Abstract
Objectives
To assess ultra-early neuroprognostic significance of GFAP, NF-L, UCH-L1, tau, and S100B concentrations, change trajectory, and combination profile after Out-of-Hospital Cardiac Arrest (OHCA).
Methods
Prospective enrollment of 22 OHCA and 10 control patients at an academic tertiary care center between May 1, 2017 and January 28, 2020. Blood was collected within one hour of return of spontaneous circulation (ROSC) (H0), at hours 6 (H6), 12, 18, 24, and daily or until discharge or death. Biomarker concentrations, multifactor score, and trajectory change were assessed and compared to final neurologic status (good vs poor Cerebral Performance Category; CPC 1–2 vs CPC 3–5, respectively).
Results
10 patients had good and 12 had poor neurologic outcomes. Poor outcome patients had higher biomarker concentrations and combined biomarker scores at early time points. The earliest significant difference between good and poor outcome patients’ serum biomarkers were at H12 for GFAP (good median: 425 pg/mL [IQR:370−630] vs poor: 5954[1712–65,055] pg/mL; p < 0.001), H12 for NF-L (64[41–69] vs 898[348–1990] pg/mL; p < 0.001), H0 for Tau (31[8–51] vs 124[53–238] pg/mL; p = 0.025), H0 for UCH-L1 (898[375–1600] vs 2475[1898–4098] pg/mL; p = 0.008), and H6 for S100B (123[70–290] vs 895[360–1199] pg/mL; p = 0.002). Four biomarker composite scores differed by H12 (78.03[52.03–111.25] vs 749 [198.46–4870.63] pg/mL; p = 0.003). Machine-learning approach also identified that four-marker score trajectory group memberships are in concordance with patient outcome.
Conclusions
Ultra-early serial serum concentrations of neuronal and astroglial biomarkers may be of neuroprognostic significance following OHCA.
Keywords: Out-of-hospital cardiac arrest, Biomarker, Neurological outcome, Cerebral performance category, Neuroprognostication, Hypoxic-ischemic injury
Introduction
Out-of-hospital cardiac arrest (OHCA) remains a major public health challenge, and while survival rates have increased, the threat of profound neurologic disability often overshadows successful return of spontaneous circulation (ROSC). Overall, approximately 10% of OHCA patients survive to hospital discharge,1 and due to neural tissues’ exquisite sensitivity to hypoxic-ischemic brain injury (HIBI),2 a high proportion of survivors sustain neurologic injury ranging from mild cognitive impairment to brain death.3, 4, 5 However, the magnitude of HIBI after cardiac arrest is initially uncertain and is influenced by multiple factors including baseline patient characteristics and adequacy of resuscitative measures.6, 7 Outcome uncertainty is further amplified in OHCA due to incomplete information regarding downtime, initial cardiac rhythm, and handoffs between multiple providers. These sources of uncertainty leave clinicians with imprecise, qualitative estimations regarding patient prognoses, which in turns hampers families’ and clinicians’ decision-making regarding goals of care and resource allocation. Currently there are no sufficiently objective clinical, radiologic, or laboratory tools for early and accurate prognostication of neurologic outcome immediately following ROSC.8, 9, 10
The ideal neuroprognostic tool in the setting of coma from HIBI would have 100% specificity for poor outcome identification, be employable early after ROSC, provide rapid results, and require few resources for implementation. Quantitative serum measurement of neural and astroglial proteins is a promising avenue for outcome prediction following OHCA11 with potential for incorporation into clinical practice. The most-studied biomarkers in the setting of HIBI are neuron specific enolase (NSE), S-100B, and neurofilament light chain (NF-L).12, 13, 14, 15, 16 NSE and S100B have shown the most clinical utility thus far, and, in conjunction with other neuroprognostication modalities, are recommended to be incorporated into neuroprognostication. However, the reliability of these biomarkers has been questioned in the setting of variable therapeutic hypothermia and post-anoxic seizure effects, inter-assay variation, hemolysis-related inaccuracy, undescribed protein kinetics, and unacceptable false positive rates despite serum concentrations greatly exceeding proposed cut-offs.8, 14, 17 Of note, a recent meta-analysis18 examined the prognostic performance of these biomarkers, finding high specificities for poor neurologic outcome (0.97 and 0.99 for S100Ba and NSE, respectively) but lower sensitivities (0.63 and 0.56, respectively). Furthermore, most studies evaluate serum NSE concentrations at time of post-OHCA NSE peak at 24−72 h13, 19, 20 or during the concentration changes leading to this time point,21, 22, 23 limiting conclusions that can be drawn in the immediate post-ROSC period. Thus, biomarker data is recommended for incorporation into multimodal neuroprognostication, but no single biomarker has proven sufficiently reliable to independently predict neurological outcomes after cardiac arrest.
The objective of this study is to demonstrate the feasibility of employing an ultra-early comprehensive panel of neuron- and glia-specific protein biomarkers within one hour of ROSC, and to explore the pharmacodynamics of these biomarkers in the characterization of short- and intermediate-term neurologic outcomes after OHCA. We hypothesized that early and serially measured blood levels of astroglial fibrillary acidic protein (GFAP), NF-L, Ubiquitin C-Terminal Hydrolase L1 (UCH-L1), Tau, and S100B immediately following ROSC has the potential to predict neurologic outcomes at time of discharge and at 6-month follow-up.
Methods
Study design and populations
This pilot study was a prospective, observational study of adult (age ≥18 years) non-traumatic OHCA patients presenting to a tertiary care academic emergency department (ED) via local emergency medical services (EMS) agencies between May 1, 2017 and January 28, 2020. OHCA cohorts consisted of a convenience sample of patients from whom initial blood sample collection was feasible within one hour from ROSC, and who had ROSC sustained for greater than 1 h. Patients who were pregnant, incarcerated, or had advance directives precluding resuscitative measures were excluded. Exclusion criteria also included preexisting medical conditions with significant probability of altering the baseline concentrations or protein kinetics of brain proteins not due to OHCA-induced brain injury. Specifically, patients with neurologic disorders (e.g. Alzheimer’s disease, multiple sclerosis, seizure disorders), brain injuries (e.g. cerebrovascular accident, traumatic brain injury), or brain cancer were excluded. End-stage liver disease (ESLD) and end-stage renal disease (ESRD) patients were also excluded given unpredictable effects of impaired renal or hepatic clearance on baseline or post-cardiac arrest biomarker levels. Using the same inclusion and exclusion criteria, the control cohort consisted of 10 age- and gender-matched ED patients admitted for chest pain observation.
Cardiac arrest resuscitation and subsequent care followed standard practices, including targeted temperature management for eligible patients. Following prehospital or ED ROSC, initial blood samples (H0; 0−59 min) were collected during ED resuscitative measures. Subsequent blood samples were collected at post-arrest hours 6, 12, 18, 24, and five days thereafter, until death, withdrawal of life-sustaining treatment (WLST), or hospital discharge. WLST criteria were multimodal, incorporating CT and/or MRI imaging, continuous EEG monitoring, somatosensory evoked potentials (SSEP), clinical neurological exams, and direction from patient’s surrogate decision-makers. No study or non-study serum protein biomarkers (e.g. NSE) were used for clinical prognostication in our patient sample. Control patients’ blood was drawn following the same time course as cardiac arrest patients.
The study was approved by the local institutional review board (IRB Project #: 201700133 Clinical trial #: NCT03112486). Delayed written informed consent was obtained within 48 h from each patient or the patient’s legally authorized representative (LAR) for healthcare decision-making if the patient did not regain medical decision-making capacity.
Outcome measures
Biomarker analyses were performed by investigators blinded to clinical data. GFAP, NF-L, UCH-L1, and Tau concentrations were measured using the same batch of reagents using a SIMOA neuro 4-plex kit in SR-X immunoassay analyzer (Quanterix Corp, Boston, MA, USA) running ultrasensitive paramagnetic bead-based enzyme-linked immunosorbent assays. Serum S100B concentrations were measured using commercial sandwich ELISA kits (cat# EZHS100B-33K, EMD Millipore, Burlington MA). All assays were conducted according to manufacturer protocols. Lab protocols are included in supplemental materials.
Patients’ neurologic outcome as defined by Cerebral Performance Category (CPC)24 was ascertained in person by research staff at time of hospital discharge or at last neurologic status before WLST or non-WLST death. Neurologic outcome was dichotomized into good (CPC 1–2) and poor (CPC 3, 4, 5) outcomes, consistent with contemporary approaches.1, 8, 25 To confirm CPC accuracy, retrospective chart review was performed by two independent investigators, including a board-certified neurointensivist (CBM) blinded to biomarker data. Re-evaluation of all survivors’ neurologic status was performed at six months via chart review or telephone call.
Statistical analysis
Biomarker values were logarithmically transformed to attain normal distribution. Descriptive statistics (i.e., mean, median, standard deviation, interquartile range) were calculated for continuous variables. Mann–Whitney U and Kruskal–Wallis tests were conducted to assess differences between groups for continuous variables. Frequencies and percentages were determined for categorical variables, and Chi-square, with Fisher’s exact test, was used to determine associations for categorical variables. The accuracy of biomarker levels to differentiate between good and poor outcome was evaluated by the receiver operating characteristic (ROC) analysis. All tests were two tailed, with a significance level set at 0.05 and the analysis was performed in Stata 15.0.
A composite biomarker profile was developed utilizing multiple biomarkers’ deviations from initial value. As GFAP, NF-L, UCH-L1, and Tau represent astroglial injury, axonal injury, neuron cell body injury, and neurodegeneration, respectively, they were used to represent potential major neuropathological pathways. S100B was not included in the combined score as it functions similarly to GFAP as an astroglial biomarker. In order to standardize individual biomarkers’ deviation from their respective initial (H0) concentrations, the serum concentration at a given time point (e.g., H6) was divided by its H0 concentration, then multiplied by 100 to obtain a marker score (Eq. 1). We then generated a composite marker score at each time point from the weighted sum of each markers’ deviations from H0 (Eq. 2). Statistical significance was assessed using Mann–Whitney U.
| Marker score at time point = (marker concentration at time point/marker at concentration at H0) × 100 | (1) |
| Composite score = (marker1 score + marker2 score + … markerN score)/N | (2) |
The predictive utility of early biomarker changes over time was assessed using trajectory analysis. Using machine learning independent of patient outcome data (described in supplemental materials), group membership into low- or high-trajectory progression was ascertained for each patient.26, 27
Results
Study population
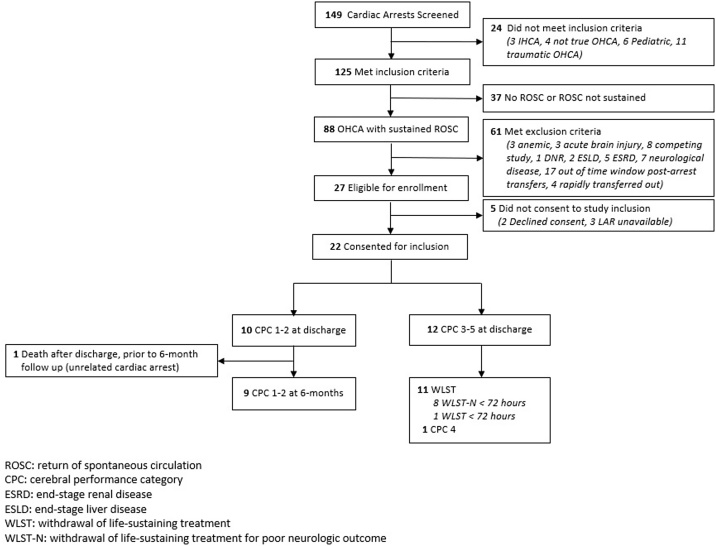
Screening, enrollment, and outcomes are depicted in Fig. 1. A total of 149 patients were screened, and 22 were included in the study. Of these, 10 were discharged with CPC 1 or 2, 1 had discharge CPC of 4, and 11 had WLST. Of the 11 WLST patients, 10 had WLST due to perceived poor neurologic prognosis, and one patient had WLST for non-neurologic poor overall prognosis. The control cohort consisted of 10 non-OHCA chest pain observation patients. Characteristics of OHCA patients and controls are shown in Table 1. Statistically significant differences between good and poor neurologic outcomes were observed in cardiac arrest type, targeted temperature management treatment, and downtime duration.
Fig. 1.
Flow diagram for out-of-hospital cardiac arrest patient enrollment and outcomes.
Abbreviations: CPC: cerebral performance category; ESRD: end-stage renal disease; ESLD: end-stage liver disease; ROSC: return of sppontaneous circulation; WLST: withdrawal of life-sustaining treatment.
Table 1.
Characteristics of control, good-, and poor-neurological outcome patients.
| Characteristics | Control (n = 10) | Poor Outcome (n = 12) | Good Outcome (n = 10) | p-value |
|---|---|---|---|---|
| Age in years, mean (SD) a | 58.3 (18.22) | 64.58 (14.10) | 55.56 (14.37) | 0.62 |
| Male sex, n (%)a | 6 (60.0) | 8 (66.67) | 7 (70.0) | 0.891 |
| Race, n (%)a | ||||
| African American | 2 (20.0) | 2 (16.67) | 1 (10.0) | 0.821 |
| White | 8 (80.0) | 10 (83.33) | 9 (90.0) | |
| Cardiac Arrest Type, n (%) | 0.005 | |||
| Asystole | 5 (41.67) | 0 | ||
| PEA | 5 (41.67) | 2 (20.0) | ||
| VF/VT | 2 (16.67) | 8 (80.0) | ||
| Witnessed, Yes, n (%) | 10 (83.33) | 9 (90.0) | 0.65 | |
| TTM, Yes, n (%) | 8(90.0) | 8 (100.0) | 0.598 | |
| Downtime (minutes), mean (SD) | 42.54 (25.37) | 21.00 (12.39) | 0.025 |
Note: p-values are calculated from Chi-square/Fishers test for categorical variables and the student-t or ANOVA test for continuous variables.
Comparing across control vs. poor vs. good outcomes.
Individual biomarker relation to neurologic outcome
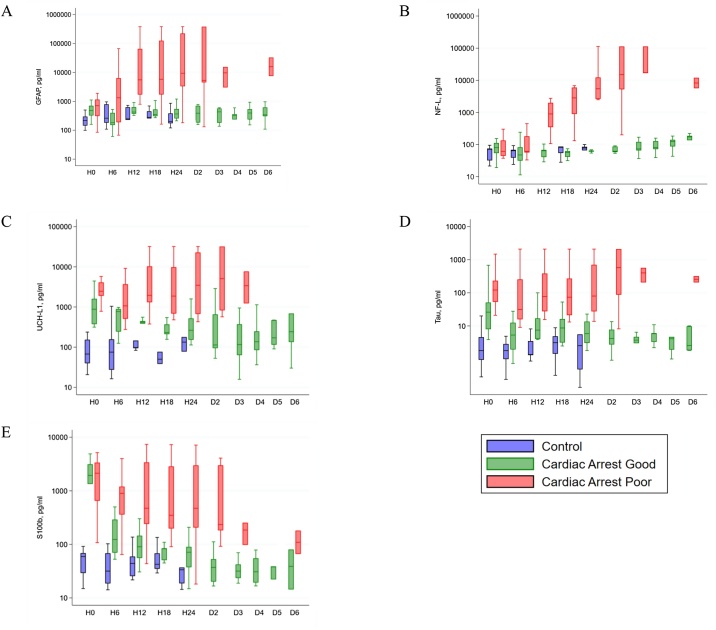
Log-scale median and interquartile range serum concentrations of (a) GFAP, (b) NF-L, (c) UCH-L1, (d) Tau, and (e) S100B over time in control, good outcome, and poor outcome patients are depicted in Fig. 2(a)–(e). Control patients’ serum biomarker concentrations remained at baseline throughout hospitalization for all biomarkers. The earliest significant difference between good and poor outcome patients’ serum biomarkers were at H12 for GFAP (good: median 425 pg/mL, IQR 370–630; poor: median 5954 pg/mL, IQR 1712–65,055 pg/ml; p < 0.001), H12 for NF-L (good: median 64 pg/mL, IQR 41–69; poor: median 898 pg/mL, IQR 348–1990 pg/ml; p < 0.001), H0 for Tau (good: median 31 pg/mL, IQR 8–51; poor: median 124 pg/mL, IQR 53–238 pg/ml; p = 0.025), H0 for UCH-L1 (good: median 898 pg/mL, IQR 375–1600; poor: median 2475 pg/mL, IQR 1898–4098 pg/ml; p = 0.008), and H6 for S100B (good: median 123 pg/mL, IQR 70–290; poor: median 895 pg/mL, IQR 360–1199 pg/ml; p = 0.002). Statistically significant differences persisted until D3 for all biomarkers. See Supplemental Table 1 for full data set.
Fig. 2.
Log-scale biomarker concentrations over time in control, good neurological outcome, and poor neurological outcome patients. Shown are median (line), upper and lower quartiles (box) and range (whiskers).
ROC curves for all biomarkers during time points H0 through D3 are depicted in Supplemental Fig. 1. To maximize utility in detection of poor neurologic outcome, cutoff values (pg/mL) were derived corresponding to 100% specificity for poor neurologic outcome. The earliest cutoff for each was GFAP 1513 pg/mL (H12; sens: 90%), NF-L 173.7 pg/mL (H12; sens: 90%), Tau 1479 pg/mL (H0; sens 8.33%), UCH-L1 4670 pg/mL (H0; sens 16.67), and S100B 509.37 pg/mL (H6; sens 72.73). Simultaneous 100% sensitivity and specificity were reached on D3 for GFAP (3019 pg/mL), NF-L (16,859 pg/mL), UCH-L1 (1228 pg/mL), and S100B (97.69). See Table 2 for full result set.
Table 2.
ROC-derived cutoffs for 100 percent sensitivity and specificity of poor neurologic outcome. Biomarker and 4 marker concentration cutoff values for 100% specificity for poor neurological outcome were derived. 100% specificity for poor outcome was reached as early as 6 h for Tau and S100B, and at 12 h for GFAP, NF-L, UCH-L1. Furthermore, serum concentrations cutoffs with both 100% sensitivity as well as specificity for poor neurological outcomes were also noted for each biomarker.
| GFAP |
NFL |
Tau |
UCH-L1 |
S100B |
4-Marker |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time point | Cutoff (pg/mL) | Sens (%) | Spec (%) | Cutoff (pg/mL) | Sens (%) | Spec (%) | Cutoff (pg/mL) | Sens (%) | Spec (%) | Cutoff (pg/mL) | Sens (%) | Spec (%) | Cutoff (pg/mL) | Sens (%) | Spec (%) | Cutoff (pg/mL) | Sens (%) | Spec (%) |
| H0 | – | – | – | – | – | – | 1479 | 8.33 | 100 | 4670 | 16.67 | 100 | – | – | – | – | – | – |
| H6 | – | – | – | – | – | – | 31.6 | 54.55 | 100 | 1031 | 63.64 | 100 | 509.37 | 72.73 | 100 | 141.3 | 45.45 | 100 |
| H12 | 1513 | 90 | 100 | 173.7 | 90 | 100 | 148.1 | 50 | 100 | 1312 | 80 | 100 | 396.97 | 70 | 100 | 569.7 | 70 | 100 |
| H18 | 1226 | 90.91 | 100 | 200.2 | 90.91 | 100 | 74.09 | 54.55 | 100 | 684.2 | 81.82 | 100 | 276.1 | 66.67 | 100 | 122.4 | 90.91 | 100 |
| H24 | 1500 | 91.67 | 100 | 2787 | 75 | 100 | 33.71 | 75 | 100 | 2488 | 66.67 | 100 | 239.9 | 75 | 100 | 605.73 | 91.67 | 100 |
| D2 | 4501 | 85.71 | 100 | 5237 | 85.71 | 100 | 87.75 | 85.71 | 100 | 3214 | 71.43 | 100 | 182.5 | 83.33 | 100 | 1438 | 85.71 | 100 |
| D3 | 3019 | 100 | 100 | 16,859 | 100 | 100 | 399.1 | 33.33 | 100 | 1228 | 100 | 100 | 97.69 | 100 | 100 | 3294 | 100 | 100 |
Composite biomarker score relation to neurologic outcome
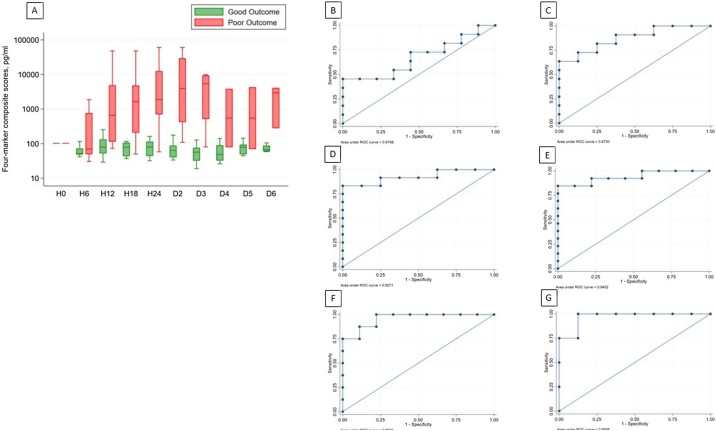
Fig. 3(a) depicts log-scale 4-marker (GFAP, NF-L, UCH-L1, Tau) standardized composite scores over time for good and poor outcome patients. For good outcome patients, the weighted composite score remained slightly negative due to down trending UCH-L1 and Tau. In contrast, the four-marker composite score for poor outcome patients rose sharply upward, reaching statistically significant difference at H12 (good: median 78.03 pg/mL, IQR 52.03–111.25; poor: median 749 pg/mL, IQR 198.46–4870.63 pg/ml; p = 0.003) and continuing to D3. ROC curves for 4-marker composite scores at 6, 12, 18, and 24 h are shown in Fig. 3(b)–(g).
Fig. 3.
Four-marker composite score differentiates neurological outcome. (a) Log-scale four-marker composite scores over time are depicted as median (line), upper and lower quartiles (box) and range (whiskers). Statistically significant difference is reached at H12 (p = 0.003; Mann-Whitney). 4 factor ROC curves for H6, H12, H18, H24, D2, and D3 are depicted in (b)–(g), respectively.
Trajectory analysis of neurologic outcome
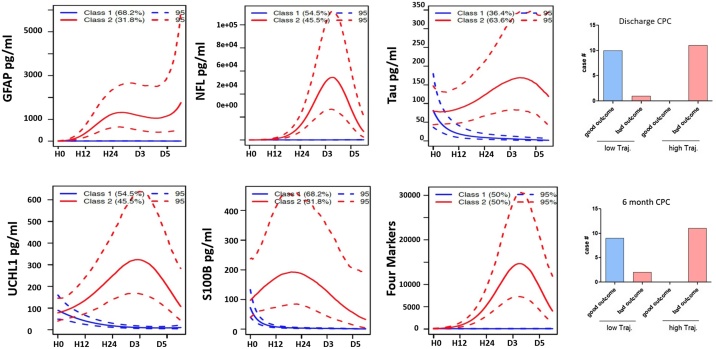
Based on machine-learning, two groups with the best fit membership for low trajectory (Class 1) and high trajectory (Class 2) marker score data were developed. Fig. 4(a)–(f) depicts low and high trajectory groups for 5 individual biomarkers and the 4-marker composite score. The percent membership for high trajectory class range from 38.9% for GFAP score to 55.6% for the four-marker score. Concordance of four-marker score trajectory group membership in independently predicting patient outcome is shown in Fig. 4(g), (h). Excluding a separate fatal cardiac arrest, identical results were obtained at 6 months. The odds ratio for poor outcome being in the composite low trajectory class is 0.0525 (95% CI: 0.0029 to 0.9719; z statistics 1.979, p = 0.0478). The two classes’ respective biomarker median and interquartile range concentrations over time are shown in Supplemental Fig. 2(a)–(e). Earliest significant class differences in biomarkers levels are shown at H0 for Tau and UCH-L1, H6 for GFAP and S100B, and H12 for NFL.
Fig. 4.
Outcome prediction derivation through trajectory analysis. Individual patients’ biomarker trajectories were independently sorted for best fit into low- or high-trajectory groups (Class 1 and Class 2, respectively; figures (a)–(e)). Serum biomarker levels for these groups are depicted (mean and 95% confidence interval depicted by solid line and dotted line, respectively). Composite score based on four biomarker trajectory assortment is depicted in (f) (mean and 95% confidence interval). Figures (g) and (h) demonstrate concordance between trajectory-derived classification and neurologic outcome.
Legend: Solid line: mean; dotted line: 95% confidence interval.
Discussion
In this pilot study of biomarker pharmacodynamics in predicting neurological outcome after OHCA, our data support our hypothesis that ultra-early and serial measurement of serum biomarkers of brain injury is both feasible and of neuroprognostic significance.
In individual biomarkers’ serial measurement, two patterns became apparent. In OHCA patients with good neurologic outcome, GFAP and NF-L remained at baseline, whereas poor outcome patients’ GFAP and NF-L levels rose over several orders of magnitude to become significantly different by H12. This suggests early elevations in GFAP and NF-L portend poor prognosis (death or functional dependence), while unchanging concentrations suggest good prognosis (independent state). In contrast, both good and poor outcome patients’ UCH-L1, Tau, and S100B concentrations became immediately elevated (H0; 0−59 min) relative to control baseline regardless of ultimate degree of injury. However, good outcome patients’ UCH-L1, Tau, and S100B levels trended downward shortly thereafter, while biomarker levels in the poor outcome group remained elevated. This divergence resulted in statistically significant difference between good and poor outcome groups at H0 for Tau and UCH-L1, and H6 in S100B (p < 0.05). Thus, for these biomarkers a downward trend suggested good outcome, while stable or further increasing levels forecast poor prognosis and may reflect ongoing secondary brain injury.
Though individual biomarkers reached thresholds for 100% specificity for poor neurologic outcome as early as 6−12 h (Tau and UCH-L1), time to concurrent maximal (100%) sensitivity and specificity was delayed until D3. Furthermore, the clinical utility of individual biomarker levels was constrained by outliers as demonstrated by substantial within-biomarker variability and range overlap between good and poor outcome patients. The combination of multiple biomarker derangements into a single, objective score mitigated this variability, and allowed the summation of various biomarkers to provide separation of good from poor outcome. One notable deviation from the overall pattern of biomarker trajectory was in the case of a subject in the poor outcome group with relatively low absolute biomarker concentrations and low trajectory changes, and yet ultimately had WLST for non-neurological medical futility. It is notable that with the exception of this patient, the multiple marker prediction model reached 100% sensitivity for poor neurological outcome at H6, the earliest sample after baseline, and all patients in the low-trajectory group had good neurologic outcome, while all high-trajectory patients had poor outcomes. This will be further discussed in the study limitations.
Our hypothesis that serial serum concentrations of neuronal and astroglial proteins could be used to prognosticate neurologic outcome was further tested using trajectory analyses and machine learning. Independent of information regarding a given patient’s outcome, trajectory analysis sorted patient biomarker data into two distinct classes, i.e. low and high trajectory groups, with high concordance between trajectory group and neurologic outcome.
Neuroprognostic uncertainty after OHCA may lead counterintuitively to either overly aggressive care despite medical futility or inappropriate WLST despite possible meaningful recovery. Clinicians may become discouraged by early subjective indications of poor neurologic outcome, and patients’ surrogate decision-makers choose early WLST in the belief that the patient would not want prolonged dependence upon life support in a neurologically devastated condition. Furthermore, despite guidelines and studies that suggest that delayed awakening (>72 h from ROSC) is not uncommon,25, 28, 29 it is estimated that in the United States alone, 1500 patients per year who would have survived with a favorable neurological outcome die due to inappropriate early WLST.30 Early, objective, quantifiable, and reliable outcome prediction tools are thus of paramount importance for patients’ post-OHCA surrogate decision-makers. Serial measurement and combined analyses of biomarkers of brain injury are promising avenues in neuroprognostication. Future large-scale studies with undifferentiated patient populations are needed to validate this approach.
Limitations
The primary limitation of this single-center pilot study was the relatively limited number of patients which represented a convenience sample for whom inclusion consent could be obtained.
Additionally, the study design excluded patients whose medical conditions might result in abnormal baseline biomarker concentrations or post-OHCA kinetics. For example, as hyperphosphorylated Tau is implicated in pathophysiology of Alzheimer’s disease, OHCA survivors with good neurologic outcomes and coincident Alzheimer’s disease might have elevated serum Tau levels relative to non-Alzheimer’s patients with poor outcomes.31 Similarly, ESLD and ESRD patients were also excluded in order to limit any differential effects of these diseases on protein kinetics. These exclusions raise the possibility of selection bias in the enrollment process.
Also as previously noted, one subject in the poor outcome group with morbid obesity (176 kg, BMI > 50) had a biomarker profile of relatively low concentrations and low change trajectories, and yet ultimately had WLST for non-neurological medical futility. This represented a marked deviation from the rest of the data. Whether this patient’s data represent inaccurate prehospital OHCA diagnosis due to morbid obesity, altered biomarker kinetics, lipid-related measurement error, unrecognized favorable neurologic status, or some other covariant is not fully explained in our data.
Other limitations arise from WLST from 11 of 12 poor outcome patients, 8 of whom had WLST before 72 h. Of the 8 subjects who had WLST before 72 h, 7 of 8 were for perceived poor neurologic prognoses. While this was uniformly in observance LAR-directed patient preferences, WLST effectively limited the sample size of patients with poor outcomes beyond the 72-h timepoint for neuroprognostication suggested by guidelines.32, 33 It is not possible that serum biomarker concentrations directly influenced decisions regarding care limitation because clinicians were blinded to their results. However, an anticipated poor outcome can introduce a “self-fulfilling prophecy” bias in which an expected poor outcome leads to care limitations and death, and thus inclusion in the poor outcomes group.8, 34 It is possible that biomarker levels correlated with other clinically used but imperfect predictors of poor outcome, and hence could fall prey to the same potential bias.8
Conclusion
The findings of this exploratory study suggest that ultra-early serial measurement of serum brain-specific proteins after OHCA may provide early, reliable, and objective information useful in neuroprognostication. Clinical utility may be enhanced through simultaneous additive evaluation multiple biomarkers. Future studies are anticipated and will need to include larger multicentered cohorts, undifferentiated patient populations, and longer-duration evaluations in order to validate these findings and account for variations in chronic disease, co-morbid conditions, and other confounding variables.
Ethics Information
The study was approved by the local institutional review board (IRB Project #: 201700133 Clinical trial #: NCT03112486). Delayed written informed consent was obtained within 48 hours from each patient or the patient’s legally authorized representative (LAR) for healthcare decision-making if the patient did not regain medical decision-making capacity.
Acknowledgment/Funding Sources/Disclosures
-
•
Internal funding was provided by the University of Florida Department of Emergency Medicine.
-
•
KW is a stockholder of Gryphon Bio Inc. and Banyan Biomarkers, Inc. that produce products relevant to the subject material.
-
•
YOE, KH, JAT, and KW are the inventors on a patent that is issued by the United States Patent and Trademark Office relevant to the material in this paper.
-
•
ZY, MC, SG, CBM, ME, TB, SC, AH, CM, TZ report no conflict of interest.
This data has not been published previously and is not under consideration elsewhere.
CRediT authorship contribution statement
Karl W. Huesgen: Conceptualization, Methodology, Investigation, Writing - original draft, Writing - review & editing, Visualization, Project administration, Supervision. Yasmeen O. Elmelige: Investigation, Data curation, Supervision, Writing - review & editing, Visualization. Zhihui Yang: Investigation, Formal analysis, Resources, Data curation, Visualization. Muhammad Abdul Baker Chowdhury: Formal analysis, Visualization. Sarah Gul: Investigation, Data curation. Carolina B. Maciel: Validation, Writing - review & editing. Marie-Carmelle Elie-Turenne: Methodology, Writing - review & editing. Torben K. Becker: Methodology, Writing - review & editing. Scott A. Cohen: Methodology, Formal analysis. Amy Holland: Investigation, Project administration. Cindy Montero: Data curation, Project administration. Tian Zhu: Investigation. Kevin K. Wang: Conceptualization, Methodology, Investigation, Resources, Writing - review & editing, Supervision, Funding acquisition. Joseph A. Tyndall: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision, Funding acquisition.
Footnotes
Prior Presentations: This work was presented at the Society for Academic Emergency Medicine Annual Meeting, Las Vegas, NV, May 16, 2019 and Society of Critical Care Medicine Annual Meeting, Orlando, FL, February 16, 2020.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.resplu.2021.100133.
Contributor Information
Karl W. Huesgen, Email: karlhuesgen@ufl.edu.
Yasmeen O. Elmelige, Email: yelmelige@ufl.edu.
Zhihui Yang, Email: zhihuiyang@ufl.edu.
Muhammad Abdul Baker Chowdhury, Email: chowdhurym@ufl.edu.
Sarah Gul, Email: sarah.gul@neurosurgery.ufl.edu.
Carolina B. Maciel, Email: carolina.maciel@neurology.ufl.edu.
Marie-Carmelle Elie-Turenne, Email: elie@ufl.edu.
Torben K. Becker, Email: t.becker@ufl.edu.
Scott A. Cohen, Email: scohen211@ufl.edu.
Amy Holland, Email: amy.holland@ufl.edu.
Cindy Montero, Email: cmont329@ufl.edu.
Tian Zhu, Email: tangtang2299@hotmail.com.
Kevin K. Wang, Email: kwang@ufl.edu.
Joseph A. Tyndall, Email: tyndall@ufl.edu.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Virani S.S., Alonso A., Benjamin E.J. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020:E139–E596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Reis C., Akyol O., Araujo C. Pathophysiology and the monitoring methods for cardiac arrest associated brain injury. Int J Mol Sci. 2017;18(1):129. doi: 10.3390/ijms18010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moulaert V.R.M.P., Verbunt J.A., van Heugten C.M., Wade D.T. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation. 2009;80(3):297–305. doi: 10.1016/j.resuscitation.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Buanes E.A., Gramstad A., Søvig K.K. Cognitive function and health-related quality of life four years after cardiac arrest. Resuscitation. 2015;89:13–18. doi: 10.1016/j.resuscitation.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Steinbusch C.V.M., van Heugten C.M., Rasquin S.M.C., Verbunt J.A., Moulaert V.R.M. Cognitive impairments and subjective cognitive complaints after survival of cardiac arrest: a prospective longitudinal cohort study. Resuscitation. 2017;120:132–137. doi: 10.1016/j.resuscitation.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Sasson C., Rogers M.A.M., Dahl J., Kellermann A.L. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circulation: Cardiovasc Qual Outcomes. 2010;3(1):63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- 7.Kempton H., Vlok R., Thang C., Melhuish T., White L. Standard dose epinephrine versus placebo in out of hospital cardiac arrest: a systematic review and meta-analysis. Am J Emerg Med. 2019;37(3):511–517. doi: 10.1016/j.ajem.2018.12.055. [DOI] [PubMed] [Google Scholar]
- 8.Zhou S.E., Maciel C.B., Ormseth C.H., Beekman R., Gilmore E.J., Greer D.M. Distinct predictive values of current neuroprognostic guidelines in post-cardiac arrest patients. Resuscitation. 2019;139:343–350. doi: 10.1016/j.resuscitation.2019.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong J.Y., Lee D.H., Oh J.H. Grey–white matter ratio measured using early unenhanced brain computed tomography shows no correlation with neurological outcomes in patients undergoing targeted temperature management after cardiac arrest. Resuscitation. 2019;140:161–169. doi: 10.1016/j.resuscitation.2019.03.039. [DOI] [PubMed] [Google Scholar]
- 10.Lee B.K., Kim W.Y., Shin J. Prognostic value of gray matter to white matter ratio in hypoxic and non-hypoxic cardiac arrest with non-cardiac etiology. Am J Emerg Med. 2016;34(8):1583–1588. doi: 10.1016/j.ajem.2016.05.063. [DOI] [PubMed] [Google Scholar]
- 11.Gul S.S., Huesgen K.W., Wang K.K., Mark K., Tyndall J.A. Prognostic utility of neuroinjury biomarkers in post out-of-hospital cardiac arrest (OHCA) patient management. Med Hypotheses. 2017;105:34–47. doi: 10.1016/j.mehy.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Stammet P., Wagner D.R., Gilson G., Devaux Y. Modeling serum level of s100β and bispectral index to predict outcome after cardiac arrest. J Am College Cardiol. 2013;62(9):851–858. doi: 10.1016/j.jacc.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 13.Stammet P., Collignon O., Hassager C. Neuron-specific enolase as a predictor of death or poor neurological outcome after out-of-hospital cardiac arrest and targeted temperature management at 33 C and 36 C. J Am College Cardiol. 2015;65(19):2104–2114. doi: 10.1016/j.jacc.2015.03.538. [DOI] [PubMed] [Google Scholar]
- 14.Moseby-Knappe M., Mattsson N., Nielsen N. Serum neurofilament light chain for prognosis of outcome after cardiac arrest. JAMA Neurol. 2019;76(1):64–71. doi: 10.1001/jamaneurol.2018.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wihersaari L., Ashton N.J., Reinikainen M. Neurofilament light as an outcome predictor after cardiac arrest: a post hoc analysis of the COMACARE trial. Intensive Care Med. 2020:1–10. doi: 10.1007/s00134-020-06218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebner F., Moseby-Knappe M., Mattsson-Carlgren N. Serum GFAP and UCH-L1 for the prediction of neurological outcome in comatose cardiac arrest patients. Resuscitation. 2020 doi: 10.1016/j.resuscitation.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Cronberg T., Brizzi M., Liedholm L.J. Neurological prognostication after cardiac arrest—recommendations from the Swedish Resuscitation Council. Resuscitation. 2013;84(7):867–872. doi: 10.1016/j.resuscitation.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Wang C.-H., Chang W.-T., Su K.-I. Neuroprognostic accuracy of blood biomarkers for post-cardiac arrest patients: A systematic review and meta-analysis. Resuscitation. 2020;148:108–117. doi: 10.1016/j.resuscitation.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Streitberger K.J., Leithner C., Wattenberg M. Neuron-specific enolase predicts poor outcome after cardiac arrest and targeted temperature management: a multicenter study on 1,053 patients. Crit Care Med. 2017;45(7):1145–1151. doi: 10.1097/CCM.0000000000002335. [DOI] [PubMed] [Google Scholar]
- 20.Luescher T., Mueller J., Isenschmid C. Neuron-specific enolase (NSE) improves clinical risk scores for prediction of neurological outcome and death in cardiac arrest patients: Results from a prospective trial. Resuscitation. 2019;142:50–60. doi: 10.1016/j.resuscitation.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Martínez-Losas P., de Sá E.L., Armada E. Neuron-specific enolase kinetics: an additional tool for neurological prognostication after cardiac arrest. Rev Esp Cardiol (Engl Ed). 2020;73(2):123–130. doi: 10.1016/j.rec.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Gillick K., Rooney K. Serial NSE measurement identifies non-survivors following out of hospital cardiac arrest. Resuscitation. 2018;128:24–30. doi: 10.1016/j.resuscitation.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Wiberg S., Hassager C., Stammet P. Single versus serial measurements of neuron-specific enolase and prediction of poor neurological outcome in persistently unconscious patients after out-of-hospital cardiac arrest–a TTM-trial substudy. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0168894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stiell I.G., Nesbitt L.P., Nichol G. Comparison of the Cerebral Performance Category score and the Health Utilities Index for survivors of cardiac arrest. Ann Emerg Med. 2009;53(2):241–248. doi: 10.1016/j.annemergmed.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Dragancea I., Wise M.P., Al-Subaie N. Protocol-driven neurological prognostication and withdrawal of life-sustaining therapy after cardiac arrest and targeted temperature management. Resuscitation. 2017;117:50–57. doi: 10.1016/j.resuscitation.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Nagin D.S., Jones B.L., Passos V.L., Tremblay R.E. Group-based multi-trajectory modeling. Stat Methods Med Res. 2018;27(7):2015–2023. doi: 10.1177/0962280216673085. [DOI] [PubMed] [Google Scholar]
- 27.Berger R.P., Bazaco M.C., Wagner A.K., Kochanek P.M., Fabio A. Trajectory analysis of serum biomarker concentrations facilitates outcome prediction after pediatric traumatic and hypoxemic brain injury. Dev Neurosci. 2010;32(5-6):396–405. doi: 10.1159/000316803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rey A., Rossetti A.O., Miroz J.-P., Eckert P., Oddo M. Late awakening in survivors of postanoxic coma: early neurophysiologic predictors and association with ICU and long-term neurologic recovery. Crit Care Med. 2019;47(1):85–92. doi: 10.1097/CCM.0000000000003470. [DOI] [PubMed] [Google Scholar]
- 29.Paul M., Bougouin W., Geri G. Delayed awakening after cardiac arrest: prevalence and risk factors in the Parisian registry. Intensive Care Med. 2016;42(7):1128–1136. doi: 10.1007/s00134-016-4349-9. [DOI] [PubMed] [Google Scholar]
- 30.Elmer J., Torres C., Aufderheide T.P. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127–135. doi: 10.1016/j.resuscitation.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattsson N., Cullen N.C., Andreasson U., Zetterberg H., Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76(7):791–799. doi: 10.1001/jamaneurol.2019.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callaway C.W., Donnino M.W., Fink E.L. Part 8: post–cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18_suppl_2):S465–S482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandroni C., Cariou A., Cavallaro F. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1816–1831. doi: 10.1007/s00134-014-3470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coppler P.J., Elmer J., Calderon L. Validation of the Pittsburgh Cardiac Arrest Category illness severity score. Resuscitation. 2015;89:86–92. doi: 10.1016/j.resuscitation.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.