oocytes of patients with PCOS due to androgen and/or insulin signaling, both of which are increased in the PCOS follicle (13, 34, 35). Although a subset of women with PCOS undergoing treatment for IVF have normal embryo development and pregnancy outcomes (36), many patients with PCOS have decreased fertilization, blastulation and implantation, and higher miscarriage rates (9, 37). The developmental competence of embryos arising from poor-quality oocytes are compromised (9, 10, 12, 38, 39). Our present study agrees with previous studies, demonstrating patients with PCOS-HA had altered early embryo development and also higher miscarriage rates. (6, 7). Although we saw no difference in live birth rates overall, given the higher miscarriage rate and the trend toward lower live birth rates, this is possibly a limitation of the sample size.
Our morphokinetic analysis demonstrated embryos from patients with PCOS develop faster to the nine-cell stage, and patients with PCOS-HA showed a faster development up to the morula stage when compared with age- and BMI-matched controls. In the human embryo, compaction is driven by complex interactions via cell-to-cell signaling. Gap junctions, desmosomes, tight junctions, and other adhesion molecules will increase to result in a tightly bound embryo devoid of distinct cell borders (40). From this important step, cellular polarity and differentiation occur, giving rise to the inner cell mass and the trophectoderm (40). Although much remains unknown regarding this process, some key proteins have demonstrated integral roles in early studies, in particular, connexins and cadherins. Connexin 43 (Cx43) has been shown to be important in both mouse and human compaction (41). Additionally, E-cadherin becomes activated at the time of compaction, although its role to date is not yet entirely understood (40). Nonetheless, there are studies showing increased expression of Cx43 and E-cadherin in PCOS, possibly explaining the differences seen in our study (41, 42).
To our present knowledge, only four smaller studies on the morphokinetics of embryos from women with PCOS undergoing IVF have been performed, with differing conclusions and several limitations. Sundvall et al. (25) showed a faster time to compaction similar to our findings, but Wissing et al. (24) showed that patients with PCOS-HA developed slower in the early stages (delayed t2, t3, t4, and t7) compared with controls. In the study performed by Sundvall et al., the patients with PCOS were younger (29 vs. 32; P=.0010), with only a small percentage of the patients having PCOS-HA, although the authors did adjust for age, BMI, male fertility diagnosis, and fertilization method. In the latter study, it must be noted that the best embryo from each patient was excluded from analysis for fresh transfer, thus limiting a full interpretation of each cohort of embryos. Neither study showed a difference in birth outcomes, with both studies reporting similar pregnancy, live birth, and miscarriage rates, although neither study was powered for such findings. This is in contrast to our study, which showed an increase in miscarriage rates in the overall analysis. Both of these studies were performed in Denmark, where the prevalence of obesity is lower than in the U.S., and the BMI values in these populations predominantly were within normal range. Furthermore, the population in Denmark is more homogenous than in the United States. Importantly, the other two studies only followed early embryo development leading up to compaction (26, 27). The study by Tabibnejad et al. (26), similar to Wissing et al. (24), found that PCOS embryos developed slower to t8 compared with patients with tubal factor. However, they only followed embryos to the eight-cell stage because they performed day 3 embryo transfers and did not adjust for age or BMI. Tam et al. (27) found no differences in regard to time points comparing patients with PCOS to controls, yet they only evaluated for 48 hours or to the six-cell stage. Taken in the context of the findings of our study, it is plausible to consider that several aspects of the PCOS phenotype contribute to these adverse outcomes, and variations among these three groups may account for the differences noted, specifically the role of HA.
In our subanalysis of the PCOS-HA group, we found faster times to compaction from t5 to morula stage, which represents an even more robust difference from controls than the primary analysis of all patients with PCOS. Although there were no differences noted in the secondary outcomes, there was a notable trend seen in the miscarriage rate of the PCOS-HA population compared with the controls (PCOS-HA 27.6% vs. control 15.7%), and it is possible that the lack of statistical significance is more indicative of a lack of power than of lack of association.
In prior work by Leary et al. it was shown that overweight and obese patients had embryos that developed to the morula stage faster than controls, with no subsequent difference in time to blastulation (43). In addition, the authors reported evidence of impaired metabolism with decreased glucose consumption at the blastocyst stage, which has been shown to correlate with embryo viability (44). Strikingly, our present study demonstrated very similar morphokinetic development results, despite controlling for BMI. In addition, prior animal work has shown impaired mitochondrial function in oocytes of a PCOS mouse model that also controlled for weight (45). These studies taken together highlight the complex and interwoven nature of metabolic dysfunction and obesity coinciding with HA. Although the mechanism of action has yet to be elucidated fully, it is clear that there is a link that requires further exploration.
Indeed, in regard to HA, studies previously have suggested that an elevated T environment can decrease rates of embryonic development as well as increased miscarriage rates in women with PCOS. Although the mechanism behind these findings still needs to be explored, it could be due to altered aromatase activity and the interaction of cumulus cells with oocytes (39). This would be an interesting avenue to explore because there has been a recent association found between embryo morphokinetics and cumulus cell gene expression in women with PCOS (46). Although the subpopulation of patients in our study with HA constituted 73% of the total PCOS group evaluated, it would be important to compare a larger sample number of patients with PCOS who have HA with those that do not to further elucidate the effects of androgens on embryo development.
Our study has several strengths. First, this is the largest study of its kind, both in number of patients as well as number of embryos analyzed. Furthermore, this was the first study to subgroup patients based on the diagnostic criteria of HA. Additionally, multiple demographic and outcome data were collected including live birth rate with comprehensive follow-up to allow further evaluation of this patient population beyond preimplantation embryo development to other clinically significant outcomes. Given that the data were collected in a single academic site, the protocols used for embryo culture, vitrification, and transfer were uniform throughout the time period studied. Importantly, all of the study patients underwent fertilization via ICSI. Finally, use of mixed modeling statistics on the morphokinetic parameters provides an appropriate evaluation of these endpoints without artificial inflation of sample size.
There are also some potential weaknesses. The retrospective nature of the study poses risk for missing data; however, because this was from a single academic site, we were able to capture all relevant data points. Furthermore, given that this data is from a single academic site, perhaps it is not as generalizable to all populations, however, the population seen is diverse in age and BMI. In the design of the study, we attempted to control purposefully for the most well-known confounders, namely, age and obesity, but we did not control for any possible affects that a higher FET rate in patients with PCOS had in terms of outcomes. We also cannot exclude the possibility of unknown confounders in our population of study skewing the significance of our findings. Finally, there is the possibility that there are other potential effect modifiers playing a role in the outcomes that we observed. Namely, in patients with PCOS there is a higher incidence of metabolic dysfunction, and thus it is possible that the outcomes measured were influenced by the concurrent presence of glucose intolerance and insulin resistance. In fact, this can affect up to 60%–80% of patients with PCOS (10). This would be true particularly in regard to pregnancy outcomes given the differences in maternal environment, independent of embryo quality. With the retrospective nature of this study, it was not possible to compare metabolic function because not all patients had evaluation in this regard, particularly in the control group. However, the 11% of the control population taking metformin was doing so for insulin resistance and or glucose intolerance rather than diabetes and elucidates that patients with PCOS do indeed have a higher rate of metabolic dysfunction. It is important to note that HA and abnormal metabolism are linked physiologically and both augment the other in the pathology of PCOS (38). To truly and completely isolate one from the other will require prospective studies that obtain metabolic testing of a control population.
In conclusion, the results of this study show direct evidence of altered embryo development in PCOS preimplantation embryos. Although the exact mechanism of faster embryonic growth and its impact of further embryo development and survival are not known, there seems to be an association with an androgen excess environment and could involve regulation via gene expression. Regardless, there appear to be worse obstetric outcomes for these patients and future studies are warranted. Further study on the mechanisms at play during this critical time in development are essential to understand and intervene potentially to optimize the health of the oocyte and the viability of future progeny.
Footnotes
N.R.C. has nothing to disclose. M.B. has nothing to disclose. J.S. has nothing to disclose. M.P. has nothing to disclose. L.Y. has nothing to disclose. H.S.-H. has nothing to disclose. W.G. has nothing to disclose. C.S.B. is a recipient of R-01 research grant (grant number DK114689) from the National Institutes of Health.
Supported by the Department of Obstetrics and Gynecology, Baylor College of Medicine.
N.R.C. and M.B. should be considered similar in author order.
Supplementary data
Supplemental Figure 1.
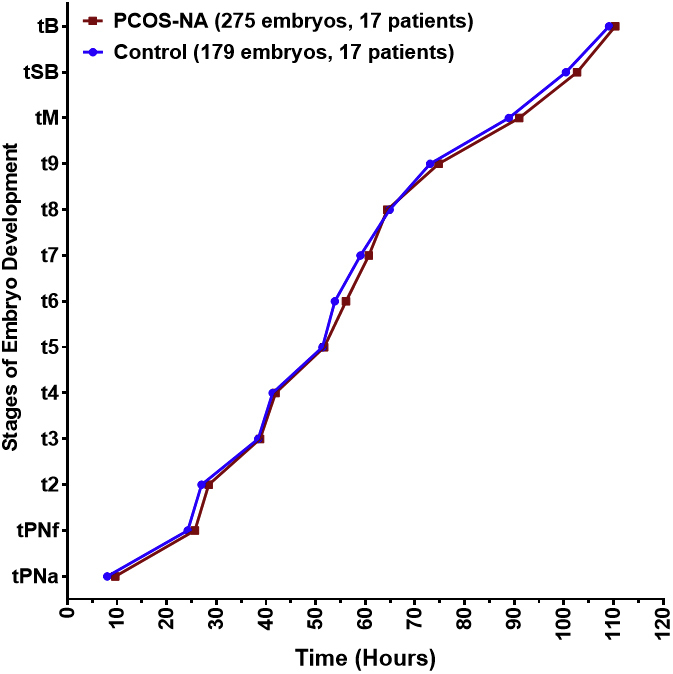
Morphokinetics of patients with PCOS-NA compared with controls. PCOS-NA patients did not show any morphokinetic differences. PCOS-NA = polycystic ovary syndrome normal androgen.
References
- 1.Azziz R. Introduction: determinants of polycystic ovary syndrome. Fertil Steril. 2016;106:4–5. doi: 10.1016/j.fertnstert.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Dumesic D.A., Abbott D.H., Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8:127–141. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodarzi M.O., Dumesic D.A., Chazenbalk G., Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 4.Rotterdam EA-SPCWG Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Baskind N.E., Balen A.H. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80–97. doi: 10.1016/j.bpobgyn.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Liu L., Tong X., Jiang L., Li T.C., Zhou F., Zhang S. A comparison of the miscarriage rate between women with and without polycystic ovarian syndrome undergoing IVF treatment. Eur J Obstet Gynecol Reprod Biol. 2014;176:178–182. doi: 10.1016/j.ejogrb.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 7.Luo L., Gu F., Jie H., Ding C., Zhao Q., Wang Q. Early miscarriage rate in lean polycystic ovary syndrome women after euploid embryo transfer - a matched-pair study. Reprod Biomed Online. 2017;35:576–582. doi: 10.1016/j.rbmo.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Norman R.J., Dewailly D., Legro R.S., Hickey T.E. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 9.Palomba S., Daolio J., La Sala G.B. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol Metab. 2017;28:186–198. doi: 10.1016/j.tem.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Palomba S., Santagni S., Falbo A., La Sala G.B. Complications and challenges associated with polycystic ovary syndrome: current perspectives. Int J Womens Health. 2015;7:745–763. doi: 10.2147/IJWH.S70314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chappell N.R., Blesson C.S. Polycystic ovary syndrome: a multifaceted enigma. Endocrinol Diabetes Open Access. 2018;1:102. [Google Scholar]
- 12.Patel S.S., Carr B.R. Oocyte quality in adult polycystic ovary syndrome. Semin Reprod Med. 2008;26:196–203. doi: 10.1055/s-2008-1042958. [DOI] [PubMed] [Google Scholar]
- 13.Wood J.R., Dumesic D.A., Abbott D.H., Strauss J.F., 3rd Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92:705–713. doi: 10.1210/jc.2006-2123. [DOI] [PubMed] [Google Scholar]
- 14.Kirkegaard K., Agerholm I.E., Ingerslev H.J. Time-lapse monitoring as a tool for clinical embryo assessment. Hum Reprod. 2012;27:1277–1285. doi: 10.1093/humrep/des079. [DOI] [PubMed] [Google Scholar]
- 15.Kaser D.J., Bormann C.L., Missmer S.A., Farland L.V., Ginsburg E.S., Racowsky C. A pilot randomized controlled trial of day 3 single embryo transfer with adjunctive time-lapse selection versus day 5 single embryo transfer with or without adjunctive time-lapse selection. Hum Reprod. 2017;32:1598–1603. doi: 10.1093/humrep/dex231. [DOI] [PubMed] [Google Scholar]
- 16.Racowsky C., Martins W.P. Effectiveness and safety of time-lapse imaging for embryo culture and selection: it is still too early for any conclusions? Fertil Steril. 2017;108:450–452. doi: 10.1016/j.fertnstert.2017.07.1156. [DOI] [PubMed] [Google Scholar]
- 17.Truong T.T., Soh Y.M., Gardner D.K. Antioxidants improve mouse preimplantation embryo development and viability. Hum Reprod. 2016;31:1445–1454. doi: 10.1093/humrep/dew098. [DOI] [PubMed] [Google Scholar]
- 18.Weinerman R., Feng R., Ord T.S., Schultz R.M., Bartolomei M.S., Coutifaris C. Morphokinetic evaluation of embryo development in a mouse model: functional and molecular correlates. Biol Reprod. 2016;94:84. doi: 10.1095/biolreprod.115.134080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkegaard K., Ahlstrom A., Ingerslev H.J., Hardarson T. Choosing the best embryo by time lapse versus standard morphology. Fertil Steril. 2015;103:323–332. doi: 10.1016/j.fertnstert.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Kirkegaard K., Ingerslev H.J. Clinical outcomes following selection of human preimplantation embryos with time-lapse monitoring: a systematic review. Hum Reprod Update. 2014;20:802. doi: 10.1093/humupd/dmu044. [DOI] [PubMed] [Google Scholar]
- 21.Kirkegaard K., Kesmodel U.S., Hindkjaer J.J., Ingerslev H.J. Time-lapse parameters as predictors of blastocyst development and pregnancy outcome in embryos from good prognosis patients: a prospective cohort study. Hum Reprod. 2013;28:2643–2651. doi: 10.1093/humrep/det300. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y.S., Thouas G.A., Gardner D.K. Developmental kinetics of cleavage stage mouse embryos are related to their subsequent carbohydrate and amino acid utilization at the blastocyst stage. Hum Reprod. 2015;30:543–552. doi: 10.1093/humrep/deu334. [DOI] [PubMed] [Google Scholar]
- 23.Basile N., Vime P., Florensa M., Aparicio Ruiz B., Garcia Velasco J.A., Remohi J. The use of morphokinetics as a predictor of implantation: a multicentric study to define and validate an algorithm for embryo selection. Hum Reprod. 2015;30:276–283. doi: 10.1093/humrep/deu331. [DOI] [PubMed] [Google Scholar]
- 24.Wissing M.L., Bjerge M.R., Olesen A.I., Hoest T., Mikkelsen A.L. Impact of PCOS on early embryo cleavage kinetics. Reprod Biomed Online. 2014;28:508–514. doi: 10.1016/j.rbmo.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Sundvall L., Kirkegaard K., Ingerslev H.J., Knudsen U.B. Unaltered timing of embryo development in women with polycystic ovarian syndrome (PCOS): a time-lapse study. J Assist Reprod Genet. 2015;32:1031–1042. doi: 10.1007/s10815-015-0488-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabibnejad N., Soleimani M., Aflatoonian A. Serum anti-Mullerian hormone and embryo morphokinetics detecting by time-lapse imaging: a comparison between the polycystic ovarian syndrome and tubal factor infertility. Int J Reprod Biomed. 2018;16:483–490. [PMC free article] [PubMed] [Google Scholar]
- 27.Tam Le M., Van Nguyen T., Thanh Nguyen T., Thanh Thi Nguyen T., An Thi Nguyen T., Huy Vu Nguyen Q. Does polycystic ovary syndrome affect morphokinetics or abnormalities in early embryonic development? Eur J Obstet Gynecol Reprod Biol X. 2019;3:100045. doi: 10.1016/j.eurox.2019.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbott D.H., Barnett D.K., Bruns C.M., Dumesic D.A. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11:357–374. doi: 10.1093/humupd/dmi013. [DOI] [PubMed] [Google Scholar]
- 29.Hickey T.E., Marrocco D.L., Amato F., Ritter L.J., Norman R.J., Gilchrist R.B. Androgens augment the mitogenic effects of oocyte-secreted factors and growth differentiation factor 9 on porcine granulosa cells. Biol Reprod. 2005;73:825–832. doi: 10.1095/biolreprod.104.039362. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y., Yu Y., Gao J., Li R., Zhang C., Zhao H. Impaired oocyte quality induced by dehydroepiandrosterone is partially rescued by metformin treatment. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarumi W., Tsukamoto S., Okutsu Y., Takahashi N., Horiuchi T., Itoh M.T. Androstenedione induces abnormalities in morphology and function of developing oocytes, which impairs oocyte meiotic competence. Fertil Steril. 2012;97:469–476. doi: 10.1016/j.fertnstert.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 32.Walters K.A. Role of androgens in normal and pathological ovarian function. Reproduction. 2015;149:R193–R218. doi: 10.1530/REP-14-0517. [DOI] [PubMed] [Google Scholar]
- 33.Yang F., Ruan Y.C., Yang Y.J., Wang K., Liang S.S., Han Y.B. Follicular hyperandrogenism downregulates aromatase in luteinized granulosa cells in polycystic ovary syndrome women. Reproduction. 2015;150:289–296. doi: 10.1530/REP-15-0044. [DOI] [PubMed] [Google Scholar]
- 34.Gill A., Jamnongjit M., Hammes S.R. Androgens promote maturation and signaling in mouse oocytes independent of transcription: a release of inhibition model for mammalian oocyte meiosis. Mol Endocrinol. 2004;18:97–104. doi: 10.1210/me.2003-0326. [DOI] [PubMed] [Google Scholar]
- 35.Hammes S.R. Steroids and oocyte maturation--a new look at an old story. Mol Endocrinol. 2004;18:769–775. doi: 10.1210/me.2003-0317. [DOI] [PubMed] [Google Scholar]
- 36.Hardy K., Robinson F.M., Paraschos T., Wicks R., Franks S., Winston R.M. Normal development and metabolic activity of preimplantation embryos in vitro from patients with polycystic ovaries. Hum Reprod. 1995;10:2125–2135. doi: 10.1093/oxfordjournals.humrep.a136247. [DOI] [PubMed] [Google Scholar]
- 37.Heijnen E.M., Eijkemans M.J., Hughes E.G., Laven J.S., Macklon N.S., Fauser B.C. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:13–21. doi: 10.1093/humupd/dmi036. [DOI] [PubMed] [Google Scholar]
- 38.Cardozo E., Pavone M.E., Hirshfeld-Cytron J.E. Metabolic syndrome and oocyte quality. Trends Endocrinol Metab. 2011;22:103–109. doi: 10.1016/j.tem.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Dumesic D.A., Padmanabhan V., Abbott D.H. Polycystic ovary syndrome and oocyte developmental competence. Obstet Gynecol Surv. 2008;63:39–48. doi: 10.1097/OGX.0b013e31815e85fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sozen B., Can A., Demir N. Cell fate regulation during preimplantation development: a view of adhesion-linked molecular interactions. Dev Biol. 2014;395:73–83. doi: 10.1016/j.ydbio.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 41.Yang M., Li J., An Y., Zhang S. Effects of androgen on immunohistochemical localization of androgen receptor and Connexin 43 in mouse ovary. Tissue Cell. 2015;47:526–532. doi: 10.1016/j.tice.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Lopes I.M., Maganhin C.C., Oliveira-Filho R.M., Simoes R.S., Simoes M.J., Iwata M.C. Histomorphometric analysis and markers of endometrial receptivity embryonic implantation in women with polycystic ovary syndrome during the treatment with progesterone. Reprod Sci. 2014;21:930–938. doi: 10.1177/1933719113519169. [DOI] [PubMed] [Google Scholar]
- 43.Leary C., Leese H.J., Sturmey R.G. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum Reprod. 2015;30:122–132. doi: 10.1093/humrep/deu276. [DOI] [PubMed] [Google Scholar]
- 44.Gardner D.K., Wale P.L., Collins R., Lane M. Glucose consumption of single post-compaction human embryos is predictive of embryo sex and live birth outcome. Hum Reprod. 2011;26:1981–1986. doi: 10.1093/humrep/der143. [DOI] [PubMed] [Google Scholar]
- 45.Chappell N.R., Zhou B., Schutt A.K., Gibbons W.E., Blesson C.S. Prenatal androgen induced lean PCOS impairs mitochondria and mRNA profiles in oocytes. Endocr Connect. 2020;9:261–270. doi: 10.1530/EC-19-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabibnejad N., Sheikhha M.H., Ghasemi N., Fesahat F., Soleimani M., Aflatoonian A. Association between early embryo morphokinetics plus cumulus cell gene expression and assisted reproduction outcomes in polycystic ovary syndrome women. Reprod Biomed Online. 2019;38:139–151. doi: 10.1016/j.rbmo.2018.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.